Summary
Background
Swallowed topical corticosteroids are prescribed for eosinophilic oesophagitis (EoE), but there is a theoretical risk of adrenal insufficiency from their use.
Aims
To determine if the use of topical corticosteroids to treat EoE is associated with the development of adrenal insufficiency.
Method
We conducted a systematic review of the published literature from January 1, 1950 to April 1, 2017 using Pubmed, Embase, Web of Science and Cochrane Central. Studies and meeting abstracts were included that described patients with EoE who received swallowed topical corticosteroids and any investigation for adrenal insufficiency.
Results
The search revealed 1610 unique publications, and 17 met inclusion criteria. There were 7 randomised controlled trials (RCTs), 6 prospective observational studies, 3 retrospective observational studies, and 1 case report. Cortisol measurements were performed on 596 individuals with EoE who received topical corticosteroids. Adrenal testing was abnormal, as defined by each study, in 94/596 patients (crude rate of 15.8%). Only 2 studies were considered to have a low risk of bias, being randomised controlled trials that estimated adrenal insufficiency in the active treatment and placebo groups, before and after treatment. None of the seven randomised controlled trials demonstrated statistically significantly different rates of adrenal insufficiency between topical corticosteroid and placebo over treatment intervals of 2–12 weeks.
Conclusion
Topical corticosteroids were associated with adrenal insufficiency in a minority of patients. Most cases came from uncontrolled observational studies, with widely varying definitions of adrenal insufficiency. Longer follow-up and larger controlled studies are needed to quantify the risk of adrenal insufficiency with maintenance topical corticosteroid therapy in EoE.
Introduction
Eosinophilic oesophagitis (EoE) is a chronic inflammatory condition thought to be caused by food or aeroallergens (1, 2). Topical corticosteroids are a first line therapy, with response rates from 50 to 90% (3, 4). Fluticasone, administered from a multi-dose inhaler to the oropharynx but then swallowed, was the first widely adopted topical corticosteroid, and recently oral viscous budesonide has been trialled with success (3, 5, 6). Optimal dose and duration are debated, although maintenance topical corticosteroids may decrease endoscopic relapse rates, strictures and food bolus impaction (7, 8). Side effects of topical corticosteroids in other settings (e.g. asthma and seasonal rhinitis) have included adrenal insufficiency, and have been reported in patients receiving topical corticosteroids for EoE (9, 10). However, risk estimates of adrenal insufficiency due to topical corticosteroids in EoE are lacking, due to recent recognition of the condition and treatment trials with short follow up periods. Consequently there are no clinical guidelines for assessment of this iatrogenic phenomenon (11–15).
When considering the use of topical corticosteroids for EoE, the balance of side effects with clinical efficacy is key. Should topical corticosteroids in EoE be notably associated with adrenal insufficiency, alternate therapies may increase in appeal, and there will be a role for assessment of adrenal function. Given the increasing use of swallowed topical corticosteroids (13, 14), we undertook a systematic review of the literature pertaining to topical corticosteroid use in both adults and children with EoE. We aimed to determine if topical corticosteroid therapy increases the risk of adrenal insufficiency, and if so, what factors were associated with adrenal insufficiency.
