Abstract
AIM
This study examined the reliability and validity of the Test of Arm Selective Control (TASC) to examine upper extremity selective voluntary motor control in children and adolescents with all types of spastic cerebral palsy (CP).
METHOD
Fifty-six participants with CP, ranging in age from 5 years 9 months to 18 years 11 months (average 11y 7mo, SD 3y 9mo; 25 males, 31 females), participated in this prospective cross-sectional study. They were evaluated using the TASC and several clinical measures.
RESULTS
TASC and Manual Ability Classification System (r = −0.529, p<0.001), TASC and ABILIHAND-Kids (r = 0.596, p<0.001), and TASC and affected extremities (r = −0.486, p=0.001) were moderately correlated. There was a weak correlation between the TASC and Gross Motor Function Classification System (r = −0.363, p=0.006) and no correlation between the TASC and age (p=0.366) or rater (p=0.713). Interrater reliability for upper extremity total score (intraclass correlation coefficient [ICC]=0.92–0.94) and upper extremity limb scores (ICC=0.92–0.96) was high for two independent rater groups (p≤0.001). Average time to administer was 16 minutes, 18 seconds.
INTERPRETATION
The TASC is a reliable and valid tool for objective assessment of selective voluntary motor control. Clinically this measure may guide the selection of medical, surgical, or therapy interventions and may improve outcome prognosis.
A deficit in selective voluntary motor control (SVMC) in the upper extremity adversely affects functional abilities in those with cerebral palsy (CP). The National Institutes of Health Taskforce on Childhood Motor Disorders defined SVMC as the ‘ability to isolate the activation of muscles in a selected pattern in response to demands of a voluntary movement or posture’.1 Reduced SVMC results from corticospinal tract damage and may present as synergistic coupling of joint motions2 resulting in decreased fluency, lack of movement, inability to reverse directions, reduced speed, or involuntary movement at other joints including mirror movements.3,4 In CP, corticospinal motor tract damage from an acute insult or injury is compounded by the persistence of corticospinal immaturity as the child ages.5,6 Maladaptive plasticity in unilateral injuries such as the preservation of the non-injured ipsilateral corticospinal tract may cause the expression of involuntary mirror movements that cannot be suppressed.7 SVMC of the upper extremity can be impacted by both unilateral and bilateral brain lesions. A child’s functional ability is dictated by their SVMC abilities, in conjunction with relative presence or absence of other common impairments observed in the upper extremity of those with CP. Clinicians have tools available to evaluate and communicate about body structure and function impairments including decreased passive range of motion (ROM), limited active ROM, decreased strength, and spasticity. However, a tool and a common language to specifically describe and measure SVMC in the upper extremities are lacking. Therefore, the relationship between deficits in SVMC and challenges in activities and participation have not been fully explored in the upper extremity.
With the positive response from the clinical and research community to the Selective Control Assessment of the Lower Extremity,8 a second working group of the National Institute of Health Taskforce on Childhood Motor Disorders was formed to translate the concept to the upper extremity. A review of tools available at the time of the meeting in 2008 that examined upper extremity motor control (Qualitative Upper Extremity Skills Test,9,10 Melbourne,11 Fugl-Myer,12,13 Shriners Hospital Upper Extremity Examination -SHUEE)14 revealed that most assessments were designed or validated in children with hemiplegic CP, required a specific set of props or video for administration, took more than 30 minutes to administer, and/or required the purchase of a manual or training course. The working group consensus was that none directly addressed the construct of SVMC as defined by the Task Force, in that it should prescribe movement speed, require reversal of directions to assess both recruitment and derecruitment of muscles, and evaluate movement at non-intended joints. Therefore, the Test of Arm Selective Control (TASC) was developed in 2010 to address the need for a clinically feasible tool to assess SVMC that was efficacious and could be performed reliably by any type of rehabilitation professional with minimal training time. Specifically, the goal of the assessment is to systematically evaluate a person’s ability to move the upper extremity with SVMC by observing movement control, coordination, fluency, mirroring, and speed at each joint, and then assigning a grade, all in ‘real time’. Constructs and operational definitions from the Selective Control Assessment of the Lower Extremity were adapted when translating the concept to the upper extremity to optimize similarity and reduce confusion amongst examiners who test SVMC in both upper and lower extremities in a clinic or research setting.
Many studies have focused on upper extremity function in children and adolescents with hemiplegia,15,16 but it is apparent that those with bilateral injury resulting in spastic quadriplegia and even diplegia also have upper extremity dysfunction.17,18 It was hypothesized that upper extremity SVMC skill relates to upper extremity function at an activity level. Therefore, this study evaluated the reliability, validity, and feasibility of the TASC to examine upper extremity SVMC in children and adolescents with all types of spastic CP, and begins to explore the relationship between upper extremity function and upper extremity SVMC.
