Abstract
Study Objective
Prior studies have identified risk factors for recurrent Clostridium difficile infection (CDI), but few studies have integrated these factors into a clinical prediction rule that can aid clinical decision making. The objective of this study was to derive and validate a CDI recurrence prediction rule to identify patients at risk for first recurrence in a national cohort of veterans.
Design
Retrospective cohort study.
Data Source
Veterans Affairs Informatics and Computing Infrastructure.
Patients
A total of 22,615 adult Veterans Health Administration beneficiaries with first-episode CDI between October 1, 2002, and September 30, 2014; of these patients, 7,538 were assigned to the derivation cohort and 15,077 to the validation cohort.
Measurements and Main Results
A 60-day CDI recurrence prediction rule was created in a derivation cohort using backward logistic regression. Those variables significant at p<0.01 were assigned an integer score proportional to the regression coefficient. The model was then validated in the derivation cohort and a separate validation cohort. Patients were then split into three risk categories, and rates of recurrence were described for each category. The CDI recurrence prediction rule included the following predictor variables with their respective point values: prior third-and fourth-generation cephalosporins (1 point), prior proton pump inhibitors (1 point), prior antidiarrheals (1 point), nonsevere CDI (2 points), and community-onset CDI (3 points). In the derivation cohort, the 60-day CDI recurrence risk for each score ranged from 7.5% (0 points) to 57.9% (8 points). The risk score was strongly correlated with recurrence (R2=0.94). Patients were split into low-risk (0-2 points), medium-risk (3-5 points), and high-risk (6-8 points) classes and had the following recurrence rates: 8.9%, 20.2%, and 35.0%, respectively. Findings were similar in the validation cohort.
Conclusion
Several CDI and patient-specific factors were independently associated with 60-day CDI recurrence risk. When integrated into a clinical prediction rule, higher risk scores and risk classes were strongly correlated with CDI recurrence. This clinical prediction rule can be used by providers to identify patients at high risk for CDI recurrence and help guide preventive strategy decisions, while accounting for clinical judgment.
Keywords: Clostridium difficile, epidemiology, prediction rule
Clostridium difficile infection (CDI) is the main cause of bacterial infectious diarrhea in nosocomial settings, accounting for 90–100% of antibiotic-associated pseudomembranous colitis cases.1 Importantly, 14–26% of individuals experience CDI recurrence despite successful treatment of the initial episode.2–5 In those patients who have already experienced one recurrence, the risk of additional recurrences may be as high as 65%.6 Recurrent CDI places a heavy burden on patients, as it increases morbidity and mortality and diminishes quality of life associated with repeated episodes of diarrhea.7, 8 Patients with recurrent CDI experience prolonged symptoms and repeated courses of antibiotics.8 This can lead to increased risk of adverse effects, rehospitalization, and development of multidrug-resistant pathogens. Additionally, patients with recurrent CDI continue to serve as a reservoir that can lead to infection in other vulnerable patients.9
Prior clinical trials identified several patient-specific factors that increase the risk for recurrent CDI.7, 10–13 These include advanced age, immunosuppression, persistent disruption of the intestinal flora, concomitant use of non-CDI antibiotics, concomitant use of gastric acid–suppressing (GAS) drugs, prolonged hospital stays, and severity of illness. Although several studies have identified risk factors for recurrent CDI, few studies have integrated these factors into a tool that can be readily used by clinicians to identify patients at low and high risk for CDI recurrence. Clinical prediction rules combine medical signs, symptoms, and other patient-specific findings into a simple rule that can be used to predict the probability of a specific disease or outcome.14 They serve as a method of translating key research findings into routine clinical practice by aiding practitioners in making better health care decisions.
Available studies provide some evidence for the effectiveness of clinical prediction rules for CDI recurrence; however, these rules have only demonstrated modest discriminatory power.4, 11, 15, 16 This is likely due to small sample size, variable selection, and study design limitations. The objective of this study was to derive and validate a clinical prediction rule to identify patients at risk for first CDI recurrence. This rule can be used to help guide clinical decision making after an initial CDI episode. It can also be used by researchers who wish to measure and balance the risk of CDI recurrence among the various groups in their studies.
