Abstract
Background
Evaluate whether modifiable cardiovascular risk conditions and lifestyle factors are temporally associated with an increased risk for ischemic heart disease and overall mortality in a cohort of hematopoietic cell transplant survivors.
Methods
≥1-year hematopoietic cell transplant survivors aged ≥20 years and transplanted from 1970–2010 at a transplant referral center were surveyed in 2010–2011 about cardiovascular health and lifestyle factors (n=3,833). Respondents (n=2,360, 61.6%) were followed to 2016 for incident ischemic heart disease and overall mortality.
Results
Of 2,360 transplant survivors (median age at baseline survey and time since transplant: 55.9 and 10.8 years, respectively), 162 (6.9%) reported ischemic heart disease at the baseline survey. Among those without ischemic heart disease at the baseline survey (n=2,198), the 5-year cumulative incidence of subsequent ischemic heart disease was 4.3%. Obesity, dyslipidemia, diabetes, and physical inactivity at baseline were associated with an increased risk for subsequent ischemic heart disease (hazard ratios ≥1.8). Greater physical activity and fruits/vegetable intake at baseline were associated with subsequent lower overall mortality (hazard ratios ≤0.7). When jointly considered, each additional cardiovascular risk condition and each adverse lifestyle factor were independently associated with subsequent ischemic heart disease (hazard ratios 1.4 [95% CI 1.0–1.9] and 1.9 [95% CI 1.2–2.9], respectively), and adverse lifestyle factors remained associated with overall mortality (1.8 [95% CI 1.5–2.3]).
Conclusions
These results support strong efforts to promote healthy lifestyle behaviors and to treat cardiovascular risk factors aggressively in hematopoietic cell transplant survivors. This may reduce future ischemic heart disease and overall mortality in this high-risk population.
MeSH Keywords: healthy lifestyle, hematopoietic stem cell transplantation, long-term survivors, mortality, myocardial ischemia
INTRODUCTION
The increasing prevalence and effectiveness of hematopoietic cell transplantation (HCT) has resulted in a growing population of long-term survivors of HCT.1 Unfortunately, HCT survivors suffer numerous long-term complications from their therapy, and cardiovascular disease is among the most significant of these.2, 3 HCT survivors experience a significantly greater incidence of cardiovascular morbidity and mortality compared with the general population.4, 5 Specifically, cardiovascular mortality maybe twice as common as expected in the general population, and in 5-year HCT survivors, accounted for 7% of excess deaths, behind new malignancies or recurrence (~50% of excess deaths), late infections (18%), chronic graft versus host disease (GVHD; 12%), and respiratory disease (8%).2 Compared with the general population, HCT survivors also have a higher burden of potentially modifiable conditions such as hypertension, dyslipidemia, and diabetes that further increase the risk of subsequent serious cardiovascular disease.5–7 Our group has previously demonstrated that adverse lifestyle factors such as low physical activity, low fruit and vegetable intake, and smoking, also remain significant modifiable risk factors associated with both serious cardiovascular events such as ischemic heart disease, and related conditions such as dyslipidemia, diabetes, and/or hypertension.8 While these cross-sectional associations between lifestyle and adverse cardiovascular conditions were significant, establishing a temporal relationship would provide stronger evidence for clinicians to treat these conditions more aggressively and to promote healthy lifestyle changes in survivors. Thus, the aim of this longitudinal study was to determine whether modifiable cardiovascular risk conditions and lifestyle factors at baseline are associated with subsequent increased risk for ischemic heart disease and overall mortality among HCT survivors. Positive findings would also provide additional evidence that interventions to improve adherence to cardiovascular risk factor treatment and lifestyle modifications are important for this high-risk population.
METHODS
Study Population
All patients who have previously undergone HCT at the Fred Hutchinson Cancer Research Center (FHCRC) and have signed informed consent for research follow-up are mailed an annual health status survey on the anniversary of their transplant. Between July 2010 and September 2011, this survey contained supplemental questions focused on cardiovascular health. As previously described, 3,833 patients aged ≥20 years were surveyed during that interval and 2,360 (61.6%) responded.8 Follow-up surveys have been sent to this group annually. As of March 31, 2016, 1,921 of the 2,360 participants (81.4%) have returned at least one subsequent survey. The FHCRC HCT program maintains a database that records demographic (age, sex, race/ethnicity) and treatment-related characteristics (underlying disease, HCT conditioning regimen including total body irradiation [TBI] and cyclophosphamide exposures, donor type [autologous, allogeneic], GVHD prophylaxis [steroids, calcineurin inhibitors, rapamycin], and acute and chronic GVHD status) of all HCT recipients.
Survey Instrument and Definitions
The baseline survey (2010–2011; available upon request) asked participants to report on their history (including age of onset) of serious cardiovascular events (e.g., ischemic heart disease, cardiomyopathy/heart failure, and stroke) and related conditions (e.g., hypertension, dyslipidemia, and diabetes), current height and weight, and family history (first degree relatives) of cardiovascular disease. Using questions adapted from the Centers for Disease Control and Prevention (CDC)’s National Health and Nutrition Examination Survey,9 the baseline survey also assessed smoking and tobacco use (never, previously but no longer, and current), weekly time spent in recreational physical activity, and average daily fruit and vegetable intake.