METHODS
Tool description
The TASC includes eight motions in each arm: shoulder flexion/extension, shoulder abduction/adduction, elbow flexion/extension, forearm supination/pronation, wrist flexion/extension, finger flexion/extension, thumb opposition (pincer grip), and metacarpophalangeal extension (key grip). The TASC uses administration and scoring rules similar to the Selective Control Assessment of the Lower Extremity.8 Each item has three segments of motion which should be completed within three seconds. TASC testing should take place in a non-distracting environment where the child can sit with their feet on the floor in a chair having the lowest level of safe upper body support that the child can maintain. A chair or bench without arms is ideal, but testing can also be done in a wheelchair if required.
During test administration the examiner explains and demonstrates each starting position and joint motion. Participants begin motions with their arm at their side (neutral shoulder rotation) in one of two positions: (1) with elbow, wrist, and fingers extended; (2) with elbow flexed to 90 degrees, forearm in a neutral position, and wrist and fingers extended. Initially, the examiner moves the participant passively through the full arc of motion to provide instruction and simultaneously assess passive ROM. The examiner should assess for the presence of spasticity or a spastic catch. Once passive motion is assessed, the participant is instructed to complete the motion, with verbal cues to maintain the correct pace (‘up, down, up’ or ‘1, 1000; 2, 1000; 3, 1000′). Instructions and verbal cues may be modified as required to optimize the participant’s performance of the motion, taking into consideration the participant’s preferred learning methods and any relevant auditory, visual, or cognitive impairments. It is suggested that testing proceed in a proximal to distal fashion. Up to three attempts are allowed for each motion. After completion of a best attempt, examiners give a score (0=no SVMC; 1=impaired SVMC; 2=intact SVMC), and check appropriate descriptors on the score sheet. Detailed definitions are provided in Table I. The TASC manual and video file include administration instructions, operational definitions, directions for scoring, and a scoresheet (Appendix S1 and Video file S1, online supporting information).
Table I.
Scoring and descriptor definitions
| Scores | Normal=2 | Normal motor control is the ability to isolate joint motion through more than 50% of the available ROM in instructed directions within a three-second verbal count. The motion occurs without accompanying motion at any other joints of either limb. In general, when descriptors are checked on the score sheet a patient cannot have normal motor control. |
| Impaired=1 | Patients with impaired motor control may be able to move the desired joint through a portion of the available ROM (≤50%) without any other joint movement, however a portion of the motion is accompanied by motion at a different joint of the same limb, or mirrored by motion on the opposite limb. | |
| Absent=0 | If a patient does not demonstrate selective voluntary motor control, they have simultaneous movement at two or more joints. For every degree of motion at the desired joint, concomitant obligatory motion occurs at another joint in the limb. This movement may occur in the defined synergy patterns, but does not have to. A score of 0 is also given if a patient is unable to actively generate any ROM at the instructed joint. | |
| Score sheet descriptors | ↓ ROM | Active motion ≤ 50% available ROM at joint being tested. |
| Slow | Motion occurs slower than verbal cues given by examiner (3 second count). | |
| Extra movement |
Movement at joints other than tested joints within the same arm, or in postural compensations at the trunk. | |
| Mirror movements |
Mirroring noted in arm opposite tested arm. | |
| No palpable contraction |
No palpable contraction of the agonist muscles to instructed joint movements. | |
| Movement one direction |
Movement in only one of the instructed directions (note motion achieved). | |
| Muscle properties | Spastic catch | Passive resistance or catch is felt when the joint is moved with increased speed while the patient is relaxed. This is assessed while the examiner is determining PROM. |
| Muscle tightness |
The joint has a contracture or PROM limitation. | |
| Notes | Flexion synergy influence |
Coupling of movements that may include some or all of the following: shoulder abduction, elbow flexion, forearm supination, and wrist/finger flexion. |
| Extension synergy influence |
Coupling of movements that may include some or all of the following: shoulder adduction, elbow extension, forearm pronation, and wrist/finger flexion. |
ROM, range of motion; PROM, passive range of motion.
Content validation
The National Institutes of Health Taskforce working group (two physical therapists, two medical doctors, and a biomedical engineer) reviewed tools available at that time for assessing aspects of SVMC (Qualitative Upper Extremity Skills Test,9 Fugl-Meyer,13 Melbourne,11 and SHUEE)14 and identified relevant motions at each joint. Upper extremity motion is particularly complex and multi-planar, so real time scoring was chosen to allow observation of movement from a perspective that appreciates all three planes of motion. With institutional review board approval, the original instructions, scoresheet, and set of tasks were piloted with an initial group of 16 consented participants (not included in Participants description, and consented under an independent protocol) who had CP. Score sheets and video recorded pilot TASC sessions were then discussed with a focus group of three occupational therapists and two physical therapists. Based on experience and feedback of these focus groups, as well as review of pilot videos, changes were recommended. The elbow task start position was defined as a fully extended elbow (start position #1), wrist task was altered to be gravity eliminated (neutral forearm), and two additional hand/finger components (finger/thumb opposition and metacarpophalangeal extension) were added to capture the higher level of manual skill required in the upper extremity. The instruction manual and score sheet (Appendix S1, online supporting information) were updated to improve clarity of operational definitions and instructions and to address areas of previous ambiguity, including: seating consistency, environmental distractions, presence of dystonia, ability to follow directions, visual gaze, postural compensations, and suggestions for pacing.
