Abstract
Our recent studies demonstrate that X-linked inhibitor of apoptosis protein (XIAP) is essential for regulating colorectal cancer invasion. Here we discovered that RhoGDIβ was a key XIAP downstream effector mediating bladder cancer (BC) invasion in vitro and in vivo. We found that both XIAP and RhoGDIβ expressions were consistently elevated in BCs of N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN)-treated mice in comparison to bladder tissues from vehicle-treated mice and human BCs in comparison to the paired adjacent normal bladder tissues. Knockdown of XIAP attenuated RhoGDIβ expression and reduced cancer cell invasion, whereas RhoGDIβ expression was attenuated in BBN-treated urothelium of RING-deletion knockin mice. Mechanistically, XIAP stabilized RhoGDIβ mRNA by its positively regulating nucleolin mRNA stability via Erks-dependent manner. Moreover, ectopic expression of GFP-RhoGDIβ in T24T(shXIAP) cells restored its lung metastasis in nude mice. Our results demonstrate that XIAP-regulated Erks/nucleolin/RhoGDIβ axis promoted BC invasion and lung metastasis.
Keywords: XIAP, RhoGDIβ, nucleolin, bladder cancer, cell invasion
Introduction
Bladder cancer (BC) is the disease with malignant growth of urinary 1, and about 74,000 new cases were diagnosed and 16,000 deaths were associated with bladder cancers, according to the report from the National Cancer Institute in 2015 2. The majority of human bladder cancer is transitional cell carcinomas derived from the urothelium, which accounts for more than 90% of bladder carcinomas 3. The depth of invasion of the bladder wall is closely associated clinic treatment of bladder cancers 4. Since high-grade muscle invasive bladder cancer (HGIBC) can progress to life threatening metastases, invasive malignant tumors contribute to nearly 100% of bladder cancer-related deaths. In light of this information, it is imperative to elucidate the mechanisms underlying bladder cancer invasion and metastasis in order to mitigate the mortality of highly invasive human bladder cancer.
X-linked inhibitor of apoptosis protein (XIAP) is a member of inhibitor of apoptosis protein (IAP) family with structure characterized by three baculoviral IAP repeat (BIR) domains and an ubiquitin associated (UBA) Ring domain 5. In addition to numerous studies elucidating the mechanisms of anti-apoptotic function of XIAP protein, our recent studies have revealed several non-apoptosis-related functions of XIAP and its RING domain, such as upregulation of Cyclin D1 with promoting bladder cancer cell growth 6 and promotion of colon cancer cell invasion via inhibiting RhoGDIα SUMOylation at lys-138 7. XIAP function as a metastatic driver can also be substituted by its activation of the NFκB via the E3 ligase activity in human prostate cancer cells 8. In contrast, several other reports depicted XIAP as a tumor suppressor due to its capable of suppressing cell migration. Notable examples include a study depicting that Caveolin-1-mediated XIAP recruiting to the α-integrin complex can enhance cell adhesion 9. Another study describes how XIAP-mediated ubiquitination regulates C-RAF kinase, which, as the effector protein of Ras, initiates MAPK cascades and thereby mediating cell growth and migration 10. Nevertheless, the overall role of XIAP in cancer progression might be dependent on cancer tissues and cell types. Our most recent studies reveal that XIAP and its RING domain was crucial for human BC invasion in vitro cell culture model and invasive bladder cancer development in mice exposed to N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) in drinking water in vivo animal model 11. Thus, the discovery of XIAP downstream effectors and evaluation of the mechanisms underlying XIAP and its RING domain modulation of human BC invasion and metastasis is of tremendous importance for understanding nature of the BC invasion and metastasis.
The RhoGDI family is consists of three members, including RhoGDIα, RhoGDIβ, and RhoGDIγ, which modulate small GTPase activity via regulating GDP/GTP exchange 12. RhoGDIα is expressed ubiquitously in cells and tissues 12, whereas RhoGDIβ commonly exists in hematopoietic, endothelial and urothelial cells 13. Particularly, the latter has been reported in bladder cancer and other cancer types 14. RhoGDIβ has been thought to act as a suppressor for both migration and metastasis in bladder, ovarian, breast and lung cancers 15. And phosphorylation of RhoGDIβ induced by Src has been reported to enhance its function as suppressor for metastasis in UMUC3 cells 16. RhoGDIβ expression level is also thought to predict prognosis of BC patients 13. However, other reports have shown that RhoGDIβ promotes tumor growth and malignant progression in gastric cancer 17, while overexpression of RhoGDIβ enhances gastric cancer cell invasion and metastasis 18. During our investigation of the contribution of XIAP overexpression to human BC invasion and metastasis, we unexpectedly found that both RhoGDIβ and XIAP were consistently elevated in most of human bladder cancer tissues and in all BBN-induced high invasive BCs. Further studies discovered that XIAP was crucial for maintaining RhoGDIβ mRNA stability and thereby increasing its protein expression and facilitating human bladder cancer cell invasion and metastasis both in vitro and in vivo.
Material and methods
Plasmids, Antibodies and Reagents
Short hairpin RNA (shRNA) specific targeting XIAP, RhoGDIβ or nucleolin, were purchased from Open Biosystems (Huntsville, AL, USA). Short hairpin RNA (shRNA) specific targeting Erk1/2 was purchased from Addgene (Cambridge, MA, USA). Human RhoGDIβ promoter-derived luciferase reporter was bought from Cyagen Biosciences Inc. (Science City, Guangzhou, China). A 2093bp section of the human RhoGDIβ promoter (from −8 to −2105 with five Ts deletion from −100 to −105) was cloned into pRP.EX2d using Kpn I and Sca II. The GFP-RhoGDIβ expression vector 19 and its scramble control were kind gift from Dr. Martin A. Schwartz (Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, Virginia). GFP-nucleolin expression vector 20 was kindly provided by Dr. Michael B. Kastan (Comprehensive Cancer Center, St. Jude Children’s Research Hospital, Memphis, USA). DN-Erk1 and HA-XIAP plasmids were described in our previous publications 7, 21. Antibodies against RhoGDIα, RhoGDIβ, HuR and the control IgG were bought from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against GFP, nucleolin and β-Actin were bought from Sigma (St. Louis, MO, USA). Antibodies specific for XIAP, VHL, Erk, p-Erk and GAPDH were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), whereas HA antibody was purchased from Covance Inc. (Princeton, NJ, USA). Cycloheximide (CHX) was bought from Calbionchem (San Diego, CA, USA). The dual luciferase assay substrate was purchased from Promega (Madison, WI, USA).
Cells and Transfectants
The human bladder cancer cell lines, TccSup and T24T, and colon cancer cell HCT116 were cultured and used as described in our previous studies 22, 23. All cell lines, including T24T, TccSup, HCT116, were authenticated every 6–12 months by testing STR loci and gender by using PowerPlex® 16 HS System by Genetica DNA Laboratories (Burlington, NC, USA) or Microread Genetics Co., Ltd (Beijing, China), and the results were 100%, 100%, and 94% matched with the data in the ATCC STR DATABASE, respectively. Transfections of shRNA specific targeting human XIAP, RhoGDIβ or Erk1/2 into TccSup or T24T cells were conducted by using PolyJet™ transfection reagent (SignaGen Laboratories, Rockville, MD, USA) according to the manufacturer’s protocol, followed up by stable transfectant selection with hygromycin (Cellgro, Manassas, VA, USA). Transfections of GFP-RhoGDIβ or shRNA specific targeting human nucleolin into T24T, TccSup and T24T(shXIAP) cells, were conducted by using the aforementioned reagent, except with puromycin (Cellgro, Manassas, VA, USA) being the selective agent. GFP-nucleolin was transiently transfected into 293T cells using PolyJet™ transfection reagent. All cell cultures and transfectants were maintained at 37°C 5% CO2 using the corresponding mediums supplemented with 1% penicillin/ streptomycin, and 2mM L-glutamine (Life Technoloies, Grand island, NY, USA).
Bladder Cancer Tissue Specimens
Thirty-two of primary human bladder cancer specimens and their paired adjacent non-tumorous bladder tissues were obtained from patients who underwent radical cystectomy at Department of Urology of the Union Hospital of Tongji Medical College between 2012 and 2013 as listed in Supplementary Data Table 1. Histological and pathological diagnoses were confirmed by a pathologist based on the 2004 World Health Organization Consensus Classification and Staging System for bladder neoplasms. All specimens were obtained with appropriate informed consent from the patients and were immediately snap-frozen in liquid nitrogen after surgical removal. Then tissues were formalin-fixed and paraffin-embedded. For IHC staining, antibodies specific against XIAP (1:100; Cat: sc-11426, Santa Cruz, CA, USA) or RhoGDIβ (1: 50; Cat: sc-271108, Santa Cruz, CA, USA) were used to be incubated at 4 °C for overnight, The staining was performed using a kit from Boster Bio-Engineering Company (Cat: SA1022, Wuhan, China), according to the manufacturer’s instructions. The results of immunostaining images were captured using the Nikon Eclipse Ni microsystems (Nikon DS-Ri2, Japan). Protein expression levels were analyzed by calculating the integrated optical density per stained area (IOD/area) using Image-Pro Plus version 6.0 (Media Cybernetics, MD, USA).
Animal experiments and bladder tissue
The C57BL/6 mice at age of 6 to 8 weeks with XIAP-WT or XIAP-ΔRING, which were generated and described in previous report24, were randomly divided into four groups as indicated: XIAP-WT mice normal control, XIAP-WT mice treated with 0.5% BBN in water, XIAP-ΔRING mice normal control, XIAP-ΔRING mice treated with 0.5% BBN in water, after 6 months, the mice were then sacrificed to evaluate the expression of RhoGDIβ and Nucleolin and/or bladder pathologic analysis.