Self-reported height and weight were converted to body-mass index (BMI) and categorized as underweight, normal, overweight, and obese (< 18.5, 18.5 to 24.9, 25.0 to 29.9, and ≥30.0 kg/m2, respectively). For recreational physical activity, the self-reported amount of time spent doing vigorous and moderate activities during the past week was summed with every two minutes of moderate activity equivalent to one minute of vigorous activity. Respondents were categorized as meeting or not meeting CDC recommendations for aerobic activities: 75 minutes per week of vigorous activity or 150 minutes per week of moderate activity, or equivalent mixture of both. We also examined physical activity time based on tertiles (respondents without any moderate or vigorous activities serving as referent). For diet, in addition to deriving the daily number of fruit and vegetable servings, respondents also were categorized as meeting (yes/no) national recommendations for at least 5 daily servings.10
Subsequent limited annual surveys assessed the development of ischemic heart disease, plus other outcomes such as new cancers and original disease recurrence, chronic GVHD (if allogeneic HCT), and infections. Vital status was prospectively tracked by FHCRC, based on annual contact with patients and families, referring providers, and periodic searches of public sources for patents without recent contact. For patients who died during the observation period, available records were reviewed to determine if death could be attributable to ischemic heart disease. The FHCRC Institutional Review Board approved all procedures.
Statistical analysis
We calculated the prevalence of baseline characteristics, adverse lifestyle factors (i.e., current smoking, <5 fruit/vegetable daily servings, not meeting CDC exercise recommendations), and cardiovascular risk conditions (i.e., hypertension, dyslipidemia, and diabetes) among the HCT survivors with and without prior ischemic heart disease. Differences between the two groups were assessed using univariate methods. The 5-year cumulative incidences of ischemic heart disease and all-cause mortality also was plotted. Limited to those without ischemic heart disease at the time of the baseline survey, a series of Cox proportional hazards models (starting at the time of the baseline survey) examined the association (hazard ratios [HR]) between each baseline cardiovascular condition or lifestyle factor with the subsequent risks of ischemic heart disease and overall mortality. Similar to our prior analysis,8 these models adjusted for sex, race/ethnicity, age at the baseline survey, HCT donor type, history of chronic GVHD, years elapsed between HCT and the baseline survey, and family history of cardiovascular and related diseases. We also separately examined the influence of TBI and cyclophosphamide conditioning, and agents used for GVHD prophylaxis.
In order to examine the cumulative effect of risk factors, variables were created that summed the number of cardiovascular risk conditions (0, 1, 2, and ≥3) and the number of adverse lifestyle factors (0, 1, and ≥2). In the analysis of ischemic heart disease, death due to other causes was treated as a competing risk event.
Given the growing role of cardiovascular risk calculators in general practice, in secondary analysis, we applied the simple non-laboratory based Framingham general cardiovascular risk scores to HCT survivors age ≥30 years at baseline.11 These risk scores were assigned based on age, sex, current smoking status, presence of obesity based on BMI, blood pressures and use of antihypertensive medications, and history of diabetes. Since we did not have actual systolic blood pressure values, we made the conservative assumption that those not treated with anti-hypertensive medications had normal blood pressures, and similarly that those treated with anti-hypertensives were well-controlled with pressures now in the normal therapeutic range. Since the number of outcomes available for analysis was limited, we categorized risk scores into <5%, 5–9%, 10–19%, and ≥20% 10-year predicted risk categories. Cox models that examined the relationship between Framingham risk score categories and subsequent ischemic heart disease and overall mortality were unadjusted because Framingham scores already account for sex and age.