Participants and testing procedures
Children and adolescents with CP were recruited using the CP Research Registry,19 through local clinicians, and by word of mouth. Inclusion criteria included a diagnosis of CP, between 4 and 18 years of age, Manual Ability Classification Scale (MACS) and Gross Motor Function Classification Scale (GMFCS) levels I–IV, and the ability to follow verbal directions. Exclusion criteria were significant ataxia or dystonia. All parents provided written informed consent for participation and children over 8 years also provided written assent. Clinicians participating in the reliability and validation testing were physical therapists, occupational therapists, and student physical therapists recruited as a sample of convenience. The study was approved by the University Institutional Review Board. Fifty-six children with spastic CP were enrolled in the study.
All testing for this study was completed in an academic facility or the participant’s local clinic. Evaluators included three pediatric occupational therapists, four physical therapists, and five student physical therapists. Before initiation of reliability (2013–2014) and validity (2013–2016) testing, each evaluator attended a brief training session to learn about the test and review the test manual and assessment procedures. During this session, videos from pilot testing were utilized to review scoring criteria and practice scoring children demonstrating different levels of SVMC.
Although TASC scoring (individual joints scores summed for left upper extremity score, right upper extremity score, and combined total scores) was done live for all participants, each assessment was also recorded. The camera was placed in front of the participant to allow for observation of the tested motion, as well as any postural compensations or mirror movements that might occur. A blinded physical therapist or medical doctor not involved in TASC scoring recorded the CP diagnosis, a MACS and GMFCS level, and administered the ABILIHAND-Kids.
Validity
Sample size for validation was derived from a similar study of SVMC8 in the lower extremity in individuals of similar ages with CP. Using a significance level of p=0.05, power to detect change of 80 percent, SD of 5.5, and a clinical difference of 3 points, the sample size of 54 was calculated.
To confirm construct validity, internal item consistency of the TASC was assessed using Cronbach’s α for all 56 assessments. Using individual item scores, Cronbach’s α was calculated separately for the eight test items for each limb (right and left arms, n=56 observations per item) and for the eight test items on both limbs together (n=112 observations per item).
Concurrent validity was assessed using correlations between the TASC and validated measures assessing upper extremity manual ability and performance, which were expected to be related to SVMC. Manual ability was classified using the MACS.20 Performance was assessed using the ABILIHAND-Kids, a self-reported measure of ability to perform daily activities with the upper extremities.21 TASC scores were also examined for relationships with upper extremity limb involvement (both upper extremities, one upper extremity, no upper extremity), GMFCS level, age of the participant, and rater. The Shapiro-Wilk test was used to examine normality of data, and relationships between tests were examined using Spearman correlation (rho) secondary to the non-parametric nature of the data.
Discriminant validity was evaluated as the ability of the TASC to detect differences in the more affected versus the less affected extremity in the subset of participants with a diagnosis of hemiplegia (n=30), where differences in upper extremity function and performance were most clearly observable. Side of diagnosis (right vs left) was used to determine more and less affected limbs, and total limb TASC score was compared using a related-samples Wilcoxon signed rank test. For discriminant validity of the entire study cohort, the impact of MACS level on total TASC scores was evaluated using the Kruskal-Wallis-H test, with Bonferroni corrections on the pairwise comparisons between MACS levels.
Interrater reliability
Two rater groups of convenience completed reliability assessment of the TASC. The first rater group was comprised of three physical therapists (mean clinical experience 17y 2mo, range 5y–35y 6mo) and the second rater group was comprised of three occupational therapists (mean clinical experience 7y 2mo, range 4y–11y 6mo). The first 17 participants with CP that were recruited participated in both reliability and validity assessments for this study. For reliability, each participant was randomly assigned to Group 1 or Group 2. At the time of testing each participant completed the TASC three times with the three different raters in a randomly assigned order. Participants were given adequate rest periods between assessments to reduce any impact of fatigue. During testing, raters were blinded to the other raters’ examinations and scores.
