Abstract
Objective
Blunted tachycardia during hypotension is a characteristic feature of patients with autonomic failure, but the range has not been defined. This study reports the range of orthostatic heart rate (HR) changes in patients with autonomic failure caused by neurodegenerative synucleinopathies.
Methods
Patients evaluated at sites of the U.S. Autonomic Consortium (NCT01799915) underwent standardized autonomic function tests and full neurological evaluation.
Results
We identified 402 patients with orthostatic hypotension (OH) who had normal sinus rhythm. Of these, 378 had impaired sympathetic activation, i.e., neurogenic OH, and based on their neurological examination were diagnosed with Parkinson disease, dementia with Lewy bodies, pure autonomic failure or multiple system atrophy. The remaining 24 patients had preserved sympathetic activation and their OH was classified as non-neurogenic, due to volume depletion, anemia or polypharmacy. Patients with neurogenic OH had twice the fall in systolic blood pressure (SBP) [−44±25 vs. −21±14 mmHg (mean±SD), p<0.0001] but only one third of the increase in HR than those with non-neurogenic OH (8±8 vs. 25±11 bpm, p<0.0001). A ΔHR/ΔSBP ratio of 0.492 bpm/mmHg had excellent sensitivity (91.3%) and specificity (88.4%) to distinguish between patients with neurogenic vs. non-neurogenic OH (AUC=0.96, p<0.0001). Within patients with neurogenic OH, HR increased more in those with multiple system atrophy (p=0.0003), but there was considerable overlap with patients with Lewy body disorders.
Interpretation
A blunted HR increase during hypotension suggests a neurogenic cause. A ΔHR/ΔSBP ratio lower than 0.5 bpm/mmHg is diagnostic of neurogenic OH.
Keywords: Heart rate, autonomic failure, neurogenic orthostatic hypotension, multiple system atrophy, Parkinson disease
INTRODUCTION
Orthostatic hypotension (OH) is defined as a sustained fall in systolic blood pressure (SBP) of at least 20 mmHg or diastolic blood pressure (DBP) of 10 mmHg or more after standing for 3 minutes.1 OH is a common problem in patients with several medical disorders that reduce cardiac output or impair vasoconstrictor mechanisms.2 When OH is due to impaired activation of sympathetic vasoconstrictor neurons, the condition is called neurogenic OH and is a hallmark feature of autonomic failure.3, 4 Diagnosing neurogenic OH is important because it indicates the presence of an underlying pathology affecting autonomic neurons and has a much worse prognosis than non-neurogenic OH.3
A characteristic difference between non-neurogenic and neurogenic OH is the associated heart rate (HR) increase upon standing.3, 4 Patients with neurogenic OH usually have little or no increase in HR in the upright position, whereas patients with non-neurogenic OH due to, for example, intravascular volume depletion or physical deconditioning, typically have marked tachycardia. Despite its importance in the differential diagnosis of OH, the range of orthostatic HR changes has not been systematically analyzed and normative data are not available. Neither the first nor the second OH consensus criteria defined HR ranges.1, 5 A recent consensus panel proposed an increase in HR of <15 beats per minute (bpm) to support the diagnosis of neurogenic OH but this proposed range was based on experts’ clinical experience rather than scientific evidence.6
Knowing the actual range of heart rate changes in patients with carefully diagnosed autonomic failure should be helpful for clinicians. Moreover, it is not known whether orthostatic HR changes differ in patients with lesions of the central or peripheral autonomic nervous system. Distinguishing both types of autonomic involvement (central vs. peripheral) is important as these two groups differ in response to treatment, comorbidities, and prognosis.
Because tachycardia when standing is largely dependent on sympathetic innervation of the sinus node,7 we hypothesized that the orthostatic HR responses will be more impaired in patients with peripheral sympathetic cardiac denervation (i.e., Parkinson disease [PD], dementia with Lewy bodies [DLB], and pure autonomic failure [PAF]) compared to those with selective central autonomic lesions (i.e., multiple system atrophy [MSA]), in whom the sympathetic postganglionic innervation of the heart is typically spared.8, 9 To test this hypothesis we describe the range of orthostatic HR changes in a large cohort of patients with carefully characterized autonomic failure and OH due to synucleinopathies.10–12 As a comparison group, we also studied a group of patients with non-neurogenic OH. These findings will help clinicians diagnose neurogenic OH.
METHODS
Subjects
Between September 2011 and January 2016 we prospectively studied 423 consecutive adult patients referred for autonomic evaluation at sites within the U.S. Autonomic Disorders Consortium (NCT01799915). The criteria to diagnose OH included: (i) a sustained fall in SBP of at least 20 mmHg or DBP of 10 mmHg or more after standing for 3 minutes.1 Patients with diabetes mellitus, lupus, congestive heart failure, isolated vasovagal syncope, autoimmune etiologies, infectious diseases,13 or amyloidosis were excluded from this study. Patients with OH that had no overshoot in BP after the Valsalva strain (i.e., phase IV of the Valsalva maneuver) were categorized as having neurogenic OH on account of impaired baroreflex-mediated sympathetic activation.14, 15 Patients who met criteria for OH who had a BP overshoot following release of the Valsalva strain and an identifiable cause for their OH (e.g., anemia, polypharmacy, varicose veins) were diagnosed as having non-neurogenic OH on the basis of their intact baroreflex-mediated sympathetic activation.