Immunohistochemistry paraffin of mouse lung specimens
Bladder tissues obtained from the sacrificed mice were formalin-fixed and paraffin-embedded. The antibodies specific against RhoGDIβ (Santa Cruz, CA, USA), Nucleolin (Abcam, MA, USA) or Ki-67 (Abcam, MA, USA) were used for immunohistochemistry (IHC) staining. The resultant immunostaining images were captured using the DM 2000 LED (1188843) microsystems (Leica, Germany). Protein expression levels were analyzed by calculating the integrated optical density per stained area (IOD/area) using Image-Pro Plus version 6.0 (Media Cybernetics, MD, USA).
Luciferase Reporter Assay
Human RhoGDIβ promoter-driven luciferase reporter or nucleolin promoter-driven luciferase reporter was transiently co-transfected together with pRL-TK into various TccSup and T24T transfectants, respectively. Twenty-four hours after transfection, cells were lysed for luciferase activity assay and internal TK assay by using the Dual-Luciferase Reporter Assay System following manufacturer’s protocol (Promega, Madison, WI, USA).
RNA-IP Assay
293T cells were cultured in 10-cm dishes until reaching 70~80% confluent, and the cells were then transiently transfected with GFP-nucleolin. After a 24 hrs post-transfection, cells were harvested after twice washing with PBS and the RNA was extracted as previously described 22. Then the RNA was subjected to reverse transcription polymerase chain reaction (RT-PCR) as described below.
Reverse Transcription-polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR
Cells were collected in 1 ml of TRIzol reagent (Invitrogen, Carlsbad, California, USA) and total RNA was extracted according to manufacturer’s instruction. RNAs were reverse transcripted and PCR was performed in our previous report 22. Specific primers of human RhoGDIβ (Forward: 5′-acc cgg ctc acc ctg gtt tgt-3′, Reverse: 5′-aca cca gtc ctg tag gtg tgc tg-3′), human nucleolin (Forward: 5′-acc taa tgc cag aag cca gcc a-3′, Reverse: 5′-ttg ccc gaa cgg agc cgt c-3′), mouse nucleolin (5′-gag gac ccc ctt cgt cgc ct-3′ and 5′-gcc tca ccg tgg gtt ttg cca-3′) and human GAPDH (Forward: 5′-gat gat ctt gag gct gtt gtc, Reverse: 5′-cag ggc tgc ttt taa ctc tg-3′) were used for PCR amplification.
ATP cell viability assay
Cells were seeded into 96-well plates at a density of 10,000 cells per well and allowed to adhere overnight. The cell culture medium was then replaced with 0.1% FBS DMEM and cultured for 12 hours. The cells were extracted with 50 μl of lysis buffer at the various time points. Cell viability was evaluated by utilizing the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA) as described in previous report 25. The results were expressed as relative proliferation rate, which was calculated as following: relative proliferation rate =ATP activity on the nth day/ATP activity on 0 day.
Western Blot
Whole cell extracts or bladder tissue extracts were collected with lysis buffer (10 mM Tris-HCl, PH 7.4, 1%SDS, 1mM Na3VO4, and proteasome inhibitor followed by sonication to fracture nucleic acids). Protein extracts were quantified using Nano Drop 2000 (Thermo Scientific, MA USA), and then subjected to Western Blot as described in our previous studies 22.
Wound Healing Assay
T24T, TccSup and their various transfectants were seeded into 6-well plates. When cell confluence reached 80~90%, wounds were created by using sterile pipette tips, and images were taken and evaluated as previous described (53).
Cell Invasion Assay
The control (uncoated) and matrigel inserts of BD BiocoatTM (BD Biosciences, Bedford, MA, USA) were used for cell invasion assay. BC Cell suspension (0.5 ml of 2.5×104 cells/ml) was added to each insert. After incubation in a humidified incubator at 37°C, 5% CO2 atmosphere for 24h, non-migrating or non-invading cells were wiped using cotton swab according to manufacturer’s instructions. Cell numbers were quantified and the images were captured under inverted microscope (Olympas, Center Valley, PA, USA) as describe in our previous study 26.
T24T Cell Lung Metastatic Assay
All animal studies were performed in the animal institute of Wenzhou Medical University according to the protocols approved by the Medical Experimental Animal Care Commission of Wenzhou Medical University. Female athymic (nu+/nu+) mice were purchased from Shanghai Silaike Experimental Animal Company, Ltd. (license No. SCXK, Shanghai 2010 0002; Shanghai, China). 72 mice at age of 5–6 weeks were randomly divided into each group, and transfectants of T24T(Nonsense), T24T(shRhoGDIβ), T24T(pEGFP), T24T(GFP-RhoGDIβ), T24T(shXIAP/pEGFP), T24T(shXIAP/GFP-RhoGDIβ), was injected into nude mice via an I.V. lateral tail vein injection (1.5–2.5×106 cells in 100 μl PBS/mouse), respectively. The mice will be evaluated and weighted twice a week. The lungs were removed by dissection after natural death or being euthanized at indicated times after injection. Six or eight lungs from indicated group were fixed in Bouin’s fixative solution (Sigma, MO, USA) for 24 hours and the numbers of lung surface metastatic lesions were counted on each lobe of every specimen. The left lungs in each group were fixed and embedded in paraffin for histopathological evaluation.
Statistical Analysis
Ordinary one-way ANOVA software was used to statistically determine the significance difference among each of experimental groups. If a significant difference was obtained by ANOVA analysis, the Tukey-Kramer multiple-comparisons T test was also used to verify the significance of the difference. Immunohistochemistry results were analyzed by Kolmogorov-Smirnov test and Spearman correlation test. The data was presented as Mean ± SD, and the differences were considered to be significant when P≤0.05.
Results
The Co-related Overexpression of RhoGDIβ and XIAP in Both Human Bladder Cancers and BBN-induced Mouse Bladder Cancers
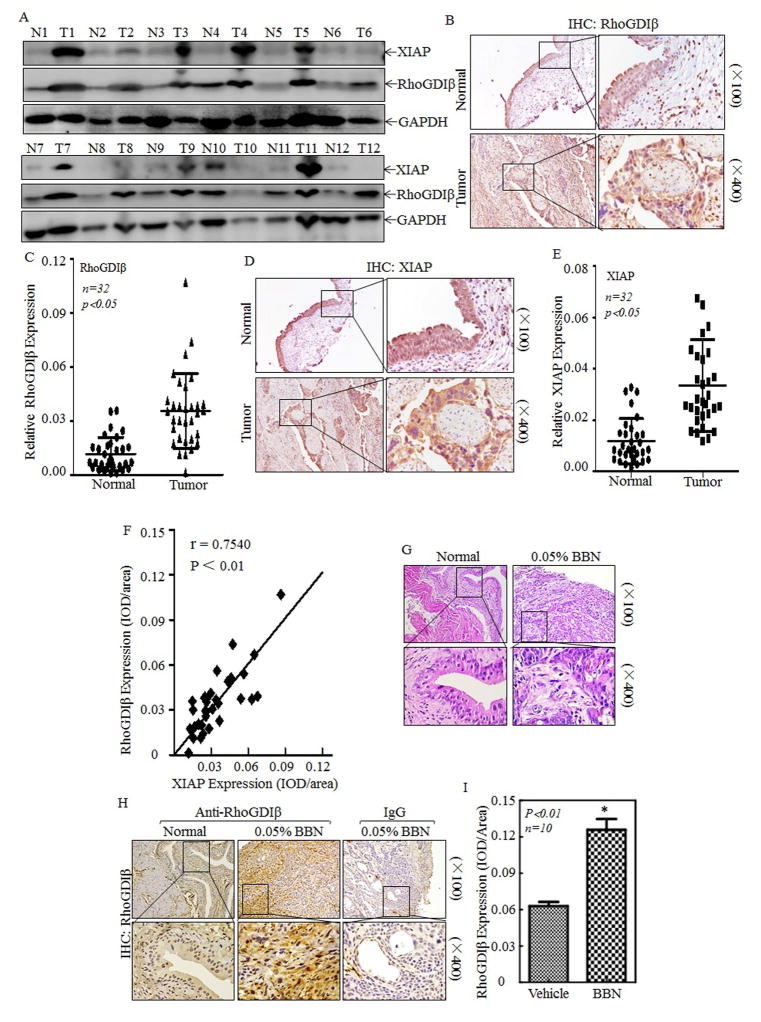
RhoGDIβ mRNA levels are reported to be downregulated in human BC patients by using the HU-133A Affymetrix array, whereas the results depicting from the Cancer Genome Atlas shows the prominent amplifications of RhoGDIβ in human BCs (TCGA, http://www.cbioportal.org/public-portal/). Since protein is the major functional carrier of the gene, we first used Western Blot to evaluate RhoGDIβ protein expression in human bladder carcinomas in comparison to their paired adjacent normal bladder tissues. The results unanticipatedly showed that RhoGDIβ protein expression was remarkably elevated in eleven of twelve (91.7%) of human BC patients (Fig. 1A), demonstrating that RhoGDIβ protein is overexpressed in most of human BC patients. Since XIAP mRNA is also upregulated in human BC tissues 27 and XIAP is crucial for colorectal cancer cell migration and invasion through its inhibition of RhoGDIα SUMOylation at L138 7, 28, we would assess the XIAP protein abundance in same BC patients for observation of RhoGDIβ protein expression. As shown in Fig. 1A, among 11 patients with RhoGDIβ overexpression, eight of them (72.7%) with XIAP protein remarkably elevated in comparison to their paired normal bladder tissues (Fig. 1A). The results obtained from immunohistochemical (IHC) staining also showed that expression of RhoGDIβ and XIAP proteins was consistently elevated in Bc tissues in comparison to the paired adjacent normal bladder tissues (Figs. 1B–1E, P<0.01, N=32). The statistic correlation analysis revealed that RhoGDIβ protein expression was highly co-related to XIAP protein expression (Fig. 1F, r=0.754, p<0.01). Chemical carcinogen N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) is able to target mouse urothelium, and induces a wide range of BC phenotypes, including hyperplasia, dysplasia, carcinoma in situ, and muscle-invasive bladder cancer. Histopathological analysis reveals that mouse BC induced by BBN mimics the progression of human bladder from non-invasive carcinoma to muscle invasive carcinoma. To evaluate the status of RhoGDIβ in BBN-induced mouse BCs, HE staining was shown in Fig. 1G, and IHC was used to determine RhoGDIβ abundances in BBN-induced mouse BCs. The results indicated that RhoGDIβ protein was significantly overexpressed in BBN-induced mouse BCs in comparison to normal mouse bladder epithelium (Figs. 1H & 1I, p<0.01, N=10). Above results obtained from in vivo studies in both human and mouse, together with our most recently findings that XIAP is overexpressed in BBN-induced mouse bladder cancers 11, leads us to investigate the potential association of XIAP overexpression with RhoGDIβ abundance.
