Abstract
Background
Obesity rates in pediatric cancer survivors (PCS) are alarmingly high. Although healthy lifestyle changes may prevent future health complications, promoting healthy behaviors in PCS is challenging, and few interventions have successfully addressed this issue.
Procedure
This randomized control trial evaluated the feasibility and preliminary effectiveness of a parent-focused 6-session intervention, NOURISH-T, compared with enhanced usual care (EUC) on the outcomes of caregiver and PCS anthropometric measurements, eating behaviors, and physical activity. Behavioral and self-report assessments of caregivers and PCS in both conditions were conducted at baseline, post-intervention, and at a 4-month follow-up.
Results
In comparison to no change among EUC caregivers, NOURISH-T caregivers showed small yet significant decreases from baseline through follow-up on BMI, waist-hip ratio, and total daily caloric intake. However there was no change with regards to daily fat and sugar intake. NOURISH-T caregivers also showed positive changes in their child feeding behaviors, including decreases in pressuring their child to eat and restricting their child’s eating and increased eating together as a family. Similarly, decreases in BMI percentile, waist-hip ratio, and sugary beverage consumption were found for NOURISH-T PCS from baseline to post-intervention. NOURISH-T PCS also significantly increased their daily steps whereas EUC PCS decreased their daily steps.
Conclusions
Results suggest that an intervention targeting parents is feasible and demonstrates preliminary effectiveness. NOURISH-T showed a longer-term effect on caregivers, and although shorter-term, a positive impact on the PCS themselves. Implications for ways to improve NOURISH-T as an intervention for increasing healthy behaviors of PCS are discussed.
Keywords: Pediatric Cancer, Pediatric Obesity, Parent Training, Randomized Clinical
Survival rates for pediatric cancer have increased dramatically over the past few decades; thus, increased attention has been given to quality of survivorship and longer-term health behaviors in pediatric cancer survivors (PCS). Although overweight and obesity rates are high in the otherwise healthy pediatric population (approximately 33%),1 PCS have higher rates of overweight and obesity, ranging from 40% to 50% five years after treatment for those treated for acute lymphobastic leukemia, lymphomas, and some sarcomas and central nervous system disease.2–8 Overwhelming evidence indicates overweight and obesity increase longer-term health risks associated with pediatric cancer, such as cardiac and endocrine functioning and bone health.7;9–12 Healthy lifestyle behaviors, such as regular physical activity (PA) and healthier eating behaviors can help reduce risk of these negative late effects.11–14 However, these healthy behaviors might be especially difficult for PCS and their families to implement, particularly because during treatment, PA and healthy eating behaviors decline dramatically.15 Consequently, families have great difficulty reversing these behaviors post-treatment.
Treatment related factors, such as cranial radiation and exposure to corticosteroids, must be considered as reasons for observed increased rates of overweight and obesity in PCS, however, evidence suggests typical obesity-related behaviors, such as eating higher calorie dense foods and lack of PA may be a more likely explanation of the post-cancer treatment weight gains observed in PCS.16 Support of this hypothesis is observed when comparing PCS patients internationally. For example, despite the similarity of cancer treatment protocols in the US and Israel, rates of overweight/obesity in Israeli PCS post-treatment are extremely low. A recent study reported almost none of the PCS were obese through two years post-treatment.17 Given the high rates of obesity in the US population,1 these findings suggest health behaviors, not cancer treatment, might better explain the overweight/obesity trajectory found in US PCS samples. The negative consequences of obesity, in combination with its high occurrence among PCS, provide support for the importance of identifying ways to reduce overweight and obesity in PCS. Despite this critical need, few interventions targeting overweight and obesity have been developed and evaluated for this vulnerable group.
Consistent with our prior work, and with the general pediatric obesity literature, the intervention implemented in this trial is based upon the rationale that targeting parents is key in effecting change in youth with overweight/obesity.18–23 The importance of parents as key change agents was also highlighted by a review of the few studies conducted on weight management interventions with PCS.24 To address this critical need, we adapted an intervention previously used with caregivers of otherwise healthy children with overweight and obesity called NOURISH (Nourishing Our Understanding of Role Modeling to Improve Support and Health).22–23 Our adaptation, NOURISH for Healthy Transitions (NOURISH-T), targets the high overweight and obesity rates in PCS by focusing on caregivers as agents for change. In NOURISH-T, caregivers are educated about the importance of modeling healthy eating and PA behaviors to promote positive health behavior change and healthy weight management in their PCS. Topics especially relevant to PCS caregivers (e.g., transitioning to survivorship) are included in the program.25 We hypothesized that caregivers and PCS in NOURISH-T would show significant improvements in healthy lifestyle behaviors, including healthy eating behavior and physical activity, as well as indicators of obesity (e.g., anthropometric measures) over time. Guided by this hypothesis, the purpose of the current study was to conduct a pilot randomized controlled trial (RCT) to test the feasibility and preliminary efficacy of NOURISH-T.