Categorizing the lesions of patients with neurogenic OH
Based on the known neuropathology of neurodegenerative synucleinopathies (Figure 1),16, 17 patients with neurogenic OH were classified clinically as having central or peripheral forms of autonomic sympathetic failure according to their neurological examination and diagnostic work-up (e.g., brain magnetic resonance imaging).18–20 As depicted in Figure 1B, central autonomic involvement included patients diagnosed clinically with probable or possible MSA based on current consensus criteria.18, 21 Peripheral autonomic involvement included patients with PAF and no evidence of CNS involvement (i.e., no REM sleep behavior disorder, olfactory dysfunction or subtle motor signs)22 as well as those fulfilling current clinical consensus criteria for a known Lewy body CNS disorder (PD, DLB).22, 23 Patients diagnosed with neurogenic OH who also had REM sleep behavior disorder, anosmia and/or subtle motor signs were categorized as prodromal PD/DLB or prodromal MSA, since longitudinal studies have shown that they are already affected by an underlying CNS neurodegenerative disorder.22, 24, 25 Since it was unclear at the time of testing whether these patients would ultimately transition into PD/DLB or MSA, they were excluded from the sub-group analysis in order to understand the impact of disease-specific lesions.
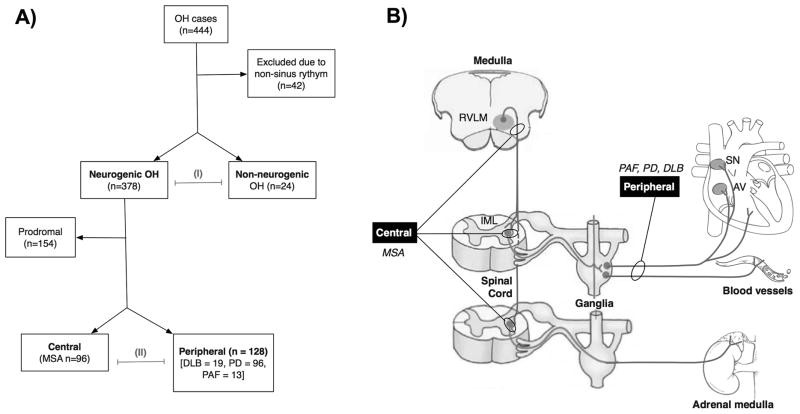
Figure 1. Patient cohort and study rationale.
A. Flow chart showing the classification of the participants. Four hundred and forty-four patients were screened. Only those in normal sinus rhythm in whom the heart rate responses could be clearly attributed to extrinsic autonomic innervation were included in the subsequent analysis. Patients were classified as having neurogenic or non-neurogenic orthostatic hypotension (OH) and both groups were compared (I). As shown in panel B, based on their neurological examination, cases of neurogenic OH were diagnosed with either probable or possible MSA, and thus classified as having central autonomic failure (due to neuronal loss in the brainstem vasomotor nuclei and intermediolateral columns of the spinal cord,49 but intact peripheral post-ganglionic sympathetic neurons innervating the heart);50 or Parkinson disease, dementia with Lewy bodies, and pure autonomic failure.22, 49, 51 The latter group was classified as having peripheral lesions, as a result of predominant involvement of peripheral postganglionic sympathetic neurons.23 Parasympathetic activity (not shown) was equally affected in patients with central and peripheral autonomic failure. The remaining patients that had autonomic failure with CNS signs, but did not meet diagnostic criteria for a defined CNS synucleinopathy were classified as having prodromal disease (see text for details). The impact of central vs. peripheral autonomic failure was compared (A II).
Sites
Recruiting sites were part of the U.S. Autonomic Disorders Consortium and supported National Institutes for Health (NIH) Rare Disease Clinical Research Network (RDCRN).22 All sites had expertise in autonomic neurology. All subjects had been referred for autonomic testing to evaluate their complaints of dizziness, lightheadedness or feeling about to faint on standing. Enrollment occurred at 5 medical centers: New York University Medical Center (New York, NY), Vanderbilt University Medical Center (Nashville, TN), Mayo Clinic (Rochester, MN), NIH Intramural Research Program (Bethesda, MD), and Beth Israel Deaconess Medical Center (Boston, MA). The National Institutes of Neurological Disorders and Stroke (NINDS) approved all recruiting sites. Each local Institutional Review Board approved the study procedures. Informed consent was obtained in all cases. A detailed manual of operations was developed to standardize the autonomic function tests across sites with full operating procedures, including timings, sampling rates, and spectral analysis guidelines. Training was provided to investigators acquiring and analyzing data. All data was reviewed, verified and audited by the RDCRN centralized Data Monitoring and Coordinating Center (DMCC Health Informatics Institute, University of South Florida). Outlying data points were flagged and discussed with investigators through email and monthly conference calls.
Protocol
Participants were free of caffeine, alcohol and nicotine from the previous evening. Subjects underwent a comprehensive medical history26 and a standardized battery of autonomic function tests, in a quiet, temperature-controlled room. An intravenous forearm catheter was inserted into the antecubital vein and subjects were transferred to the tilt-table for instrumentation to complete a 10-min head up tilt and collection of supine and 60 head-up tilt plasma norepinephrine samples. Continuous electrocardiographic monitoring included RR interval recording from 3 precordial electrodes. Beat-to beat BP was measured with finger plethysmography with the hand supported at heart level. Intermittent BP was also measured at 1-minute intervals with a validated automated cuff sphygmomanometer over the brachial artery. All signals were acquired and digitized and sampled at a minimum rate of 500 Hz. Subjects in non-sinus rhythm were excluded from further analysis.