Interrater reliability for each rater group was assessed using intraclass correlation coefficient (ICC, 2,1) and corresponding 95 percent confidence intervals (CI) for total score and for limb score. To help with interpretation of ICC reliability, we used general guidelines for reliability coefficients with values lower than 0.40 indicating poor agreement, 0.40 to 0.75 indicating good agreement, and greater than 0.75 indicating excellent agreement.22,23
Post hoc analyses of participant videos from the reliability sample were conducted to determine if variability of select components (visual gaze and rate of cuing) of TASC administration affected scores. Videos taken with a digital video camera were viewed to determine if the participant was looking ahead or at their tested limb, and the total time taken to complete all three segments of the motion was measured and converted to segments or beats per minute. Partial correlations were examined between the observed behavior (coded as a binary value for gaze [ahead or at limb] or scalar value for speed [measured in beats per minute]) and the score for that joint, while controlling for the participant.
Feasibility
Average time to administer, from beginning of instruction to final scoring completion, was assessed to determine feasibility of use in the clinic setting. Average time to administer (in minutes) was determined by adding all test times and dividing by the number of tests to get the average test time.
All statistical analyses were performed using SPSS v24 (IBM Corp., Armonk, NY, USA) and significance was established for p-values less than 0.05.
RESULTS
Participants’ ages ranged from 5 years 9 months to 18 years 11 months (mean=median 11y 7mo, SD 3y 9mo). There were 25 males and 31 females. Demographic and clinical characteristics of the participants in the study (n=56) are further described in Table II.
Table II.
Participant characteristics
| Reliability OT group (n=8) | Reliability PT group (n=9) | Validity sample (n=56) | ||
|---|---|---|---|---|
|
| ||||
| Age y:mo | Mean (SD) | 11:1 (2:6 | 14:1 (3:6) | 11:7 (3:9) |
| Range | 6:6–14:9 | 10:3–18:9 | 5:9–18:11 | |
|
| ||||
|
AH-Kids logit score |
Mean (SD) | 1.88 (1.93) | 1.49 (2.71) | 2.36 (2.29) |
| Range | −0.843 to 5.043 | −2.33 to 6.68 | −2.33 to 6.68 | |
|
| ||||
| Sex | Males n (%) | 1 (12.5%) | 7 (77.8%) | 25 (44.6%) |
| Females n(%) | 7 (87.5%) | 2 (22.2%) | 31 (55.4%) | |
|
| ||||
| MACS | I n(%) | 2 (25.0%) | 2 (22.2%) | 12 (21.4%) |
| II n(%) | 5 (62.5%) | 3 (33.3%) | 34 (37.5%) | |
| III n(%) | 1 (12.5%) | 3 (33.3%) | 9 (16.1%) | |
| IV n(%) | 0 (0%) | 1 (11.1%) | 1 (1.8%) | |
|
| ||||
| GMFCS | I n(%) | 2 (25.0%) | 1 (11.1%) | 18 (32.1%) |
| II n(%) | 3 (37.5%) | 3 (33.3%) | 21 (37.5%) | |
| III n(%) | 3 (37.5%) | 4 (44.4%) | 11 (19.6%) | |
| IV n(%) | 0 (0%) | 1 (11.1%) | 6 (10.7%) | |
|
| ||||
| CP type | Left hemiplegia n(%) | 0 (0%) | 5 (55.6%) | 16 (28.6%) |
| Right hemiplegia n(%) | 4 (50.0%) | 1 (11.1%) | 14 (25.5%) | |
| Diplegia n(%) | 1 (12.5%) | 1 (11.1%) | 15 (26.8%) | |
| Quadriplegia n(%) | 3 (37.5%) | 2 (22.2%) | 11 (19.6%) | |
|
| ||||
|
Upper extremities affected |
Zero n(%) | 1 (12.5%) | 1 (11.1%) | 15 (26.8%) |
| One n(%) | 4 (50.0%) | 6 (66.7%) | 30 (53.6%) | |
| Two n(%) | 3 (37.5%) | 2 (22.2%) | 11 (19.6%) | |
|
| ||||
| TASC total score | Mean (SD) | 17.12 (6.87) | 19.33 (6.42) | 20.93 (6.66) |
| Range | 3–24 | 10–29 | 3–32 | |
OT, occupational therapist; PT, physical therapist; y, years; m, months; SD, standard deviation; AH-Kids, ABILHAND-Kids; MACS, Manual Ability Classification Scale; GMFCS, Gross Motor Function Classification Scale; CP, cerebral palsy; TASC, Test of Arm Selective Control.
Validity
Internal item consistency was high when assessed for the eight test items on the right limb alone (α=0.946), left limb alone (α=0.940), and both limbs together (α=0.943), suggesting a unidimensional construct for test items and content validity.
