Abstract
Background
Rotavirus vaccine was introduced into the Extended Program on Immunization in Madagascar in May 2014. We analyzed trends in prevalence of all cause diarrhea and rotavirus hospitalization in children < 5 years of age before and after vaccine introduction and assessed trend rotavirus genotypes circulating at Centre Hospitalier Universitaire Mère Enfant Tsaralalàna (CHU MET).
Methods
From January 2010 to December 2016, we reviewed the logbook admission to observe the rate of hospitalization caused by gastroenteritis among 19619 children < 5 years of age admitted to hospital In June 2013–Dec 2016. Active rotavirus surveillance was also conducted at CHUMET with support from WHO. Rotavirus was detected by EIA from stool specimen of children with gastroenteritis eligible surveillance at sentinel site laboratory and rotavirus positive specimens were further genotyped at Regional Reference Laboratory by PCR.
Results
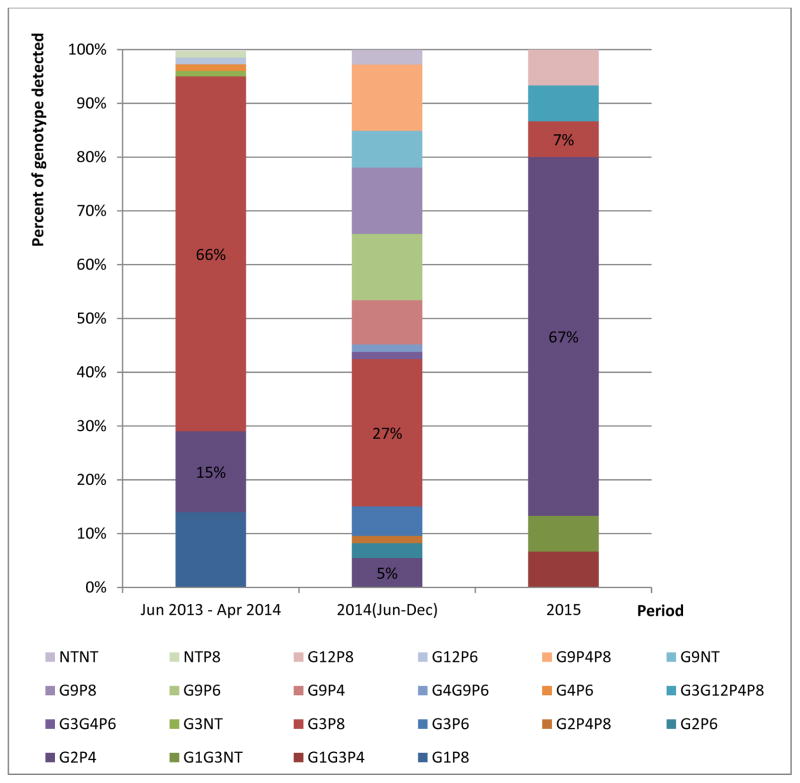
Diarrhea hospitalizations decreased after rotavirus vaccine introduction. The median proportion of annual hospitalizations due to diarrhea was 26% (range: 31% to 22%) before vaccine introduction; the proportion was 25% the year of vaccine introduction, 17% in 2015 and 16% in 2016. Rotavirus positivity paralleled patterns observed in diarrhea. Before vaccine introduction, 56% of stool specimens tested positive for rotavirus; the percent positive was 13% in 2015, 12% in 2016. 21 distinct genotypes were detected in the pre-vaccine period; the most common were G3P[8] (n=53; 66%), G2P[4] (n=12; 15%), and G1P[8] (n=11; 14%). 6 distinct genotypes were found in 2015; the most common genotype was G2P[4] (n=10; 67%).
Conclusions
Following rotavirus vaccine introduction all-cause diarrhea and rotavirus-specific hospitalizations declined dramatically.