Methods
Study Design
This was a national, retrospective cohort study of all patients with CDI receiving care at any of the approximately 150 Veterans Health Administration (VHA) hospitals and 820 VHA clinics in the United States. Data for this study were obtained from the Veterans Affairs (VA) Informatics and Computing Infrastructure (VINCI), which includes administrative, clinical, laboratory, and pharmacy data repositories that are linked using unique patient identifiers. All data collection and analyses were performed at the South Texas Veterans Health Care System, Audie L. Murphy Memorial VA Hospital (San Antonio, TX). This study was approved by the Institutional Review Boards at UT Health San Antonio and the South Texas Veterans Health Care System Research and Development Committee.
Study Population
The cohort was created by including all adult patients (aged 18–89 years) with any inpatient or outpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for CDI (008.45) plus any positive laboratory value (e.g., glutamate dehydrogenase, enzyme immunoassay, polymerase chain reaction) for CDI during the visit or within 7 days of the visit from October 1, 2002, through September 30, 2014. The cohort was limited to patients with first-episode CDI by excluding those patients with an ICD-9-CM code for CDI (008.45) in the year prior to study inclusion. We also excluded those who died within 60 days of the end of treatment discontinuation for the initial episode to capture only those patients at risk for 60-day recurrence.
Study Definitions
A first recurrence was defined as a second outpatient or inpatient visit during which a patient received an ICD-9-CM code for CDI, plus a minimum 3-day gap between the visit and the end of active CDI therapy for the initial episode. For those in whom CDI therapy was not provided, the gap was defined from the day of the outpatient encounter or hospital discharge to a second outpatient or inpatient visit. Recurrent CDI typically occurs within one to three weeks post-treatment discontinuation, but late recurrences, occurring up to 60 days post-treatment discontinuation, are frequent.2 The mean relapse time is approximately 14.5 days, whereas mean reinfection time is 42.5 days.2 We chose 60-day recurrence as our primary dependent variable because it is likely to capture the majority of CDI recurrences. This definition has been used as the primary outcome in other studies evaluating CDI recurrence risk.11
Patient demographics included age during the initial CDI episode, sex, race, and ethnicity. Sex, race, and Hispanic ethnicity were defined as the most frequent reporting of each characteristic over the study period. Principal CDI was defined as ICD-9-CM code 008.45 in the first position. This often indicates that CDI was the primary contributor to hospitalization. Secondary CDI was defined as ICD-9-CM code 008.45 in any position except first. Community-onset CDI was defined based on the presence of CDI therapy initiated in the outpatient setting or on days 1 or 2 of hospitalization. Hospital-onset CDI was defined based on the presence of CDI therapy beginning on day 3 or later of hospitalization.
Data on the Charlson Comorbidity Index score as modified by Deyo et al.,17 Charlson comorbidities, and other relevant diagnoses were collected in the year prior to the first CDI episode (Appendix 1). Data on other markers of CDI severity that occurred at any time during a CDI encounter (from hospital admission or outpatient visit to end of CDI therapy) were also captured, including intensive care unit admission, sepsis/septicemia, shock, acute renal failure, megacolon, prolonged ileus, perforated intestine, colectomy, and white blood cell count, and C-reactive protein, serum creatinine, and albumin concentrations. For prediction rule simplicity, these were combined into one “severe CDI” category, meaning the patient experienced at least one of these severity indicators.
Data on prior (90 days prior to a CDI encounter) non-CDI antibiotics (excluding oral vancomycin, metronidazole, fidaxomicin, rifaximin, and nitazoxanide since they may also be used to treat CDI), GAS drugs (antacids, histamine2 receptor antagonists, proton pump inhibitors), antidiarrheal medications, narcotics, and laxatives and stool softeners were collected.
Data and Statistical Analyses
Data extraction and variable creation were conducted by using SAS version 9.2 (SAS Corp., Cary, NC). All other data and statistical analyses were conducted by using JMP 13.0 (SAS Corp.).
