Abstract
The optimal threshold of controlled attenuation parameter (CAP) for the detection of hepatic steatosis using both M and XL probe is unknown in nonalcoholic fatty liver disease (NAFLD). Magnetic resonance imaging proton-density-fat-fraction (MRI-PDFF) is an accurate and precise method to detect presence of hepatic steatosis and is better than CAP. Thus, the aim of this study was to evaluate the diagnostic accuracy and the optimal threshold of CAP for the detection of hepatic steatosis as defined by MRI-PDFF ≥ 5%. This cross-sectional study included 119 adults (59% women), prospectively recruited with and without NAFLD who underwent MRI-PDFF and CAP using either M probe or XL probe when indicated within a six-month period at the NAFLD Research Center, UCSD. Mean (±standard deviation) age and BMI were 52.4 (±15.2) years and 29.9 (±5.5) kg/m2, respectively. The prevalence of NAFLD (MRI-PDFF≥5%) and MRI-PDFF≥ 10% was 70.6% and 47.1%, respectively. The area under the ROC (AUROC) of CAP for the detection of MRI-PDFF ≥ 5% was 0.80 (95%CI:0.70–0.90) at the cut-point of 288 dB/m and of MRI-PDFF ≥10% was 0.87 (95%CI:0.80–0.94) at the cut-point of 306 dB/m. When stratified by IQR of CAP, we observed that an IQR below median (30 dB/m) had a robust AUROC compared to IQR above median ([0.92, 95%CI:0.85-1.00] vs. [0.70, 95%CI:0.56-0.85], p-value=0.0117), and these differences were statistically and clinically significant.
Conclusion
The cut-point of CAP for presence of hepatic steatosis (MRI-PDFF ≥ 5%) was 288 dB/m. The diagnostic accuracy of CAP for the detection of hepatic steatosis is more reliable when IQR of CAP is <30 dB/m. These novel data have implications for clinical utility of CAP in the assessment of NAFLD.
Keywords: NAFLD, hepatic steatosis, controlled attenuation parameter, MRI-PDFF
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is being recognized as one of the most prevalent causes of chronic liver disease worldwide (1, 2). In the United State, NAFLD is estimated to affect approximately one-third of the adult population and its prevalence is strongly associated with obesity, type 2 diabetes and metabolic syndrome (1, 3, 4). NAFLD is currently the second leading etiology for the indication of liver transplants in the United States (5–7) and yet early stage of NAFLD, such as simple hepatic steatosis, remains broadly underdiagnosed although it can potentially progress to NASH, leading to liver fibrosis, cirrhosis and hepatocellular carcinoma (8, 9).
Although liver biopsy is considered as the reference method for the diagnosis of NAFLD, it encounters important limitations. Its accuracy has been questioned due to sampling errors and its interpretation and quantitative scoring is limited by significant inter- and intra-observer variability (10–15). In addition, liver biopsy is an expensive and invasive procedure which limits its use for the screening of population (12). Thus, there is an increasing interest in developing non-invasive imaging techniques that can clinically assess hepatic steatosis in NAFLD. Although conventional ultrasonography is widely used as first-line assessment of hepatic steatosis, it is limited by a lack of quantitative accuracy and is operator dependent (16, 17); computerized tomography is limited by radiation exposure and inaccurate quantification of steatosis(18). Magnetic resonance imaging (MRI) such as Magnetic resonance spectroscopy (MRS) has emerged as leading noninvasive modalities for steatosis quantification in NAFLD in terms of sensitivity, specificity and reliability (18, 19). MRI that measure the proton density fat fraction (MRI-PDFF) has been proven to correlate well with MRS (20, 21) and histology-proven steatosis grade from contemporaneous liver biopsies(22–25). However, similar to liver biopsies, MRI is expensive and not routinely accessible.
The controlled attenuation parameter (CAP) is a novel technique based on the properties of ultrasonic signals developed to quantify ultrasound attenuation during measurement of liver stiffness vibration controlled elastography acquired by the Fibroscan (26). Although CAP is less accurate than MRI-PDFF in detecting all grades of hepatic steatosis (27, 28), CAP has been shown to correlate with histological grade of hepatic steatosis in several studies (26, 29–32). Moreover, CAP allows a rapid, non-invasive, bed-side assessment of hepatic steatosis and it is less expensive and more accessible than MRI. However, the use of CAP for the diagnosis of NAFLD in routine clinical practice is limited due to the lack of optimal threshold of CAP for the detection of hepatic steatosis and the absence of indicator of the quality of CAP measurements. Recently, Karlas and colleagues performed an individual patient meta-analysis on CAP accuracy for the grading of hepatic steatosis to better define relevant threshold of CAP for the stage of hepatic steatosis. However, this study included patients with heterogeneous etiology of chronic liver diseases, mainly viral hepatitis and a minority of NAFLD. Furthermore, CAP were exclusively measured using M probe which use is limited in obese patients due to a high failure rate (33) while obesity is a frequent characteristic of NAFLD patients. The use of the XL probe equipped with CAP has been shown to reduce the failure rate in obese patients providing improvement of CAP utility for the diagnosis of NAFLD (34, 35). Studies including NAFLD patients have reported different thresholds of CAP using M and XL probe for the grade of steatosis using liver biopsy as reference (27, 36). However, to really provide a relevant quantitative threshold of CAP for the detection of hepatic steatosis, measurement using a quantitative modality should be used and non-NAFLD controls should be included.” So far, the optimal threshold of CAP has not been assessed with head to head comparison with another quantitative measure of hepatic steatosis and this study will fill that gap in knowledge.
