Abstract
Background
In persons with chronic kidney disease (CKD), hemoglobin A1c (HbA1c) may be a problematic measure of glycemic control. Glycated albumin and fructosamine have been proposed as better markers of hyperglycemia in CKD. We investigated associations of HbA1c, glycated albumin, and fructosamine with fasting glucose by CKD categories.
Methods
Cross-sectional analysis of 1,665 Atherosclerosis Risk in Communities Study participants with diagnosed diabetes aged 65 years or older. We compared Spearman’s rank correlations (r) and used Deming regression to obtain root mean square errors (RMSEs) for the associations across CKD categories defined using estimated glomerular filtration rate and urine albumin-to-creatinine ratio.
Results
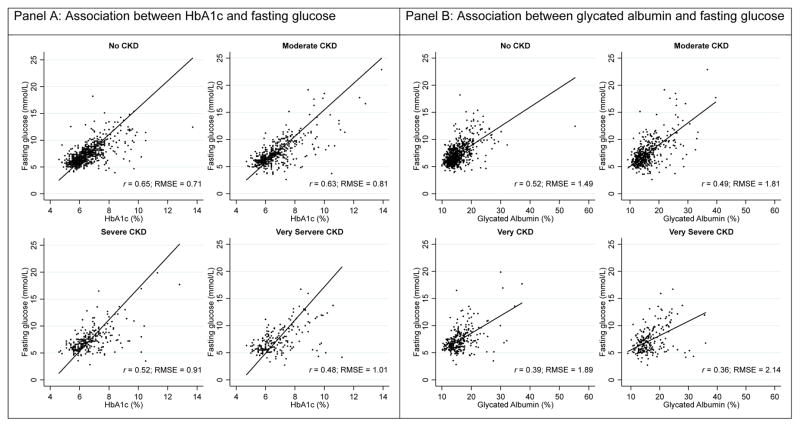
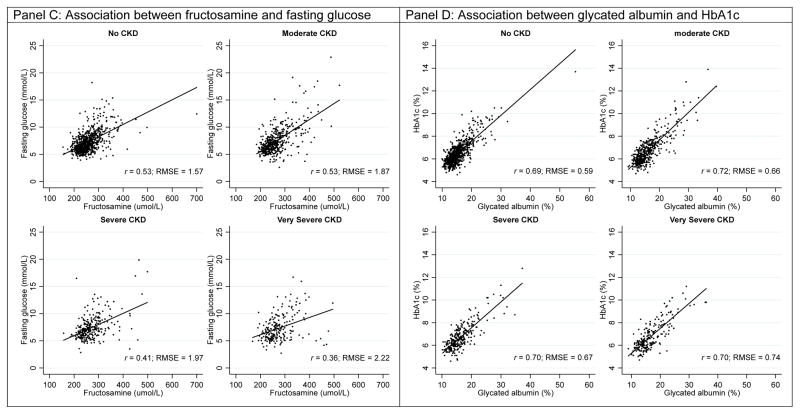
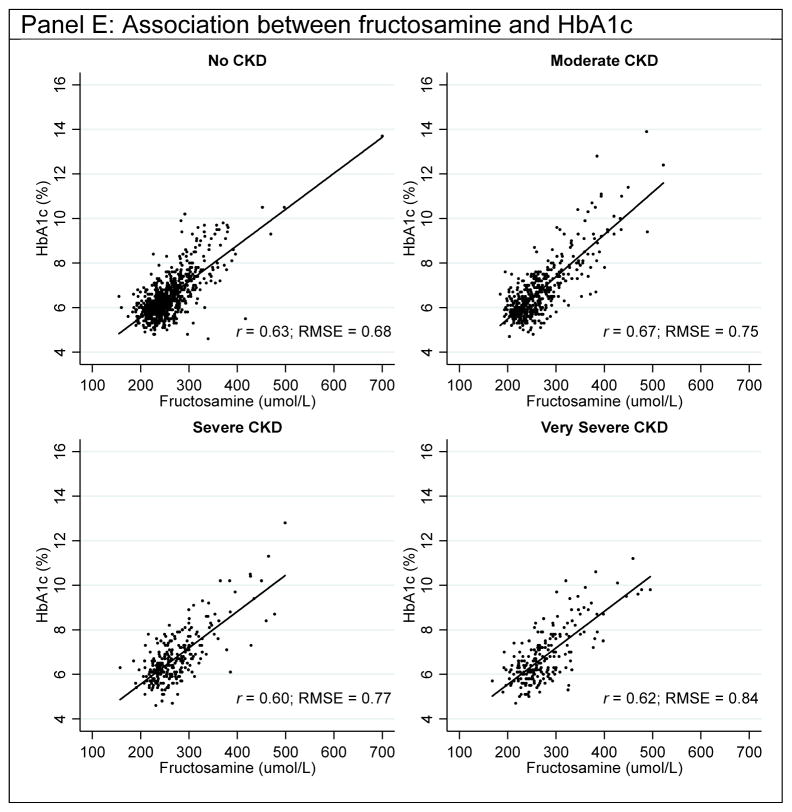
Correlations of HbA1c, glycated albumin, and fructosamine with fasting glucose were lowest in persons with severe CKD (HbA1c r=0.52, RMSE=0.91; glycated albumin r=0.39; RMSE=1.89; fructosamine r=0.41; RMSE=1.87) and very severe CKD (r=0.48 and RMSE=1.01 for HbA1c; r=0.36 and RMSE=2.14 for glycated albumin; r=0.36 and RMSE=2.22 for fructosamine). Associations of glycated albumin and fructosamine with HbA1c were relatively similar across CKD categories.
