Abstract
Background
We performed a phase I trial evaluating a combination of cisplatin, gemcitabine, and escalating doses of veliparib in patients with untreated advanced pancreas adenocarcinoma (PDAC) in two cohorts: germline mutated BRCA 1/2 (BRCA+) and wild-type BRCA (BRCA−). Aims were to determine the safety, dose-limiting toxicities (DLT’s), maximum tolerated dose, recommended phase II dose (RP2D) of veliparib combined with cisplatin and gemcitabine and to assess antitumor efficacy (RECIST 1.1) and overall survival (OS).
Methods
Gemcitabine and cisplatin were dosed at 600 mg/m2 and 25 mg/m2 over 30 minutes on days 3 and 10 of a 21-day cycle. Four dose levels (DL) of veliparib were evaluated: (DL0) 20 mg, (DL1) 40 mg, and (DL2) 80 mg, given orally BID days 1–12, and DL2A 80 mg BID days 1–21.
Results
17 patients were enrolled: 9 BRCA+; 7 BRCA− and 1 unknown status. DLTs were reached at DL2A: 80 mg BID days 1–21. Two of 5 (40%) patients in this cohort experienced grade 4 neutropenia and thrombocytopenia. Two grade 5 events occurred on protocol. The objective response rate in the BRCA+ cohort was 7/9 (77.8%). Median OS for BRCA+ patients was 23.3 months (95% CI 3.8–30.2). Median OS for BRCA− patients was 11 months (95% CI 1.5–12.1).
Conclusion
The RP2D of veliparib is 80 mg po BID days 1–12 combined with cisplatin and gemcitabine; the DLT was myelosuppression. Substantial antitumor activity was seen in BRCA+ PDAC. A randomized phase II trial is currently evaluating cisplatin, gemcitabine +/− veliparib in BRCA+ PDAC (NCT #01585805).
Keywords: Pancreas cancer, BRCA, veliparib, cisplatin, germline
Introduction
Pancreatic adenocarcinoma (PDAC) is the fourth leading cause of cancer-related mortality worldwide and has an overall 5-year survival rate of 8%1. Multi-agent cytotoxic combinations such as FOLFIRINOX and gemcitabine/nab-paclitaxel have improved the outcomes for advanced PDAC, nonetheless, overall survival for most patients is less than a year2–4. Advances in identification of genomic alterations, specifically the ability to accurately identify relevant genetic mutations, has ushered in an era of unprecedented precision medicine in oncology in general. In PDAC, germline BRCA 2 and 1 mutations, which occur in up to 5–7% of patients, incur an increased risk of developing PDAC estimated to be 2.5 –3.5 fold higher than the general population5,6. In addition to known increased sensitivity to DNA-damaging chemotherapy in women with breast and ovarian cancer, mutations in BRCA genes have generated much attention following the discovery of their synthetically lethal interaction with poly-ADP ribose (PARP)7,8. PARP are a family of enzymes, two of which, PARP1 and 2, are key components of the DNA repair mechanism for cells with single-strand DNA breaks and nucleoside base damage9. PARP inhibitor therapy (PARPi) has been effective in tumors with defects in homologous recombination (HR) such as breast and ovarian cancers with BRCA1 or 2 mutations10,11. In vitro work in the PDAC cell line BRCA2 mutated CaPan-1 demonstrated single-agent activity for the PARPi, KU-0058684, and in combination with cytotoxic agents12,13. Clinical trials have demonstrated the viability of exploiting this deficiency in DNA repair in other cancers such as breast, ovary, as well as PDAC10,11,14.
Given the rationale for evaluating DNA-damaging agents and PARPi in germline BRCA mutated patients (BRCA+), we designed a series of trials in PDAC. The specific goals were to evaluate the: (1) The appropriate dose and schedule for combining the PARPi, veliparib, with cisplatin and gemcitabine; (2) to evaluate the activity of single-agent veliparib in BRCA+ PDAC (results reported in a related manuscript) and based on (1) to design a random assignment phase II trial to assess the efficacy of cisplatin plus gemcitabine with/without veliparib in advanced BRCA/PALB2+ PDAC. Herein we report the phase I evaluation of cisplatin, gemcitabine and veliparib in two cohorts of patients with PDAC, one with, and one without, a germline BRCA 1,2 mutation. We specifically choose to evaluate a combination regimen where the delivery of the PARPi preceded cytotoxic therapy to maximize the potential for synergistic synthetic lethality and further choose to evaluate a 12-day and 21-day continuous dosing schedule of veliparib recognizing upfront that myelosuppression was anticipated to be dose-limiting. This study was designed in conjunction with the Cancer Therapeutics and Evaluation Program (NCI CTEP) and the Lustgarten Foundation.