Figure 1. RhoGDIβ was overexpressed in BC of human patients and BBN-induced mice.
(A) Total protein lysates were prepared from the cancerous (T) and their paired adjacent normal (N) tissues of human BC patients were collected as described in “Methods”, the total proteins were extracted and then subjected to Western blotting analyses, and specific antibodies against XIAP and RhoGDIβ were used to determine the level of XIAP and RhoGDIβ proteins. (B–F) Rho-GDIβ and XIAP proteins level was evaluated by using IHC staining in bladder cancer tissues in comparison to the paired normal bladder tissues (n=32). IHC images were captured under microscopy. (B&D), and the quantitative (C&E) and correlation of XIAP expression with RhoGDIβ level was analyzed and presented (F). (G–I) Upon 23 weeks treatment of mice with or without BBN, the bladder tissues from indicated mice were pathologically analyzed by H&E staining (G) and by IHC staining to evaluate RhoGDIβ expression (H). Homotypic IgG was used as negative control (H) and the optical density was calculated as described in “Methods” (I). The results were presented as mean ± SD from at least triplicate experiments and asterisk (*) indicated a significant difference (P<0.05)
XIAP Positively Regulated RhoGDIβ Expression and Invasion of BCs
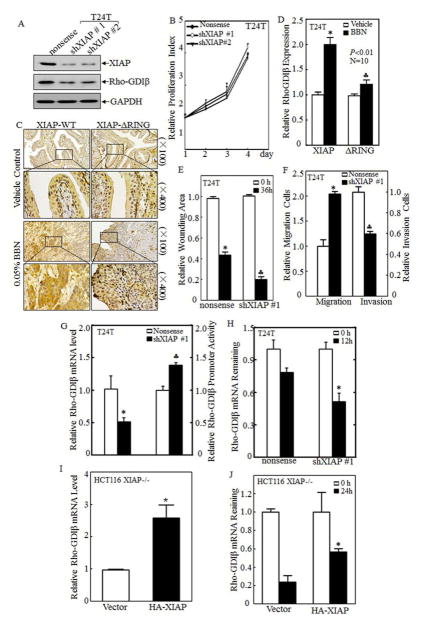
To elucidate the relationship between XIAP and RhoGDIβ in BCs, we used shRNA specific targeting human XIAP to knockdown XIAP expression in human BC T24T and TccSup cell lines and evaluated the effect of XIAP knockdown on RhoGDIβ protein abundance in both cell lines. The results showed that knockdown of XIAP attenuated RhoGDIβ protein expression in both T24T (Fig. 2A) and TccSup (Fig. S1A) cells, while it did not show observable effect on cell growth of BC cells (Fig. 2B). Our most recent studies reveal that XIAP and its RING domain was crucial for human BC invasion in vitro cultured cells and invasive BC formation in in vivo mice 11. To extend this novel in vitro discovery of XIAP positive regulation of RhoGDIβ to in vivo, we compared RhoGDIβ abundances in mouse bladder epithelium between wild-type (WT) mice and XIAPΔRING knockin mice following BBN exposure for 23 weeks. The results indicated that RhoGDIβ abundance in bladder epithelium following BBN exposure was almost completely abolished in XIAP-ΔRING mice as compared with that in WT mice (Figs. 2C & 2D). These in vivo results are consistent with findings in vitro cultured cells and strongly reveal that XIAP and its RING domain are crucial for BBN-induced RhoGDIβ expression. We next assessed the role of XIAP in BC cell migration and invasion. The results showed that knockdown of XIAP significantly inhibited BC cell invasion (Fig. 2E & S1B), while it unexpectedly enhanced BC cell migration in both T24T (Fig. 2F) and TccSup (Fig. S1C) cell lines, suggesting that XIAP behaviors a differential functions in regulation of BC cell migration and invasion, and such biological functions are distinct from our previous observation in colorectal cancer HCT116 cells and prostate cancer cells 7. The results reveal that XIAP overexpression is not only crucial for RhoGDIβ expression, but also specifically contributes to BC cell invasion in vitro and might be associated with invasive BC formation in vivo.
Figure 2. XIAP promoted RhoGDIβ expression and invasion of BC cells.
(A) Short hairpin RNA specific to XIAP (shXIAP) and its scramble nonsense was stably transfected into BC cells T24T. XIAP and RhoGDIβ proteins expression were then detected in its paired cells by Western blot. (B) The proliferative activity of T24T cell transfectants was measure using ATPase activity assay. (C&D) WT and XIAPΔRING mice were treated with or without BBN for 180 days, IHC was used to evaluate RhoGDIβ expression in bladder tissues from indicated mice (C), and the optical density was calculated as described in “Methods” (D). (E) The quantification of wound healing area of T24T transfectants was analyzed using CMA software and presented as relative wounding area. (F) Relative migrating cells (left pane) and invading cells (right panel) of T24T transfectants were quantitated and shown as relative migration or relative invasion. (G) The RhoGDIβ mRNA level was evaluated in paired T24T/nonsense and T24T/shXIAP transfectants by using real-time PCR (left panel). And RhoGDIβ promoter-driven luciferase reporter and pRL-TK was transiently co-transfected into T24T cells as indicated. The results were normalized by corresponding internal TK activity (right panel). (H) T24T transfectants as indicated were seeded into 6-well plate and cultured till 75–80% confluence. The cells were than treated with 5μM of Act D for indicated time periods, Real-time PCR assay was employed to analyze the stability of RhoGDIβ mRNA. (I) RhoGDIβ mRNA expression in HCT116 XIAP deficient (XIAP−/−) cells and its HA-XIAP reconstitutive transfectants, XIAP−/−(HA-XIAP), were determined by real-time PCR assay. (J) RhoGDIβ mRNA stability in XIAP−/−(vector) and XIAP−/− (HA-XIAP) cells was evaluated upon the presence of 5μM ActD treatment for indicated time by Real-time PCR assay. The results were presented as mean ± SD from three independent experiments and the asterisk (*) indicated a significant difference (P< 0.05).
XIAP Was Crucial for Stabilization of RhoGDIβ mRNA
Above results suggest the XIAP upregulation of RhoGDIβ abundance in human bladder epithelium both in vitro and in vivo. To elucidate the mechanisms underlying such novel XIAP biological function, the mRNA abundance of RhoGDIβ in XIAP knockdown and its nonsense transfectants was evaluated by using real-time PCR. Consistent with RhoGDIβ protein, RhoGDIβ mRNA was significantly decreased in T24T(shXIAP) cells (Fig. 2F, left panel) and TccSup(shXIAP) cells (Fig. S1D, left panel) in comparison to their corresponding nonsense transfectants. The RhoGDIβ promoter transcriptional activity was also evaluated by transient transfection of RhoGDIβ promoter-driven luciferase reporter together with TK as an internal control. The results showed that RhoGDIβ promoter transcription activity was increased in T24T(shXIAP) transfectants (Fig. 2F, right panel) and TccSup(shXIAP) transfectants (Fig. S1D, right panel). These results excluded XIAP regulation of RhoGDIβ at transcription, further suggesting that XIAP might regulate RhoGDIβ mRNA stability. Consequently, actinomycin D (Act D), a RNA synthesis inhibitor, was used to block new mRNA synthesis for determining effect of XIAP on RhoGDIβ mRNA stability in T24T(Nonsense) vs. T24T(shXIAP), and TccSup(Nonsense) vs. TccSup(shXIAP) cells. The results indicated that the remaining level of RhoGDIβ mRNA detected with Real-time PCR was profoundly lower in either T24T(shXIAP) (Fig. 2G) or TccSup(shXIAP) transfectants (Fig. S1E) in comparison to their corresponding nonsense transfectants. Consistently, stable transfection of HA-XIAP into XIAP-deficient HCT116 cells, XIAP−/ − (HA-XIAP), resulted in increasing RhoGDIβ mRNA expression (Fig. 2H) and RhoGDIβ mRNA stability (Fig. 2I) as compared with those in XIAP−/ − (Vector) transfectant. Taken together, our results demonstrate that XIAP positively modulates RhoGDIβ abundance by upregulating its mRNA stability.