Keywords: Rotavirus vaccine, rotavirus, surveillance, genotype
Background
Diarrheal diseases remain one of the most common causes of child mortality in the world, with the majority of this burden in developing countries (1). In 2009, the World Health Organization (WHO) recommended all countries include rotavirus vaccine in their routine immunizations programs (2, 3, 4). In Madagascar, the two-dose oral monovalent human rotavirus vaccine, Rotarix(RV1, GSK Biologics), was introduced into the routine Expanded Program on Immunization (EPI) in May 2014 with support from WHO and funding from Gavi. Madagascar’s vaccine schedule recommends rotavirus vaccine administration to infants at 2 and 4 months of age. Rotarix contains a G1P[8] human rotavirus strain and has been found to be effective in preventing severe rotavirus disease caused by the vaccine strain as well as genotypes not included in the vaccine (5, 6,7).
Laboratory-based rotavirus gastroenteritis sentinel surveillance was initiated in Madagascar in June 2013 at one sentinel hospital site in the capital city of Antananarivo. Diarrhea and rotavirus surveillance was important for making decisions regarding whether to introduce rotavirus vaccine into a country’s routine immunization program and is essential to enable monitoring of vaccine impact and for selective pressure of rotavirus genotypes by the vaccine. The circulating genotypes can change over seasons and regions. Rotavirus vaccine impact evaluations in many countries have shown significant reduction of rotavirus hospitalizations since licensure in 2006including: Brazil, US, Panama, Mexico, Austria, Australia, Belgium, El Salvador, Rwanda, Ghana (8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18). Little is known about rotavirus disease burden and the impact of rotavirus vaccine introduction in Madagascar.
The objective of this analysis is to describe the impact of rotavirus vaccine introduction on the burden of severe all-cause diarrhea and rotavirus hospitalizations among children ≤59 months of age admitted to one sentinel surveillance hospital site in Madagascar. We also describe the trends in circulating rotavirus genotypes causing severe disease in Madagascar among children ≤59 months of age before and after rotavirus vaccine introduction.
Methods
Centre Hospitalier Universitaire Mère Enfant Tsaralalàna (CHUMET), an 82 bed public referral pediatric hospital located in the capital of Madagascar, primarily serves local patients, though some patients come from elsewhere in the country. CHUMET is the only rotavirus gastroenteritis surveillance site in Madagascar that reports to the WHO sentinel hospital surveillance network.
Retrospective hospital log book review
We reviewed hospital admission logbooks from CHUMET for children <5 years of age hospitalized for acute gastroenteritis with or without dehydration from January 1, 2010 to December 31, 2016; we compared diarrhea hospitalizations from the pre-vaccine period (January 2010 to December 2013 with the post-vaccine period (January 2015 to December 2016).
Active Rotavirus surveillance
Rotavirus surveillance-eligible stool specimens collected at CHUMET from children ≤59 months of age were tested for rotavirus antigen by enzyme immunoassay (EIA). Children were included if they were admitted with acute gastroenteritis defined as 3 or more watery, non-bloody stools within a 24 hour period lasting less than 7 days, and stool was collected within 48hours of admission. Surveillance data for this reported was collected from June 2013 to December 2016; we compared rotavirus prevalence detected by EIA from June 2013 to April 2014 as the pre-vaccine period with January 2015 to December 2016 as the post-vaccine period.
Samples submitted to surveillance site laboratory were screened for group A rotavirus using EIA prospect TM kit. Rotavirus antigen-positive fecal samples were stored at −20 °C until transfer to the regional reference laboratory (RRL) in Medunsa, South Africa for confirmation by polymerase chain reaction (PCR) and genotyping.
At the RRL, rotavirus antigen-positive samples were tested for determination of G and P types, using semi-nested multiplex PCR method performed with consensus- and genotype-specific primers. The first-round PCR products were purified; the sequencing was performed using consensus primers for the VP7 and VP4 genes and the sequence data were analyzed with the bioinformatics software packages. The rotavirus 11 genomic segments were separated by polyacrylamide gel electrophoresis (PAGE) to display two major distinctive RNA migration patterns. Genotyping results are presented for three periods: June 2013–April 2014, May–December 2014, and January–December 2015.
Analysis
All analyses were performed and figures generated using Microsoft Excel 2013. The Pearson chi square results were calculated using R 3.2.4.