As a measure of parasympathetic function, subjects were coached to breathe at 6 cycles per minute, and respiratory sinus arrhythmia during deep paced breathing was calculated from the average of the 3 longest RR intervals during expiration divided by the average the 3 shortest RR intervals during inspiration (i.e., expiratory: inspiratory [E:I] ratio).14 A 300-second segment of spontaneous breathing in the supine position was selected and processed to detect RR intervals from the electrocardiogram and continuous BP. Beat-to-beat HR variability in the time- and frequency-domain were measured following standards outlined in the manual of opperations.27 Venous blood was sampled through the indwelling catheter after at least 15 minutes supine to assay plasma norepinephrine levels by high-performance liquid chromatography.22
Subjects then performed a standardized Valsalva maneuver,15 maintaining an expiratory pressure of >30-mmHg for at least 10 seconds. If BP was not higher than baseline within 10 seconds after release of the Valsalva strain (i.e., phase IV), the overshoot in BP was considered absent, indicative of sympathetic (autonomic) failure. Cases with partial recovery of the blood pressure in phase IV, suggesting some preservation of baroreflex-mediated sympathetic activation, were not included. Pressure recovery time was measured as the time (in seconds) taken for the systolic BP to return to baseline (pre-strain) values following release of the Valsalva strain.15, 28 Subjects were then tilted upright to an angle of 60-degrees and instructed to remain immobile. After 10 minutes upright, a second set of blood samples for plasma catecholamine measurements were acquired. Patients also underwent a complete blood count to screen for anemia and metabolic panel to screen for dehydration or electrolyte imbalances.
The procedure for defining the orthostatic HR response was standardized across all sites, average heart rate over the 5 minutes of supine rest during spontaneous breathing was used to define baseline (pre-tilt) resting supine value. Orthostatic HR responses were calculated as the difference between heart rate at baseline and at 3 minutes of passive upright tilt. Cardiac baroreflex gain was assessed with 2 methods: (i) by calculating the slope of the regression line relating changes in systolic BP against RR intervals during baseline, phase I, II (lowest BP and shortest RR interval during the straining phase), and IV of the Valsalva maneuver and expressed as ms/mmHg, as previously described;29 and (ii) by dividing the change (Δ) in heart rate by the fall (Δ) in SBP at the 3 minute mark upright and expressed as bpm/mmHg (ΔHR/ΔSBP ratio).
Statistical analysis
Data were first tested for normality using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Parametric and non-parametric evaluations were used as appropriate. Supine hypertension was defined as BP >140/90 mmHg in the horizontal position.30 Linear regression analysis and Pearson correlation coefficients were used to assess the relationship between RR intervals and hemodynamic parameters. Differences between groups were compared using 2-way ANOVA with multiple comparisons or Kruskal-Wallis test. To account for multiple comparison errors, we applied a Bonferroni correction. Patients were stratified a priori as having autonomic failure due to lesion in the central or peripheral autonomic nervous system. The impact of central vs. peripheral neurodegenerative lesions on heart rate responses were examined with ANCOVA and post-hoc multiple comparisons.
We calculated the sensitivity and the specificity of the orthostatic HR increase to distinguish between neurogenic vs. non-neurogenic OH, and between neurogenic OH of central vs. peripheral origin using receiver-operating characteristic (ROC) curves assuming nonparametric conditions.31 With a similar method, we also calculated the sensitivity and specificity of the ΔHR/ΔSBP ratio, a marker of cardiac baroreflex gain, to distinguish between neurogenic vs. non-neurogenic OH, and between central vs. peripheral neurogenic OH. Differences between groups were further assessed with ANCOVA using age as a covariant.
Data were analyzed with SPSS 19.0 (SPSS, Chicago, IL, USA) and Prism (Graph-Pad Software, Inc., La Jolla, CA, USA) and expressed in mean ± standard deviation (SD) unless otherwise specified. Significance was set at p<0.05.
RESULTS
Subject characteristics
We identified 444 patients who met criteria for OH (Figure 1A). Of these, 42 patients (9.4%) were excluded because they were not in normal sinus rhythm at the time of testing (remaining cohort n=402). This sub-group of excluded patients with non-sinus rhythm was similar to those with normal sinus rhythm in age (p=0.247), distribution of diagnosis, and fall in BP after 3 minutes of head-up tilt (SBP p=0.895, DBP p=0.478).
As shown in Table 1, 378 cases had sympathetic and parasympathetic autonomic failure and were diagnosed as having neurogenic OH. The remaining 24 cases had preserved sympathetic and parasympathetic reflexes and were diagnosed with non-neurogenic OH. The age of the neurogenic OH cohort was 72±10 years (mean±SD, range: 98-44 years), similar to the non-neurogenic cohort. In the supine position BP was higher in the neurogenic than in the non-neurogenic cohort. The prevalence of supine hypertension in the neurogenic OH cohort was 71% (n=269/378).
Table 1.
Age, sex and autonomic features of patients with neurogenic and non-neurogenic orthostatic hypotension.
| Non-neurogenic OH | Neurogenic OH | Central | Peripheral | Prodromal | |
|---|---|---|---|---|---|
| n | 24 | 378 | 96 | 128 | 154 |
| Age, y | 68±10 | 72±10 | 65±1 | 76±7d | 72±12 |
| Sex, M:F | 13:11 | 231:147 | 52:44 | 81:47 | 98:56 |
| SBP supine, mmHg | 132±16 | 157±28a | 149±25 | 163±25d | 157±30 |
| SBP 3-min tilt, mmHg | 95±11 | 99±24 | 102±22 | 98±21 | 94±28 |
| DBP supine, mmHg | 74±12 | 85±16c | 84±16 | 85±14 | 85±18 |
| DBP 3-min tilt, mmHg | 48±3 | 64±14 | 65±15 | 64±9 | 64±14 |
| Heart rate supine, bpm | 70±13 | 71±12 | 76±12 | 70±11e | 68±12 |
| Heart rate 3-min tilt, bpm | 102±27 | 82±14a | 87±14 | 77±9 d | 77±13 |
| E:I ratio | 1.23±0.17 | 1.11±0.10a | 1.10±0.10 | 1.11±0.01 | 1.11±0.11 |
| HF HRV, mmHg2 | 495±942 | 128±23b | 140±233 | 108±191 | 151±284 |
| Valsalva ratio | 1.28±0.23 | 1.10±0.10a | 1.11±0.18 | 1.09±0.13 | 1.18±0.22 |
| Valsalva overshoot, ΔmmHg | 22±14 | −15±18a | −18±17 | −14±15 | −14±21 |
| Pressure recovery time, s | 8±6 | 27±18b | 26±15 | 26±15 | 31±24 |
| Baroreflex gain, ms/mmHg | 4.0±2.2 | 2.1±1.6c | 2.3±2.0 | 2.1±0.9 | 2.0±1.7 |
All data are mean±SD. Central lesions included cases of probable or possible MSA (49% from NYU, 43% from Mayo Clinic Rochester, 8% from Vanderbilt University). Peripheral lesions included patients with PAF, PD and DLB (88% from NYU, 8% from Vanderbilt University, 3% from Mayo Clinic Rochester, 1% from intramural NIH). 154 cases were classified as having prodromal CNS involvement based on clinical findings indicating and underlying central neurodegenerative process (REM behavior disorder with or without anosmia), but were excluded from lesion analysis, as the precise diagnosis was undetermined at the time of testing (82% from NYU, 11% from Mayo Clinic Rochester, 6% from Vanderbilt University, 1% from Beth Israel Deaconess Medical Center). Differences between non-neurogenic vs. neurogenic cohorts were assessed with non-parametric Mann-Whitney test to account for differences in the sample size.