All analyses were performed using STATA (version 14; STATA, College Station, TX). Global tests of proportionality based on Schoenfeld residuals ensured that proportional hazards assumptions were met (p≥0.05); covariates with nonproportional hazards were treated as time-varying.12
RESULTS
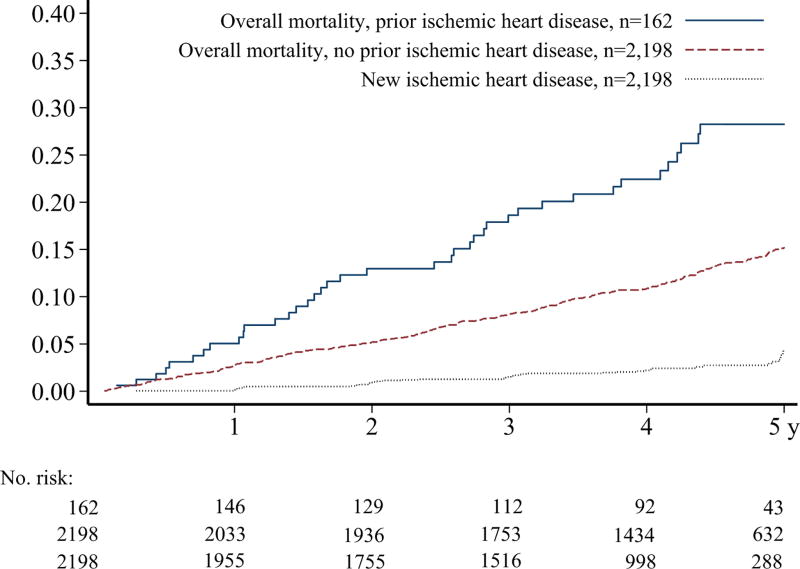
The 2,360 HCT survivors analyzed were a median of 10.8 years (range 0.9–40.4 years) since HCT at the time of our baseline cardiovascular survey. Of these, 162 (6.9%) already had a history of ischemic heart disease. Compared with those without prior ischemic heart disease at the time of the baseline survey, those affected were more often male, older at time of both transplant and baseline study assessment, and more likely to have a history of chronic GVHD (Table 1). Those with prior ischemic heart disease also had a significantly greater baseline burden of hypertension, dyslipidemia, and diabetes (all p<0.05). While baseline smoking rates and fruit/vegetable intake were similar in these two groups, those with prior ischemic heart disease were much less physically active. They also were more likely to die during follow-up (24.1% vs. 12.6%; p<0.001). At 5 years, the cumulative incidence of overall mortality was 28.2% vs. 15.2% between these two groups (Figure 1; p<0.0001 for difference). Among surviving participants, the median duration of follow-up after the baseline survey was similar between those with and without prior ischemic heart disease (4.8 vs. 4.7 years [range 0.1–5.9]).
TABLE 1.
Characteristics of Hematopoietic Cell Transplantation (HCT) Survivors With and Without Prior Ischemic Heart Disease at Baseline Survey
| Characteristics, n (%) | No prior disease N=2,198 |
With prior disease N=162 |
P-value | ||
|---|---|---|---|---|---|
| Female | 1,061 | (48) | 42 | (26) | <.001 |
| White, non-Hispanic | 1,959 | (89) | 145 | (90) | .88 |
| Median age at HCT (range), years | 43.6 | (0.4–78.8) | 48.9 | (10.0–78.9) | <.001 |
| Median age at cohort entry (range), years | 55.5 | (20.2–83.3) | 62.4 | (22.0–83.0) | <.001 |
| Original disease | .25 | ||||
| Leukemia / myelodysplastic syndrome | 1,265 | (58) | 102 | (63) | |
| Lymphoma | 481 | (22) | 26 | (16) | |
| Multiple myeloma | 234 | (11) | 19 | (12) | |
| Other cancer | 60 | (3) | 1 | (1) | |
| Non-malignant condition | 158 | (7) | 14 | (9) | |
| Donor type | .42 | ||||
| Autologous | 635 | (29) | 42 | (26) | |
| Allogeneic | 1,563 | (71) | 120 | (74) | |
| History of chronic graft vs. host disease | 967 | (62)* | 95 | (79)* | <.001 |
| Body mass index categories, kg/m2 | .046 | ||||
| <18.5 | 66 | (3) | 2 | (1) | |
| 18.5–24.9 | 945 | (44) | 55 | (34) | |
| 25–29.9 | 744 | (34) | 67 | (42) | |
| ≥30 | 411 | (19) | 36 | (23) | |
| Hypertension | 568 | (26) | 90 | (56) | <.001 |
| Dyslipidemia | 673 | (31) | 126 | (78) | <.001 |
| Diabetes | 281 | (13) | 55 | (34) | <.001 |
| Current smoker | 157 | (8) | 17 | (10) | .18 |
| Fruit/vegetable intake | |||||
| No. servings/day, median (interquartile range) | 3.4 | (2.1–5.2) | 3.2 | (2.0–4.6) | .23 |
| ≥5 servings/day | 509 | (28) | 29 | (22) | .11 |
| Physical activity, minutes/week† | .023 | ||||
| <90 | 611 | (38) | 59 | (48) | |
| 90–239 | 511 | (31) | 40 | (32) | |
| ≥240 | 504 | (31) | 25 | (20) | |
| Met national activity recommendations‡ | 1,079 | (66) | 67 | (54) | .005 |
| Died during follow-up period | 277 | (13) | 39 | (24) | <.001 |
Among allogeneic recipients.
In terms of vigorous physical activity; comparable time spent doing moderate activity was divided by two.
150 minutes of moderate activity per week or 75 minutes of vigorous activity per week, or equivalent mixture of both
FIGURE 1.
Cumulative incidence of all-cause mortality over 5 years, stratified by presence or absence of ischemic heart disease at baseline survey, and cumulative incidence of new onset ischemic heart disease. Difference in mortality by ischemic heart disease history, log-rank p<0.0001.