Methods
Our RCT compared NOURISH-T to a one-session control intervention (Enhanced Usual Care/EUC) at two pediatric cancer clinics, John Hopkins All Children’s Hospital located in St. Petersburg, FL and University of Pittsburgh and Children’s Hospital of Pittsburgh. IRB approval was obtained at both sites. Families at each clinic were randomly assigned to either NOURISH-T or EUC and completed assessments at baseline, post-intervention and a 4-month post-intervention follow-up. Caregivers were the primary target for participation in the program.
A more detailed description of the two conditions can be found elsewhere.25 NOURISH-T emphasizes environmental, personal and behavioral factors; while also incorporating specific cognitive skills guided by both Social Cognitive and Cognitive Behavioral Theories.26–29 NOURISH-T involved 6 manualized phone sessions with each caregiver in which topics relevant to changing eating and PA behaviors in the context of developing a “new normal” post treatment were discussed. Each psycho-educational session involved establishing weekly goals, problem solving, homework and review. NOURISH-T families also received relevant print and web-based resources throughout the program to supplement and reinforce session content. All NOURISH-T participants were re-contacted approximately 2-months after post-intervention for a “booster/check-in” session. Caregivers assigned to EUC were provided a one-hour wellness session addressing the role of diet and exercise in pediatric overweight using material from the publically available We Can! Manual.30 In addition to this session, EUC participants received nationally available web-based information on wellness issues at two additional times over the course of 6-weeks by mail. Interventionists conducted a booster session with these caregivers approximately two months post-intervention. The sessions were recorded for research purposes in order to guarantee quality of implementation. The facilitators did not have access to the recordings and recordings were not used within the delivery of the intervention.
Participants
Inclusion/Exclusion Criteria. Caregivers were considered eligible if they were: 1) parents of PCS, 2) 18 years or older, and 3) fluent in English; and ineligible if they: 1) were non-ambulatory, 2) pregnant, or 3) did not reside with the PCS at least 50% of the time. Eligible PCS were: 1) between 5–13 years old at enrollment, 2) off treatment for 6 months to 4 years, 3) living with a participating caregiver, 4) able to engage in PA tailored to current medical status, 5) not taking medications affecting body weight (e.g., steroids or psychostimulants) within 6 months of enrollment, 6) not language/developmentally delayed, and 7) ≥ 85th body mass index (BMI) percentile.31 Both caregiver and PCS were required to meet eligibility criteria. PCS who relapsed during the intervention were excluded.
Enrollment. A total of 53 caregiver/child dyads meeting criteria were enrolled in the study (31-All Children’s Hospital, 22-University of Pittsburgh). A randomization scheme utilizing a random number generator with a 1:1 ratio was used to assign participants either to NOURISH-T (n=27) or EUC (n=26). A portion of these participants only completed the baseline assessment; leaving a subset of 37 dyads (18 NOURISH-T, 19 EUC) dyads with post-intervention assessment data. Another 14 families consented to the study and provided some baseline assessment data but did not participate beyond the initial consent process (i.e., did not do the intervention) and were not included in any of the main analyses. Of these 14 families, three were determined to be ineligible due to language and developmental delay barriers, two PCS relapsed, four families moved out of the area and five decided to withdraw from the study.
Measures
Pre-, post- and follow-up assessments were completed by both caregivers and PCS and included anthropometric indicators, a dietary recall, step counts, and self-report eating and PA behavior instruments. Data obtained for both the caregiver and PCS included the anthropometric indictors, dietary recall, and step counts. Anthropometric Measures: Height, weight, waist and hip circumferences were measured via standardized equipment and were used to calculate waist-to-hip ratio (WHR), caregiver BMI, and child BMI percentile. Measures were obtained in-person at a clinic visit or from medical charts (PCS only). If in-person assessments were not possible at post- or follow-up, caregivers were instructed on how to take their own measurements at home. Dietary Recall. Caregivers completed the Automated Self-administered 24-Hour Dietary Recall-2011 (ASA24) for themselves and their PCS.32 We specifically examined total caloric, sugar, and fat intake. Step Counts. Each PCS and caregiver wore a pedometer consecutively for 7 days prior to the pre-, post- and 4-month follow-up assessments. Step counts were used to calculate daily-steps averaged over a week.
Caregiver Only Measures. Child Feeding Questionnaire.33;34 This 31-item questionnaire assesses parental approaches to and attitudes about feeding their children. The restriction and pressure to eat subscales were examined specifically as these have been consistently related to obesity.35 The CFQ yields reliable and valid scores. Family Eating and Exercise Behaviors. This 28-item questionnaire assesses eating, exercise and weight-related habits of families (e.g., frequency of family meals, fast food consumption, television use during meals, fruit and vegetable and sugar sweetened beverage availability, encouragement of healthy food consumption, PA, and dieting in their children). These items were previously found to have adequate reliability.22;36;37 Satisfaction/Exit Surveys. Caregivers complete a 20-item survey assessing what they liked/disliked about the intervention, as well as what was/wasn’t useful or helpful in reaching health goals.