Patients and Methods
Study Design and Treatment
This was a phase I single arm study. The primary endpoint was determination of the maximum tolerated dose (MTD) and the dose-limiting toxicity (DLT) for the combination of veliparib with gemcitabine and cisplatin and to identify the recommended phase II dose (RP2D) for the triplet. Secondary endpoints were to determine the safety and oncologic outcomes (response rate, progression-free (PFS) and overall survival (OS)) for this combination. This trial was reviewed by the Institutional Review and Privacy Board at all sites. All patients provided written informed consent.
It was estimated that a minimum of six and maximum of 24 patients would be studied. Three-6 patients were treated in each veliparib dose level. Treatment was administered in 21 day cycles, with fixed doses of gemcitabine and cisplatin give on the 3rd and 10th day. Gemcitabine was dosed at 600 mg/m2 IV and cisplatin was dosed at 25 mg/m2 IV, both infused over 30 minutes. Veliparib was given orally twice daily, with the duration of dosing dependent on the dose level (DL) of the patient group (Table 1). Six dose levels were planned: DL0 20 mg BID (twice daily), DL1 40 mg BID, and DL2 80 mg, by mouth days 1–12, and DL2A 80 mg BID days 1–21, DL3 140 mg BID, DL4 200 mg BID days 1–21. Germline genetic testing for BRCA status was conducted at Myriad Genetics (USA) or other equivalent diagnostic companies in the US and abroad.
Table 1.
Baseline Patient Characteristic
| Table 1. Baseline Patient Characteristics and Treatment Dosage Information
| |||||||
|---|---|---|---|---|---|---|---|
| Dose level | Dose of Veliparib | Age | Sex | Disease Stage | ECOG PS | BRCA Mutation | Founder |
| 0–2A | 58 (41–71) | 10 M 7 F | III–IV | 0–1 | N/A | N/A | |
|
| |||||||
| 0 | 20 mg BID | 70 | M | IV | 0 | BRCA1: 185delAG | Founder (AJ) |
| 0 | 62 | M | IV | 1 | BRCA2: 6174delT | Founder (AJ) | |
| 0 | 66 | M | IV | 1 | |||
|
| |||||||
| 1 | 40 mg BID | 60 | M | IV | 1 | ||
| 1 | 64 | M | IV | 1 | BRCA2: 6174delT | Founder (AJ) | |
| 1 | 57 | M | III | 1 | Unknown | Unknown | |
|
| |||||||
| 2 | 80 mg BID | 70 | F | IV | 1 | BRCA1: 2800delAA | N |
| 2 | 56 | F | IV | 1 | BRCA1: 187delAG | Founder (AJ) | |
| 2 | 58 | M | IV | 1 | BRCA2: 6174delT | Founder (AJ) | |
| 2 | 51 | F | IV | 0 | BRCA2: c.8177A>G | N | |
| 2 | 55 | M | IV | 1 | |||
| 2 | 50 | F | IV | 1 | BRCA1: 5385insC | Founder (AJ) | |
|
| |||||||
| 2A | 80 mg BID cont | 71 | F | III | 0 | ||
| 2A | 58 | M | IV | 1 | |||
| 2A | 50 | F | IV | 1 | BRCA2: D281N(1069G>A) | N | |
| 2A | 70 | M | IV | 1 | |||
| 2A | 41 | F | IV | 1 | |||
Fifteen patients had stage IV disease and 2 patients had stage III. One patient in dose level 1 had an unknown BRCA status, but was included in the BRCA− group. Gemcitabine is fixed at 600 mg/m2 IV and cisplatin is fixed at 25 mg/m2 IV.
Patient Population
Patients with untreated, inoperable stage III or IV PDAC who were known carriers of a germline BRCA1, 2, or PALB2 mutation (BRCA+), or patients with a family history who were susceptible for such mutations (e.g., personal/family history of breast, pancreas, ovary, endometrial, prostate, or other related malignancy) were eligible. Other key eligibility criteria included measurable or evaluable disease (RECIST 1.1), no prior platinum or PARPi therapy, and an Eastern Cooperative Oncology Group (ECOG) score 0–1, ≥18 years of age, life expectancy ≥ 3 months, and normal organ and bone marrow function as defined by: absolute neutrophil count (ANC) ≥ 1,500/mcL, hemoglobin ≥ 9.0 mg/dl, platelets ≥ 100,000/mcL, total bilirubin ≤ 2 X institutional upper limit of normal (ULN), AST/ALT ≤ 2.5 X institutional ULN, unless evidence of liver metastases, in which case AST/ALT must be ≤ 5 X institutional ULN, and creatinine ≤ 1.5 X ULN. Females of child-bearing potential required a negative pregnancy test. Patients with a known active infection, e.g. hepatitis B/C, or HIV positive patients who did not have evidence of significant immune compromise, were eligible.
