Abstract
Background
Small tumor diagnostic tools including ultrasound-guided fine-needle aspiration (US-guided FNA) and computed tomography (CT) could be causing rising and racially/ethnically different thyroid cancer incidence rates due to variable overdiagnosis of indolent tumors. Papillary tumors and <40mm tumors are most likely to be overdiagnosed as indolent tumors by FNA and CT.
Methods
Age-adjusted incidence rates (AAIRs) for years 2007-2014 were calculated for race/ethnicity (white, Hispanic, Asian, African American, Native American) by patient/tumor characteristics for microscopically confirmed malignant thyroid cancer cases in SEER 18 (N=93,607). Multivariate analysis determined cancer patients’ odds ratios (ORs) of diagnosis with papillary thyroid carcinoma (vs. other histologies) and tumors <40mm (vs. ≥40mm).
Results
For both males and females, there were statistically significant differences in incidence rates between race/ethnicity with whites having the highest AAIRs and African Americans the lowest AAIRs. Among thyroid cancer patients, tumor size and histology differed significantly by race and insurance coverage, after controlling for gender, age, stage, and tumor sequence. Non-whites with thyroid cancer (vs. whites) were less associated with small tumors (ORs 0.51-0.79; all P<0.0001). Medicaid and uninsured patients with thyroid cancer were less associated with tumors <40mm (OR 0.55-0.71, 95% CI=0.49-0.76) and papillary carcinoma (OR 0.86, 95% CI=0.80-0.93)
Conclusion
Diagnosis of small tumors is occurring at greater rates in whites (relative to non-whites) and insured (vs. Medicaid and uninsured) patients; consequently, these groups may be vulnerable to unnecessary tests and treatments, or potentially aided with early detection. Guidelines may be needed that define post-detection interventions to limit overtreatment of indolent, small-sized, papillary carcinomas.
Keywords: Thyroid neoplasms; Tomography; Fine-Needle; Multivariate Analysis; Carcinoma, Papillary; Insurance Coverage; Medicaid; African American; Hispanic American; Indians, North American
Introduction
Within the United States, thyroid cancer is the eighth most common cancer with an estimated 56,870 new diagnoses in 2017 and an estimated prevalence of 726,646 confirmed persons living with thyroid cancer in 2014.1,2 While thyroid cancer makes up just 3.4% of new cancers diagnosed, incidence rates have increased dramatically over the last thirty years in all age groups making thyroid cancer rates an important health concern.3,4 Epidemiologists believe this increase will lead to thyroid cancer becoming the fourth most prevalent cancer in the United States by 2030.3,5 The etiology of increasing incidence in the United States is unknown.6–8 Worldwide risk factors for thyroid cancer include inadequate and excess dietary iodine, thyroid problems (goiter, benign-nodules, adenomas), and radiation exposure.3,7–9
Researchers have attributed rising rates of thyroid cancer in the United States to the improvement of diagnostic techniques, specifically ultrasound-guided fine-needle aspiration (US-guided FNA) and computed tomography (CT).3,10 The introduction of US-guided FNA into the healthcare system of the United States in the 1990s provided a new ability to detect tumors not easily discovered by palpation.3,10 According to the AJCC 8th edition, stage I tumors are <40mm and have no metastasis outside of the thyroid; thus, they should be the most difficult to detect by palpation alone.11–13 Stage I tumors (vs. stages II-IV) and papillary carcinoma (vs. medullary, follicular, anaplastic) are currently the two categories of thyroid cancer that are most overdiagnosed by US-guided FNA.14 Due to potential unequal access to US-guided FNA, overdiagnosis (i.e., detecting tumors that have not produced clinical symptoms and may never become a health concern) may vary between populations in that some populations have greater access to preventive healthcare and therefore greater access to early-detection diagnostic techniques.
Diagnosis of small tumors through greater access to technology could be beneficial in some cases because it may detect early cancers before metastasis and local expansion. On the other hand, overdiagnosis with this technology could be harmful since it may lead to unnecessary biopsy, surgery, radiation, and interventions, all of which have associated health risks. Although many researchers have suggested overdiagnosis is the cause of rising thyroid cancer incidence rates in the United States, epidemiologists have yet to rule out an actual increase in incidence rates, and furthermore have suggested alternate explanations for the rising rates, including biological or genetic factors, environmental exposure, comorbidity, and lack of treatment adherence.3,4,9,15–20
Thyroid cancer incidence varies by race/ethnicity.18,20 According to SEER 18, from 2000-2014, whites had the highest estimated thyroid cancer incidence rate followed by Hispanics, Asians, Native Americans, and African Americans.21 In contrast, many other cancer sites, such as colorectal, gastric, head and neck, lung, and prostate, have racial/ethnic disparities that show the highest incidence rates in African Americans.18,22 Descriptive papers have suggested that thyroid cancer’s racial/ethnic differences could have arisen from dissimilar access to US-guided FNA and CT, a manifestation of variances in socioeconomic status and insurance coverage.10,23–26 If this is true, age-adjusted incidence rates (AAIRs) and odds ratios (ORs) would vary by race/ethnicity primarily in stage I papillary carcinomas that are <40 mm in size. The purpose of this study is to investigate recent age-adjusted incidence rates (AAIRs) of thyroid cancer by race/ethnicity across tumor and patient characteristics to determine their potential relationship to diagnostic technology, and to exam the association between race/ethnicity and tumor size and histology among patients with thyroid cancer.
Materials and Methods
Using the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 18 Registries Database program, AAIRs were calculated for each racial/ethnic group from 2007-2014.27 Race/ethnicity was categorized into 5 groups: white Hispanics (hereby referred to as Hispanic), white non-Hispanic (hereby referred to as white), Black non-Hispanic (hereby referred to as African American), Asian and Pacific Islander (hereby referred to as Asian), and Alaskan Native/American Indian (hereby referred to as Native American). SEER 18 provides cancer surveillance information for about 28% of the United States’ population. Race/ethnicity-specific AAIRs were calculated for microscopically confirmed, malignant thyroid cancers diagnosed from 2007-2014. Patients aged 0-14 years were excluded due to low incidence. Thyroid cancer AAIRs were calculated in relation to other demographic and cancer characteristic variables. With quality control as a focus of the SEER Program, annual evaluations have been conducted in each SEER registry to insure quality and completeness of data collection being reported.28
The histology codes were grouped into five categories using ICD-O-3 codes: papillary carcinoma (8050, 8260, 8340-8344, 8450, 8452, 8453, 8460), follicular carcinoma (8290, 8330-8332, 8335), medullary carcinoma (8345-8347, 8510), anaplastic carcinoma (8012, 8020-8021, 8030-8032), and other (thyroid cancers of other histologies). Tumor stage codes (I, II, III, IV) were grouped based on the Derived American Joint Committee on Cancer (AJCC) 6th edition (2004+), while tumor size codes (0-19mm, 20-29mm, 30-39mm, 40-49mm, 50-59mm, 60+mm) were grouped on the new AJCC 8th edition cutoff of 40mm (for stage I tumors).13,14 Tumor size codes 990, 991, and 992 were included in 0-19mm; 993 in 20-29mm; 994 in 30-39mm; and 995 in 40-49mm. Tumor size code 996 was excluded. Known age at diagnosis categories were 15-39, 40-54, 55-64, 65-74, 75-84, and ≥85 years. Insurance coverage was grouped as insured (private and Medicare), Medicaid, and uninsured. Incidence rates were also calculated for tumor sequence number at diagnosis where thyroid cancer was either the first cancer diagnosed or was the second or otherwise (hereby referred to as second and higher) diagnosed.
