Abstract
Objective
Evidence suggests disordered breathing is critically involved in Sudden Unexpected Death in Epilepsy (SUDEP). To that end, evaluating structures that are activated by seizures and can activate brain regions that produce cardiorespiratory changes can further our understanding of the pathophysiology of SUDEP. Prior preclinical studies have shown that electrical stimulation of the human amygdala induces apnea, suggesting a role for the amygdala in controlling respiration. In this study, we aimed to both confirm these findings in a larger group of patients with intractable temporal lobe epilepsy (TLE) and also further explore the anatomical and cognitive properties of this effect.
Methods
Seven surgical TLE patients had depth electrodes implanted in the amygdala that were used to deliver electrical stimulation during functional mapping prior to resection. Real-time respiratory monitoring was performed in each patient to confirm apnea.
Results
Our data confirm that amygdala stimulation reliably induces apnea (occurring in all seven patients) and further suggest that apnea can be overcome by instructing the patient to inhale, and can be prevented entirely by breathing through the mouth prior to electrical stimulation. Finally, stimulation-induced apnea occurred only when stimulating the medial most amygdalar contacts located in the central nucleus.
Interpretation
These findings confirm a functional connection between the amygdala and respiratory control in humans. Moreover, they suggest specific amygdalar nuclei may be critical in mediating this effect and that attentional state is critical to apnea mediated by amygdala activation - perhaps alluding to future development of strategies for the prevention of SUDEP.
Keywords: amygdala stimulation, apnea, SUDEP
Introduction
The autonomic nervous system acts largely unconsciously to regulate essential bodily functions such as heart rate, digestion, respiratory rate and pupillary response. Dysfunction of this system is observed in many disparate neuropsychiatric disorders including Parkinson’s Disease, anxiety, depression and epilepsy1–4. While the peripheral components of the autonomic nervous system are well-understood, networks involved in central control of the autonomic nervous system are less clear5. In particular, very little is known about the conscious control of breathing in humans. Unlike most autonomic functions, respiration is highly susceptible to conscious intentional manipulation: we can easily hold our breath, alter our breathing rate, and intentionally sniff to sample smells in the environment. We have such good conscious control of respiration that we can even use intentional breathing modulations to perform complex tasks, such as control of a computerized wheelchair6.
A deeper understanding of human respiratory regulation has particular importance to patients with epilepsy, given its potential relevance to SUDEP. SUDEP is the most frequent cause of death in epilepsy patients, with accumulating evidence strongly suggesting respiratory involvement7–9. Much of this research in the past decade extends from the seminal retrospective study of mortality in epilepsy monitoring units (MORTEMUS)9. A consistent sequence of events occurred after each seizure resulting in SUDEP: rapid breathing followed the seizure, followed by apnea, followed by bradycardia and postictal generalized electroencephalogram (EEG) suppression with terminal apnea preceding the terminal asystole9, 10. Thus in all recorded cases of SUDEP, the initial pathological symptom was respiratory dysfunction (this information was inferred from scalp EEG and surface ECG recordings). One possibility is that seizures spreading to brain structures involved in higher order control of brainstem function, including breathing, can lead to respiratory dysfunction, and therefore SUDEP. This emphasizes the importance of elucidating the neural substrates underlying central respiratory control in humans.
Animal studies suggest that subnuclei of the amygdala may be particularly important for respiratory control11–14; however, until recently this has been largely unstudied in humans. In the human amygdala, local field potential oscillations align with inspiration during natural nasal breathing, indicating the region tracks respiratory phase15. Furthermore, two recent studies showed that electrical stimulation of the amygdala induces apnea (studies included 2 and 3 patients)7, 16. These findings combine to suggest a potential role for the amygdala in respiratory control - potentially mediated through the heavy interconnections with the extended amygdala17, 18 which has afferent connections to brainstem nuclei critical to mediating breathing19, 20. Combined, these data suggest a potential role for the amygdala in SUDEP - activation of the amygdala by seizures may produce prolonged, potentially fatal apnea. How this respiratory control is affected by route of inhalation and consciousness is yet unknown and may provide new avenues of therapeutic intervention.
In this study, we aimed to confirm previous findings of amygdala-stimulation-induced apnea in a larger group of seven patients with TLE. Additionally, we sought to test the hypotheses that stimulation-induced apnea can be prevented with behavioral interventions, is dependent on route of airflow and is specific to mesial sub-regions.
Materials and Methods
Participants and electrodes
Participants included seven patients with intractable TLE who underwent surgical stereotactic EEG evaluations in the epilepsy monitoring unit at Northwestern Memorial Hospital. All methods were approved by the Northwestern Feinberg School of Medicine’s Institutional Review Board and all participants gave written informed consent prior to participation in the study. This study included data from both retrospectively identified patients and actively recruited patients. For the retrospective analysis, we conducted a review of all patients ever admitted for surgical EEG evaluations. In our review of all patients since our first surgical case in 2010, we identified 4 patients whose electrode coverage included the amygdala, and whose breathing was monitored during clinical protocols involving electrical stimulation. Retrospectively identified patients included P1, P3, P4 and P6. The only inclusion criteria for patients were those who, as part of the clinically determined surgical plan, had depth electrodes implanted inside the amygdala, and in whom respiration was also monitored during electrical stimulation protocols. In addition to our retrospective analysis, we recruited three patients who were prospectively identified and consented to participate, including P2, P5 and P7 (Table 1).