PCS Only Measures. Child Sugar Sweet Beverage and Fast Food Intake. This 13-item questionnaire assesses child intake of sugar sweetened beverages, breakfast and dinner habits, as well as frequency of fast food intake, and has been used with children and young adolescents.22 Physical Activity Questionnaire for Children (PAQ-C). This 9-item PA recall questionnaire reliably assesses children’s PA preference and frequency over a 7-day period.38;39 Rating of Medical Late Effects.40 Clinic staff evaluated PCS on their level of activity limitations.
Analysis
First independent sample t-tests and Fishers exact tests was conducted to investigate baseline differences between NOURISH-T and EUC participants. We then conducted a series of repeated-measures ANOVAs to compare each outcome measure from baseline to post-intervention and follow-up. Evaluation results of all assessments are reported for the participating caregivers; however, only pre- to post-intervention changes are reported for PCS due to missing data at follow-up for several self-report measures. Due to the missing data or incomplete response the ASA-24 could not be evaluated for PCS. Other researchers have also reported this inconsistency and inaccuracy of responses.41 To derive a more independent assessment, we controlled for caregiver BMI change and PCS BMI percentile at baseline when evaluating PCS anthropometric change.
Results
Demographic Characteristics
Across both conditions, PCS were on average 9.9 years old, 52.1% male, and obese (95.6% BMI) at baseline. Over half were diagnosed with acute lymphobastic leukemia or another type of lymphoma and were off-treatment for an average of 1.96 years. Most caregivers were overweight or obese with an average BMI at baseline of 32.5; 80% had a BMI over 25. Most PCS (80.0%) were classified as having no late medical effect limitations due to treatment and could engage in PA. There were no demographic differences between NOURISH-T and EUC among the full sample; however, there were some group differences among the subset that completed post-intervention assessments with regards to PCS and caregiver age, time off-treatment, and BMI at diagnosis (see Table 1).
Table 1.
Demographic and Baseline Obesity Characteristics
| All Participants (n=53) | Subset (n=37)a | |||||
|---|---|---|---|---|---|---|
| NOURISH-T (n=27) | EUC(n=26) | p-value | NOURISH-T(n=18) | EUC(n=19) | p-value | |
| PCS Demographic Characteristics | ||||||
| Age1 | 9.4 (2.5) | 10.5 (2.5) | .17 | 9.4 (2.7) | 11.6 (2.9) | .02* |
| Gender - Male2 | 58.3 (14) | 45.8 (11) | .28 | 52.9% (n=9) | 40% (8) | .32 |
| Race/Ethnicity | ||||||
| Caucasian1 | 74.1 (20) | 65.4 (17) | .35 | 77.8 (14) | 73.7 (14) | .54 |
| Hispanic1 | 14.8 (4) | 15.4 (4) | .63 | 16.7 (3) | 21.1 (4) | .53 |
| Other/Unknown1 | 11.1 (3) | 19.2 (5) | .33 | 5.6 (1) | 5.3 (1) | .74 |
| PCS Cancer Characteristics | ||||||
| Age at Diagnosis2 | 4.6 (3.3) | 5.9 (3.5) | .22 | 4.6 (3.4) | 7.0 (3.0) | .04* |
| Cancer Diagnosis | ||||||
| Lymphoma or ALL1,3 | 55.6 (15) | 53.8 (14) | .56 | 55.6 (10) | 52.6 (10) | .56 |
| Sarcoma1 | 7.4 (2) | 19.2 (5) | .19 | 11.1 (2) | 26.3 (5) | .23 |
| Brain Cancer1 | 18.5 (5) | 7.7 (2) | .23 | 22.2 (4) | 10.5 (2) | .30 |
| Other/Unknown1 | 18.5 (5) | 19.2 (5) | .61 | 11.1 (2) | 10.5 (2) | .68 |
| BMI at Diagnosis2 | 73.7 (29.1) | 68.8 (31.4) | .68 | 88.0 (13.6) | 61.2 (29.2) | .01** |