Methods
We performed a systematic review to assess the occurrence of adrenal insufficiency in patients with EoE who received topical corticosteroids using methods consistent with PRISMA guidelines (16). We identified published reports of topical corticosteroid use in patients with EoE using PUBMED, EMBASE, Web of Science, and Cochrane CENTRAL from January 1, 1950 to April 1, 2017. The search terms included EoE, topical corticosteroids and any term relevant to adrenal insufficiency in humans. Specifically, the terms were: eosinophilic AND ('oesophagitis'/exp OR oesophagitis) AND ('steroids'/exp OR steroids OR 'corticosteroids'/exp OR corticosteroids OR 'fluticasone'/exp OR fluticasone OR 'budesonide'/exp OR budesonide OR 'ciclesonide'/exp OR ciclesonide OR 'adrenal'/exp OR adrenal) AND [humans]/lim. In addition, the reference lists of all articles included in the final analysis and in previous reviews were hand-searched to identify other relevant studies. We also hand-searched all scientific abstracts presented at four pertinent scientific meetings (Digestive Diseases Week – DDW, United European Gastroenterology Week – UEGW, American Academy of Allergy, Asthma and Immunology – AAAAI, American College of Gastroenterology -- ACG) from 2013–2017. Indexed publications in any language were eligible for inclusion; review articles, editorials and data previously published in another form were excluded. Studies of any design were included if they described patients with EoE who took at least one dose of topical corticosteroids, and recorded at least one objective measure of adrenal function. EoE was defined as >15 eosinophils per high power field (HPF) following oesophageal biopsy. Each abstract and title was reviewed by two authors (equal task allocation, such that between CR, HP, and MD each article received dual review), with disagreements included for full text review. Any discrepancies regarding study inclusion after full text review were resolved by consensus among HP, MD, and CR, and in case of persistent irresolution, adjudicated by the senior author (ESD). Two authors (MD and CR) extracted the data from included studies into tables, which were checked by the first author (HP). We updated data from abstracts with those of full publications as they became available.
Risk of bias of each included study for detecting adrenal insufficiency was independently assessed by at least two authors, using the National Institutes of Health/National Heart, Lung and Blood Institute quality assessment tools. (17) Discrepancies regarding quality ratings were resolved by group discussion, adjudicated by the senior author (ESD). Authors with potential conflicts of interest with a particular article did not review that article or participate in discussions surrounding inclusion. The primary outcome assessed was the percentage of individuals with EoE who received topical corticosteroids and had biochemical adrenal insufficiency, as defined by either (1) low morning cortisol using each study’s defined threshold, or (2) insufficient cortisol response to adrenocorticotropic hormone (ACTH) stimulation, using the thresholds of the study in which each was tested. Patient (age) and treatment (duration, formulation and type of topical corticosteroid) characteristics were evaluated as covariates.
Results
Search results and overall results of adrenal testing
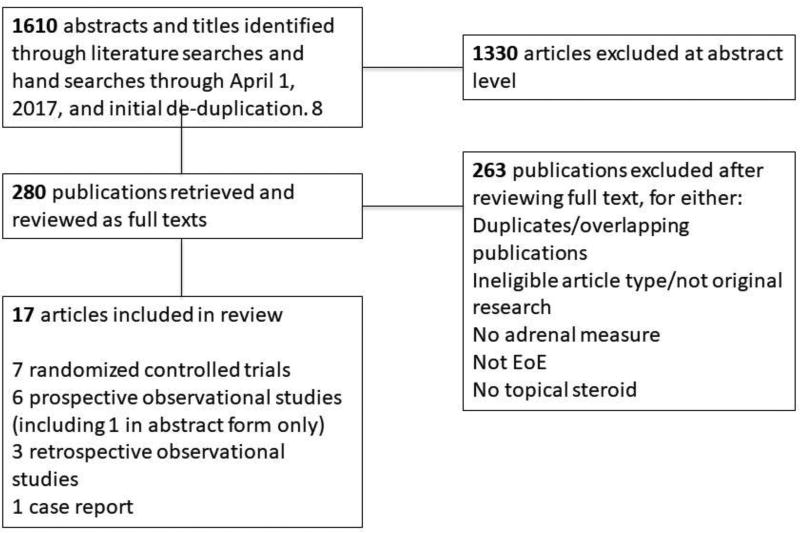
We identified 1610 unique reports, and 17 met inclusion criteria (Tables 1 and 2) (5, 18–33). There were 7 randomised controlled trials, 6 prospective observational studies, 3 retrospective observational studies, and 1 case report (see Figure 1). These included cortisol measurements from 596 individuals with EoE who received swallowed topical corticosteroids. The majority came from observational studies (n=346, see Table 2). The remaining 250 patients came from 6 randomised placebo-controlled trials and a single randomised controlled trial (RCT) that compared budesonide formulations (Table 1). The crude rate of adrenal insufficiency amongst those receiving topical corticosteroids in randomised controlled trials was 12/250 (4.8%), compared to placebo, 2/117 (1.7%, Table 1). Amongst the observational studies, the crude rate of adrenal insufficiency was 82/346 (23.7%, Table 2)
Table 1.