Using a well-characterized, prospective cohort of American adults with NAFLD and non-NAFLD controls, we conducted a cross-sectional analysis to evaluate the diagnostic accuracy and the optimal threshold of CAP using M and XL probe for the detection of hepatic steatosis as defined by MRI-PDFF ≥ 5%.
MATERIAL AND METHODS
Study participant and design
This was a cross-sectional analysis of participant derived consecutively from a prospective cohort aimed at assessing the diagnostic accuracy and optimal threshold of CAP to diagnose hepatic steatosis (defined as MRI-PDFF ≥ 5%) and non-NAFLD controls (defined as MRI-PDFF< 5%). We followed the Standards for Reporting of Diagnostic Accuracy STARD guidelines in this study of CAP in detecting hepatic steatosis (Supplemental Table 1). Please see supplemental methods for further details.
Study participants were recruited at the NAFLD Research Center at the University of California, San Diego (UCSD) between July 2014 and May 2017; 157 potential eligible participants were screened and 156 participants were deemed eligible for the study, 119 participants complied with the study protocol and underwent MRI-PDFF and CAP assessment within a six-month period (Supplementary Figure 1). All participants underwent a careful evaluation for other causes of hepatic steatosis and liver disease and were invited for a clinical research visit with standardized history, physical and anthropometric exam, fasting biochemical testing, transient elastography and CAP assessment at the UCSD NAFLD Research Center (20, 21, 27, 37–42), advanced magnetic resonance imaging (MRI) based phenotyping at the UCSD MR3T Research Laboratory. This study was Health Insurance Portability and Accountability Act (HIPAA) compliant, informed written consent was obtained from all patients and this study was approved by the UCSD Institutional Review Board.
Inclusion/Exclusion criteria
Inclusion criteria were as follows: at least 18 years of age, willing and able to complete all procedures and observations specified in the protocol, fully informed, and had signed the Informed Consent/Assent and Health Insurance Portability and Accountability Act provisions.
Exclusion criteria were as follows: history of significant alcohol intake within 2 years of recruitment (≥14 drinks/week for men or ≥7 drinks/week for women); any evidence of secondary causes of hepatic steatosis including nutritional, iatrogenic, or infectious etiology or HIV infection; evidence of liver diseases other than NAFLD, which include viral hepatitis (screened by positive serum hepatitis B surface antigen and hepatitis C RNA assays), autoimmune hepatitis, genetic or acquired disorders such as hemochromatosis, Wilson’s disease, glycogen storage disease, alpha-1 antitrypsin deficiency, and cholestatic or vascular liver disease; evidence of decompensated liver disease (defined as Child-Pugh score > 7 points); active substance use; major systemic illnesses; contraindication(s) to MRI; pregnant or trying to be pregnant; or any other conditions believed by the principal investigator to affect patient’s competence, compliance, or completion of the study.
Clinical Research Evaluation
All patients underwent a standardized clinical evaluation, detailed history, anthropometric exam, and fasting biochemical tests at the UCSD NAFLD Research Center. Detailed information from history and anthropometric exam included age, sex, height, weight, body mass index, ethnic background, vital signs were collected by a trained clinical investigator. Alcohol consumption was documented in prior clinical visit and confirmed in the research clinic using the Alcohol Use Disorders Identifications Test and the Skinner questionnaire. Other causes of liver diseases and secondary causes of hepatic steatosis such as steatogenic medications were ruled out systematically using history and biochemical tests. Biochemical tests included aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, total bilirubin, direct bilirubin, albumin, hemoglobin A1c, fasting glucose, insulin, prothrombin time, international normalized ratio, fasting lipid panel, platelet count and ferritin.
Outcome measures
The primary outcome was the presence of hepatic steatosis defined as MRI-PDFF ≥ 5%. The secondary outcome was the detection of hepatic fat content ≥ 10% defined as MRI-PDFF ≥ 10% as this threshold has been used in several therapeutic trials as inclusion criteria.
Transient Elastography and CAP measurement
Transient elastography was performed by a trained technician, blinded to clinical and MRI results, using the FibroScan® 502 Touch model (M Probe; XL Probe; Echosens, Paris, France). Detailed methods have been previously-described in references (43, 44). Briefly, TE measurement was obtained in the supine position with the right arm fully adducted by scanning the area of abdomen at the location of the right liver lobe during a 10 seconds breath hold. Participants were asked to fast at least 3 hours prior to the exam. The procedure included a minimum of 10 measurements to determine the median valid liver stiffness measurements in kilopascals (kPa) and the interquartile range (IQR). According to the manufacturer protocol, all patients were first scanned using the M probe (3.5 MHz) and when indicated by the equipment upon initial assessment, patients were re-scanned using the XL probe (2.5 MHz). The CAP value in dB/m was simultaneously measured for the assessment of liver steatosis measurements, co-localized to the valid liver stiffness measurements. All CAP data were collected prospectively. Each participant underwent two consecutives readings of LSM and CAP by the same FibroScan. Unreliable liver stiffness was defined as success rate (ratio of the number of successful measurements to the total number of acquisitions) <60% and/or number of valid measurement <10 and/or IQR/med >30%.(45)
Magnetic Resonance Imaging
MRI-PDFF Advanced magnetic resonance imaging (MRI) based phenotyping was performed at the UCSD MR3T Research Laboratory using the 3T research scanner (GE Signa EXCITE HDxt; GE Healthcare, Waukesha, WI) with all participants in the supine position. MRI-PDFF was used to measure hepatic steatosis defined as MRI-PDFF≥5%. The details of the MRI protocol have been previously described in references methods (22, 23, 46, 47). The median time between MRI-PDFF and CAP was 8 days. The image analysts were blinded to all clinical and biochemical data.