XIAP Mediated Nucleolin Expression and Consequent Stabilized mRNA of RhoGDIβ
RNA binding proteins (RBPs), such as human antigen R (HuR), Von Hippel-Lindau (VHL) and nucleolin, serve pivotal role with regarding to regulating mRNA stability 29–31. Given above results showing that XIAP regulates RhoGDIβ mRNA stability, the expression levels of these potential targets were analyzed in XIAP knockdown and nonsense transfectants. Western blot analysis showed that nucleolin expression was profoundly decreased in T24T (Fig. 3A) and Tccsup (Fig. S2A) XIAP knockdown transfectants, while HuR and VHL expression only showed minimal alteration between the paired transfectants (Fig. 3A & S2A ). Consistently, the similar results were observed in XIAP−/ − (Vector) cells in comparison to its parental HCT116-WT cells, while re-constitutional expression of HA-XIAP in XIAP−/ − cells, XIAP−/ − (HA-XIAP), restored nucleolin expression (Fig. 3B). It was important to note that the nucleolin protein was remarkably overexpressed in BBN-induced mouse high invasive bladder cancers (Figs. 3C & 3D). Consistent with RhoGDIβ downregulation in BBN-treated mouse bladder epithelium in XIAPΔRING knockin mice, nucleolin abundance was also attenuated in XIAPΔRING knockin mice following BBN exposure (Figs. 3E & 3F), revealing that XIAP and its RING domain play an important role in nucleolin expression in bladder epithelium both in vitro and in vivo. RBPs could directly bind to their target mRNA, by which increases its stability 32. Thus, to determine whether nucleolin can bind to RhoGDIβ mRNA, GFP-nucleolin was transiently transfected into 293T cells followed by RNA-IP. RT-PCR analysis showed that RhoGDIβ mRNA could be pulled down by nucleolin antibody (Fig. 3G), indicating that nucleolin did bind to RhoGDIβ mRNA. To test whether this binding activity enhanced RhoGDIβ mRNA stability and subsequently increased its protein expression, shRNA specific targeting nucleolin was stably transfected into T24T and mouse embryonic fibroblast (MEF) cells, respectively. The results showed that compared with nonsense transfectants, nucleolin knockdown attenuated RhoGDIβ protein expression in both T24T and MEF cells (Fig. 3H). In addition, RhoGDIβ mRNA level in T24T(shNCL) transfectants (Fig. 3I) and MEF(shNCL) (Fig. 3J) was dramatically reduced as compared with their corresponding nonsense transfectants, suggesting that nucleolin is not only able to bind to RhoGDIβ mRNA, but also is pivotal for RhoGDIβ mRNA and protein expression.
Figure 3. Nucleolin protein was implicated in XIAP stabilizing RhoGDIβ mRNA.
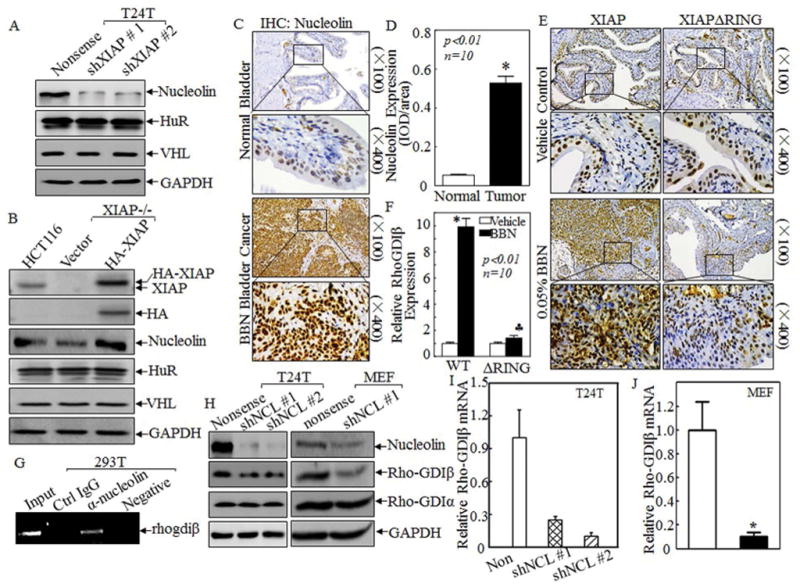
(A&B) The RNA binding proteins, nucleolin, HuR and VHL, were detected in T24T XIAP knockdown transfectants in comparison with those in their corresponding nonsense transfectants (A) or HCT116(vector), XIAP−/−(vector) and XIAP−/−(HA-XIAP) cells (B) by Western blotting. (C–F) IHC was carried out to evaluate Nucleolin expression in bladder tissues from above indicated mice (C&E) and the optical density was calculated as described in “Methods” (D&F). (G) 293T cells were transiently transfected with expression vector of GFP-nucleolin for 36 hrs. The cells were extracted with polysome lysis buffer, and the cell extracts were used for RNA-IP as described in “Methods”. RT-PCR was used to detect RhoGDIβ mRNA expression in the pull-down complex. (H) shRNA specific targeting nucleolin plasmid or its nonsense vector was transfected into T24T and MEF cells, respectively. Nucleolin, RhoGDIβ and RhoGDIα proteins expression were determined by Western Blot. (I&J) The RhoGDIβ mRNA expression was evaluated in T24T(nonsense) and T24T(shNCL) (I) or in MEF (nonsense) and MEF(shNCL) transfectants by real-time PCR (J). The results were presented as mean ± SD from three independent experiments and the asterisks (*), (♣) indicated a significant difference (P<0.05).
Erks Activation was Crucial for XIAP Upregulation of Nucleolin mRNA Stability
Above results indicate that nucleolin protein expression is downregulated in XIAP knockdown BC cell lines and in mouse urothelium of XIAPΔRING knockin mice. To elucidate the molecular mechanism underlying XIAP regulation of nucleolin protein, the nucleolin mRNA expression levels were evaluated in XIAP knockdown transfectants using real time-PCR assay. As shown in the left panel of Fig. 4A and Fig. S3A, knockdown of XIAP resulted in a reduction of nucleolin mRNA levels in both T24T and TccSup cells. The results from subsequent testing in HCT116 cells also showed that nucleolin mRNA level was diminished in XIAP-deficient cells as compared with that in HCT116-WT or XIAP−/ − (HA-XIAP) (Fig. 4B). A nucleolin promoter-driven luciferase reporter was then used to determine whether transcription or post-transcription regulation resulted in this mRNA alteration. The results revealed that the relative nucleolin promoter transcriptional activity in either T24T(shXIAP) (Fig. 4A, right panel) or TccSup(shXIAP) (Fig. S3A, right panel) cells was significantly increased in comparison to their corresponding scramble transfectants, excluding possibility of XIAP upregulating nucleolin transcription and further suggesting that XIAP might regulate nucleolin mRNA stability. To test this notion, the cells were treated by 5μM Act D for 2 hrs or 4 hrs, and total RNA was then extracted and subjected to real-time PCR for evaluation of nucleolin mRNA levels. Treatment of T24T(shXIAP) and TccSup (shXIAP) transfecatnts with Act D for 4 hrs, there was only about 40% and 20% nucleolin mRNA remaining, which was significantly decreased in comparison to nonsense cells (about 60% nucleolin mRNA remaining) under the same experimental conditions (Fig. 4C & Fig. S3B), respectively. Our results reveal that XIAP promotes nucleolin expression through lengthening the half-life of nucleolin mRNA.
Figure 4. XIAP regulated nucleolin mRNA stability in Erk-dependent manner.
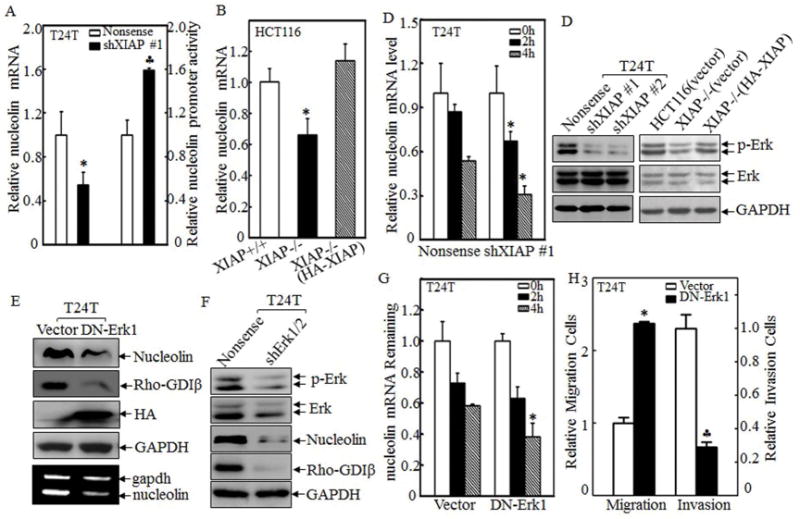
(A&B) The nucleolin mRNA expression was evaluated in paired T24T/nonsense and T24T/shXIAP transfectants (A, left panel) or XIAP+/+, XIAP−/−(vector) and XIAP−/−(HA-XIAP) cells (B) by real-time PCR assay. Nucleolin promoter-driven luciferase reporter and pRL-TK was transiently co-transfected into T24T cells as indicated, and then the luciferase activity was evaluated (A, right panel). (C) T24T transfectants as indicated were seeded into 6-well plate and cultured till 75–80% confluence. The cells were then treated with 5μM of Act D for indicated time periods, Real-time PCR assay was employed to analyze the stability of nucleolin mRNA. (D) The cell extracts of T24T and HCT116 cell transfectants as indicated were subjected to Western blotting for determination of Erk phosphorylation. (E&F) The plasmid of HA-tagged DN-Erk1, shErk1/2, and their control vectors were transfected into T24T cells. The cell extracts were subjected to Western Blotting using specific antibodies as indicated and total RNA from the transfectants was used to determine nucleolin mRNA levels by using RT-PCR assay. (G) T24T transfectants as indicated were seeded into 6-well plate and cultured till 75–80% confluence. The cells were than treated with 5μM of Act D for indicated time periods. Real-time PCR assay was employed to analyze the stability of nucleolin mRNA. (H) Cell migration (left panel) and invasion (right panel) of transfectants was evaluated and quantitated by using transwell assay. All the results were presented as mean ± SD from three independent experiments and the asterisks (*), (♣) indicated a significant difference (P <0.05).