For concurrent validity assessment, TASC scores were moderately positively correlated with ABILHAND-Kids logit scores (r=0.596, p<0.001), and moderately inversely (as expected) correlated with MACS levels (r=−0.529, p<0.001). Given the low number of participants in MACS level IV, the correlation was also run with that participant removed; the moderate correlation remained (r=−0.561, p<0.001). The TASC also demonstrated a fair correlation with the number of upper extremities involved (r=−0.486, p=0.001). There was a low correlation between the TASC and GMFCS levels (r=−0.362, p=0.006) and no correlation between the TASC and age (p=0.366) or TASC and rater (p=0.713). All data were complete, with the exception of one missing ABILIHAND-Kids questionnaire. Box plots for categorical variables are shown in Figure 1, and scatter plots for ABILHAND-Kids that include CP type and dominant limbs are available in Figure S1 (online supporting information).
Figure 1. TASC validity.
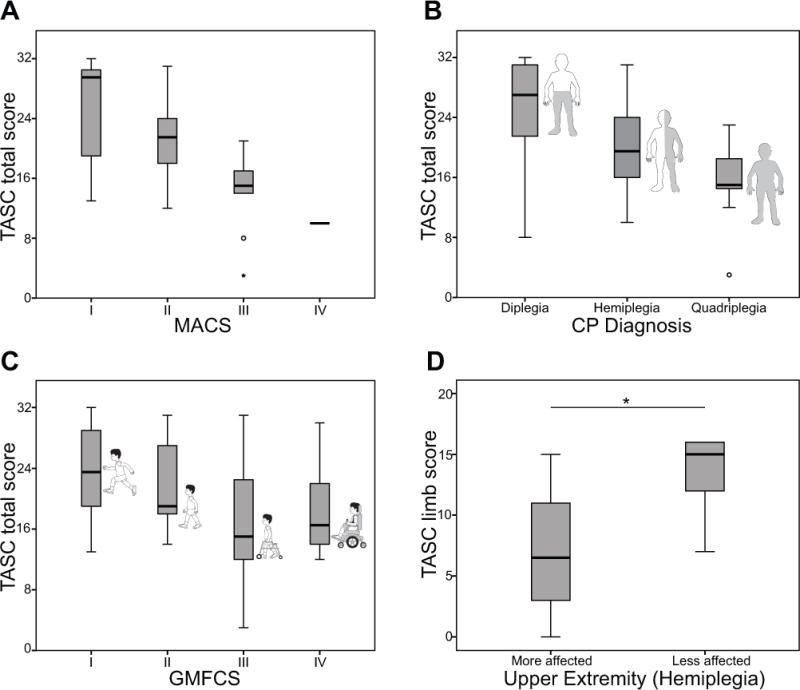
(a) Boxplot of total TASC scores by MACS classification. There was a correlation between MACS level and TASC score (r=−0.529, p<0.001), demonstrating concurrent validity with an activity measure. (b) Boxplot of total TASC scores by limb distribution, or the total number of upper extremities affected according to diagnosis. This was correlated to the total TASC score (r=−0.486, p=0.001). (c) Boxplot of total TASC scores by GMFCS classification. GMFCS level demonstrated a weak correlation to the TASC score (r=−0.362, p=0.006). (d) Discriminate validity between more and less affected limbs in hemiplegia. *Indicates significant (p<0.001) difference between the TASC limb score between sides. TASC, Test of Arm Selective Control; MACS, Manual Ability Classification System; CP, cerebral palsy; GMFCS, Gross Motor Functional Classification System.
With regards to discriminant validity, there was a significant difference in TASC scores between the most and least affected upper extremity for participants with hemiplegia (z=435, p<0.001), as shown in Figure 1. There was also a significant effect of MACS level on total TASC score across all participants (H(3)=6.86, p=0.001), with significant pairwise comparisons between MACS levels I and III (p=0.001) and MACS levels II and III (p=0.021).
Reliability
Interrater reliability for upper extremity total score (ICC=0.94) and upper extremity limb scores (ICC=0.92–0.96) was excellent for both rater groups (all p<0.001), shown in Table III.
Table III.
ICC (2,1) values for interrater reliability
| Rater group |
TASC score | (n) | ICC | 95% CI | p value |
|---|---|---|---|---|---|
| PT | L limb only | 9 | 0.93 | 0.77,0.95 | <0.001 |
| PT | R limb only | 9 | 0.96 | 0.87,0.99 | <0.001 |
| PT | Combined limb scores | 18 | 0.94 | 0.88,0.98 | <0.001 |
| PT | Total score | 9 | 0.94 | 0.82,0.99 | <0.001 |
| OT | L limb only | 8 | 0.92 | 0.72,0.98 | <0.001 |
| OT | R limb only | 8 | 0.92 | 0.73,0.98 | <0.001 |
| OT | Combined limb scores | 16 | 0.93 | 0.84,0.98 | <0.001 |
| OT | Total score | 8 | 0.94 | 0.81,0.99 | <0.001 |
ICC, intraclass correlation coefficient; TASC, Test of Arm Selective Control; CI, confidence interval; PT, physical therapist; OT, occupational therapist; L, left; R, right;.