| Type of Treatment | ||||||
| Chemotherapy Only1 | 50.0 (11) | 50.0 (11) | 1.0 | 46.7 (7) | 44.4 (8) | .59 |
| Chemotherapy & Surgery1 | 18.2 (4) | 31.8 (7) | .24 | 20.0 (3) | 38.9 (7) | .21 |
| Chemotherapy, Surgery, & Radiation1 | 31.8 (7) | 18.2 (4) | .24 | 33.3 (5) | 16.7 (3) | .24 |
| Time off Treatment2 | 2.2 (1.5) | 2.2 (1.1) | .99 | 2.4 (1.0) | 1.7 (0.9) | .02* |
| Late Medical Effects - No Limitations1 | 76.9 (10) | 83.3 (10) | .54 | 72.7 (8) | 81.8 (9) | .50 |
| Caregiver Demographic Characteristics | ||||||
| Age1 | 38.1 (7.5) | 42.5 (10.0) | .09 | 36.8 (6.1) | 42.6 (10.2) | .04* |
| Gender - Male2 | 25.0 (6) | 25.0 (6) | .63 | 29.4 (5) | 25.0 (5) | .53 |
| Annual Income - Over $60,0001 | 43.5 (10) | 54.2 (13) | .33 | 37.5 (6) | 55.0 (11) | .24 |
| Marital Status - Married1 | 78.3 (18) | 83.3 (20) | .47 | 81.3 (13) | 90.9 (18) | .39 |
| Education - College Degree + 1 | 43.5 (10) | 50.0 (12) | .44 | 50.0 (8) | 50.0 (10) | 1.0 |
Notes:
p<.05;
p<.01
Subset of participants with post-intervention data;
[mean(SD)];
[%(n)];
Acute Lymphoblastic Leukemia
Caregiver Outcomes
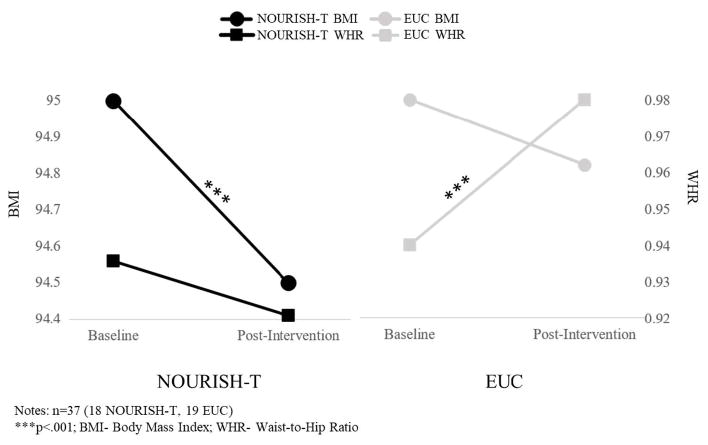
There were several differences on caregiver outcomes over time as a function of intervention condition, specifically on the anthropometric and measures of eating behaviors. Outcomes related to PA (daily steps averaged over a week) did not reach significance, however, caregiver BMI changes were found (Figure 1). Caregivers in NOURISH-T showed significant decreases in average BMI from 37.8 (SD=19.9) at baseline to 35.0 (SD=11.7) at follow-up (F=97.5, p<.001). In contrast, caregivers in EUC, although having lower BMI levels across all assessment points, remained virtually the same from 30.1 (SD=7.1) at baseline to 30.3 at both post-intervention and follow-up (post-SD=6.9; follow-up-SD=6.7). Similarly, WHR changes were found for caregivers over time as a function of intervention condition, where WHR decreased significantly for NOURISH-T caregivers from baseline to post-intervention (F=4471.2, p<.001), with a continued decreasing trajectory through follow-up; EUC caregivers showed an increasing, although not significant, trend in WHR from baseline to follow-up.
Figure 1.
Caregiver Anthropometric Outcomes from Baseline to Follow-Up
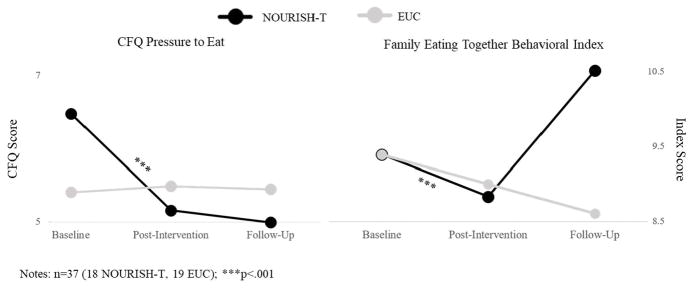
NOURISH-T caregivers significantly reduced their daily caloric intake from baseline through follow-up, whereas EUC caregivers significantly increased their intake across the 3 assessments (F=169.8, p<.001). Results for total fat and overall sugar intake were not significant. NOURISH-T caregivers showed a decrease in their pressuring of their child to eat (F=222.9, p<.001), whereas EUC caregivers remained at the same level across assessments (Figure 2). For restriction of eating, NOURISH-T caregivers showed a significant decrease over time and EUC caregivers manifested a non-significant decrease. Caregivers in both groups showed a similar pattern of behavior from baseline to post-intervention with respect to eating together as a family; however, NOURISH-T caregivers increased this pattern of eating together as a family behavior from post-intervention to follow-up (F=1561.4, p<.001) whereas EUC caregivers showed a (albeit non-significant) decline in this behavior (Figure 2).
Figure 2.