TABLE 1.
| Patient | Age, y | Sex | Handedness | Duration of Epilepsy, y | Epileptogenic Zone | Etiology | Brian MRI | Comorbidities |
|---|---|---|---|---|---|---|---|---|
| 1. P1 | 34 | M | Right | 6 | Right mestial temporal | Unknown | Normal | None |
| 2. P2 | 36 | M | Right | 31 | Right mesial temporal | Perinatal ischemia | Right MTS, Right encephalomalacia | ADHD |
| 3. P3 | 48 | M | Right | 12 | Right mesial temporal | Unknown | Normal | OSA, Depression (Sertraline) |
| 4. P4 | 35 | F | Right | 3 | Right mesial temporal/right amygdala | FCD | Right MTS | None |
| 5. P5 | 27 | F | Right | 5 | Left mesial temporal | FCD | Left MTS | Anxiety (Sertraline) |
| 6. P6 | 57 | F | Right | 3 | Left anterior temporal | Left tentorial meningioma | Left tentorial meningioma | Depression and Anxiety (Fluoxetine) |
| 7. P7 | 36 | F | Right | 36 | Left mesial temporal | Unknown | Left MTS | Depression and Anxiety |
The surgically implanted electrodes were used both to record local field potential (LFP) data with the intent of recording seizures and interictal abnormalities as well as to deliver electrical stimulation with the intent of functional mapping and seizure induction prior to resection. Electrodes for this study included 8-contact depth electrodes (Integra Epilepsy) utilizing the Leksell frame (Elekta, Sweden) and Brainlab planning system (iPlan Stereotaxy 3.0; Brainlab, Feldkirchen, Germany). Electrodes had 5-mm center-to-center spacing between adjacent electrode contacts. Preoperative structural MR images were acquired on all patients with either a 1.5T or 3T MRI scanner. CT scans were acquired postoperatively with depth electrodes in place, clearly showing electrode positions with respect to skull geometry. Electrodes were localized using pre-operative structural MRI scans and post-operative computed tomography (CT) scans with FSL’s registration tool FLIRT22. The individual CT image was registered to the MRI image using a degree-of-freedom of 6 with a cost function of mutual information, which was followed by an affine registration with a degree-of-freedom of 12. The individual MRI image was registered to the standard MNI152 1mm brain (degree-of-freedom of 12). Finally, the transformation matrix generated above was combined to create a transformation from the individual CT image to standard MNI coordinates. Notably, normalization of P2’s brain onto the standard MNI coordinates was not possible due to abnormal anatomy in this patient (suspected perinatal ischemic injury and extensive encephalomalacia of the entire right hemisphere). In this case, anatomical landmarks were used (notably the internal capsule and anterior commissure) to determine that the electrode location in the anterior amygdala. Finally, the electrodes were localized by thresholding the raw CT image, and the un-weighted mass center of each electrode was calculated and converted into standard MNI space using Matlab and FSL (Fig. 1).
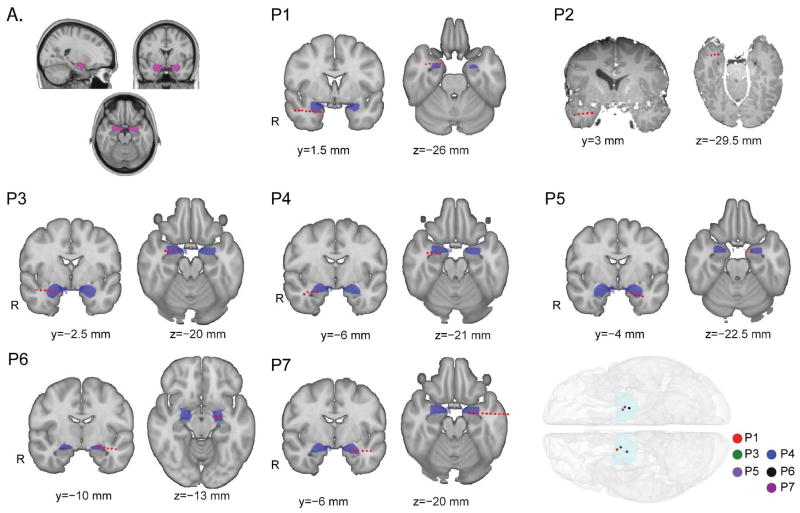
Figure 1.
Individual patients’ electrode placement. Electrode localization in each patient was achieved using pre-operative structural MRI scans and post-operative CT scans. Each panel (P1-7) shows an individual patients amygdala-destined depth wire (red circles) overlaid onto a standard MNI-152 1mm brain. The amygdala is filled in blue, and each red circle is one electrode contact on the depth wire. In each panel, the brain on the left shows a coronal slice that best illustrates the amygdala depth wire placement for that particular patient, while the brain image on the right shows an axial slice that best illustrates the amygdala depth wire for each patient. Notably, normalization of P2’s brain onto the standard MNI coordinates was not possible due to abnormal anatomy in this patient (suspected perinatal ischemic injury and extensive encephalomalacia of the entire right hemisphere). For this reason, we show P2’s individual pre-operative MRI with amygdala wire contacts overlaid as in the other patients. Lower Inset. A whole brain image showing the mesial-most amygdala electrode location for each patient. Because P2’s brain could not be normalized to MNI space, this patient is not represented in the whole brain image. Upper Inset A. Image showing the amygdala mask used with FSL’s available Harvard-Oxford human brain Atlas, which we used to define the amygdala.
Within the FSL software, several human brain atlases that provide MNI-coordinate-translated regional masks are available. We used this feature to locate the amygdala in each patient. To this end, we used the Harvard-Oxford Atlas which is included in FSL to map the amygdala (Fig. 1).
Electrical stimulation
Electrical stimulation is routinely conducted in surgical epilepsy patients in order to map functional cortical brain areas and also to induce seizures as part of standard clinical protocols. Electrical stimulation of the amygdala was administered using either a Grass S-88X Stimulator (Astro-Med, Inc., West Warwick, RI) or the Nihon Kohden cortical stimulator (Nihon Kohden Corp, Tokyo, Japan). Amygdala stimulation was conducted with standard clinical mapping parameters used for bedside cortical stimulation (bipolar stimulation except when noted, 50Hz, pulse duration 0.3s) with train durations from 5 to 30 seconds. In case a seizure was induced, IV lorazepam and resuscitation equipment was always kept in close proximity to the patient. In each patient, amygdala stimulation was initiated at the lowest strength (1mA) and slowly increased in 1mA increments until apnea (or any other clinical symptom) was observed. All patients exhibited apnea beginning at 4mA strength of electrical stimulation. The duration of stimulation was initially set at 5 or 10 seconds and increased to a maximum of 30 seconds if tolerated by the patient. To begin the experimental session, the patient was instructed to sit quietly and relax. After the patient was breathing regularly for at least 30 seconds, a bipolar electrical stimulation train was administered to the two mesial-most amygdala depth contacts. Stimulation was administered with no visual or auditory warnings or cues so the patient was unaware when it was delivered. Apnea and hypopnea were confirmed by real time monitoring of respiratory signals on the acquisition computer, and a reduction in respiratory rate, as discussed below. A patient who stopped breathing for the duration of electrical stimulation is reported to have apnea, while a patient whose rate slowed but did not stop was reported to have hypopnea (described in the results below, and can be seen in Figure 2). The prospectively identified patients who participated in the mouth breathing experiment were asked to breathe through their mouth but otherwise relax and sit quietly. Electrical stimulation was also administered to electrodes implanted in other clinically important brain structures, including the hippocampus, which did not induce apnea in any patients, in contrast to a recent study that did find hippocampal-stimulation-induced apnea16. Hippocampal stimulation functioned as a negative control region in our experiment.