Rationale for using MRI-PDFF for hepatic steatosis quantification as gold standard
MRI-PDFF was used as a gold standard for the following reasons. First, to really provide a relevant quantitative threshold of CAP for the detection of hepatic steatosis, measurement using a quantitative modality should be used. MRI-PDFF is a quantitative method that has been shown to be a highly precise, accurate, and reproducible non-invasive biomarker for the quantification of liver fat content (48, 49). It has been proven to correlate well with magnetic resonance spectroscopy (r2=0.99, P < 0.001) (20, 21) and histology-proven steatosis grade from contemporaneous liver biopsies (22–25). In addition, MRI-PDFF has been demonstrated to be superior to ultrasound, computed tomography and CAP for quantification of liver fat content (19, 27). Second, in the future many therapeutic trials in NAFLD will require a liver biopsy which is an invasive and expensive procedure. Likewise MRI-PDFF is expensive, thus an optimal threshold of CAP to approximate MRI-PDFF ≥ 5% for the screening of patients with and without NAFLD would reduce the therapeutic trials cost. In addition, several trials used an MRI-PDFF ≥10% as inclusion criteria and thus, an optimal threshold of CAP to approximate MRI-PDFF ≥ 10% was chosen as secondary outcome. Third, to be able to assess the diagnostic accuracy of CAP for the detection of hepatic steatosis as defined by MRI-PDFF ≥ 5%, participant with NAFLD and non-NAFLD are needed and it would be unethical to perform a liver biopsy in normal participants who do not have a clinical indication of performing a liver biopsy.
Statistical Analyses
Patients’ demographic data, laboratory, and imaging data were summarized with mean and standard deviation for continuous variables or median and interquartile range (IQR) and with numbers and percentages for categorical variables. Mean and frequency were compared using an independent samples t-test or Wilcoxon Rank Sum Test or Chi-square test or Fisher’s Exact Test, where appropriate. The Kruskal-Wallis test was used to compare CAP and different category of hepatic fat content assessed with MRI-PDFF.
Main analyses
Receiver operating characteristic (ROC) curve analyses were used to assess the diagnostic accuracy of CAP for the detection of hepatic steatosis (MRI-PDFF ≥ 5%) and of hepatic fat content ≥ 10%. For each ROC analysis, the area under the ROC curve (AUROC), the optimal thresholds, and the following performance parameters were calculated: sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The optimal threshold of each modality was determined using the Youden index.(50)
Sample size estimation
Given the previously described superiority of MRI-PDFF compared to CAP for the detection of hepatic steatosis in NAFLD biopsy-proven cohort (27), an AUROC of CAP of 0.85 (0.75–0.96) for the detection of hepatic steatosis and a correlation between MRI-PDFF and CAP approximately of 0.50, a projected sample size of 102 people are needed to assess the diagnostic accuracy of CAP for the detection of hepatic steatosis using MRI-PDFF as a gold standard with an alpha of 0.05 and a power of 0.80.
Sensitivity analyses
Sensitivity analyses were conducted to further assess the impact of covariates on the accuracy of CAP for detection of hepatic steatosis as defined by MRI-PDFF ≥ 5%. The AUROC of CAP were compared using the method by Hanley and McNeil (51).
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) or SPSS (IBM, Chicago, IL). A two-tailed p-value ≤0.05 was considered statistically significant.
RESULTS
Baseline characteristics
In this prospective study, 119 participants (58.8% female) with MRI-PDFF and CAP were consecutively enrolled. The mean (± standard deviation) age and body mass index (BMI) were 52.4 (±15.2) years and 29.9 (±5.5) kg/m2, respectively. Baseline cohort characteristics are summarized in Table 1. CAP were assessed using either M probe (n= 82, 68.9%) or XL probe (n=37, 31.1%) when appropriate. The prevalence of NAFLD (MRI-PDFF≥5%) and MRI-PDFF≥ 10% was 70.6% (n=84) and 47.1% (n=56), respectively. A total of 156 patients were eligible for the study, although 22 patients were excluded because CAP was not performed and 15 patients were excluded because MRI-PDFF was not performed (Supplementary Figure 1).
Table 1.