Conclusions
In older adults with severe or very severe CKD, HbA1c, glycated albumin, and fructosamine were not highly correlated with fasting glucose. Our results suggest there may be no particular advantage of glycated albumin or fructosamine over HbA1c for monitoring glycemic control in CKD.
Keywords: epidemiology, older adults, biomarkers, chronic kidney disease
Introduction
Chronic kidney disease (CKD) is a common condition in older adults with diabetes 1. HbA1c has long been the standard clinical measure of glycemic control in persons with diabetes regardless of CKD status. HbA1c is formed when glucose binds to the N-terminal of the beta chain of hemoglobin in erythrocytes, reflecting glycemic exposure over the past 2–3 months. The interpretation of HbA1c can be problematic when erythrocyte turnover is altered. Impaired erythrocyte turnover often presents as anemia and is common in CKD, even in early stages 2, and thus, concerns have been raised about the performance of HbA1c as a measure of glycemic control in the setting of CKD 3.
Non-traditional markers of hyperglycemia such as glycated albumin and fructosamine have been advocated as better measures of glycemic status as compared to HbA1c in the setting of CKD 4–6. Glycated albumin and fructosamine are ketoamines that are produced when glucose binds to serum albumin and total serum proteins indiscriminately, respectively. Both markers reflect average glycemia over the past 2–3 weeks 7. Prior studies have suggested that HbA1c underestimates hyperglycemia in advanced CKD, but the majority of these studies have been conducted among individuals with advanced stages of kidney disease, including dialysis patients 5,8–10. However, the vast majority of persons with CKD will develop advanced CKD requiring renal replacement therapy and it is unclear whether HbA1c underestimates glycemia in people with early CKD. To our knowledge, no studies have conducted head-to-head comparisons of HbA1c, glycated albumin, and fructosamine with fasting glucose. Furthermore, data comparing HbA1c, glycated albumin, and fructosamine with fasting glucose in the setting of the early stages of CKD are lacking.
The objective of this study was to evaluate if the associations of HbA1c, glycated albumin, and fructosamine with fasting glucose varied by CKD stage and/or anemia status in persons with diabetes.
Methods
Study population
We conducted a cross-sectional analysis using data from the Atherosclerosis Risk in Communities (ARIC) Study, an ongoing prospective cohort study of men and women from four U.S. communities (Minneapolis, Minnesota; Washington County, Maryland; Forsyth County, North Carolina; and Jackson, Mississippi). Participants were recruited in 1987–1989 (visit 1) and had subsequent in-person visits in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). For the present analysis, we included participants who completed the most recent visit (visit 5). Of the 6,538 participants who attended visit 5, we excluded participants without a history of diagnosed diabetes defined by self-report of physician diagnosis and no use of glucose lowering medication (n=4,391), non-white or non-black participants (n=4), black participants from Minneapolis, Minnesota and Washington County, Maryland (n=14), prevalent end-stage-renal-disease (n=33), missing estimated glomerular filtration rate (eGFR, n=46), missing albuminuria (n=179), use erythropoietin stimulating hormone or iron supplementation (n=15), fasting for less than 8 hours (n=89), and missing any of the glycemic markers (fasting glucose, HbA1c, glycated albumin, or fructosamine, n=112) Our final analytic sample was 1,665 participants with diagnosed diabetes.
All study protocols were reviewed and approved by the institutional review boards at all participating institutions and written documentation of informed consent was obtained from all participants.
Classification of Chronic Kidney Disease
CKD categories were defined using the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guidelines 11 using eGFR and albuminuria. We used the 2012 CKD-EPI (CKD Epidemiology Collaboration) equation using serum creatinine and cystatin C, age, sex, and race to estimate GFR 12. Serum creatinine was measured on a Roche Modular P Chemistry Analyzer (Roche Diagnostics) using the creatinase enzymatic method and standardized to isotope-dilution mass spectrometry-traceable reference method. Serum cystatin C was measured using the Gentian immunoassay turbidimetric method (Gentian, Moss, Norway), calibrated and standardized to International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) reference. Albuminuria was assessed as a ratio of urine albumin to urine creatinine (ACR) from spot urine samples expressed as mg of albumin per gram of creatinine. Urine albumin was measured using the nephelometric methods on either the Dade Behring BN100 or the Beckman Image Nephelometer. Urine creatinine was measured using the Jaffe method. Estimated GFR was categorized in these GFR categories: G1, eGFR ≥ 90 mL/min/1.73 m2; G2, eGFR 60–89 mL/min/1.73 m2; G3a, eGFR 45–59 mL/min/1.73 m2; G3b, eGFR 30–44 mL/min/1.73 m2; G4, eGFR 15–29 mL/min/1.73 m2; and G5, eGFR <15 mL/min/1.73 m2. Albuminuria was categorized into these stages: A1, ACR <30 mg/g; A2, ACR 30–300 mg/g; and A3, ACR >300 mg/g. Estimated GFR and albuminuria categories were combined to define four CKD risk categories: low risk (G1/G2 and A1); moderately high-risk (G3a and A1; G1/G2 and A2); high risk (G3b and A1; G3a and A2; G1/G2 and A3); or very high-risk (G3a and A3; G3b and A2/A3; G4/G5). For interpretability, we will refer to the risk categories as “no CKD” (for low risk), “moderate CKD” (for moderately high risk), “severe CKD” (for high risk), and “very severe CKD” (for very high risk).
