Abstract
The thalamus is a key sensorimotor relay area that is implicated in autism spectrum disorder (ASD). However, it is unknown how the thalamus and white-matter structures that contain thalamo-cortical fiber connections (e.g., the internal capsule) develop from childhood into adulthood and whether this microstructure relates to basic motor challenges in ASD. We used diffusion weighted imaging in a cohort-sequential design to assess longitudinal development of the thalamus, and posterior- and anterior-limbs of the internal capsule (PLIC and ALIC, respectively) in 89 males with ASD and 56 males with typical development (3–41 years; all verbal). Our results showed that the group with ASD exhibited different developmental trajectories of microstructure in all regions, demonstrating childhood group differences that appeared to approach and, in some cases, surpass the typically developing group in adolescence and adulthood. The PLIC (but not ALIC nor thalamus) mediated the relation between age and finger-tapping speed in both groups. Yet, the gap in finger-tapping speed appeared to widen at the same time that the between-group gap in the PLIC appeared to narrow. Overall, these results suggest that childhood group differences in microstructure of the thalamus and PLIC become less robust in adolescence and adulthood. Further, finger-tapping speed appears to be mediated by the PLIC in both groups, but group differences in motor speed that widen during adolescence and adulthood suggest that factors beyond the microstructure of the thalamus and internal capsule may contribute to atypical motor profiles in ASD.
Keywords: Thalamus, Internal Capsule, Autism Spectrum Disorder, Diffusion Magnetic Resonance Imaging, White Matter
Introduction
The thalamus is commonly referred to as the relay center of the brain because of its abundant connections with the cortex and involvement in multimodal sensory processing. Lesions of the thalamic nuclei have been associated with complex-movement deficits (Lee & Marsden, 1994), somatosensory challenges (Graff-Radford et al., 1985), and visuomotor impairments (Fabre & Buser, 1980). Yet, we know very little about how the thalamus develops in autism spectrum disorder (ASD), a disorder in which sensorimotor symptoms are commonly reported (Baranek et al., 2014; Fournier et al., 2010; Ming et al., 2007).
A growing body of evidence has begun to implicate the thalamus in ASD neuropathology. While there is still uncertainty about whether there are volumetric abnormalities of the thalamus (see Herbert et al., 2003; Lin et al., 2015; Tamura et al., 2010; Tsatsanis et al., 2003; Waiter et al., 2004; or in contrast, Bigler et al., 2010; Hardan et al., 2007, 2006; Haznedar et al., 2006; Lange et al., 2015; Schuetze et al., 2016), functional magnetic resonance imaging studies have been more consistent in demonstrating patterns of atypical activation of sensorimotor thalamic networks in ASD (Cerliani et al., 2015; Green et al., 2016; Nair et al., 2015, 2013; Woodward et al., 2017). This pattern appears to be primarily characterized by thalamic hyperconnectivity. For example, functional connectivity between the motor regions of the thalamus and the cortex in individuals with ASD was found to be hyperconnected during a visuomotor task (Mizuno et al., 2006). In another study, motor-thalamic hyperconnectivity related to lower IQ in ASD (Woodward et al., 2017), and increased pulvinar-amygdala hyperconnectivity related to greater sensory over reactivity in ASD (Green et al., 2016). Combined, these morphometric and functional neuroimaging data support the role of the thalamus and its projections in ASD symptomatology. However, when and why these thalamic atypicalities develop remains unclear.
Irregularities in thalamic functional connectivity in ASD may be related to microstructural atypicalities in both the thalamus and white matter connections between the thalamus and other brain regions. Diffusion tensor imaging (DTI) measures the restricted Brownian motion of water diffusion that is sensitive to microstructural tissue properties of the human brain. Traditional DTI measures include fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD), and these measures provide complementary information about the microstructure of tissues throughout the brain. In ASD, previous DTI research has shown aberrant microstructural properties in white-matter areas including the corpus callosum, superior temporal gyrus, and left arcuate fasciculus (for a review, see Travers et al., 2012). Multiple studies suggest microstructural atypicalities in both the anterior and posterior limbs of the internal capsule (ALIC and PLIC), white-matter tracts that interface with the thalamus. In the ALIC and PLIC, the results suggest reduced FA and increased MD and RD in children and adolescents with ASD (Brito et al., 2009; Cheng et al., 2010; Ogur and Boyunaga, 2015; Shukla et al., 2011; Vogan et al., 2016). Other white-matter tracts that interface with the thalamus are also reported as atypical in ASD. Nair and colleagues (2013, 2015) reported increased MD and RD in thalamic projections to the somatosensory and motor cortices in adolescents with ASD, as well as trend-level group differences in the MD and RD of the thalamus proper. While the DTI measurements cannot specify the underlying white-matter pathology, this pattern of atypical microstructure (i.e., decreased FA and increased MD and RD) has previously been associated with myelination atypicalities (Alexander et al., 2011).
The atypical microstructure reported in white matter that interfaces with the thalamus may be intimately related to core ASD symptoms. For example, evidence suggests that reduced FA values in thalamic-related, white-matter projections were associated with autism symptom severity (Cheon et al., 2011) and self-injurious behaviors (Duerden et al., 2014). Further, FA of the ALIC has been associated with stereotypic behavior, social withdrawal, and social responsiveness scores (Cheon et al., 2011; Ogur & Boyunaga, 2015). However, while the underlying microstructure undergoes rapid development across the lifespan (Lebel & Beaulieu, 2011), little is known regarding how the microstructural properties of the thalamus and surrounding white matter develop and change with age in ASD. An examination into the microstructural development of the thalamus and key white matter tracts that interface with the thalamus may provide insights into the underlying neurobiology of ASD and underpinnings of sensorimotor difficulties in this population. While the thalamus interfaces with a number of different white matter tracts, it may be particularly important to examine the developmental trajectories of the superior ALIC and PLIC, as these tracts have been commonly reported as having atypical microstructure in ASD (Brito et al., 2009; Cheng et al., 2010; Ogur & Boyunaga, 2015; Shukla et al., 2011; Vogan et al., 2016) and because these tracts previously have been associated with ASD symptoms (Cheon et al., 2011; Ogur & Boyunaga, 2015).