Baseline characteristics of the cohort stratified by NAFLD status
| Characteristics | Total Patients (n=119) |
MRIPDFF <5% (n=35) |
MRIDPFF ≥ 5% (n=84) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 52.4 (15.2) | 50.2 (17.6) | 53.3 (14.1) | 0.3254 |
| Female, n (%) | 70 (58.8) | 17 (48.6) | 53 (62.4) | 0.1424 |
| White, n (%) | 59 (51.8) | 17 (48.6) | 42 (53.2) | 0.6508 |
| Hispanic or Latino, n (%) | 37 (32.5) | 16 (45.7) | 21 (28.6) | 0.0442 |
| Clinical | ||||
| Type 2 Diabetes, n (%) | 49 (41.2) | 11 (31.4) | 38 (45.2) | 0.1631 |
| BMI (kg/m2) | 29.9 (5.5) | 26.8 (4.9) | 31.2 (5.2) | <0.0001 |
| Waist circumference (cm) | 101.0 (14.4) | 90.8 (13.1) | 105.3 (12.7) | <0.0001 |
| Obesity, n (%) | 52 (43.7) | 8 (22.9) | 44 (52.4) | 0.0031 |
| Biological data | ||||
| AST (U/L) | 36.4 (24.7) | 29.3 (13.7) | 39.4 (27.7) | 0.0101 |
| ALT (U/L) | 48.6 (39.2) | 27.9 (14.2) | 57.3 (43.0) | <0.0001 |
| Alk P (U/L) | 76.3 (33.0) | 71.5 (25.7) | 78.3 (35.6) | 0.2478 |
| GGT (U/L) | 48.1 (53.9) | 41.9 (49.9) | 50.6 (55.6) | 0.4630 |
| Total Bilirubin (mg/dL) | 0.7 (1.0) | 1.0 (1.8) | 0.5 (0.3) | 0.1050 |
| Direct Bilirubin (mg/dL) | 0.2 (0.1) | 0.2 (0.2) | 0.2 (0.1) | 0.3342 |
| Albumin (g/dL) | 4.4 (0.4) | 4.4 (0.6) | 4.4 (0.3) | 0.5911 |
| Glucose (mg/dl) | 116.5 (45.6) | 112.2 (46.4) | 118.3 (45.5) | 0.5104 |
| Hemoglobin A1c (%) | 6.0 (1.1) | 5.8 (1.1) | 6.1 (1.1) | 0.2201 |
| Insulin (U/ml) | 26.9 (29.8) | 14.8 (14.5) | 31.4 (32.8) | 0.0025 |
| HOMA-R | 8.1 (12.9) | 3.5 (3.3) | 9.9 (14.7) | 0.0027 |
| Triglycerides (mg/dL) | 156.1 (103.6) | 98.1 (34.2) | 180.7 (113.2) | <0.0001 |
| Total cholesterol (mg/dL) | 180.9 (34.9) | 171.0 (31.8) | 185.1 (35.5) | 0.0479 |
| HDL-cholesterol (mg/dL) | 49.3 (13.5) | 54.9 (14.3) | 47.0 (12.5) | 0.0038 |
| LDL-cholesterol (mg/dL) | 102.7 (31.8) | 96.5 (30.8) | 105.4 (32.0) | 0.1759 |
| Platelet count (109/L) | 228.0 (70.6) | 207.8 (75.3) | 236.3 (67.4) | 0.0468 |
| Prothrombin time | 11.4 (2.5) | 11.8 (1.8) | 11.2 (2.7) | 0.1878 |
| INR | 1.3 (2.3) | 1.1 (0.2) | 1.4 (2.8) | 0.3960 |
| Clinical Prediction Rules | ||||
| AST/ALT | 0.9 (0.3) | 1.1 (0.4) | 0.8 (0.3) | <0.0001 |
| APRI | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.4) | 0.8344 |
| BARD, median (IQR) | 2 (2) | 2 (1) | 2 (2) | 0.0485 |
| FIB-4 | 1.5 (1.4) | 1.9 (2.1) | 1.3 (0.8) | 0.1471 |
| NAFLD Fibrosis Score | −1.3 (1.7) | −1.3 (2.0) | −1.3 (1.5) | 0.9887 |
| Imaging data | ||||
| MRI-PDFF (%) | 10.8 (7.7) | 2.8 (1.2) | 14.1 (6.8) | <0.0001 |
| Liver stiffness (kPa) | ||||
| Median, median (IQR) | 6.0 (4.4) | 4.5 (2.8) | 6.5 (3.8) | 0.0011 |
| IQR, median (IQR) | 0.9 (1.1) | 0.6 (0.9) | 1.0 (1.0) | 0.0092 |
| IQR/M, median (IQR) | 0.2 (0.1) | 0.1 (0.1) | 0.2 (0.1) | 0.2067 |
| Total number of measurements | 13.4 (7.0) | 15.7 (11.7) | 12.4 (3.2) | 0.1098 |
| Total number of valid measurement | 10.2 (1.0) | 10.7 (1.2) | 10.0 (0.8) | 0.0079 |
| Success rate <60%, n (%) | 11 (9.2) | 4 (11.4) | 7 (8.3) | 0.7294 |
| Unreliable liver stiffness*, n (%) | 13 (10.9) | 5 (14.3) | 8 (9.5) | 0.5218 |
| CAP (dB/m) | ||||
| Median, median (IQR) | 305 (80.0) | 235 (75.0) | 315 (52.0) | <0.0001 |
| IQR, median (IQR) | 30 (19.0) | 32 (13.0) | 28.5 (19.5) | 0.0831 |
| IQR/M, median (IQR) | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | <0.0001 |
| Probe size, n (%) | 0.0915 | |||
| Use of M probe | 82 (68.9) | 28 (80.0) | 54 (64.3) | |
| Use of XL probe | 37 (31.1) | 7 (20.0) | 30 (35.7) |
Continuous variable are expressed in mean with standard deviation in parentheses or median, unless otherwise noted as median with interquartile range (IQR) in parentheses or n (%). BMI: body mass index, Obesity was defined as BMI≥30 kg/m2, HbA1c: glycated hemoglobin, ALT: alanine aminotransferase, AST: aspartate aminotransferase, INR: International Normalized Ratio, APRI: AST to platelet ratio, HDL: High Density Lipoprotein, LDL: Low Density Lipoprotein, Alk P: Alkaline Phosphatase, MRI-PDFF: magnetic resonance imaging proton-density fat fraction, CAP: controlled attenuation parameter. Success rate was defined as the ratio of the number of valid measurements to the total number of measurements.
Unreliable liver stiffness was defined as success rate <60% and/or number of valid measurement <10 and/or IQR/med >30%.44
P-value determined by comparing characteristics of patients without NAFLD (MRI-PDFF<5%) and with NAFLD (MRI-PDFF≥5%) using an independent samples t-test, Wilcoxon Two-Sample test or a Chi-Square or Fishers exact test as appropriate. Bold indicates significant P-values <0.05.
Diagnostic accuracy of CAP for the detection of hepatic steatosis (MRI-PDFF≥5%)
The distribution of CAP measurements across different category of hepatic fat content assessed with MRI-PDFF is illustrated in Figure 1. The AUROC of CAP for the detection of hepatic steatosis (MRI-PDFF ≥ 5%) was 0.80 (95%Confidence of interval (CI): 0.70-0.90) at the cut-point of 288 dB/m Figure 2A. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) of CAP for the detection of hepatic steatosis (MRI-PDFF≥5%) was 75.0%, 77.1%, 88.7%, 56.2% respectively Table 2.