p<0.0001,
p<0.001,
p<0.01. Central vs. peripheral lesions
p<0.0001,
p<0.001.
OH = orthostatic hypotension, M=male, F=female, SBP = systolic blood pressure, DBP = diastolic blood pressure, E:I = expiratory:inspiratory, HF HRV = high frequency heart rate variability. Data includes only patients with normal sinus rhythm.
Autonomic features
Also shown in Table 1, resting HR was almost the same in the neurogenic and non-neurogenic groups. All patients with neurogenic OH had evidence of baroreflex impairment with a profound decrease in SBP during phase II of the Valsalva strain (−54±29 mmHg) accompanied by only a small shortening of RR intervals (−116±121 ms). Pressure recovery time was prolonged in all cases confirming autonomic failure (Table 1).32, 33 The average baroreflex “cardiac gain” calculated with regression analysis using data from all phases of the Valsalva maneuver was lower in patients with neurogenic vs. non-neurogenic OH (p=0.0017), and lower than in age-matched healthy subjects.34
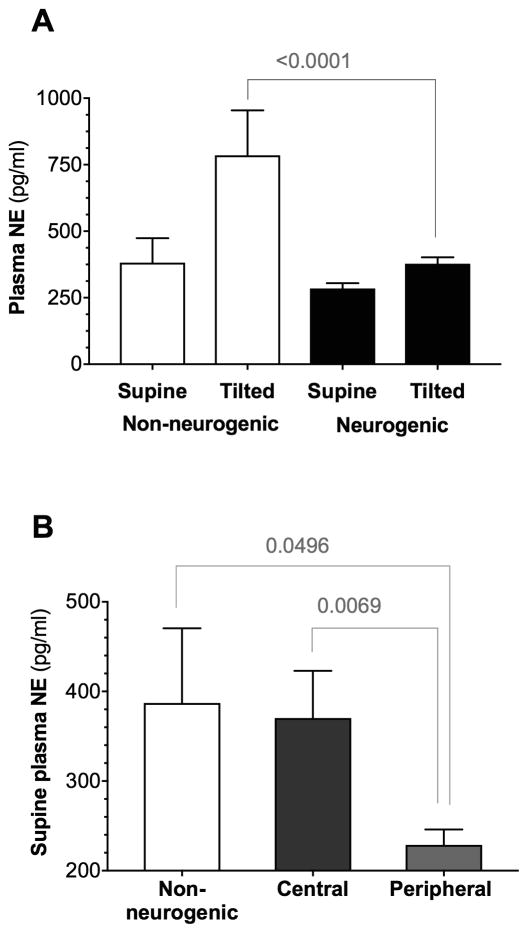
As shown in Figure 2, the normal increase in the plasma concentration of norepinephrine (NE) in the upright position was reduced in patients with neurogenic OH confirming impaired baroreflex-mediated sympathetic activation (+53±78% increase in NE, Δ%). In contrast, plasma norepinephrine concentration more than doubled with upright tilt in patients with non-neurogenic OH (+115±67 Δ%, p=0.015), confirming their fully functional baroreflex-mediated sympathetic activation.
Figure 2. Norepinephrine profiles in neurogenic and non-neurogenic orthostatic hypotension.
A. Plasma norepinephrine levels supine and after 10 minutes of upright tilt in patients with neurogenic vs. non-neurogenic orthostatic hypotension (OH). Note the preserved increase (~ Δ100%) in plasma norepinephrine levels in patients with non-neurogenic OH, indicating intact baroreflex-mediated sympathetic activation. B. Supine plasma norepinephrine levels in central and peripheral autonomic failure compared to non-neurogenic OH. Norepinephrine levels were lowest in patients with peripheral lesions (i.e., Parkinson disease, dementia with Lewy bodies, and pure autonomic failure), indicating severe involvement of post-ganglionic sympathetic neurons in this group. Differences assessed with ANOVA.