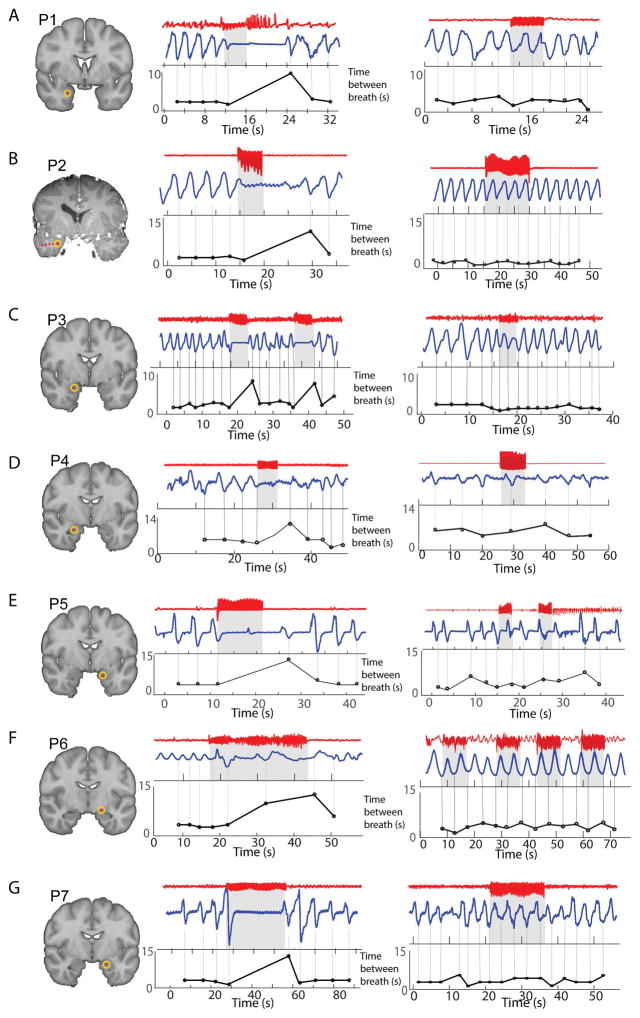
Figure 2.
Electrical stimulation of the amygdala induces apnea. A – G. Each panel shows one individual patient’s electrode location on the left. The middle panel shows an example of amygdala stimulation for each patient. The iEEG signal from the amygdala wire is shown in red, on top, with the respiratory signal in blue below. In each patient, there is a clear disruption in the respiratory signal at the moment electrical stimulation begins. Electrical stimulation beginning and ending is represented by the light gray square with the dark black line above it. The plot below shows the time between breaths over the time course of the epoch. Each black circle represents one breath, and the y-axis is the time since the previous breath in seconds. A large increase can be seen following electrical stimulation onset in each patient (a larger value indicates a slower breathing rate). The right panel shows an example of electrical stimulation of a control region (the control region was a mesial electrode in the hippocampus). In each patient, hippocampal stimulation did not induce apnea.
Physiological recordings
As standard clinical protocol at NMH, respiratory monitoring is routinely conducted for all surgical epilepsy patients. To monitor breathing during the hospital stay, chest and abdominal excursions were recorded using respiratory inductance plethysmography belts (Ambu, Ballerup, Denmark). One belt is placed around the chest and the other around the abdomen. Respiratory belts provide a measure of tidal volume during breathing. To measure air flow through the nose, patients are also fitted with a nasal cannula attached to a piezoelectric sensor (Salter Labs, Carlsbad, California). While chest and abdominal belts provide a measure of chest and abdominal expansions and contractions, nasal cannula provide a measure of nasal airflow. These two different measures result in markedly different respiratory waveform patterns. While airflow is zero during breath holding (with air held either inside or outside the lungs), the same is not true for chest expansions and contractions. Thus breathing belt signals are stationary during breath holding, whereas cannula signals return to zero. Not all patients were compliant with the nasal cannula. In analyzing respiratory data for the purposes of our study, we chose nasal cannula data when available because it has been shown to be more sensitive and accurate than belt data21. Across patients, the respiratory signal used was as follows: P1, belt; P2, cannula; P3, belt; P4, cannula; P5, cannula; P6, belt; P7, cannula. All respiratory signals are directly connected into the Nihon Kohden EEG acquisition system and filtered between 0.08Hz and 30Hz, with the same sampling rate as the ongoing EEG data acquisition. This setup provides perfect synchronization between respiratory and EEG signals. To look for respiratory modulations resulting from electrical stimulation of the amygdala, respiratory rate values for each patient were entered into a 2-way ANOVA with stimulation presence (before versus after stimulation onset) and stimulation location (amygdala versus hippocampus) as factors.
Results
Electrical stimulation of the amygdala reduces respiratory rate
Electrical stimulation of the amygdala reliably induced hypopnea or apnea in 7 out of 7 patients tested (Fig. 2) (ANOVA main effect of stimulation on respiratory rate: F(1,24)=9.82; P = 0.0045). This effect was only present during electrical stimulation of the amygdala, and did not occur during electrical stimulation of a control region, as evidenced by a significant interaction between stimulation presence and stimulation location (F(1,24)=13.64; P = 0.0011). T-tests confirmed a significant reduction in breathing rate following stimulation of the amygdala (T(6)=4.7; P = 0.003) but not following stimulation of a control region (T(6)=−1.38; P = 0.21). Individual patients’ responses to amygdala stimulation differed in terms of whether they exhibited apnea or hypopnea, whether the apnea persisted following termination of electrical pulses and whether or not the patient was aware of the presence of apnea.