The county level variables, poverty and education, were also included in our multivariate analysis as proxy socioeconomic variables. The poverty variable was based on the county in which subjects resided at the time of their thyroid cancer diagnosis, and each county was categorized as having <20% or ≥20% of the population living below 200% of the poverty level based on the Census American Community Survey (ACS) 5-year files for 2010-2014. Similarly, the education variable was based on county, and categorized each county as having <10% vs. ≥10% of the population having less than a high school education.
Population-based AAIRs and 95% confidence intervals were calculated for the 8-year period of 2007-2014 using the 2000 United States’ standard population reported per 100,000 person-years. Data and software used to obtain these measures for patient, tumor, and socioeconomic variables were provided through SEER*Stat version 8.3.4.27 Thyroid cancer AAIRs were only reported if at least 25 patients existed within a category.
Multivariate logistic regression analysis via SAS (version 9.4, SAS Institute, Cary, NC) was used to calculate odds ratios (ORs) to determine the independent factors associated with tumor size and histology among patients with thyroid cancer. Model 1 compared the factors associated with diagnosis of papillary carcinoma, the least lethal and smallest carcinoma that saw improved diagnosis with US-guided FNA, versus diagnosis with follicular, medullary, and anaplastic carcinomas.29–32 Model 2 compared the factors associated with diagnosis of tumors <40mm, the size range whose diagnosis is aided by US-guided FNA and is within the stage I cancer range in AJCC 8th edition, compared to tumors ≥40mm.13,17 Results examined the associations between race/ethnicity and tumor size/histology of thyroid cancer patients while adjusting for age, sex, insurance status, tumor sequence at diagnosis, poverty level of county, and education level of county. These models also included bivariate interaction terms. Missing data and groupings of less than 25 patients were excluded from the multivariate analysis. Statistical significance was set at p-value <0.05. The c-statistic was used to select the models presented herein.
The University of Iowa Human Subjects’ Office determined this study did not meet their criteria for human subjects’ research because it was limited to the analysis of de-identified data.
Results
Between 2007 and 2014, there were 93,607 microscopically confirmed, malignant thyroid cancers diagnosed in the United States with the majority involving whites, followed by Hispanics, Asians, African Americans, and Native Americans (68%, 15%, 10%, 7%, 1%, respectively) (Table 1). Race/ethnicity was statistically different over time in chi-square tests producing p-values <0.0001. The AAIRs per 100,000 person-years increased for all races over the eight-year period (Table 2). whites (15.1) had the highest incidence rates followed by Asians (13.6), Hispanics (12.2), Native Americans (10.1), and African Americans (8.4).
Table 1.
Thyroid Cancer Frequency and Percent Distributions for Tumor and Patient Characteristics by Race/Ethnicity, SEER18 (2007-2014)
| Total | White | African American | Asian | Hispanic | Native American | Determined by chi-squared test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| N | N | % | N | % | N | % | N | % | N | % | |||
| 93,607 | 63,277 | 68 | 6,389 | 7 | 9,532 | 10 | 13,878 | 15 | 531 | 1 | |||
|
| |||||||||||||
| Year of Diagnosis | 2007 | 9495 | 6639 | 10 | 604 | 9 | 920 | 10 | 1291 | 9 | 41 | 8 | <0.0001 |
| 2008 | 10491 | 7305 | 12 | 654 | 10 | 1003 | 11 | 1474 | 11 | 55 | 10 | ||
| 2009 | 11404 | 7818 | 12 | 765 | 12 | 1140 | 12 | 1620 | 12 | 61 | 11 | ||
| 2010 | 11532 | 7820 | 12 | 780 | 12 | 1178 | 12 | 1683 | 12 | 71 | 13 | ||
| 2011 | 12117 | 8222 | 13 | 851 | 13 | 1232 | 13 | 1748 | 13 | 64 | 12 | ||
| 2012 | 12545 | 8371 | 13 | 830 | 13 | 1328 | 14 | 1932 | 14 | 84 | 16 | ||
| 2013 | 12935 | 8576 | 14 | 895 | 14 | 1400 | 15 | 1989 | 14 | 75 | 14 | ||
| 2014 | 13088 | 8526 | 13 | 1010 | 16 | 1331 | 14 | 2141 | 15 | 80 | 15 | ||
|
| |||||||||||||