Figure 1. Distribution of CAP measurements stratified by hepatic fat content (MRI-PDFF).
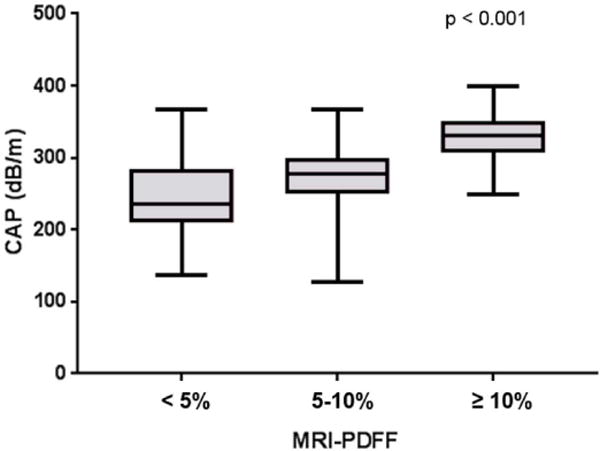
CAP measurements increase with increase of liver fat content assessed by MRI-PDFF (Kruskal–Wallis test P < 0.001): MRI-PDFF <5% n=35, MRI-PDFF 5-10% n=28, MRI-PDFF≥10% n=56.
Figure 2. Diagnostic accuracy of CAP for the detection of hepatic steatosis.
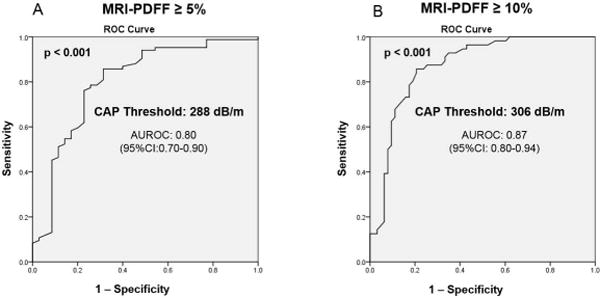
Receiver operating curves (ROC) and area under the ROC A. for the detection of hepatic steatosis defined by MRI-PDFF ≥ 5% B. for the detection of hepatic fat content ≥ 10% defined as MRI-PDFF ≥ 10%
Table 2.
Diagnostic accuracy of CAP for the detection of hepatic steatosis
| AUROC (95%CI) | Cutoff (dB/m) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Primary analysis: MRI-PDFF ≥ 5% | ||||||
| CAP (dB/m) | ||||||
| Optimal threshold | 0.80 (0.70–0.90) | 288 | 75.0 | 77.1 | 88.7 | 56.2 |
| Threshold for 100% sensitivity | 128 | 100 | 0 | 70.6 | 0 | |
| Threshold for 100% specificity | 369 | 8.3 | 100 | 100 | 31.2 | |
| Secondary analysis: MRI-PDFF ≥ 10% | ||||||
| CAP (dB/m) | ||||||
| Optimal threshold | 0.87 (0.80–0.94) | 306 | 78.6 | 82.5 | 80.0 | 81.2 |
| Threshold for 100% sensitivity | 250 | 100 | 38.1 | 58.9 | 100 | |
| Threshold for 100% specificity | 369 | 12.5 | 100 | 100 | 56.2 | |
MRI-PDFF: magnetic resonance imaging proton-density fat fraction, CAP: controlled attenuation parameter, PPV: positive predictive value, NPV: negative predictive value.
Diagnostic accuracy of CAP for the detection of hepatic fat content ≥10% (MRI-PDFF≥ 10%)
The AUROC of CAP for the detection of hepatic fat content ≥10% (MRI-PDFF≥ 10%) was 0.87 (95%CI: 0.80–0.94) at the cut-point of 306 dB/m. Figure 2B. The sensitivity, specificity, PPV and NPV of CAP for the detection of hepatic fat content ≥10% (MRI-PDFF≥ 10%) was 78.6%, 82.5%, 80.0%, 81.2% respectively Table 2. Additional analysis assessing the diagnostic accuracy of CAP for the detection of hepatic fat content ≥15% (MRI-PDFF≥ 15%) and hepatic fat content ≥20% (MRI-PDFF≥20%) are provided in Supplemental Table 2.
Sensitivity analyses of the performance of CAP for the detection of hepatic steatosis
When stratified by M probe or XL probe, CAP measurements were significantly higher using XL probe compared to M probe in the lower grade of hepatic fat content with a mean (+/− standard deviation) of 300.14 (+/−35.14) dB/m versus 230.25 (+/−57.7) dB/m, p = 0.005 respectively when MRI-PDFF was below 5%; and 295.0 (+/−47.00) dB/m versus 254.47 (+/−46.60) dB/m, p=0.034 when MRI-PDFF was between 5 and 10% Figure 3, Supplemental Table 3.
Figure 3. Higher CAP value using XL compared to M probe when MRI-PDFF < 10%.
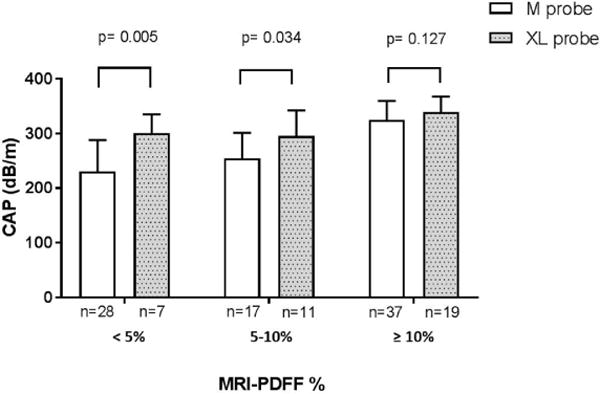
CAP measurements and standard deviation are presented using M probe (pink bar) and XL probe (blue bar) stratified by hepatic fat content assessed by MRI-PDFF. CAP measurements were significantly higher using XL probe compared to M probe in the lower grade of hepatic fat content. p-value were determined using independent two-tailed t- test.