Orthostatic heart rate and blood pressure changes
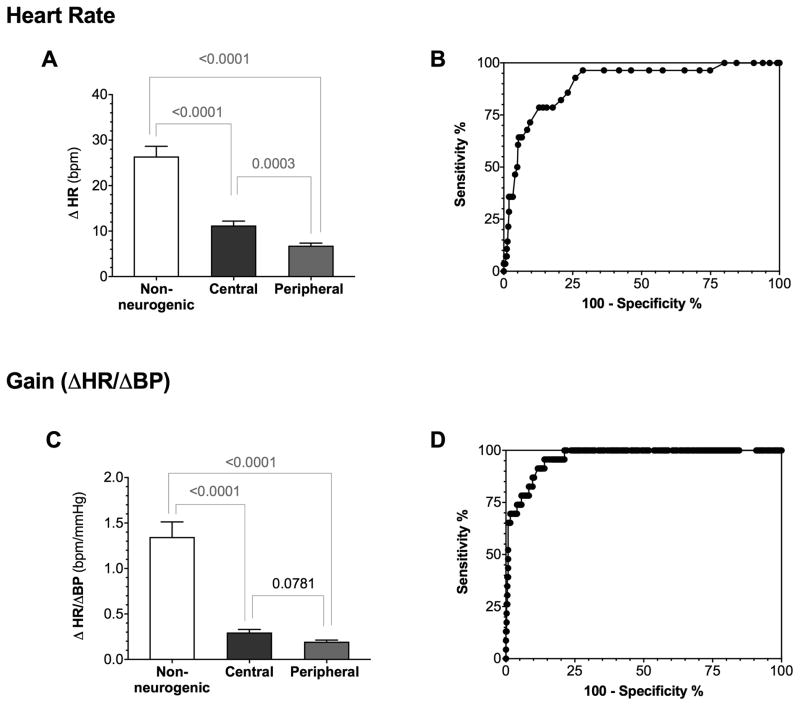
After 3 minutes of upright tilt, the BP fall was significantly more pronounced in the cohort with neurogenic OH (SBP: −43±25 vs. non-neurogenic −21±14 mmHg, p<0.0001). Despite the marked fall in BP in the neurogenic OH group, the HR increase was much lower than in the non-neurogenic group (+8±8 bpm vs. non-neurogenic +25±11 bpm, p<0.0001). In patients with neurogenic OH, the orthostatic HR response was unrelated to measures of parasympathetic function in the time-domain (pnn50 p=0.648; RMSSD p=0.131; SDNN p=0.738) or frequency-domain (high frequency [HF] HRV p=0.852).
The simple bedside measure of cardiac baroreflex gain calculated as the ratio of HR to SBP changes at 3-minutes of tilt (ΔHR/ΔSBP ratio) was significantly reduced in patients with neurogenic vs. non-neurogenic OH (0.227±0.256 vs. 1.347±0.794, p<0.0001, Figure 3C). This simple measurement of baroreflex function correlated (R2=0.1101; p=0.0001) with the more complex baroreflex sensitivity index calculated using the blood pressure and heart rate changes during the Valsalva maneuver.
Figure 3. Orthostatic heart rate changes in neurogenic vs. non-neurogenic orthostatic hypotension.
A. The orthostatic heart rate (HR) increase was significantly more pronounced in patients with non-neurogenic orthostatic hypotension (OH) compared to patients with neurogenic central or peripheral OH. Patients with peripheral neurogenic OH (i.e., Parkinson disease, dementia with Lewy bodies and pure autonomic failure) had significantly less HR increase than those with central neurogenic OH (i.e., multiple system atrophy, ANCOVA controlling for age p=0.023). While the orthostatic heart rate changes were more preserved in central autonomic failure, neither of these measurements provided a robust method to discriminate between central vs. peripheral autonomic failure. Panel B shows the ROC curve of the orthostatic HR changes revealing only moderate sensitivity and specificity to distinguish non-neurogenic vs. neurogenic OH. C. The ΔHR/ΔSBP ratio was significantly higher in patients with non-neurogenic compared to those with neurogenic OH but did not distinguish among patients with neurogenic OH. D. ROC curve showing the sensitivity and specificity of the ΔHR/ΔSBP ratio to distinguish between non-neurogenic and neurogenic OH, considerably better than using the orthostatic HR increase alone (B).
The orthostatic HR increase alone had poor discriminatory capacity to distinguish between neurogenic and non-neurogenic OH in individual patients. In this regard, a HR increase of < 17 bpm had only moderate sensitivity (79%) and specificity (87%) (AUC=0.89 95%CI: 0.83–0.95, p<0.0001) (Figure 3B). The ΔHR/ΔSBP ratio had a considerably better sensitivity and specificity to discriminate between patients with neurogenic and non-neurogenic OH than the HR increase alone. A ΔHR/ΔSBP ratio of 0.492 bpm/mmHg yielded the best-combined sensitivity (91.3%) and specificity (88.4%) to distinguish between neurogenic and non-neurogenic OH (AUC=0.96 95%CI: 0.95–0.99, p<0.0001, Figure 3D).
Impact of central vs. peripheral autonomic failure
The cohort included 96 patients with central autonomic failure (MSA) and 128 patients with peripheral autonomic failure (PAF=13, DLB=19, PD=96, Figure 1 and Table 1). The 13 patients with PAF included in the peripheral category had symptomatic neurogenic OH and no clinical signs of CNS deficits (no REM sleep behavior disorder, normal olfaction, and no subtle signs of motor impairment).22 One hundred and fifty-one patients with neurogenic OH also had anosmia or REM sleep behavior disorder and were thus classified as probable prodromal PD/DLB or MSA.22 Since these “prodromal” patients did not fit current diagnostic consensus criteria and it was unclear at the time of testing whether they would evolve into central (MSA) or peripheral (PD/DLB) forms of autonomic failure, they were excluded from the sub-group analysis to understand the impact of disease-specific lesions on HR.
Table 1 shows the age, sex, and autonomic features of the cohorts with central and peripheral lesions and a third group of patients considered to have probable prodromal disease. Parasympathetic function was equally impaired in patients with central vs. peripheral autonomic failure [RMSSD p=0.2554, pnn50 p=0.6701, HF HRV p=0.4519, and E:I ratio p=0.7038].
Among the patients with neurogenic OH, as shown in Figure 2, supine plasma norepinephrine levels were highest in patients with central autonomic failure (370±284 pg/ml), reflecting preserved post-ganglionic sympathetic neurons. Resting HR, HR in the upright tilt position, and the orthostatic HR change were higher in patients with central autonomic failure (Table 1 and Figure 3).