The cohort was randomized, using a random number generator, to either the derivation or validation cohort. The derivation cohort consisted of one third of the total cohort, whereas the remaining two thirds were assigned to the validation cohort. The validation cohort was assigned a larger sample size to more accurately test the generalizability of the prediction rule. Similar methodology was used by Fine et al18 for deriving and validating a prediction rule for community-acquired pneumonia.
Each variable collected was first tested for its relationship with 60-day CDI recurrence using bivariable analyses (χ2, Fisher exact test, t test, or Wilcoxon rank sum test). A backward stepwise logistic regression model was then performed using all candidate variables that were present in at least 5% of the sample. Antibiotics and GAS drugs were entered into the model in classes rather than combined groups. Only variables with a p value <0.01 were retained in the final model.
Each significant predictor variable was assigned an integer score proportional to the regression coefficient derived in the final model. We assigned one point to the smallest regression coefficient, using it as the least common denominator for assigning point values for the score items. All other variables were assigned integer scores proportional to the regression coefficient.19, 20 Patients were then assigned a risk score according to the variables present and their respective integer scores.
The Spearman rank correlation test was used to test the correlation between patient scores and recurrence rates. Next, scores were divided into thirds to represent low-, medium-, and high-risk groups; recurrence rates were calculated for each group.
In the separate validation cohort, predictor variables were collected, and each patient was assigned a risk score. The analyses described above were repeated in the validation cohort. Finally, because the time to recurrence varied, we conducted sensitivity analyses. We used the prediction rule derived for 60-day recurrence and repeated the correlation analysis of risk score with 30- and 90-day recurrence as the dependent variables.
Results
Overall, 22,615 patients met study inclusion criteria, of whom 7,538 were assigned to the derivation cohort and 15,077 to the validation cohort. Baseline characteristics of patients randomized to the derivation and validation cohorts were similar; no significant differences were noted in demographics, CDI characteristics, comorbidities, concomitant infections, medications, or CDI severity.
Table 1 describes the patients’ baseline characteristics stratified by presence or absence of 60-day CDI recurrence. Baseline characteristics of patients with 60-day CDI recurrence significantly differed from those of patients without a recurrence with respect to demographics, CDI characteristics, comorbidities, medications, and CDI severity.
Table 1.
Association between Baseline Characteristics and 60-Day CDI Recurrence in the Derivation Cohort (n=7538)
| Characteristic | No 60-Day CDI Recurrence (n=6315) |
60-Day CDI Recurrence (n=1223) |
p Value |
|---|---|---|---|
| Age (yrs), median (IQR) | 65 (59 – 76) | 66 (60 – 76) | 0.1195 |
| Age ≥ 65 yrs | 3347 (53.0) | 681 (55.7) | 0.0845 |
| Male sex | 6031 (95.5) | 1151 (94.1) | 0.0357 |
| Race-ethnicity | 0.0007 | ||
| Non-Hispanic white | 4092 (64.8) | 863 (70.6) | |
| Non-Hispanic black | 1459 (23.1) | 248 (20.3) | |
| Hispanic | 379 (6.0) | 48 (3.9) | |
| Other | 221 (3.5) | 37 (3.0) | |
| Missing | 164 (2.6) | 26 (2.1) | |