Patients were excluded if they had received prior adjuvant therapy with a platinum drug, and if PDAC recurred within ≤ 6 months of adjuvant therapy or if CNS metastases were stable for ≤ 3 months. Patients with contraindications to platinum agents or who were receiving any other investigational agents were excluded. Patients with a history of seizures or allergic reactions attributed to compounds of similar composition to veliparib or other agents were also excluded. Additionally, patients with uncontrolled infection, cardiovascular disease, or psychiatric illness were ineligible.
Patients were removed from study treatment if they experienced disease progression, intercurrent illness that precluded treatment administration, had unacceptable toxicity, experienced a > 2-week delay in initiating cycle #2, or voluntarily chose to change therapy. Up to 3 dose reductions per drug was permitted per patient.
Dose-Limiting Toxicity and Biostatistical Design
The primary endpoints were to determine the MTD, DLT and RP2D for veliparib combined with cisplatin and gemcitabine. The DLT assessment period applied to the first cycle of treatment (21 days). Toxicities were assessed according to Common Terminology Criteria for Adverse Events (CTCAE) version 4. Hematologic dose-limiting toxicities included grade 4 (G4) thrombocytopenia or neutropenia, grade 3 (G3) thrombocytopenia with bleeding, or febrile neutropenia (fever > 38.5° C and neutrophil count < 0.5). Non-hematologic DLTs included G3 diarrhea, nausea and vomiting refractory to loperamide or anti-emetic therapy and unable to be corrected to grade 1 (G1) or less within 24 hours. G4 metabolic toxicities (except hyperglycemia) were DLTs regardless of duration.
Dose escalation was conducted via a 3 + 3 design. Three-six patients were treated per dose level. If < 1/3 or < 2/6 patients experienced DLT, the dose was escalated to the next level. If 1/3 experienced a DLT the cohort was expanded to 6. This continued until the above criteria were exceeded. The maximum-tolerated dose (MTD), and the RP2D, were 1 dose level below DLT.
Toxicity was assessed with CTCAE v 4.0. Radiologic re-staging (RECIST 1.1) was conducted every 6 weeks (2 cycles) from the start of therapy with a window period of +/− 5 days. All patients were followed for survival until death.
A number of correlative studies designed to evaluate the presence/absence of loss of heterozygosity, identification reversion BRCA mutations, gene expression signatures were also included in this phase I trial and will be reported in a separate manuscript.
Results
Patient Characteristics and Treatment
Baseline patient characteristics are shown in Table 1. Seventeen (10 male, 7 female) patients, median age 58 years (range 41–71) were enrolled between February 2012 and October 2013 at 3 sites: Memorial Sloan Kettering Cancer Center, Princess Margaret Cancer Centre, and University of Chicago Medical Center. The cut off for data analysis was June 30, 2017. Fifteen (88.2%) patients had stage IV disease, 2 (11.8%) patients had stage III disease (both BRCA−). Nine (52.9%) patients were BRCA+ and seven (41.2%) patients were BRCA−. One patient had undetermined BRCA status and was included in the BRCA− group.
Four dose levels were evaluated. DL0, DL1, and DL2 had no DLTs after which, a new DL2A was added. DL2A had 2/5 patients experience DLTs. This led to the recommended dose of veliparib for combination therapy with gemcitabine and cisplatin to be dose level 2: 80 mg BD days 1–12 combined with fixed doses of cisplatin and gemcitabine.
Toxicities and Dose Reductions
Hematologic Toxicities
Hematologic toxicities, as captured during cycle 1, the DLT assessment period, are shown in Table 2. During the DLT period, DL0 and DL 1, 2 were well-tolerated. DL2A had 7 G3 events, and 6 G4 events. Of 6 G4 events, 4 were DLTs (neutropenia, thrombocytopenia, n=2); both of these patients had dose reductions and continued on study. Beyond the DLT period, 2 patients experienced G4 neutropenia (DL2), and 3 patients experienced G4 thrombocytopenia (DL2). One patient in DL2A developed febrile neutropenia.
Table 2.
