Abstract
Background & Aims
Nonalcoholic Fatty Liver Disease (NAFLD) is an epidemic. Identifying modifiable risk factors for NAFLD development is essential to design effective prevention programs. We tested whether 25-year patterns of body mass index (BMI) change are associated with midlife NAFLD.
Methods
4423 participants from CARDIA, a prospective population-based biracial cohort (age 18–30), underwent BMI measurement at baseline (1985–1986) and three or more times over 25 years. At Year 25, 3115 had liver fat assessed by noncontrast CT. NAFLD was defined as liver attenuation ≤40 Hounsfield Units after exclusions. Latent mixture modeling identified 25-year trajectories in BMI percent change (%Δ) from baseline.
Results
We identified 4 distinct trajectories of BMI%Δ: stable (26.2% of cohort, 25-year BMI %Δ = 3.1%), moderate increase (46.0%, BMI%Δ = 21.7%), high increase (20.9%, BMI%Δ = 41.9%), and extreme increase (6.9%, BMI%Δ = 65.9%). Y25 NAFLD prevalence was higher in groups with greater BMI%Δ: 4.1%, 9.3%, 13.0%, and 17.6%, respectively (P-trend <0.0001). In multivariable analyses, participants with increasing BMI%Δ had increasingly greater odds of NAFLD compared to the stable group: OR: 3.35 (95% CI, 2.07–5.42), 7.80 (4.60–13.23), and 12.68 (6.68–24.09) for moderate, high and extreme BMI increase, respectively. Associations were only moderately attenuated when adjusted for baseline or Y25 BMI.
Conclusions
Trajectories of weight gain during young adulthood are associated with greater NAFLD prevalence in midlife independent of metabolic covariates and baseline or concurrent BMI highlighting the importance of weight maintenance throughout adulthood as a target for primary NAFLD prevention.
Keywords: obesity, NAFLD, NASH, prevention
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in both children and adults, affecting approximately 30% of the population.1,2 NAFLD represents a spectrum of liver conditions that are associated with differential risk for the development of cirrhosis,3 liver cancer,4 need for liver transplantation5 and cardiovascular disease.6 In addition, mortality is up to 69% higher among persons with NAFLD compared to persons without NAFLD.7 Thus, improving our understanding of how to prevent NAFLD has the potential to save millions of lives worldwide.
NAFLD is the hepatic manifestation of the metabolic syndrome, and being overweight or obese is associated with greater odds of having NAFLD.3,8 Weight gain throughout adulthood, as opposed to weight at a single time point, has been reported to be a risk factor for various lifestyle-related diseases including diabetes,9 and cardiovascular diseases.10–12 However, little is known about objective long-term weight patterns and NAFLD prevalence, especially among a young, contemporary and racially diverse population.13,14 It remains unclear whether long-term patterns of weight gain through younger adulthood, regardless of concurrent body mass index (BMI) or weight category (e.g., normal weight, overweight or obese), adversely influence NAFLD prevalence in middle age. Recognition of patterns of weight change over time may allow earlier identification of patients who are at risk for developing NAFLD and optimize strategies aimed at primary NAFLD prevention.
We therefore sought to examine the association of BMI trajectories (e.g., patterns of change over time) among young adults (age 18–30 years) over 25 years with NAFLD defined by computed tomography (CT) in middle age (43–55 years). We hypothesized that multiple different trajectories of BMI change exist throughout adulthood, and that those groups with greater BMI increase throughout adulthood would have a higher prevalence of NAFLD in middle age, independent of baseline or concurrent BMI.
METHODS
Study Sample
The Coronary Artery Risk Development in Young Adults (CARDIA) study is an ongoing longitudinal cohort study of 5,115 biracial men and women from four metropolitan populations. Participants were 18–30 years of age at enrollment (1985–1986, exam year 0). Recruitment was balanced within each center by sex, age, race, and education. Participants have been followed at 9 examinations for more than 30 years with collection of detailed clinical data, including non-contrast CT measurement of liver fat at year 25 (2010–2011). Retention rates among survivors for the in-person examinations have been high (Y2, 90%; Y5, 86%; Y7, 81%; Y10, 77%; Y15, 74%; Y20, 72%; Y25, 72%; Y30, 71%) and > 90% of initial participants have maintained contact over time.15 Participants provided written informed consent at each examination, and institutional review boards from each field center (University of Alabama at Birmingham, Birmingham, Alabama; Northwestern University, Chicago, Illinois; University of Minnesota, Minneapolis, Minnesota; and Kaiser Permanente, Oakland, California) approved the study annually.