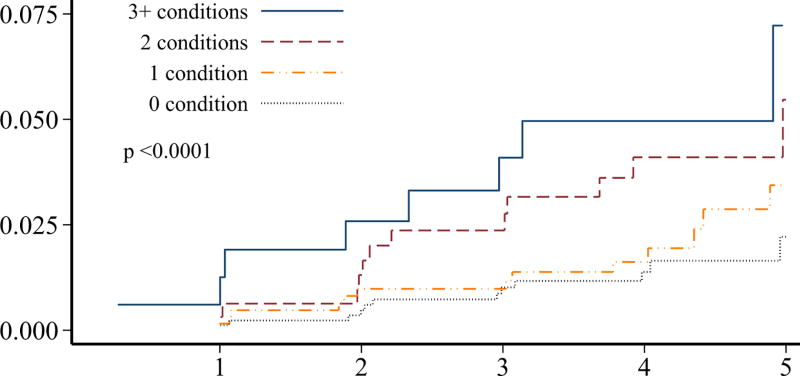
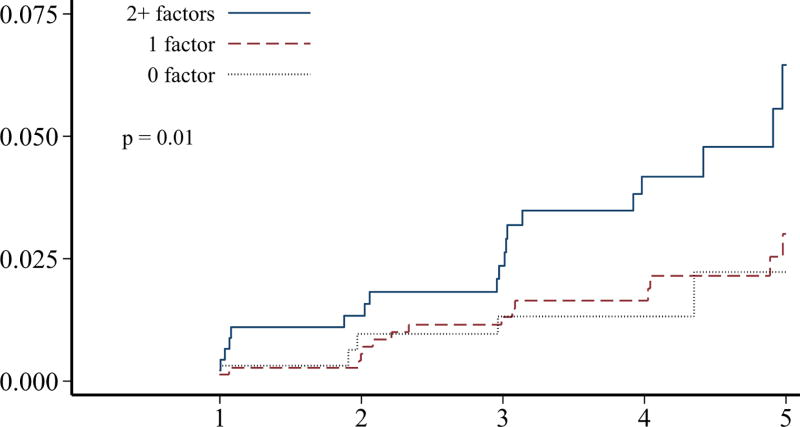
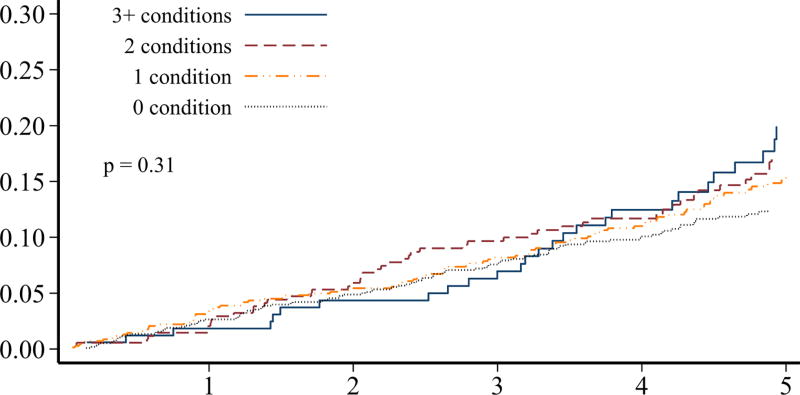
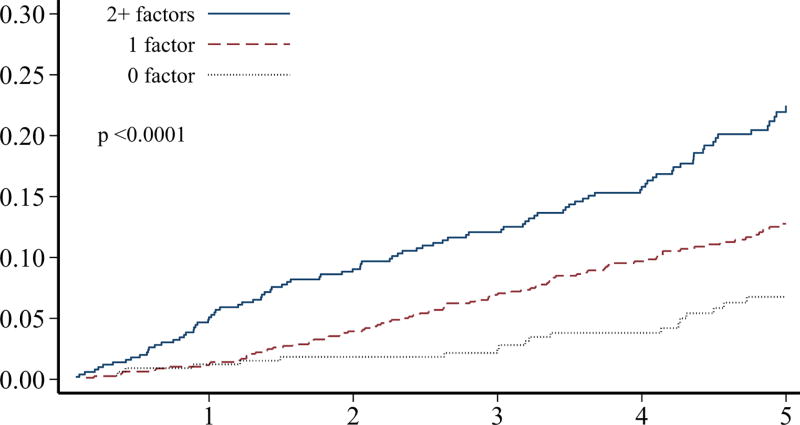
Although individuals without prior ischemic heart disease (n=2,198) at the baseline survey had a significantly lower burden of adverse cardiovascular conditions, more than 30% of these survivors still had ≥2 adverse lifestyle factors present (Table 2). Over 50% of these survivors had a predicted 10-year general cardiovascular risk of at least 10%. The 5-year cumulative incidence of ischemic heart disease in survivors without prior ischemic heart disease was 4.3% (95% CI 3.0–6.0%). When the cumulative incidence rates of ischemic heart disease and overall mortality were plotted, stratified by numbers of adverse cardiovascular conditions and numbers of lifestyle factors, these characteristics were generally associated with a differential risk of both outcomes (p<0.01 for all comparisons except number of cardiovascular conditions and mortality; Figure 2). Although our follow-up was limited, the cumulative incidence rates increased without evidence of a plateau.