Results
Retrospective hospital log book review
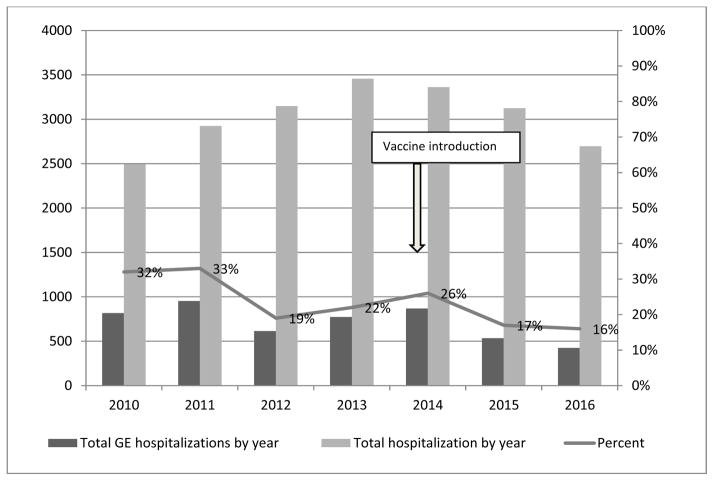
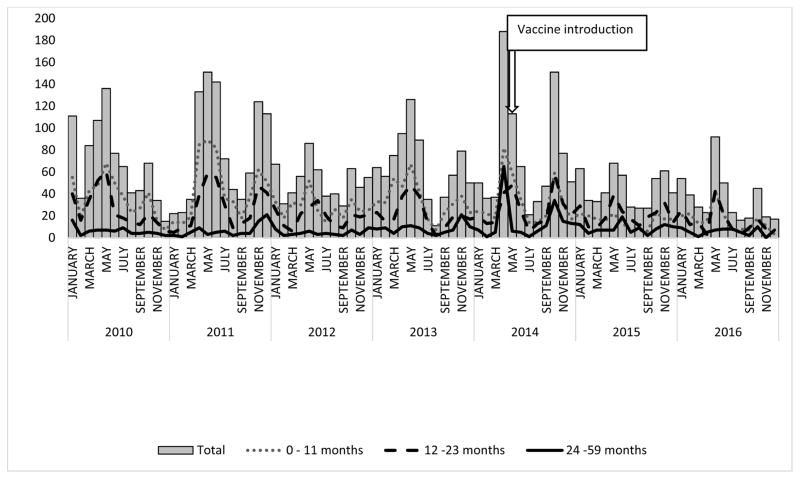
From January 2010 to December 2013, 12,026 children ≤59 months were admitted to hospital; diarrhea hospitalizations represented a median of 26% (range: 31% to 22%) of annual hospitalizations during this period (Figure 1). In 2014, the year of rotavirus vaccine introduction, 3,362 children ≤59 months were admitted to hospital, of which 25% were due to diarrhea. In the years following vaccine introduction, 5821 children were admitted to hospital with 16% due to diarrhea, compared with the pre-vaccine median, this is a 36% (p<0.001) reduction in the percent diarrhea hospitalizations in the post vaccine period. We noted increased diarrhea hospitalizations during two annual seasons: April–June and October-November. In 2015 and 2016, two seasonal peaks in diarrhea admissions are observable but reduced compared to earlier years (Figure 2). Before rotavirus vaccine introduction, 54%, 36%, and 10% of diarrhea hospitalizations included in this analysis were among children 0–11 months, 12–23 month, and 24–59 months of age, respectively (Figure 2); after rotavirus vaccine introduction, this distribution changed to 39%, 42%, and 19% of diarrhea hospitalizations, respectively.
Figure 1.
Number of all diarrhea hospitalizations and total hospital admissions, and percent of total hospital admissions attributed to diarrhea among children ≤59 months by year 2010–2016, Madagascar.
Figure 2.
Total and age group-specific number of diarrhea hospitalizations among children ≤59 months of age at sentinel hospital from January 2010 to December 2016, Madagascar.