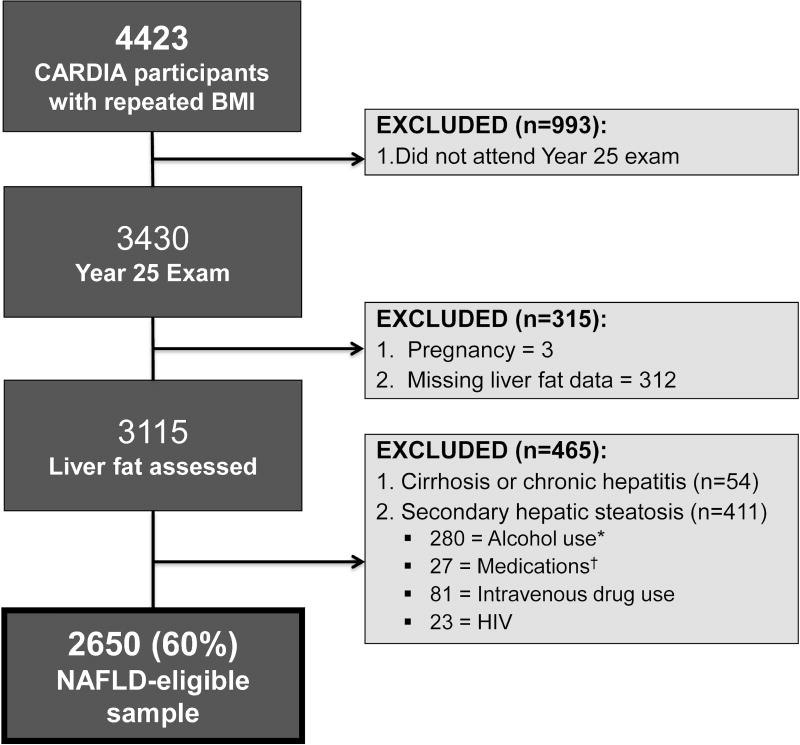
Trajectories of percent change (%Δ) in BMI relative to baseline were modeled among all 4423 participants with BMI measured at baseline and at 3 or more follow-up examinations. BMI values at examinations at which the participant was pregnant (n=266) were excluded. Of the 3430 participants with repeat BMI measures at Y25, 3115 had liver fat assessed. We excluded those with self-reported cirrhosis or viral hepatitis (n=54), risk factors for chronic liver disease (e.g. intravenous drug use, n=81) or causes of secondary hepatic steatosis: alcohol consumption ≥ 7 drinks/week in women and ≥ 14 drinks/week in men (n=280),16 human immunodeficiency virus (n=23), and medications known to cause hepatic steatosis (e.g. valproic acid, methotrexate, tamoxifen, steroids, amiodarone) (n=27). The remaining 2650 formed the NAFLD-eligible sample population (Figure 1).
Figure 1.
Study population. Abbreviations: CT, computed tomography; HIV, human immunodeficiency virus. *Alcohol use was defined as ≥ 7 drinks/week in women, ≥ 14 drinks/week in men. †Medications = valproic acid, methotrexate, tamoxifen, steroids and amiodarone.
Measurements
Standardized protocols for data collection were used across study centers and have previously been described.15,17 Weight and height were measured with participants wearing light clothes and no shoes at each of the 8 examinations. Body weight was measured to the nearest 0.2 kg with a calibrated balance-beam scale. Height was measured with a vertical ruler to the nearest 0.5 cm. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured in duplicate to the nearest 0.5 cm around the minimal abdominal girth identified laterally midway between the iliac crest and the lowest portion of the rib cage and anteriorly midway between the xiphoid process and the umbilicus parallel to the floor. Hip circumference was measured in duplicate to the nearest 0.5cm at the level of the pubis symphysis anteriorly and posteriorly at the level of the maximal protrusion of the gluteal muscles. Participants were asked to fast for 12 hours and to avoid smoking and heavy physical activity for 2 hours before each examination. Overweight was defined as BMI 25–29.9 kg/m2, class I obesity as BMI 30–34.9 kg/m2, class II as BMI 35–39.9 kg/m2 and class III obesity as BMI ≥ 40 kg/m2. The metabolic syndrome was defined according to Adult Treatment Panel III criteria.18 To quantify physical activity (reported as exercise units (EU)), the CARDIA physical activity history questionnaire was used, which was an interviewer-based self-report of duration and intensity of participation in 13 categories of exercise over the previous 12 months.17 As a reference, 300 EU approximates 150 minutes of moderate-intensity activity per week or 30 minutes of moderate-intensity activity five days per week.17 All CARDIA protocols are publicly available at: http://www.cardia.dopm.uab.edu.
The CT protocol included the heart and abdomen using a non-contrast CT scan performed using GE (GE 750HD 64 and GE LightSpeed VCT 64 Birmingham and Oakland Centers, respectively; GE Healthcare, Waukesha, WI) or Siemens (Sensation 64, Chicago and Minneapolis Centers; Siemens Medical Solutions, Erlangen, Germany) multi-detector CT scanners and has been described previously.19 Quality control and image analysis was performed at a core reading center (Wake Forest University Health Sciences, Winston-Salem, NC).
The primary outcome was NAFLD at Y25 defined as liver attenuation (LA) ≤ 40 Hounsfield Units (equivalent to moderate-severe fat)20 after exclusion of other causes of liver fat (Figure 1).19 LA was measured in the right lobe of the liver and was reported as the average of nine measurements on three CT slices using circular regions of interest of 2.6 cm2. The intraclass correlation coefficient between different readers on a random selected sample of 156 participants was 0.975 for LA, indicating high reproducibility of CT measured LA.
Statistical Analysis
Trajectories in BMI, waist circumference (WC) and waist-to-hip ratio (WHR) %Δ were modeled among all participants (N=4423) using data from each examination attended. The %Δ was modeled rather than absolute values of increase since %Δ more appropriately represents baseline and relative change values. We used latent class models to identify subgroups that share a similar trajectory in BMI, WC or WHR %Δ.21 The optimal number of trajectory classes was determined using the Bayesian information criterion such that no group included less than 5% of the population. To estimate the association of trajectory group with prevalent NAFLD, trajectory group membership was included as an independent variable in a logistic regression model examining predictors of Y25 NAFLD. To account for the uncertainty in BMI %Δ trajectory group assignment, we calculated the posterior predicted probability for each individual of being a member in each of the classes.22 Participants were assigned to the trajectory group for which they had the greatest posterior predictive probability. Models were sequentially adjusted a priori for potential confounders including demographics (baseline age, sex, race, education, center), cumulative burden of metabolic risk factors (cumulative systolic blood pressure (SBP), number of visits with blood pressure medications, cumulative triglycerides, cumulative years of diabetes, pack-years of cigarette smoking exposure, cumulative alcohol use (drinks/day), cumulative physical activity (exercise units per year)), and BMI at baseline (Y0) or at the time of NAFLD assessment (Y25). Cumulative SBP, alcoholic beverages, physical activity and triglycerides were calculated by summing the product of the average SBP (or triglycerides or alcohol or physical activity) and the time interval (in years) between 2 consecutive examinations over the 25 years. Interaction terms were assessed between trajectory group membership and race and sex. We compared the predictive utility of BMI %Δ trajectory group compared with other adiposity measures (WC or WHR) using the C statistic derived from the logistic regression models. Missing data were excluded from analyses. All variables analyzed had < 1% missing data. All analyses were completed using SAS software version 9.4 (SAS Institute Inc.). Two-sided P<0.05 was considered statistically significant.