Measurement of Glucose, HbA1c, Glycated Albumin, and Fructosamine
Glucose was measured from plasma using the hexokinase method. HbA1c was measured in EDTA whole blood using a Tosoh G7 Automated HPLC Analyzer (Tosoh Bioscience, Inc). This instrument uses a non-porus ion-exchange high performance liquid chromatography to accurately and precisely separate stable HbA1c (HbA with glucose bound to the N-terminus of the beta chain) from other hemoglobin components. The percentage of HbA1c is calculated by the analyzer when the separated hemoglobin components pass through the LED photometer flow cell. Glycated albumin and fructosamine were measured in serum using a Roche Modular P800 Chemistry Analyzer (Roche Diagnostics Corporation). Glycated albumin was measured by the Asahi Kasei Pharma method, which separately measures total albumin and glycated albumin. The inter-assay coefficient of variation for HbA1c was 1.9% at a HbA1c value of 5.36 %-points. The inter-assay coefficient of variation for glycated albumin was 2.3% at a concentration of 1.579 g/dL and 2.8% at a concentration of 0.426 g/dL. Fructosamine was measured using a colorimetric assay (Roche Diagnostics Corp). The inter-assay coefficient of variation for fructosamine was 3.2% at a concentration of 212.6 μmol/L and 2.5% at a concentration of 856.7 μmol/L.
Measurement of Other Covariates
Sex and race was self-reported at the baseline examination, all others were collected at visit 5. Hypertension was defined as a mean systolic blood pressure of 140 mm Hg or higher, a diastolic blood pressure of 90 mm Hg or higher, or current use of hypertension medication. Anemia status was defined using World Health Organization’s sex-specific cut-points, hemoglobin <13.0 g/dl for men and <12 g/dl for women 13. Body mass index was calculated using weight and height. Diabetes duration was calculated as the time between the first report of doctor-diagnosed diabetes or self-reported use of glucose-lowering medication and visit 5. Medication use for diabetes, hypertension, cholesterol, and anemia (erythropoietin stimulating agents or iron supplementation) was determined by the medication inventory
Statistical Analysis
Study participant characteristics were summarized according to CKD category at baseline. We evaluated the associations between HbA1c, glycated albumin, and fructosamine with fasting glucose using Spearman’s rank correlation coefficients (r) and used Deming regression to generate regression statistics and root mean square errors (RMSEs) overall and by CKD categories. Ordinary regression requires one variable to be the dependent variable whereas in Deming regression both variables have the same status 14,15. When the slope is equal to 1, then the dependent variable and the independent variable are the same; otherwise, the two variables are different from each other. When the distributions of variables are different between groups, the RMSE is more relevant because the Spearman’s rank correlation coefficient is a function of the distribution of the explanatory variable. The RSME reflects the deviations from the regression line. A lower RMSE value suggests better fit and RMSEs that are consistent across groups indicate that linear associations between the explanatory and independent variables are similar. We graphically displayed the associations between the glycemic markers using scatterplots fitted with Deming regression lines. We further stratified the CKD groups by anemia status to examine whether the associations were different by anemia status.
We conducted sensitivity analyses using CKD defined using eGFR only and albuminuria only. Glycated albumin and fructosamine may also have abnormal values in the setting of abnormal albumin metabolism. We therefore excluded persons with low serum albumin (<3.4 mg/dL). To quantify the association between HbA1c, glycated albumin, and fructosamine with fasting glucose or HbA1c ignoring CKD status, we stratified by the anemia status only. In these analyses, we defined anemia as a 3-level variable: no anemia refers to hemoglobin values 13 g/dL or higher in men and 12.0 g/dL or higher in women; mild anemia refers to hemoglobin values 11.0–12.9 g/dL in men and 11.0–11.9 g/dL in women; and moderate anemia refers to hemoglobin values less than 11.0 g/dL in men and women. Anti-diabetic medication can alter glucose metabolism in the short and long-term so we stratified CKD categories by any or no anti-diabetic medication use. We also further stratified CKD categories by race and sex to evaluate if associations of HbA1c, glycated albumin, and fructosamine with fasting glucose differed by these factors. Postprandial hyperglycemia has been postulated to influence the correlation between fasting glucose and HbA1c by diabetes severity 16, therefore we also stratified HbA1c (less than 7%, 7 to less than 8%, and 8% or greater).
Results
In our study population of 1,665 participants with diagnosed diabetes, the prevalence of moderate CKD was 27.9%, severe CKD was 16.1%, and very severe CKD was 12.6%. Older age, hypertension, obesity, duration of diabetes, and anemia were more common at more severe CKD stages (Table 1). The prevalence of anemia was 21.0% in people without CKD, 30.6% in moderate CKD, 43.7% in severe CKD, and 54.6% in very severe CKD. The medians of HbA1c, glycated albumin, and fructosamine were 6.4%, 14.9%, and 254.1 mmol/dL, respectively. The median of these glycemic markers was lowest in persons with no CKD and highest in persons with very severe CKD.