Details of respiratory modulations in response to electrical stimulation of the amygdala in each individual patient follow:
P1 exhibited apnea upon electrical stimulation of the amygdala (Fig. 2A, left panel). P1’s respiratory rate prior to electrical stimulation was 0.4Hz, and reduced to 0Hz during stimulation. Amygdala stimulation in this patient produced after-discharges. Interestingly, after electrical stimulation was stopped, the apnea persisted for the duration of the after-discharges. Electrical stimulation of the amygdala was repeated two times, with apnea observed on both occasions. Electrical stimulation of the anterior hippocampus did not produce any apnea (Fig. 2A, right panel).
P2 exhibited apnea upon electrical stimulation of the amygdala (Fig. 2B). P2’s respiratory rate prior to stimulation was 0.25Hz and reduced to 0Hz during stimulation. Apnea in this patient tended to persist for several seconds after stimulation was stopped, a behavior that was observed in at least one other patient. This behavior could potentially indicate reduced ability to recover from disruption of normal amygdala activity, and is therefore of interest to explore as a potential sign or marker of predisposition to SUDEP in future studies. Amygdala stimulation was performed 4 times and apnea occurred every time.
P3 also exhibited apnea upon electrical stimulation of the amygdala (Fig. 2C). P3’s respiratory rate prior to electrical stimulation was 0.32Hz, and reduced to 0Hz during stimulation. Electrical stimulation of the amygdala was repeated 8 times, and apnea was induced on all 8 occasions.
P4 exhibited hypopnea upon electrical stimulation of the amygdala (Fig. 2D). P4’s respiratory rate prior to electrical stimulation was 0.31Hz, and reduced to 0.11Hz during stimulation. Tidal volume also decreased during stimulation. Amygdala stimulation was performed one time only.
P5 exhibited hypopnea upon electrical stimulation of the amygdala (Fig. 2E). Amygdala stimulation was performed twice and hypopnea was observed both times. P5’s respiratory rate prior to stimulation was 0.28Hz and reduced to 0.17Hz during stimulation. Tidal volume was also decreased during stimulation. Hypopnea persisted for 10s following termination of stimulation.
P6 exhibited hypopnea upon electrical stimulation of the amygdala (Fig. 2F). Amygdala stimulation was performed once. This patient reported feeling unable to breathe during stimulation. P6’s respiratory rate prior to electrical stimulation was 0.38Hz, and reduced to 0.08Hz during stimulation.
P7 exhibited apnea upon electrical stimulation of the amygdala (Fig. 2G). P7’s respiratory rate prior to stimulation was 0.22Hz and reduced to 0Hz during stimulation. Amygdala stimulation was performed three times and apnea was observed every time.
Stimulation-induced apnea does not occur during mouth breathing
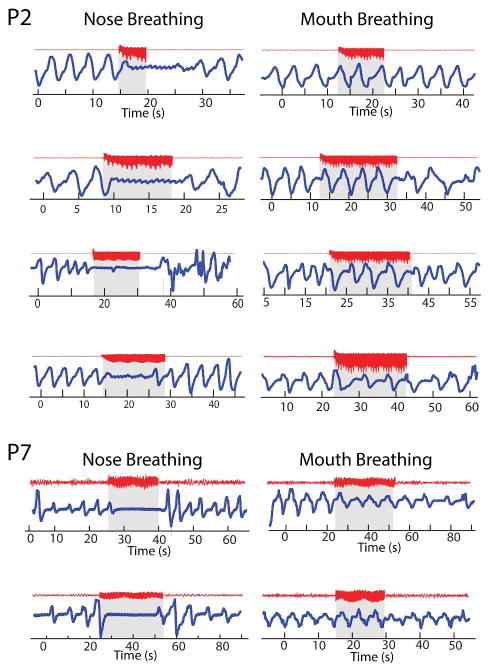
Data from humans and rodents suggest that respiratory entrainment of local field potential oscillations in limbic brain regions is dependent on air flow through the nasal cavities suggesting specificity to the nasal breathing route15, 23. This suggests the potential for different cortical mechanisms of respiratory control during nose and mouth breathing. To test whether differences in stimulation-induced apnea could be observed during mouth breathing, we asked two patients (P2 and P7, both of whom exhibited amygdala-induced apnea) to breathe through their mouth while relaxing quietly. After 30 seconds of consistent mouth breathing, electrical stimulation was delivered exactly as in the nasal breathing condition. In both patients, amygdala stimulation did not induce apnea or hypopnea when administered while the patient was breathing through the mouth (Fig. 3). In P2, this finding replicated 4 out of 4 times, and in P7, this finding replicated 2 out of 2 times. Neither patient had any reduction in respiratory rate or tidal volume during electrical stimulation of the amygdala while breathing through the mouth.
Figure 3.
Amygdala-stimulation-induced apnea is specific to the nasal breathing route. Examples of amygdala stimulation delivered during nasal and oral breathing conditions in two patients (P2 and P7). Each row shows the respiratory response during a repetition for nose and mouth breathing. Thus, P2 performed 4 repetitions and P7 performed 2 repetitions.
Stimulation-induced apnea can be interrupted by prompting to breathe
Given the potential importance of our findings to life-threatening apnea and SUDEP, we wanted to know if we could prompt patients to overcome the induced apnea by instructing them to breathe during electrical stimulation of the amygdala. To test this hypothesis, we instructed 3 patients (P4, P5, and P7) to take a breath during electrical stimulation of the amygdala, after apnea had begun. All three patients were able to overcome the apnea during electrical stimulation of the amygdala (Fig. 4). Patient P5 performed this task a single time, while P7 and P2 were both asked to breathe during stimulation on 4 separate occasions. Both patients were able to overcome apnea and inhale each time they were asked.
Figure 4.