| Principal CDI diagnosis | 1768 (28.0) | 433 (35.4) | <0.0001 |
| Hospital LOS ≥14 days | 3094 (49.0) | 632 (51.7) | <0.00763 |
| Community-onset CDI | 2463 (39.0) | 795 (65.0) | <0.0001 |
| Comorbidities | |||
| Hypertension | 4913 (77.8) | 936 (76.5) | 0.3043 |
| Dyslipidemia | 3524 (55.8) | 733 (59.9) | 0.0062 |
| Obesity | 1175 (18.6) | 247 (20.2) | 0.2055 |
| Prior myocardial infarction | 701 (11.1) | 125 (10.2) | 0.3446 |
| Congestive heart failure | 1522 (24.1) | 295 (24.1) | 0.9921 |
| Peripheral vascular disease | 1194 (18.9) | 230 (18.8) | 0.8928 |
| Cerebrovascular disease | 1250 (19.8) | 220 (18.0) | 0.1213 |
| Dementia | 208 (3.3) | 27 (2.2) | 0.0429 |
| COPD | 2235 (35.4) | 461 (37.7) | 0.1196 |
| Rheumatologic disease | 158 (2.5) | 42 (3.4) | 0.0766 |
| Peptic ulcer disease | 278 (4.4) | 49 (4.0) | 0.5038 |
| Liver disease | 417 (6.6) | 84 (6.9) | 0.6607 |
| Diabetes mellitus | 2633 (41.7) | 495 (40.5) | 0.4277 |
| Hemiplegia or paraplegia | 284 (4.5) | 38 (3.1) | 0.0222 |
| Renal disease | 1661 (26.3) | 333 (27.2) | 0.4892 |
| Cancer | 1604 (25.4) | 300 (24.5) | 0.5207 |
| HIV/AIDS | 114 (1.8) | 21 (1.7) | 0.8537 |
| GERD | 1749 (27.7) | 388 (31.7) | 0.0048 |
| Transplant | 114 (1.8) | 34 (2.8) | 0.0270 |
| Inflammatory bowel disease | 139 (2.2) | 57 (4.7) | <0.0001 |
| Irritable bowel syndrome | 76 (1.2) | 20 (1.6) | 0.2793 |
| Charlson Comorbidity Index score, median (IQR) | 3 (1 – 6) | 3 (1 – 5) | |
| Any severe CDI | 4098 (64.9) | 564 (46.1) | <0.0001 |
| CDI severity indicators | |||
| ICU admission | 158 (2.5) | 12 (1.0) | 0.0004 |
| Sepsis/septicemia | 871 (13.8) | 108 (8.8) | <0.0001 |
| Shock | 189 (3.0) | 21 (1.7) | 0.0070 |
| Acute renal failure | 1711 (27.1) | 227 (18.6) | <0.0001 |
| Megacolon | 19 (0.3) | 1 (0.1) | 0.1292 |
| Prolonged ileus | 272 (4.3) | 32 (2.6) | 0.0022 |
| Perforated intestine | 25 (0.4) | 4 (0.3) | 0.6744 |
| White blood cell count ≥ 15 × 103/mm3 | 2305 (36.5) | 286 (23.4) | <0.0001 |
| C-reactive protein ≥ 160 mg/L | 95 (1.5) | 7 (0.6) | 0.0027 |
| Albumin < 2.5 g/dL | 1749 (27.7) | 146 (11.9) | <0.0001 |
| Serum creatinine >1.5 mg/dL | 1377 (21.8) | 249 (20.4) | 0.2477 |
| Colectomy | 6 (0.1) | 0 (0.0) | 0.2129 |
| Prior medications | |||
| Antibiotics | 3404 (53.9) | 758 (62.0) | <0.0001 |
| Penicillins | 1478 (23.4) | 338 (27.6) | 0.0018 |
| First- and second-generation cephalosporins | 644 (10.2) | 149 (12.2) | 0.0021 |
| Third- and fourth-generation cephalosporins | 720 (11.4) | 196 (16.0) | <0.0001 |
| Carbapenems | 227 (3.6) | 56 (4.6) | 0.0932 |
| Macrolides | 512 (8.1) | 122 (10.0) | 0.0372 |
| Fluoroquinolones | 1718 (27.2) | 408 (33.4) | <0.0001 |
| Aminoglycosides | 145 (2.3) | 43 (3.5) | 0.0191 |
| Tetracyclines | 202 (3.2) | 45 (3.7) | 0.4141 |
| Clindamycin | 474 (7.5) | 114 (9.3) | 0.0298 |
| Other | 1465 (23.2) | 330 (27.0) | 0.0044 |
| High-risk antibiotics | 2248 (35.6) | 543 (44.4) | <0.0001 |
| Narcotics | 2450 (38.8) | 505 (41.3) | 0.1073 |
| Antidiarrheals | 461 (7.3) | 150 (12.3) | <0.0001 |
| Laxatives | 2431 (38.5) | 488 (39.9) | 0.3428 |
| GAS | 3499 (55.4) | 459 (62.1) | <0.0001 |
| PPIs | 2924 (46.3) | 669 (54.7) | <0.0001 |
| H2RAs | 916 (14.5) | 179 (14.6) | 0.9080 |
| Other | 701 (11.1) | 158 (12.9) | 0.0729 |
| CDI therapies | |||
| Metronidazole | 4831 (76.5) | 631 (51.6) | <0.0001 |
| Vancomycin | 2172 (34.4) | 350 (28.6) | <0.0001 |
| Fidaxomicin | 44 (0.7) | 49 (4.0) | 0.1788 |
| Probiotics | 1572 (24.9) | 171 (14.0) | <0.0001 |
Data are no. (%) of patients unless otherwise specified.