Given that XIAP does not directly bind to nucleolin mRNA, we anticipate that XIAP utilizes an intermediary mechanism that can regulate nucleolin mRNA stability. The extracellular regulated protein kinases (Erks) has been reported to be capable of lengthening the half-life of nucleolin mRNA 33. We therefore, next determine the potential effect of XIAP in regulation of Erks activation. As shown in left panel of Fig. 4D and Fig. S3C, Erks phosphorylation in T24T(shXIAP) or TccSup(shXIAP) cells was dramatically suppressed in comparison to their scramble control transfectants, T24T(Nonsense) or TccSup(Nonsense). The results from XIAP-deficient HCT116 cells also indicated that Erks phosphorylation was inhibited in XIAP−/ − cells in comparison to either HCT116(Vector) cells, or HA-XIAP reconstitutive expressed XIAP−/− cells, XIAP−/−(HA-XIAP) cells (Fig. 4D, right panel). To determine role of Erks in regulation of nucleolin abundance, HA-tagged dominant negative Erk1 (DN-Erk1) or shErk1/2 plasmids were transfected into T24T cell, respectively. And the stable transfectants were used to analyze the expression of nucleolin. The results showed that nucleolin protein (Fig. 4E & 4F) and mRNA (Fig. 4G, low panel) were remarkably suppressed with attenuation of RhoGDIβ expression upon DN-Erk1 or shErk1/2 transfection. Subsequently, nucleolin mRNA stability was also decreased in T24T(DN-Erk1) cells as compared with that observed in T24T(Vector) cells (Fig. 4G). Consistently, inhibition of Erks in T24T cells led to an increase in cell migration and a decrease in BC invasion (Fig. 4H). Taken together, our results demonstrate that XIAP mediates Erks activation, which in turn stabilizes nucleolin mRNA and increases its abundance, consequently leading to RhoGDIβ mRNA stabilization and protein expression, as well as promoting invasion in human BC cells. Erk-dependent regulatory effect of XIAP on necleolin was also supported by the results showing that overexpression of HA-XIAP was not able to recuse the nucleolin level in T24T(DN-ERk1) transfectants (Fig. S3D).
XIAP-Mediated RhoGDIβ Was Crucial for T24T Cell Invasion in vitro
To define the effect of RhoGDIβ in BC cell migration and invasion, pEGFP-RhoGDIβ and its scramble vector pEGFP were transfected into T24T and TccSup cells, respectively. The results obtained from Western Blot indicated that GFP-RhoGDIβ was successfully transfected and ectopically expressed in both T24T (Fig. 5A) and TccSup (Fig. S4A) cells. A follow-up trans-well assay showed that both T24T and TccSup cells overexpressing GFP-RhoGDIβ, T24T(pEGFP-RhoGDIβ) and TccSup(pEGFP-RhoGDIβ), had a remarkably promotive effect on cell invasion although cell migration capacity was significantly decreased as compared with those in the corresponding scramble vector transfectants (Figs. 5B & S4B), suggesting that RhoGDIβ promotes BC cell invasion. Moreover, we used shRNA specific targeting human RhoGDIβ to stably knock down RhoGDIβ expression in T24T cells (Fig. 5C), and inhibiting RhoGDIβ protein expression also had no obvious effect on regulating T24T cell proliferation (Fig. 5D). Then its effect on T24T cell invasion was then evaluated. As shown in Figs. 5E, knockdown of RhoGDIβ profoundly inhibited invasion ability of T24T cells. To specific define whether RhoGDIβ is XIAP downstream effector for its mediation of XIAP regulation of BC invasion, we constitutively expressed RhoGDIβ in T24T(shXIAP) cells (Fig. 5F). The results indicated that ectopic expression of RhoGDIβ significantly reversed the inhibition of T24T cell invasion due to specific knockdown of XIAP (Fig. 5G). Our results clearly demonstrate that RhoGDIβ is a key XIAP downstream effector for mediating the invasion of high grade BCs.
Figure 5. RhoGDIβ promoted invasion and inhibited migration of BC cells.
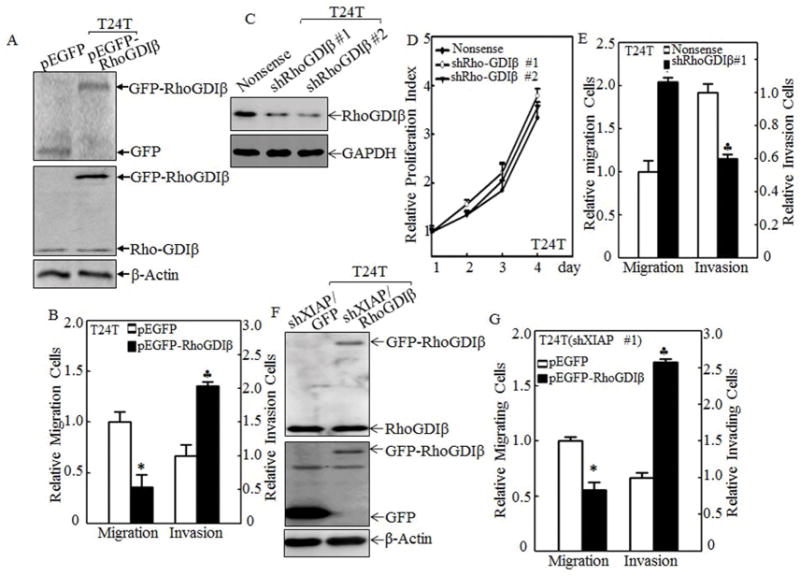
(A, C&F) pEGFP-RhoGDIβ or its control vector pEGFP were transfected into T24T cells (A), T24T(shXIAP) cells (F), and shRhoGDIβ or its nonsense control plasmids were transfected into T24T cells (C), respectively. The transfectants were identified using Western blot assay. (D) The relative cell proliferation rate of indicated T24T transfectants was measured using ATPase activity assay. (B, E&G) The cell migration (left panel) and invasion (right panel) of T24T (B, E), and T24T(shXIAP) (G) transfectants as indicated were determined and quantitated by using transwell assay. The results were presented as mean ± SD from at least triplicate experiments and the asterisks (*), (♣) indicated a significant difference (P <0.05).
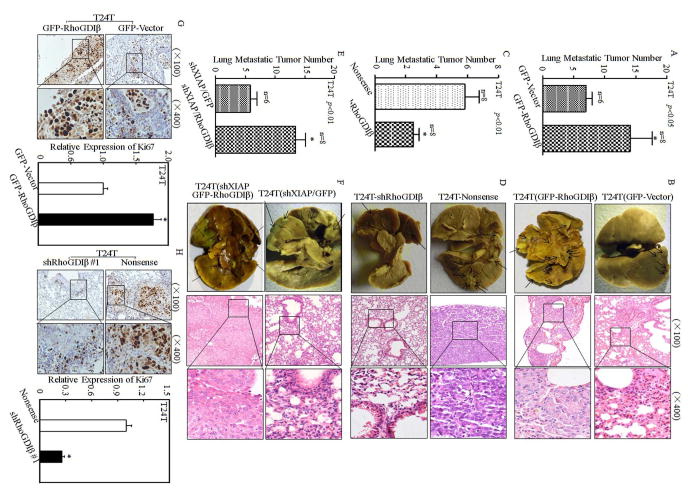
RhoGDIβ Expression Was Critical for T24T Lung Metastasis in vivo Nude Mice
To evaluate the contribution of RhoGDIβ to mediation of BC lung metastasis, T24T(GFP-vector) and T24T(GFP-RhoGDIβ) stable transfectants were injected into nude mice via an I.V. lateral tail vein and the lung metastatic abilities of two transfectants were evaluated. The results showed that ectopic expression of RhoGDIβ in T24T cells, T24T(GFP-RhoGDIβ), exhibited a reduction of mouse survival rate in comparison to the nude mice injected with T24T(GFP-vector) cells (Table S2). Consistent with mouse survival rate, the number and size of lung metastatic tumors in mice injected with T24T(GFP-RhoGDIβ) cells was remarkably increased (Table S3 & Figs. 6A & 6B). Those results clear indicate that RhoGDIβ might be a positive regulator for BC lung metastasis although it inhibited cell migration in same human BC cells. Moreover, knockdown of RhoGDIβ in T24T cells, T24T(shRhoGDIβ), attenuated its in vivo lung metastatic ability with significantly prolonger of nude mouse survival (Table S3 & Figs. 6C & 6D), revealing that RhoGDIβ is crucial for T24T cell lung metastasis. To test whether restoration of RhoGDIβ expression in T24T(shXIAP) cells could rescue their lung metastatic ability due to XIAP knockdown, T24T(shXIAP/GFP-RhoGDIβ) and its scramble transfectant T24T(shXIAP/GFP) was injected into nude mice via an I.V. lateral tail vein and the lung metastatic abilities of two transfectants were evaluated. As shown in Table S4 and Figs. 6E & 6F, compared with the mice injected with T24T(shXIAP/GFP), the survival rate of nude mice injected with T24T(shXIAP/GFP-RhoGDIβ) was decreased and the number of lung metastatic tumor was remarkably increased. Moreover, the results obtained from IHC staining for expression level of Ki-67, a well-known cell proliferative marker, revealed that Ki-67 expression was remarkably elevated by ectopic expression of Rho-GDIβ (Fig. 6G), and knockdown of Rho-GDIβ red to a dramatically reduction of Ki-67 expression (Fig. 6H). It is noted that XIAP knockout promotes cancer cell motility through regulating β-actin polymerization in Rho-GDIα SUMOylation-dependent manner in human colon HCT116 cells 28, whereas XIAP knockout inhibited RhoGDIβ expression and human invasive bladder cancer cell invasion with promotion of cancer cell migration. While detailed mechanisms underlying the differences are still under exploring in our group, we anticipate that the biological functional differences might be associated with the expression ratio of RhoGDIβ/RhoGDIα between human colon cancer and bladder cancer cells. As shown in Fig. S5, RhoGDIβ was highly expressed in both human bladder cancer cell lines (T24T and TccSup), whereas it was barely detectable in human colon HCT116 cells. In contrast, RhoGDIα expression in HCT116 cells was remarkably higher than that in T24T and TccSup cells (Fig. S5). Thus, we anticipate that XIAP mainly interacts with RhoGDIα and regulates its SUMOylation, as well as its function in regulation of cell motility in human colon cancer cells, while in bladder cancer cells, XIAP mainly targets Rho-GDIβ expression and its function in cancer cell invasion. Collectively, our results demonstrate that the forced expression of RhoGDIβ in T24T(shXIAP) cells can reverse the inhibition of T24T cell lung metastasis in nude mice due to XIAP knockdown, further revealing that RhoGDIβ is a key XIAP downstream effector being responsible for its mediating BC lung metastatic ability.