To date, there are no reports of how DTI microstructural measures of the thalamus and internal capsule develop from childhood to mid-adulthood in ASD and whether these measures appear to be related to sensorimotor challenges commonly reported in this population. Therefore, the aims of this study were to: (1) compare the longitudinal development of the thalamus and internal capsule microstructure between the ASD and typically developing groups and (2) examine if the microstructure of the thalamus and internal capsule relates to sensorimotor measures. The thalamus subserves many sensorimotor functions, but for the present analyses, the finger-tapping task was selected as our behavioral sensorimotor measure for two reasons: 1) studies in typical development and individuals with Parkinson’s disease show this task engages the thalamus (Moritz et al., 2000; Wurster et al., 2015) and 2) atypical development of finger-tapping speed previously has been reported in ASD (Duffield et al., 2013; Travers et al., 2017). We hypothesized that microstructure in the thalamus and surrounding PLIC and ALIC tracts would have a different developmental trajectory in ASD compared to typical development. Further, we hypothesized that these microstructural measures would relate to motor speed challenges in ASD.
Methods
Design
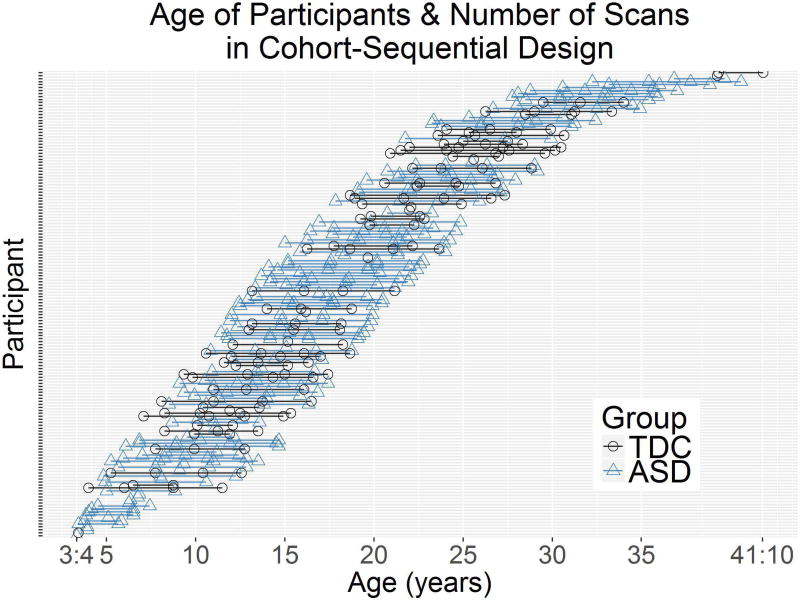
These data were part of a larger longitudinal study aimed at understanding brain development in ASD from childhood through adulthood (Lange et al., 2015; Travers et al., 2015; Zielinski et al., 2014). Using a cohort sequential design (i.e., accelerated longitudinal design) (Nesselroade and Baltes, 1979), DTI and behavioral measures were collected 1–4 times over a nine-year period from participants of multiple age cohorts (Figure 1). We longitudinally examined microstructural changes in the bilateral thalamus and in the white matter of the bilateral PLIC and ALIC. We then examined whether these microstructural measurements related to finger-tapping speed at Time 1.
Figure 1.
Depiction of cohort-sequential design, showing each participant’s age at scan and number of scans. Color denotes whether the participant was in the group with autism spectrum disorder (ASD) or in the group with typically developing controls (TDC).
Participants
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. There were 145 male participants 3–41 years of age (89 with ASD; 56 with typical development [TDC]). Based on complete DTI scans and behavioral data, the participants were selected from the broader longitudinal study (110 ASD and 78 TDC) and were required to have an IQ test that corresponded with the imaging data. The participants with ASD were diagnosed based on the Autism Diagnostic Observation Scale-General (ADOS-G) (Lord et al., 2012), Diagnostic Statistical Manual-IV-TR (APA, 2000), Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994), and International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria. Typically developing participants were evaluated based on IQ testing, standardized psychiatric assessment, ADOS-G testing, neuropsychological assessment, and past history to ensure typical development. The ASD and TDC groups were matched on age (p = .46), but not full scale IQ (p < .001). All participants had English as their first language and were verbal at the time of testing. Forty-eight participants with ASD reported being on a centrally active medication (i.e., stimulant, antidepressant, neuroleptic, atypical neuroleptic, or anti-anxiety medication) during at least one of the scans (36 at Time 1, 26 at Time 2, 19 at Time 3, and 10 at Time 4). See Table 1 for more detailed participant information.
Table 1.
Demographic characteristics of the longitudinal study sample and p-values for group comparisons.
| ASD | TDC | p-value | ||
|---|---|---|---|---|
| Number of subjects | 89 | 56 | -- | |
| Scans per subject, Mean (SD) | 2.9 (1.1) | 2.6 (1.1) | .18 | |
| Scans at Time 1 | 77 | 43 | -- | |
| Scans at Time 2 | 62 | 20 | -- | |
| Scans at Time 3 | 65 | 41 | -- | |
| Scans at Time 4 | 46 | 23 | ||
| Total Number of Scans | 250 | 127 | -- | |
| Number of participants with 4 scans | 33 | 11 | -- | |
| Number of participants with 3 scans | 23 | 13 | -- | |
| Number of participants with 2 scans | 16 | 12 | -- | |
| Number of participants with 1 scan | 17 | 20 | -- | |
| Interscan Interval Range | 1.7–3.9 | 1.8–4.4 | -- | |
| FSIQa Mean (SD) | 99.1 (17.4) | 116.9 (13.2) | <.001 | |
| FSIQa range | 53–138 | 89–153 | -- | |
| VIQa Mean (SD) | 94.7 (20.6) | 114.3 (11.8) | <.001 | |
| VIQa range | 34–145 | 87–151 | -- | |
| PIQa Mean (SD) | 101.5 (16.8) | 115.4 (13.3) | <.001 | |
| PIQa range | 62–140 | 87–155 | -- | |
ASD= Autism Spectrum Disorder; FSIQ = Full scale IQ; PIQ= Performance IQ, TDC = Typically developing controls; VIQ= Verbal IQ.
Assessments
Finger-Tapping Speed
Following the Halstead-Reitan Battery (Reitan Laboratories: www.reitanlabs.com; Heaton et al., 1991), finger tapping was operationally defined as the number of taps executed over a time period of 10 seconds by the pointer finger of each hand. Participants were instructed to tap their pointer finger as quickly as possible until instructed to stop. More detailed information about finger tapping procedures and the longitudinal data for finger tapping scores in this group has been previously reported (Travers et al., 2017).