Table 1.
Characteristics of study participants with diagnosed diabetes by chronic kidney disease (CKD) categories, ARIC Visit 5 (2011–2013)
| Variable | CKD Categories | |||
|---|---|---|---|---|
|
| ||||
| No CKD | Moderate CKD | Severe CKD | Very Severe CKD | |
| N=724 | N=464 | N=268 | N=209 | |
| Age (years), mean (SD) | 74.9 (4.7) | 76.4 (5.1) | 77.2 (5.4) | 78.3 (5.6) |
| Female, % | 52.2 | 56.3 | 58.6 | 49.3 |
| Black, % | 30.5 | 28.2 | 24.1 | 27.3 |
| Body mass index (kg/m2), % | ||||
| <25 | 17.4 | 13.5 | 14.5 | 13.2 |
| 25–<30 | 35.7 | 38.8 | 32.2 | 35.6 |
| ≥30 | 46.9 | 47.7 | 53.3 | 51.2 |
| Hypertension†, % | 80.3 | 88.1 | 89.9 | 95.1 |
| Cholesterol lowering med, % | 69.6 | 71.8 | 73.5 | 75.1 |
| Diabetes duration (years), mean (SD) | 9.4 (5.5) | 10.6 (6.3) | 12.2 (6.2) | 13.2 (6.8) |
| Diabetes medication use, % | ||||
| None | 41.3 | 37.5 | 33.7 | 32.7 |
| Oral medication only | 49.0 | 45.9 | 44.8 | 41.5 |
| Insulin only | 3.2 | 10.0 | 8.8 | 13.7 |
| Oral and insulin use | 6.0 | 6.4 | 12.3 | 11.2 |
| Hemoglobin (g/l), mean (SD) | 133.9 (13.6) | 130.7 (14.3) | 127.7 (24.4) | 123.1 (16.3) |
| Anemia‡, % | 21.0 | 30.6 | 43.7 | 54.6 |
| Fasting glucose (mmol/L), median (Q1–Q3) | 6.8 (6.0–8.1) | 6.9 (5.9–8.3) | 7.0 (6.0–8.3) | 6.8 (5.6–8.5) |
| HbA1c (%), median (Q1–Q3) | 6.3 (5.8–6.9) | 6.4 (5.9–7.2) | 6.5 (6.0–7.3) | 6.4 (6.0–7.4) |
| Glycated albumin (%), median (Q1–Q3) | 14.5 (13.1–16.6) | 14.9 (13.2–17.3) | 15.4 (13.8–17.7) | 15.7 (13.7–19.1) |
| Fructosamine (umol/L), median (Q1–Q3) | 249.8 (229.1–277.3) | 251.8 (230.5–282.6) | 259.5 (236.5–291.8) | 264.4 (240.2–297.4) |
Hypertension was defined as a mean systolic blood pressure greater than 140 mmHg or mean diastolic blood pressure greater than 90 mmHg or current anti-hypertension medication use.
Anemia was defined using sex-specific cut-points of hemoglobin 130 g/L for men and 120 g/l for women.
Note: Chronic kidney disease staging was done using the KDIGO 2012 guidelines with eGFR and albumin-to-creatinine ratio.
In the overall study population, the association between HbA1c with fasting glucose was stronger (r=0.60, RMSE=0.82) than the association between glycated albumin and fructosamine with fasting glucose (respectively, r=0.46; RMSE=1.75 and r=0.48; RMSE=1.83, Table 2). The associations of HbA1c, glycated albumin, and fructosamine with fasting glucose slightly differed across CKD categories. The association between HbA1c and fasting glucose was strongest in persons without CKD (r=0.65) and weakest in persons with very severe CKD (r=0.48). The RMSE was approximately 40% larger in the very severe CKD group compared to the no CKD group (very severe CKD: RMSE=1.01 vs. no CKD: RMSE=0.71). This trend was also observed when comparing glycated albumin and fructosamine with fasting glucose. By contrast, when glycated albumin and fructosamine were compared to HbA1c, the Spearman’s rank correlation coefficients were similar across CKD categories and the RMSE was only moderately higher in people with very severe CKD compared to people without CKD. Similar results were observed with CKD defined using eGFR only (Supplemental Table 1), albuminuria only (Supplemental Table 2), and when excluding persons with very low serum albumin (Supplemental Table 3). Scatterplots of HbA1c, glycated albumin, and fructosamine with fasting glucose or HbA1c with the Deming regression line by CKD categories are presented in Figure 1.
Table 2.