Hematological Toxicities
| Table 2. Hematologic Toxicities (Max Grade per Patient)
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 (DLT period: 0–21 days) | ||||||||||||||||
|
|
||||||||||||||||
| Dose 0 | Dose 1 | Dose 2 | Dose 2A | |||||||||||||
| 20 mg po BID day 1–12 | 40 mg po BID day 1–12 | 80 mg po BID day 1–12 | 80 mg po BID day 1–21 | |||||||||||||
| Hematologic Toxicity | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | ||||
| Leukopenia | 1 | 2 | 2 | 1 | ||||||||||||
| Neutropenia | 1 | 1 | 1 | 1 | 2* | |||||||||||
| Lymphopenia | 2 | 1 | ||||||||||||||
| Anemia | 1 | 2 | 2 | 2 | ||||||||||||
| Thrombocytopenia | 1 | 1 | 2* | |||||||||||||
| DLTs | 0 | 0 | 0 | 2 | ||||||||||||
|
|
|
|
|
|||||||||||||
| Cycle 2 and beyond | ||||||||||||||||
|
| ||||||||||||||||
| Dose 0 | Dose 1 | Dose 2 | Dose 2A | |||||||||||||
| Hematologic Toxicity | G2 | G3 | G4 | G5 | G2 | G3 | G4 | G5 | G2 | G3 | G4 | G5 | G2 | G3 | G4 | G5 |
| Leukopenia | 3 | 2 | 1 | 4 | 1 | 2 | 1 | |||||||||
| Neutropenia | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 1 | 2 | |||||||
| Lymphopenia | 1 | 1 | 3 | 4 | ||||||||||||
| Anemia | 1 | 2 | 1 | 1 | 4 | 2 | 2 | |||||||||
| Thrombocytopenia | 1 | 2 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | |||||||
| Febrile Neutropenia | 1 | |||||||||||||||
| Acute Leukemia | 1** | |||||||||||||||
Abbreviations: G, toxicity grade (according to Common Terminology Criteria for Adverse Events version 4).
2 patients experienced both G4 neutropenia and thrombocytopenia.
1 patient experienced acute myeloid leukemia
Non-Hematologic Toxicities and Grade 5 Events
Non-hematologic toxicities are summarized in Table 3. There were no dose-limiting non-hematologic toxicities. DL0 had one G4 event of elevated bilirubin. In DL2A there was 1 G4 biliary infection in the context of a known biliary stent; this patient also experienced several other G3 biliary infections during the treatment course.
Table 3.
Non-hematological Toxicities
| Table 3. Non-Hematologic Toxicities (Max Grade per Patient)
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose 0 | Dose 1 | Dose 2 | Dose 2A | |||||||||||
| Non-hematologic Toxicity | G2 | G3 | G4 | G5 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | |
| Nausea | GI | 2 | 1 | 3 | 1 | |||||||||
| Diarrhea | 2 | 1 | ||||||||||||
| Vomitting | 1 | 2 | ||||||||||||
| Hyperglycemia | Metabolic | 3 | 1 | 2 | 3 | |||||||||
| Hypomagnesemia | 1 | 2 | 2 | |||||||||||
| Hypokalemia | 1 | 2 | ||||||||||||
| Hyponatremia | 1 | 2 | ||||||||||||
| Hypophosphatemia | 1 | 1 | 2 | 1 | 1 | |||||||||
| Alkaline-Phosphate | LFTs | 1 | 1 | 2 | 1 | 2 | ||||||||
| AST | 1 | 1 | 1 | |||||||||||
| ALT | 1 | 1 | 2 | 1 | 2 | |||||||||
| Bilirubin | 1 | 1 | 1 | 1 | ||||||||||
| Creatinine | 1 | 1 | 1 | |||||||||||
| Fever (non-neutropenic) | 1 | 1 | 1 | 1 | ||||||||||
| Fatigue | 2 | 1 | 1 | 3 | 1 | 2 | 1 | |||||||
| Dyspnea | 1 | |||||||||||||
| Dysgeusia | 1 | |||||||||||||
| Neuropathy | 1 | 1 | ||||||||||||
| Pain (abdominal) | 2 | 1** | 1*** | 2 | ||||||||||
| Pain (other) | 2** | 2*** | 1 | 1 | ||||||||||
| Biliary Infections | 1 | 1**** | 1**** | |||||||||||
| Infections (other) | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1**** | ||||||
| Colon Perforation | 1* | |||||||||||||
1 patient experienced a fatal colonic perforation while on treatment
1 patient experienced both G2 abdominal pain and G2 bone pain
1 patient experienced both G3 abdominal pain and G2 non-specific pain
1 patient experienced G3, G4 biliary infections, and G3 cholecystitis
There were two G5 events on study. One patient developed acute myeloid leukemia (AML). This patient was a 60-year male with stage IV PDAC BRCA2+ with a family history significant for BRCA-related cancers and AML. The patient had a complete response per RECIST in metastatic PDAC but developed AML 2.5 years into treatment and died shortly thereafter of AML. Cytogenetic analysis identified that this was likely a therapy-related event, with deletions of 5q31 and 7q31 detected in 66% and 61.3% of cells respectively. A second patient, a 70-year old male with stage IV PDAC BRCA1+ with a personal history significant for esophageal perforation and hernia repair, experienced a fatal colonic perforation. Evaluation during the event indicated a malignant vs diverticular stricture. The event occurred at the end of cycle #3 and may have been related to therapy response, versus considered more likely related to a diverticular stricture. The patient was non-neutropenic at the time.