Active Rotavirus surveillance
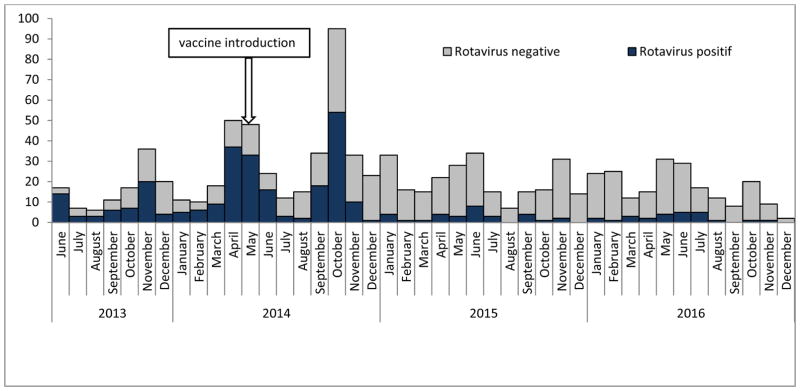
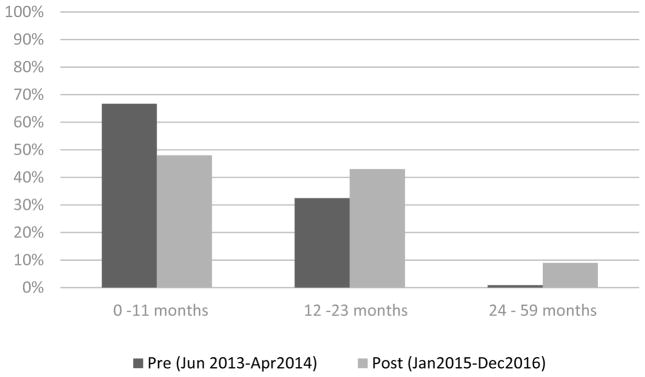
The number of stool specimens tested and EIA rotavirus positive results are shown by month from June 2013 to December 2016 in Figure 3; the seasonality and reduction observed in the number of stool specimens and rotavirus positivity parallels patterns observed in diarrhea hospitalizations (Figure 2). During this period of rotavirus surveillance, we collected and analyzed by EIA 203 (30% of all diarrhea admissions) stool specimens before rotavirus vaccine introduction and 449 (47% of all diarrhea admissions) stool specimens after rotavirus vaccine introduction. The remaining children did not meet the case definition for rotavirus or were not able to provide a stool specimen. From June 2013 to April 2014, rotavirus was detected in 114 (56%) stool specimens. The proportion of rotavirus positive stool specimens was 12% (n=56) in the post vaccine period (2015–2016), a reduction of 78% (p<0.01). Before vaccine introduction, 67%, 32%, and 1% of all rotavirus detected in stool specimens was among children aged 0–11 months, 12–23 months, and 24–59 months. In contrast, 48%, 43%, and 9% of all rotavirus detected in stool specimens was among children aged 0–11 months, 12–23 months, and 24–59 months after vaccine introduction, respectively (Figure 4).
Figure 3.
Number of rotavirus positive stool samples among children ≤59 months of age by month from June 2013 to December 2016, Madagascar.
Figure 4.
Percent of total rotavirus positive samples by age group before and after rotavirus vaccine introduction, Madagascar.
