Abstract
The vitamin folic acid has been recognized as a crucial environmental factor for nervous system development. From the early fetal stages of the formation of the presumptive spinal cord and brain to the maturation and maintenance of the nervous system during infancy and childhood, folate levels and its supplementation have been considered influential in the clinical outcome of infants and children affected by neurological diseases. Despite the vast epidemiological information recorded on folate function and neural tube defects, neural development and neurodegenerative diseases, the mechanisms of folate action in the developing neural tissue have remained elusive. Here we compiled studies that argue for a unique role for folate in nervous system development and function and its consequences to neural disease and repair.
Keywords: Folic acid, neural tube defects, folate receptor
INTRODUCTION
For more than 50 years epidemiological studies have demonstrated that periconceptional folate supplementation reduces the occurrence of one of the most common serious birth defects, called neural tube defects (NTDs). These defects manifest when the neural tube fails to close and form properly during the first four weeks of pregnancy (Wallingford et al., 2013). It is believed that neural tissue exposed to the amniotic fluid eventually degenerates (Meuli and Moehrlen, 2014), leading to severe neurological deficits that are lethal in anterior NTDs (anencephaly), or result in paralysis or motor disability when they occur at the spinal cord (spina bifida).
Strikingly, detailed understanding of the mechanisms of folate action during neural tube formation has remained elusive. Folate belongs to the vitamin B family and it participates in methylation reactions such as those necessary for nucleotide synthesis. Hence, folate supports rapid growth by enabling DNA synthesis in proliferating cells. It has been shown that one of the cellular processes involved in normal formation of the neural tube in some vertebrates is oriented cell division (Schoenwolf and Alvarez, 1989; Schoenwolf and Yuan, 1995; Sausedo et al., 1997). This allows the developing neural tissue to acquire critical mass, and provides the preferred rostrocaudal orientation so that these divisions contribute to the elongation of the developing neural tube in chick and mouse embryos (Schoenwolf and Alvarez, 1989; Schoenwolf and Yuan, 1995; Sausedo et al., 1997; Keller, 2006). On the other hand, in other vertebrates like Xenopus laevis, the necessity for cell divisions during neural tube formation has been ruled out by demonstrating that blocking DNA synthesis does not interfere with neurulation (Harris and Hartenstein, 1991). Nevertheless, folate action is necessary during Xenopus laevis neural tube formation, as recently demonstrated by our study (Balashova et al., 2017), suggesting that folate might play additional functions during neural tube formation besides its role as a vitamin for DNA synthesis. Moreover, many knockout mice for enzymes directly involved in folate metabolism did not exhibit increases in NTD incidence (Watanabe et al., 1995; Chen et al., 2001; Swanson et al., 2001). In contrast, knocking out one of the folate uptake systems, folate receptor 1 (Folr1/folate binding protein 1/folate receptor α) leads to severe NTDs in mice (Piedrahita et al., 1999; Finnell et al., 2002; Spiegelstein et al., 2004; Wallingford et al., 2013). Similarly, mutations in folate uptake systems have been reported in families with higher incidence of NTDs than the general population, including 2 SNPs in FOLR1 (O’Byrne et al., 2010). These studies strongly indicate that folate acts as an essential regulator of neural tube morphogenesis, probably independent of nucleotide synthesis.
Here, we review the findings that address the role of folate in the development of the nervous system and folate’s effects on neural function and repair. These studies argue for specialized functions of folate in neural tissue and propose new questions that require further investigation to be answered on the mechanisms of folate action in nervous system development, disease and regeneration.
FOLATE AND FOLATE UPTAKE SYSTEMS
Folic acid/folate (pteroyl-L-glutamic acid and pteroyl-L-glutamate, respectively) belongs to the B9 family of vitamins. Chemically, folic acid is a protonated form of folate anion present in solution; therefore the terms are used interchangeably. However, in the field of nutrition the term “folates” is frequently used to refer to the chemically diverse mixture of naturally occurring folic acid derivatives - pteroylglutamates. Pteroylglutamates may vary in one-carbon donor groups, degree of pteridine ring reduction and number of glutamate residues (O’Broin et al., 1975; Lewis et al., 1999).
In animals, folate cannot be synthesized de novo. Dietary folate is obtained in the form of dihydrofolate, tetrahydrofolate or 5-methyltetrahydrofolate, which are naturally present in a variety of foods, or as synthetic folic or folinic acid added to fortified food (O’Broin et al., 1975; Eitenmiller and Landen, 1999; Lewis et al., 1999). Folate is needed to carry one-carbon groups for methylation reactions and nucleotide base synthesis, and is therefore indispensable for DNA replication and repair, as well as RNA synthesis (Figueiredo et al., 2009). Folate is also involved in histone and DNA methylation, methionine production and homocysteine remethylation reactions (Stover, 2009). Thus, folates are especially important during early embryogenesis, which is characterized by rapid cell divisions. In addition, folate is necessary for aminoacid metabolism, neurotransmitter and phospholipid biosynthesis (Desai et al., 2016; Mentch and Locasale, 2016). Numerous clinical studies have established a correlation between low folate levels and increased risk of NTDs (Milunsky et al., 1989, 1991a; 1991b; Simpson et al., 1991), cardiovascular disorders (Bazzano et al., 2006; Wang et al., 2006; Wang et al., 2007), cognitive malfunction (Mitchell et al., 2014) and certain types of cancer (Kim, 1999; Sanjoaquin et al., 2005; Cole et al., 2007; Johansson et al., 2008) highlighting the importance of understanding the mechanisms of folate action.
Folates are hydrophilic molecules whose cellular uptake employs three transport systems: the proton-coupled folate transporter (PCFT), reduced-folate carrier 1 (RFC1), and folate receptors (FOLRs, also called folate binding proteins) (Matherly and Goldman, 2003; Kamen and Smith, 2004; Zhao and Goldman, 2007) (Table 1). PCFT is the main transporter for intestinal absorption of folate and plays a major role in folate homeostasis in humans (Zhao et al., 2009a; Zhao et al., 2009b). RFC1 is a typical anion antiporter that utilizes the gradient of organic phosphate across the cell membrane to transport folate into the cells at neutral pH (Goldman, 1971; Sirotnak and Tolner, 1999). FOLRs are high affinity folate binding proteins. In mammals, there are four known genes for FOLR. FOLR1, 2 and 4 are linked to the plasma membrane by a carboxy terminus - glycosylphosphatidylinositol (GPI) anchor (Kamen and Caston, 1986; Ratnam et al., 1989; Wang et al., 1992; Shen et al., 1994). FOLR1 is expressed in a wide variety of cell types and tissues including epithelial tissues (Weitman et al., 1992; Antony, 1996; Smith et al., 1999; Chancy et al., 2000). FOLR2 expression is restricted to hematopoietic tissues (Reddy et al., 1999). FOLR4 (also known as Juno) is found in the membrane of egg cells, where it facilitates fertilization via recognition of complementary sperm protein (Bianchi et al., 2014). FOLR3 is a secreted protein (Shen et al., 1995).