| Age (years) | 15–29 | 8697 | 5379 | 9 | 442 | 7 | 827 | 9 | 1981 | 14 | 68 | 13 | <0.0001 |
| 30–44 | 24750 | 15362 | 24 | 1682 | 26 | 2933 | 31 | 4604 | 33 | 169 | 32 | ||
| 45–54 | 22248 | 15118 | 24 | 1556 | 24 | 2228 | 23 | 3230 | 23 | 116 | 22 | ||
| 55–64 | 19088 | 13637 | 22 | 1464 | 23 | 1752 | 18 | 2139 | 15 | 96 | 18 | ||
| 65–74 | 12261 | 8889 | 14 | 872 | 14 | 1167 | 12 | 1281 | 9 | 52 | 10 | ||
| 75–84 | 5272 | 3906 | 6 | 294 | 5 | 504 | 5 | 542 | 4 | 26 | 5 | ||
| ≥85 | 1291 | 986 | 2 | 79 | 1 | 121 | 1 | 101 | 1 | 4 | 1 | ||
|
| |||||||||||||
| Gender | Male | 22682 | 16720 | 26 | 1205 | 19 | 2056 | 22 | 2585 | 19 | 116 | 22 | <0.0001 |
| Female | 70925 | 46557 | 74 | 5184 | 81 | 7476 | 78 | 11293 | 81 | 415 | 78 | ||
|
| |||||||||||||
| Insurance Status | Insured | 80312 | 57034 | 90 | 4964 | 78 | 7944 | 83 | 10019 | 72 | 351 | 66 | <0.0001 |
| Medicaid | 8303 | 3483 | 6 | 929 | 15 | 1105 | 12 | 2635 | 19 | 151 | 28 | ||
| Uninsured | 2474 | 1019 | 2 | 303 | 5 | 253 | 3 | 892 | 6 | 7 | 1 | ||
| Unknown | 2518 | 1741 | 3 | 193 | 3 | 230 | 2 | 332 | 2 | 22 | 4 | ||
|
| |||||||||||||
| Histology at Diagnosis | Papillary | 82445 | 55692 | 88 | 5218 | 82 | 8597 | 90 | 12474 | 90 | 464 | 87 | <0.0001 |
| Follicular | 6707 | 4555 | 7 | 746 | 12 | 547 | 6 | 815 | 6 | 44 | 8 | ||
| Medullary | 1659 | 1171 | 2 | 151 | 2 | 99 | 1 | 230 | 2 | 8 | 2 | ||
| Anaplastic | 801 | 541 | 1 | 63 | 1 | 93 | 1 | 100 | 1 | 4 | 1 | ||
| Other | 1995 | 1318 | 2 | 211 | 3 | 196 | 2 | 259 | 2 | 11 | 2 | ||
|
| |||||||||||||
| AJCC 6th Ed. Stage at Diagnosis | I | 62314 | 42320 | 67 | 4212 | 66 | 6085 | 64 | 9352 | 67 | 345 | 65 | <0.0001 |
| II | 7136 | 5103 | 8 | 619 | 10 | 571 | 6 | 806 | 6 | 37 | 7 | ||
| III | 11680 | 7878 | 12 | 716 | 11 | 1325 | 14 | 1701 | 12 | 60 | 11 | ||
| IV | 7830 | 4948 | 8 | 474 | 7 | 1020 | 11 | 1333 | 10 | 55 | 10 | ||
| Unknown/NA | 4647 | 3028 | 5 | 368 | 6 | 531 | 6 | 686 | 5 | 34 | 6 | ||
|
| |||||||||||||
| Tumor Size at Diagnosis (mm) | 0–19 | 56660 | 39943 | 63 | 3475 | 54 | 5525 | 58 | 7448 | 54 | 269 | 51 | <0.0001 |
| 20–29 | 13932 | 9103 | 14 | 817 | 13 | 1560 | 16 | 2361 | 17 | 91 | 17 | ||
| 30–39 | 7782 | 4929 | 8 | 555 | 9 | 857 | 9 | 1391 | 10 | 50 | 9 | ||
| 40–49 | 4610 | 2849 | 5 | 428 | 7 | 485 | 5 | 811 | 6 | 37 | 7 | ||
| 50–59 | 2639 | 1574 | 2 | 248 | 4 | 309 | 3 | 486 | 4 | 22 | 4 | ||
| ≥60 | 3670 | 2123 | 3 | 492 | 8 | 348 | 4 | 679 | 5 | 28 | 5 | ||
| Unknown | 4314 | 2756 | 4 | 374 | 6 | 448 | 5 | 702 | 5 | 34 | 6 | ||
|
| |||||||||||||
| Tumor Sequence | First Cancer | 80955 | 53763 | 85 | 5576 | 87 | 8577 | 90 | 12575 | 91 | 464 | 87 | <0.0001 |
| Second Cancer | 12652 | 9514 | 15 | 813 | 13 | 955 | 10 | 1303 | 9 | 67 | 13 | ||
|
| |||||||||||||
| Poverty County | <20% | 7519 | 6233 | 10 | 300 | 5 | 476 | 5 | 502 | 4 | 8 | 2 | <0.0001 |
| ≥20% | 86088 | 57044 | 90 | 6089 | 95 | 9056 | 95 | 13376 | 96 | 523 | 98 | ||
|
| |||||||||||||
| Education County | <10% | 24642 | 19500 | 31 | 1018 | 16 | 2574 | 27 | 1324 | 10 | 226 | 43 | <0.0001 |
| ≥10% | 68949 | 43767 | 69 | 5371 | 84 | 6958 | 73 | 12548 | 90 | 305 | 57 | ||
Data were missing for 16 patients
Abbreviations: AJCC, American Joint Committee on Cancer; NA, not available; SEER 18, Surveillance, Epidemiology, and End Results Program 18 database
Table 2.
Thyroid Cancer Age-Adjusted Incidence Rates (AAIRs) per 100,000 Person-Years for Tumor and Patient Characteristics by Race/Ethnicity, SEER18 (2007-2014)
| White | Africian American |
Asian | Hispanic | Native American | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Characteristic | Category | AAIR | 95% CI | AAIR | 95% CI | AAIR | 95% CI | AAIR | 95% CI | AAIR | 95% CI |
| Year of Diagnosis | 2007–2014 | 15.1 | 15.0–15.3 | 8.4 | 8.2–8.6 | 13.6 | 13.3–13.8 | 12.2 | 12.0–12.5 | 10.1 | 9.2–11.0 |
| 2007–2010 | 14.2 | 14.1–14.4 | 7.6 | 7.3–7.9 | 12.9 | 12.5–13.3 | 11.4 | 11.1–11.7 | 8.8 | 7.7–10.1 | |
| 2011–2014 | 16.0 | 15.9–16.2 | 9.1 | 8.8–9.5 | 14.2 | 13.8–14.6 | 13.0 | 12.7–13.3 | 11.3 | 10.0–12.6 | |
|
| |||||||||||
| Age (years) | 15–39 | 12.9 | 12.7–13.1 | 5.0 | 4.8–5.3 | 10.3 | 9.9–10.7 | 8.8 | 8.6–9.1 | 9.4 | 8.1–10.9 |