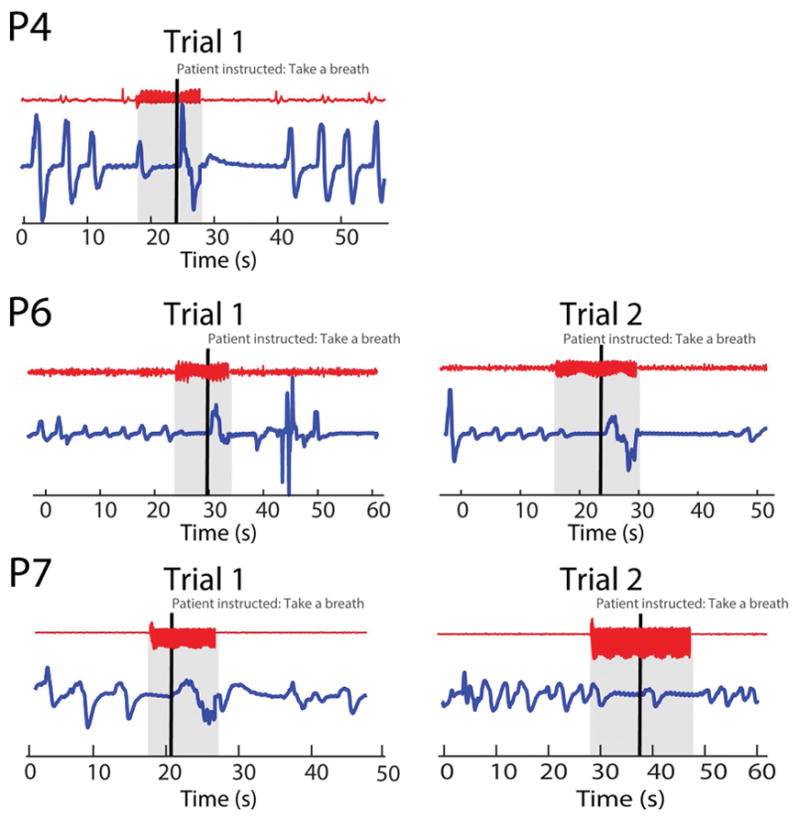
Amygdala-stimulation-induced apnea can be interrupted by instructing the patient to take a breath. Examples of apnea during amygdala stimulation that is interrupted by instructing the patient to inhale. Each repetition for each patient is shown as labeled. During stimulation, the patient was instructed to take a breath (thick black vertical line), and interruption of apnea can be seen.
Stimulation-induced apnea occurs during sleep
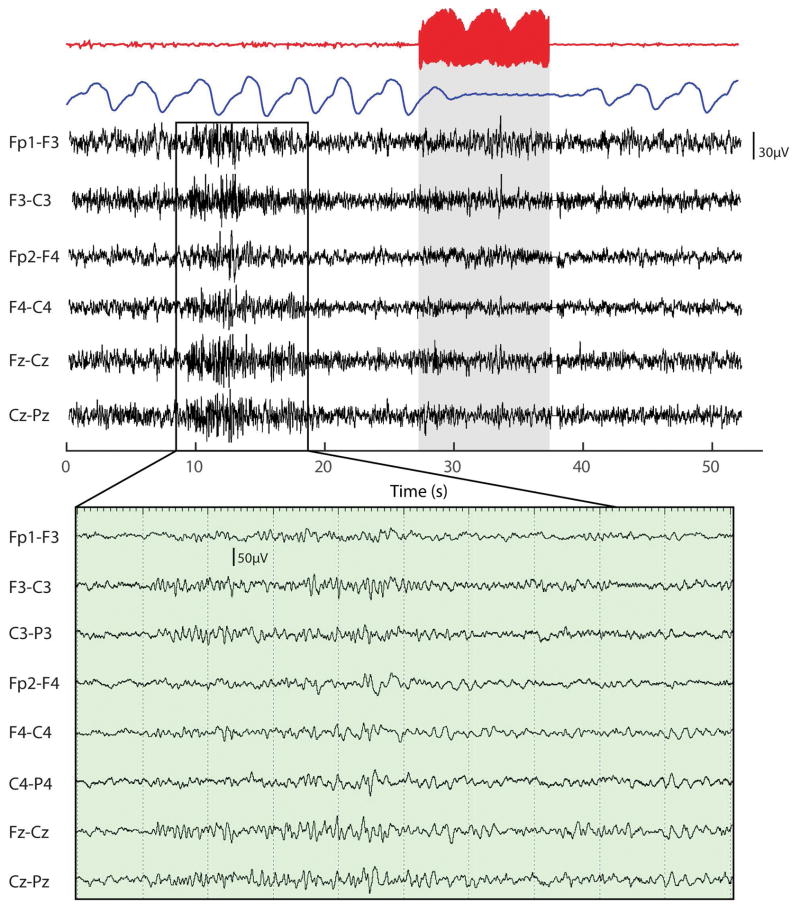
Given that SUDEP occurs more frequently during sleep, we wanted to test whether electrical stimulation still induced apnea during sleep. To test this hypothesis, we electrically stimulated the amygdala of one patient (P2) during sleep. Sleep was confirmed using standard sleep scoring techniques from surface electrodes placed on the patient’s scalp. When the patient was confirmed to be in stage 2 sleep for 5 continuous minutes, electrical stimulation was administered and apnea was induced (Fig. 5). Apnea occurred on four separate occasions of electrical stimulation administered during sleep in P2.
Figure 5.
Electrical stimulation of the amygdala induces apnea during stage 2 sleep. Electrical stimulation was delivered to the amygdala of one patient during stage-2 sleep. The red trace is iEEG data from the amygdala showing the stimulation, with the blue trace showing the respiratory signal. Below is simultaneously recorded surface EEG data from the frontocentral electrodes, with an inset below of a magnified 10 second period prior to stimulation, showing the presence of sleep spindles and confirming stage 2 sleep. Clear apnea can be observed in the blue trace following electrical stimulation of the amygdala during sleep.