Table 1.
| PCFT | RFC1 | FOLR1 | FOLR2 | FOLR3 | FOLR4 | |
|---|---|---|---|---|---|---|
| Tissue | Intestine, liver, kidney, choroid plexus | Ubiquitously expressed | Choroid plexus, neural plate, placenta, retina, renal tubules, dorsal root ganglia neurons, dopaminergic neuronal precursors | Hemato-poietic tissues, placenta | Secreted | Oocyte |
| Subcellular localization | Apical brush border of small intestine epithelial cells/basal membrane of hepatocytes | Trans-membrane carrier | Apical surface neural plate cells | Unclear | NA | GPI-anchored at the oocyte cell membrane |
| Kinetics/mode of folate uptake | High affinity/highly efficient transporter at low pH | Low affinity/highly efficient phosphate antiporter at neutral pH | High affinity/endocytosis | High affinity/endocytosis | High affinity/endocytosis | Does not bind folate |
| Role | Intestinal folate absorption Folate homeostasis |
Major route of folate delivery into cell of systemic tissues | Apical constriction of neural plate cells during neural tube formation | Unclear | Folate transport | Sperm-egg fusion |
The field of cancer research has elucidated some aspects of the biochemistry and molecular function of folate transporters, since these transporters are often overexpressed in tumors and cancer cells, and have been used as conduits for anticancer drug therapeutics. From these studies we have learned that folate uptake by FOLRs and FOLR recycling in cancer-derived and immortalized mammalian cell lines occurs through endocytosis. The exact mechanisms of FOLR endocytosis vary depending on the experimental model used (Rothberg et al., 1990; Rijnboutt et al., 1996; Mayor et al., 1998; Kamen and Smith, 2004; Bhagatji et al., 2009; Elnakat et al., 2009). Acidification of the endosome interior to a pH of 6.5, as occurs in early endosomes, is required for dissociation of folate from FOLRs (Yang et al., 2007). Some studies support a model for FOLR1 internalization upon ligand binding through a Cdc42-regulated actin-dependent pathway, which is shared by the majority of GPI-anchored proteins (GPI-APs). Others have shown that FOLR1 is endocytosed in cholesterol and sphingolipid-enriched cell surface invaginations (Kamen et al., 1988) and once incorporated into vesicles, proceeds into GPI-AP-enriched early endosomal compartment (Mayor et al., 1998; Sabharanjak et al., 2002). This is followed by microtubular delivery to the recycling endosomal compartment via Rab7/dynein and Rab 4/kinesin I-dependent trafficking as demonstrated in KB and HeLa cells (Chen et al., 2008).
FOLRs subcellular localization also varies based on the cell type. FOLR1 is found in the apical cell surfaces of choroid plexus, placenta, brush-border membrane of proximal renal tubular cells and superficial neural plate cells (Weitman et al., 1992; Antony, 1996; Parker et al., 2005; Balashova et al., 2017) and basolateral membrane of retinal pigment epithelium (Smith et al., 1999; Chancy et al., 2000). Its GPI post-translational modification is found in organisms ranging from protozoans to mammals and serves to anchor a great variety of functionally different proteins to the outer leaflet of membranes (Lakhan et al., 2009). The polarized nature of FOLR1 subcellular localization is a characteristic of GPI-APs (Paladino et al., 2006), which are also known to be recruited to lipid rafts and to elicit downstream signaling triggered by GPI-AP endocytosis (Watanabe et al., 2007; Watanabe et al., 2009; Tassew et al., 2012). In addition, a number of GPI-APs have been shown to interact with transmembrane signaling molecules, including those involved in cell-cell and cell-matrix adhesion (Davy et al., 1999; Monnier et al., 2002; Paratcha et al., 2003; Matsunaga et al., 2004; Tassew et al., 2012). These properties of GPI-APs predict that FOLRs might play additional roles besides folate uptake.
FOLATE ACTION DURING NEURAL TUBE FORMATION AND IN NEURAL TUBE DEFECTS
The prevailing model for folate action during neural tube formation is centered on folate metabolism enabling nucleic acid biosynthesis, cell proliferation and growth. Knockout mice on some enzymes participating in folate metabolism support this model. For instance, mice lacking the mitochondrial monofunctional 10-formyl-tetrahydrofolate synthetase (Mthfd1l) exhibit developmental delay and aberrant neural tube closure (Momb et al., 2013). The NTD phenotype is partially rescued by formate supplementation, suggesting that formate derived from mitochondria is necessary for adequate development and formation of the neural tube (Momb et al., 2013). Supporting the metabolic function of folate in neural tube defects, the incidence of NTDs is elevated in cases where either the infant or the mother are homozygous for methylenetetrahydrofolate reductase (van der Put et al., 1995; van der Put et al., 1996; Blom et al., 2006), although not every ethnic group studied exhibited this association (Papapetrou et al., 1996; Wilcken and Wang, 1996; Mornet et al., 1997; Speer et al., 1997; Koch et al., 1998). However, this model is thrown into question by mice in which several enzymes involved in folate metabolism are disrupted but do not show NTDs (Watanabe et al., 1995; Chen et al., 2001; Swanson et al., 2001), as well as screenings in humans that found cases with innate errors in folate metabolism which are not associated with increased incidence of NTDs (Blom et al., 2006).
One of the first folate-related mouse models of neural tube defects was the Folbp1 (mouse FOLR1) knockout, which is embryonic lethal and associated with NTDs (Piedrahita et al., 1999; Tang et al., 2005), indicating that FOLR1 is necessary for neurulation. Although FOLR1 is able to bind folate with high affinity, transport through an endocytotic mechanism is less efficient for providing adequate levels of folate to the cell compared to the reduced folate carrier, as shown in leukemia cells (Sierra et al., 1995; Spinella et al., 1995). This suggests that folate transport may not be the primary function of FOLR1. At present the physiological role of FOLR1 in normal tissues is unclear, although disruption of Folbp1 gene results in misexpression of a number of essential signaling molecules, including upregulation of the ventralizing signal sonic hedgehog in abnormally expanded midbrain floor plate (Tang et al., 2005). Endocytosis of FOLR1 in the neuroepithelium appears to be dependent on the multifunctional endocytic receptor complex formed, among others, by Megalin, also known as LRP2. When LRP2 is knocked out, the neuroepithelium is deficient in folate and mice exhibit rostral neural tube closure defects (Kur et al., 2014).