| 40–54 | 24.8 | 24.4–25.1 | 13.4 | 12.9–14.0 | 22.3 | 21.6–23.1 | 19.0 | 18.5–19.6 | 14.5 | 12.4–16.9 | |
| 55–64 | 25.9 | 25.4–26.3 | 7.8 | 16.9–18.7 | 23.5 | 22.4–24.6 | 22.7 | 21.7–23.7 | 16.0 | 12.9–19.5 | |
| 65–74 | 27.1 | 26.5–27.6 | 20.2 | 18.9–21.6 | 27.1 | 25.6–28.7 | 26.9 | 25.4–28.4 | 17.3 | 12.9–22.7 | |
| 75–84 | 20.1 | 19.4–20.7 | 13.9 | 12.4–15.6 | 21.5 | 19.7–23.5 | 21.9 | 20.1–23.8 | 19.2 | 12.5–28.1 | |
| ≥85 | 10.8 | 10.2–11.5 | 10.1 | 8.0–12.5 | 13.7 | 11.4–16.4 | 12.1 | 9.8–14.7 | |||
|
| |||||||||||
| Gender | Male | 7.9 | 7.8–8.0 | 3.6 | 3.4–3.9 | 6.4 | 6.2–6.7 | 5.1 | 4.9–5.4 | 4.5 | 3.7–5.5 |
| Female | 22.4 | 22.2–22.6 | 12.5 | 12.1–12.8 | 19.8 | 19.3–20.2 | 19.3 | 18.9–19.6 | 15.2 | 13.7–16.8 | |
|
| |||||||||||
| Insurance Status | Insured | 13.6 | 13.5–13.7 | 6.6 | 6.4–6.7 | 11.2 | 11.0–11.5 | 8.8 | 8.7–9.0 | 6.7 | 6.0–7.5 |
| Medicaid | 0.9 | 0.8–0.9 | 1.2 | 1.1–1.3 | 1.7 | 1.6–1.8 | 2.4 | 2.3–2.5 | 2.8 | 2.4–3.3 | |
| Uninsured | 0.3 | 0.2–0.3 | 0.4 | 0.3–0.4 | 0.3 | 0.3–0.4 | 0.7 | 0.7–0.8 | |||
| Unknown | 0.4 | 0.4–0.4 | 0.3 | 0.2–0.3 | 0.3 | 0.3–0.4 | 0.3 | 0.3–0.3 | |||
|
| |||||||||||
| Histology at Diagnosis | Papillary | 13.4 | 13.3–13.5 | 6.8 | 6.6–7.0 | 12.1 | 11.9–12.4 | 10.8 | 10.6–11.0 | 8.8 | 8.0–9.6 |
| Follicular | 0.6 | 0.6–0.6 | 0.4 | 0.4–0.5 | 0.4 | 0.4–0.5 | 0.4 | 0.4–0.5 | 0.5 | 0.3–0.8 | |
| Medullary | 0.3 | 0.3–0.3 | 0.2 | 0.2–0.2 | 0.1 | 0.1–0.2 | 0.2 | 0.2–0.3 | |||
| Anaplastic | 0.1 | 0.1–0.1 | 0.1 | 0.1–0.1 | 0.2 | 0.1–0.2 | 0.1 | 0.1–0.2 | |||
|
| |||||||||||
| AJCC 6th Ed. Stage at Diagnosis | I | 14.9 | 14.7–15.1 | 6.5 | 6.3–6.8 | 12.3 | 11.9–12.7 | 10.3 | 10.0–10.5 | 10.5 | 9.2–11.9 |
| II | 1.9 | 1.8–1.9 | 1.4 | 1.2–1.5 | 1.4 | 1.2–1.5 | 1.5 | 1.4–1.7 | 1.6 | 1.1–2.4 | |
| III | 3.1 | 3.0–3.2 | 1.9 | 1.7–2.1 | 3.9 | 3.6–4.1 | 3.6 | 3.4–3.8 | 2.3 | 1.6–3.2 | |
| IV | 1.8 | 1.8–1.9 | 1.3 | 1.1–1.4 | 2.8 | 2.6–3.0 | 3.0 | 2.8–3.2 | 2.3 | 1.6–3.1 | |
| Unknown/NA | 0.7 | 0.6–0.7 | 0.5 | 0.4–0.6 | 0.8 | 0.7–0.8 | 0.7 | 0.6–0.7 | 0.7 | 0.4–0.9 | |
|
| |||||||||||
| Tumor Size at Diagnosis (mm) | 0–19 | 11.8 | 11.7–12.0 | 5.5 | 5.3–5.7 | 9.7 | 9.5–10.0 | 8.8 | 7.9–8.3 | 6.1 | 5.4–7.0 |
| 20–29 | 2.8 | 2.8–2.9 | 1.3 | 1.2–1.4 | 2.8 | 2.7–2.9 | 2.5 | 2.4–2.6 | 2.2 | 1.7–2.7 | |
| 30–39 | 1.5 | 1.5–1.6 | 0.9 | 0.8–1.0 | 1.5 | 1.4–1.7 | 1.5 | 1.4–1.6 | 1.1 | 0.8–1.5 | |
| 40–49 | 0.9 | 0.8–0.9 | 0.7 | 0.6–0.8 | 0.9 | 0.8–1.0 | 0.9 | 0.8–0.9 | 0.9 | 0.8–1.2 | |
| 50–59 | 0.5 | 0.4–0.5 | 0.4 | 0.4–0.5 | 0.6 | 0.5–0.6 | 0.6 | 0.5–0.6 | |||
| ≥60 | 0.6 | 0.6–0.6 | 0.9 | 0.8–1.0 | 0.7 | 0.6–0.7 | 0.9 | 0.8–0.9 | 0.7 | 0.5–1.0 | |
| Unknown | 0.6 | 0.6–0.7 | 0.7 | 0.6–0.7 | 0.7 | 0.6–0.7 | 0.5 | 0.5–0.6 | 0.7 | 0.5–1.0 | |
|
| |||||||||||
| Tumor Sequence | First Cancer | 13.1 | 13.0–13.2 | 7.3 | 7.1–7.5 | 12.2 | 11.9–12.4 | 10.8 | 10.6–11.0 | 8.7 | 7.9–9.5 |
| Second Cancer | 2.0 | 2.0–2.1 | 1.1 | 1.1–1.3 | 1.4 | 1.3–1.5 | 1.4 | 1.3–1.5 | 1.4 | 1.1–1.8 | |
Abbreviations: AJCC, American Joint Committee on Cancer; AAIR, age-adjusted incidence rate; CI, confidence interval; NA, not available; SEER 18, Surveillance, Epidemiology, and End Results Program 18 database. AAIRs are not provided if there were <25 cases available to use in the calculation.
For both males and females, there were statistically significant differences in incidence rates between race/ethnicity with whites having the highest AAIRs and African Americans the lowest AAIRs (Table 2). The ratio of female to male rates was 3:1 to 4:1 in each race/ethnicity group. The female and male AAIRs decreased from whites (22.4; 7.9), Asians (19.8; 6.4), Hispanics (19.3; 5.1),Native Americans (15.2; 4.5), to African Americans (12.5; 3.6), respectively.
For those diagnosed from the ages of 15-54 years, there were statistically significant differences in incidence rates decreasing in order from white to Asian, Hispanic, Native American, and African American. For those diagnosed from 55 to 84 years old, African Americans maintained significantly lower rates than all other race/ethnicity groups. For ≥85, there were no statistically significant differences in incidence rates between races/ethnicities.