RESULTS
Fat Distribution and Metabolic Characteristics
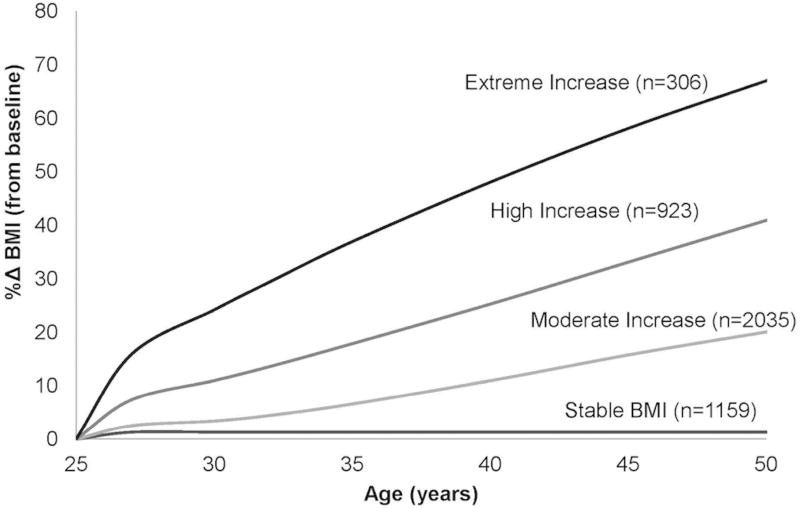
Four discrete trajectories in BMI %Δ from young adulthood to middle age were identified (Figure 2). In general, BMI increased over time in the majority of participants: only 26.2% of the cohort (n=1159) maintained stable BMI %Δ throughout follow-up (stable; BMI %Δ=3.1% ± 9.1% (mean±SD)); 46.0% (n=2035) had moderate BMI increase (moderate increase; BMI %Δ= 21.7% ± 9.7%), 20.9% (n=923) had high BMI increase (high increase; BMI %Δ= 41.8% ± 13.8%), and 6.9% (n=306) had an extreme BMI increase (extreme increase; BMI %Δ= 65.9% ± 19.3%), with a notable early rapid %Δ in BMI. The observed BMI %Δ corresponded to an average weight gain over 25 years of 1.9 ± 8.0, 15.3 ± 7.5, 29.0 ± 11.0, and 43.8 ± 14.1 kg in the stable, moderate, high and extreme increase groups, respectively.
Figure 2.
Percent Change (%Δ) Body Mass Index (BMI) Trajectories by Age in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Total N=4423.
Participant characteristics at the baseline examination according to BMI %Δ trajectory group are presented in Table 1. Individuals with a higher BMI %Δ trajectory were younger and more likely to be female, black and have lower education than the stable group. At Y25, groups with greater increases in BMI had a higher prevalence of components of the metabolic syndrome manifested by higher diabetes prevalence, fasting glucose, HOMA-IR and triglycerides and lower HDL levels (Table 2).
TABLE 1.
Baseline Characteristics at Year 0 by Body Mass Index Percent Change (%Δ) Trajectory Group
| Demographic Characteristics* |
Stable N=1159 |
Moderate Increase N=2035 |
High Increase N=923 |
Extreme Increase N=306 |
P value† |
|---|---|---|---|---|---|
| Age, years | 26.1 (3.2) | 25.5 (3.4) | 23.4 (3.5) | 21.6 (3.2) | <0.0001 |
| Race | |||||
| Blacks | 440 (38.0) | 949 (46.6) | 576 (62.4) | 214 (69.9) | <0.0001 |
| Whites | 719 (62.0) | 1086 (53.4) | 347 (37.6) | 92 (30.1) | |
| Sex | |||||
| Women | 605 (52.2) | 1058 (52.0) | 546 (59.2) | 223 (72.9) | <0.0001 |
| Men | 554 (47.8) | 997 (48.0) | 377 (40.9) | 83 (27.1) | |
| Education, years | 14.4 (2.4) | 14.0 (2.3) | 13.3 (1.9) | 12.8 (1.7) | <0.0001 |
| Current smoker | 338 (29.3) | 585 (28.9) | 262 (28.6) | 83 (27.3) | 0.08 |
| Alcohol drinker | 780 (67.6) | 1272 (62.8) | 493 (53.7) | 144 (47.1) | <0.0001 |
| Alcohol use, g/day | 12.1 (20.2) | 9.5 (15.6) | 8.1 (18.9) | 5.0 (10.2) | <0.0001 |
| Physical activity, exercise units | 444.2 (297.8) | 397.6 (292.1) | 420.6 (301.6) | 365.6 (284.9) | <0.0001 |
| Weight, kg | 73.8 (18.3) | 70.8 (15.4) | 69.8 (15.5) | 66.8 (13.2) | <0.0001 |
| Height, cm | 171.2 (9.5) | 170.6 (9.4) | 169.6 (9.7) | 167.7 (8.9) | 0.001 |
| BMI, kg/m2 | 25.2 (5.9) | 24.3 (4.7) | 24.3 (4.7) | 23.8 (4.1) | <0.0001 |
| Waist circumference, cm | 79.9 (13.2) | 77.8 (10.8) | 76.2 (10.6) | 74.1 (8.6) | <0.0001 |
| Waist-hip ratio | 0.79 (0.07) | 0.78 (0.07) | 0.76 (0.06) | 0.75 (0.07) | <0.0001 |
| Systolic BP, mm Hg | 111.1 (11.2) | 110.5 (10.9) | 109.6 (10.4) | 109.0 (11.6) | 0.001 |
| Diastolic BP, mm Hg | 69.6 (9.9) | 68.8 (9.6) | 67.6 (9.3) | 66.6 (9.2) | <0.0001 |
| Hypertension | 62 (5.4) | 73 (3.6) | 31 (3.4) | 11 (3.6) | 0.06 |
| Fasting glucose, mg/dl | 84.5 (21.2) | 82.3 (14.3) | 80.7 (8.4) | 80.2 (9.3) | <0.0001 |
| Diabetes | 22 (1.9) | 21 (1.0) | 3 (0.3) | 2 (0.7) | 0.005 |
| Total cholesterol level | 179.9 (33.7) | 178.4 (33.2) | 173.4 (31.1) | 168.2 (32.9) | <0.0001 |