HR increased significantly more in patients with central than in those with peripheral autonomic failure. When age was used as a covariant, the orthostatic HR response remained significantly higher in patients with MSA compared to those with Lewy body disorders (ANCOVA, p=0.023). Because of overlap, however, the HR increase had no discriminatory capacity to distinguish between central and peripheral neurogenic OH in individual patients. A HR increase of < 10 bpm had poor sensitivity (52%) and specificity (65%) to do so (AUC=0.61 95%CI: 0.533–0.69, p=0.0084).
The ΔHR/ΔSBP ratio was not different between the central and peripheral groups. Similarly, the baroreflex gain index based on hemodynamic changes during the Valsalva maneuver also failed to show differences in patients with central vs. peripheral autonomic failure (Table 1).
DISCUSSION
Our results show that neurogenic OH can be diagnosed accurately based on the reduced increase in HR in relation to the fall in SBP. A ΔHR/ΔSBP ratio after 3 minutes in the standing position lower than 0.5 bpm/mmHg can discriminate patients with neurogenic from those with non-neurogenic OH with excellent sensitivity (91.3%) and specificity (88.4%).
In contrast, the distinction between central and peripheral causes of neurogenic OH is more challenging. HR rises significantly less in patients with Lewy body disorders (i.e., peripheral autonomic failure) than in patients with MSA (i.e., central autonomic failure, Figure 3A) but there was substantial overlap in the values. Using the ΔHR/ΔSBP ratio does not improve diagnostic accuracy. Nevertheless, differences in the HR rise are of physiological interest, and combined with other markers,22, 35 might help in the differential diagnosis of patients with neurogenic OH.
Patients with autonomic failure due to disorders that affect postganglionic sympathetic fibers innervating the heart (PAF, PD and DLB) had the smallest rise in HR during tilt (p=0.0002) while the largest increases in HR were recorded in patients with MSA in whom postganglionic sympathetic neurons and their axons innervating the heart are mostly spared.23, 36 Given that parasympathetic modulation of the heart was equally affected in central and peripheral lesions, the data suggests that it is the difference in residual cardiac sympathetic outflow that drives the greater HR rise. Indeed there was a weak, but significant association between the release of norepinephrine upright and the orthostatic heart rate rise (R2=0.072, p=0.0016). This is consistent with the observation that there is a biphasic mode of tachycardia elicited by the upright posture: initially it depends on parasympathetic withdrawal, but sympathetic stimulation is the predominant mechanism when stabilization in the orthostatic position is attained.7
Other reports have also showed a more preserved HR increase in patients with MSA compared to patients with PD,37 with chronotropic insufficiency having been described in the latter group.38, 39 Similarly, we have recently shown that patients with MSA typically have a HR increase >10 bpm, patients with PD/DLB have an average increase < 9 bpm, whereas those with PAF have the smallest increase of < 6 bpm.22 This supports the hypothesis that the extent of peripheral sympathetic denervation is the main determinant of the ability to increase HR on standing.
Patients with MSA have loss of autonomic neurons in the brainstem and the spinal cord with relative preservation of post-ganglionic sympathetic innervation.21, 40 Using stringent criteria to define PAF in the absence of signs indicating CNS involvement such as REM sleep behavior disorder and anosmia, we were able to select a cohort of patients with truly isolated peripheral autonomic failure.22, 41 Those with apparent prodromal disease will continue to be followed to ascertain the predictive value of the HR changes and the prognostic implications.
In patients with neurodegenerative autonomic failure as a result of abnormal deposition of the protein α-synuclein, preservation of the peripheral sympathetic nerves and less blunted HR responses to tilt were indicative of a worse prognosis (i.e., a diagnosis of MSA22). In line with this, the usefulness of the HR rise was recently validated prospectively as an autonomic biomarker in discriminating the future risk of a patient that presents with isolated nOH in the prodromal phase, later being diagnosed with MSA or PD/DLB, when other causes of impaired cardiovagal function, including type-2 diabetes mellitus,42 have been ruled out.22 Specifically, all patients presenting with neurogenic OH who later developed MSA had an increase in HR of more than 10 bpm during head-up tilt, whereas all those who eventually developed DLB/PD had a rise of less than 11 bpm.
The cohort here described included 154 prodromal patients who were classified as having early, prodromal PD/DLB or prodromal MSA. Most of these patients would have classically been considered as having PAF, but on account of them having subtle motor signs, anosmia, and/or REM behavior disorder, they had already signs of early CNS involvement. Since at the time of writing, it was unclear whether these patients would evolve into PD/DLB or MSA, we could not ascertain unequivocally whether they had a central or peripheral lesion. We suspect that given that the prodromal cohort is currently older in age, have slower resting HR, and their ΔHR/ΔSBP ratio is significantly lower compared to patients with central autonomic failure (p=0.020), the vast majority will eventually be diagnosed with PD or DLB, rather than MSA. This is consistent with our recently reported data on the natural history of PAF, where only 24% of patients with autonomic failure and biomarkers of early CNS involvement phenoconverted to MSA within 4 years.22 Thus, finding a central autonomic lesion in a patient with autonomic failure has prognostic value.