The aforementioned studies strongly argue for a role of folate and FOLR1 in neural tube formation. Our recently published study was aimed at identifying the specificity of their function in terms of cellular processes regulated by folate action and the identity of targeted tissues dependent on FOLR1 role during neurulation (Balashova et al., 2017). We found that from the early stages of neural plate folding in Xenopus laevis, FOLR1 is expressed in superficial neural plate cells, where it localizes to the apical membrane. Similarly, in mouse embryos FOLR1 colocalizes with LRP2 in the dorsal neuroepithelial midline, which corresponds to the site where Closure 2 is initiated, and in the ventral midline of the midbrain neuroepithelium (Kur et al., 2014). Interestingly, knocking down FOLR1 in Xenopus neural plate cells only, elicits NTDs, while FOLR1 knockdown from non-neural ectoderm or mesoderm does not (Balashova et al., 2017), suggesting a specific requirement for FOLR1 in neural plate cells, essential for the formation of the neural tube.
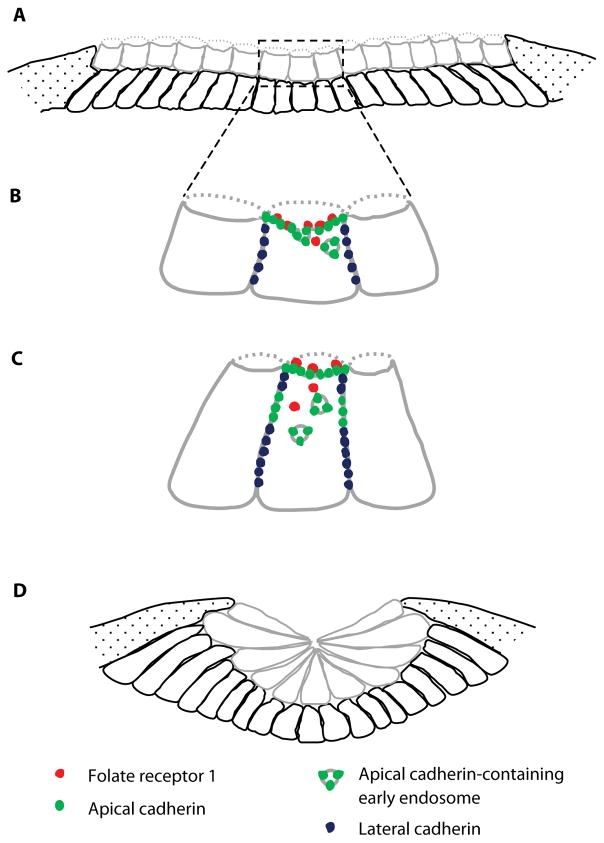
We demonstrated that FOLR1 interacts with apically enriched components of adherens junctions like C-cadherin, the most abundant cadherin in the neural plate (Nandadasa et al., 2009), and β-catenin (Balashova et al., 2017), suggesting potential roles for FOLR1 in cell adhesion and cytoskeletal organization. One of the early and crucial cellular processes during neural plate bending is the apical constriction of neural plate cells, which occurs in frog, chick and mouse embryos, although at varying mediolateral and anteroposterior regions of the neuroepithelium. The Agarwala lab has demonstrated that the spatiotemporal regulation of apicobasal polarity in the folding neural plate by bone morphogenetic protein and transforming growth factor beta signaling drives the apical constriction of neural plate cells in the middle hinge point of neurulating chick embryos (Eom et al., 2011; Eom et al., 2012; Amarnath and Agarwala, 2017). Similarly, in frog embryos the medial and anterior localizations of planar cell polarity molecules at the neural plate midline are required for the changes in cell shape and movement necessary for neural plate folding and neural tube formation (Ossipova et al., 2014; Ossipova et al., 2015). In turn, neural cell apical constriction is postulated to be driven by the contraction of the actomyosin network anchored at the apical adherens junctions (Haigo et al., 2003; Nishimura and Takeichi, 2008; Martin et al., 2009). The precise dynamics of the proteins that form the adherens junctions during this process remain to be determined. Nevertheless, studies from the Sokol lab have demonstrated that Rab11-containing recycling endosomes accumulate in the apical adherens junctions of medial neural plate cells through a planar cell polarity- and Rho family GTPase GEF-H1-dependent mechanism, and this apical Rab11 localization is necessary and sufficient for apical constriction (Itoh et al., 2014; Ossipova et al., 2014). We discovered that FOLR1 is necessary for apical constriction of neural plate cells and C-cadherin endocytosis, which is apparent in constricting neural cells (Balashova et al., 2017); the necessity of this process becomes obvious when considering the dramatic reduction in cell apical surface occurring during apical constriction, which has to be accompanied by removal of the excess of apical membrane and transmembrane proteins, including those mediating cell-cell adhesion (Fig. 1). Indeed, it has been shown that endocytosis is involved in apical membrane removal during contraction of cell apical surface in a number of model systems (Lee and Harland, 2010; Mateus et al., 2011). We propose that endocytosis of adhesion proteins facilitated by FOLR1 may be needed to enable reduction of apical cell circumference and redistribution of adhesion molecules from cell apexes to lateral intercellular contacts to accommodate for dynamic cell shape changes during neurulation (Fig. 1). Interestingly, a model of neurulation named “cortical tractor” has been proposed in which recycling of cell adhesion proteins from the apical circumference to the cell basolateral surface is required for cell shape changes (Jacobson et al., 1986; Jacobson, 1994). More recently it has been demonstrated that collectively migrating astrocytes utilize a mechanism similar to the cortical tractor to recycle N-cadherin molecules (Peglion et al., 2014).
Figure 1.
Model of the role of folate receptor 1 in neural plate folding. (A) Two layers (grey – superficial, black – deep) of Xenopus laevis neural plate cells at the beginning of folding show a cuboidal and non-polarized morphology. Speckled regions represent non-neural ectoderm. (B) In superficial neural plate cells folate receptor 1 (red) and cadherins (green) localize to the apical membrane. Cadherins are also enriched in the cell lateral border (blue). Circles inside the cell represent early endosomes containing apical cadherin. (C) Folate receptor 1-dependent cadherin endocytosis results in redistribution of adhesion molecules and membrane from the cell apex to the lateral cell border, facilitating constriction of the apical pole and elongation of the neural plate cell. (D) Apical constriction along with other morphogenic behaviors such as cell elongation and intercalation lead to folding of the neural plate. Model derived from Balashova et al., 2017.
Our study (Balashova et al., 2017) suggests that the role of folate and FOLR1 during neural tube formation may not be related to its metabolic function as a vitamin (Fig. 2). Intriguingly, folate is a chemoattractant for the model unicellular organism Dictyostelium discoideum, in which it elicits changes in the cytoskeleton that enable the amoeba to crawl towards the food source (McRobbie and Newell, 1983; Yumura, 1994). The mechanisms of the chemoattraction involve the amoeba’s folate receptor, which is a G-protein coupled receptor (Wang et al., 1988; Pan et al., 2016) that elicits calcium transients, which in turn modify cytoskeletal protein dynamics (Europe-Finner and Newell, 1985; Nebl and Fisher, 1997). The identification of the signaling mechanisms that link folate/FOLR1 with regulators of the cytoskeleton and cell adhesion remodeling during neural tube formation in vertebrates demands further investigation.