Imaging protocol
DTI and structural magnetic resonance scans were conducted with participants at Times 1–4. The DTI procedures are detailed in Travers et al. (2015). In total, 377 scans were acquired on a Siemens Trio 3.0 T Scanner (127 TDC; 250 ASD). A spin-echo, echo planar imaging (EPI), single-shot pulse sequence with diffusion weighting was used by the DTI acquisition along with bipolar gradients with dual-echo refocusing to reduce eddy currents for all time points (Reese et al., 2003). Image distortions from magnetic field inhomogeneities were reduced by parallel imaging with a geometric reduction factor of two. Diffusion weighted images with 12 non-collinear diffusion encoding directions with a single b0 image and b= 1000 s/mm2 were retrieved for each slice. Over the cerebellum and cerebrum, 60 contiguous, 2.5 mm thick axial slices were retrieved (echo time [TE] = 84 ms at Time 1 and 91 ms at Times 2, 3, and 4; repetition time [TR] = 7000 ms; pixel bandwidth = 1346 Hz; matrix= 128 × 128; Field of view [FOV] = 256mm; and resolution= 2 × 2 × 2.5 mm, nex= 4). An update in scanner hardware and software occurred between Times 1 and 2 (change from 8-channel to 12-channel head coil with corresponding change in echo time). To statistically model the degree to which the DTI measures were affected by the update, we performed linear regressions examining DTI values as a function of the scanner update. This regression was modeled only in adults in order to try to dissociate biological development occurring between Times 1 and 2 with the scanner update occurring across the same time span, as developmental changes in adults during this time frame would be minimal compared to that in children/adolescents. These adjusted metrics were then used across all participants in the statistical analyses to account for DTI changes as a function of the scanner update. To further determine that the present longitudinal results were not solely due to noise from this update, all patterns of results were tested by dropping Time 1 and using only Times 2, 3, and 4 data (see supplementary materials Figure S3 and Table S2). Similar strategies were successfully employed in our longitudinal DTI study of the corpus callosum (Travers et al., 2015).Further, a subset of participants with ASD were sedated at Time 1 (32 participants) and Time 2 (15 participants). To determine that the present results were not driven by the scans collected under sedation, we performed all analyses without the sedated scans (supplementary materials Figure S4 and Table S3).
DTI image analysis
Quality control checks looked for motion-induced FA hyperintensities, blurring, slice intensity banding, and frontal lobe distortions. All 377 scans met the quality control checks. DTI images were edited for translation, distortion, and rotation caused by head movements, and for eddy currents using an affine registration tool provided by the fMRIB software library (FSL) (Jenkinson et al., 2012). A mask that excluded the upper 1% apparent diffusion coefficient and lower 1% average b0 maps and average diffusion-weighted map was used to prevent inclusion of extreme intensity regions before processing. Rotation corrections from head motion were also applied to the gradient orientations (Leemans and Jones, 2009). A 2D Gaussian Kernel with 1.88 mm full width at half maximum (FWHM) was used to smooth images in the axial plane. Then, the images were skull stripped and each dataset was fit for a tensor using the robust estimation of tensors by outlier rejection (RESTORE) algorithm (Chang et al., 2005) provided within the Camino software package. To create a population-specific template, the tensors were recursively aligned by affine and diffeomorphic diffusion tensor registration implemented in DTI-TK (Zhang et al., 2006) as follows: we first averaged the multiple native space scans of each participant to make a participant-specific template. From these data, we randomly selected participants from the ASD group to match the number of participants with scans in the typically developing group in order to create a template from equal-sized groups. Any additional scans were subsequently registered and warped to the template (but did not go into template creation).
The quantitative maps calculated from the estimated diffusion tensors were fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD or λ3), and axial diffusivity (AD or λ1), where λ1, λ2, and λ3 are the diffusion tensor eigenvalues. The units for the diffusivity measurements were mm2/sec and scaled at 10−3.
The population masks for the bilateral thalamus were created from the JHU EVE template (Oishi et al., 2009), while population masks for the PLIC and ALIC were created from the JHU ICBM-DTI-81 atlas (Mori et al., 2005). It is important to note that while definitions of the PLIC can include the brainstem corticospinal tract, the JHU’s definition of the PLIC is superior to the corticospinal tract. These anatomical atlases were co-registered to the study-specific population template using ANTS diffeomorphic registration (Avants et al., 2008). Anatomical masks were aligned to each subject’s native image space using the transformations found during affine and diffeomorphic alignment and applying the inverse warp (Faria et al., 2011). Anatomical regions of interest were defined and threshold masks were applied to each individual’s FA, MD, RD, and AD map. Once the masks were applied, we took steps to ensure that we were measuring the microstructure of the thalamus proper (and not elements of adjacent white matter or cerebrospinal fluid). Specifically, to remove adjacent white matter from the thalamus delineation, we applied an FA < 0.5 upper threshold. To remove surrounding cerebrospinal fluid, we applied an MD < 1.02 × 10−3 mm2/s upper bound threshold. For the PLIC and ALIC white matter, we applied an FA > 0.2 lower bound threshold and an MD < 1.74 × 10−3 mm2/s upper bound threshold. All ROIs in native space were inspected for accuracy by the first author and verified by BGT and ALA for a few cases. The medians of the DTI measures for each ROI were calculated for each individual at each time point.
To account for head motion, the total motion index (TMI) was calculated (Benner et al., 2011; Yendiki et al., 2013) and used as a covariate in all analyses, even though TMI was not correlated with age, r = −.02, p = .80, nor different between groups, t = −1.78, p = .08.
Statistical analysis
All statistical analyses were performed in R version 3.2.2 (R Core Team, 2015). To examine longitudinal development of the thalamus, PLIC, and ALIC, linear mixed-effects models assessed the effects of age (quadratically modeled), diagnosis, and hemisphere (left-versus-right laterality) on the tensor coefficients, after accounting for head motion (i.e., TMI) and full scale IQ (FSIQ). Age, head motion, and FSIQ were mean centered to reduce multicollinearity. Thalamus, ALIC, and PLIC were assessed in separate models. Bonferroni correction was used to control for multiple comparisons, with statistical significance defined as α<.0042 (p<.05, corrected for 12 separate models assessed).
To examine how Time 1 DTI measures of the bilateral thalamus, PLIC, and ALIC differentially affected Time 1 finger-tapping speed, we performed a moderated mediation, structural equation path analysis using the Latent Variable Analysis (lavaan) package in R (Rosseel, 2012). This analysis was selected because of the large age range of the sample and the known effect of age on both brain microstructure and finger-tapping speed. The moderator was diagnostic group (ASD or TDC). The independent variable was age, and the dependent variable was finger-tapping speed. Three mediators were included and allowed to co-vary: thalamus microstructure, PLIC microstructure, and ALIC microstructure. To arrive at these mediators, we performed data reduction using a principal component analyses (PCA) to find a single measure that represented the common covariance among the microstructural properties (FA, MD, RD, and AD) of each combined bilateral brain region (i.e., one measure for the thalamus, one for the PLIC, and one for the ALIC). The PCA analyses created eight principal components for each region of interest, but only the largest principal component (PC1) was used in the path analysis, as this represented the greatest common variance among the metrics for each region. We also examined the rotational loadings of each diffusion measure on PC1 to see how heavily each measure contributed to PC1. To assess potential laterality effects, we compared this moderated-mediation model to a model where left-hemisphere brain regions mediated age-related changes in right-handed finger-tapping speed. However, this laterality model was a poorer fit than the bilateral model according to AIC (Akaike, 1974). Therefore, we only report the bilateral model.