Our study has limitations. Diagnosis was made clinically and pathological confirmation was lacking. The HR changes were measured in response to tilt (i.e., passive standing) and not in response to active standing, which may be more widely used in the clinical setting, although it should be expected to be similar.43 A blunted HR response to standing has also been described in patients with OH due to diabetic autonomic neuropathy.42, 44 We specifically excluded patients with diabetes mellitus, however, because of their frequent cardiovascular comorbidities, dehydration, prevalence of antihypertensive medication, and other factors that would make it more difficult to interpret the HR changes. While we did capture a range of severities of autonomic failure in the cohort, it may also be possible that owing to referral bias, the results are skewed towards more severe cases of autonomic failure, which were referred to specialized autonomic clinics. The number of cases with non-neurogenic OH was smaller, but did allow for non-parametric statistical comparisons. We did not include a comparison group of normal controls, as this would have required provocation to induce blood pressure changes, and would not be directly comparable. Nevertheless, reports in the literature show baroreflex gain in healthy elderly subjects is > 2.0 bpm/mmHg, and therefore considerably higher than the ones we here describe in patients with neurogenic OH.34, 45, 46 Calculating baroreflex gain from the hemodynamic changes in the upright posture may involve inputs from other afferent systems than those involved during the Valsalva maneuver, it nevertheless appears to be a robust and simple physiological measure that can be obtained at the bedside without the need for extensive testing.
By virtue of MSA having an earlier age at onset, patients with central autonomic failure were younger than those with peripheral autonomic failure owing to Lewy body disorders. Nevertheless, when age was used as a covariant, the difference in the orthostatic HR response remained significant (ANCOVA, p=0.023) with patients with MSA having a rise of +11 bpm compared to +7 bpm in patients with Lewy body disorders. Adding other clinical features, like a younger age at onset and early bladder involvement22, 47, 48 might help further define phenoconversion to MSA based on the central localization of the lesion responsible for autonomic failure.
In conclusion, a diagnosis of neurogenic OH is best established using the ΔHR/ΔSBP ratio. HR increased significantly more in patients with neurogenic OH with central autonomic lesions due to MSA than in those with peripheral autonomic lesions due to Lewy body disorders, but there was considerable overlap. In patients with OH, reduced HR responses to the upright position with a ΔHR/ΔSBP ratio < 0.5 bpm/mmHg require additional screening for primary and/or secondary causes of autonomic failure, such as a neurodegenerative synucleinopathy. Early identification of neurogenic OH may reduce delays in treatment, decrease the need for expensive testing, and could provide information to assist in defining prognosis, especially when used in combination with additional discriminatory biomarkers.
Acknowledgments
Supported by the NIH Rare Disease Clinical Research Network (U54-NS065736, all authors).
Footnotes
Author Contributions
Conceptualized and designed the study: H.K., L.N.-K., I.B., P.A.L., D.S.G.
Acquisition and data analysis: H.K., L.N.-K., J.-A.P., I.B., P.A.L., W.S., D.S.G., A.C.P.,C.A.S., C.H.G., R.F.
Drafted the initial version of the manuscript and figures: H.K., L.N.-K., J.-A.P.
Potential Conflicts of Interest
Nothing to report.
References
- 1.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2011 Apr;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 2.Palma JA, Kaufmann H. Epidemiology, Diagnosis, and Management of Neurogenic Orthostatic Hypotension. Mov Disord Clin Pract. 2017 May-Jun;4(3):298–308. doi: 10.1002/mdc3.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman R. Clinical practice. Neurogenic orthostatic hypotension. The New England journal of medicine. 2008 Feb 7;358(6):615–24. doi: 10.1056/NEJMcp074189. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann H, Palma JA. Neurogenic orthostatic hypotension: the very basics. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017 Jul;27(Suppl 1):39–43. doi: 10.1007/s10286-017-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 1996 Apr;6(2):125–6. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. Journal of neurology. 2017 Aug;264(8):1567–82. doi: 10.1007/s00415-016-8375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Convertino VA. Neurohumoral mechanisms associated with orthostasis: reaffirmation of the significant contribution of the heart rate response. Frontiers in physiology. 2014;5:236. doi: 10.3389/fphys.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufmann H, Goldstein DS. Autonomic dysfunction in Parkinson disease. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 117. 2013. pp. 259–78. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DS. Dysautonomia in Parkinson disease. Comprehensive Physiology. 2014 Apr;4(2):805–26. doi: 10.1002/cphy.c130026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Movement disorders : official journal of the Movement Disorder Society. 2015 Apr 15;30(5):639–45. doi: 10.1002/mds.26079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alafuzoff I, Hartikainen P. Alpha-synucleinopathies. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 145. 2017. pp. 339–53. [DOI] [PubMed] [Google Scholar]
- 12.Fanciulli A, Wenning GK. Multiple-system atrophy. The New England journal of medicine. 2015 Jan 15;372(3):249–63. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- 13.Carod-Artal FJ. Infectious diseases causing autonomic dysfunction. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017 Jul 20; doi: 10.1007/s10286-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DS, Low PA. Clinical evaluation of the autonomic nervous system. Continuum (Minneap Minn), Autonomic Disorders. 2007;13(6):33–49. [Google Scholar]
- 15.Goldstein DS, Cheshire WP., Jr Beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017 Dec;27(6):361–7. doi: 10.1007/s10286-017-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isonaka R, Sullivan P, Jinsmaa Y, Corrales A, Goldstein DS. Spectrum of abnormalities of sympathetic tyrosine hydroxylase and alpha-synuclein in chronic autonomic failure. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2018 doi: 10.1007/s10286-017-0495-6. [DOI] [PubMed] [Google Scholar]
- 17.Coon EA, Cutsforth-Gregory JK, Benarroch EE. Neuropathology of autonomic dysfunction in synucleinopathies. Movement disorders : official journal of the Movement Disorder Society. 2018 Jan 3; doi: 10.1002/mds.27186. [DOI] [PubMed] [Google Scholar]