AAIRs varied significantly between race/ethnicity groups for histology (papillary, follicular, medullary). Papillary carcinoma incidence rates among all races/ethnicities varied far more widely by race/ethnicity compared to all other histologies, with whites having the highest AAIR (13.4), and African Americans having the lowest (6.8) (Table 2). With regard to follicular and medullary carcinoma, whites had statistically higher AAIRs than Hispanics, Asians, or African Americans.
AAIRs varied significantly by tumor size at diagnosis (0-39mm) and tumor stage at diagnosis (I-IV) (Table 2). Whites had a higher incidence rate than all other races when looking at tumors ≤20mm. For tumors 20mm-39mm, whites had significantly higher incidence than African Americans. When tumors were ≥40mm, there were no statistically significant differences between whites and the other races.
Stage I AAIRs decreased from white (14.9) to Asian (12.3), Native American (10.5), and Hispanic (10.3), to African American (6.5). While whites had significantly higher incidence for stage II, Asians and Hispanics had significantly higher incidence rates for stages III and IV.
Incidence rates varied significantly by race/ethnicity for insured and Medicaid patients. AAIRs (white, African American, Asian, Hispanic) for the insured patients were over 6-fold greater than AAIRs for patients with Medicaid or no insurance. Among insured persons, whites had significantly greater AAIRs of thyroid cancer than other race/ethnicity groups (Figure 1). Conversely, white Medicaid patients had significantly lower AAIRs than all other race/ethnicity Medicaid patients. Hispanics had the greatest AAIRs among those uninsured; otherwise, there was no significant difference between the AAIRs of the other races/ethnicities, including whites and African Americans, among those uninsured.
Figure 1.
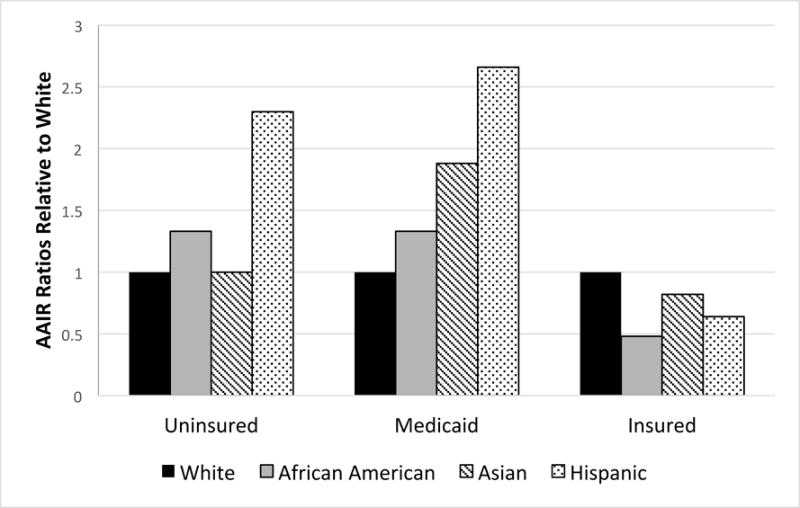
Thyroid cancer age-adjusted incidence rate ratios of each race/ethnicity relative to whites by insurance coverage for SEER18 (2007-2014)
AAIRs of thyroid cancer as a first cancer decreased over an almost 2-fold range across race/ethnicity with whites (13.1) having the highest and African Americans the lowest (7.3). As a second cancer, the incidence rates of thyroid cancer were also greatest in whites (2.0) and lowest in African Americans (1.1).
The multivariate analysis of patients with thyroid cancer (Table 3) showed race/ethnicity groups vary (P<0.0001) in their odds of being diagnosed with papillary carcinoma (compared to medullary, anaplastic, and follicular) and small tumors (<40mm) (vs. large tumors). Compared to whites, Asians and Hispanics had 40% and 37%, greater odds of being diagnosed with papillary thyroid carcinoma, respectively, while African Americans had 28% lower odds. Non-whites were a quarter to a half less likely to have smaller tumors (<40mm) compared to whites.
Table 3.
Multivariate Analysis of Thyroid Cancer Patients with Papillary Carcinoma or Tumors <40mm for Tumor and Patient Characteristics, SEER 18 (2007-2014)
| DISTRIBUTION | MODEL 1: PAPILLARY (vs. Other Histologies) | MODEL 2: TUMOR <40mm (vs. ≥40mm) | |||||
|---|---|---|---|---|---|---|---|
| (N=93,607) | (N=93,607) | (N=89,293) | |||||
|
|
|||||||
| N | % | OR* | 95% CI | OR* | 95% CI | ||
| Race | White | 63277 | 68 | 1.00 | Referent | 1.00 | Referent |
| African American | 6389 | 7 | 0.72 | (0.67, 0.78) | 0.51 | (0.47, 0.55) | |
| Asian | 9532 | 10 | 1.40 | (1.29, 1.51) | 0.79 | (0.73, 0.85) | |
| Hispanic | 13878 | 15 | 1.37 | (1.28, 1.47) | 0.64 | (0.60, 0.68) | |
| Native American | 531 | 1 | 1.16 | (0.88, 1.54) | 0.58 | (0.46, 0.75) | |
|
| |||||||
| Year of Diagnosis | 2007 | 9495 | 10 | 1.00 | Referent | 1.00 | Referent |
| 2008 | 10491 | 11 | 1.00 | (0.91, 1.09) | 1.07 | (0.98, 1.17) | |
| 2009 | 11404 | 12 | 0.99 | (0.91, 1.08) | 1.10 | (1.01, 1.21) | |
| 2010 | 11532 | 12 | 1.10 | (1.01, 1.20) | 1.00 | (0.91, 1.09) | |
| 2011 | 12117 | 13 | 1.15 | (1.06, 1.26) | 1.06 | (0.97, 1.16) | |
| 2012 | 12545 | 13 | 1.23 | (1.12, 1.34) | 1.08 | (0.99, 1.18) | |
| 2013 | 12935 | 14 | 1.30 | (1.19, 1.42) | 1.08 | (0.99, 1.18) | |
| 2014 | 13088 | 14 | 1.28 | (1.17, 1.39) | 1.03 | (0.95, 1.13) | |
|
| |||||||
| Age (years) | 15–29 | 8697 | 9 | 2.89 | (2.47, 3.38) | 2.46 | (2.09, 2.90) |
| 30–44 | 24750 | 26 | 2.76 | (2.39, 3.19) | 3.70 | (3.16, 4.33) | |
| 45–54 | 22248 | 24 | 2.85 | (2.49, 3.26) | 4.35 | (3.72, 5.09) | |
| 55–64 | 19088 | 20 | 2.41 | (2.11, 2.76) | 4.20 | (3.59, 4.92) | |
| 65–74 | 12261 | 13 | 1.77 | (1.55, 2.03) | 3.13 | (2.67, 3.67) | |
| 75–84 | 5272 | 6 | 1.46 | (1.27, 1.68) | 1.88 | (1.59, 2.22) | |
| ≥80 | 1291 | 1 | 1.00 | Referent | 1.00 | Referent | |
|
| |||||||
| Gender | Male | 22682 | 24 | 1.00 | Referent | 1.00 | Referent |
| Female | 70925 | 76 | 1.05 | (1.00, 1.10) | 2.42 | (2.31, 2.53) | |
|
| |||||||
| Insurance Status | Insured | 80312 | 86 | 1.00 | Referent | 1.00 | Referent |
| Medicaid | 8303 | 9 | 0.86 | (0.80, 0.93) | 0.71 | (0.66, 0.76) | |
| Uninsured | 2474 | 3 | 0.93 | (0.82, 1.06) | 0.55 | (0.49, 0.62) | |
| Unknown | 2518 | 3 | 0.88 | (0.79, 1.00) | 1.01 | (0.86, 1.18) | |
|
| |||||||
| Histology at Diagnosis | Papillary | 82445 | 88 | – | – | 1.00 | Referent |