Results
Longitudinal Trajectories
We tested for case-control differences in the tensor coefficients (FA, MD, RD, AD) and their longitudinal trajectories in the bilateral thalamus, ALIC, and PLIC, while controlling for head motion, FSIQ, and assessing laterality differences. Figure 2 shows graphs of these relations, and Table 2 reports the results from the linear mixed-effects models. Importantly, the longitudinal trajectories of the thalamus, PLIC, and ALIC in the typically developing group of the present study are visually concordant with the cross-sectional developmental trajectories of a previous study (Lebel et al., 2008), providing evidence that our typically developing group followed a typical trajectory in these regions. There were laterality effects across the majority of the models. However, there were no significant group-by-hemisphere interactions, suggesting that both groups demonstrated similar laterality effects.
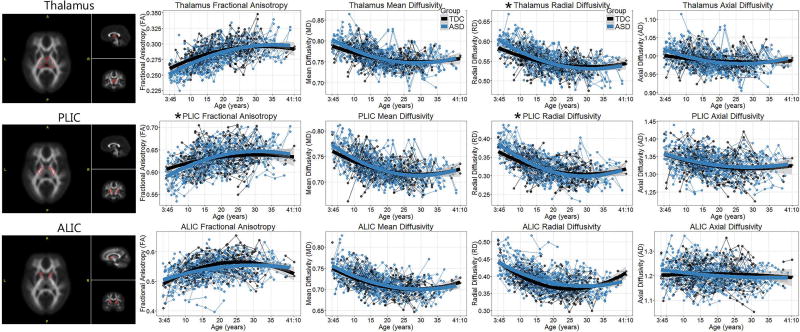
Figure 2.
DTI metrics of the thalamus, posterior limb of the internal capsule (PLIC) and anterior limb of the internal capsule (ALIC) plotted as a function of age in the group with autism spectrum disorder (ASD) and the typically developing controls (TDC). Data have been adjusted for the head coil change, total head motion, and full scale IQ. Group-by-age interactions that remained significant after controlling for multiple comparisons are indicated by *.
Table 2.
Results of linear mixed effects models using diagnostic group status, age (linear and quadratic term), brain hemisphere (right- or left-side) to predict DTI metrics (fractional anisotropy [FA], mean diffusivity [MD], radial diffusivity [RD], and axial diffusivity [AD]) for the thalamus, posterior limb of the internal capsule (PLIC), and anterior limb of the internal capsule (ALIC). These analyses controlled for head motion and full scale IQ.
| Group | Age | Age2 | Hemisphere | Age × Group | Age2 × Group | Hemisphere × Group |
Group × Age × Hemisphere |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p- value |
β | p- value |
β | p- value |
β | p- value |
β | p- value |
β | p- value |
β | p- value |
β | p- value |
||
| Thalamus | |||||||||||||||||
| FA | −0.0049 | <.05 | 0.0011 | <.001 | −4.39E-05 | .01 | 0.0043 | .009 | 0.0005 | .04 | 1.67E-05 | .36 | −1.63E-03 | .37 | 4.25E-05 | .86 | |
| MD | 0.0014 | .66 | −0.001 | .002 | 3.99E-05 | .08 | 0.0041 | .12 | −0.0009 | .01 | 4.29E-05 | .09 | −9.01E-04 | .76 | 6.72E-05 | .86 | |
| RD | 0.0037 | .31 | −0.001 | <.001 | 4.44E-05 | .08 | −0.0039 | .16 | −0.0012 | .001 | 2.98E-05 | .30 | 7.56E-04 | .81 | −1.39E-04 | .73 | |
| AD | −0.0017 | .65 | −0.0002 | .53 | −4.75E-06 | .86 | 0.0130 | <.001 | −0.0009 | .03 | 7.38E-05 | .01 | −3.75E-03 | .26 | −1.45E-04 | .74 | |
| PLIC | |||||||||||||||||
| FA | −0.0013 | .77 | 0.0010 | .002 | −6.58E-05 | .01 | 0.0125 | <.001 | 0.0014 | <.001 | −3.59E-06 | .90 | 3.18E-04 | .90 | −9.06E-05 | .79 | |
| MD | 0.0025 | .43 | −0.0015 | <.001 | 7.05E-05 | <.001 | −0.0048 | .06 | −0.0008 | .02 | 1.46E-05 | .57 | 7.73E-04 | .79 | −3.02E-05 | .94 | |
| RD | 0.0014 | .74 | −0.0021 | <.001 | 1.10E-04 | <.001 | −0.0070 | .009 | −0.0015 | <.001 | 2.55E-05 | .42 | 1.97E-03 | .52 | 1.08E-04 | .78 | |
| AD | 0.0030 | .61 | −0.0009 | .03 | −3.00E-05 | .37 | 0.0098 | .001 | −0.0002 | .69 | 3.75E-05 | .33 | 3.20E-04 | .92 | −6.73E-05 | .88 | |
| ALIC | |||||||||||||||||
| FA | −0.0099 | .06 | 0.0016 | <.001 | −1.33E-04 | <.001 | 0.0330 | <.001 | −0.0001 | .87 | 7.18E-05 | .06 | −2.48E-03 | .50 | −9.39E-04 | <.05 | |
| MD | 0.0028 | .43 | −0.0007 | .01 | 4.30E-05 | .06 | 0.0059 | 0.01 | −0.0008 | .02 | 1.90E-05 | .47 | −1.93E-03 | .46 | 4.37E-04 | .19 | |
| RD | 0.0095 | <.05 | −0.0014 | <.001 | 1.40E-04 | <.001 | −0.0168 | <.001 | −0.0009 | .06 | −3.68E-05 | .29 | 1.35E-03 | .69 | 8.72E-04 | <.05 | |
| AD | −0.0040 | .56 | 0.0006 | .26 | −6.84E-05 | .12 | 0.0552 | <.001 | −0.0010 | .15 | 8.62E-05 | .09 | −8.41E-03 | .09 | −6.55E-04 | .30 | |
Bolded effects indicate values that hold after Bonferroni correction.