Spearman’s correlation coefficients and summary statistics from linear (Deming) regression (unadjusted) for HbA1c or fasting glucose on glycated albumin or fructosamine stratified by chronic kidney disease (CKD) categories*
| CKD Category | Spearman’s r | Deming Regression | |||
|---|---|---|---|---|---|
|
| |||||
| Intercept | Slope | RMSE | |||
| HbA1c, % (X) and fasting glucose, mmol/L (Y) | Overall | 0.60 | −9.85 | 2.60 | 0.82 |
| No CKD | 0.65 | −9.00 | 2.51 | 0.71 | |
| Moderate CKD | 0.63 | −8.91 | 2.44 | 0.81 | |
| Severe CKD | 0.52 | −12.20 | 2.92 | 0.91 | |
| Very Severe | 0.48 | −13.45 | 3.06 | 1.01 | |
|
| |||||
| Glycated albumin, % (X) and fasting glucose, mmol/L (Y) | Overall | 0.46 | 1.85 | 0.35 | 1.75 |
| No CKD | 0.52 | 1.86 | 0.35 | 1.49 | |
| Moderate CKD | 0.49 | 1.02 | 0.40 | 1.81 | |
| Severe CKD | 0.39 | 2.13 | 0.32 | 1.89 | |
| Very Severe | 0.36 | 2.87 | 0.26 | 2.14 | |
|
| |||||
| Fructosamine, umol/L (X) and fasting glucose, mmol/L (Y) | Overall | 0.48 | 1.29 | 0.02 | 1.83 |
| No CKD | 0.53 | 1.42 | 0.02 | 1.57 | |
| Moderate CKD | 0.53 | −0.30 | 0.03 | 1.87 | |
| Severe CKD | 0.41 | 1.89 | 0.02 | 1.97 | |
| Very Severe | 0.36 | 2.93 | 0.02 | 2.22 | |
|
| |||||
| Glycated albumin, % (X) and HbA1c, % (Y) | Overall | 0.70 | 2.95 | 0.23 | 0.64 |
| No CKD | 0.69 | 2.99 | 0.23 | 0.59 | |
| Moderate CKD | 0.72 | 2.82 | 0.24 | 0.66 | |
| Severe CKD | 0.70 | 2.99 | 0.23 | 0.67 | |
| Very Severe | 0.70 | 3.09 | 0.22 | 0.74 | |
|
| |||||
| Fructosamine, umol/L (X) and HbA1c, % (Y) | Overall | 0.63 | 2.12 | 0.02 | 0.74 |
| No CKD | 0.63 | 2.33 | 0.02 | 0.68 | |
| Moderate CKD | 0.67 | 1.70 | 0.02 | 0.75 | |
| Severe CKD | 0.60 | 2.30 | 0.02 | 0.77 | |
| Very Severe | 0.62 | 2.27 | 0.02 | 0.84 | |
Note: Chronic kidney disease staging was done using the KDIGO 2012 guidelines with eGFR and albumin-to-creatinine ratio.
Figure 1.
Scatterplots of HbA1c, glycated albumin, and fructosamine with fasting glucose or HbA1c by CKD categories
Solid black line is the Deming linear regression (Table 2); dashed line is the Lowess line.
Note: Chronic kidney disease staging was done using the KDIGO 2012 guidelines with eGFR and albumin-to-creatinine ratio.
In analyses assessing at anemia within CKD status, the weakening in the association between HbA1c, glycated albumin, and fructosamine with fasting glucose was more pronounced in people with anemia than in people without anemia by CKD category (Table 3). Similar results were observed when stratifying by anemia status only. In analyses stratified by anemia status only, the associations between HbA1c with fasting glucose were strongest in people without anemia and weakest in people with moderate anemia (Supplemental Table 4). The associations between glycated albumin and fructosamine with fasting glucose were different by anemia status as indicated by the different Deming regression slopes. In analyses that compared glycated albumin and fructosamine to fasting glucose or HbA1c, we observed lower Spearman’s rank correlation coefficients in people with moderate anemia compared to people without anemia but the RMSEs were relatively preserved.
Table 3.
Spearman’s correlation coefficients and summary statistics from linear (Deming) regression (unadjusted) for HbA1c or fasting glucose on glycated albumin or fructosamine stratified by CKD category and anemia status†
| CKD categories | With anemia N=525 |
Without anemia N=1,140 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Spearman’s r | Deming Regression | Spearman’s r | Deming Regression | ||||||
|
|
|
||||||||
| Intercept | Slope | RMSE | Intercept | Slope | RMSE | ||||
| HbA1c, % (X) and fasting glucose, mmol/L (Y) | No CKD | 0.59 | −12.84 | 3.00 | 0.72 | 0.70 | −7.93 | 2.36 | 0.69 |
| Moderate CKD | 0.62 | −9.33 | 2.48 | 0.72 | 0.64 | −8.57 | 2.40 | 0.85 | |
| Severe CKD | 0.53 | −16.20 | 3.48 | 0.89 | 0.52 | −10.11 | 2.65 | 0.91 | |
| Very Severe CKD | 0.35 | −12.78 | 2.89 | 1.15 | 0.67 | −12.60 | 3.02 | 0.76 | |
|
| |||||||||
| Glycated albumin, % (X) and fasting glucose, mmol/L (Y) | No CKD | 0.48 | 0.12 | 0.44 | 1.52 | 0.56 | 2.11 | 0.34 | 1.47 |
| Moderate CKD | 0.51 | 2.12 | 0.32 | 1.57 | 0.50 | 0.46 | 0.45 | 1.87 | |
| Severe CKD | 0.40 | 3.27 | 0.23 | 1.67 | 0.41 | 0.50 | 0.45 | 1.90 | |
| Very Severe CKD | 0.29 | 3.71 | 0.19 | 2.09 | 0.53 | −1.23 | 0.57 | 1.79 | |
|
| |||||||||
| Fructosamine, umol/L (X) and fasting glucose, mmol/L (Y) | No CKD | 0.49 | 0.90 | 0.02 | 1.68 | 0.54 | 1.59 | 0.02 | 1.55 |
| Moderate CKD | 0.54 | 1.13 | 0.02 | 1.62 | 0.54 | −1.06 | 0.03 | 1.95 | |