| Follicular | 6707 | 7 | – | – | 0.18 | (0.17, 0.19) | |
| Medullary | 1659 | 2 | – | – | 0.49 | (0.43, 0.56) | |
| Anaplastic | 801 | 1 | – | – | 0.03 | (0.02, 0.04) | |
| Other | 1995 | 2 | – | – | 0.18 | (0.16, 0.21) | |
|
| |||||||
| AJCC 6th Ed. Stage at Diagnosis | I | 62314 | 67 | 2.17 | (1.99, 2.36) | – | – |
| II | 7136 | 8 | 0.87 | (0.80, 0.94) | – | – | |
| III | 11680 | 12 | 2.25 | (2.08, 2.44) | – | – | |
| IV | 7830 | 8 | 1.00 | Referent | – | – | |
| Unknown/NA | 4647 | 5 | 1.55 | (1.40, 1.72) | – | – | |
|
| |||||||
| Tumor size at Diagnosis (mm) | 0–19 | 56660 | 61 | 11.85 | (10.83, 12.97) | – | – |
| 20–29 | 13932 | 15 | 5.15 | (4.70, 5.64) | – | – | |
| 30–39 | 7782 | 8 | 3.46 | (3.14, 3.81) | – | – | |
| 40–49 | 4610 | 5 | 2.08 | (1.89, 2.29) | – | – | |
| 50–59 | 2639 | 3 | 1.40 | (1.26, 1.57) | – | – | |
| ≥60 | 3670 | 4 | 1.00 | Referent | – | – | |
| Unknown | 4314 | 5 | 1.66 | (1.49, 1.86) | – | – | |
|
| |||||||
| Tumor Sequence | First Cancer | 80955 | 86 | – | – | 0.69 | (0.64, 0.74) |
| Second Cancer | 12652 | 14 | – | – | 1.00 | Referent | |
|
| |||||||
| Poverty County | <20% | 7519 | 8 | 1.00 | Referent | 1.00 | Referent |
| ≥20% | 86088 | 92 | 1.15 | (1.07, 1.25) | 0.82 | (0.75, 0.90) | |
All bold face ORs are statistically significant using a P < .05
Abbreviations: AJCC, American Joint Committee on Cancer; NA, not available; SEER 18, Surveillance, Epidemiology, and End Results Program 18 database.
A dash (—) indicates a variable that was omitted from each model.
All ORs provided were adjusted for other variables in the table.
The sample size was smaller in model 2 because 4314 cases were excluded for unknown tumor sizes.
In addition to race/ethnicity, other variables including gender, age at diagnosis, insurance status, histology, and tumor sequence were significantly associated with tumor size (Table 3). Specifically, age at diagnosis between 15 and 84 was associated with smaller tumor size (<40mm) compared to the ≥85 age group. The odds ratios of having a smaller tumor size (vs. larger tumor size) peaked in the 45-54 age range (OR, 4.35). Compared to insured patients, Medicaid patients diagnosed with thyroid cancer were less likely to have papillary carcinoma (OR, 0.86) and small tumors (OR, 0.71). Uninsured patients were also less likely to have small tumors (OR, 0.55). Females had 2.4 greater odds of being diagnosed with smaller tumors (vs. larger tumors) compared to males. Finally, second or higher tumor sequence was associated with smaller tumors compared to first cancers.
Interaction terms were tested, but minimally impacted the value of the c-statistic. The c-statistic for Model 1 was 0.788 and for Model 2 was 0.736. In counties with higher proportions of residents living below 200% of the poverty level, patients were more likely to have papillary carcinoma (OR, 1.15; 95% confidence interval [CI], 1.07-1.25) and less likely to have a small tumor (OR, 0.82; 95% CI, 0.76-0.90).
Discussion
Our analysis updates and quantifies previous descriptive studies of thyroid cancer incidence trends in the United States, particularly regarding our comparison of thyroid cancer incidence rates by race/ethnicity in the United States across personal and tumor characteristics from 2007-2014 in SEER 18, and our multivariate analysis of thyroid cancer patients diagnosed with papillary carcinoma (vs. other histologies) or with small tumors (<40mm) (vs. large tumors) from 2007-2014. Previous studies have suggested that racial/ethnic differences in AAIRs and increasing trends of AAIRs were due to dissimilarities in access to technology and resultant overdiagnosis of sub-clinical thyroid cancers.3,10,15,23 AAIRs for the period 2007-2014 showed thyroid cancer incidence trends similar to those of past studies, with whites having the highest incidence and African Americans the lowest. Consistent with previous studies, we conclude the differences in AAIRs could be due to dissimilar access to technology especially since insurance AAIRs differed greatly between whites and African Americans. The AAIRs of thyroid cancer in insured patients are highest in whites and lowest in African Americans, but AAIRs are greater in African American Medicaid patients (vs. white Medicaid patients), and there was no difference in incidence in uninsured patients.
Adding to past studies, our multivariate analysis of thyroid cancer patients found that whites (vs. non-whites) with thyroid cancer have greater odds of having tumors <40mm. This finding is likely due to variability between races in the diagnosis of indolent disease (tumors that have not begun to produce symptoms or create clinical apparent disease) with the aid of new technology, a likely result of variability between races in their access to this technology. Our findings could mean that whites with thyroid cancer are more likely to be subjected to unnecessary tests, biopsies, and medical interventions because they have higher odds (than other cancer patients) of being diagnosed when their tumors are small and possibly indolent. However, it is also possible that with greater diagnosis of small tumors, white thyroid cancer patients (vs. non-white) could potentially be having clinically significant tumors advantageously diagnosed before they metastasize or grow larger.