The thalamus and PLIC exhibited a very similar patterns of results. There were group-by-age interactions for all DTI coefficients of the thalamus and all but AD of the PLIC. However, only the RD of the thalamus and PLIC and FA of the PLIC withstood Bonferroni correction. No age2-by-group interaction withstood the multiple-comparisons correction.
The pattern of results in the ALIC was distinct from both the thalamus and PLIC. In the ALIC, no significant group-by-age interaction effects withstood the multiple-comparisons correction. A three-way interaction among age, group, and laterality were observed in FA and RD. However, once again this did not withstand multiple-comparison correction. Further, a graphical examination of these three-way interactions (supplementary materials Figure S1) suggested that group differences appeared in left-hemisphere but not right-hemisphere ALIC values and only in the oldest participants. Because there were relatively few participants in these older age ranges, these three-way interactions should be interpreted with caution.
Differential Analyses
Our analyses controlled for FSIQ because IQ has been previously associated with thalamic connectivity in ASD (Woodward et al., 2017). However, differential analyses were performed to examine group differences in thalamic developmental trajectories without co-varying for FSIQ. See supplementary materials Table S1 and Figure S2 for the results. Intriguingly, when we did not account for IQ, group-by-age interactions were muted and only remained for the PLIC RD and ALIC MD.
To ascertain that the developmental trajectories were not attributable to the hardware change that occurred between Times 1 and 2, follow-up analyses examined the same models while omitting Time 1 data, which was collected before the hardware change. As can be seen in supplementary materials (Figure S3 and Table S2), similar beta coefficients were found when omitting Time 1 data, although statistical power was reduced with the loss of Time 1 data. Similar results were found when omitting the scans collected under sedation (Figure S4 and Table S3).
Finger Tapping Analyses
To examine how DTI measures of the bilateral thalamus, PLIC, and ALIC predicted motor dexterity (finger-tapping speed), we first created composite DTI values using PCA of the thalamus (left/right FA, MD, RD, and AD of the thalamus), of the PLIC (left/right FA, MD, RD, and AD of the PLIC), and of the ALIC (left/right FA, MD, RD, and AD of the ALIC). The first principal component (PC1; measuring the greatest common variance among these measures) was used in all of the following analyses to represent the combined measure of the DTI coefficients of each region. Higher numbers for PC1 were indicative of higher FA, lower MD, lower RD, and lower AD. We examined the rotational loadings of each variable to determine the degrees to which each DTI metric contributed to PC1. For the thalamus, all DTI coefficients equally contributed to the PC1. For the PLIC, FA, MD, and RD (but not AD) equally contributed to PC1. For the ALIC, FA and RD were the largest contributors to PC1.
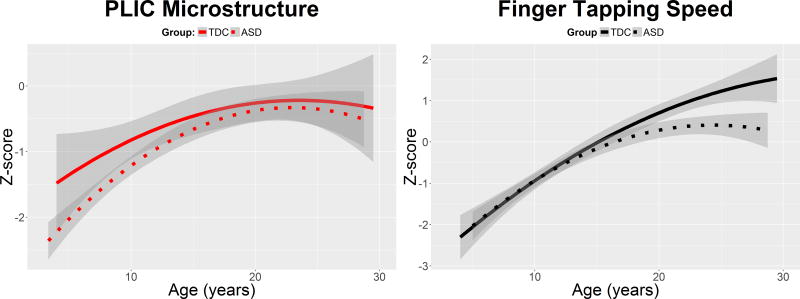
Moderated, mediation analyses were performed using structural equation modeling path analysis to examine how the principal component DTI measure of each region (thalamus, PLIC, and ALIC) mediated the relation between age and finger-tapping speed in both diagnostic groups at Time 1. The model showed excellent fit: Comparative Fit Index > .95; Root mean square error of approximation < 0.001. The results of this model are shown in Figure 3. A test of the indirect effects demonstrated that the PLIC significantly mediated the relation between age and finger-tapping speed in the ASD group, b = 0.14 [0.02–0.26], SE = 0.06, p = .03, and in the typically developing group, b = 0.20 [0.03–0.36], SE = 0.09, p = .02. However, the indirect effect was not significant for the ALIC or thalamus in either group (all p’s > .40). To better visualize the PLIC mediating the effect of age on finger-tapping speed, we plotted the quadratic trajectory of PLIC microstructure development and the quadratic trajectory of finger-tapping speed as a function of age in both groups (Figure 4). PLIC microstructure and finger-tapping speed were each normalized with z-scores to enhance comparability of the graphs. From Figure 4, we see that even though the PLIC mediates the relation between age and finger-tapping speed in both groups, the microstructural group differences in PLIC are observed to be most prominent at younger ages, whereas the group differences in finger-tapping speed are most prominent at older ages.
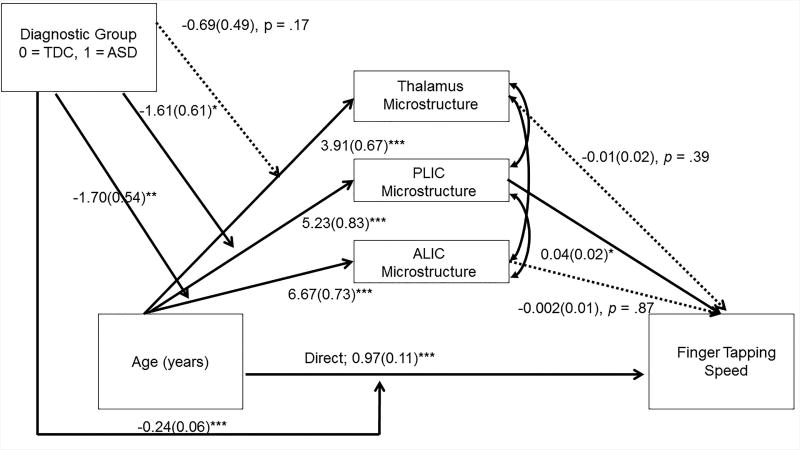
Figure 3.
Results of moderated mediation using a structural equation path analysis. The analysis examined the microstructural properties of the thalamus, PLIC, and ALIC as potential mediators of the relation between age and finger-tapping speed. Diagnostic group was examined as a potential moderator. Unstandardized regression coefficients (standard errors) are reported. The model fit indices were good: Comparative Fit Index > .95; Root mean square error of approximation < 0.001. The indirect effects indicated that the PLIC was a significant mediator between age and finger-tapping speed similarly in both the ASD and typically developing groups. Consistent with the effect sizes of the age-by-group interactions reported in the linear mixed-effects analyses, diagnostic group significantly moderated the effect of age on the PLIC and thalamus, but not the ALIC. Diagnostic group status also significantly moderated the direct effect between age and finger-tapping speed, consistent with previous reports of atypical age-related changes in finger-tapping speed in ASD (Travers et al., 2017). However, the indirect effects were similar in both groups, suggesting that group status was not a moderator of the indirect effects. *p < .05, **p < .01, ***p < .001.