| HDL cholesterol, mg/dl | 52.9 (13.3) | 52.8 (13.0) | 54.2 (13.4) | 53.8 (11.8) | 0.04 |
| Triglyceride, mg/dl | 80.9 (64.4) | 73.1 (43.1) | 65.5 (35.6) | 59.7 (25.6) | <0.0001 |
All results are presented as mean (SD) or n (%)
All variables have <1% missing
Chi square test for categorical variables and ANOVA for continuous variables
Abbreviations: SD, standard deviation; n, number; BMI, body mass index; BP, blood pressure; HDL, high density lipoprotein
TABLE 2.
Follow Up Characteristics at Year 25 by Body Mass Index Percent Change (%Δ) Trajectory Group
| Demographic Characteristics* | Stable BMI N=917 |
Moderate Increase N=1574 |
High Increase N=716 |
Extreme Increase N=221 |
P value† |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 51.3 (3.2) | 50.7 (3.4) | 48.6 (3.6) | 46.7 (3.3) | <0.01 |
| Race, n (%) | |||||
| Blacks | 440 (38.0) | 949 (46.6) | 576 (62.4) | 214 (69.9) | <0.0001 |
| Whites | 719 (62.0) | 1086 (53.4) | 347 (37.6) | 92 (30.1) | |
| Sex | |||||
| Women | 605 (52.2) | 1058 (52.0) | 546 (59.2) | 223 (72.9) | <0.0001 |
| Men | 554 (47.8) | 977 (48.0) | 377 (40.9) | 83 (27.1) | |
| Current smoker | 178 (19.4) | 263 (16.7) | 104 (14.5) | 30 (13.6) | 0.11 |
| Alcohol drinker | 594 (64.8) | 859 (54.6) | 328 (45.8) | 85 (38.5) | <0.0001 |
| Alcohol use, g/day | 440 (38.0) | 949 (46.6) | 576 (62.4) | 214 (69.9) | <0.0001 |
| Physical Activity, exercise units | 402.2 (283.3) | 287.5 (244.8) | 340.5 (281.1) | 222.3 (222.9) | <0.0001 |
| BMI, kg/m2 | 25.9 (5.7) | 29.5 (5.8) | 34.3 (7.2) | 38.9 (7.3) | <0.0001 |
| Waist circumference, cm | 85.4 (14.7) | 93.9 (13.7) | 102.2 (15.2) | 109.5 (13.8) | <0.0001 |
| Waist-to-hip ratio | 0.83 (0.09) | 0.86 (0.09) | 0.87 (0.09) | 0.86 (0.08) | <0.0001 |
| Systolic BP, mm Hg | 116.8 (17.1) | 119.5 (15.2) | 122.8 (16.1) | 121.6 (16.6) | <0.0001 |
| Diastolic BP, mm Hg | 71.6 (11.3) | 74.6 (10.8) | 78.1 (11.0) | 78.9 (11.0) | <0.0001 |
| Hypertension | 239 (26.1) | 510 (32.4) | 300 (41.9) | 97 (43.9) | <0.0001 |
| Fasting glucose, mg/dl | 97.8 (33.5) | 99.1 (25.6) | 101.3 (28.4) | 102.4 (26.1) | 0.04 |
| HOMA-IR | 3.3 (3.8) | 4.2 (3.5) | 5.0 (3.9) | 6.1 (5.1) | <0.0001 |
| Diabetes | 95 (10.4) | 193 (12.3) | 97 (13.5) | 37 (16.7) | 0.04 |
| Total cholesterol level | 192.1 (36.3) | 193.2 (37.8) | 193.4 (36.5) | 183.1 (34.0) | 0.002 |
| HDL cholesterol, mg/dl | 65.6 (21.2) | 56.3 (16.2) | 54.2 (15.7) | 51.4 (13.0) | <0.0001 |
| Triglyceride, mg/dl | 99.2 (75.6) | 117.2 (91.0) | 126.1 (95.2) | 113.0 (64.4) | <0.0001 |
| Liver Attenuation, HU | 58.2 (9.4) | 55.2 (11.8) | 53.2 (13.5) | 51.5 (12.7) | <0.0001 |
All results are presented as mean (SD) or n (%)
All variables have <1% missing
Chi square test for categorical variables and ANOVA for continuous variables
Abbreviations: SD, standard deviation; n, number; BMI, body mass index; BP, blood pressure; HOMA-IR, Homeostatic model assessment for insulin resistance; HDL, high density lipoprotein; HU, Hounsfield units
At baseline, the majority of participants were normal weight (> 62%) and the extreme increase group had the smallest proportion of obesity (5.6%, Supplementary Table 1). However, over time, the proportion of overweight/obesity increased substantially, particularly among groups with substantial %Δ in BMI. Mean BMI at Y25 was 25.9 ± 5.7, 29.5 ± 5.8, 34.3 ± 7.2, and 38.9 ± 7.3 kg/m2 in the stable, moderate, high increase and extreme increase groups, respectively (Supplementary Table 1).