Family Eating Patterns from Baseline to Follow-Up
PCS Outcomes
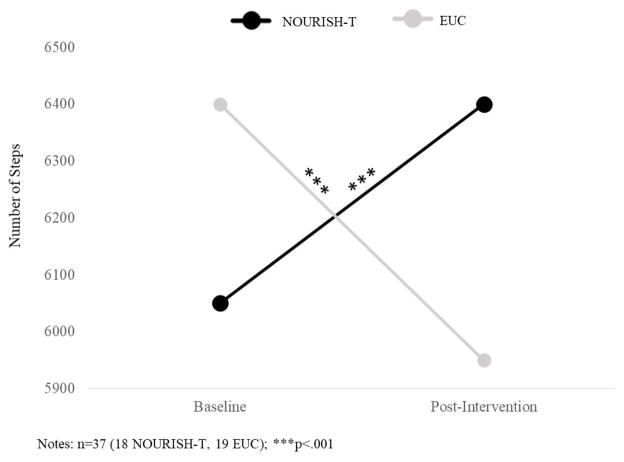
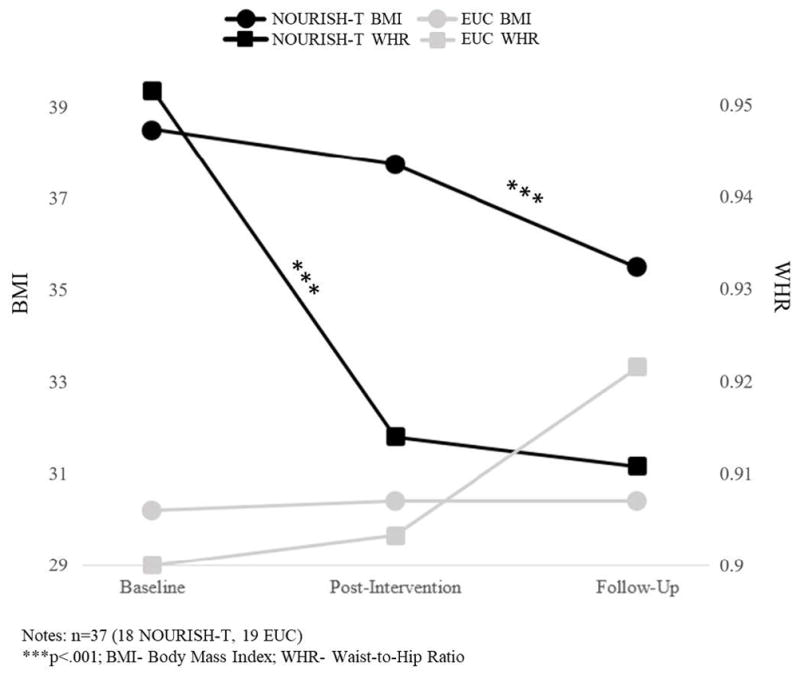
When controlling for caregiver BMI and BMI percentile at baseline, there was a decrease in BMI percentile from baseline to post-intervention for those PCS in NOURISH-T we were able to obtain follow-up data (F=2125.1, p<.001) and a non-significant decrease for PCS in EUC (Figure 3). Additionally, a significant increase in WHR was evident for PCS in EUC from baseline to post-intervention (F=4471.2, p<.001), compared with a non-significant decrease for PCS in NOURISH-T (Figure 3). PCS in NOURISH-T significantly increased their daily steps averaged over a week from baseline to post-intervention (F=151.2, p<.001). In contrast, PCS in EUC decreased their averaged daily steps from baseline to post-intervention (Figure 4). A similar pattern emerged for overall PA (via PAQ-C). PCS at baseline reported much less PA than PCS in EUC. However, from baseline to post-intervention PCS in NOURISH-T increased their activity significantly between the two time-points, whereas PCS in EUC decreased their activity slightly (p<.05). PCS in NOURISH-T decreased their sugared beverage consumption over time, (from an average of 2 drinks per week at post-intervention to just 1 drink per week at follow-up, F=9.8, p<.027). PCS in EUC consumed fewer sugared drinks at all time points than those in NOURISH-T; however, they remained at the same level across all 3 assessments with an average of 1 drink per week.
Figure 3.
PCS Anthropometric Outcomes from Baseline to Post-Intervention
Figure 4.
PCS Daily Steps Averaged Over a Week from Baseline to Post-Intervention
Intervention Satisfaction
Overall, NOURISH-T caregivers reported sessions were enjoyable, content was relevant, and they used the resources and handouts provided. Furthermore, compared with EUC caregivers, NOURISH-T caregivers were significantly more comfortable with the sessions, felt content were more relevant, and reported being more likely to use the handouts. Interestingly, caregivers in EUC reported they wanted more information overall; when asked about what type of information would be most helpful, they indicated the type of information provided to caregivers in the NOURISH-T condition.
Discussion
The primary aim of this trial was to document the feasibility and preliminary effectiveness of NOURISH-T, a weight-management intervention for caregivers of PCS, a group especially vulnerable to overweight/obesity. Overall, results support the feasibility of NOURISH-T. Specifically, a pattern of findings emerged supporting our main hypotheses showing positive changes in health behaviors among caregivers in NOURISH-T compared with EUC caregivers. While improvements were small in scale, caregivers showed significant changes over time on a number of key anthropometric and dietary indices, including lower BMI levels, lower WHRs, and decreases in caloric consumption. Notably, EUC caregivers either showed no change or decreased in the health indices assessed over time. Results provide preliminary evidence of efficacy and support for a larger, multi-site trial to assess for clinically significant impacts.