Figure 4.
PLIC microstructure and finger-tapping speed as a function of age in both the group with autism spectrum disorder (ASD) (dotted line) and the group with typical development (TDC) (solid line). These data correspond to the cross-sectional (Time 1) data used in the moderated mediation analysis. To maximize comparison between the graphs, the PLIC microstructure and finger-tapping speed were normalized using z-scores. The graphs demonstrate that the group differences in PLIC are most prominent at younger ages, whereas the group differences in finger-tapping speed are most prominent at older ages.
Discussion
The purpose of this study was to examine the developmental trajectories of the thalamus and internal capsule (i.e., PLIC and ALIC) from childhood to mid-adulthood in ASD and to investigate whether deficits in simple motor coordination correlated with abnormal microstructure. First, we found distinct longitudinal trajectories in the ASD group that differed when compared to the TDC group in the thalamus and PLIC across the majority of the DTI metrics. These trajectories were characterized by small-sized group differences in microstructure during childhood that approached the typically developing group with age and, in some cases, surpassed the typically developing group during adolescence and adulthood. In contrast to the thalamus and PLIC, the developmental trajectories of the ALIC were similar across both groups for the most part, except for different trajectories observed in MD. Second, the PLIC was the only region found to successfully mediate the relation between age and finger-tapping speed, and the PLIC was a successful mediator in both groups. These findings support differential microstructural development of the thalamus and PLIC in individuals with ASD who are verbal but similar relations between the PLIC and motor speed in both groups.
Longitudinal Development of the Thalamus, PLIC, and ALIC
The present study revealed that the group with ASD had atypical thalamic and PLIC microstructure during childhood that appeared to approach or intersect with the typically developing group in adolescence and adulthood. These findings are consistent with previous reports of FA, MD, and RD group differences in thalamus-related white matter of children and adolescents with ASD (Brito et al., 2009; Nair et al., 2015, 2013; Ogur and Boyunaga, 2015; Shukla et al., 2011; Vogan et al., 2016). Further, the findings of the group differences in microstructure of the thalamus proper are consistent with the trend-level group differences in MD and RD observed by Nair et al. (2013). The developmental trajectory identified by the present study may help explain why these group differences were only at trend level, given that the thalamic values showed less of a group difference during adolescence.
The present finding of a narrowing group difference with age in thalamic microstructure in ASD may shed light on the recent findings of thalamic hyperconnectivity in ASD (Mizuno et al., 2006; Woodward et al., 2017). Increased thalamic hyperconnectivity has been shown to relate to lower IQs (Woodward et al., 2017) and sensory over reactivity (Green et al., 2016). While DTI is non-specific to the type of white matter change occurring, the present results are consistent with increasing thalamo-cortical connectivity with age in ASD.
The observed DTI trajectory differences also raise interesting questions regarding brain development in ASD. Extant literature of older children, adolescents, and adults generally report reduced FA and increased diffusivity measures in ASD (Aoki et al., 2013; Travers et al., 2015); however, recent studies in infants and younger children have suggested patterns of increased FA and decreased diffusivity (for review see Conti et al, 2015 and Wolff et al., 2017). This suggests the existence of early-in-life brain changes in which the underlying microstructural properties become altered compared to that of typically developing individuals. In terms of thalamic regions, Wolff et al. (2012) reported that thalamocortical projections, including the anterior thalamic radiations and posterior and anterior internal capsules, already displayed reductions of FA in the ASD toddlers by 20 months of age. Consistent with this finding, the present results suggest reduced FA of the thalamus and PLIC in the youngest individuals with ASD in our sample, compared to typically developing controls. Our data further suggest that these thalamic area group differences that appear early in life may not persist, and there may be another developmental period later in life (~20 years), where ASD white matter characteristics undergo an additional shift compared to that of the controls.
The present findings also suggest that the ASD group and typically developing groups demonstrated similar laterality effects in the microstructure of the thalamus, PLIC, and ALIC. Previous studies have found larger-sized group differences in the right hemisphere PLIC and ALIC compared to the left hemisphere (Cheon et al., 2011; Ogur and Boyunaga, 2015; Vogan et al., 2016), which is not consistent with the present study. While group differences in average laterality were not detected, it is possible that there is a wide range of individual differences in laterality within the ASD group that are not represented by the average. Indeed, previous network analyses within this sample suggest that left-right hemisphere microstructure of the internal capsule structures may be more weakly related in ASD compared to typical development (Dean et al., 2016). Therefore, future investigations should continue to account for potential laterality differences in ASD.
Differences in the developmental trajectories of the PLIC compared to the ALIC are also intriguing. The PLIC and ALIC have different developmental trajectories in typical development, such that the PLIC microstructure appears to develop more quickly than the ALIC during infancy (Qiu et al., 2015), but the ALIC microstructure appears to change more rapidly than the PLIC during childhood and adolescence (Lebel et al., 2008). In a study of infants at high risk for ASD, both the PLIC and ALIC showed a distinct developmental trajectory in the infants who went on to receive an ASD diagnosis compared to those who did not (Wolff et al., 2012), suggesting that both of these tracts may show developmental differences in ASD very early in life. In the present study, it was in the earlier-developing PLIC where we found atypical developmental trajectories in the ASD group. Therefore, our results are consistent with very early brain changes in ASD that may alter developmental trajectories across the life span (Johnson et al., 2015). However, age-increasing connectivity in the thalamus in ASD is particularly intriguing. Specifically, the microstructure of the thalamus, PLIC, and ALIC have group differences that narrow in adolescence and adulthood, while other key white matter tracts, like the corpus callosum, show persistently robust group differences (Travers et al., 2015). It is unclear why some brain areas would show smaller group differences with age, while others do not in ASD. However, understanding this pattern may elucidate the developmental neural underpinning of ASD, and future research is needed to better understand how individual brain differences in infancy lead to brain changes throughout the life span in ASD.
Motor Speed and Thalamic Microstructure
The relation between finger-tapping speed and age was specifically mediated by PLIC microstructure in both groups. These findings are consistent with the PLIC primarily projecting to the motor and sensory cortices and the ALIC primarily projecting to the prefrontal cortices (Behrens et al., 2003). A study examining the specific functions of the internal capsule found FA of the inferior genu to be related to fine finger movement, the posterior limb to be related to fluency, and the anterior limb to be related to cognitive flexibility (Sullivan et al., 2010). Together, these findings may speak to a pattern of specialization for finger-tapping speed within the brain that appears to be similar in typical development and ASD. Future research with higher resolution partitioning of the internal capsule and thalamus will be needed to confirm specialization between simple motor coordination and corresponding brain circuits in ASD relative to typical development.