Stimulation-induced apnea is specific to the mesial portion of the amygdala
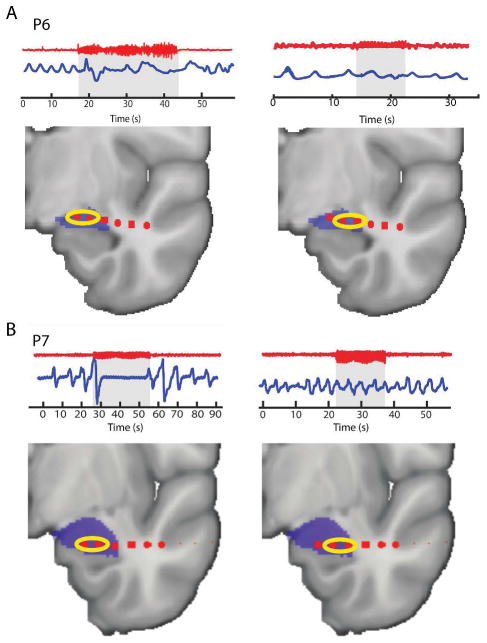
The mechanism underlying amygdala involvement in respiratory control is unknown. Anatomically, the amygdala is functionally heterogeneous, and can be divided into lateral and medial subdivisions25. The extended amygdala is a basal forebrain continuum that runs from the medial dorsal amygdala that contains the central and medial nucleus of the amygdala (CeA and MeA) and extends rostrally to the bed nuclei of the stria terminalis (BNST) and the shell of the nucleus accumbens26. The extended amygdala has a powerful influence over the autonomic network, with direct connections to the brainstem. One mechanism by which amygdalar involvement in seizures could induce apnea is via the central subdivision and its direct connections to these control regions. In support of this hypothesis, data from rodents suggest that the central nucleus of the amygdala is involved in respiratory control14. To test whether human amygdala-stimulation-induced apnea is specific to medial (including the CeA) as opposed to lateral amygdala subregions, we stimulated more laterally along the depth wire inserted into the amygdala, and observed elimination of apnea effects in lateral amygdala locations in two patients (Fig. 6). In patient P6, bipolar electrical stimulation of the two medial-most contacts of the amygdala reliably induced apnea, whereas bipolar stimulation of the neighboring pair just lateral did not (Fig. 6A). In patient P7, the same was true (Fig. 6B). These results suggest that apnea can only be induced during stimulation of the medial subdivision of the human amygdala, which we surmise are likely within the bounds of the CeA25.
Figure 6.
Amygdala-stimulation-induced apnea is specific to the medial sub-regions of the amygdala. Two patients had electrode coverage spanning the mesial and lateral portions of the amygdala, providing the opportunity to test whether the effect differed across the medial and lateral amygdala sub-regions. In two patients (P6 and P7), we delivered bipolar stimulation to the mesial-most and lateral-most contacts within the amygdala and in both patients, we found that apnea only occurred during stimulation of the medial amygdala. The red traces show the iEEG data from the amygdala, with the blue traces showing the respiratory signal. Below each example is the individual patient’s amygdala electrode locations. The yellow circles indicate which two electrodes were stimulated in each instance.
Discussion
In line with recent studies7, 16, we found that electrical stimulation of the human amygdala consistently induces apnea. By including data from a larger group of patients, our study solidifies the already existing evidence suggesting a role for the amygdala in respiratory control. It should be noted that our study, like the previous reports, also suffers from selection and observational biases inherent to case studies. Beyond observation of amygdala-induced apnea in a larger group of patients, our data contribute several interesting new aspects to the recent emerging literature on this topic which may begin to uncover new avenues of research into preventative measures for seizure-related respiratory irregularities. We found that amygdala-stimulation-induced apnea could be prevented by asking patients to breathe through their mouths several minutes prior to delivering stimulation. We also found that apnea could be interrupted by directly instructing patients to take a breath during stimulation once apnea had begun. We found that apnea is only induced when the medial subregion (specifically the central nucleus) of the amygdala is stimulated, with no apnea occurring as a result of lateral amygdala stimulation. Previous studies implicate a more lateral apnea-inducing stimulation site. However, the location of the most medial stimulation site when noted in these studies seems to juxtapose the mesial amygdalar nuclei7. In animal studies the lateral and basolateral amygdala (BLA) lack an afferent connection to brainstem respiratory centers, unlike the medial subregions31, 32. Thus our finding that stimulation-induced apnea is selective to medial amygdala stimulation dovetails with anatomical connections between the amygdala and respiratory control centers27, 28. We cannot exclude the possibility that descending fibers tracts are stimulated during our trials, rather than neuronal populations within the amygdala, however, this is less likely as no large fiber bundles are known to be present in the location of our stimulation and our stimulation intensity is unlikely to directly affect tissues a substantial distance away 25, 29.
Overall, we suggest a mechanism whereby the amygdala exhibits control over breathing via extensive connections to the extended amygdala. The extended amygdala, comprising chiefly of the central amygdala (CeA) and the bed nucleus of the stria terminalis (BNST), is a part of the central autonomic network (CAN) that is interconnected between higher order cortical areas, brainstem respiratory centers such as the ventral lateral medulla (VLM), nucleus of the solitary tract (NTS), periaqueductal gray (PAG), the parabrachial nucleus (PBN) and hypothalamic networks, raising the possibility that it could be the missing link in the mediation of SUDEP14, 30. The extended amygdala is activated by seizures, and its activation can have powerful effects on heart rate, blood pressure and respiratory function30–32. There is controversy regarding whether cardiac failure or respiratory arrest is more important as the primary cause of death in SUDEP33. Activation of this region could also be partly responsible for general autonomic nervous system dysfunction with seizures in people with longstanding epilepsy4, 34.
In addition to finding that amygdala stimulation induces apnea, studies have also found that breathing is inhibited when seizures spread to the amygdala7, 16. Insofar as these data suggest a potential amygdalar role in SUDEP, it follows that discovering ways to prevent this effect could prevent SUDEP. In light of this, we found a key attentional component to stimulation-induced apnea and potential techniques that can prevent and interrupt stimulation-induced apnea. In 2 out of 2 patients, with multiple repetitions, amygdala-stimulation-induced apnea did not occur during mouth breathing and in 3 of 3 patients it could be interrupted with a prompt to take a breath. Additionally, we found amygdala-stimulation-induced apnea occurred during stage 2 sleep, suggesting that this is not a state-dependent phenomenon, and demonstrating the vulnerability of epilepsy patients to apneas following seizures during sleep, when the patient’s attention is diminished, and when there may be no one else present to bring the patient’s attention to breathing.