AIDS=acquired immune deficiency syndrome; CDI=Clostridium difficile infection; CI=confidence interval; COPD=chronic obstructive pulmonary disease; GAS=gastric acid–suppressing; GERD=gastroesophageal reflux disease; HIV=human immunodeficiency syndrome; H2RAs=histamine2 receptor antagonists; ICU=intensive care unit; IQR=interquartile range; LOS=length of stay; PPIs=proton pump inhibitors.
In the logistic regression model, the following were identified as independent predictors of 60-day recurrence and assigned points (Table 2): prior third- and fourth-generation cephalosporins (1 point), prior proton pump inhibitors (1 point), prior antidiarrheals (1 point), nonsevere CDI (2 points), and community-onset CDI (3 points). The total score ranged from 0–8 points.
Table 2.
Results of Backward Stepwise Logistic Regression Model and Integer Score Assignment
| Variable | Regression coefficient | p value | Integer score |
|---|---|---|---|
| Prior third- or fourth-generation cephalosporins | 0.1346 | 0.0030 | 1 |
| Prior proton pump inhibitors | 0.1460 | 0.0002 | 1 |
| Prior antidiarrheals | 0.1889 | <0.0001 | 1 |
| Nonsevere CDI | 0.3277 | <0.0001 | 2 |
| Community-onset CDI | 0.4761 | <0.0001 | 3 |
CDI = Clostridium difficile infection.
In the derivation cohort, the 60-day CDI recurrence risk for each score ranged from 7.5% (0 points) to 57.9% (8 points). The risk score was strongly correlated with 60-day recurrence (R2=0.94) (Figure 1). Correlation was similar in the sensitivity analysis for 30-day (R2=0.95) and 90-day (R2=0.94) recurrence. Patients were split into low-risk (0-2 points), medium-risk (3-5 points), and high-risk (6-8 points) classes and had the following recurrence rates: 8.9% in the low-risk class, 20.2% in the medium-risk class, and 35.0% in the high-risk class (Figure 2). Findings were similar in the validation cohort and when the derivation and validation cohorts were combined to reflect the overall population.
Figure 1.
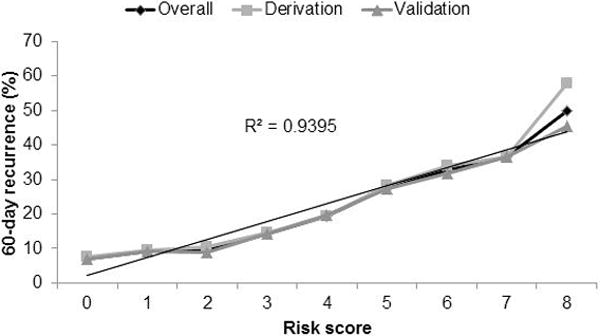
Correlation of risk score and 60-day Clostridium difficile infection recurrence in the derivation (n=7538) and validation (n=15,077) cohorts, and in the overall population (derivation and validation cohorts combined).
Figure 2.