For administration parameters, there was not a significant effect of participant gaze (p=0.637) on TASC score, but there was a weak but significant inverse relationship between speed of the movement and TASC score (r=−0.199, p=0.038).
Feasibility
Fifty-three participants had administration times recorded. The average time to administer the test from instruction to scoring was 16 minutes, 18 seconds (range 5–30 minutes). There were no participants that met inclusion criteria that were unable to complete the assessment. Any clinician wishing to rate SVMC can read and use the free manual and scoring forms (Appendix S1, online supporting information) and/or watch the video instruction file (Video file S1, online supporting information). The images and participants in these supplemental files are demonstrating the intended postures for each of the items to aid in administration training. It is worth noting that the models of convenience that were used in the manual and videos do not have CP.
DISCUSSION
The results of this study support the content and construct validity and interrater reliability of the TASC to measure SVMC in children and young adults with CP, providing the opportunity to further our understanding of the relationship between SVMC and arm/hand function. Validity and reliability were like the similarly constructed measure of lower extremity SVMC, the Selective Control Assessment of the Lower Extremity, despite differences in the requirements for function in upper and lower extremity function. There was a lower correlation than may have been hypothesized when considering the importance of SVMC (TASC score) to function (ABILHAND-Kids), but this may represent redundancy in the system and opportunities for alternative strategies to complete both unilateral and bilateral task items in the upper limb. CP diagnoses commonly associated with more upper extremity involvement (hemiplegia, quadriplegia) were inversely correlated with TASC scores suggesting that those with more upper extremity involvement have lower overall SVMC. The TASC was able to discriminate between more and less affected arms for children with hemiplegia and across MACS levels globally, but not between every MACS level in post hoc comparisons. Additionally, TASC scores were not influenced by age or rater and, as expected, were not substantially correlated with functional scales assessing domains unrelated to upper extremity functional ability, such as the GMFCS. Taken together, these results indicate that the TASC was evaluating motor control of the upper extremity in the study cohort without undue influence of confounding factors. These findings should be further confirmed with a larger cohort, and with the addition of a test of unimanual activity in order to further examine the relationship between the body function of SVMC and unimanual activity capacity within a limb.
After brief training, raters demonstrated excellent reliability in assessing upper extremity SVMC in a group of children with hemiplegic, diplegic, and quadriplegic CP using the TASC. The in-person training initially utilized in this study has been translated to a user manual freely available to any clinician. Future work includes development of a secure and web-accessible library of videos to provide examples of different scores for each test item to provide additional reference to clinicians learning the tool.
The TASC is a simple tool that is feasible for use in both the clinic and research setting to objectively measure SVMC for children and adolescents with spastic CP. The TASC can stand alone and may be self-taught using the detailed training manual, although a more formal webinar training is planned for the future. Another recently reported tool, the Selective Control of the Upper Extremity Scale also seeks to quantify SVMC in those with hemiplegia.24 In addition to confining the patient population to hemiplegia only, the Selective Control of the Upper Extremity Scale also requires scoring from a videotaped session. This prevents clinicians from reviewing performance or scoring during direct patient care time and presents additional challenges of video storage compliance per local protocol. In addition to these practical considerations, it can be easier to fully appreciate tri-planar motion and complex postural compensations when viewing in real time compared to a two-dimensional video image, which is the rationale for the choice of real time scoring for the TASC. In addition, descriptors such as tone and ROM and general impression comments may be easier to make note of at the time of TASC performance. While both the TASC and Selective Control of the Upper Extremity Scale have been developed simultaneously to address a similar unmet assessment need (upper extremity SVMC), there are key differences that clinicians and researchers should consider when choosing the more appropriate SVMC evaluation tool for their setting (see Table S1, online supporting information, for a detailed summary).
Video review may have value beyond participant scoring. An area where novice and experienced raters alike can use videos is to audit administration technique. In this study, we assessed the role that participant gaze and speed of movement played on performance. We found visual gaze irrelevant to score, but speed of movement was important, as is implied by the definition of SVMC. Therefore, the administrator should pay careful attention to the speed at which they are asking participants to move, and aim to be consistent with each administration.
The objective and systematic measure of SVMC may be used in the future to guide the selection of medical, surgical, or therapy interventions and broader implementation across clinical settings should facilitate improved understanding of SVMC in the context of clinical care. As clinicians and researchers more systematically record SVMC, we may also gain a better understanding of whether interventions may impact and improve SVMC allowing for isolated joint movement with increased speed, fluency, and coordination and ultimately improved functional ability. Specifically, the tool requires future assessment to determine its ability to predict change related to intervention. Examples of interventions that are postulated to improve SVMC or unmask underlying SVMC include intensive therapy programs, surgical interventions, and pharmacological interventions aimed to reduce excessive tone such as botulinum neurotoxin injections. Evaluation of these scientific questions would benefit from a multi-center study to extend the TASC sample size across a larger diversity of participants.