Figure 6. RhoGDIβ was essential for promoting T24T cell lung metastasis.
The T24T/pEGFP, T24T/pEGFP-RhoGDIβ, T24T/nonsense, T24T/shRhoGDIβ, T24T(shXIAP/pEGFP), T24T(shXIAP/pEGFP-RhoGDIβ) cells were intravenously inoculated into nude mice as described in “Methods”. (A, C&E) The lung metastatic tumor numbers of T24T/pEGFP, T24T/pEGFP-RhoGDIβ (A); T24T/nonsense, T24T/shRhoGDIβ (C); and T24T(shXIAP/pEGFP), T24T(shXIAP/pEGFP-RhoGDIβ) (E) were analyzed. (B, D&F) Representative images of the lungs and lung surface metastatic foci as indicated were shown after fixation in a neutral-buffered formalin/Bouin’s fixative solution (left panel), and the histologic appearance of lung metastases were analyzed using H&E staining (right panel), respectively. (G&H) Ki-67 protein expression of lung metastatic tumor tissues was detected using immunohistochemical staining analysis (G&H, left panel) and the quantitative analysis was done indicated paired groups (G&H, right panel). The results were presented as mean ± SD from at least triplicate experiments and asterisk (*) indicated a significant difference (P <0.05).
Discussion
The function of XIAP in regulating cancer cell motility varies upon cancer types 7–9. Although our most recent studies indicate that BCs of human patients and BBN-exposed mice exhibit overexpression of XIAP, which mediates human BC cell invasion in vitro and invasive BC development in vivo, nothing is known about XIAP-regulated downstream effector(s) that mediates BC invasion and metastasis. In current studies, we un-anticipatively found that XIAP and RhoGDIβ protein abundances were consistently elevated in most of cases of (72.7%) human BCs and all (100%) of BBN-induced mouse high invasive BCs, which is distinct from previous report that RhoGDIβ is downregulated in human BCs 13. The results obtained from IHC staining showed that XIAP overexpression was highly related to RhoGDIβ expression in human BCs. The investigation from lost-expression studies indicated that XIAP/its RING domain were crucial for maintaining RhoGDIβ protein levels in human BC cell lines and in mouse BC tissues. We also discovered that RhoGDIβ was a key XIAP downstream effector that mediated invasion in vitro cultured human BC cells and BC cell lung metastasis in vivo nude mice injected with T24T cells. Mechanistic studies showed that XIAP was required for RhoGDIβ mRNA stabilization by its RING positive regulating nucleolin mRNA stability via Erks-dependent manner. Consistently, XIAP-regulated Erks/nucleolin/RhoGDIβ axis was crucial for its promotion of BC cell invasion in vitro, while knockdown of RhoGDIβ in T24T cells attenuated the lung metastasis of T24T cells in nude mice. Moreover, ectopic expression of GFP-RhoGDIβ in XIAP-knocked down T24T cells, T24T(shXIAP/GFP-RhoGDIβ), completely rescued their lung metastatic ability in nude mice. Our results clearly demonstrate that XIAP-mediated Erk activation upregulates nucleolin expression, which in turn bound to and stabilize RhoGDIβ mRNA, and subsequently promotes human BC invasion in vitro and lung metastasis in vivo.
XIAP functions as an inhibitor of cell apoptosis primarily by inhibiting caspase activity 34. Consequently, clinical studies demonstrate that XIAP is overexpressed in rental, prostate, breast, and bladder cancer tissues coupled with studies depicting that XIAP inhibition sensitizes cancer cells to apoptosis, the nature of XIAP in cancer progression are also reported 35, 36. Recent studies from our or other laboratories demonstrate that XIAP regulates cell migration and metastasis in colon cancer HCT116 cells via attenuating RhoGDIα SUMOylation 7. Also, the complex of XIAP and survivin can activate the NF-κB transcription factor, in turn promoting cancer cell migration and metastasis in human prostate cancer cells 8. Other studies report that XIAP can bind to and promotes Rac1 or C-RAF degradation via enhancing their poly-ubiquitination, while the attenuation of XIAP expression stabilizes Rac1 or C-RAF and in turn elevates cell migration 10, 37. Those studies reveal that function of XIAP in regulation of cancer cell migration, invasion and metastasis is cell type-dependent manner. Our current studies found that XIAP was overexpressed in 66.7% of BC tissues in comparison to their paired adjacent normal bladder tissues. The results obtained from IHC staining showed that XIAP was also overexpressed in human BCs in comparison to the paired normal bladder tissues. The studies by using knockdown approach showed that XIAP expression was crucial for the invasion of human high grade BC TccSup and T24T cells although it inhibited the migration of both cell lines. Our results demonstrate that XIAP plays differential roles in regulation of migration and invasion of human BC cells, providing additional evidence supporting that XIAP behavior in regulation of cancer cell migration and invasion is cancer- and cell-type specific.
RhoGDIβ is a recently identified regulator of cancer cell motility and metastasis 19, 38. The results from study in breast cancer show that RhoGDIβ is involved in promoting cancer cell invasion 39, and RhoGDIβ overexpression has also been found in human cancer tissues from patients with colorectal and hepatocellular carcinoma in comparison with corresponding adjacent normal tissues 40, 41. The clinical studies show that the RhoGDIβ expression levels are associated with prognosis of colorectal cancer patients 41. The patients with higher level of RhoGDIβ expression have poor overall survival than those with low RhoGDIβ expression relative to their adjacent normal gastric cancer and colorectal cancers 18, 41. On other hand, RhoGDIβ expression is downregulated in lung cancer tissues 42, and forced RhoGDIβ expression reduces lung metastasis in mice 43. RhoGDIβ has been thought to be a suppressor for invasion and metastasis in human BCs 13, 14. However, in current study, the overexpression of RhoGDIβ is found in 11 of 12 bladder cancer tissues as compared with that in their paired adjacent normal tissues, and the results obtained from IHC staining also revealed the overexpression of RhoGDIβ in human BCs in comparison to the paired normal bladders. These findings are distinct from previous reports 13, 14. The explanation for the differential conclusions between the studies might be caused by methodology for evaluation and comparison. Since protein is the functional carrier, in current study we determined RhoGDIβ protein expression in human BCs using a specific antibody against RhoGDIβ with Western Blot, in which only showed a specific antibody-recognized protein band at 27 Kd, rather than determination of RhoGDIβ mRNA levels or immunohistochemistry of BC Tissue Microarray. Moreover, we compared RhoGDIβ protein expression in BCs in comparison to their adjacent normal tissues side by side, which resulted in more reliable results and conclusion. Our notion of RhoGDIβ overexpression in BCs was also greatly supported by the clear data obtained from the studies of BBN-induced mouse high invasive BCs.