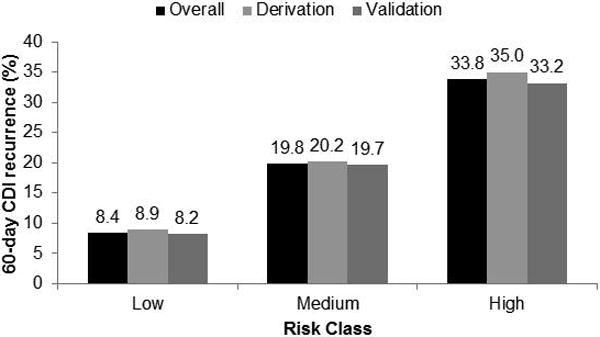
Sixty-day Clostridium difficile infection (CDI) recurrence by risk class in the derivation (n=7538) and validation (n=15,077) cohorts, and in the overall population (derivation and validation cohorts combined).
Discussion
CDI recurrence is a common and challenging health problem. Prior studies have identified several clinical and host risk factors for CDI recurrence; however, few have integrated these factors into a tool that can help guide clinical decision making. Our study developed a clinical prediction rule for recurrent CDI using a robust split-cohort design and, to our knowledge, the largest CDI cohort for this purpose. Antibiotic and proton pump inhibitor use have been widely reported as a predictor of recurrent CDI,11, 15, 21–24 whereas other predictor variables have not been extensively studied.
Antidiarrheals have not been previously associated with CDI recurrence; however, these agents are largely avoided during active CDI due to the risk of worsening clinical outcomes.25, 26 Antidiarrheals, such as loperamide, slow fecal transit time,27 which could delay elimination of C. difficile through the bowel and result in absorption of C. difficile toxins into the gut mucosa. This could increase the likelihood of recurrent episodes due to relapse and accumulation of C. difficile in the gastrointestinal tract.
The remaining variables included in the prediction rule all seem to reflect “healthier” patients with CDI. Patients with community-onset, nonsevere CDI might be discharged on outpatient therapy more quickly than those who develop CDI in the hospital or who have other comorbid conditions that necessitate inpatient treatment.28 CDI treatment regimens typically include antibiotics taken 3–4 times daily, which could limit patient adherence in the outpatient setting, thus reducing the likelihood of C. difficile eradication. Furthermore, strict precautions are taken to prevent the spread of spores in a patient’s hospital environment compared to home, which could play a role in the association between duration of hospitalization and CDI recurrence.6
To our knowledge, four prior prediction rules for CDI recurrence have been published. Similar to our study, antibiotic use11, 15 and community-onset CDI4, 15 have been previously included in these rules. Variables that were commonly found in other rules, but not our rule, included older age and severe CDI as measured by subjective or objective measures.4, 11, 15, 16 The fact that these variables were not significant predictors of recurrence in our population could reflect the generally older age and poorer health of the VHA population, which would limit the variation in these variables and the likelihood of detecting differences between those who did and did not have 60-day CDI recurrence.
The use of a simple, objective clinical prediction rule for recurrent CDI has several possible implications for public health. The rule could potentially define a high-risk population in whom awareness of the risk would facilitate more prompt recognition, diagnosis, and treatment of recurrent CDI. These patients could also be targeted for specific interventions aimed at preventing recurrence, including infection control precautions, judicious use of antibiotics, appropriate duration of antibiotic therapy, and use of other preventive measures. Additionally, certain risk factors might also be mitigated prior to CDI recurrence, thus limiting the risk of recurrence. Lastly, a prediction rule could be of value in selecting high-risk patients for clinical trials of novel agents to prevent recurrent CDI, such as fidaxomicin.