The implication from these observations is that altered breathing prior to stimulation onset has the potential to stop apnea; forced mouth breathing could potentially prevent hypoventilation and SUDEP. In individuals who naturally breathe through their noses, sustained mouth breathing can bring attentional focus to respiration. While our experimental design required switching between nasal and oral breathing, effectively bringing attention to both nasal and oral breathing conditions (and yet apnea still only occurred during nasal breathing conditions), we cannot eliminate the possibility that the lack of apnea during mouth breathing was the result of attentional factors, rather than a reflection of the anatomical difference between the two breathing routes. The attentional component of both the prompted breathing and the mouth breathing exercises adds to the hypothesis that decreased consciousness and a prolonged postictal state following a seizure contribute to SUDEP, and strategies that can regain the patient’s attention have the potential to arrest apnea35, 36.
The fact that amygdala-stimulation-induced apnea can be overcome with attentional interventions raises an interesting question regarding the implications of amygdalectomy and whether electrical stimulation of the amygdala regions represents activation or disruption of ongoing amygdala activity. For example, if stimulation represents an interruption of normal amygdala activity, then this would suggest necessity of an intact amygdala for normal breathing. If this were the case, we would expect to see respiratory dysfunction, including apnea, following amygdala resection. While some effects of this sort, including reduced respiratory rates, have been reported37, they do not explain the severity of symptoms we observed. However, unilateral amygdalectomy would leave one amygdala intact, raising the possibility that it could take over. Alternatively, if stimulation represents activation, as opposed to interruption of the amygdala, then this would suggest necessity of intact amygdala for respiratory pause. There are no studies to date that have conducted a detailed examination of respiratory patterns in patients with resected amygdalas, and given our findings, this would be of high interest for future studies. Regardless, it is clear that patients who undergo amygdala resection are able to breathe, thus limiting the clinical impact of our findings to some degree. One potential explanation for these apparent discrepancies is that the amygdala may not be directly involved in respiratory control; rather it is the extensive connections between the amygdala and autonomic centers in the brainstem that cause the observed effects. Only with sustained abnormal activation from these amygdala regions, be it from seizures or stimulation, does propagation lead to apnea. That said, it is possible that amygdala resection patients could show deficits in established respiratory modulations in response to unpleasant and fearful stimuli, especially odors38. This remains to be tested in future studies.
Notably, the amygdala’s role in respiratory control could relate to its close anatomical and functional relationship with the olfactory system. The amygdala receives direct input from the olfactory bulb (just one synapse from olfactory sensory neurons in the nose) and olfactory sampling is inextricably related to breathing- it is impossible to encounter a smell without first inhaling through the nose. Beyond delivery of odor stimuli to the nose, olfactory sampling networks perform rapid adaptive control over sniffing when potentially threatening chemicals are detected. In humans, behavioral data suggest the olfactory system achieves odor-specific sniff reductions within 160ms of sniff onset, suggesting a subcortical mechanism38, 40. The amygdala, with its direct connections to the olfactory bulb41–43, and autonomic control centers via the BNST44, 45 combined with its well-established role in threat detection39, 46–48, is a prime candidate for mediating fast protective respiratory modifications in response to potentially threatening chemical stimuli.
In humans, the continuous, involuntary rhythm of breathing is generated within the brainstem49, however, very little is known about the conscious control of breathing24. This study demonstrates a role for the amygdala in voluntary respiratory control and allows for further study of this pathway in dysfunctional breathing states - not only in those with epilepsy but also infants with developmental abnormalities and in neurodegenerative disorders. It is our hope that understanding how we can prevent amygdala-stimulation-induced apnea will pave the way for research into potential avenues to explore for the prevention of SUDEP whether by more detailed examination into these neuronal pathways and alterations induced by seizures or by preventing SUDEP by more acute interventions such as wearable convulsive seizure detectors to aide in arousing patients as an early intervention50.
Acknowledgments
Thanks to Jeremy Eagles and Navid Shadlou for technical help and support. This work was supported by a grant to C.Z. from the National Institute on Deafness and Other Communication Disorders (R00DC012803), and by a grant to N.B. from Citizens United for Research In Epilepsy (CURE) (Taking Flight Award).
Footnotes
Author Contributions:
WPN, CZ, SS, JT and JR were responsible for conception and design of the study. CZ, GL, and GZ were responsible for acquisition and analysis of data. WPN and CZ were responsible for drafting the manuscript and figures.
Potential Conflict of Interest:
Nothing to report
References
- 1.Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016 Mar;41(2):89–104. doi: 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zesiewicz TA, Baker MJ, Wahba M, Hauser RA. Autonomic Nervous System Dysfunction in Parkinson’s Disease. Curr Treat Options Neurol. 2003 Mar;5(2):149–60. doi: 10.1007/s11940-003-0005-0. [DOI] [PubMed] [Google Scholar]
- 3.Vazomotor İJEHA, Fonksiyonu S. Abnormal Vasomotor System Function in Idiopathic Generalized Epileptic Patients. 2014. [Google Scholar]
- 4.Toth V, Hejjel L, Fogarasi A, et al. Periictal heart rate variability analysis suggests long-term postictal autonomic disturbance in epilepsy. Eur J Neurol. 2010;17(6):780–7. doi: 10.1111/j.1468-1331.2009.02939.x. [DOI] [PubMed] [Google Scholar]
- 5.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002 Mar 25;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 6.Plotkin A, Sela L, Weissbrod A, et al. Sniffing enables communication and environmental control for the severely disabled. Proceedings of the National Academy of Sciences. 2010 Aug 10;107(32):14413–8. doi: 10.1073/pnas.1006746107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. J Neurosci. 2015 Jul 15;35(28):10281–9. doi: 10.1523/JNEUROSCI.0888-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman LM, Spitz M, Seyal M. Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: report on two deaths in video-EEG-monitored patients. Epilepsia. 2010 May;51(5):916–20. doi: 10.1111/j.1528-1167.2009.02513.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013 Oct 1;12(10):966–77. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 10.Surges R, Sander JW. Sudden unexpected death in epilepsy: mechanisms, prevalence, and prevention. Curr Opin Neurol. 2012 Apr;25(2):201–7. doi: 10.1097/WCO.0b013e3283506714. [DOI] [PubMed] [Google Scholar]