NOURISH-T suggests the importance of caregivers serving as role models of healthy eating and exercise to facilitate family changes in these behaviors. Focusing on caregivers to effect change in their PCS is also consistent with recommendations made recently in a systematic review of the few extant interventions targeting obesity in PCS.24 The prevention of overweight and obesity in PCS is especially important, as this group manifests relatively high rates of these post-treatment.
Although some positive outcomes were evident among PCS in NOURISH-T from baseline to post-intervention, results were less consistent by follow-up for PCS due to lower consistent response rate. Given the difficulty of obtaining follow-up information for PCS due to the nature of this pilot, e.g., relatively small sample size and available resources to ensure follow-up assessments, we must caution that the clinical significance of our pilot findings should be considered in this light. These factors suggest the need for further exploration that identifies those assessments most reliable to evaluate intervention efficacy for PCS with obesity.
Despite limitations of this pilot with regard to clinical significance, results for PCS in NOURISH-T were encouraging. Specifically, BMI percentile decreased significantly and waist-to-hip ratio decreased (although not significantly) for PCS whose caregivers were in NOURISH-T, whereas waist-to-hip ratio increased significantly from for PCS in EUC. Findings for daily steps averaged over a week were noteworthy as NOURISH-T PCS significantly increased their averaged daily steps at post-assessment, while those of EUC PCS decreased. Future research must use more reliable tools that are less obtrusive than pedometers (e.g., wrist accelerometers); nonetheless, these preliminary results are promising and show PA can be significantly improved in a relatively short period of time (over 6 weeks), suggesting this intervention has the potential to make a change in the lives of PCS.
Significant findings for dietary changes in PCS were limited to one measure of sugared drink consumption, partially due to the challenges of administering the ASA24 to children (e.g., lengthy measure, extensive detailed information needed to complete the measure),41 again suggesting the need for more reliable tools and more resources to ensure follow-up assessment. Despite the limitations of our pilot, our results generally support the efficacy of NOURISH-T, and suggest testing our intervention across several pediatric clinics on a larger scale is warranted.
Based on the findings of this study, we have identified several questions and limitations to address in future research. One question we had hoped to address, but were unable to given the limitations of our study is at what time point over the course of treatment and survivorship might our intervention be best implemented. Moreover, without adequate clinic staff time and resources, securing follow-up assessment data from participating families often proved challenging. Because this was a pilot, PCS anywhere from 6-months to 4-years off treatment were included. The average time since treatment ended of those choosing to participate (1.96 years) might suggest when the timing to initiate our intervention would be most efficient. We found trying to recruit families soon after treatment ended (6-months post-treatment) was extremely difficult as families often are ‘trying to forget’ their cancer and hospital experiences, and/or trying to ‘move on with their lives.’42 Similarly, too long after treatment ends (e.g., after 3 years) families are likely to have created a ‘new normal’ and see their issues as the same as any other family dealing with childhood obesity. The importance of engaging in an intervention related to longer term cancer prevention issues seems less clear to families as the time since treatment ended increases, despite significant evidence suggesting the particular importance of a healthy lifestyle for PCS.7;12;14;43 A larger scale study with sufficient staff and resources might better address the question of timing as the current trial’s relatively small sample size precluded an evaluation of the influence of variables such as time since treatment ended. Finding differences between those PCS in NOURISH-T vs EUC who followed through with assessments as a function of PCS age and BMI at diagnosis might also be important factors to consider in future research. Additionally, tracking progress towards individually identified weekly goals throughout the intervention, may provide a wraparound approach and improve our ability to assess intervention efficacy.
Intervention format must also be considered. There are several benefits in face-to-face group interventions for families of children with obesity. However, this in-person approach is often impractical for families with a PCS.24 Many families travel from long distances to get to a cancer clinic, making coordinating a face-to-face group difficult, if not impossible. Practical strategies for targeting this population should be addressed in future research.
Our goal was to have as many families participate as possible; therefore, reducing participant burden became paramount. However, it could also be argued by offering an individualized intervention for each family, this pilot intervention was potentially more intensive than provided in prior child obesity studies,22;23;44 and might prove more translatable to actual pediatric cancer clinic practice. One recent small-scale study evaluated a tailored weight management intervention for PCS with overweight and obesity used web-based and phone modalities exclusively to implement their intervention, and found positive health behavior changes.45 Further research is needed, however, to examine the relative efficacy of group vs. individual intervention format in a larger scale study, as well as whether parents should be targeted exclusively as opposed to in conjunction with their children.21;24
We acknowledge there were limitations with regard to the measures in this study. Besides problems in using more obtrusive and less reliable tools to assess PA (i.e., pedometers), we found families, and particularly children, had difficulty in completing the highly time consuming and detailed ASA-24, a web-based tool designed to evaluate dietary intake. Future research should consider alternative ways to assess dietary intake in this group.