Randomised controlled trials of tCS for EoE
| Author Year Study design |
Number of patients with adrenal function test |
Age (years, mean) |
Medication formulation and daily dose |
Duration (weeks) |
Test Modality |
Result (adrenal insufficiency, %, authors conclusion) |
Quality rating |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Dellon 2017 RPCT | T=51 | 22.3 | Budesonide Suspension 4mg | 12 | SC (pre- and post) | T=3/51=5.8% | Good |
| P=42 | P=2/42=4.7% | ||||||
| No | |||||||
|
| |||||||
| Miehlke 2016 RPCT | T=57 | 40 | Budesonide (viscous and dispersible) 2–4mg | 2 | SC (pre- and post) | T=1/57=1.7% | Good |
| P=19 | P=0/19 | ||||||
| No | |||||||
|
| |||||||
| Gupta 2015 RPCT | T=53 | 8.9 | Budesonide (viscous) 0.35–4mg† | 12 | SC (pre- and post) | T=1/53=1.9% | Fair |
| P=18 | P=0 | ||||||
| No | |||||||
|
| |||||||
| Butz 2014 RPCT | T=28 | 12.2 | Fluticasone (aerolised and swallowed) 880 to 1760mcg† | 24 | SC or SALC | T=7/28 (28.6%) | Fair |
| P=14 | P=0 | ||||||
|
| |||||||
| Alexander 2012 RPCT | T=21 | 37.5 | Fluticasone (aerolised and swallowed) 1760mcg | 6 | 24-hour urine cortisol | T=0 | Fair |
| P=15 | P=0 | ||||||
|
| |||||||
| Dellon 2012 RCT | T=25 | 35 | Budesonide (aerolised or oral viscous) 2mg† | 8 | Full-dose ACTH stimulation test | T=0 | Fair |
|
| |||||||
| Dohil 2010 RPCT | T=15 | 7.8 | Budesonide (viscous) 1mg to 2mg† | 12 | SC | T=0 | Fair |
| P=9 | P=0 | ||||||
ACTH, adrenocorticotropic hormone; RPCT, randomised placebo controlled trial; RCT, randomised controlled trial, no placebo; T, treatment group; P, placebo group; SALC, salivary cortisol; SC, serum cortisol
Co-intervention with topical corticosteroids e.g. for asthma was allowed.
Table 2.