Genotype testing
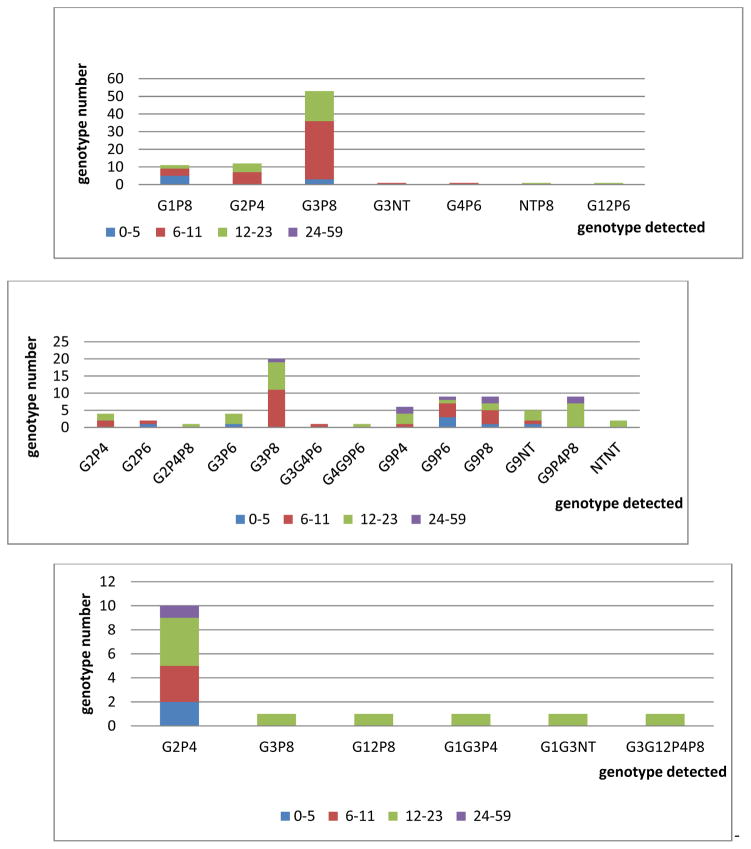
A total of 195 (69%) rotavirus positive specimens were genotyped at RRL (Table 1). Indeed, a limited number of rotavirus positive specimens were further tested for genotype according to RRL and WHO recommendations; the rest (n= 317) were not tested. We detected 21 distinct genotypes during the surveillance period. Before vaccine introduction, 7 different genotypes were detected in 80 specimens (Figure 5). The most commonly detected genotypes were G3P[8] (n=53; 66%), G2P[4] (n=12; 15%), and G1P[8] (n=11; 14%). Other genotypes were detected in the additional 4 specimens (5%). Of the genotyped specimens from the pre-vaccine introduction period, 68% were from children 0–11 months of age (Figure 6). During the first eight months after rotavirus vaccine introduction in 2014, 13 rotavirus genotypes were detected in 100 specimens. The most common detected genotypes were G3P[8] (n=20, 20%) and G9P[6], G9P[8], and G9P[4]P[8] with 9 specimens each. There were 9 additional genotypes detected in 53 specimens. In 2015, 6 distinct rotavirus genotypes were found in 15 specimens. The most common genotype was G2P[4] (n=10, 67%). The remaining 5, G3P[8], G9P[4]P[8], G1G3P[4], G1G3P[NT], G3G12P[4]P[8], had one positive specimen each. Compared with the genotypes detected pre-vaccination period, G1P[8], G3P[NT], G4P[6], G12P[6], and GNTP[8] were not detected in 2015. Of the genotyped specimens from 2015, 60% were from children 12–23 months of age.
Table 1.
Rotavirus Antigen-positive detected by EIA and genotyping, Madagascar.
| Pre Jun 2013–Apr 2014 |
Introduction May 2014–Dec 2014 |
Post Jan 2015–Dec 2016 |
Total | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
|
|
||||||||
| Specimen tested | 203 | 244 | 446 | 893 | ||||
| Rotavirus+ (EIA) | 114 | 56% | 137 | 56% | 56 | 13% | 307 | 34% |
| Genotyping at RRL | 80 | 70% | 100 | 73% | 15 | 27% | 195 | 64% |
Figure 5.
Rotavirus genotype distribution by year, Madagascar.
Figure 6.
Rotavirus genotype according to age groups during pre-vaccine introduction period, in Jun–Dec 2014 and in 2015, Madagascar.
Discussion
Our analysis of retrospective diarrhea hospitalization admissions and laboratory-confirmed rotavirus from a sentinel hospital surveillance site Madagascar following rotavirus vaccine introduction showed an important decrease diarrhea hospitalizations and rotavirus positive stool specimens, particularly in the 0–11 month age group. Prior to vaccine introduction, we identified two annual peaks for diarrhea hospitalizations in April–June and October–November. In the first 2 years following rotavirus vaccine introduction, a peak in diarrheal hospitalizations was observed in June, but with much fewer hospitalizations than the previous years and a less distinct peak was observed later in the year. Although the number of months of laboratory surveillance from before vaccine introduction are limited, the decrease in rotavirus hospitalizations are dramatic and concur with those from diarrhea hospital admissions. To the best of our knowledge, this is the first published description of severe diarrhea and rotavirus disease burden in the pre- and post-rotavirus vaccine introduction in Madagascar.