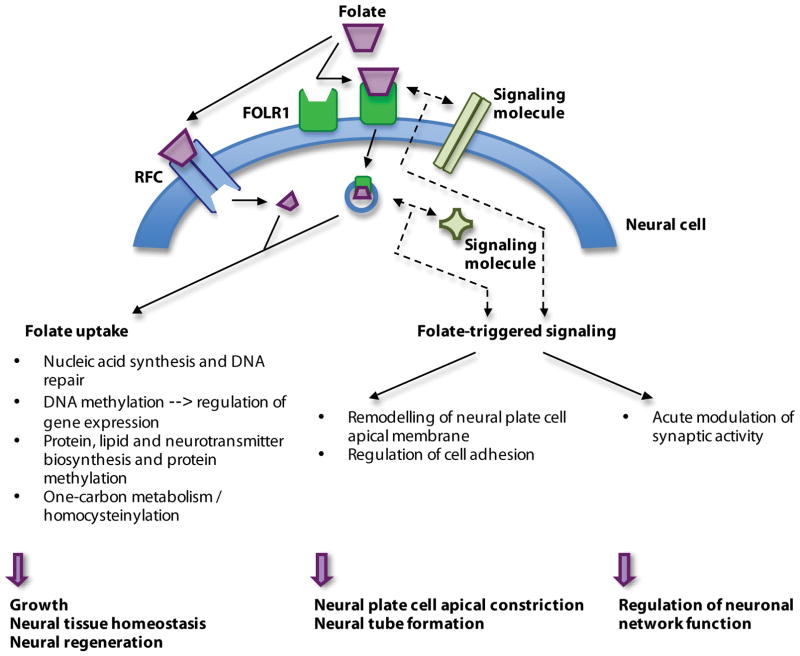
Figure 2.
Mechanisms of folate action in the developing and mature nervous system. Folate is taken into neural cells through folate receptor 1 (FOLR1) or reduced folate carrier (RFC) to be used in DNA and RNA biosynthesis, DNA repair and one-carbon metabolic reactions. These roles of folate as a vitamin are needed for neural cell division during periods of rapid growth and neural tissue repair. Alternatively, folate interacts with its receptor, triggering specific signaling mechanisms that enable the shaping of the neural plate during neural tube formation and modulation of synaptic activity to facilitate neural function.
FOLATE AND NEURAL FUNCTION
The involvement of folate in nervous system development and function transcends the early stages of formation of the neural tube. For instance, a dietary folic acid deficiency that results in increased homocysteine blood levels due to the deficient conversion of homocysteine into methionine, leads to a decrease in the number of proliferating neural progenitors in the dentate gyrus, affecting the neurogenesis necessary for learning and memory tasks (Kruman et al., 2005). Similarly, in vitro studies have shown that folate-depleted culture media results in decreased cell proliferation, differentiation and survival in the rat H19-7 hippocampal cell line (Akchiche et al., 2012). These studies argue for a mechanism whereby folate deficiency leads to an excess of homocysteine (Kruman et al., 2005; Akchiche et al., 2012), which in turn leads to aberrant homocysteinylation of neural proteins, interfering with normal neuronal development and survival (Akchiche et al., 2012).
Despite the fact that most studies have focused on the metabolism and nucleic acid synthesis-dependent mechanisms for folate action in the nervous system, other studies have found that folate also engages acute signaling in neurons. Folate induces epileptiform activity when applied in the brain of mice and rats (Spector, 1972; Baxter et al., 1973; Hill and Miller, 1974), and enhances neuronal excitability in the cat cerebral cortex (Davies and Watkins, 1973). Acute application of folate in rat hippocampal slices inhibits GABAergic transmission, enhancing excitation by blocking the postsynaptic response to GABA (Otis et al., 1985) as well as by inhibiting presynaptic GABA receptors in the rat cuneate nucleus (Hill and Miller, 1974).
These effects on neuronal excitability argue for a specific action of folate in neural tissue and acute folate-triggered signaling independent of gene expression. Altogether these findings support a model in which folate triggers a specific signaling pathway in the neural tissues that is necessary for neural tube formation, and later may result in modulation of synaptic activity (Fig. 2).
FOLATE ACTION IN NEURODEGENERATIVE DISEASE AND NERVOUS SYSTEM REPAIR
Neural tube defects are not the only neurodevelopmental pathology associated with folate levels and function. The inherited disorder of brain-specific folate deficiency is a result of mutations in FOLR1 that manifests in late infancy and leads to developmental regression, motor dysfunction and epilepsy (Steinfeld et al., 2009; Perez-Duenas et al., 2010; Perez-Duenas et al., 2011). These FOLR1 mutants are defective in their binding to folate, and lead to inositol and choline insufficiencies, which in turn result in perturbed myelination (Steinfeld et al., 2009). This phenotype is similar to the disturbed myelination in 6-week-old rats fed with a folate-deficient diet (Hirono and Wada, 1978). Mutant forms of FOLR1 result in mislocalization of the protein and loss of FOLR1 from the cell membrane. However, the clinical severity of affected patients did not show a straightforward correlation with the altered folate binding and transport properties of FOLR1 mutants, suggesting that other factors might contribute to the clinical outcome of cerebral folate transport deficiency (Grapp et al., 2012).
Folate deficiency and an excess of homocysteine have also been associated with neurodegenerative diseases like Alzheimer’s and Parkinson’s disease in the adult and aging brain (Mattson and Shea, 2003; Miranda-Morales et al., 2017). High homocysteine and low folate levels in hippocampal cultures result in neuronal cell death correlated with uracil misincorporation and increased susceptibility to oxidative cell damage. Similarly, in vivo, mice on a folate-deficient diet exhibit increased DNA damage and hippocampal neurodegeneration (Kruman et al., 2002). Moreover, a folate-deficient diet for 3 months in uracil DNA glycosylase knockout mice leads to higher susceptibility to neurodegeneration of hippocampal pyramidal neurons, along with disturbances in neurotrophin and neurotransmitter metabolism that contribute to impaired spatial learning (Kronenberg et al., 2008).
The counterpoint of folate deficiency and neurodegeneration is evidence supporting a role for folate in the repair and regeneration of injured neural tissue (Fig. 2). Folate enhances regrowth of sensory spinal axons in vivo after bilateral lesion of rat dorsal root ganglia (Iskandar et al., 2004). Intraperitoneal injections with folic acid in injured rats result in enhanced axon regeneration of damaged spinal cord and optic nerves as well as functional recovery following spinal cord contusion (Iskandar et al., 2004; Miranpuri et al., 2017). The mechanism underlying folate action in axon regeneration depends on FOLR1, and is correlated with changes in DNA methylation (Iskandar et al., 2010). Matrix metalloproteinases, such as matrix metalloproteinase-2 (MMP2), are upregulated upon spinal cord injury, and not only interfere with functional recovery but also contribute to the associated neuropathic pain (Kawasaki et al., 2008; Miranpuri et al., 2016). Folate treatment managed to reduce MMP2 expression through methylating its promoter, reducing neuropathic pain and increasing functional recovery after spinal cord contusion (Miranpuri et al., 2017).