Observational studies of tCS for EoE
| Author Year Study design |
Number of patients with adrenal function assessment |
Age (years, mean) |
Medication formulation and daily dose |
Mean Duration (weeks) |
Test modality |
Result (adrenal insufficiency, %, threshold†) |
Quality rating |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Bose 2017 REC | 37 | 9.4 | Fluticasone or Budesonide suspension Unknown dose | 93 | SC and then LDST | 19/37 (51%) low SC (<276nmol/L [10mcg/dl]); | Poor |
| 2/10 (20%) low LDST | |||||||
|
| |||||||
| Oliva 2017 POL | 36 | 12.3 | Budesonide Suspension 2mg to 4mg | 12 | SC | 0 (decrease from pre- to post-treatment) | Poor |
|
| |||||||
| Andreae 2016 POL | 20 | 5.5 | Fluticasone 172 to 880mcg‡ | variable | SC | 0 (decrease from pre- to post-treatment) | Poor |
|
| |||||||
| Ahmet 2016 POL | 29 | 14.1 | Fluticasone 125 to 250mcg budesonide 1–2mg‡ | 14 | SC and LDST | 5/29 (17%) low SC (<185nmol/L [6.7 mcg/dL]); | Poor |
| 19/29 (65.5%) low LDST | |||||||
|
| |||||||
| Hsu 2016 POL | 106 | 8.4 | 88–880 mcg fluticasone, 0.25–2mg budesonide, 320–960 ciclesonide, 160–320 mcg beclomethasone‡ | 78 | SC and LDST | 32/106 (30.2%) single low SC (<139 nmol/L [5 mcg/dL]), | Poor |
| 5/106 (4.7%) low LDST§ | |||||||
|
| |||||||
| Harel 2015 REC | 14 | 10.6 | Budesonide viscous 0.5 to 2mg daily‡ | 80 | LDST | 6/14 (43%) low LDST | Poor |
|
| |||||||
| Golekoh 2015 REC | 58 | 12.9 | Budesonide viscous 0.5 to 2mg or fluticasone 440 to 1760mcg | 192 | LDST | 6/58 (10%) low LDST; | Poor |
| 9/58 (15%) “abnormal” LDST (<552 nmol/L [20 mcg/dL]) | |||||||
|
| |||||||
| Philla 2015 POL | 25 | 1.1 | Budesonide viscous 0.5 to 1mg or fluticasone 220 to 880mcg | 17 | SC | 0 (decrease from pre- to post-treatment) | Poor |
|
| |||||||
| Krishna 2011 Case report | 1 | 28 | Budesonide 1mg‡ | 52 | SC | 0 (decrease from pre- to post-treatment) | Poor |
|
| |||||||
| Aceves 2007 REC | 20 | 5.5 | Budesonide viscous 1–2mg‡ | 14 | SC | 0 (normal = 55–469 nmol/L [2–17 mcg/dL]) | Poor |
|
| |||||||
| TOTAL | 346 | 82¶ | |||||
ACTH, adrenocorticotropic hormone; RPCT, randomised placebo controlled trial; RCT, randomised controlled trial, no placebo; REC, retrospective cohort study; POL, prospective open label study; NR, not reported; T, treatment group; P, placebo group; SALC, salivary cortisol; SC, serum cortisol; LDST, low dose adrenocorticotropin stimulation test
All LDST’s performed with 1 mcg adrenocorticotropin with insufficient adrenal response defined as a peak serum cortisol <500nmol/L [18 mcg/dL] at either 20 or 30 minutes post-stimulation, unless otherwise specified.
Co-intervention with topical corticosteroids e.g. for asthma was allowed.
Only 10 subjects received LDST testing (7 of whom had two low morning serum cortisol).
Defined as those with EITHER low morning serum cortisol or low LDST response.
Figure 1.
Flow diagram depicting selection of studies
The modality of adrenal axis assessment varied between studies (serum cortisol, urine cortisol, salivary cortisol), as did the definition of adrenal insufficiency. Morning cortisol was used as a stand-alone diagnostic test in ten studies, one of which used salivary cortisol for some subjects (22). Several studies used the low dose adrenocorticotropin (ACTH) stimulation test (LDST), either as a stand-alone test (four studies) (21, 26, 28, 30), or after serum cortisol measurement, to identify the ‘at risk’ subgroup that should receive LDST (two studies) (24, 25). These latter studies used different pre-stimulation cortisol cut-offs (138nmol/L [5mcg//dL] (24) and 276 nmol/L [10 mcg/dL](25), which differed still from cut-offs of 55nmol/L [2mcg/dL] (32) and 185nmol/L [6.7 mcg/dL] (28) used for unstimulated serum cortisol in other studies. All studies used a cut-off of 500nmol/L [18 mcg/dL] to diagnose adrenal insufficiency after low dose ACTH testing. One study used 24-hour urine cortisol to assess the adrenal axis (20). Due to the heterogeneity of study designs and outcome measures, formal meta-analysis was not pursued.