Limitations
This study was a heterogeneous representation of individuals with CP, and a relatively modest sample size of those demonstrating greater upper extremity impairment. While the TASC is moderately correlated with several tests at the activity/performance level of the International Classification of Function framework, the TASC is actually a body structure and function level tool. As such, SVMC may be influenced by other body structure and function issues common in those with CP, such as strength, spasticity, and active ROM, and it will be of value to compare TASC scores to impairment level tests in future studies to ascertain that upper extremity SVMC is a truly independent construct.25
Longitudinal use of the TASC before and after interventions will also allow the assessment of the tool’s minimal clinically important difference, sensitivity to change, and/or its’ ability to predict outcomes. Additionally, further study across multiple institutions, across populations with greater impairment levels, and with the addition of a unimanual activity measure, will add to our understanding of the psychometric properties of the tool, while furthering the assessment of feasibility and efficacy for use in both clinical and research settings.
CONCLUSION
Evidence of reliability and validity (construct and discriminant) provides initial confidence that the TASC can be used clinically to systematically observe and evaluate SVMC in the upper extremity. Ongoing research using the TASC is focused on SVMC impairment at individual joints and the impact of mirroring. Additional studies of test-retest reliability, stability of scores over time, and changes with intervention may provide support for the TASC’s utility for measuring progress and assisting with clinical decision-making for those with spastic CP. Continued development and clinical use of the TASC has the potential to further improve our understanding of the impact of upper extremity SVMC on function.
Supplementary Material
Appendix S1: Test of Arm Selective Control administration manual.
Figure S1: Scatterplots of TASC scores versus ABILHAND-Kids Logit scores.
Table SI: Comparison between Test of Arm Selective Control and Selective Control of Upper Extremity Scale.
Video S1: Video of Test of Arm Selective Control administration with an adolescent with typical development (intact selective voluntary motor control) to demonstrate standardized instructions and testing procedures.
What this paper adds.
The Test of Arm Selective Control (TASC) demonstrates a high degree of reliability and multiple aspects of validity when assessing upper extremity selective control in those with cerebral palsy.
The TASC is an upper limb companion to the Selective Control Assessment of the Lower Extremity.
Acknowledgments
This study was supported by the National Institutes of Health Task Force on Childhood Motor Disorders (U13NS061384). We gratefully acknowledge all participants and their families; Dr.Terence Sanger for leading the National Institute of Health Taskforce and supporting this project, and TASC team collaborators: Eileen Fowler, Darcy Fehlings, Katherine Block Denlinger, Erin Salzman, Jessica Barnum, Alexandrea Nichols, Erin Cameron, Elizabeth Byrne, Shannon Murphy, Andrew Robertson, and Katie Warner.
ABBREVIATIONS
- ICC
Intraclass correlation coefficient
- MACS
Manual Ability Classification Scale
- ROM
Range of motion
- SVMC
Selective voluntary motor control
- TASC
Test of Arm Selective Control
Footnotes
SUPPORTING INFORMATION
The following additional material may be found online:
References
- 1.Sanger TD, Chen D, Delgado MR, et al. Definition and classification of negative motor signs in childhood. Pediatrics. 2006;118:2159–67. doi: 10.1542/peds.2005-3016. [DOI] [PubMed] [Google Scholar]
- 2.Sukal-Moulton T, Krosschell KJ, Gaebler-Spira DJ, Dewald JP. Motor impairments related to brain injury timing in early hemiparesis. Part II: abnormal upper extremity joint torque synergies. Neurorehabil Neural Repair. 2014;28:24–35. doi: 10.1177/1545968313497829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler E, Staudt L. Are we being too ‘selective’ about motor control? Dev Med Child Neurol. 2014;56:509–10. doi: 10.1111/dmcn.12375. [DOI] [PubMed] [Google Scholar]