TABLE 2.
Cardiovascular Risk Conditions, Lifestyle Factors, and Conventional Predicted 10-year Cardiovascular Risk Among HCT Survivors With and Without Prior Ischemic Heart Disease
| Characteristic, n (%) | No prior disease N=2,198 |
With prior disease N=162 |
P-value | ||
|---|---|---|---|---|---|
| No. cardiovascular risk conditions* | <.001 | ||||
| 0 | 965 | (45) | 17 | (11) | |
| 1 | 687 | (32) | 46 | (29) | |
| 2 | 349 | (16) | 49 | (31) | |
| ≥3 | 165 | (8) | 48 | (30) | |
| No. adverse lifestyle factors† | .020 | ||||
| 0 | 329 | (20) | 21 | (17) | |
| 1 | 787 | (49) | 49 | (40) | |
| ≥2 | 500 | (31) | 53 | (43) | |
| 10-year predicted cardiovascular risk‡ | |||||
| <5% | 401 | (19) | - | ||
| 5–9% | 536 | (26) | - | ||
| 10–19% | 646 | (31) | - | ||
| ≥20% | 492 | (24) | - | ||
Obesity, hypertension, dyslipidemia, and/or diabetes; incomplete data for 32 and 2 HCT recipients without and with prior ischemic heart disease.
Smoking, <5 fruit and vegetable servings per day, and/or not meeting national exercise recommendations; incomplete data for 582 and 39 HCT recipients without and with prior ischemic heart disease.
Based on the Framingham general cardiovascular risk score; incomplete data for 123 HCT recipients without prior ischemic heart disease.
FIGURE 2.
Among hematopoietic cell transplant survivors without prior ischemic heart disease (n=2,198), the cumulative incidence of ischemic heart disease over 5-years, stratified by the number of A) cardiovascular risk conditions and B) adverse lifestyle factors, and the cumulative incidence of overall mortality stratified by the number of C) cardiovascular risk conditions and D) adverse lifestyle factors. Global differences in incidence determined by Fine-Gray method (ischemic heart disease) or log-rank (overall mortality).
Among those without ischemic heart disease at the baseline survey, obesity, hypertension, dyslipidemia, and diabetes were associated with an increased risk for subsequent ischemic heart disease, with an approximate doubling of the hazards associated with each condition (p<0.0001 for trend; Table 3). The association between these cardiovascular conditions and overall mortality was less striking, although underweight BMI status and diabetes were significantly associated with mortality risk.
TABLE 3.
Association of Baseline Characteristics with Subsequent Ischemic Heart Disease and Overall Mortality Among HCT Survivors Without Prior Ischemic Heart Disease (n=2,198)
| Characteristic | Ischemic Heart Disease, N=58 |
All-Cause Mortality, N=277 |
||
|---|---|---|---|---|
|
|
||||
| HR* | (95% CI) | HR* | (95% CI) | |
| Cardiovascular risk conditions | ||||
| Body mass index, kg/m2 | ||||
| <18.5 | 2.5 | (0.6–11.0) | 4.6 | (2.7–7.8) |
| 18.5–24.9 | 1.0 | (ref) | 1.0 | (ref) |
| 25–29.9 | 1.0 | (0.5–1.9) | 0.9 | (0.7–1.2) |
| ≥30 | 2.0 | (1.0–3.9) | 1.0 | (0.7–1.5) |
| Hypertension vs. none | 1.7 | (0.97–2.9) | 1.2 | (0.9–1.5) |
| Dyslipidemia vs. none | 1.8 | (1.0–3.0) | 0.8 | (0.6–1.1) |
| Diabetes vs. none | 2.3 | (1.2–4.2) | 1.5 | (1.1–2.0) |
| No. risk conditions† | ||||
| 0 | 1.0 | (ref) | 1.0 | (ref) |
| 1 | 1.3 | (0.6–2.6) | 1.1 | (0.8–1.5) |
| 2 | 2.1 | (0.97–4.5) | 1.1 | (0.8–1.5) |
| ≥3 | 4.4 | (2.0–9.6) | 1.2 | (0.8–1.8) |
| Lifestyle factors | ||||
| Current smoker | 1.2 | (0.4–3.2) | 1.3 | (0.8–2.1) |
| Fruit/vegetable intake | ||||
| No. servings/day | 0.8 | (0.5–1.4) | 0.7 | (0.6–0.9) |
| ≥5 vs. <5 servings/day | 0.9 | (0.5–1.7) | 0.7 | (0.5–0.9) |
| Physical activity, minutes/week‡ | ||||
| <90 | 1.0 | (ref) | 1.0 | (ref) |
| 90–239 | 0.5 | (0.3–0.97) | 0.4 | (0.3–0.5) |
| ≥240 | 0.2 | (0.1–0.6) | 0.4 | (0.3–0.6) |
| Met national activity recommendations vs. not | 0.3 | (0.2–0.6) | 0.4 | (0.3–0.5) |
| No. adverse lifestyle factors§ | ||||
| 0 | 1.0 | (ref) | 1.0 | (ref) |
| 1 | 1.4 | (0.5–3.5) | 1.8 | (1.1–3.0) |
| ≥2 | 3.1 | (1.2–7.6) | 3.5 | (2.2–5.7) |
| Risk conditions & lifestyle factors** | ||||
| Per additional risk condition (0 to ≥3) | 1.4 | (1.0–1.9) | 1.0 | (0.9–1.2) |
| Per additional adverse lifestyle factor (0 to ≥2) | 1.9 | (1.2–2.9) | 1.8 | (1.5–2.3) |
| 10-year predicted cardiovascular risk†† | ||||
| <5% | 1.0 | (ref) | 1.0 | (ref) |
| 5–9% | 5.3 | (1.2–23.5) | 1.3 | (0.8–2.2) |
| 10–19% | 6.6 | (1.5–28.1) | 2.3 | (1.5–3.6) |
| ≥20% | 8.7 | (2.0–36.9) | 3.7 | (2.4–5.7) |
BMI, body mass index; HCT, hematopoietic cell transplantation