| Severe CKD | 0.43 | 3.29 | 0.01 | 1.71 | 0.41 | 0.35 | 0.03 | 2.10 | |
| Very Severe CKD | 0.28 | 3.90 | 0.01 | 2.16 | 0.50 | −0.72 | 0.03 | 2.08 | |
|
| |||||||||
| Glycated albumin, % (X) and HbA1c, % (Y) | No CKD | 0.67 | 3.01 | 0.23 | 0.63 | 0.68 | 2.97 | 0.23 | 0.57 |
| Moderate CKD | 0.72 | 3.44 | 0.20 | 0.63 | 0.72 | 2.50 | 0.27 | 0.63 | |
| Severe CKD | 0.63 | 3.47 | 0.19 | 0.68 | 0.74 | 2.43 | 0.27 | 0.62 | |
| Very Severe CKD | 0.72 | 2.95 | 0.22 | 0.75 | 0.71 | 2.91 | 0.24 | 0.69 | |
|
| |||||||||
| Fructosamine, umol/L (X) and HbA1c, % (Y) | No CKD | 0.61 | 2.79 | 0.01 | 0.72 | 0.64 | 2.22 | 0.02 | 0.66 |
| Moderate CKD | 0.68 | 2.65 | 0.02 | 0.70 | 0.67 | 1.19 | 0.02 | 0.74 | |
| Severe CKD | 0.55 | 3.09 | 0.01 | 0.75 | 0.64 | 1.61 | 0.02 | 0.79 | |
| Very Severe CKD | 0.64 | 2.05 | 0.02 | 0.83 | 0.62 | 2.59 | 0.02 | 0.85 | |
Anemia was defined using sex-specific cut-points of hemoglobin 130 g/l for men and 120 g/l for women.
Note: Chronic kidney disease staging was done using the KDIGO 2012 guidelines with eGFR and albumin-to-creatinine ratio.
In sensitivity analyses, we did not observe major differences by anti-diabetic medication use (Supplemental Table 5), race (Supplemental Table 6), or sex (Supplemental Table 7). In analyses that stratified CKD categories by HbA1c, the RMSE was highest in persons with HbA1c >8%. Additionally, the RMSE tends to increase by severity of CKD, however the differences are very modest (Supplemental Table 8). These values are likely unstable and should be interpreted with caution given the limited sample size after the additional stratification by HbA1c, particularly in persons with very severe CKD.
Discussion
In this cohort of older adults with diagnosed diabetes, CKD was common and anemia was prevalent across all CKD categories. In people with very severe CKD, HbA1c, glycated albumin, and fructosamine were all poorly correlated with fasting glucose. However, the associations between glycated albumin and fructosamine with HbA1c were relatively preserved across CKD categories, regardless of anemia status. Our findings suggest that HbA1c performs similarly to glycated albumin and fructosamine in persons with CKD.
HbA1c is the standard measure recommended for use for monitoring glycemic control in persons with diabetes, regardless of CKD status 17,18. The American Diabetes Association and the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Clinical Guidelines state that HbA1c may be limited in the setting of CKD but that there is insufficient evidence to recommend use of glycated albumin or fructosamine over HbA1c.
There are a number of factors that can interfere with the measurement or interpretation of HbA1c. Factors associated with shortened erythrocyte survival or decreases in mean erythrocyte age may affect the interpretation of HbA1c because these factors reduce the time for the chemical reaction between hemoglobin and glucose. HbA1c is particularly problematic in the setting of dialysis because of the high prevalence of severe anemia potentially from blood loss (i.e., treatment-related phlebotomy), uremic-induced inhibitors of erythropoiesis, and shortened erythrocyte survival. Concerns have also been raised about the performance of HbA1c as a measure of glycemic control in the setting of earlier stages of CKD because anemia is common in early stages of CKD.
Glycated albumin and fructosamine are glycated serum proteins that are attractive as alternatives to HbA1c because they are not affected by hemoglobin-related factors or erythrocyte turnover. Glycated albumin and fructosamine are also vulnerable to conditions that alter their values independent of average glucose. Indeed, the interpretation of glycated albumin and fructosamine may be limited in the setting of CKD due to proteinuria and impaired protein homeostasis 19
Previous studies that have examined the associations of HbA1c and glycated albumin with fasting glucose have shown weaker correlations in people on dialysis compared to those not on dialysis, and postulated that HbA1c underestimates glycemic control in participants with diabetes on dialysis 8,9. Because of the perceived superiority of glycated albumin over HbA1c in the setting of dialysis, some countries, including Japan and Korea, recommend using glycated albumin over HbA1c to monitor glycemic control in dialysis patients. We found, however, that HbA1c was consistently associated with glycated albumin and fructosamine across CKD categories, suggesting that, at least outside the setting of dialysis, all three biomarkers may be performing similarly as measures of hyperglycemia.