%Δ BMI and NAFLD
NAFLD prevalence at Y25 was higher with increasing BMI change group and was present in 4.1% (n=38), 9.3% (n=146), 13.0% (n=93), and 17.6% (n=39) in the stable, moderate increase, high increase, and extreme increase groups, respectively (p-trend <0.0001, Supplementary Figure 1A). In comparison with individuals in the stable group, those in trajectory groups with patterns of increasingly severe BMI %Δ had progressively greater odds of having NAFLD even when adjusted for demographics, education and cardiovascular risk factors (ORs 3.35 [95% CI 2.07–5.43], 7.80 [4.60–13.23], and 12.68 [6.68–24.09]) for moderate, high and extreme increase groups, respectively, Table 3). These associations were only moderately attenuated when adjusted for baseline BMI (Table 3). Associations were attenuated more substantially, but remained statistically significant when adjusted for Y25 BMI (Table 3).
TABLE 3.
Odds Ratios for the Association of BMI %Δ Trajectory Group with Prevalent NAFLD in Middle Age in CARDIA
| BMI %Δ Group |
Unadjusted | Base Model* |
Base Model + Y0 BMI |
Base Model + Y25 BMI |
||||
|---|---|---|---|---|---|---|---|---|
| N=2615 | N=2615 | N=2615 | N=2611 | |||||
| OR (95% CI) |
P value | OR (95% CI) |
P value | OR (95% CI) |
P value | OR (95% CI) |
P value | |
| Stable | Reference | |||||||
| Moderate Increase | 2.65 (1.71–4.09) | 0.005 | 3.35 (2.07–5.43) | 0.04 | 4.40 (2.66–7.31) | 0.03 | 2.56 (1.55–4.21) | 0.69 |
| High Increase | 4.27 (2.71–6.73) | 0.0002 | 7.80 (4.60–13.23) | <0.0001 | 11.38 (6.51–19.89) | <0.0001 | 3.73 (2.13–6.52) | 0.001 |
| Extreme Increase | 5.51 (3.22–9.42) | <0.0001 | 12.68 (6.68–24.09) | <0.0001 | 21.18 (10.75–41.72) | <0.0001 | 3.68 (1.83–7.41) | 0.04 |
Results presented as Odds Ratio (95% Confidence Interval)
NAFLD = liver attenuation ≤ 40 HU after exclusion for secondary causes of liver fat
Base model adjusted for baseline age, sex, race, education, center, cumulative systolic blood pressure (mm Hg-years), number of years with blood pressure medications, cumulative triglycerides (mg/dl-years), cumulative years with diabetes, cumulative alcohol use (drinks/day), physical activity level (exercise units-year) and pack-years of cigarette smoking exposure
Abbreviations: BMI, body mass index; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval
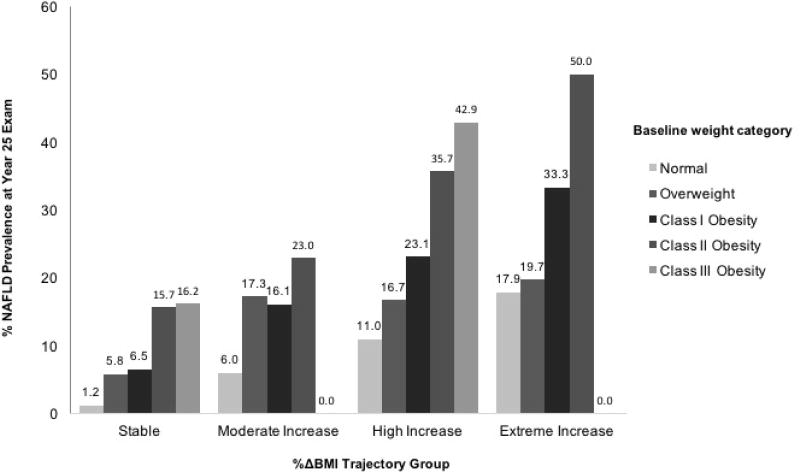
Figure 3 demonstrates Y25 NAFLD prevalence stratified by weight category at baseline according to BMI %Δ trajectory group. Within each trajectory group, NAFLD prevalence increased with increasing weight category group. However, participants with class I obesity who maintained a stable BMI over time were significantly less likely to have prevalent NAFLD at Y25 compared with those who were normal weight at baseline but who had an extreme increase in BMI %Δ over time (e.g., 6.5% vs. 17.9%, p < 0.0001). Associations were similar, though less pronounced, for participants with baseline class II (15.7%) and class III (16.2%) obesity who maintained stable weight compared with those who were normal weight at baseline with extreme BMI increase over time (p<0.05 for both, respectively).
Figure 3.
Year 25 NAFLD Prevalence Stratified by Baseline Weight Category and Percent Change (%Δ) in BMI Trajectory Group (Total N at Year 25 exam = 2650). Class I obesity was defined as body mass index (BMI) 30–34.9 kg/m2, class II obesity as BMI 35–39.9 kg/m2, class III obesity as BMI ≥ 30 kg/m2, overweight as BMI 25–29.9 kg/m2 and normal weight as BMI < 25 kg/m2.