- 11.Zhou G, Tsai H-W, Davenport P. Respiratory activation of neurons in the central nucleus of the amygdala (CeA) and effect of CeA neuron stimulation on respiration (1178.5) The FASEB Journal. 2014 Apr 1;28(1 Supplement) [Google Scholar]
- 12.Harper RM, Frysinger RC, Trelease RB, Marks JD. State-dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Res. 1984 Jul 23;306(1–2):1–8. doi: 10.1016/0006-8993(84)90350-0. [DOI] [PubMed] [Google Scholar]
- 13.Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983 Sep;31(3):353–60. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JX, Harper RM, Frysinger RC. Respiratory modulation of neuronal discharge in the central nucleus of the amygdala during sleep and waking states. Exp Neurol. 1986 Jan;91(1):193–207. doi: 10.1016/0014-4886(86)90037-3. [DOI] [PubMed] [Google Scholar]
- 15.Zelano C, Jiang H, Zhou G, et al. Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J Neurosci. 2016 Dec 7;36(49):12448–67. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology. 2017 Jan 13; doi: 10.1212/WNL.0000000000003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oler JA, Birn RM, Patriat R, et al. Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. Neuroimage. 2012 Jul 16;61(4):1059–66. doi: 10.1016/j.neuroimage.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. BNST neurocircuitry in humans. Neuroimage. 2014 May 1;91:311–23. doi: 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006 Jan 1;494(1):75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha S, Batten TF, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience. 2000;99(4):613–26. doi: 10.1016/s0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BN, Russell C, Khan RM, Sobel N. A comparison of methods for sniff measurement concurrent with olfactory tasks in humans. Chem Senses. 2006 Nov;31(9):795–806. doi: 10.1093/chemse/bjl021. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002 Oct;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 23.Yanovsky Y, Ciatipis M, Draguhn A, Tort AB, Brankack J. Slow oscillations in the mouse hippocampus entrained by nasal respiration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014 Apr 23;34(17):5949–64. doi: 10.1523/JNEUROSCI.5287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, Wheless JW, Papanicolaou AC, Ruszinkó M, Sokolov Y, Kozma R. Breathing as a fundamental rhythm of brain function. Front Neural Circuits. 2017 Jan 12;10:115. doi: 10.3389/fncir.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Entis JJ, Doerga P, Barrett LF, Dickerson BC. A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra-high resolution MRI. Neuroimage. 2012 Apr 2;60(2):1226–35. doi: 10.1016/j.neuroimage.2011.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999 Jun 29;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- 27.Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981 Nov 1;202(3):335–56. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- 28.Nagy FZ, Paré D. Timing of impulses from the central amygdala and bed nucleus of the stria terminalis to the brain stem. J Neurophysiol. 2008 Dec;100(6):3429–36. doi: 10.1152/jn.90936.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miocinovic S, Lempka SF, Russo GS, et al. Experimental and theoretical characterization of the voltage distribution generated by deep brain stimulation. Experimental neurology. 2009 Mar;216(1):166–76. doi: 10.1016/j.expneurol.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol. 2013 Mar;11(2):141–59. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samoriski GM, Piekut DT, Applegate CD. Differential spatial patterns of Fos induction following generalized clonic and generalized tonic seizures. Exp Neurol. 1997 Feb;143(2):255–68. doi: 10.1006/exnr.1996.6368. [DOI] [PubMed] [Google Scholar]
- 32.Saha S, Drinkhill MJ, Moore JP, Batten TFC. Central nucleus of amygdala projections to rostral ventrolateral medulla neurones activated by decreased blood pressure. Eur J Neurosci. 2005 Apr;21(7):1921–30. doi: 10.1111/j.1460-9568.2005.04023.x. [DOI] [PubMed] [Google Scholar]
- 33.Ravindran K, Powell KL, Todaro M, O’Brien TJ. The pathophysiology of cardiac dysfunction in epilepsy. Epilepsy Res. 2016 Aug 11;127:19–29. doi: 10.1016/j.eplepsyres.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Chouchou F, Bouet R, Pichot V, Catenoix H, Mauguière F, Jung J. The neural bases of ictal tachycardia in temporal lobe seizures. Clin Neurophysiol. 2017 Jun 26; doi: 10.1016/j.clinph.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Poh MZ, Loddenkemper T, Reinsberger C, et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology. 2012 Jun 5;78(23):1868–76. doi: 10.1212/WNL.0b013e318258f7f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014 May;10(5):271–82. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masaoka Y, Hirasawa K, Yamane F, Hori T, Homma I. Effects of left amygdala lesions on respiration, skin conductance, heart rate, anxiety, and activity of the right amygdala during anticipation of negative stimulus. Behavior modification. 2003 Oct;27(5):607–19. doi: 10.1177/0145445503256314. [DOI] [PubMed] [Google Scholar]
- 38.Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. Journal of neurophysiology. 2003 Aug;90(2):1084–94. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- 39.Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005 Nov;30(10):953–8. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Bensafi M, Pouliot S, Sobel N. Odorant-specific patterns of sniffing during imagery distinguish ‘bad’ and ‘good’ olfactory imagers. Chemical senses. 2005 Jul;30(6):521–9. doi: 10.1093/chemse/bji045. [DOI] [PubMed] [Google Scholar]
- 41.Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. The Journal of comparative neurology. 1994 Aug 15;346(3):403–34. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- 42.LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual review of neuroscience. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Experimental brain research. 1985;58(2):379–91. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- 45.Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain research bulletin. 1991 Nov;27(5):651–62. doi: 10.1016/0361-9230(91)90041-h. [DOI] [PubMed] [Google Scholar]
- 46.Mendez-Bertolo C, Moratti S, Toledano R, et al. A fast pathway for fear in human amygdala. Nature neuroscience. 2016 Aug;19(8):1041–9. doi: 10.1038/nn.4324. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen WS, Balderston NL, Miskovich TA, Belleau EL, Helmstetter FJ, Larson CL. The effects of stimulus novelty and negativity on BOLD activity in the amygdala, hippocampus, and bed nucleus of the stria terminalis. Social cognitive and affective neuroscience. 2017 May 01;12(5):748–57. doi: 10.1093/scan/nsw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrmann MJ, Boehme S, Becker MP, et al. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human brain mapping. 2016 Mar;37(3):1091–102. doi: 10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006 Mar;7(3):232–42. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onorati F, Regalia G, Caborni C, et al. Multicenter clinical assessment of improved wearable multimodal convulsive seizure detectors. Epilepsia. 2017 Nov;58(11):1870–9. doi: 10.1111/epi.13899. [DOI] [PubMed] [Google Scholar]