The function of RhoGDIβ in regulating cancer cell motility is also dependent on cancer types and experimental systems. RhoGDIβ induces the metastatic capacity of MDA-MB-231 breast cancer cells 44, and suppressing expression of RhoGDIβ significantly attenuates migration of ovarian or gastric cancer cells 18, 38. Knockdown RhoGDIβ expression increases the activation of matrix metallopeptidase 9 (MMP-9) and induces lung cancer cell A549 cell invasion 45. Other reports demonstrate that RhoGDIβ is a suppressor of bladder cancer cell metastasis 13, and the phosphorylation of RhoGDIβ plays a role with regards to regulating its function 46. RhoGDIβ phosphorylation at Tyr153 mediated by Src enhances its inhibitory effect on bladder cancer cell metastasis 16, however, protein kinase C alpha (PKCα) induces RhoGDIβ phosphorylation at Ser31 deactivates its GDI function via disrupting its interaction with Rac1 46, whereas same group also reports that RhoGDIβ overexpression increases Rac1 activity in human UMUC3 cells and such Rac1 activation is not associated with RhoGDIβ regulation of BC cell migration and BC cell metastasis in UMUC3 cells 19. It has also reported that repressing RhoGDIβ expression enhances endothelin-1 signaling pathway which plays a critical role in promoting BC invasion and metastasis 47. Our study here demonstrates that RhoGDIβ was upregulated by XIAP in Erk/nucleolin-dependent manner with the diverse effects on regulation of BC cell migration and invasion. We found that XIAP-regulated RhoGDIβ was able to promote human BC cell invasion, while it inhibited human BC cell migration in same BC cells. We also showed that XIAP was crucial factor for Erk activation and nucleolin protein expression, and that nucleolin subsequently stabilized RhoGDIβ mRNA and elevated RhoGDIβ protein expression. Collectively, XIAP-regulated RhoGDIβ expression plays an important role in promoting human BC cell invasion and lung metastasis although it inhibits BC cell migration, in which potential mechanism underlying of RhoGDIβ function might be distinct from RhoGDIβ in regulating activation of Rac1 and MMP9 that has been reported in previous studies 19, 48. This notion was supported by our most recent finding that migration and invasion in human BC cells are differential regulated by Superoxide dismutase (SOD) and matrix metalloproteinase-2 (MMP-2), respectively 22. This novel discovery contributes to understanding of the molecular relationship between XIAP and RhoGDIβ in mediating human BC invasion, and also provides value information for potential clinical therapy of patients with human invasive BCs.
Although migration and invasion are complimentary stages in cancer metastasis, several studies demonstrate that some migration related proteins had no effect on cell invasion. For example, Ets-1 promotes both migration and invasion of melanoma and Hela cells, whereas it regulates prostate PC3 cell migration, but not invasion 49. In colon cancer cells, α-catenin is shown to be essential for cancer cell migration, but serves no role in cancer cell invasion 50. Our results revealed the promoting effect of XIAP in in BC cell invasion, while XIAP inhibited cell migration in same human BC cell lines T24T and TccSup. These results are consistent with our most recent findings that an inverse relationship between cell migration and invasion in human BC T24T and T24 cells 22. We demonstrate that cell migration related proteins CDC42 and Rac1 were suppressed in highly metastatic T24T cells, and their cell migration is inhibited in comparison to that of the less metastatic parental T24 cells 22. Our further studies reveal that highly metastatic T24T cells have increased MMP-2 expression, which upregulates T24T invasion, whereas overexpression of SOD2 mediates the inhibition of cell migration of T24T cells 22. Moreover, we have elucidated that nucleolin overexpression and translocation into the nucleus enhances mmp-2 mRNA stability, thus promoting T24T cell invasion; while Sp1 overexpression and activation plays an important role in SOD2 transcription and protein overexpression, as well as inhibition of cell migration inT24T cells 22. Although our studies found that RhoGDIβ served as XIAP downstream effector that is responsible for promotion of cancer cell invasion with inhibition of migration, the further identifying RhoGDIβ downstream targets that mediate these diverse functions of RhoGDIβ in regulation of human BC invasion and migration will warrant of high significances in understanding nature of RhoGDIβ in modulation of BC cell motility and metastasis.
Mammalian nucleolin protein consists of three domains: N-terminus, C-terminus, and central domain, and there are four RNA binding domains locate in central domain, which can bind to AU-rich elements in 3′-UTR of target mRNA 51. In this study, we found that RhoGDIβ mRNA was stabilized by RNA binding protein nucleolin. Nucleolin directly bound to RhoGDIβ mRNA was identified using RNA immunoprecipitation, which provides evidence that nucleolin might be involved in enhancing RhoGDIβ mRNA stability. Further study indicated that XIAP expression was crucial for maintaining nucleolin mRNA stability in BC cells. Erk has been reported to be implicated in stabilizing nucleolin mRNA in cells exposed to phorbol 12-myristate 13-acetate 33. The results obtained from our study indicated that attenuated XIAP expression led to suppression of Erk phosphorylation and nucleolin mRNA stability, whereas inhibition of Erk activation also impaired nucleolin mRNA stability, demonstrating that in BC cells, XIAP promotes Erk activation, which in turn stabilizes nucleolin mRNA and subsequently resulting in increases in RhoGDIβ mRNA stability and expression. In conclusion, our study shows that XIAP is critical for promoting nucleolin expression, which in turn stabilizes RhoGDIβ mRNA, subsequently regulating BC cell invasion and lung metastasis. The identification of the XIAP/Erk/nucleolin/RhoGDIβ pathway in BCs provides a significant insight into understanding of the diverse roles of XIAP beyond its conventional anti-apoptosis function. This study leads to a significant change in current dogma regarding the importance of RhoGDIβ in BC invasion and metastasis, which could lead to the development of new approaches that target RhoGDIβ and/or XIAP for BC prevention and therapy.
Supplementary Material
Novelty and Impact statements.
The novel discovery of highly abundant RhoGDIβ expression in high-grade invasive BCs of human specimens, mouse models and cell lines, as a key XIAP downstream mediator being responsible for overexpressed XIAP promotion of BC invasion and lung metastasis provides a highly insight into understand nature of BC invasion and metastasis, which in turn lead to a significant change in current dogma regarding the importance of RhoGDIβ in BC invasion and metastasis, as well as development of new approaches that target RhoGDIβ and/or XIAP for improving the outcome of patients with invasive BC.
Acknowledgments
We want to thank Dr. Martin A. Schwartz (Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, Virginia) and Dr. Michael B. Kastan (Comprehensive Cancer Center, St. Jude Children’s Research Hospital, Memphis) for their kind gifts of GFP-RhoGDIβ and GFP-nucleolin, respectively.
Abbreviation
- XIAP
X-link inhibitor of apoptosis protein
- Rho-GDI
RhoGDP dissociation inhibitor
- BBN
N-butyl-N-(4-hydroxybutyl)-nitrosamine
- Erk
extracellular regulated protein kinases
- GFP
green fluorescent protein
- BC
bladder cancer
- VHL
Von Hippel-Lindau
- HuR
human antigen R
Footnotes
Conflicts of Interest: No potential conflicts of interest to disclose.
Financial Disclosure: This work was partially supported by grants from NIH/NCI CA165980, CA177665 and CA112557, as well as NIH/NIEHS ES000260; Innovation Team of Zhejiang Province (2013TD10) and the Natural Science Foundation of China (NSFC81229002).
References
- 1.Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society: Cancer Facts and Figures. 2015 Atlanta, Ga: American Cancer Society; 2015. [Google Scholar]
- 3.Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The National Cancer Data Base report on bladder carcinoma. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996;78:1505–13. doi: 10.1002/(sici)1097-0142(19961001)78:7<1505::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Whelan P. Survival from bladder cancer in England and Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S90–2. doi: 10.1038/sj.bjc.6604600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duckett CS, Li F, Wang Y, Tomaselli KJ, Thompson CB, Armstrong RC. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol Cell Biol. 1998;18:608–15. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Zhang R, Li J, Huang H, Zhang D, Zhang J, Gao J, Chen J, Huang C. X-linked inhibitor of apoptosis protein (XIAP) regulation of cyclin D1 protein expression and cancer cell anchorage-independent growth via its E3 ligase-mediated protein phosphatase 2A/c-Jun axis. J Biol Chem. 2013;288:20238–47. doi: 10.1074/jbc.M112.448365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Zhang D, Luo W, Yu Y, Yu J, Li J, Zhang X, Zhang B, Chen J, Wu XR, Rosas-Acosta G, Huang C. X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J Biol Chem. 2011;286:15630–40. doi: 10.1074/jbc.M110.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Ahn S, Ko YG, Boo YC, Chi SG, Ni CW, Go YM, Jo H, Park H. X-linked inhibitor of apoptosis protein controls alpha5-integrin-mediated cell adhesion and migration. Am J Physiol Heart Circ Physiol. 2010;299:H300–9. doi: 10.1152/ajpheart.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogan T, Harms GS, Hekman M, Karreman C, Oberoi TK, Alnemri ES, Rapp UR, Rajalingam K. X-linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility. Nat Cell Biol. 2008;10:1447–55. doi: 10.1038/ncb1804. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, Xu J, Guo X, Huang H, Li J, Peng M, Zhu J, Tian Z, Wu XR, Tang MS, Huang C. XIAP RING domain mediates miR-4295 expression and subsequently inhibiting p63alpha protein translation and promoting transformation of bladder epithelial cells. Oncotarget. 2017;7:56540–57. doi: 10.18632/oncotarget.10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Zhang Y, Dagher MC, Shacter E. Rho GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Res. 2005;65:6054–62. doi: 10.1158/0008-5472.CAN-05-0175. [DOI] [PubMed] [Google Scholar]