This study has some potential limitations. First, we used a retrospective cohort study design that includes data collection from electronic medical records. Cohort studies might be subject to misclassification bias and confounding by unmeasured variables. Additionally, electronic medical data are created for the purpose of patient care, rather than research, which could lead to information bias. Similarly, administrative codes might not entirely capture all of a given patient’s comorbidities and, therefore, cannot be considered equivalent to a medical chart review. Although we included several CDI recurrence risk factors, there might be other factors that are not included that could aid in the predictive model of CDI recurrence. These include processes of care, patient frailty, medication adherence, and infecting C. difficile strain. CDI therapies could have also impacted recurrence risk; however, we did not include these in our model, such that the rule can be used to guide initial selection of therapy in high-risk patients. Our predominately elderly, male veteran CDI population might not be representative of all CDI populations; thus, potentially limiting the generalizability of our epidemiologic findings to other settings. Validation of the prediction rule in a cohort more inclusive of women, who are generally at higher risk for initial CDI, is needed. Next, some CDI episodes could have been missed, as veterans could have been treated at non-VHA facilities, especially those with severe CDI who might have needed a higher level of care. We attempted to limit study survival bias by excluding patients with CDI who died prior to assessing for recurrence; however, survival bias cannot be ruled out. It is also important to note the large population of patients who received laxatives/stool softeners prior to the initial CDI episode. This could have increased the misclassification of CDI due to colonization, especially in patients who were diagnosed by the more sensitive nucleic acid amplification tests.
Prediction rules themselves also have inherent limitations. They are developed using clinical information to predict an outcome in a population of patients; therefore, application of the rule to an individual patient could be problematic. Because of this limitation, the prediction rule is intended to supplement, not supersede, a clinician’s clinical judgment. Our prediction rule is designed to predict CDI recurrence, not to drive decisions on implementing specific preventive or treatment measures. Whether this rule can be applied in conjunction with different management strategies to improve clinical outcomes and health service utilization requires further study.
Conclusion
Several CDI and patient-specific factors were independently associated with 60-day CDI recurrence risk. When integrated into a clinical prediction rule, increasing risk score was strongly correlated with CDI recurrence. This clinical prediction rule can be used by providers to identify patients at high risk for CDI recurrence and help guide preventive strategy decisions, while accounting for clinical judgment.
Acknowledgments
The authors would like to thank Eric H. Young and Samuel J. Palka for editing this manuscript.
Research support: This study is the result of work supported with resources and the use of facilities at the Audie L. Murphy Memorial VA Hospital, San Antonio, Texas. This study was supported, in part, by an American College of Clinical Pharmacy Research Institute Futures Grant. Dr. Reveles is supported by the National Institutes of Health/National Institute on Aging San Antonio Claude D. Pepper Older Americans Independence Center (grant no. 1P30AG044271-01A1) Career Development (KL2) Program. Dr. Frei is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant no. UL1 TR001120).
Appendix 1. Study Comorbidity Definitions
| Comorbidity | ICD-9-CM code(s) |
|---|---|
| Comorbidities in year prior to CDI encounter | |
| Hypertension | 401-405 |
| Dyslipidemia | 272 |
| Obesity | 278 |
| Myocardial infarction | 410, 412 |
| Congestive heart failure | 428 |
| Peripheral vascular disease | 441, 443.9, 785.4, V43.4 |
| Cerebrovascular disease | 430-438 |
| Dementia | 290 |
| COPD | 490-496, 500-505, 506.4 |
| Rheumatologic disease | 710.0-710.1, 710.4, 714.0-714.2, 714.81, 725 |
| Peptic ulcer disease | 531.0-531.9, 532.0-532.9, 533.0-533.9, 534.0-534.9 |
| Liver disease | 571.2, 571.4, 571.5, 571.6, 572.2-572.8, 456.0-456.21 |
| Diabetes | 250.0-250.3, 250.4, 250.5, 250.6, 250.7, 250.8, 250.9 |
| Hemiplegia or paraplegia | 342, 344.1 |
| Renal disease | 582, 583, 585, 586, 588 |
| Neoplastic disease | 140-172, 174-208 |
| HIV/AIDS | 42-44, V08 |
| Bacteremia | 790.7 |
| Pneumonia | 480.0-483.99, 485–487 |
| Skin infection | 680-686 |
| Endocarditis | 421.0, 421.1, 421.9, 424.9 |
| Urinary tract infection | 590-599 |
| Device-related infection | 996.31, 996.62, 996.64, 999.31 |
| Acute respiratory infection | 460-466 |
| GERD | 530.11, 530.81 |