As rotavirus vaccine was introduced to Madagascar’s national immunization schedule in 2014, we expected a reduction in diarrhea and rotavirus illness among children 0–11 months of age in 2015 and 2016, since this cohort was eligible for vaccination. We also expected a reduction in diarrhea and rotavirus illness children 12–23 months of age in 2016, since they would have been eligible for vaccination as infants the previous year. The nation-wide coverage of rotavirus vaccination estimated by WHO/UNICEF was 39% in 2014 and 69% in 2015(19). We observed the greatest reduction in diarrhea and rotavirus illness in age groups targeted for rotavirus vaccination. These findings are similar to several other countries that have introduced rotavirus vaccine, including Ghana, Rwanda, Malawi, South Africa, Brazil, Mexico, Panama and El Salvador (8, 9, 11, 12, 13, 17, 18, 19, 21, 22, 23, 23). Reductions in diarrhea and rotavirus in age groups not eligible for vaccination, have also been reported by several high-income and middle-income countries, including the USA, Australia, Austria, and El Salvador (10, 11, 17, 25, 26, 27). Rwanda showed also indirect protection of groups not eligible for vaccination two years only after introduction, although indirect effects were not found in Zambia and South Africa (17, 22, 29). In Madagascar, there were very few hospitalized children ages 24–59 months and we did not observed a reduction in diarrhea hospitalizations or rotavirus stools in the 12–23 and 24–59 month old age group. This cohort was not targeted for vaccination during this surveillance period.
Before vaccine introduction, the most common genotypes were G1P[8], G2P[4], andG3P[8], which is similar to findings from the USA and the majority of the countries in Europe (29, 30, 31). In 2015, G2P[4] became the most common genotype in Madagascar. Rotarix has been demonstrated to have high levels of protection against homotypic (G1P[8]) and partially heterotypic G1 strains or P[8]-expressing strains; many studies have also found high levels of protection against heterotypic strains ( 35,36, 37, 38, 39, 40, 41, 42, 43, 44, 45). While this analysis has limited data following vaccine introduction, the rotavirus genotype G1P[8] was not observed after vaccine introduction and genotype G3P[8] also decreased in first year after vaccine implementation. Ongoing surveillance of circulating rotavirus genotypes is important to observe for selective pressure, which could lead to diminished vaccine effectiveness.
This descriptive analysis has several limitations. First, during the surveillance period, not all children hospitalized for diarrhea were tested for rotavirus (758/2168); as decisions about stool testing were made based on the rotavirus case definition, we do not believe this will bias the results. Second, there was only one surveillance sentinel site in the country located in the capital so our results may not be representative of all children in Madagascar. Third, laboratory sentinel hospital surveillance was initiated only 11 months prior to rotavirus vaccine introduction, consequently we are limited in the information we have about rotavirus disease burden prior to vaccine introduction. Additionally, 11 months of surveillance is an imperfect comparison for 12 month periods; nonetheless, we did observe significant reductions in rotavirus positive stools. Finally, data about molecular epidemiology of rotavirus are limited, making it difficult to draw conclusions about the post-vaccine introduction situation in Madagascar.
In summary, hospital admissions for diarrhea and rotavirus declined during the first two years after introduction of rotavirus vaccine in Madagascar among groups targeted for vaccination. These findings are similar to those reported by other countries that had previously introduced rotavirus vaccine. These promising results may be built upon as Madagascar works to improve routine vaccination coverage in future years and as the cohorts of vaccine-eligible children ages.