In summary, PCS are at increased risk for overweight/obese and associated health complications. Our work examining the trajectory of weight gain cross-culturally suggested becoming overweight post-treatment may be attributed to lifestyle behavior changes. This study demonstrated that our intervention, NOURISH-T, which targets eating and PA change, is feasible and yields positive effects in PCS and their caregivers. These findings must be translatable for use in pediatric oncology aftercare clinics thus further increasing their potential impact.
Acknowledgments
ROLE OF FUNDING SOURCES
This research was supported by funding from the National Institutes of Health (R21CA167259-A1, Stern, PI; ClinicalTrials.gov,#NCT02815982). NIH had no role in the study design, writing of the manuscript, or the decision to submit the paper for publication.
Abbreviations
- PCS
Pediatric Cancer Survivors
- PA
Physical Activity
- NOURISH-T
NOURISH for Healthy Transitions
- RCT
Randomized Control Trial
- EUC
Enhanced Usual Care
- BMI
Body Mass Index
- WHR
Waist-to-Hip Ratio
- CFQ
Child Feeding Questionnaire
- PAQ-C
Physical Activity Questionnaire for Children
- ASA24
Automated Self-administered 24-Hour Dietary Recall
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
Reference List
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110(10):2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 4.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103(8):1730–1739. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Skylar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 6.Robison LL, Green DM, Hudson M, Meadows AT, Mertens AC, Parker RJ. Long- term outcomes of adult survivors of childhood cancer: results from the Childhood Cancer Survivor Study. Cancer. 2005;104(S11):2557–2564. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]
- 7.Robison L, Hudson M. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Revs Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sklar CA, Mertens AC, Walter A, et al. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: role of cranial irradiation. Med Pediatr Oncol. 2000;35:91–95. doi: 10.1002/1096-911X(200008)35:2<91::AID-MPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 9.Amankwah E, Saenz A, Hale G, Brown P. Association between body mass index at diagnosis and pediatric leukemia mortality and relapse: a systematic review and meta-analysis. Leukemia and Lymphoma. 2016;57:1140–1148. doi: 10.3109/10428194.2015.1076815. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the childhood cancer survivor study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers and Prev. 2010;19:170–181. doi: 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang FF, Parsons SK. Obesity in childhood cancer survivors: call for early weight management. Adv Nutr: Int Rev J. 2015;6:611–619. doi: 10.3945/an.115.008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruble K, Scarvalone S, Gallicchio L, Davis C, Wells D. Group physical activity intervention for childhood cancer survivors: a pilot study. J Phys Act Health. 2016;13:352–3599. doi: 10.1123/jpah. [DOI] [PubMed] [Google Scholar]
- 14.Cox CL, Montgomery M, Oeffinger KC, et al. Promoting physical activity in childhood cancer survivors: results from the Childhood Cancer Survivor Study. Cancer. 2009;115(3):642–654. doi: 10.1002/cncr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern M, Lamanna J, Russell C, et al. Adaptation of an obesity intervention program for pediatric cancer survivors. Clin Pract Pediatr Psychol. 2013;1:264–275. doi: 10.1037/cpp0000023. [DOI] [Google Scholar]
- 16.Green DM, Cox CL, Zhu L, et al. Risk factors for obesity in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2012;30:246–55. doi: 10.1200/JCO.2010.34.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern M, Bachar E, Ronen Ackerman E, Rancourt D, Bonne O, Weintraub M. Weight trajectories of Israeli pediatric cancer survivors. J Pediatr Psychol. 2017;42(5):588–597. doi: 10.1093/jpepsy/jsw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golan M, Crow S. Targeting parents exclusively in the treatment of childhood obesity: long- term results. Obes Res. 2004;12(2):357–361. doi: 10.1038/oby.2004.45. [DOI] [PubMed] [Google Scholar]
- 19.Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. Br J Nutr. 2006;95(5):1008–1015. doi: 10.1079/BJN20061757. [DOI] [PubMed] [Google Scholar]
- 20.Janicke DM, Sallinen BJ, Perri MG, et al. Comparison of parent-only vs. family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janicke DM, Steele RG, Gayes LA, et al. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. J Pediatr Psychol. 2014;39(8):809–825. doi: 10.1093/jpepsy/jsu023. [DOI] [PubMed] [Google Scholar]
- 22.Mazzeo SE, Kelly NR, Stern M, et al. Nourishing Our Understanding of Role Modeling to Improve Support and Health (NOURISH): design and methods. Contemp Clin Trials. 2012;33:512–522. doi: 10.1016/j.cct.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzeo S, Gow R, Stern M, Gerke C. Developing an intervention for parents of overweight children. Int J Child Adolesc health. 2008;1(4):355–363. [Google Scholar]
- 24.Raber M, Swartz MC, Santa Maria D, et al. Parental involvement in exercise and diet interventions for childhood cancer survivors: a systematic review. Pediatr Rev. 2016;80:338–346. doi: 10.1038/pr.2016.84. [DOI] [PubMed] [Google Scholar]
- 25.Stern M, Ewing L, Davila E, Thompson A, Hale G, Mazzeo S. Design and rationale for NOURISH-T: A randomized control trial targeting parents of overweight children with cancer transitioning off treatment. Contemp Clin Trials. 2015;41:227–237. doi: 10.1016/j.cct.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandura A. Social foundations of thought and action. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 27.Austin SB. Prevention research in eating disorders: theory and new directions. Psychol Med. 2000;30(6):1249–1262. doi: 10.1017/s0033291799002573. [DOI] [PubMed] [Google Scholar]
- 28.Steiner-Adair C, Sjostrom L, Franko DL, et al. Primary prevention of risk factors for eating disorders in adolescent girls: learning from practice. Int J Eat Disord. 2002;32(4):401–411. doi: 10.1002/eat.10089. [DOI] [PubMed] [Google Scholar]
- 29.Kendall PC, Choudhury MS. Children and adolescents in cognitive-behavioral therapy: some past efforts and current advances, and challenges in our future. Cognit Ther Res. 2003;27(1):89–105. http://dx.doi.org/10.1023/A:1022542814822. [Google Scholar]
- 30.U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute. [Accessed October 4, 2014];Families finding the balance: a parent handbook. (NIH Publication No. 05-5273). http://www.nhlbi.nih.gov/health/educational/wecan/tools-resources/nutrition.htm. Published June 2005. Updated November 8 2013.