| Transplant | V42, E878.0 |
| Inflammatory bowel disease | 555, 556 |
| CDI severity indicators | |
| Shock | 639.5, 785.52, 785.59 |
| Sepsis/septicemia | 020.2, 038.0-038.9, 995.91, 995.92 |
| Perforation of intestine | 569.83 |
| Prolonged ileus | 560.1 |
| Megacolon | 558.2, 564.7 |
| Acute renal failure | 584, 586 |
AIDS = acquired immune deficiency syndrome; CDI = Clostridium difficile infection; COPD = chronic obstructive pulmonary disease; GERD = gastroesophageal reflux disease; HIV = human immunodeficiency syndrome; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Footnotes
DR KELLY R REVELES (Orcid ID : 0000-0001-8880-4879)
Publisher's Disclaimer: Disclaimer: The contents of this article do not necessarily represent the views of the United States Department of Veterans Affairs or the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis. 1989;2:340–3. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]
- 3.Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012:S93–103. doi: 10.1093/cid/cis499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyre DW, Walker AS, Wyllie D, et al. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis. 2012:S77–87. doi: 10.1093/cid/cis356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluml BM. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc (2003) 2005;5:566–72. doi: 10.1331/1544345055001274. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;7:1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;6:403–10. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 8.DuPont HL. The search for effective treatment of Clostridium difficile infection. N Engl J Med. 2011;5:473–5. doi: 10.1056/NEJMe1013236. [DOI] [PubMed] [Google Scholar]
- 9.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;3:324–33. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 10.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;4:298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Hu MY, Katchar K, Kyne L, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;4:1206–14. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 12.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;3:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 13.Nitzan O, Elias M, Chazan B, Raz R, Saliba W. Clostridium difficile and inflammatory bowel disease: role in pathogenesis and implications in treatment. World J Gastroenterol. 2013;43:7577–85. doi: 10.3748/wjg.v19.i43.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group JAMA. 2000;1:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Zilberberg MRK, Olsen M, et al. Development and validation of a recurrent Clostridium difficile risk-prediction model. J Hosp Med. 2014;7:418–23. doi: 10.1002/jhm.2189. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB, Sr, Collins SH, Pencina KM, Kean Y, Gorbach S. Risk estimation for recurrent Clostridium difficile infection based on clinical factors. Clin Infect Dis. 2014;10:1386–93. doi: 10.1093/cid/ciu107. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;6:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;4:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 19.Martinez D, Heudebert G, Seas C, et al. Clinical prediction rule for stratifying risk of pulmonary multidrug-resistant tuberculosis. PLoS One. 2010;8:e12082. doi: 10.1371/journal.pone.0012082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;3:165–71. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 21.Shivashankar R, Khanna S, Kammer PP, et al. Clinical predictors of recurrent Clostridium difficile infection in out-patients. Aliment Pharmacol Ther. 2014;5:518–22. doi: 10.1111/apt.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tschudin-Sutter S, Tamma PD, Milstone AM, Perl TM. The prediction of complicated Clostridium difficile infections in children. Infect Control Hosp Epidemiol. 2014;7:901–3. doi: 10.1086/676874. [DOI] [PubMed] [Google Scholar]
- 23.Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;4:452–60. doi: 10.1017/ice.2014.88. [DOI] [PubMed] [Google Scholar]
- 24.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;7:1011–9. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 25.Brown JW. Toxic megacolon associated with loperamide therapy. JAMA. 1979;5:501–2. [PubMed] [Google Scholar]
- 26.Kato H, Kato H, Iwashima Y, Nakamura M, Nakamura A, Ueda R. Inappropriate use of loperamide worsens Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;2:194–5. doi: 10.1016/j.jhin.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Church JM, Fazio VW. A role for colonic stasis in the pathogenesis of disease related to Clostridium difficile. Dis Colon Rectum. 1986;12:804–9. doi: 10.1007/BF02555349. [DOI] [PubMed] [Google Scholar]
- 28.Lubbert C, Zimmermann L, Borchert J, Horner B, Mutters R, Rodloff AC. Epidemiology and recurrence rates of Clostridium difficile infections in Germany: a secondary data analysis. Infect Dis Ther. 2016;4:545–54. doi: 10.1007/s40121-016-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