- 4.Sukal-Moulton T, Murray TM, Dewald JP. Loss of independent limb control in childhood hemiparesis is related to time of brain injury onset. Exp Brain Res. 2013;225:455–63. doi: 10.1007/s00221-012-3385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahill-Rowley K, Rose J. Etiology of impaired selective motor control: emerging evidence and its implications for research and treatment in cerebral palsy. Dev Med Child Neurol. 2014;56:522–8. doi: 10.1111/dmcn.12355. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Vikingstad EM, Huttenlocher PR, Towle VL, Levin DN. Functional magnetic resonance studies of the reorganization of the human hand sensorimotor area after unilateral brain injury in the perinatal period. Proc Natl Acad Sci U S A. 1994;91:9612–6. doi: 10.1073/pnas.91.20.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyre JA, Smith M, Dabydeen L, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- 8.Fowler EG, Staudt LA, Greenberg MB, Oppenheim WL. Selective Control Assessment of the Lower Extremity (SCALE): development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Dev Med Child Neurol. 2009;51:607–14. doi: 10.1111/j.1469-8749.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 9.DeMatteo C, Law M, Russell D, Pollock N, Rosenbaum P, Walter S. The reliability and validity of Quality of Upper Extremity Skills Test. Phys Occup Ther Pediatr. 1993;13:1–18. [Google Scholar]
- 10.DeMatteo C, Law M, Russell D, Pollock N, Rosenbaum P, Walter S. Quality of Upper Extremity Skills Test. Hamilton, Ontario, CA: CanChild Centre for Disabilty Research; 1992. [Google Scholar]
- 11.Spirtos M, O’Mahony P, Malone J. Interrater reliability of the Melbourne Assessment of Unilateral Upper Limb Function for children with hemiplegic cerebral palsy. Am J Occup Ther. 2011;65:378–83. doi: 10.5014/ajot.2011.001222. [DOI] [PubMed] [Google Scholar]
- 12.Singer B, Garcia-Vega J. The Fugl-Meyer Upper Extremity Scale. J Physiother. 2017;63:53. doi: 10.1016/j.jphys.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Woodbury ML, Velozo CA, Richards LG, Duncan PW, Studenski S, Lai SM. Dimensionality and construct validity of the Fugl-Meyer Assessment of the upper extremity. Arch Phys Med Rehabil. 2007;88:715–23. doi: 10.1016/j.apmr.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Davids JR, Peace LC, Wagner LV, Gidewall MA, Blackhurst DW, Roberson WM. Validation of the Shriners Hospital for Children Upper Extremity Evaluation (SHUEE) for children with hemiplegic cerebral palsy. J Bone Joint Surg Am. 2006;88:326–33. doi: 10.2106/JBJS.E.00298. [DOI] [PubMed] [Google Scholar]
- 15.Chen YP, Pope S, Tyler D, Warren GL. Effectiveness of constraint-induced movement therapy on upper-extremity function in children with cerebral palsy: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2014;28:939–53. doi: 10.1177/0269215514544982. [DOI] [PubMed] [Google Scholar]
- 16.Wallen M, Stewart K. Grading and quantification of upper extremity function in children with spasticity. Semin Plast Surg. 2016;30:5–13. doi: 10.1055/s-0035-1571257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnefoy-Mazure A, Sagawa Y, Jr, Lascombes P, De Coulon G, Armand S. A descriptive analysis of the upper limb patterns during gait in individuals with cerebral palsy. Res Dev Disabil. 2014;35:2756–65. doi: 10.1016/j.ridd.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Buckon CE, Sienko Thomas S, Aiona MD, Piatt JH. Assessment of upper-extremity function in children with spastic diplegia before and after selective dorsal rhizotomy. Dev Med Child Neurol. 1996;38:967–75. doi: 10.1111/j.1469-8749.1996.tb15057.x. [DOI] [PubMed] [Google Scholar]
- 19.Hurley DS, Sukal-Moulton T, Msall ME, Gaebler-Spira D, Krosschell KJ, Dewald JP. The cerebral palsy research registry: development and progress toward national collaboration in the United States. J Child Neurol. 2011;26:1534–41. doi: 10.1177/0883073811408903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliasson AC, Krumlinde-Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–54. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 21.Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND-Kids: a measure of manual ability in children with cerebral palsy. Neurology. 2004;63:1045–52. doi: 10.1212/01.wnl.0000138423.77640.37. [DOI] [PubMed] [Google Scholar]
- 22.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 23.Portnoy L, Watkins M. Foundations of clinical research: applications to practice. Upper Saddle River, NJ: Prentice Hall; 2008. [Google Scholar]
- 24.Wagner LV, Davids JR, Hardin JW. Selective Control of the Upper Extremity Scale: validation of a clinical assessment tool for children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2016;58:612–7. doi: 10.1111/dmcn.12949. [DOI] [PubMed] [Google Scholar]
- 25.Gordon AM. What does selective motor control of the upper extremity in cerebral palsy tell us? Dev Med Child Neurol. 2016;58:536–7. doi: 10.1111/dmcn.13028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Test of Arm Selective Control administration manual.
Figure S1: Scatterplots of TASC scores versus ABILHAND-Kids Logit scores.
Table SI: Comparison between Test of Arm Selective Control and Selective Control of Upper Extremity Scale.
Video S1: Video of Test of Arm Selective Control administration with an adolescent with typical development (intact selective voluntary motor control) to demonstrate standardized instructions and testing procedures.