FUTURE DIRECTIONS
Folates are pivotal molecules for the development and maintenance of all tissues. However, the nervous system seems particularly sensitive to folate action throughout development, as well as in the adult and aging brain. This argues for a potentially specific need for folate action that is different than its ubiquitous role in DNA synthesis and cell proliferation. The GPI-AP folate receptor is molecularly equipped to elicit signaling upon folate binding by partnering with transmembrane proteins and/or intracellular proteins once it has been endocytosed. Hence, the spatiotemporal profile of folate receptor expression and its interactions with distinct proteins might contribute to provide specificity to folate action in the nervous system (Fig. 2). Future studies will be needed to determine the signaling mechanisms mediated by folate and folate receptors, and the relevance of these pathways to nervous system development, maturation and disease.
Acknowledgments
We thank Dr. Andrew M. Hamilton for critical comments on this manuscript. The work in the lab has been supported by the Basil O’Connor Starter Scholar Research Award Grant 5-FY09-131 from the March of Dimes Foundation, the Klingenstein Foundation Award in Neuroscience 2008, NSF 1120796, NIH-NINDS R01NS073055 and Shriners Hospital for Children 86500-NCA, 85220-NCA and 85300-NCA grants to LNB and Shriners Hospital for Children Postdoctoral Fellowship to OAB.
Footnotes
The authors declare that they do not have any conflict of interest.
References
- Folic acid and neural tube defects. Lancet. 1991a;338:153–154. [PubMed] [Google Scholar]
- Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991b;338:131–137. [PubMed] [Google Scholar]
- Akchiche N, Bossenmeyer-Pourie C, Kerek R, Martin N, Pourie G, Koziel V, Helle D, Alberto JM, Ortiou S, Camadro JM, Leger T, Gueant JL, Daval JL. Homocysteinylation of neuronal proteins contributes to folate deficiency-associated alterations of differentiation, vesicular transport, and plasticity in hippocampal neuronal cells. FASEB J. 2012;26:3980–3992. doi: 10.1096/fj.12-205757. [DOI] [PubMed] [Google Scholar]
- Amarnath S, Agarwala S. Cell-cycle-dependent TGFbeta-BMP antagonism regulates neural tube closure by modulating tight junctions. J Cell Sci. 2017;130:119–131. doi: 10.1242/jcs.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony AC. Folate receptors. Annu Rev Nutr. 1996;16:501–521. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- Balashova OA, Visina O, Borodinsky LN. Folate receptor 1 is necessary for neural plate cell apical constriction during Xenopus neural tube formation. Development. 2017;144:1518–1530. doi: 10.1242/dev.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Miller AA, Webster RA. Some studies on the convulsant action of folic acid. Br J Pharmacol. 1973;48:350P–351P. [PMC free article] [PubMed] [Google Scholar]
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296:2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- Bhagatji P, Leventis R, Comeau J, Refaei M, Silvius JR. Steric and not structure-specific factors dictate the endocytic mechanism of glycosylphosphatidylinositol-anchored proteins. J Cell Biol. 2009;186:615–628. doi: 10.1083/jcb.200903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, Roon P, Ganapathy V, Smith SB. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- Chen H, Yang J, Low PS, Cheng JX. Cholesterol level regulates endosome motility via Rab proteins. Biophys J. 2008;94:1508–1520. doi: 10.1529/biophysj.106.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER Polyp Prevention Study G. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- Davies J, Watkins JC. Facilitatory and direct excitatory effects of folate and folinate on single neurones of cat cerebral cortex. Biochem Pharmacol. 1973;22:1667–1668. doi: 10.1016/0006-2952(73)90034-8. [DOI] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Sequeira JM, Quadros EV. The metabolic basis for developmental disorders due to defective folate transport. Biochimie. 2016;126:31–42. doi: 10.1016/j.biochi.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Eitenmiller RR, Landen WO. Vitamin analysis for the health and food sciences. Boca Raton: CRC Press; 1999. p. 518. [Google Scholar]
- Elnakat H, Gonit M, Salazar MD, Zhang J, Basrur V, Gunning W, Kamen B, Ratnam M. Regulation of folate receptor internalization by protein kinase C alpha. Biochemistry. 2009;48:8249–8260. doi: 10.1021/bi900565t. [DOI] [PubMed] [Google Scholar]
- Eom DS, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development. 2011;138:3179–3188. doi: 10.1242/dev.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom DS, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate hinge point formation during neural tube closure by dynamic modulation of apicobasal polarity. Birth Defects Res A Clin Mol Teratol. 2012;94:804–816. doi: 10.1002/bdra.23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Europe-Finner GN, Newell PC. Calcium transport in the cellular slime mould Dictyostelium discoideum. FEBS Lett. 1985;186:70–74. doi: 10.1016/0014-5793(85)81341-7. [DOI] [PubMed] [Google Scholar]
- Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, Burke CA, McKeown-Eyssen GE, Baron JA. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101:432–435. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell RH, Spiegelstein O, Wlodarczyk B, Triplett A, Pogribny IP, Melnyk S, James JS. DNA methylation in Folbp1 knockout mice supplemented with folic acid during gestation. J Nutr. 2002;132:2457S–2461S. doi: 10.1093/jn/132.8.2457S. [DOI] [PubMed] [Google Scholar]
- Goldman ID. The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Ann N Y Acad Sci. 1971;186:400–422. doi: 10.1111/j.1749-6632.1971.tb46996.x. [DOI] [PubMed] [Google Scholar]
- Grapp M, Just IA, Linnankivi T, Wolf P, Lucke T, Hausler M, Gartner J, Steinfeld R. Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain. 2012;135:2022–2031. doi: 10.1093/brain/aws122. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Harris WA, Hartenstein V. Neuronal determination without cell division in Xenopus embryos. Neuron. 1991;6:499–515. doi: 10.1016/0896-6273(91)90053-3. [DOI] [PubMed] [Google Scholar]
- Hill RG, Miller AA. Antagonism by folic acid of presynaptic inhibition in the rat cuneate nucleus. Br J Pharmacol. 1974;50:425–427. doi: 10.1111/j.1476-5381.1974.tb09619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono H, Wada Y. Effects of dietary folate deficiency on developmental increase of myelin lipids in rat brain. J Nutr. 1978;108:766–772. doi: 10.1093/jn/108.5.766. [DOI] [PubMed] [Google Scholar]