- 31.Barlow S. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(S4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. [Accessed October 4, 2014];Automated Self-Administered 24-Hour Recall (ASA24) http://appliedresearch.cancer.gov/asa24/. Published 2011. Updated April 25 2017.
- 33.Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36(6):201–210. doi: 10.1006/appe.2001.0398. [DOI] [PubMed] [Google Scholar]
- 34.Davidson KK, Birch LL. Child and parent characteristics as predictors of change in girls’ body mass index. Int J Obes. 2001;25(12):1834–1842. doi: 10.1038/sj.ijo.0801835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worobey J, Borrelli A, Espinosa C, Worobey HS. Feeding practices of mothers from varied income and racial/ethnic groups. Early Child Dev Care. 2013;183(11):1661–1668. doi: 10.1080/03004430.2012.752735. http://dx.doi.org/10.1080/03004430.2012.752735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallis JF, McKenzie TL, Alcaraz JE. Habitual physical activity and health-related physical fitness in fourth-grade children. Am J Dis Child. 1993;147:890–896. doi: 10.1001/archpedi.1993.02160320092025. [DOI] [PubMed] [Google Scholar]
- 37.Neumark-Sztainer D, Eisenberg ME, Fulkerson JA, Story M, Larson NI. Family meals and disordered eating in adolescents: longitudinal findings from project EAT. Arch Pediatr Adolesc Med. 2008;162(1):7–22. doi: 10.1001/archpediatrics.2007.9. [DOI] [PubMed] [Google Scholar]
- 38.Gross LD, Sallis JF, Buono MJ, Roby JJ, Nelson J. A reliability of interviewers using the seven-day physical activity recall. Res Q Exerc Sport. 1990;61:321–325. doi: 10.1080/02701367.1990.10607495. [DOI] [PubMed] [Google Scholar]
- 39.Terwee CB, Mokkink LB, van Poppel MN, Chinapaw MJ, van Mechelen W, de Vet HC. Qualitative attributes and measurement properties of physical activity questionnaires: a checklist. Sports Med. 2010;40(7):525–537. doi: 10.2165/11531370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Meck MM, Leary M, Sills RH. Late effects in survivors of childhood cancer. Pediatr Rev. 2006;27(7):257–262. doi: 10.1542/pir.27-7-257. [DOI] [PubMed] [Google Scholar]
- 41.Diep C, Hingle M, Chen T, et al. The automated self-administered 24-hour dietary recall for children, 2012 Version, for youth aged 9 to 11 years: a validation study. J Acad Nutr Diet. 2015;115:1591–1598. doi: 10.1016/j.jand.2015.02.021. https://doi.org/10.1016/j.jand.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern M, Krivoy E, Foster R, Bitsko M, Toren A, Ben Arush M. Psychosocial functioning and career decision-making in Israeli adolescent cancer survivors. Pediatr Blood Cancer. 2010;55:708–713. doi: 10.1002/pbc.22642. [DOI] [PubMed] [Google Scholar]
- 43.Children’s Oncology Group. [Accessed July 7 2017];Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 4. http://www.survivorshipguidelines.org. Published October 2014. Updated 2014.
- 44.Bean MK, Mazzeo SE, Stern M, et al. Six-month dietary changes in ethnically diverse, obese adolescents participating in a multidisciplinary weight management program. Clin Pediatr. 2011;50(5):408–416. doi: 10.1177/0009922810393497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang JS, Dillon L, Terrones L. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatr Blood Cancer. 2014;61:894–900. doi: 10.1002/pbc.24937113. [DOI] [PMC free article] [PubMed] [Google Scholar]