Anemia is common in older adults and in people with diabetes, particularly in people with CKD 22,23, and is thought to pose major problems for the interpretation of HbA1c as a measure of hyperglycemia. Nonetheless, findings for the performance of HbA1c in the setting of anemia have been mixed and may be attributed to differences in the cause of anemia between populations 24,25. In our study, the association between HbA1c and fasting glucose was strongest in people without anemia and weakest in people with anemia (Supplemental Table 3). Regardless of anemia status, we observed poor associations between HbA1c, glycated albumin, and fructosamine with fasting glucose in people with severe or very severe CKD. By contrast, we observed consistently concordant associations of glycated albumin, fructosamine, and HbA1c with each other across CKD categories and anemia status. HbA1c, glycated albumin, and fructosamine all reflect chronic (average) glucose exposure and, as measures of glycated proteins, are more biologically similar to each other as compared to fasting glucose. It is notable that the Spearman’s rank correlation was higher in people without anemia compared to people with moderate anemia. This may be because the range of glycated albumin and fructosamine is wider in people with no anemia compared to people with moderate anemia in our analytic sample.
There is a large body of literature demonstrating a strong link between HbA1c and clinical complications including in persons with CKD 26–30. In randomized clinical trials, reducing HbA1c in people with CKD have been associated with fewer microvascular complications 31,32. There is a growing literature demonstrating the prognostic value of glycated albumin and fructosamine with clinical outcomes 33–36, but there have been few studies on the performance of these biomarkers in persons with CKD 10,37–39. There are presently no clinical trial data demonstrating the effectiveness of glycated albumin or fructosamine as glycemic targets in persons with moderate CKD. The overwhelming body of evidence for monitoring glycemic control in CKD is derived from studies of HbA1c, which remains the gold standard measure of glycemic control regardless of CKD status.
The poor correlations of HbA1c, glycated albumin, and fructosamine with fasting glucose may partially reflect difficulties in the interpretation of fasting glucose in this population. Fasting glucose has higher within-person variability compared to the other three biomarkers 40,41. In our study population, 62% of the participants were on glucose-lowering medications, suggesting that a single fasting measure in this population may be discordant with measures of “usual” glycemic control. Although, in analyses stratified for glucose-lowering medication use, we observed similar results. In CKD specifically, fasting glucose values may be influenced by drug clearance and impaired gluconeogenesis. Thus, fasting glucose may be a problematic reference standard in the setting of CKD. Additionally, a single measure of fasting glucose does not reflect postprandial hyperglycemia; HbA1c, glycated albumin, and fructosamine are all influenced by non-fasting as well as fasting glucose concentrations.
Our results should be interpreted in the context of our study limitations. We cannot definitively determine which biomarker is “best” for monitoring glycemic control in the setting of CKD absent an ideal measure of hyperglycemia. An additional limitation of our study was the limited sample size of persons with severe or very severe CKD, reflecting the community-based study population.
Strengths of our study included the ability to conduct head-to-head comparisons of multiple glycemic markers in the setting of a diverse community-based study population of older adults with diabetes. In addition, CKD status was rigorously characterized with the availability of creatinine, cystatin C, and urine albumin and creatinine.
In our cohort of community-based participants with diagnosed diabetes, HbA1c, glycated albumin, and fructosamine were inconsistently associated with fasting glucose in people with severe to very severe CKD. However, glycated albumin and fructosamine were similarly associated with HbA1c across CKD categories, regardless of anemia status. Our results in an older population suggest that glycated albumin and fructosamine have similar performance to HbA1c and may not necessarily overcome limitations of HbA1c in persons with CKD. Future studies with prospective follow-up for clinical outcomes are needed to help determine the best biomarker for glycemic control in older adults with diabetes and CKD. The use of continuous blood glucose monitors to estimate average glucose also has potential for helping to address these questions as direct measures of circulating average glucose could provide a better reference standard. In the context of the current evidence, our study supports the American Diabetes Association and Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines and suggests that it is premature to recommend glycated albumin or fructosamine over HbA1c as measures of glucose control in adults with moderate to severe CKD.
Supplementary Material
Highlights.
Glycated albumin and fructosamine were similarly associated with hemoglobin A1c but not fasting glucose across chronic kidney disease stages in older adults with diagnosed diabetes.
Our data suggests that the limitations of hemoglobin A1c may not be overcome by glycated albumin or fructosamine in the setting of chronic kidney disease.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). MJ and BW were supported by NIH/NHLBI grant T32 HL007024. TS was supported by R03-DK-104012 and R01-HL-132372-01. This research was supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK089174 and K24DK106414, awarded to ES. CMR was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782). We thank the staff and participants of the ARIC study for their important contributions. Reagents for the glycated albumin assays were donated by the Asahi Kasei Pharma Corporation. Reagents for the fructosamine assays were donated by Roche Diagnostics.
Footnotes
Disclosure. No potential conflicts of interest relevant for this article were reported.