We identified a significant interaction between BMI %Δ trajectory group with both race and sex on the odds of Y25 NAFLD. Despite this statistical interaction, among all race-sex groups the odds of NAFLD increased with increasing BMI %Δ trajectories and whereas we observed different magnitudes of association across race-sex groups, the direction of the association did not differ. The prevalence of NAFLD was low (<5%) in several subgroups, particularly in white females and black males with stable BMI (Supplementary Table 2); thus, the point estimates for odds of NAFLD by BMI %Δ trajectory group had poor precision in analyses stratified by race and sex (e.g., 95% confidence interval: 14.8–427.4, Supplementary Table 3).
%Δ Waist Circumference and Waist-to-Hip Ratio
Overall patterns of change throughout adulthood were similar when we examined the trajectories of %Δ in WC or WHR separately (Supplementary Figure 2). Changes in WC and WHR trajectory groups were highly to moderately correlated with BMI %Δ groups (Spearman r =0.82 for WC and 0.39 for WHR, P<0.0001 for both). Both WC and WHR %Δ were similarly associated in a dose-response fashion with NAFLD at Y25 (Supplementary Figures 1B and 1C). As expected, associations with measures of central adiposity %Δ and prevalent NAFLD demonstrated similar trends as those models that used BMI %Δ (Supplementary Tables 4 and 5). In the multivariable adjusted model, WC %Δ discriminated prevalent NAFLD comparably to BMI %Δ (C statistic, 0.784 for WC and 0.780 for BMI) and slightly better than WHR %Δ (C statistic, 0.754).
DISCUSSION
In a large, population-based, prospective study of biracial adults followed for 25 years, we identified 4 trajectories of BMI change that were significantly associated with prevalent NAFLD in midlife. We found that those groups with greater BMI increase from young adulthood to middle age have the greatest odds of having NAFLD in midlife, regardless of demographics, the cumulative burden of clinical covariates, and weight status at baseline or concurrently. Indeed, those who had baseline obesity and had major BMI increases through young adulthood had NAFLD prevalence of 28% to 40%; conversely, those who had baseline obesity but maintained stable BMI had NAFLD prevalence of only 16%. These findings highlight the importance of weight maintenance throughout young adulthood, regardless of baseline weight, as a critical target for the primary prevention of NAFLD.
The current study provides unique insights into long-term patterns of change in BMI during early adulthood. Importantly, participants at baseline had similar mean BMI values, yet their patterns over time diverged markedly. In CARDIA, a large proportion of participants developed incident obesity over time with increases in BMI in all groups to varying degrees. We identified heterogeneous patterns of BMI change as separate trajectory groups, thus providing increased understanding of lifetime trends in weight. Knowledge of these trajectories throughout adulthood may allow us to potentially attenuate the NAFLD epidemic, highlighting the period of transition from young adulthood to middle age as a prime target for health promotion, primordial prevention, and long-term disease prevention.
The association between adult weight gain and NAFLD was independent of multiple metabolic risk factors associated with histologic progression of NAFLD. Body weight gain in earlier adulthood (age 25–40 years) has been associated with increased markers of insulin resistance compared with later adulthood weight gain (age >40 years).23 In a cross-sectional study of 1119 Chinese participants (mean age 47), earlier increases in insulin resistance mediated the relationship between adult weight gain and NAFLD.13 In addition, BMI increase in earlier adulthood is more strongly associated with unfavorable levels of obesity biomarkers (e.g., adiponectin) and markers of liver damage (e.g., gamma-glutamyl transferase), than BMI gain in later adulthood.23 Therefore, the increased NAFLD prevalence in midlife among adults with a steep, early increase in BMI (as was observed in our extreme increase trajectory group) may in fact be mediated by the systemic influences of visceral adiposity. This is supported by the finding that increased WC, a marker of visceral adiposity, was highly predictive of prevalent NAFLD.
We observed significant differences in the strength of the association of BMI trajectory and NAFLD prevalence by race and sex. NAFLD prevalence was highest in white men followed by white women, black men, and black women, whereas obesity prevalence was highest among black women and black men. There are significant differences in NAFLD prevalence between racial-ethnic groups with a higher risk of severe disease in Hispanics and Whites, and a surprisingly low risk in African Americans, for reasons that are not entirely known.8,24 Future studies are needed to assess the potential impact of adult weight gain among various racial-ethnic populations who may be at differential risk for NAFLD despite a high prevalence of obesity.
Strengths and Limitations
Our study has several strengths. The CARDIA study is well-positioned to examine longitudinal trends in BMI and its relationship to NAFLD since the cohort began at the start of the obesity epidemic (1985–86), prior to the onset of the NAFLD epidemic, and involves a biracial population with a wealth of rigorously measured covariates. In addition to our BMI findings, we saw similar trajectory patterns with measures of central fat distribution (WC, WHR) with greater NAFLD prevalence associated with greatest increases in these measures, independent of cumulative comorbidities. Finally, we applied innovative statistical methods to examine patterns of changes in adiposity in a large, well-characterized cohort of black and white Americans.