While both groups showed that age-related changes in finger-tapping speed were mediated by the PLIC, finger-tapping speed is only one measure of motor function, and it is widely accepted that the thalamus and internal capsule contribute to a number of sensorimotor functions, many of which we were unable to examine in this study. Examining the links among microstructure of the thalamus and its projections with a diversity of sensory and motor features in ASD is a key area of future research.
The similar PLIC-speed relations in both groups raises the question of whether the narrowing of group differences in the PLIC with age confers improvements in motor speed in ASD. The narrowing of the group differences in the thalamus and PLIC microstructure is not consistent with previous reports in this same longitudinal sample of an early plateau during adolescence of finger-tapping speed and grip strength in ASD (Travers et al., 2017). Further, the plotting of the age-related PLIC changes in comparison to the finger-tapping speed changes showed distinct trajectories. Therefore, at the ages when the thalamic microstructure in ASD converged with thalamic microstructure in typical development, the motor behaviors in ASD diverged from motor behaviors in typical development. This divergence could be due to the contributions of other brain structures that were not measured in the present study, such as atypicalities in structures that are known to relate to motor skills such as the cerebellum, brainstem, or motor cortex.
While the present study has unique strengths, there are several key limitations. As a longitudinal study, the DTI protocol was kept at 12 directions over the course of the entire study, but despite best efforts to keep the protocol consistent, there was a hardware change needed between Times 1 and 2. To address this change, we incorporated a covariate in all statistical analyses, which appeared to significantly mitigate this potential bias. The analyses without Time 1 data (i.e., without the different protocol) also demonstrated similar results, suggesting that the protocol change was not driving the present findings. Another limitation is that the participants in this study were all males who were verbal and without co-occurring intellectual disability, which prevents these findings from being generalizable to females with ASD, to those who are minimally verbal, and to those with IQ’s less than 70. More studies are needed to examine these effects in females, individuals who are minimally verbal, and more heterogeneous populations of individuals with ASD. Finally, while our mediation analysis was able to examine brain-based mediators of age-related changes in finger-tapping speed, we did not have the sample size to be able to examine if this mediation happens differently at different ages. Therefore, finger-tapping relations with the thalamus and internal capsule should be further examined in narrower age ranges.
In summary, these findings suggest that the thalamus and internal capsule microstructure undergo an atypical developmental trajectory in ASD that is suggestive of increasing connectivity from childhood through adolescence and adulthood. Microstructure of the PLIC was involved in motor speed in both groups, with age-related changes in finger-tapping speed being mediated by the PLIC. However, future studies are needed to examine the behavioral correlates of this atypical development across multiple sensory and motor domains.
Supplementary Material
Acknowledgments
We sincerely thank the children, adolescents, and adults with autism and with typical development, and all the families who participated in this study. We thank Danica Samsin for her contributions to this work. This work was supported by the National Institute of Mental Health (RO1 MH080826 to J.E.L., A.L.A., N.L., E.D.B.; RO1 MH084795 to J.E.L., N.L.; RO1 MH097464 to J.E.L., N.L., A.L.A.; K08 MH100609 to B.A.Z., KO8 MH092697 to J.S.A., and K99 MH110596 to D.C.D.), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32 HD007489 to D.C.D., B.G.T., and P30 HD003352 and U54 HD090256 to the Waisman Center), the Poelman Foundation (to E.D.B.), the Primary Children’s Foundation (Early Career Development Award to B.A.Z.), the Brain and Behavior Research Foundation (NARSAD Young Investigator Award to B.G.T), and the Hartwell Foundation (to B.G.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Child Health and Development, or the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp DPM, Zakszewski E, Field AS. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity. 2011;1:423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- Aoki Y, Abe O, Nippashi Y, Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Molecular Autism. 2013;4:1–17. doi: 10.1186/2040-2392-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT, Little LM, Diane Parham L, Ausderau KK, Sabatos-DeVito MG. Sensory features in autism spectrum disorders. In: Volkmar FR, Rogers SJ, Paul R, Pelphrey KA, editors. Handbook of autism and pervasive developmental disorders. 4. Hoboken, New Jersey: John Wiley & Sons; 2014. pp. 378–407. [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CaM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Benner T, van der Kouwe AJW, Sorensen AG. Diffusion imaging with prospective motion correction and reacquisition. Magnetic Resonance in Medicine. 2011;66:154–167. doi: 10.1002/mrm.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie JA, Johnson M, Lange N, Chipman J, Lu J, McMahon W, Lainhart JE. Volumetric and voxel-based morphometry findings in autism subjects with and without macrocephaly. Developmental Neuropsychology. 2010;35:278–295. doi: 10.1080/87565641003696817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Rodrigues Lde S, Gasparetto EL, Calcada CA. Diffusion tensor imaging findings in school-aged autistic children. Journal of Neuroimaging. 2009;19:337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiatry. 2015;72:767–777. doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine. 2005;53:1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, Herrington J, Nair A, Koh YJ, Jang DP, Kim YB, Leventhal BL, Cho ZH, Castellanos FX, Schultz RT. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: a diffusion tensor imaging study. Brain Research. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Conti E, Calderoni S, Marchi V, Muratori F, Cioni G, Guzzetta A. The first 1000 days of the autistic brain: A systematic review of diffusion imaging studies. Frontiers in Human Neuroscience. 2015;9:159. doi: 10.3389/fnhum.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, Travers BG, Adluru N, Tromp DPM, Destiche DJ, Samsin D, Prigge MB, Zielinski BA, Fletcher PT, Anderson JS, Froehlich AL, Bigler ED, Lange N, Lainhart JE, Alexander AL. Investigating the microstructural correlation of white matter in autism spectrum disorder. Brain Connectivity. 2016;6:415–433. doi: 10.1089/brain.2015.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Card D, Roberts SW, Mak-Fan KM, Chakravarty MM, Lerch JP, Taylor MJ. Self-injurious behaviours are associated with alterations in the somatosensory system in children with autism spectrum disorder. Brain Structure and Function. 2014;219:1251–1261. doi: 10.1007/s00429-013-0562-2. [DOI] [PubMed] [Google Scholar]
- Duffield TC, Trontel HG, Bigler ED, Froehlich A, Prigge MB, Travers B, Green RR, Cariello AN, Cooperrider J, Nielsen J, Alexander A, Anderson J, Fletcher PT, Lange N, Zielinski B, Lainhart J. Neuropsychological investigation of motor impairments in autism. Journal of Clinical and Experimental Neuropsychology. 2013;35:867–881. doi: 10.1080/13803395.2013.827156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre M, Buser P. Structures involved in acquisition and performance of visually guided movements in the cat. Acta Neurobiologiae Experimentalis. 1980;40:95–116. [PubMed] [Google Scholar]