Unless otherwise noted, separate models adjusted for each characteristic listed, plus sex, race, age at baseline survey, time since HCT, HCT donor type, history of chronic GVHD, and family history of cardiovascular disease.
Obesity (body mass index ≥30), hypertension, dyslipidemia, and/or diabetes.
In terms of vigorous physical activity; comparable time spent doing moderate activity was divided by two.
Current smoker, <5 fruits/vegetables servings per day, not meeting national activity recommendations.
Model adjusted jointly for the number of cardiovascular risk conditions and the number of adverse lifestyle factors, in addition to sex, race, age at baseline questionnaire, time since HCT, HCT donor type, history of chronic GVHD, and family history of cardiovascular disease.
Based on Framingham general cardiovascular risk score; HR estimates were unadjusted since Framingham score itself already accounts for sex, baseline age, BMI, smoking, hypertension, and diabetes status.
In contrast, increased levels of physical activity were associated with at least a 50% lower hazard of both ischemic heart disease and overall mortality (Table 3). While estimates for greater fruit/vegetable intake and current smoking did not meet statistical significance in all instances, they were associated with lower and higher hazards of outcomes, respectively. This included a 30% lower risk of death associated with greater fruit/vegetable intake. When all three lifestyle factors were analyzed in combination, survivors who had multiple adverse lifestyle factors at baseline were significantly more likely to experience subsequent ischemic heart disease and death from any cause (HRs >3 if two or more factors present vs. none; p<0.01 for trend). When the numbers of adverse cardiovascular risk conditions and lifestyle factors were analyzed jointly, both remained significantly associated with a higher hazard of subsequent ischemic heart disease (HR 1.4, 95% CI 1.0–1.9, and HR 1.9, 95% CI 1.2–2.9, respectively), while lifestyle factors remained associated with a higher hazard of overall mortality (HR 1.8, 95% CI 1.5–2.3) but not cardiovascular risk conditions (HR 1.0, 95% CI 0.9–1.2).
Although HRs for ischemic heart disease were increased for males, older age at time of the baseline survey, longer time since transplant, autologous recipients, and history of chronic GVHD, none of these associations were statistically significant (Supplemental Table 1). While statistical power to identify an association may be limited for some of these exposures, we also did not find TBI, cyclophosphamide-containing conditioning regimens, or specific agents used for GVHD prophylaxis (steroids, calcineurin inhibitors, or rapamycin) to be associated with a subsequent risk of ischemic heart disease (data not shown).
Higher Framingham risk scores also were associated with greater risks of both ischemic heart disease and overall mortality, although the association appeared to be stronger for ischemic heart disease (Table 3). To address the possibility that the associations between cardiovascular conditions, lifestyle factors, and subsequent ischemic heart disease and overall mortality could be influenced by survivors with a history of post-HCT relapse, we conducted sensitivity analyses that excluded those with post-HCT relapse (n=255; median time of relapse was 7.3 years before the baseline survey). An increasing number of adverse lifestyle factors remained significantly associated with subsequent ischemic heart disease and overall mortality (HRs 1.6 for each additional factor present, p<0.05 for both outcomes).
Finally, we examined the characteristics of those who returned a subsequent survey following our baseline assessment versus those who did not. Those without a subsequent survey response following baseline were more likely to die during follow-up (30.5% vs. 9.5%). These included 63 who died within one year of the baseline survey, and would not have had an opportunity to return a subsequent survey. Among those who survived ≥1-year following baseline, those without a subsequent survey response (n=376) were more likely male (58.0% vs. 51.7%) and slightly younger at baseline (median 51.8 vs. 56.5 years) compared with responders. While these non-responders were less likely to report ≥2 adverse cardiovascular conditions at baseline (21.3% vs 27.3%), they were more likely to have ≥2 adverse lifestyle factors at baseline (39.5% vs. 29.8%).