Dose-Reductions
Outside of the DLT period twelve (70.6%) patients had reductions in gemcitabine and cisplatin, 9 (75%) due to recurrent thrombocytopenia, 2 (16.7%) due to neutropenia, and 1 (8.3%) due to biliary infections. Two (11.8%) patients discontinued cisplatin, 1 (50%) because of thrombocytopenia and another due to neuropathy. One (5.9%) patient discontinued gemcitabine due to recurrent thrombocytopenia. Three (17.6%) patients had a reduction of veliparib dose, 2 related to thrombocytopenia and 1 due to nausea and fatigue. For one patient who discontinued cytotoxic therapy they were maintained on single-agent veliparib for an extended time period with durable disease control (>2 years).
Treatment
Treatment responses are summarized in Table 4. Seven patients (41.2%; all BRCA+) had a RECIST response, 6 (85.7%) partial responses (PR), 1 (14.3%) complete response (CR). In all, BRCA+ had CR or PR rate of 77.8%. Eight patients (47.1%) had stable disease (SD). Two (11.8%) patients had progression of disease (POD) as best response. No objective responses were seen in BRCA−/unknown patients.
Table 4.
Best Response to Treatment
| Table 4. Best Response to Treatment
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | All patients | Dose 0 (BRCA+) | Dose 0 (BRCA−) | Dose 1 (BRCA+) | Dose 1 (BRCA−) | Dose 2 (BRCA+) | Dose 2 (BRCA−) | Dose 2A (BRCA+) | Dose 2A (BRCA−) |
| No. Patients | 17 | 2 | 1 | 1 | 2 | 5 | 1 | 1 | 4 |
| Complete response | 1 (5.9%) | 1 | |||||||
| Partial response | 6 (35.3%) | 1 | 1 | 3 | 1 | ||||
| Stable disease | 8 (47.1%) | 1 | 1 | 2 | 1 | 3 | |||
| Progressive disease | 2 (11.8%) | 1 | 1 | ||||||
|
| |||||||||
| Overall response rate | 7 (41.2%) | 1 | 1 | 4 | 1 | ||||
The patient with unknown BRCA status was in dose 1 and their disease status was SD. 1 patient in dose level 2A withdrew from study before finishing cycle 1.
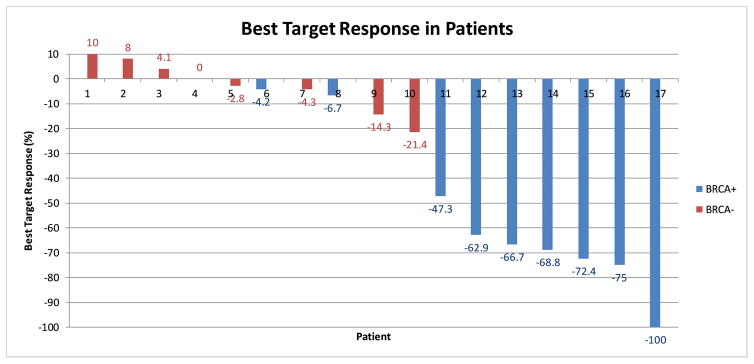
Best target response is shown in Figure 1a. For responders, the median time to best response was 7.4 months (95% CI 5.1–15.7). For the entire cohort of BRCA+ patients (N=9), the median best response to treatment was a 66.7% reduction in total tumor volume (95% CI 35.2% – 76.8%). For BRCA− patients, the median best response was a 1.4% reduction by RECIST (95% CI 5.4% increase to 10.6% decrease).
Figure 1.
Figure 1a. Best Target Response in Patients
Waterfall plot depicting best target response in all patients on study. Seven patients had a response and 8 patients had stable disease. Individuals with BRCA mutations overwhelmingly had a response compared to those without mutations. The patient with unknown BRCA status had a 21.4% reduction per RECIST 1.1.
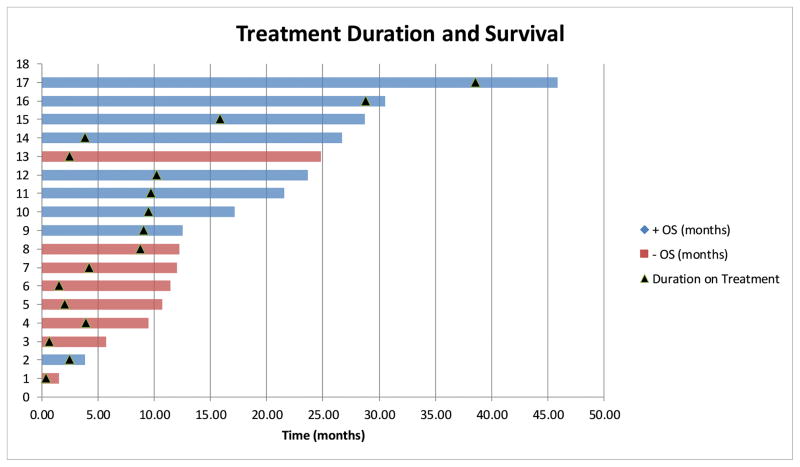
Figure 1b. Overall Survival and Treatment Duration
Swimmers plot depicting overall survival and indicating treatment duration. Note that BRCA+ patients remained on treatment for a longer duration and had more favorable survival. The patient with unknown BRCA status was on treatment for 8.8 months and had an OS of 12.3 months.