Acknowledgments
We thank the following people:
Medical staff, medical trainees, samples collector, staff in laboratory from CHU MET, Antananarivo
CICM-Madagascar, Ankatso
WHO
Staff in laboratory MRC/Medunsa Diarrheal Pathogens Research Unit, University of Limpopo, Pretoria, South Africa
Staff in laboratory from National Institute for Communicable Disease of the National Health Laboratory Service in Johannesburg, South Africa
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention (CDC).
References
- 1.World Health 0rganization. Weekly epidemiological record No 47. 2008;83:421–4282. [Google Scholar]
- 2.Babji S, Kang G. Rotavirus vaccination in developing countries. Curr Opin Virol. 2012;2:443–448. doi: 10.1016/j.coviro.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009;84:533–540. [PubMed] [Google Scholar]
- 4.Rotavirus vaccines. WHO position paper—January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- 5.Glass RI, et al. Rotavirus vaccines: Current prospects and challenges. Lancet. 2006;368:323–332. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 7.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(January 4):289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 8.doCarmo GM, Yen C, Cortes J, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011:8. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurgel RG, Bohland AK, Vieira SC, et al. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology. 2009;137:1970–1975. doi: 10.1053/j.gastro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 10.Gastañaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA. 2013;310:851–853. doi: 10.1001/jama.2013.170800. [DOI] [PubMed] [Google Scholar]
- 11.Molto Y, Cortes JE, De Oliveira LH, et al. Reduction of diarrhea-associated hospitalizations among children aged <5 years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2011;30:S16–S20. doi: 10.1097/INF.0b013e3181fefc68. [DOI] [PubMed] [Google Scholar]
- 12.Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations among children < 5 years of age in Mexico. Pediatr Infect Dis J. 2011;30:S11–S15. doi: 10.1097/INF.0b013e3181fefb32. [DOI] [PubMed] [Google Scholar]
- 13.Paulke-Korinek M, Kundi M, Rendi-Wagner P, et al. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine. 2011;29:2791–2796. doi: 10.1016/j.vaccine.2011.01.104. [DOI] [PubMed] [Google Scholar]
- 14.Raes M, Strens D, Vergison A, et al. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J. 2011;30:e120–e125. doi: 10.1097/INF.0b013e318214b811. [DOI] [PubMed] [Google Scholar]
- 15.Zeller M, Rahman M, Heylen E, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine. 2010;28:7507–7513. doi: 10.1016/j.vaccine.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Yen C, Armero Guardado JA, Alberto P, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30:S6–10. doi: 10.1097/INF.0b013e3181fefa05. [DOI] [PubMed] [Google Scholar]
- 17.Ngabo Fidele, Tate Jacqueline E, Gatera Maurice, Rugambwa Celse, Donnen Philippe, Lepage Philippe, Mwenda Jason M, Binagwaho Agnes, Umesh D Parashar. Effect of pentavalent rotavirus vaccine introduction on hospital admissions for diarrhoea and rotavirus in children in Rwanda. The Lancet Global Health. 2016;4(2):e129–136. doi: 10.1016/S2214-109X(15)00270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enweronu-Laryea Christabel C, Boamah Isaac, Sifah Eric, Diamenu Stanley K, Armah George. Decline in severe diarrhea hospitalizations after the introduction of rotavirus vaccination in Ghana: a prevalence study. BMC Infectious Diseases. 2014;14:431. doi: 10.1186/1471-2334-14-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO and UNICEF estimates of immunization coverage: 2015 revision – DRAFT. May, 2016. [Google Scholar]
- 20.Bar-Zeev N, Kapanda L, Tate JE … and the VacSurv Consortium. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis. 2015;15:422–428. doi: 10.1016/S1473-3099(14)71060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groome MJ, Page N, Cortese MM, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14:1096–1104. doi: 10.1016/S1473-3099(14)70940-5. [DOI] [PubMed] [Google Scholar]
- 22.Mismang VM, Page N, Groome MJ, et al. Impact of rotavirus vaccine on childhood diarrheal hospitalization after introduction into the South African public immunization program. Pediatr Infect Dis J. 2013;32:1359–1364. doi: 10.1097/INF.0b013e3182a72fc0. [DOI] [PubMed] [Google Scholar]