When stratified by IQR of CAP, the direct comparison of the AUROC of CAP for the detection of hepatic steatosis (MRI-PDFF ≥ 5%), using the Hanley and McNeil test showed that CAP measurement with IQR of CAP below median (30 dB/m) was significantly more accurate than CAP measurement with IQR of CAP above median (30 dB/m) for the detection of hepatic steatosis (MRI-PDFF≥5%) with an AUROC of 0.92 (95%CI: 0.85–1.00) versus 0.70 (95%CI: 0.56–0.85), p-value=0.0117 Figure 4. In the subgroup of individuals with IQR of CAP <30 dB/m, there was no significant difference in the performance of CAP between unadjusted and adjusted model when either BMI or type 2 diabetes status was included in the models. The optimal strategy for the screening of NAFLD using CAP and IQR of CAP as validity criteria is detailed in Figure 5.
Figure 4. The diagnostic accuracy of CAP increase when IQR of CAP is <30 dB/m.
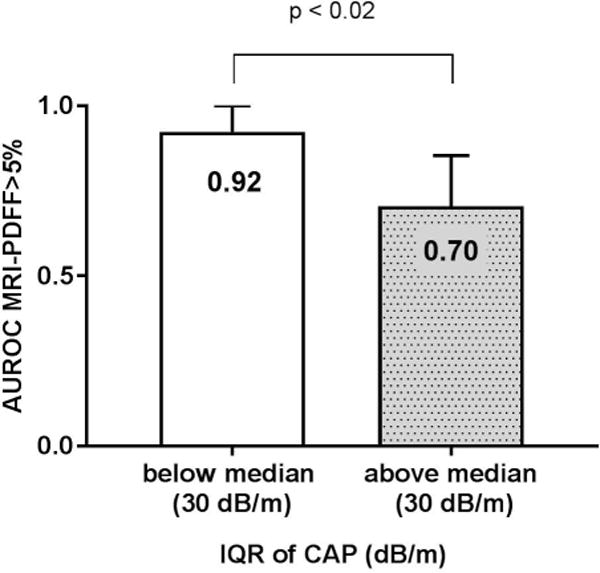
Area under the receiver operating curves (AUROC) of CAP and 95 % confidence of interval for the detection of hepatic steatosis defined by MRI-PDFF ≥ 5% was significantly higher when IQR of CAP was below median (30 dB/m) n= 60 compared to AUROC of CAP when IQR of CAP n=59 was above median, p value 0.017 determined using the method by Hanley and McNeil.
Figure 5. Optimal strategy for the screening of NAFLD using CAP measurements and its IQR as validity criteria.
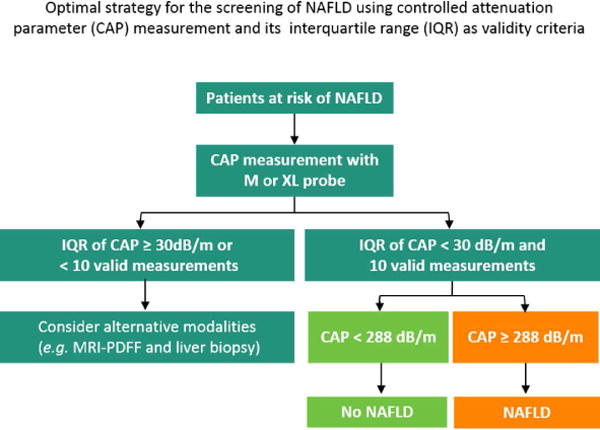
CAP measurements are considered valid when IQR of CAP is below 30 dB/m and 10 valid measurements are achieved. If the valid CAP measurement is below the optimal threshold the patient is considered as non-NAFLD.
DISCUSSION
Main findings
Using a well-characterized, prospective cohort of American adults with and without NAFLD, this study demonstrates that the optimal threshold of CAP for the detection of hepatic steatosis as defined by MRI-PDFF ≥ 5% is 288 dB/m with a good diagnostic accuracy (AUROC: 0.80, 95%CI: 0.70-0.90). Furthermore, the secondary analysis shows that the optimal threshold of CAP for the detection of hepatic fat content ≥10% (MRI-PDFF≥ 10%) is 306 dB/m with an AUROC of 0.87 (95%CI: 0.80–0.94) which could be integrated in future clinical trial design as inclusion criteria. The key novelty of this study is to provide estimates of the diagnostic accuracy and optimum thresholds of CAP measurements, using M or XL probes, for the detection of hepatic steatosis by an accurate, and quantitative standard using MRI-PDFF in a Western population with NAFLD and non-NAFLD controls. These novel data have important implications for the clinical utility of CAP in the assessment of NAFLD and would help developing an optimal clinical approach for non-invasive diagnosis of NAFLD. Furthermore, CAP may have utility in longitudinal follow-up of anti-steatosis therapeutic interventions in clinical routine practice. In addition, this study demonstrates that the diagnostic accuracy of CAP for the detection of hepatic steatosis is more reliable when IQR of CAP is <30 dB/m, providing reliable quality indicator that would help clinicians in interpreting the CAP measurements. Ultimately, the use of these optimal thresholds for the quantitative diagnosis of hepatic fat may modify the clinical trials design for the treatment of NAFLD and reduce their costs by reducing screen failure rates for the trials that use an MRI-PDFF of 10% or higher for inclusion into a trial. In future, patients with a certain level of CAP values may only move forward for MRI-PDFF assessment in these clinical trials thereby reducing the number of MRI scans needed to enroll patients into the trial. However, further studies are needed to determine the clinical relevance and cost-effectiveness of CAP for the diagnosis of hepatic steatosis in NAFLD.