- 13.Theodorescu D, Sapinoso LM, Conaway MR, Oxford G, Hampton GM, Frierson HF., Jr Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clin Cancer Res. 2004;10:3800–6. doi: 10.1158/1078-0432.CCR-03-0653. [DOI] [PubMed] [Google Scholar]
- 14.Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, Frierson HF, Conaway MR, Theodorescu D. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62:6418–23. [PubMed] [Google Scholar]
- 15.Cho HJ, Baek KE, Yoo J. RhoGDI2 as a therapeutic target in cancer. Expert Opin Ther Targets. 2010;14:67–75. doi: 10.1517/14728220903449251. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Moissoglu K, Wang H, Wang X, Frierson HF, Schwartz MA, Theodorescu D. Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proc Natl Acad Sci U S A. 2009;106:5807–12. doi: 10.1073/pnas.0810094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho HJ, Baek KE, Park SM, Kim IK, Nam IK, Choi YL, Park SH, Im MJ, Choi J, Ryu J, Kim JW, Lee CW, et al. RhoGDI2 confers gastric cancer cells resistance against cisplatin-induced apoptosis by upregulation of Bcl-2 expression. Cancer Lett. 2011;311:48–56. doi: 10.1016/j.canlet.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Cho HJ, Baek KE, Park SM, Kim IK, Choi YL, Nam IK, Hwang EM, Park JY, Han JY, Kang SS, Kim DC, Lee WS, et al. RhoGDI2 expression is associated with tumor growth and malignant progression of gastric cancer. Clin Cancer Res. 2009;15:2612–9. doi: 10.1158/1078-0432.CCR-08-2192. [DOI] [PubMed] [Google Scholar]
- 19.Moissoglu K, McRoberts KS, Meier JA, Theodorescu D, Schwartz MA. Rho GDP dissociation inhibitor 2 suppresses metastasis via unconventional regulation of RhoGTPases. Cancer Res. 2009;69:2838–44. doi: 10.1158/0008-5472.CAN-08-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Ma WY, Ryan CA, Dong Z. Proteinase inhibitors I and II from potatoes specifically block UV-induced activator protein-1 activation through a pathway that is independent of extracellular signal-regulated kinases, c-Jun N-terminal kinases, and P38 kinase. Proc Natl Acad Sci U S A. 1997;94:11957–62. doi: 10.1073/pnas.94.22.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin H, Yu Y, Hu Y, Lu C, Li J, Gu J, Zhang L, Huang H, Zhang D, Wu XR, Gao J, Huang C. Divergent behaviors and underlying mechanisms of cell migration and invasion in non-metastatic T24 and its metastatic derivative T24T bladder cancer cell lines. Oncotarget. 2015;6:522–36. doi: 10.18632/oncotarget.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y, Yu Y, Hou Q, Zheng X, Zhang M, Zhang D, Li J, Wu XR, Huang C. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP. J Biol Chem. 2012;287:35234–43. doi: 10.1074/jbc.M112.389494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schile AJ, Garcia-Fernandez M, Steller H. Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 2008;22:2256–66. doi: 10.1101/gad.1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Zhang D, Luo W, Yu J, Li J, Yu Y, Zhang X, Chen J, Wu XR, Huang C. E3 ligase activity of XIAP RING domain is required for XIAP-mediated cancer cell migration, but not for its RhoGDI binding activity. PloS one. 2012;7:e35682. doi: 10.1371/journal.pone.0035682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo Z, Che X, Wang Y, Li B, Li J, Dai W, Lin CP, Huang C. High mobility group Box-1 inhibits cancer cell motility and metastasis by suppressing activation of transcription factor CREB and nWASP expression. Oncotarget. 2014;5:7458–70. doi: 10.18632/oncotarget.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Bi Y, Zeng F, Zheng L, Tong Q. Expression of X-linked inhibitor of apoptosis protein and its effect on chemotherapeutic sensitivity of bladder carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2007;27:285–7. doi: 10.1007/s11596-007-0317-5. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Zhang D, Liu J, Li J, Yu Y, Wu XR, Huang C. RhoGDI SUMOylation at Lys-138 increases its binding activity to Rho GTPase and its inhibiting cancer cell motility. J Biol Chem. 2012;287:13752–60. doi: 10.1074/jbc.M111.337469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng SS, Chen CY, Xu N, Shyu AB. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–70. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuen JS, Cockman ME, Sullivan M, Protheroe A, Turner GD, Roberts IS, Pugh CW, Werner H, Macaulay VM. The VHL tumor suppressor inhibits expression of the IGF1R and its loss induces IGF1R upregulation in human clear cell renal carcinoma. Oncogene. 2007;26:6499–508. doi: 10.1038/sj.onc.1210474. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Bhatia D, Xia H, Castranova V, Shi X, Chen F. Nucleolin links to arsenic-induced stabilization of GADD45alpha mRNA. Nucleic Acids Res. 2006;34:485–95. doi: 10.1093/nar/gkj459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukong KE, Chang KW, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends Genet. 2008;24:416–25. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Westmark CJ, Malter JS. Up-regulation of nucleolin mRNA and protein in peripheral blood mononuclear cells by extracellular-regulated kinase. J Biol Chem. 2001;276:1119–26. doi: 10.1074/jbc.M009435200. [DOI] [PubMed] [Google Scholar]
- 34.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–52. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 35.Xie Y, Tobin LA, Camps J, Wangsa D, Yang J, Rao M, Witasp E, Awad KS, Yoo N, Ried T, Kwong KF. MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in cancer cells. Oncogene. 2012 doi: 10.1038/onc.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang Y, Yu Y, Hou Q, Zheng X, Zhang M, Zhang D, Li J, Wu XR, Huang C. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by downregulating overexpression of antiapoptotic protein XIAP. J Biol Chem. 2012 doi: 10.1074/jbc.M112.389494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberoi TK, Dogan T, Hocking JC, Scholz RP, Mooz J, Anderson CL, Karreman C, Meyer zu Heringdorf D, Schmidt G, Ruonala M, Namikawa K, Harms GS, et al. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 2012;31:14–28. doi: 10.1038/emboj.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens EV, Banet N, Onesto C, Plachco A, Alan JK, Nikolaishvili-Feinberg N, Midkiff BR, Kuan PF, Liu J, Miller CR, Vigil D, Graves LM, et al. RhoGDI2 antagonizes ovarian carcinoma growth, invasion and metastasis. Small GTPases. 2012;2:202–10. doi: 10.4161/sgtp.2.4.17795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Zhang B. D4-GDI, a Rho GTPase regulator, promotes breast cancer cell invasiveness. Cancer Res. 2006;66:5592–8. doi: 10.1158/0008-5472.CAN-05-4004. [DOI] [PubMed] [Google Scholar]
- 40.Fang Y, Yi J, Lizhi L, Qiucheng C. Rho GDP dissociation inhibitor beta promotes cell proliferation and invasion by modulating the AKT pathway in hepatocellular carcinoma. DNA Cell Biol. 2014;33:781–6. doi: 10.1089/dna.2014.2545. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Wang J, Zhang X, Zeng Y, Liang L, Ding Y. Overexpression of RhoGDI2 correlates with tumor progression and poor prognosis in colorectal carcinoma. Ann Surg Oncol. 2011;19:145–53. doi: 10.1245/s10434-011-1944-4. [DOI] [PubMed] [Google Scholar]
- 42.Niu H, Li H, Xu C, He P. Expression profile of RhoGDI2 in lung cancers and role of RhoGDI2 in lung cancer metastasis. Oncol Rep. 2010;24:465–71. doi: 10.3892/or_00000880. [DOI] [PubMed] [Google Scholar]
- 43.Said N, Sanchez-Carbayo M, Smith SC, Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. 2012;122:1503–18. doi: 10.1172/JCI61392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, Shokrgozar MA. Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of beta1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol. 2012;29:2512–8. doi: 10.1007/s12032-011-0113-8. [DOI] [PubMed] [Google Scholar]
- 45.Niu H, Wu B, Peng Y, Jiang H, Zhang Y, Wang J, He P. RNA interference-mediated knockdown of RhoGDI2 induces the migration and invasion of human lung cancer A549 cells via activating the PI3K/Akt pathway. Tumour Biol. 2015;36:409–19. doi: 10.1007/s13277-014-2671-9. [DOI] [PubMed] [Google Scholar]
- 46.Griner EM, Churchill ME, Brautigan DL, Theodorescu D. PKCalpha phosphorylation of RhoGDI2 at Ser31 disrupts interactions with Rac1 and decreases GDI activity. Oncogene. 2012 doi: 10.1038/onc.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Titus B, Frierson HF, Jr, Conaway M, Ching K, Guise T, Chirgwin J, Hampton G, Theodorescu D. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res. 2005;65:7320–7. doi: 10.1158/0008-5472.CAN-05-1403. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh WT, Yeh WL, Cheng RY, Lin C, Tsai CF, Huang BR, Wu CY, Lin HY, Huang SS, Lu DY. Exogenous endothelin-1 induces cell migration and matrix metalloproteinase expression in U251 human glioblastoma multiforme. J Neurooncol. 2014;118:257–69. doi: 10.1007/s11060-014-1442-1. [DOI] [PubMed] [Google Scholar]
- 49.Shaikhibrahim Z, Langer B, Lindstrot A, Florin A, Bosserhoff A, Buettner R, Wernert N. Ets-1 is implicated in the regulation of androgen co-regulator FHL2 and reveals specificity for migration, but not invasion, of PC3 prostate cancer cells. Oncol Rep. 2011;25:1125–9. doi: 10.3892/or.2011.1156. [DOI] [PubMed] [Google Scholar]
- 50.Andre F, Janssens B, Bruyneel E, van Roy F, Gespach C, Mareel M, Bracke M. Alpha-catenin is required for IGF-I-induced cellular migration but not invasion in human colonic cancer cells. Oncogene. 2004;23:1177–86. doi: 10.1038/sj.onc.1207238. [DOI] [PubMed] [Google Scholar]
- 51.Sengupta TK, Bandyopadhyay S, Fernandes DJ, Spicer EK. Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J Biol Chem. 2004;279:10855–63. doi: 10.1074/jbc.M309111200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.