- 23.Sáfadi MA, Berezin EN, Munford V, et al. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in São Paulo, Brazil. Pediatr Infect Dis J. 2010;29:1019–1022. doi: 10.1097/INF.0b013e3181e7886a. [DOI] [PubMed] [Google Scholar]
- 24.Lambert SB, Faux CE, Hall L, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust. 2009;191:157–160. doi: 10.5694/j.1326-5377.2009.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 25.Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalisations in South Australian children following the introduction of rotavirus vaccination. Vaccine. 2011;29:4663–4667. doi: 10.1016/j.vaccine.2011.04.109. [DOI] [PubMed] [Google Scholar]
- 26.Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006–2009. Clin Infect Dis. 2011;53:245–253. doi: 10.1093/cid/cir307. [DOI] [PubMed] [Google Scholar]
- 27.Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204:980–986. doi: 10.1093/infdis/jir492. [DOI] [PubMed] [Google Scholar]
- 28.Mpabalwani Evans M, Simwaka Chibumbya J, Mwenda Jason M, Mubanga Cynthia P, Monze Mwaka, Matapo Belem, Parashar Umesh D, Tate Jacqueline E. Impact of Rotavirus Vaccination on Diarrheal Hospitalizations in Children Aged <5 Years in Lusaka. Zambia. 2016;1(62Suppl 2):S183–7. doi: 10.1093/cid/civ1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentsch JR, Hull J, Teel E, et al. G and P types of circulating rotavirus strains in the United States during 1996–2005: nine years of pre-vaccine data. J Infect Dis. 2009 doi: 10.1086/605038. In press. [DOI] [PubMed] [Google Scholar]
- 30.Iturriza-Gómara M, Dallman T, Bányai K, Böttiger B, Buesa J, Diedrich S, et al. Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol Infect. 2011;139(6):895–909. doi: 10.1017/S0950268810001810. [DOI] [PubMed] [Google Scholar]
- 31.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 32.Midgley S, Böttiger B, Jensen TG, Friis-Møller A, Person LK, Nielsen L, et al. Human group A rotavirus infections in children in Denmark; detection of reassortant G9 strains and zoonotic P(14) strains. Infect Genet Evol. 2014;27:114–120. doi: 10.1016/j.meegid.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Anca IA, Furtunescu FL, Pleţca D, Streinu-Cercel A, Rugină S, Holl K. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children below five years of age in Romania. Germs. 2014;4(2):30–40. doi: 10.11599/germs.2014.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boula A, Waku-Kouomou D, NjikiKinkela M, Esona MD, Kemajou G, Mekontso D, et al. Molecular surveillance of rotavirus strains circulating in Yaoundé, Cameroon, September 2007–December 2012. Infect Genet Evol. 2014;28:470–475. doi: 10.1016/j.meegid.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linhares AC, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Palacios GM, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 37.Tregnaghi MW, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30:e103–e108. doi: 10.1097/INF.0b013e3182138278. [DOI] [PubMed] [Google Scholar]
- 38.Phua KB, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009;27:5936–5941. doi: 10.1016/j.vaccine.2009.07.098. [DOI] [PubMed] [Google Scholar]
- 39.Lau YL, et al. Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine. 2013;31:2253–2259. doi: 10.1016/j.vaccine.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Vesikari T, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 41.Correia JB, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201:363–369. doi: 10.1086/649843. [DOI] [PubMed] [Google Scholar]
- 42.Steele AD, et al. Human rotavirus vaccine Rotarix provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect Dis. 2012;12:213. doi: 10.1186/1471-2334-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkwood CD, Boniface K, Barnes GL, Bishop RF. Distribution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix® and RotaTeq®, into the National Immunization Program of Australia. Pediatr Infect Dis J. 2011;30(1, Suppl):S48–S53. doi: 10.1097/INF.0b013e3181fefd90. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho-Costa FA, et al. Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerg Infect Dis. 2009;15:95–97. doi: 10.3201/eid1501.071136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurgel RG, et al. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology. 2009;137:1970–1975. doi: 10.1053/j.gastro.2009.07.046. [DOI] [PubMed] [Google Scholar]