In context with published literature
This is the first prospective study to assess the optimal threshold of CAP for the diagnosis of hepatic steatosis using M and XL probe in a in a well-characterized cohort of American adults with NAFLD and non-NAFLD controls using advanced MRI-PDFF as the gold standard. This study provides also the first estimates of the diagnostic accuracy and optimal threshold of CAP for the detection of hepatic fat content ≥10% which is used as inclusion criteria in therapeutic trials (NCT02912260, NCT02781584). We report a good diagnostic accuracy of CAP for the detection of hepatic steatosis in NAFLD with an AUROC of 0.80 (95%CI: 0.70–0.90) consistent with previous studies (26, 29–32). Furthermore, we have found that an IQR of CAP < 30dB/m is a quality criteria for CAP measurement which is also consistent with a recent study by Wong et al. showing the validity of CAP for the diagnosis of fatty liver is lower if the IQR of CAP is ≥ 40 dB/m using M probe in a cohort of patients with different liver diseases.(32)
In a recent study, Karlas and colleagues have proposed an optimal threshold of CAP for the detection of histological steatosis grade above S0 of 248db/m (237–261) based on an individual patient meta-analysis including heterogeneous etiology of chronic liver disease. (31) Interestingly, the authors have identified that the etiology of the liver disease, and features highly associated with NAFLD such as diabetes and BMI needs consideration when interpreting CAP.(31) These latter observation, highlight the utmost need to assess CAP in the setting of well-characterized NAFLD cohorts. Indeed, our cohort demographical characteristics such as higher BMI (29.9 ± 5.5 kg/m2) and higher prevalence of type 2 diabetes may have reflected a more accurate assessment of the diagnostic performances and thresholds of CAP in a Western population with NAFLD and non-NAFLD controls. Therefore, these covariates may at least partially account for the different threshold found in our study and this meta-analysis: 288 dB/m versus 248 dB/m, respectively.
Fewer studies including small cohorts have assessed the diagnostic accuracy of CAP using XL probe (34, 35, 44). The sensitivity analyses shows that CAP measurements were significantly higher using XL probe compared to M probe in the lower grade of hepatic fat content. Similarly, Chan et al. have shown significant higher value of CAP using XL probe compared to M probe in an Asian NAFLD cohort(35). Likewise, a recent study by Vuppalanchi et al. in patients with NAFLD in a multicenter setting, have reported a significant higher CAP values measured with XL probe compared to M probe in an adjusted model for BMI. In this study, only 4.2 % of the total cohort did not have NAFLD as opposed to 29.4% in the current study. In addition the liver biopsy was used as the reference standard and only a minority of patients had steatosis grade 0 (which would equate with a MRI-PDFF of less than 5%). Therefore, our study is complimentary to this previous study and provides a more robust assessment of CAP for detection of presence of hepatic steatosis at a threshold of MRI-PDFF of 5%. We believe that the reference standard that is required to establish the optimal threshold of CAP to detect presence of hepatic steatosis in the clinical practice would have to be quantitative, reproducible, and valid across the entire dynamic range of liver fat content (liver fat content typically ranges between 0.2% to 50% on MRS) rather than a subjective estimate of liver fat on an ordinal scale using histologic grade of steatosis. Although, direct comparison of the performance of CAP using XL probe compared to M probe have shown similar diagnostic accuracy (34, 35), further studies using both probes on the same patients are needed to compare CAP measurement using M and XL probe.
Hepatic steatosis is an important clinical feature in NAFLD that can progress to NASH, fibrosis, cirrhosis and hepatocellular carcinoma (8, 9). Therefore, early diagnosis and screening of hepatic steatosis before the progression to NASH and severe liver fibrosis may benefit patients at risk of NAFLD. Contrary to viral hepatitis, the impact of hepatic steatosis on accelerating the disease progression to fibrosis and cirrhosis is unclear in NAFLD. Future longitudinal studies designed to determine the prognostic significance of hepatic steatosis in long-term outcomes are needed.
Strengths and limitations
There are several notable strengths of this study including the prospectively well-characterized recruited cohort by experienced investigators at a dedicated research center that is specialized for both clinical and radiologic research in NAFLD. All participants underwent a systematic and standardized liver disease assessment to exclude for other causes of liver disease before inclusion in the study, and detailed advanced MRI of the liver.
However, we acknowledge following limitations of this study. This is a single center study conducted at a highly specialized tertiary care center using advanced MRI techniques that may not be available in other clinical center. Thus the generalizability of these findings in other clinical settings is unknown. In addition, the cross-sectional design of the study did not allow the assessment of CAP for monitoring longitudinal changes in hepatic fat content. Liver biopsy was not performed in this study as the study was designed to assessed the optimal threshold of CAP which is a quantitative biomarker for the detection of hepatic steatosis and therefore a quantitative modality should be used a gold standard. We used the most accurate non-invasive quantitative modality which has emerged as a novel standardized biomarker for assessing hepatic steatosis(52). MRI-PDFF has been proven to correlate well with magnetic resonance spectroscopy (20, 21) and histology-proven steatosis grade (22–24). Additionally, MRI-PDFF performance has been reported to be higher than histology in quantifying changes in steatosis in longitudinal studies (20, 48).