Figure 1b shows OS and duration on treatment. The median duration on treatment for the entire cohort was 4.2 months (95% CI 4–13.8). For the BRCA+ patients, the median duration on treatment was 9.7 months (95% CI 6.4–22). For the BRCA− patients, the median duration on treatment was 2.3 months (95% CI 1 to 5).
Patient Survival
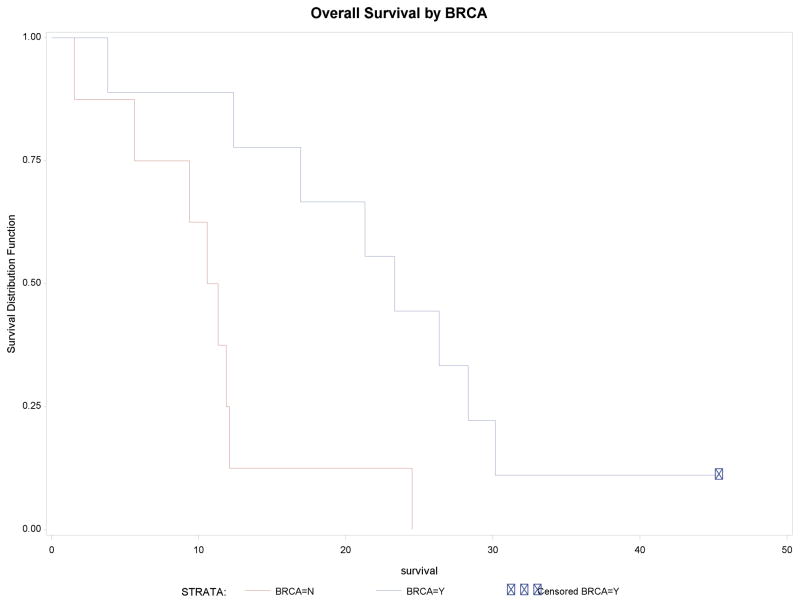
Figure 2 shows the overall survival of the entire cohort identified by BRCA status. BRCA+ patients had a median survival of 23.3 months (95% CI 3.8–30.2). BRCA− patients had a median survival of 11 months (95% CI 1.5–12.1). One BRCA+ patient remains alive, with recent discontinuation of protocol therapy (late 2016) after >3 years of disease control.
Figure 2.
Kaplan-Meier overall survival curves based on BRCA status. Median survival for BRCA+ patients was 23.3 months, 95% CI 3.8–30.2 months. Median survival for BRCA- patients is 11 months, 95% CI 1.5–12.1 months. Note that 1 patient who is BRCA+ remains alive after >3 years of disease control.
Discussion
Treatment for advanced PDAC is evolving and therapy refinement and patient selection are emerging realities in small subsets of patients with this disease15. Increasing evidence indicates that germline BRCA+ is an important such subgroup. We chose to evaluate in a prospective fashion the utility of platinum based therapy combined with a PARPi in subgroups of patients with PDAC where the concept of synthetic lethality could be evaluated and potentially exploited.
The combination of gemcitabine and cisplatin has been evaluated at various doses and schedules in patients with sporadic PDAC, however, no optimal dose and schedule has been confirmed. For the most part, antitumor efficacy has been modest in randomized trials evaluating the addition of cisplatin or oxaliplatin to gemcitabine, compared to gemcitabine alone in unselected patients16–20. A randomized phase III trial with gemcitabine and cisplatin for advanced biliary cancers supported using gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 on days 1 and 8 every 3 weeks, however lower doses of gemcitabine have previously been demonstrated to have activity in advanced PDAC21,22. We chose to evaluate a fixed cisplatin dose of 25 mg/m2, a fixed gemcitabine dose of 600mg/m2 and to dose-escalate veliparib. While cisplatin plus gemcitabine may have limited activity in PDAC absent a HR defect, we hypothesized that it would be an effective regimen in BRCA+ and in an enriched patient population (surrogate family/personal history of cancer). We chose this dose and schedule in order to: (1) preserve the platinum dose, (2) minimize the myelosuppressive impact of the combination by reducing the gemcitabine dose, (3) permit the addition of the PARPi, and, (4) to allow for a treatment that could be administered for a prolonged period. Despite the lower dose of gemcitabine, the dose-intensity of gemcitabine when calculated, was comparable to standard approaches22. A key principle underpinning the hypothesis is that a combination strategy would delay the emergence of resistance by overcoming the limitations of a single-agent targeted approach.