- Iskandar BJ, Nelson A, Resnick D, Skene JH, Gao P, Johnson C, Cook TD, Hariharan N. Folic acid supplementation enhances repair of the adult central nervous system. Ann Neurol. 2004;56:221–227. doi: 10.1002/ana.20174. [DOI] [PubMed] [Google Scholar]
- Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee RV, Skene JH, Nelson A, Patel N, Gherasim C, Simon K, Cook TD, Hogan KJ. Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J Clin Invest. 2010;120:1603–1616. doi: 10.1172/JCI40000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Ossipova O, Sokol SY. GEF-H1 functions in apical constriction and cell intercalations and is essential for vertebrate neural tube closure. J Cell Sci. 2014;127:2542–2553. doi: 10.1242/jcs.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AG. Normal neurulation in amphibians. Ciba Found Symp. 1994;181:6–21. doi: 10.1002/9780470514559.ch2. discussion 21–24. [DOI] [PubMed] [Google Scholar]
- Jacobson AG, Oster GF, Odell GM, Cheng LY. Neurulation and the cortical tractor model for epithelial folding. J Embryol Exp Morphol. 1986;96:19–49. [PubMed] [Google Scholar]
- Johansson M, Appleby PN, Allen NE, Travis RC, Roddam AW, Egevad L, Jenab M, Rinaldi S, Kiemeney LA, Bueno-de-Mesquita HB, Vollset SE, Ueland PM, Sanchez MJ, Quiros JR, Gonzalez CA, Larranaga N, Chirlaque MD, Ardanaz E, Sieri S, Palli D, Vineis P, Tumino R, Linseisen J, Kaaks R, Boeing H, Pischon T, Psaltopoulou T, Trichopoulou A, Trichopoulos D, Khaw KT, Bingham S, Hallmans G, Riboli E, Stattin P, Key TJ. Circulating concentrations of folate and vitamin B12 in relation to prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition study. Cancer Epidemiol Biomarkers Prev. 2008;17:279–285. doi: 10.1158/1055-9965.EPI-07-0657. [DOI] [PubMed] [Google Scholar]
- Kamen BA, Caston JD. Properties of a folate binding protein (FBP) isolated from porcine kidney. Biochem Pharmacol. 1986;35:2323–2329. doi: 10.1016/0006-2952(86)90458-2. [DOI] [PubMed] [Google Scholar]
- Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004;56:1085–1097. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Kamen BA, Wang MT, Streckfuss AJ, Peryea X, Anderson RG. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988;263:13602–13609. [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Mechanisms of elongation in embryogenesis. Development. 2006;133:2291–2302. doi: 10.1242/dev.02406. [DOI] [PubMed] [Google Scholar]
- Kim YI. Folate and cancer prevention: a new medical application of folate beyond hyperhomocysteinemia and neural tube defects. Nutr Rev. 1999;57:314–321. doi: 10.1111/j.1753-4887.1999.tb06905.x. [DOI] [PubMed] [Google Scholar]
- Koch MC, Stegmann K, Ziegler A, Schroter B, Ermert A. Evaluation of the MTHFR C677T allele and the MTHFR gene locus in a German spina bifida population. Eur J Pediatr. 1998;157:487–492. doi: 10.1007/s004310050860. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Harms C, Sobol RW, Cardozo-Pelaez F, Linhart H, Winter B, Balkaya M, Gertz K, Gay SB, Cox D, Eckart S, Ahmadi M, Juckel G, Kempermann G, Hellweg R, Sohr R, Hortnagl H, Wilson SH, Jaenisch R, Endres M. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J Neurosci. 2008;28:7219–7230. doi: 10.1523/JNEUROSCI.0940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Mouton PR, Emokpae R, Jr, Cutler RG, Mattson MP. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16:1055–1059. doi: 10.1097/00001756-200507130-00005. [DOI] [PubMed] [Google Scholar]
- Kur E, Mecklenburg N, Cabrera RM, Willnow TE, Hammes A. LRP2 mediates folate uptake in the developing neural tube. J Cell Sci. 2014;127:2261–2268. doi: 10.1242/jcs.140145. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Sabharanjak S, De A. Endocytosis of glycosylphosphatidylinositol-anchored proteins. J Biomed Sci. 2009;16:93. doi: 10.1186/1423-0127-16-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Harland RM. Endocytosis is required for efficient apical constriction during Xenopus gastrulation. Curr Biol. 2010;20:253–258. doi: 10.1016/j.cub.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CJ, Crane NT, Wilson DB, Yetley EA. Estimated folate intakes: data updated to reflect food fortification, increased bioavailability, and dietary supplement use. Am J Clin Nutr. 1999;70:198–207. doi: 10.1093/ajcn.70.2.198. [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus AM, Gorfinkiel N, Schamberg S, Martinez Arias A. Endocytic and recycling endosomes modulate cell shape changes and tissue behaviour during morphogenesis in Drosophila. PLoS One. 2011;6:e18729. doi: 10.1371/journal.pone.0018729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403–456. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Tauszig-Delamasure S, Monnier PP, Mueller BK, Strittmatter SM, Mehlen P, Chedotal A. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6:749–755. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Mayor S, Sabharanjak S, Maxfield FR. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobbie SJ, Newell PC. Changes in actin associated with the cytoskeleton following chemotactic stimulation of dictyostelium discoideum. Biochem Biophys Res Commun. 1983;115:351–359. doi: 10.1016/0006-291x(83)91011-2. [DOI] [PubMed] [Google Scholar]
- Mentch SJ, Locasale JW. One-carbon metabolism and epigenetics: understanding the specificity. Ann N Y Acad Sci. 2016;1363:91–98. doi: 10.1111/nyas.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuli M, Moehrlen U. Fetal surgery for myelomeningocele is effective: a critical look at the whys. Pediatr Surg Int. 2014;30:689–697. doi: 10.1007/s00383-014-3524-8. [DOI] [PubMed] [Google Scholar]
- Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, Rothman KJ, Willett W. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA. 1989;262:2847–2852. doi: 10.1001/jama.262.20.2847. [DOI] [PubMed] [Google Scholar]