- Faria AV, Hoon A, Stashinko E, Li X, Jiang H, Mashayekh A, Akhter K, Hsu J, Oishi K, Zhang J, Miller MI, van Zijl PC, Mori S. Quantitative analysis of brain pathology based on MRI and brain atlases--applications for cerebral palsy. Neuroimage. 2011;54:1854–1861. doi: 10.1016/j.neuroimage.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Graff-Radford NR, Damasio H, Yamada T, Eslinger PJ, Damasio AR. Nonhaemorrhagic thalamic infarction. Clinical, neuropsychological and electrophysiological findings in four anatomical groups defined by computerized tomography. Brain, Part2. 1985;108:485–516. doi: 10.1093/brain/108.2.485. [DOI] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, Dapretto M. Reduced modulation of thalamocortical connectivity during exposure to sensory stimuli in ASD. Autism Research. 2016;10:801–809. doi: 10.1002/aur.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Keshavan MS, Minshew NJ. Abnormal brain size effect on the thalamus in autism. Psychiatry Research: Neuroimaging. 2006;147:145–151. doi: 10.1016/j.pscychresns.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Melhem NM, Keshavan MS, Minshew NJ. Brief report: abnormal association between the thalamus and brain size in asperger’s disorder. Journal of Autism and Developmental Disorders. 2007;38:390–394. doi: 10.1007/s10803-007-0385-1. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. The American Journal of Psychiatry. 2006;163:1252–1263. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proceedings of the National Academy of Science of the Unites States of America. 2008;105:3593–3598. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Jones EJH, Gliga T. Brain adaptation and alternative developmental trajectories. Developmental Psychopathology. 2015;27:425–442. doi: 10.1017/S0954579415000073. [DOI] [PubMed] [Google Scholar]
- Lange N, Travers BG, Bigler ED, Prigge MBD, Froehlich AL, Nielsen JA, Cariello AN, Zielinski BA, Anderson JS, Fletcher PT, Alexander AA, Lainhart JE. Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Research. 2015;8:82–93. doi: 10.1002/aur.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Movement Disorders. 1994;9:493–507. doi: 10.1002/mds.870090502. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Lin H-Y, Ni H-C, Lai M-C, Tseng W-YI, Gau SS-F. Regional brain volume differences between males with and without autism spectrum disorder are highly age-dependent. Molecular Autism. 2015;6:29. doi: 10.1186/s13229-015-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter A, LeCouteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule–2nd edition (ADOS-2) Los Angeles, CA: Western Psychological Corporation; 2012. [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain and Development. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Research. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher L, Van Zijl PC. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Moritz CH, Haughton VM, Cordes D, Quigley M, Meyerand ME. Whole-brain functional mr imaging activation from a finger-tapping task examined with independent component analysis. American Journal of Neuroradiology. 2000;21:1629–1635. [PMC free article] [PubMed] [Google Scholar]
- Nair A, Carper RA, Abbott AE, Chen CP, Solders S, Nakutin S, Datko MC, Fishman I, Müller R-A. Regional specificity of aberrant thalamocortical connectivity in autism. Human Brain Mapping. 2015;36:4497–4511. doi: 10.1002/hbm.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesselroade JR, Baltes PB. Longitudinal research in the study of behavior and development. New York: Academic Press; 1979. [Google Scholar]
- Ogur T, Boyunaga OL. Relation of behavior problems with findings of cranial diffusion tensor MRI and MR spectroscopy in autistic children. International Journal of Clinical and Experimental Medicine. 2015;8:5621–5630. [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PCM, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Mori S, Miller MI. Diffusion tensor imaging for understanding brain development in early life. Annual Review of Psychology. 2015;66:853–876. doi: 10.1146/annurev-psych-010814-015340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magnetic Resonance in Medicine. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. Lavaan: an R package for structural equation modeling. Journal of Statistical Software. 2012;48:1–36. [Google Scholar]
- Schuetze M, Park MTM, Cho IY, MacMaster FP, Chakravarty MM, Bray SL. Morphological alterations in the thalamus, striatum, and pallidum in autism spectrum disorder. Neuropsychopharmacology. 2016;41:2627–2637. doi: 10.1038/npp.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52:286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48:4155–4163. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R, Kitamura H, Endo T, Hasegawa N, Someya T. Reduced thalamic volume observed across different subgroups of autism spectrum disorders. Psychiatry Research: Neuroimaging. 2010;184:186–188. doi: 10.1016/j.pscychresns.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp DPM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, Alexander AL. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Research. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Duffield TC, Prigge MDB, Froehlich AL, Lange N, Alexander AL, Lainhart JE. Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Developmental Science. 2017;20(4) doi: 10.1111/desc.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Tromp DPM, Adluru N, Lange N, Destiche D, Ennis C, Nielsen JA, Froehlich AL, Prigge MBD, Fletcher PT, Anderson JS, Zielinski BA, Bigler ED, Lainhart JE, Alexander AL. Atypical development of white matter microstructure of the corpus callosum in males with autism: a longitudinal investigation. Molecular Autism. 2015;6:15. doi: 10.1186/s13229-015-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biological Psychiatry. 2003;53:121–129. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- Vogan VM, Morgan BR, Leung RC, Anagnostou E, Doyle-Thomas K, Taylor MJ. Widespread white matter differences in children and adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2016;46:2138–2147. doi: 10.1007/s10803-016-2744-2. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J, Jacob S, Elison J. The journey to autism: Insights from neuroimaging studies of infants and toddlers. Development and Psychopathology. 2017:1–17. doi: 10.1017/S0954579417000980. Epub ahead of print 20 June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N, Giraldo-Chica M, Cascio CJ. Thalamocortical dysconnectivity in autism spectrum disorder: an analysis of the autism brain imaging data exchange. Biological Psychiatry. 2017;2:76–84. doi: 10.1016/j.bpsc.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster CD, Graf H, Ackermann H, Groth K, Kassubek J, Riecker A. Neural correlates of rate-dependent finger-tapping in Parkinson’s disease. Brain Structure and Function. 2015;220:1637–1648. doi: 10.1007/s00429-014-0749-1. [DOI] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2013;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Medical Image Analysis. 2006;10:764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MBD, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N, Alexander AL, Bigler ED, Lainhart JE. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.