Several limitations should also be considered when interpreting our study results. NAFLD prevalence in CARDIA is somewhat lower than what has been reported in other U.S. cohorts. Our NAFLD outcome was measured by LA on non-contrast CT which is a relatively insensitive tool for liver fat assessment compared to magnetic resonance imaging (e.g., MR-PDFF or spectroscopy), which are not available in CARDIA.20,25 We chose our LA cutoff based on previous studies correlating liver attenuation with histology, which showed excellent specificity but lower sensitivity for the detection of NAFLD.20 Maintaining high specificity minimizes the impact of measurement bias, however our LA cutoff could not detect lesser degrees of pathologic steatosis between 5% and 30%. Second, CARDIA included a biracial cohort of adults and we observed a lower NAFLD prevalence in blacks compared to whites consistent with other studies.24 Our cohort also did not ask about Hispanic ethnicity or obtain data on genetic polymorphisms including patatin-like phospholipase domain containing protein 3 (PNPLA3), which partially explain racial differences in NAFLD. Thus, our findings cannot be generalized to other ethnic groups. Contemporaneous or baseline laboratory data on hepatic function are not available in CARDIA. However, there is no laboratory test for NAFLD and serum aminotransferases are often normal despite the presence of liver injury.26 NAFLD was also not assessed in CARDIA prior to the Y25 follow up examination and thus, we do not know when during adulthood NAFLD may have developed. However, since NAFLD is primarily an asymptomatic disease, detection in midlife mirrors clinical practice when NAFLD is commonly incidentally found on imaging performed for other reasons.27 It is also possible that some CARDIA participants had undiagnosed NAFLD at the baseline examination. However, over 62% of NAFLD participants were normal weight at baseline. Finally, viral hepatitis status was obtained by self-report, raising the possibility of undiagnosed hepatitis, particularly Hepatitis C, within our cohort.
Clinical and Public Health Implications
Weight loss has been associated with biochemical,28 radiographic,29,30 and histologic31,32 improvements in NAFLD, but initial weight loss may be a challenge and maintaining lower weight after weight loss is difficult over time.33 Weight loss and physical activity are the recommended treatments for NAFLD.16 However, efficacy of lifestyle intervention is poor due to a lack of patient adherence, programs designed specifically for patients with liver disease, and financial support from payers to sustain these programs long-term.16,34 Finally, lifestyle interventions in early adult life are more likely to be successful than interventions attempted later in life once lifestyle habits and diseases have further progressed.35 In addition to weight loss as a treatment for obesity (e.g., secondary prevention), our data suggest that primary prevention strategies aimed at weight maintenance through young adulthood are likely to have a significant impact at preventing NAFLD and its consequences. Thus, maintaining weight throughout adulthood regardless of starting point (e.g., normal weight, overweight or obese), may reduce NAFLD risk in middle age.
CONCLUSION
Our findings imply that the trajectory of BMI change throughout early adulthood to midlife—independent of baseline and concurrent BMI—provides additional information about the risk of developing NAFLD. These associations were independent of key comorbidities and metabolic risk factors. This novel characterization of change in BMI trajectories across a critical period for significant weight gain highlights young adulthood as an important target for behavior and lifestyle interventions for primordial prevention of NAFLD. Prevention programs that target weight maintenance in early adulthood, regardless of starting weight or weight category, may be more effective for NAFLD prevention than programs that target weight loss after the disease has developed in later life.
Supplementary Material
Supplementary Figure 1. Year 25 NAFLD Prevalence stratified by A) %Δ Body Mass Index (BMI) B) Waist Circumference (WC) or C) Waist-to-Hip Ratio (WHR) Trajectory Group. P-trend <0.0001 for trajectory group membership for all adiposity measures. Total N at Year 25 exam = 2650.
Supplementary Figure 2. Percent Change (%Δ) Trajectories by Age. Waist Circumference (A) and Waist to Hip Circumference Ratio (B). Total N=4423.
Supplementary Table 1. BMI and weight categories at each CARDIA examination by BMI percent change (%Δ) trajectory group
Supplementary Table 2. Prevalence (%) of NAFLD Stratified by Race, Sex and BMI %Δ Trajectory Group
Supplementary Table 3. Association of BMI Percent Change (%Δ) Trajectory Group with Prevalent NAFLD in Middle Age Stratified by Race and Sex
Supplementary Table 4. Odds Ratios for the Association of Waist Circumference (WC) Percent Change (%Δ) Trajectory Group with Prevalent Nonalcoholic Fatty Liver Disease (NAFLD) in Middle Age in CARDIA
Supplementary Table 5. Odds Ratios for the Association of Waist to Hip Ratio (WHR) Percent Change (%Δ) Trajectory Group with Prevalent Nonalcoholic Fatty Liver Disease (NAFLD) in Middle Age in CARDIA
KEY POINTS.
NAFLD is an epidemic associated with increased liver and non-liver related morbidity and mortality.
Overweight and obesity weight categories are highly associated with NAFLD and early identification of modifiable risk factors is an important strategy to decrease the NAFLD burden.
Increasing patterns of weight change throughout adulthood are associated with differential risk for NAFLD in midlife independent of starting or concurrent weight or weight category.
Strategies aimed at weight maintenance through young adulthood, rather than weight loss attempts later, are likely to be most successful at preventing NAFLD.
Acknowledgments
The authors thank the participants of the CARDIA study for their long-term commitment and contributions to the study.
Financial Support: The CARDIA Study is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). Dr. VanWagner is supported by the National Center for Advancing Translational Sciences (KL2TR001424). Dr. Carr is supported by the NIH (R01-HL-098445).