DISCUSSION
Our prospective longitudinal study showed that potentially modifiable cardiovascular risk conditions and adverse lifestyle factors remain associated with the subsequent risk of ischemic heart disease and overall mortality in a HCT survivor population. For both risk conditions and lifestyle factors, we also identified a significant dose-response relationship with ischemic heart disease. A dose-response relationship also was observed between adverse lifestyle factors and overall mortality. Our results suggest that the influence of adverse lifestyle factors on both ischemic heart disease and overall mortality among HCT survivors could be as significant, if not more so, than even cardiovascular risk conditions. Importantly, these associations were observed with only 5-years of follow-up. The development of clinical interventions to control cardiovascular risk conditions and promote healthier lifestyles could improve outcomes in this high-risk population.
Among HCT survivors free of ischemic heart disease at baseline, we observed an approximate 4% cumulative incidence of new ischemic heart disease after 5-years. Although not all data were available for assignment of Framingham general cardiovascular risk scores,11 at least 50% of our study population free of ischemic heart disease at baseline had a predicted 10% or greater risk of having a serious cardiovascular event within the next 10-years. This risk may be underestimated, given our conservative assumption that those who were treated with anti-hypertensives had good blood pressure control and that no HCT survivor had undiagnosed hypertension. Cardiovascular risk scores developed for the general population also do not consider cancer treatments such as radiation and select chemotherapy that may further increase the risk of cardiovascular disease.13, 14 At the same time, our prior research suggests that HCT survivors reported healthier lifestyle habits than a matched general population sample, which may attenuate cardiovascular disease risk.8
Multiple studies have now demonstrated that HCT survivors have a greater burden of potentially modifiable cardiovascular risk conditions compared with the general population.5–7, 15 Furthermore, HCT survivors who are affected by these conditions before transplant or who develop them in the years after transplant, experience a greater risk of a subsequent serious CV event.5, 7 While dyslipidemia and other metabolic abnormalities are common after transplant, often as a side effect of immunosuppressive treatment for GVHD, some of these abnormalities may resolve after immunosuppressive treatment has ended.7, 16 Nevertheless, laboratory data from HCT survivors’ one-year post-transplant visits suggest that those with higher total cholesterol and triglyceride serum concentrations may be more likely to experience a subsequent serious cardiovascular event.7 Since it is difficult to predict in advance whether hypertension, dyslipidemia, or diabetes that develops soon after HCT will later self-resolve, tighter control of these cardiovascular risk conditions soon after they manifest may be more appropriate than watchful waiting. Certainly, HCT survivors who have pre-existing cardiovascular risk conditions should continue to be monitored closely and treated for these conditions. Studies of breast cancer patients treated in a large health maintenance organization suggest that control of cardiovascular conditions often worsens during cancer treatment and adherence to cardiovascular medications may not return to pre-cancer levels even after completion of active cancer treatment.17, 18
The association of adverse lifestyle factors with subsequent cardiovascular disease has not been well studied in cancer survivors. A multi-institutional European study of over 500 HCT survivors found that those who experienced subsequent arterial events were less physically active and more likely to have continued smoking after HCT.15 In other studies, obesity and physical inactivity were linked to the subsequent development of cardiovascular disease in colorectal cancer survivors,19 and higher levels of vigorous activity were associated with overall better health in testicular cancer survivors.20 Increased physical activity at baseline also has been found to be protective against the subsequent risk of serious cardiovascular events in childhood Hodgkin lymphoma survivors in a dose-dependent fashion.21 Other lifestyle factors such as diet may also be influential. For example, increased adherence to a healthier diet (e.g., Mediterranean diet pattern) was associated with lower adiposity and a lower risk of metabolic syndrome in previously irradiated childhood leukemia survivors.22 Finally, overall increased adherence to lifestyle-related cancer prevention recommendations (e.g., maintaining lean body weight, being physically active, eating a healthier diet) has been associated with lower overall mortality in older female cancer survivors.23 Like our study, these studies also relied on self-report.