This trial evaluated 17 patients with advanced PDAC. The RP2D of veliparib for the combination with cisplatin 25 mg/m2, gemcitabine 600mg/m2 is 80 mg BD days 1–12 every 21 days. Target inhibition is known to be achieved at this dose level of veliparib23. Predictably, hematologic toxicity was dose-limiting. While we could not directly compare the frequency or severity of toxicities between BRCA+ and BRCA− due to unequal distribution of the two cohorts by dose level, we subjectively did not appreciate a significant different in toxicity.
A key point of the study was to identify if an active regimen such as this could be administered longitudinally. Feasibility was met in this regard. One early toxicity with the fatal colonic perforation was suspected to be of benign etiology and unrelated to therapy. One late event was notable – acute myeloid leukemia. Myelosuppression is well documented with gemcitabine and cisplatin combinations24,25. Veliparib has been shown to enhance the myelosuppression of cytotoxic agents, and given the patient’s extended treatment duration (2.5 years) we speculate that cumulative myelosuppression and exposure to this triplet combination therapy may have led to AML26,27. Furthermore, therapy-related AML is a rare but well documented complication of both PARPi therapy and cytotoxic therapy28. Additionally, pre-clinical data has shown that genetic ablation of PARP increases the incidence of cancer, suggesting a tumor suppressor function for PARP29. This possible role may be more pronounced in patients with prolonged PARP inhibition, such as with our aforementioned patient. Substantial activity for the triplet combination was identified in BRCA+ patients. For the study overall, 7/17 (41.2%) patients had a RECIST response; all of these patients were BRCA+. Specifically, for the BRCA+ cohort the response rate was 7/9 (77.8%) and these responses were deep by RECIST (Figure 1a). No BRCA− patients had an objective tumor response. BRCA+ patients had a median OS of 23.3 months (95% CI 3.8–30.2), and BRCA− patients had a median OS of 11 months (95% CI 1.5–12.1). Figure 1b further supports this therapy’s effectiveness in the BRCA+ population. While the median on-treatment duration for BRCA− patients was 2.3 months (95% CI 1–5), median on treatment duration for BRCA+ patients was 9.7 months (95% CI 6.4–22). This four-fold difference in treatment duration suggests that response to therapy is both significant and durable in BRCA+ patients. A variety of subsequent therapies were administered following discontinuation of protocol therapy. FOLFIRINOX (N=3), gemcitabine/nab-paclitaxel (N=2) and both regimens (N=4) were most common. Additionally, 4 (23.5%) patients had a combination of platinum and other agent, and 1 (5.9%) patient had palliative radiation. Three (17.6%) patients received no further cancer-directed therapy.
While proof-of-concept trials have demonstrated effectiveness of PARPi in breast and ovarian cancers, information regarding its potential in combination therapy in PDAC is limited30,31. Our trial is the first prospective study to evaluate cisplatin-based therapy in a BRCA+ patient population.
We acknowledge the limitations of a small, non-randomized study with regard to the conclusions drawn. The efficacy data, while limited, clearly supports further development of a synthetic-lethality based therapy in genomically-selected PDAC. We further draw the conclusion that family history in our data set does not appear to be an effective surrogate for predicting utility to platinum-based therapy nor significant enrichment for BRCA+ status, however, we do recognize that other retrospective data sets suggest the presence of a family history of cancer may be a surrogate for platinum sensitivity32–34. Our cohort of BRCA− patients had family histories notable for potential BRCA− related cancers, however, their treatment outcome (Figure 1a) and duration on treatment (Figure 1b) resembled outcome typical of a sporadic PDAC setting.
Our results do provide prospective, robust evidence to existing pre-clinical data that suggest the actionability of BRCA+ status in PDAC35. The exact reasoning for improvement in outcome is unclear, with synthetic lethality, platinum, or likely the combination of all 3 drugs, being plausible explanations for activity along with the potential contribution of other genes involved in DNA damage response and PDAC susceptibility, e.g., ATM36. A randomized phase II trial of cisplatin/gemcitabine with/without veliparib is ongoing and will provide more insight on the therapeutic potential of this triplet in patients with BRCA+ PDAC.
In conclusion, the combination of cisplatin, gemcitabine and veliparib can be safely administered in advanced PDAC. Hematologic toxicity is dose-limiting. Substantial activity is observed in a genomically-selected subgroup of germline BRCA+ PDAC.
Acknowledgments
Funding Support
Lustgarten Foundation
National Cancer Institute
David M. Rubenstein Center for Pancreatic Cancer Research
Cancer Support Grant P30 CA008748
National Cancer Institute of the National Institutes of Health: R25CA020449
Robert Mayer, Anne Fusco, Talal Khawaja, Sloane C. Smith, Erica Kaufmann
Footnotes
Data from this trial has been presented at the American Society Clinical Oncology 2015
Conflict of Interest: The authors declare no known conflict of interest.
All authors contributed to the preparation, writing, review and editing of the manuscript.