Given that non-papillary tumors are generally more aggressive compared to papillary tumors, it is unexpected that our regression analysis of thyroid cancer patients showed African Americans have higher odds of non-papillary thyroid cancer compared to whites, but our analysis of the general population showed that African Americans do not have higher AAIRs for more advanced stage cancer (II-IV) compared to whites. On the other hand, Asians and Hispanics with thyroid cancer have higher odds of having papillary thyroid carcinoma compared to whites, yet have higher AAIRs for more advanced stage cancer compared to whites.13,21 While it is encouraging that African Americans do not have higher rates of stage III/IV thyroid cancer compared to whites, especially given their higher odds of having non-papillary thyroid cancers, it is concerning that Asians and Hispanics are being diagnosed with higher rates of stage II/IV thyroid cancer given their higher odds of papillary carcinoma. This warrants further investigation.
All non-whites with thyroid cancer have lower odds of tumors <40mm compared to whites after controlling for other tumor and patient factors. In addition, the differences between AAIRs by tumor size became substantially more comparable between races once tumors were ≥40mm. Thus, it therefore appears that compared to non-whites, and especially to African Americans, whites have greater exposure to the healthcare system and US-guided FNA when tumors are small and likely non-palpable, but once tumors grow to a size ≥40mm and are likely palpable, the various races/ethnicities seek care more similarly to one another.
Our multivariate analysis of patients with thyroid cancer found that insured patients (compared to Medicaid) were 29% more associated with small tumors and 14% more associated with papillary carcinoma after accounting for other patient factors. Insured patients (vs. uninsured) were 45% more associated with small tumors. Within each race, insured patients had 5- to 13-fold greater AAIRs than those with Medicaid, and a 15- to 40-fold greater AAIRs than uninsured patients. Among insured patients, whites had the highest AAIRs; conversely, in the Medicaid and uninsured categories, whites had the lowest AAIRs (Figure 1). Of notice, a study conducted within the Department of Defense on members of the armed forces and their dependents, who all had similar benefits and access to health care, found no differences in subclinical detection between whites and African Americans.26 Furthermore, Hispanics without insurance had AAIRs 2-3 times greater than those of whites without insurance; while insured Hispanics had incidence rates 35% lower than insured whites. These findings suggest race/ethnicity and insurance status together affect the AAIRs of thyroid cancer.
Our multivariate analysis of thyroid cancer patients also revealed that women with thyroid cancer and second cancer patients were more likely to be diagnosed with small tumors (<40mm) compared to men. The 2013 Kaiser survey suggested that women visit healthcare providers more frequently and receive general check-ups more often than males, so it is possible that at least part of the gender difference in tumor size is related to health care system use.33 Our analysis of the general population showed that women had much higher AAIRs of thyroid cancer compared to men. Further investigation is warranted to better understand the extent to which higher incidence among females is related to health care use versus biological, autoimmune, hormonal, or genetic factors.15
Finally, our multivariate analysis of thyroid cancer patients showed that second cancer patients were more likely to be diagnosed with small tumors (<40mm). It is possible that cancer survivors and those with past tumor diagnoses could be accessing US-guided FNA and other diagnostic examinations in association with more frequent follow-up surveillance.
A limitation to our study is that SEER is retrospective and has a limited number of reportable variables; this did not allow us to examine differences in known thyroid cancer risk factors between race/ethnicity, such as radiation exposure. Furthermore, errors in reporting race and ethnicity in medical records and death certificates may underestimate incidence rates in non-white populations. Race and ethnicity categories are not homogenous; differences may exist in subgroups within each race/ethnicity and thus, overall race/ethnicity trends may not be representative of all subsets. Moreover, some categories have low patient representation, particularly the Native American category, which may lead to imprecise rates. Increased analysis of Native American thyroid cancer AAIRs is needed to gain a more complete understanding of their risk. Finally, a limitation of the study is that we had to use county level education and poverty variables.
Our study has a number of strengths and novel components. First, this analysis included more recent SEER data including insurance information that was not available prior to 2006 cases. We are therefore among the first to examine the interplay between thyroid cancer incidence, race/ethnicity and insurance coverage using population-based SEER data. We are also among the first to evaluate population-based incidence trends applying the new AJCC 8th edition tumor stage I size limits to our analysis, which allowed us to newly comment on incidence under these new guidelines. The large sample size of SEER 18 allowed for more precise risk estimates than previous studies that used SEER 9 and 12; this allowed us to include several potential covariates in our multivariate analysis.
Our analysis of the most recent years of data available in the SEER database indicates that white race and private insurance coverage are significantly associated with smaller tumors and papillary carcinoma. This suggests that greater access to US-guided FNA in some populations is a driver of race/ethnicity differences in the incidence of thyroid cancer, and may lead to increased diagnosis of thyroid cancer. Diagnosis of small tumors could leave those with greater access to US-guided FNA more vulnerable to unnecessary tests and treatments, or potentially could aid these populations with early detection before metastasis or further tumor growth. Guidelines may be needed that define post-detection interventions to limit overtreatment of indolent, small-sized, papillary carcinomas. Ongoing clinical trials evaluating active surveillance of small papillary thyroid cancers can hopefully inform these guidelines.24,34,35 In addition, educating patients about thyroid cancer overdiagnosis and the risks associated with potential overtreatment of thyroid cancer may allow patients to be more engaged in shared decision making so they can help determine their best course of action.14,34,35
Acknowledgments
Details on funding:
This project was funded in part with funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN261201300020I and by training grant 5 T32 GM007337, Medical Scientist Research Training program, University of Iowa. No conflicts of interests to disclose for any authors.
Footnotes
Author Contribution
Kristin S. Weeks: Conceptualization, data curation, methodology, writing original draft, review and edit. Amanda R. Kahl: Data curation, formal analysis, methodology, review and edit. Charles F. Lynch: Conceptualization, methodology, supervision, review andedit. Mary E. Charlton: Conceptualization, methodology, supervision, review and edit.
Conflict of Interest:
All authors have read and approved the manuscript. This manuscript is not under consideration elsewhere.
Contributor Information
Kristin S. Weeks, University of Iowa, Carver College of Medicine, Medical Scientist Training Program, MD/PhD candidate.
Amanda R. Kahl, University of Iowa College of Public Health, Department of Epidemiology.
Charles F. Lynch, University of Iowa College of Public Health, Department of Epidemiology.