- Miranda-Morales E, Meier K, Sandoval-Carrillo A, Salas-Pacheco J, Vazquez-Cardenas P, Arias-Carrion O. Implications of DNA Methylation in Parkinson’s Disease. Front Mol Neurosci. 2017;10:225. doi: 10.3389/fnmol.2017.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranpuri GS, Meethal SV, Sampene E, Chopra A, Buttar S, Nacht C, Moreno N, Patel K, Liu L, Singh A, Singh CK, Hariharan N, Iskandar B, Resnick DK. Folic Acid Modulates Matrix Metalloproteinase-2 Expression, Alleviates Neuropathic Pain, and Improves Functional Recovery in Spinal Cord-Injured Rats. Ann Neurosci. 2017;24:74–81. doi: 10.1159/000475896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranpuri GS, Schomberg DT, Alrfaei B, King KC, Rynearson B, Wesley VS, Khan N, Obiakor K, Wesley UV, Resnick DK. Role of Matrix Metalloproteinases 2 in Spinal Cord Injury-Induced Neuropathic Pain. Ann Neurosci. 2016;23:25–32. doi: 10.1159/000443553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ES, Conus N, Kaput J. B vitamin polymorphisms and behavior: Evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci Biobehav Rev. 2014;47C:307–320. doi: 10.1016/j.neubiorev.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Momb J, Lewandowski JP, Bryant JD, Fitch R, Surman DR, Vokes SA, Appling DR. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc Natl Acad Sci U S A. 2013;110:549–554. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Macchi P, Deitinghoff L, Andersen JS, Mann M, Flad M, Hornberger MR, Stahl B, Bonhoeffer F, Mueller BK. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419:392–395. doi: 10.1038/nature01041. [DOI] [PubMed] [Google Scholar]
- Mornet E, Muller F, Lenvoise-Furet A, Delezoide AL, Col JY, Simon-Bouy B, Serre JL. Screening of the C677T mutation on the methylenetetrahydrofolate reductase gene in French patients with neural tube defects. Hum Genet. 1997;100:512–514. doi: 10.1007/s004390050544. [DOI] [PubMed] [Google Scholar]
- Nandadasa S, Tao Q, Menon NR, Heasman J, Wylie C. N- and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development. 2009;136:1327–1338. doi: 10.1242/dev.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl T, Fisher PR. Intracellular Ca2+ signals in Dictyostelium chemotaxis are mediated exclusively by Ca2+ influx. J Cell Sci. 1997;110(Pt 22):2845–2853. doi: 10.1242/jcs.110.22.2845. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–1502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- O’Broin JD, Temperley IJ, Brown JP, Scott JM. Nutritional stability of various naturally occurring monoglutamate derivatives of folic acid. Am J Clin Nutr. 1975;28:438–444. doi: 10.1093/ajcn/28.5.438. [DOI] [PubMed] [Google Scholar]
- O’Byrne MR, Au KS, Morrison AC, Lin JI, Fletcher JM, Ostermaier KK, Tyerman GH, Doebel S, Northrup H. Association of folate receptor (FOLR1, FOLR2, FOLR3) and reduced folate carrier (SLC19A1) genes with meningomyelocele. Birth Defects Res A Clin Mol Teratol. 2010;88:689–694. doi: 10.1002/bdra.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Kim K, Lake BB, Itoh K, Ioannou A, Sokol SY. Role of Rab11 in planar cell polarity and apical constriction during vertebrate neural tube closure. Nat Commun. 2014;5:3734. doi: 10.1038/ncomms4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Kim K, Sokol SY. Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. Biol Open. 2015;4:722–730. doi: 10.1242/bio.201511676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis LC, Madison DV, Nicoll RA. Folic acid has a disinhibitory action in the rat hippocampal slice preparation. Brain Res. 1985;346:281–286. doi: 10.1016/0006-8993(85)90861-3. [DOI] [PubMed] [Google Scholar]
- Paladino S, Pocard T, Catino MA, Zurzolo C. GPI-anchored proteins are directly targeted to the apical surface in fully polarized MDCK cells. J Cell Biol. 2006;172:1023–1034. doi: 10.1083/jcb.200507116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M, Xu X, Chen Y, Jin T. Identification of a Chemoattractant G-Protein-Coupled Receptor for Folic Acid that Controls Both Chemotaxis and Phagocytosis. Dev Cell. 2016;36:428–439. doi: 10.1016/j.devcel.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou C, Lynch SA, Burn J, Edwards YH. Methylenetetrahydrofolate reductase and neural tube defects. Lancet. 1996;348:58. doi: 10.1016/s0140-6736(05)64382-6. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Peglion F, Llense F, Etienne-Manneville S. Adherens junction treadmilling during collective migration. Nat Cell Biol. 2014;16:639–651. doi: 10.1038/ncb2985. [DOI] [PubMed] [Google Scholar]
- Perez-Duenas B, Ormazabal A, Toma C, Torrico B, Cormand B, Serrano M, Sierra C, De Grandis E, Marfa MP, Garcia-Cazorla A, Campistol J, Pascual JM, Artuch R. Cerebral folate deficiency syndromes in childhood: clinical, analytical, and etiologic aspects. Arch Neurol. 2011;68:615–621. doi: 10.1001/archneurol.2011.80. [DOI] [PubMed] [Google Scholar]
- Perez-Duenas B, Toma C, Ormazabal A, Muchart J, Sanmarti F, Bombau G, Serrano M, Garcia-Cazorla A, Cormand B, Artuch R. Progressive ataxia and myoclonic epilepsy in a patient with a homozygous mutation in the FOLR1 gene. J Inherit Metab Dis. 2010;33:795–802. doi: 10.1007/s10545-010-9196-1. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, Finnell RH. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Ratnam M, Marquardt H, Duhring JL, Freisheim JH. Homologous membrane folate binding proteins in human placenta: cloning and sequence of a cDNA. Biochemistry. 1989;28:8249–8254. doi: 10.1021/bi00446a042. [DOI] [PubMed] [Google Scholar]
- Reddy JA, Haneline LS, Srour EF, Antony AC, Clapp DW, Low PS. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood. 1999;93:3940–3948. [PubMed] [Google Scholar]
- Rijnboutt S, Jansen G, Posthuma G, Hynes JB, Schornagel JH, Strous GJ. Endocytosis of GPI-linked membrane folate receptor-alpha. J Cell Biol. 1996;132:35–47. doi: 10.1083/jcb.132.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Ying YS, Kolhouse JF, Kamen BA, Anderson RG. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990;110:637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005;113:825–828. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- Sausedo RA, Smith JL, Schoenwolf GC. Role of nonrandomly oriented cell division in shaping and bending of the neural plate. J Comp Neurol. 1997;381:473–488. [PubMed] [Google Scholar]
- Schoenwolf GC, Alvarez IS. Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development. 1989;106:427–439. doi: 10.1242/dev.106.3.427. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Yuan S. Experimental analyses of the rearrangement of ectodermal cells during gastrulation and neurulation in avian embryos. Cell Tissue Res. 1995;280:243–251. doi: 10.1007/BF00307795. [DOI] [PubMed] [Google Scholar]
- Shen F, Ross JF, Wang X, Ratnam M. Identification of a novel folate receptor, a truncated receptor, and receptor type beta in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry. 1994;33:1209–1215. doi: 10.1021/bi00171a021. [DOI] [PubMed] [Google Scholar]
- Shen F, Wu M, Ross JF, Miller D, Ratnam M. Folate receptor type gamma is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification: protein characterization and cell type specificity. Biochemistry. 1995;34:5660–5665. doi: 10.1021/bi00016a042. [DOI] [PubMed] [Google Scholar]