E.M. O’Reilly, M.A. Lowery and D.P. Kelsen contributed to conceptualization, design, conduct of the clinical investigation, funding support, data collection, interpretation, manuscript writing, review and editing.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Ma H, Hong G, et al. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981–2010. Sci Rep. 2014;4:6747. doi: 10.1038/srep06747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005;7:60. [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch HT, Deters CA, Snyder CL, et al. BRCA1 and pancreatic cancer: pedigree findings and their causal relationships. Cancer Genet Cytogenet. 2005;158:119–25. doi: 10.1016/j.cancergencyto.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 8.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 9.Sandhu SK, Yap TA, de Bono JS. Poly(ADP-ribose) polymerase inhibitors in cancer treatment: a clinical perspective. Eur J Cancer. 2010;46:9–20. doi: 10.1016/j.ejca.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 11.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 12.Giovannetti E, Mey V, Danesi R, et al. Synergistic cytotoxicity and pharmacogenetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines. Clin Cancer Res. 2004;10:2936–43. doi: 10.1158/1078-0432.ccr-03-0520. [DOI] [PubMed] [Google Scholar]
- 13.McCabe N, Lord CJ, Tutt AN, et al. BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of Poly (ADP-Ribose) polymerase: an issue of potency. Cancer Biol Ther. 2005;4:934–6. doi: 10.4161/cbt.4.9.2141. [DOI] [PubMed] [Google Scholar]
- 14.Bendell J, O’Reilly EM, Middleton MR, et al. Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Ann Oncol. 2015;26:804–11. doi: 10.1093/annonc/mdu581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowery MA, Jordan EJ, Basturk O, et al. Real Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-0899. [DOI] [PubMed] [Google Scholar]
- 16.Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–51. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 17.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–52. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 18.Kulke MH, Niedzwiecki D, Tempero MA, et al. A randomized phase II study of gemcitabine/cisplatin, gemcitabine fixed dose rate infusion, gemcitabine/docetaxel, or gemcitabinefirinotecan in patients with metastatic pancreatic cancer (CALGB 89904) Journal of Clinical Oncology. 2004;22:316s–316s. doi: 10.1200/JCO.2009.22.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–16. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–85. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 22.Khalil MA, Qiao W, Carlson P, et al. The addition of erlotinib to gemcitabine and cisplatin does not appear to improve median survival in metastatic pancreatic cancer. Invest New Drugs. 2013;31:1375–83. doi: 10.1007/s10637-013-9967-2. [DOI] [PubMed] [Google Scholar]
- 23.Kummar S, Kinders R, Gutierrez ME, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–11. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheruku R, Hussain M, Tyrkus M, et al. Myelodysplastic syndrome after cisplatin therapy. Cancer. 1993;72:213–8. doi: 10.1002/1097-0142(19930701)72:1<213::aid-cncr2820720138>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Starlinger P, Brugger P, Schauer D, et al. Myelosuppression of thrombocytes and monocytes is associated with a lack of synergy between chemotherapy and anti-VEGF treatment. Neoplasia. 2011;13:419–27. doi: 10.1593/neo.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isakoff SJ, Overmoyer B, Tung NM, et al. A phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancer. Journal of Clinical Oncology. 2010;28:1019–1019. [Google Scholar]
- 27.Kummar S, Chen A, Ji J, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–34. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong WM, Ohgaki H, Huang H, et al. Null mutation of DNA strand break-binding molecule poly(ADP-ribose) polymerase causes medulloblastomas in p53(−/−) mice. Am J Pathol. 2003;162:343–52. doi: 10.1016/S0002-9440(10)63825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogelman DR, Wolff RA, Kopetz S, et al. Evidence for the efficacy of Iniparib, a PARP-1 inhibitor, in BRCA2-associated pancreatic cancer. Anticancer Res. 2011;31:1417–20. [PubMed] [Google Scholar]
- 31.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–50. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan E, Lowery MA, Wong W, et al. Prospective assessment for pathogenic germline alterations (PGA) in pancreas cancer (PAC) Journal of Clinical Oncology. 2017;35:4102–4102. [Google Scholar]
- 33.Peters MLB, Brand R, Borazanci EH, et al. Germline genetic testing in unselected pancreatic ductal adenocarcinoma (PDAC) patients. Journal of Clinical Oncology. 2017;35:1501–1501. [Google Scholar]
- 34.Fogelman D, Sugar EA, Oliver G, et al. Family history as a marker of platinum sensitivity in pancreatic adenocarcinoma. Cancer Chemotherapy and Pharmacology. 2015;76:489–498. doi: 10.1007/s00280-015-2788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohse I, Borgida A, Cao P, et al. BRCA1 and BRCA2 mutations sensitize to chemotherapy in patient-derived pancreatic cancer xenografts. Br J Cancer. 2015;113:425–32. doi: 10.1038/bjc.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]