Mary E. Charlton, University of Iowa College of Public Health, Department of Epidemiology.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. 2015. [DOI] [PubMed] [Google Scholar]
- 3.Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: A global overview. International Journal of Cancer. 2015;136(9):2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 4.Krook KA, Fedewa SA, Chen AY. Prognostic indicators in well differentiated thyroid carcinoma when controlling for stage and treatment. The Laryngoscope. 2015;125(4):1021–1027. doi: 10.1002/lary.25017. [DOI] [PubMed] [Google Scholar]
- 5.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research. 2014 Jun 1;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 6.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the US, 1985-1995. Cancer. 1998;83(12):2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Yu GP, Li JCL, Branovan D, McCormick S, Schantz SP. Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid. 2010;20(5):465–473. doi: 10.1089/thy.2008.0281. [DOI] [PubMed] [Google Scholar]
- 8.Meza R, Chang JT. Multistage carcinogenesis and the incidence of thyroid cancer in the US by sex, race, stage and histology. BMC public health. 2015;15(1):1–9. doi: 10.1186/s12889-015-2108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konturek A, Barczyński M, Stopa M, Nowak W. Trends in Prevalence of Thyroid Cancer Over Three Decades: A Retrospective Cohort Study of 17,526 Surgical Patients. World Journal of Surgery. 2015;40(3):538–544. doi: 10.1007/s00268-015-3322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zevallos JP, Hartman CM, Kramer JR, Sturgis EM, Chiao EY. Increased thyroid cancer incidence corresponds to increased use of thyroid ultrasound and fine-needle aspiration: A study of the Veterans Affairs health care system. Cancer. 2015;121(5):741–746. doi: 10.1002/cncr.29122. [DOI] [PubMed] [Google Scholar]
- 11.Cesur M, Corapcioglu D, Bulut S, Gursoy A, Yilmaz AE, Erdogan N, Kamel N. Comparison of palpation-guided fine-needle aspiration biopsy to ultrasound-guided fine-needle aspiration biopsy in the evaluation of thyroid nodules. Thyroid. 2006 Jun 1;16(6):555–61. doi: 10.1089/thy.2006.16.555. [DOI] [PubMed] [Google Scholar]
- 12.Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RJ, Williamson MR, Blumhardt R, Bauman JM, Tekkel M. Thyroid palpation versus high resolution thyroid ultrasonography in the detection of nodules. Journal of Ultrasound in Medicine. 1998 Aug 1;17(8):487–96. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle MR, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer: What Changed and Why? 2017:751–756. doi: 10.1089/thy.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA otolaryngology–head & neck surgery. 2014 Apr 1;140(4):317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 15.La Vecchia C, Negri E. Thyroid cancer: The thyroid cancer epidemic overdiagnosis or a real increase? Nature Reviews Endocrinology. 2017 Jun;13(6):318–319. doi: 10.1038/nrendo.2017.53. [DOI] [PubMed] [Google Scholar]
- 16.Davies L, Morris LG, Haymart M, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocrine Practice. 2015;21(6):686–696. doi: 10.4158/EP14466.DSCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Wang W. Increasing incidence of thyroid cancer in Shanghai, China, 1983-2007. Asia-Pacific Journal of Public Health. 2015;27(2):223–229. doi: 10.1177/1010539512436874. [DOI] [PubMed] [Google Scholar]
- 18.Magreni A, Bann DV, Schubart JR, Goldenberg D. The effects of race and ethnicity on thyroid cancer incidence. JAMA Otolaryngology–Head & Neck Surgery. 2015;141(4):319–323. doi: 10.1001/jamaoto.2014.3740. [DOI] [PubMed] [Google Scholar]
- 19.Keegan TH, Grogan RH, Parsons HM, et al. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid. 2015;25(6):635–648. doi: 10.1089/thy.2015.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suresh R, Sethi S, Ali S, Giorgadze T, Sarkar FH. Differential Expression of MicroRNAs in Papillary Thyroid Carcinoma and Their Role in Racial Disparity. Journal of Cancer Science & Therapy. 2015;7(5):145. doi: 10.4172/1948-5956.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fast Stat from SEER18. National Cancer Institute; Online Accessed 4 December, 2017< https://seer.cancer.gov/faststats/>. [Google Scholar]
- 22.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA: a Cancer Journal for Clinicians. 2016;66(4):290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 23.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013 Jul 1;23(7):885–91. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roche AM, Fedewa SA, Chen AY. Association of Socioeconomic Status and Race/Ethnicity with Treatment and Survival in Patients with Medullary Thyroid Cancer. JAMA Otolaryngology–Head & Neck Surgery. 2016:1–9. doi: 10.1001/jamaoto.2016.1051. [DOI] [PubMed] [Google Scholar]
- 25.Morris LG, Sikora AG, Myssiorek D, et al. The basis of racial differences in the incidence of thyroid cancer. Annual Surgery Oncology. 2008;15:1169–1176. doi: 10.1245/s10434-008-9812-6. [DOI] [PubMed] [Google Scholar]
- 26.Brown SR, Lee S, Brown TA, Waddell BE. Effect of race on thyroid cancer care in an equal access healthcare system. The American Journal of Surgery. 2010;199(5):685–689. doi: 10.1016/j.amjsurg.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 27.SEER Research Data 1973-2014 when Using SEER*Stat: Surveillance, Epidemiology, and End Results (SEER) Program. https://www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2015 Sub (1973-2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016 submission.
- 28.National Cancer Institute. SEER Quality Improvement. 2016 Sep; https://seer.cancer.gov/about/factsheets/SEER_QI_Fact_Sheet.pdf. Accessed December 4, 2017.
- 29.Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism. 2006;91(1):313–319. doi: 10.1210/jc.2005-1322. [DOI] [PubMed] [Google Scholar]
- 30.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. Cancer. 1997;79(3):564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Adam MA, Pura J, Goffredo P, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. Journal of Clinical Oncology. 2015;33(21):2370–2375. doi: 10.1200/JCO.2014.59.8391. [DOI] [PubMed] [Google Scholar]
- 32.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Annals of surgery. 2007;246(3):375–384. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salganicoff A, Ranji U, Beamesderfer A, Kurani N. Women and Health Care in the Early Years of the Affordable Care Act: Key findings from the 2013 Kaiser Women’s health survey. Report of Kaiser Family Foundation. 2014 May; Online Accessed 4 December 2017. https://kaiserfamilyfoundation.files.wordpress.com/2014/05/8590-women-and-health-care-in-the-early-years-of-the-affordable-care-act.pdf.
- 34.Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surgery. 2010;34(1):28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 35.Nikiforov Y, Seethala R, Tallini G, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma. JAMA Oncology. 2016;2(8):1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]