- Sierra EE, Brigle KE, Spinella MJ, Goldman ID. Comparison of transport properties of the reduced folate carrier and folate receptor in murine L1210 leukemia cells. Biochem Pharmacol. 1995;50:1287–1294. doi: 10.1016/0006-2952(95)94097-y. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Mills JL, Rhoads GG, Cunningham GC, Hoffman HJ, Conley MR. Vitamins, folic acid and neural tube defects: comments on investigations in the United States. Prenat Diagn. 1991;11:641–648. doi: 10.1002/pd.1970110823. [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr. 1999;19:91–122. doi: 10.1146/annurev.nutr.19.1.91. [DOI] [PubMed] [Google Scholar]
- Smith SB, Kekuda R, Gu X, Chancy C, Conway SJ, Ganapathy V. Expression of folate receptor alpha in the mammalian retinol pigmented epithelium and retina. Invest Ophthalmol Vis Sci. 1999;40:840–848. [PubMed] [Google Scholar]
- Spector RG. Influence of folic acid on excitable tissues. Nat New Biol. 1972;240:247–249. doi: 10.1038/newbio240247b0. [DOI] [PubMed] [Google Scholar]
- Speer MC, Worley G, Mackey JF, Melvin E, Oakes WJ, George TM. The thermolabile variant of methylenetetrahydrofolate reductase (MTHFR) is not a major risk factor for neural tube defect in American Caucasians. The NTD Collaborative Group. Neurogenetics. 1997;1:149–150. doi: 10.1007/s100480050022. [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Mitchell LE, Merriweather MY, Wicker NJ, Zhang Q, Lammer EJ, Finnell RH. Embryonic development of folate binding protein-1 (Folbp1) knockout mice: Effects of the chemical form, dose, and timing of maternal folate supplementation. Dev Dyn. 2004;231:221–231. doi: 10.1002/dvdy.20107. [DOI] [PubMed] [Google Scholar]
- Spinella MJ, Brigle KE, Sierra EE, Goldman ID. Distinguishing between folate receptor-alpha-mediated transport and reduced folate carrier-mediated transport in L1210 leukemia cells. J Biol Chem. 1995;270:7842–7849. doi: 10.1074/jbc.270.14.7842. [DOI] [PubMed] [Google Scholar]
- Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P, Wevers R, Grosso S, Gartner J. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet. 2009;85:354–363. doi: 10.1016/j.ajhg.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover PJ. One-carbon metabolism-genome interactions in folate-associated pathologies. J Nutr. 2009;139:2402–2405. doi: 10.3945/jn.109.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson DA, Liu ML, Baker PJ, Garrett L, Stitzel M, Wu J, Harris M, Banerjee R, Shane B, Brody LC. Targeted disruption of the methionine synthase gene in mice. Mol Cell Biol. 2001;21:1058–1065. doi: 10.1128/MCB.21.4.1058-1065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LS, Santillano DR, Wlodarczyk BJ, Miranda RC, Finnell RH. Role of Folbp1 in the regional regulation of apoptosis and cell proliferation in the developing neural tube and craniofacies. Am J Med Genet C Semin Med Genet. 2005;135C:48–58. doi: 10.1002/ajmg.c.30053. [DOI] [PubMed] [Google Scholar]
- Tassew NG, Charish J, Seidah NG, Monnier PP. SKI-1 and Furin generate multiple RGMa fragments that regulate axonal growth. Dev Cell. 2012;22:391–402. doi: 10.1016/j.devcel.2011.11.022. [DOI] [PubMed] [Google Scholar]
- van der Put NM, Steegers-Theunissen RP, Frosst P, Trijbels FJ, Eskes TK, van den Heuvel LP, Mariman EC, den Heyer M, Rozen R, Blom HJ. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet. 1995;346:1070–1071. doi: 10.1016/s0140-6736(95)91743-8. [DOI] [PubMed] [Google Scholar]
- van der Put NM, van den Heuvel LP, Steegers-Theunissen RP, Trijbels FJ, Eskes TK, Mariman EC, den Heyer M, Blom HJ. Decreased methylene tetrahydrofolate reductase activity due to the 677C-->T mutation in families with spina bifida offspring. J Mol Med (Berl) 1996;74:691–694. doi: 10.1007/s001090050073. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Niswander LA, Shaw GM, Finnell RH. The continuing challenge of understanding, preventing, and treating neural tube defects. Science. 2013;339:1222002. doi: 10.1126/science.1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Van Haastert PJ, Devreotes PN, Schaap P. Localization of chemoattractant receptors on Dictyostelium discoideum cells during aggregation and down-regulation. Dev Biol. 1988;128:72–77. doi: 10.1016/0012-1606(88)90268-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Demirtas H, Xu X. Homocysteine, B vitamins, and cardiovascular disease. N Engl J Med. 2006;355:207–209. author reply 209–211. [PubMed] [Google Scholar]
- Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- Wang X, Shen F, Freisheim JH, Gentry LE, Ratnam M. Differential stereospecificities and affinities of folate receptor isoforms for folate compounds and antifolates. Biochem Pharmacol. 1992;44:1898–1901. doi: 10.1016/0006-2952(92)90089-2. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Bianco C, Strizzi L, Hamada S, Mancino M, Bailly V, Mo W, Wen D, Miatkowski K, Gonzales M, Sanicola M, Seno M, Salomon DS. Growth factor induction of Cripto-1 shedding by glycosylphosphatidylinositol-phospholipase D and enhancement of endothelial cell migration. J Biol Chem. 2007;282:31643–31655. doi: 10.1074/jbc.M702713200. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nagaoka T, Lee JM, Bianco C, Gonzales M, Castro NP, Rangel MC, Sakamoto K, Sun Y, Callahan R, Salomon DS. Enhancement of Notch receptor maturation and signaling sensitivity by Cripto-1. J Cell Biol. 2009;187:343–353. doi: 10.1083/jcb.200905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- Wilcken DE, Wang XL. Relevance to spina bifida of mutated methylenetetrahydrofolate reductase. Lancet. 1996;347:340. doi: 10.1016/s0140-6736(96)90526-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen H, Vlahov IR, Cheng JX, Low PS. Characterization of the pH of folate receptor-containing endosomes and the rate of hydrolysis of internalized acid-labile folate-drug conjugates. J Pharmacol Exp Ther. 2007;321:462–468. doi: 10.1124/jpet.106.117648. [DOI] [PubMed] [Google Scholar]
- Yumura S. Rapid translocation of myosin II in vegetative Dictyostelium amoebae during chemotactic stimulation by folic acid. Cell Struct Funct. 1994;19:143–151. doi: 10.1247/csf.19.143. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. The molecular identity and characterization of a Proton-coupled Folate Transporter--PCFT; biological ramifications and impact on the activity of pemetrexed. Cancer Metastasis Rev. 2007;26:129–139. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]
- Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009a;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem. 2009b;284:4267–4274. doi: 10.1074/jbc.M807665200. [DOI] [PMC free article] [PubMed] [Google Scholar]