Implications for clinical care and future research
Using a prospective study, we confirmed the good diagnostic accuracy of CAP for the detection of hepatic steatosis as defined by MRI-PDFF ≥ 5% and we provided a novel optimal threshold of 288 dB/m using XL probe when appropriate in an American cohort of well-characterized individuals with NAFLD and non-NAFLD controls. The use of this new threshold, higher than previous threshold, is more accurate and would decrease the screen failure rate in clinical trials. Furthermore, the use of this optimal threshold may enable the use of CAP for non-invasive diagnosis of NAFLD in routine clinical practice. Future studies are necessary to assess the clinical utility of CAP for diagnosis of hepatic steatosis in a multicenter, longitudinal design, both in observational and intervention studies. The cost-effectiveness of utilizing CAP versus other modalities available must also be evaluated to develop optimal diagnostic strategies for diagnosing NAFLD.
Supplementary Material
Acknowledgments
Grant support: The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303. CS and RL serve as co-PIs on the grant R01-DK106419. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.CC is supported by grants from the Société Francophone du Diabète (SFD), the Philippe Foundation and Monahan Foundation
All authors approved the final version of this article.
Abbreviations
- AUROC
area under the receiver operator characteristic curve
- BMI
body mass index
- CAP
controlled attenuation parameter
- CI
confidence interval
- MRI-PDFF
magnetic resonance imaging-proton density fat fraction
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TE
transient elastography
- UCSD
University of California at San Diego
Footnotes
Guarantor(s) of the article: Rohit Loomba
Conflict of interests: Dr. Sirlin consults, advises, and is on the speakers’ bureau for Bayer. He received grants from GE Healthcare. Dr Loomba have received grant from Siemens and GE Healthcare. All other authors report no other conflict of interests.
Author contributions:
Cyrielle Caussy: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission.
Mosab H. Alquraish: data collection, critical revision of the manuscript, approved final submission.
Phirum Nguyen: patient visits, data collection, critical revision of the manuscript, approved final submission
Carolyn Hernandez: patient visits, data collection, critical revision of the manuscript, approved final submission.
Sandra Cepin: data collection, critical revision of the manuscript, approved final submission.
Lynda Fortney: patient visits, data collection, critical revision of the manuscript, approved final submission.
Veeral Ajmera: analysis and interpretation of data, critical revision of the manuscript, approved final submission.
Ricki Bettencourt: statistical analysis, critical revision of the manuscript, approved final submission
Summer Collier: data collection, critical revision of the manuscript, approved final submission.
Jonathan Hooker: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Ethan Sy: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Emily Rizo: patient visits, critical revision of the manuscript, approved final submission
Lisa Richards: patient visits, critical revision of the manuscript, approved final submission
Claude B. Sirlin: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Rohit Loomba: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–1025. doi: 10.1016/j.metabol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 8.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. e641–649. doi: 10.1016/j.cgh.2014.04.014. quiz e639–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD, American Association for the Study of Liver D Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 11.Asrani SK. Incorporation of Noninvasive Measures of Liver Fibrosis Into Clinical Practice: Diagnosis and Prognosis. Clin Gastroenterol Hepatol. 2015;13:2190–2204. doi: 10.1016/j.cgh.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 13.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. doi: 10.3748/wjg.v20.i2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 15.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 16.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 17.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560–1566. doi: 10.3748/wjg.v16.i13.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767–775. doi: 10.1148/radiol.13121360. [DOI] [PubMed] [Google Scholar]
- 25.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598–607.e592. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637.e627. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 29.Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim DY, Ahn SH, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102–109. doi: 10.1111/liv.12282. [DOI] [PubMed] [Google Scholar]
- 31.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, Kumar M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Wong VW, Petta S, Hiriart JB, Camma C, Wong GL, Marra F, Vergniol J, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Foucher J, Castera L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411–412. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 34.de Ledinghen V, Hiriart JB, Vergniol J, Merrouche W, Bedossa P, Paradis V. Controlled Attenuation Parameter (CAP) with the XL Probe of the Fibroscan(R): A Comparative Study with the M Probe and Liver Biopsy. Dig Dis Sci. 2017 doi: 10.1007/s10620-017-4638-3. [DOI] [PubMed] [Google Scholar]
- 35.Chan WK, Nik Mustapha NR, Wong GL, Wong VW, Mahadeva S. Controlled attenuation parameter using the FibroScan(R) XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017;5:76–85. doi: 10.1177/2050640616646528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, Neuschwander-Tetri BA, et al. Performance Characteristics of Vibration-Controlled Transient Elastography for Evaluation of Non-Alcoholic Fatty Liver Disease. Hepatology. 2017 doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, Nguyen P, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015;149:1784–1793. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, Hofflich H, Bettencourt R, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016;43:83–95. doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel NS, Peterson MR, Brenner DA, Heba E, Sirlin C, Loomba R. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:630–639. doi: 10.1111/apt.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarrinpar A, Gupta S, Maurya MR, Subramaniam S, Loomba R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut. 2016;65:1546–1554. doi: 10.1136/gutjnl-2015-309456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui J, Chen CH, Lo MT, Schork N, Bettencourt R, Gonzalez MP, Bhatt A, et al. Shared genetic effects between hepatic steatosis and fibrosis: A prospective twin study. Hepatology. 2016;64:1547–1558. doi: 10.1002/hep.28674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, Choi PC, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–1871. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 45.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, Clark L, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–425. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen CH, Ikhwan MA, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest. 2017 doi: 10.1172/JCI93465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeder SB. Emerging quantitative magnetic resonance imaging biomarkers of hepatic steatosis. Hepatology. 2013;58:1877–1880. doi: 10.1002/hep.26543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 51.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 52.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