- 18.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008 Aug 26;71(9):670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases [see comments] Journal of neurology, neurosurgery, and psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017 Jul 4;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jellinger KA. Neuropathology of multiple system atrophy: new thoughts about pathogenesis. Movement disorders : official journal of the Movement Disorder Society. 2014 Dec;29(14):1720–41. doi: 10.1002/mds.26052. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann H, Norcliffe-Kaufmann L, Palma JA, et al. Natural history of pure autonomic failure: A United States prospective cohort. Annals of neurology. 2017 Feb;81(2):287–97. doi: 10.1002/ana.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Annals of internal medicine. 2000 Sep 5;133(5):338–47. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 24.Iranzo A, Stefani A, Serradell M, et al. Characterization of patients with longstanding idiopathic REM sleep behavior disorder. Neurology. 2017 Jul 18;89(3):242–8. doi: 10.1212/WNL.0000000000004121. [DOI] [PubMed] [Google Scholar]
- 25.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2015 Oct;30(12):1600–11. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein DS, Cheshire WP., Jr The autonomic medical history. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017 Aug;27(4):223–33. doi: 10.1007/s10286-017-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Electrophysiology TFotESoCtNASoP. Heart Rate Variabilty: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 28.Levin AB. A simple test of cardiac function based upon the heart rate changes induced by the Valsalva maneuver. The American journal of cardiology. 1966;18(1):90–9. doi: 10.1016/0002-9149(66)90200-1. [DOI] [PubMed] [Google Scholar]
- 29.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010 Nov 23;75(21):1904–11. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA : the journal of the American Medical Association. 2014 Feb 5;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 31.Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristic curves. Critical care. 2004 Dec;8(6):508–12. doi: 10.1186/cc3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrezenmaier C, Singer W, Swift NM, Sletten D, Tanabe J, Low PA. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Archives of neurology. 2007 Mar;64(3):381–6. doi: 10.1001/archneur.64.3.381. [DOI] [PubMed] [Google Scholar]
- 33.Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005 Nov 22;65(10):1533–7. doi: 10.1212/01.wnl.0000184504.13173.ef. [DOI] [PubMed] [Google Scholar]
- 34.Norcliffe-Kaufmann L, Kaufmann H, Martinez J, Katz SD, Tully L, Reynolds HR. Autonomic Findings in Takotsubo Cardiomyopathy. The American journal of cardiology. 2016 Jan 15;117(2):206–13. doi: 10.1016/j.amjcard.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Palma JA, Kaufmann H. Autonomic disorders predicting Parkinson’s disease. Parkinsonism & related disorders. 2014 Jan;20( Suppl 1):S94–8. doi: 10.1016/S1353-8020(13)70024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orimo S, Uchihara T, Nakamura A, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008 Mar;131(Pt 3):642–50. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 37.Pilleri M, Levedianos G, Weis L, et al. Heart rate circadian profile in the differential diagnosis between Parkinson disease and multiple system atrophy. Parkinsonism & related disorders. 2014 Feb;20(2):217–21. doi: 10.1016/j.parkreldis.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Palma JA, Carmona-Abellan MM, Barriobero N, et al. Is cardiac function impaired in premotor Parkinson’s disease? A retrospective cohort study. Movement disorders : official journal of the Movement Disorder Society. 2013 May;28(5):591–6. doi: 10.1002/mds.25431. [DOI] [PubMed] [Google Scholar]
- 39.DiFrancisco-Donoghue J, Elokda A, Lamberg EM, Bono N, Werner WG. Norepinephrine and cardiovascular responses to maximal exercise in Parkinson’s disease on and off medication. Movement disorders : official journal of the Movement Disorder Society. 2009 Sep 15;24(12):1773–8. doi: 10.1002/mds.22612. [DOI] [PubMed] [Google Scholar]
- 40.Cykowski MD, Coon EA, Powell SZ, et al. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015 Aug;138(Pt 8):2293–309. doi: 10.1093/brain/awv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufmann H, Norcliffe-Kaufmann L, Palma JA Autonomic Disorders C. Reply to “Pure autonomic failure vs. Manifest CNS synucleinopathy: Relevance of stridor and autonomic biomarkers”. Annals of neurology. 2017 Jun;81(6):910–1. doi: 10.1002/ana.24949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa A, Bosone D, Ramusino MC, et al. Twenty-four-hour blood pressure profile, orthostatic hypotension, and cardiac dysautonomia in elderly type 2 diabetic hypertensive patients. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2016 Dec;26(6):433–9. doi: 10.1007/s10286-016-0381-7. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka H, Sjoberg BJ, Thulesius O. Cardiac output and blood pressure during active and passive standing. Clin Physiol. 1996 Mar;16(2):157–70. doi: 10.1111/j.1475-097x.1996.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 44.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007 Jan 23;115(3):387–97. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 45.Scheen AJ, Philips JC. Squatting test: a dynamic postural manoeuvre to study baroreflex sensitivity. Clin Auton Res. 2012 Feb;22(1):35–41. doi: 10.1007/s10286-011-0140-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhang R, Claassen JA, Shibata S, et al. Arterial-cardiac baroreflex function: insights from repeated squat-stand maneuvers. American journal of physiology Regulatory, integrative and comparative physiology. 2009 Jul;297(1):R116–23. doi: 10.1152/ajpregu.90977.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKay JH, Cheshire WP. First symptoms in multiple system atrophy. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2018 Jan 8; doi: 10.1007/s10286-017-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakakibara R, Tateno F, Yamamoto T, Uchiyama T, Yamanishi T. Urological dysfunction in synucleinopathies: epidemiology, pathophysiology and management. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017 Nov 9; doi: 10.1007/s10286-017-0480-0. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann H, Oribe E, Miller M, Knott P, Wiltshire-Clement M, Yahr MD. Hypotension-induced vasopressin release distinguishes between pure autonomic failure and multiple system atrophy with autonomic failure. Neurology. 1992 Mar;42(3 Pt 1):590–3. doi: 10.1212/wnl.42.3.590. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003 Apr 22;60(8):1327–32. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein DS, Orimo S. Cardiac sympathetic neuroimaging: summary of the First International Symposium. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2009 Jun;19(3):137–48. doi: 10.1007/s10286-009-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]