List of Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- CT
computed tomography
- HU
Hounsfield units
- LA
liver attenuation
- EU
exercise units
- WC
waist circumference
- WHR
waist-to-hip circumference ratio
- SBP
systolic blood pressure
- OR
odds ratio
- CI
confidence interval
- %Δ
percent change
- NASH
nonalcoholic steatohepatitis
- HDL-C
high density lipoprotein cholesterol
- HOMA-IR
homeostatic model assessment of insulin resistance
- MR
magnetic resonance imaging
Footnotes
Disclosures
The authors have no conflicts of interest. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58(11):1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 4.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md) 2010;51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 5.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 7.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology (Baltimore, Md) 2010;51(2):595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 8.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology (Baltimore, Md) 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the 'normal' weight range. Jama. 1995;273(6):461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 11.Rexrode KM, Hennekens CH, Willett WC, et al. A prospective study of body mass index, weight change, and risk of stroke in women. Jama. 1997;277(19):1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 12.Wakasugi M, Narita I, Iseki K, et al. Weight gain after 20 years of age is associated with prevalence of chronic kidney disease. Clin Exp Nephrol. 2012;16(2):259–268. doi: 10.1007/s10157-011-0565-3. [DOI] [PubMed] [Google Scholar]
- 13.Du S, Wang C, Jiang W, et al. The impact of body weight gain on nonalcoholic fatty liver disease and metabolic syndrome during earlier and later adulthood. Diabetes Res Clin Pract. 2016;116:183–191. doi: 10.1016/j.diabres.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Deshpande GA, Urayama KY, Masuda K, Fukui T, Matsuyama Y. Association of weight gain since age 20 with non-alcoholic fatty liver disease in normal weight individuals. J Gastroenterol Hepatol. 2015;30(5):909–917. doi: 10.1111/jgh.12861. [DOI] [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 16.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology (Baltimore, Md) 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 17.Parker ED, Schmitz KH, Jacobs DR, Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA study. American journal of public health. 2007;97(4):703–709. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertens I, Van Gaal LF. New International Diabetes Federation (IDF) and National Cholesterol Education Program Adult Treatment panel III (NCEP-ATPIII) criteria and the involvement of hemostasis and fibrinolysis in the metabolic syndrome. J Thromb Haemost. 2006;4(5):1164–1166. doi: 10.1111/j.1538-7836.2006.01919.x. [DOI] [PubMed] [Google Scholar]
- 19.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: The Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235(2):599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188(5):1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 21.Nagin DS, Odgers CL. Group-Based Trajectory Modeling (Nearly) Two Decades Later. J Quant Criminol. 2010;26(4):445–453. doi: 10.1007/s10940-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. Jama. 2014;311(5):490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montonen J, Boeing H, Schleicher E, Fritsche A, Pischon T. Association of changes in body mass index during earlier adulthood and later adulthood with circulating obesity biomarker concentrations in middle-aged men and women. Diabetologia. 2011;54(7):1676–1683. doi: 10.1007/s00125-011-2124-6. [DOI] [PubMed] [Google Scholar]
- 24.Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology (Baltimore, Md) 2005;41(2):372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 25.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 26.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology (Baltimore, Md) 2003;37(6):1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 27.Blais P, Husain N, Kramer JR, Kowalkowski M, El-Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. The American journal of gastroenterology. 2015;110(1):10–14. doi: 10.1038/ajg.2014.134. [DOI] [PubMed] [Google Scholar]
- 28.Cazzo E, Jimenez LS, Pareja JC, Chaim EA. Effect of Roux-en-Y gastric bypass on nonalcoholic fatty liver disease evaluated through NAFLD fibrosis score: a prospective study. Obes Surg. 2015;25(6):982–985. doi: 10.1007/s11695-014-1489-2. [DOI] [PubMed] [Google Scholar]
- 29.Winder JS, Dudeck BS, Schock S, Lyn-Sue JR, Haluck RS, Rogers AM. Radiographic Improvement of Hepatic Steatosis After Laparoscopic Roux-en-Y Gastric Bypass. Obes Surg. 2016 doi: 10.1007/s11695-016-2299-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HJ, He J, Pan LL, et al. Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. JAMA internal medicine. 2016 doi: 10.1001/jamainternmed.2016.3202. [DOI] [PubMed] [Google Scholar]
- 31.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367–378. e365. doi: 10.1053/j.gastro.2015.04.005. quiz e314–365. [DOI] [PubMed] [Google Scholar]
- 32.Lassailly G, Caiazzo R, Buob D, et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015;149(2):379–388. doi: 10.1053/j.gastro.2015.04.014. quiz e315–376. [DOI] [PubMed] [Google Scholar]
- 33.Wing RR, Phelan S. Long-term weight loss maintenance. The American journal of clinical nutrition. 2005;82(1 Suppl):222s–225s. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 34.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology (Baltimore, Md) 2010;51(1):121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson MA, Gluckman PD, Ma RC, Matzen P, Biesma RG. Early life opportunities for prevention of diabetes in low and middle income countries. BMC Public Health. 2012;12:1025. doi: 10.1186/1471-2458-12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Year 25 NAFLD Prevalence stratified by A) %Δ Body Mass Index (BMI) B) Waist Circumference (WC) or C) Waist-to-Hip Ratio (WHR) Trajectory Group. P-trend <0.0001 for trajectory group membership for all adiposity measures. Total N at Year 25 exam = 2650.
Supplementary Figure 2. Percent Change (%Δ) Trajectories by Age. Waist Circumference (A) and Waist to Hip Circumference Ratio (B). Total N=4423.
Supplementary Table 1. BMI and weight categories at each CARDIA examination by BMI percent change (%Δ) trajectory group
Supplementary Table 2. Prevalence (%) of NAFLD Stratified by Race, Sex and BMI %Δ Trajectory Group
Supplementary Table 3. Association of BMI Percent Change (%Δ) Trajectory Group with Prevalent NAFLD in Middle Age Stratified by Race and Sex
Supplementary Table 4. Odds Ratios for the Association of Waist Circumference (WC) Percent Change (%Δ) Trajectory Group with Prevalent Nonalcoholic Fatty Liver Disease (NAFLD) in Middle Age in CARDIA
Supplementary Table 5. Odds Ratios for the Association of Waist to Hip Ratio (WHR) Percent Change (%Δ) Trajectory Group with Prevalent Nonalcoholic Fatty Liver Disease (NAFLD) in Middle Age in CARDIA