Although the interpretation of our results is strengthened by the prospective, longitudinal design, and large sample size, our study also has some limitations. Most cases of ischemic heart disease were self-reported. However, a previous study has shown that HCT survivors can report ischemic heart disease reasonably accurately when compared with medical record review.24 Our questions regarding lifestyle factors were all adapted from well-validated national surveys. While it is possible that results could be subject to response bias, the overall response rate during the follow-up period was high (>80%) and our mortality analysis should be relatively free from such bias. Finally, while the overall duration of follow-up was only 5 years, we already observed a robust association between cardiovascular risk conditions, lifestyle factors, and subsequent ischemic heart disease during this time. Other studies of HCT and other cancer survivors with longer follow-up do not suggest that the increased burden of cardiovascular and other late effects plateaus over time.4, 25–27
Overall, our results support the importance of healthy lifestyle choices for cancer survivors, including those treated with HCT, and more aggressive screening and treatment of potentially modifiable cardiovascular risk conditions.28–30 Patients may use the cancer experience as motivation to adopt healthier lifestyles.31 Clinicians should leverage this opportunity to foster these changes. A growing body of evidence suggests that interventions designed to improve diet and increase physical activity in cancer survivors can lead to short- and medium-term improvements.32–35 More research is needed to determine whether such improvements can persist longer-term with meaningful impacts on subsequent health and quality of life.
Supplementary Material
Acknowledgments
Funding: The National Institutes of Health (CA15704, CA18029, CA151775, and CA167451) provided funding for this study.
Footnotes
Disclosures: MED Flowers reports consulting/advisory roles with Pharmacyclics LLC & CSL Behring, and research funding with Pharmacyclics LLC; PJ Martin reports equity with Procter & Gamble, honoraria from Pfizer and Incyte, and research funding from Fresenius; SJ Lee reports advisory roles with Amgen, Kadmon, and BMS, serving on a steering committee for a clinical trial sponsored by Incyte, and honorarium from Mallinckrodt for an invited lecture.
| Author | Concept | Data curation |
Formal analysis |
Funding | Investigation | Methodology | Administration | Resources | Software | Supervision | Validation | Visualization | Writing- original |
Writing- review/edit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leger | X | X | X | X | X | X | X | |||||||
| Baker | X | X | X | X | X | |||||||||
| Cushing-Haugen | X | X | X | X | X | X | X | |||||||
| Flowers | X | X | X | X | X | |||||||||
| Leisenring | X | X | X | X | ||||||||||
| Martin | X | X | X | X | X | X | ||||||||
| Mendoza | X | X | X | X | ||||||||||
| Reding | X | X | X | |||||||||||
| Syrjala | X | X | X | |||||||||||
| Lee | X | X | X | X | X | X | X | X | ||||||
| Chow | X | X | X | X | X | X | X | X | X | X | X | X |
References
- 1.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19:1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow EJ, Wong K, Lee SJ, et al. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:794–800. doi: 10.1016/j.bbmt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) - Questionnaires, Datasets, and Related Documentation. [accessed November 29, 2017]; Available from URL: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 10.Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Prev Chronic Dis. 2008;5:A35. [PMC free article] [PubMed] [Google Scholar]
- 11.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 12.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 13.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am. Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow EJ, Chen Y, Hudson MM, et al. Prediction of Ischemic Heart Disease and Stroke in Survivors of Childhood Cancer. [Accessed November 29, 2017];J Clin Oncol. doi: 10.1200/JCO.2017.74.8673. Available from URL: http://ascopubs.org/doi/10.1200/JCO.2017.74.8673. [DOI] [PMC free article] [PubMed]
- 15.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 16.Majhail NS, Challa TR, Mulrooney DA, Baker KS, Burns LJ. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1100–1107. doi: 10.1016/j.bbmt.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Calip GS, Boudreau DM, Loggers ET. Changes in adherence to statins and subsequent lipid profiles during and following breast cancer treatment. Breast Cancer Res Treat. 2013;138:225–233. doi: 10.1007/s10549-013-2424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calip GS, Hubbard RA, Stergachis A, Malone KE, Gralow JR, Boudreau DM. Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment. Pharmacoepidemiol. Drug Saf. 2015;24:75–85. doi: 10.1002/pds.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer. 2011;47:267–276. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Fung C, Sesso HD, Williams AM, et al. Multi-Institutional Assessment of Adverse Health Outcomes Among North American Testicular Cancer Survivors After Modern Cisplatin-Based Chemotherapy. J Clin Oncol. 2017;35:1211–1222. doi: 10.1200/JCO.2016.70.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32:3643–3650. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24:313–321. doi: 10.1007/s10552-012-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR Guidelines for Cancer Prevention Is Associated with Lower Mortality among Older Female Cancer Survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:792–802. doi: 10.1158/1055-9965.EPI-13-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 27.van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 28.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow EJ, Anderson L, Baker KS, et al. Late effects surveillance recommendations among survivors of childhood hematopoietic cell transplantation: a Children's Oncology Group report. Biol Blood Marrow Transplant. 2016;22:782–795. doi: 10.1016/j.bbmt.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armenian SH, Lacchetti C, Barac A, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 31.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiskemann J, Dreger P, Schwerdtfeger R, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011;117:2604–2613. doi: 10.1182/blood-2010-09-306308. [DOI] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30:2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajotte EJ, Yi JC, Baker KS, Gregerson L, Leiserowitz A, Syrjala KL. Community-based exercise program effectiveness and safety for cancer survivors. J Cancer Surviv. 2012;6:219–228. doi: 10.1007/s11764-011-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.