References
- 1.National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2010. 2010. [Google Scholar]
- 2.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little RR, Tennil ALW, England JD, et al. Can glycohemoglobin (GHB) be used to accurately assess glycemic control in patients with chronic renal failure (CRF)? Diabetes. 1999;48(5):SA84–SA84. [Google Scholar]
- 4.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18(3):896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 5.Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus. 2011;4(6):368–375. doi: 10.1093/ndtplus/sfr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology. 2012;17(2):182–188. doi: 10.1111/j.1440-1797.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 7.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14(11):1–10. doi: 10.1007/s11892-014-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A1c levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73(9):1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 9.Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int. 2010;30(1):72–79. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 10.Chen CW, Drechsler C, Suntharalingam P, Karumanchi SA, Wanner C, Berg AH. High Glycated Albumin and Mortality in Persons with Diabetes Mellitus on Hemodialysis. Clin Chem. 2016 doi: 10.1373/clinchem.2016.258319. clinchem-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):e150. [Google Scholar]
- 12.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izaks GJ, Westendorp RGJ, Knook DL. The definition of anemia in older persons. Jama. 1999;281(18):1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 14.Cornbleet PJ, Gochman N. Incorrect least-squares regression coefficients in method-comparison analysis. Clin Chem. 1979;25(3):432LP-438. http://clinchem.aaccjnls.org/content/25/3/432.abstract. [PubMed] [Google Scholar]
- 15.Linnet K. Evaluation of regression procedures for methods comparison studies. Clin Chem. 1993;39(3):424LP-432. http://clinchem.aaccjnls.org/content/39/3/424.abstract. [PubMed] [Google Scholar]
- 16.Monnier L, Colette C, Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12(Supplement 1):42–46. doi: 10.4158/EP.12.S1.42. [DOI] [PubMed] [Google Scholar]
- 17.Foundation NK. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Association AD. 6. Glycemic targets. Diabetes Care. 2017;40(Supplement 1):S48–S56. doi: 10.2337/dc17-S009. [DOI] [PubMed] [Google Scholar]
- 19.Schleicher ED, Olgemöller B, Wiedenmann E, Gerbitz KD. Specific glycation of albumin depends on its half-life. Clin Chem. 1993;39(4):625–628. [PubMed] [Google Scholar]
- 20.Chen H-S, Wu T-E, Lin H-D, et al. Hemoglobin A 1c and fructosamine for assessing glycemic control in diabetic patients with CKD stages 3 and 4. Am J kidney Dis. 2010;55(5):867–874. doi: 10.1053/j.ajkd.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23(2):77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G. Unrecognized anemia in patients with diabetes. Diabetes Care. 2003;26(4):1164–1169. doi: 10.2337/diacare.26.4.1164. [DOI] [PubMed] [Google Scholar]
- 23.Eisenstaedt R, Penninx BWJH, Woodman RC. Anemia in the elderly: current understanding and emerging concepts. Blood Rev. 2006;20(4):213–226. doi: 10.1016/j.blre.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2010;33(4):780–785. doi: 10.2337/dc09-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Min W-K, Chun S, Lee W, Park H. Glycated albumin may be a possible alternative to hemoglobin A1c in diabetic patients with anemia. Clin Chem Lab Med. 2011;49(10):1743–1747. doi: 10.1515/CCLM.2011.646. [DOI] [PubMed] [Google Scholar]
- 26.Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909–913. doi: 10.2337/diacare.24.5.909. [DOI] [PubMed] [Google Scholar]
- 27.Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis. Diabetes Care. 2006;29(7):1496–1500. doi: 10.2337/dc05-1887. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez SPB, McCullough KP, Thumma JR, et al. Hemoglobin A1c Levels and Mortality in the Diabetic Hemodialysis Population. Diabetes Care. 2012;35(12):2527–2532. doi: 10.2337/dc12-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill CJ, Maxwell AP, Cardwell CR, et al. Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Am J Kidney Dis. 2014;63(1):84–94. doi: 10.1053/j.ajkd.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Trivin C, Metzger M, Haymann J-P, et al. Glycated hemoglobin level and mortality in a nondiabetic population with CKD. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.08540814. CJN-08540814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 32.The Relationship of Glycemic Exposure (HbA1c) to the Risk of Development and Progression of Retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44(8):968LP-983. http://diabetes.diabetesjournals.org/content/44/8/968.abstract. [PubMed] [Google Scholar]
- 33.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279–288. doi: 10.1016/S2213-8587(13)70199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selvin E, Rawlings AM, Lutsey P, et al. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation. 2015;132(4):269–277. doi: 10.1161/CIRCULATIONAHA.115.015415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmström H, Walldius G, Grill V, Jungner I, Hammar N. Fructosamine is a risk factor for myocardial infarction and all-cause mortality–Longitudinal experience from the AMORIS cohort. Nutr Metab Cardiovasc Dis. 2015;25(10):943–950. doi: 10.1016/j.numecd.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Shurraw S, Hemmelgarn B, Lin M, et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171(21):1920–1927. doi: 10.1001/archinternmed.2011.537. [DOI] [PubMed] [Google Scholar]
- 37.Freedman BI, Andries L, Shihabi ZK, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol. 2011;6(7):1635–1643. doi: 10.2215/CJN.11491210. [DOI] [PubMed] [Google Scholar]
- 38.Shafi T, Sozio SM, Plantinga LC, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2012 doi: 10.2337/dc12-1896. DC_121896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafi T, Sozio SM, Plantinga LC, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2013;36(6):1522–1533. doi: 10.2337/dc12-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 41.Parrinello CM, Lutsey PL, Couper D, et al. Total Short-term Variability in Biomarkers of Hyperglycemia in Older Adults. Clin Chem. 2015;61(12):1540–1541. doi: 10.1373/clinchem.2015.246231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.