Results from blinded, randomised trials
Only 2 studies were considered to have a low risk of bias. These were randomised controlled trials that estimated adrenal insufficiency in the active treatment and placebo groups, excluded concomitant use of other steroid formulations, used a single modality to define adrenal insufficiency, and incorporated baseline measurements (rather than post-treatment measures alone) (5, 18). Dellon et al found adrenal insufficiency in 3/51 patients receiving topical corticosteroids (5.9%), compared to 2/42 receiving placebo (4.8%), whilst Miehlke et al demonstrated adrenal insufficiency in 1/57 (1.8%) topical corticosteroids, compared to 0/19 receiving placebo (Table 1).
No adverse clinical events related to exogenous steroid exposure (such as a change in body weight, growth rate in children, or adrenal crisis) were reported in any clinical trial. All were efficacy trials that evaluated adrenal insufficiency as a secondary safety endpoint. Adrenal insufficiency was no more common in patients receiving topical corticosteroids than patients receiving placebo in all 6 placebo-controlled randomised controlled trials, comprising 3 adult and 3 paediatric studies (Table 1). Treatment-related changes in mean cortisol did not significantly differ between treatment and placebo groups in the four studies reporting this outcome (5, 18, 19, 23). Rates adrenal insufficiency were estimated following a relatively short treatment course, with 6 of the 7 randomised controlled trials testing at 12 weeks or less, the study by Butz et al being the exception at 6 months (22). This paediatric study demonstrated a non-statistically significant trend (p=0.15) toward increased adrenal insufficiency in those treated with high-dose (1760 mcg/day) fluticasone (22), though 5/7 cases of decreased cortisol were from salivary cortisol measurements. Three of the 7 randomised controlled trials (5, 18, 20) specifically excluded patients with concomitant inhaled topical corticosteroid use (for a condition other than EoE); the remainder did not specify this as an exclusion criterion (19, 21–23).
Results from observational studies
Observational studies evaluated longer durations of treatment (up to 3.7 years, Table 2). Mean duration of topical corticosteroid use prior to investigation (when stated) was 22 months (range 10–43 months) (24–26, 28, 30, 31). Adrenal insufficiency was the primary endpoint in 6 of 10 studies (24–26, 28, 30, 31). All were paediatric studies, and 4 of 6 were retrospective (see Table 2).
Adrenal insufficiency was defined heterogeneously. Five studies used LDST (24–26, 28, 30), measuring peak cortisol at different intervals from 15 to 60 minutes post-stimulation. Thresholds for adrenal insufficiency by unstimulated serum cortisol varied from 55 to 276 nmol/L [2 to 10 mcg/dL] (24, 25, 28, 34). Of studies using both tests, 2 demonstrated that a minority of those with low unstimulated cortisol had abnormal LDSTs (24, 25), while a third demonstrated the opposite trend, with nearly 4 times as many abnormal LDSTs as unstimulated cortisol assays (28). Six studies sought clinical evidence of exogenous steroid exposure, including history of fracture (24), growth restriction (24, 25, 27, 29), adrenal crisis (24, 28, 30). None demonstrated an increase in clinical events, though one patient had a history of fracture (24), and Bose et al reported a lower body mass index in patients with an abnormal morning cortisol than those without (p=0.04) (25).
Incomplete data was common in the observational studies. For example, Hsu et al were only able to measure cortisol in 106/225 (47%) of their cohort (24). Andreae et al tested the adrenal axis only “when prompted by parental anxiety” (27). Notably, all observational studies with any cases of adrenal insufficiency lacked pre-treatment assessment of adrenal function (24–26, 28, 30). Of those that assessed change in adrenal measures from pre- to post-treatment, no cases of decreased adrenal function were reported (0/102 patients) (27, 29, 31, 33, 34).
The approach to concomitant use of other topical corticosteroids for non-EoE indications (usually inhaled formulations) also varied. Concomitant use was an exclusion criterion in 3 observational studies, which reported rates of adrenal insufficiency of 0%, 0%, and 10% (26, 29, 31). In contrast, when concomitant corticosteroid use was permitted, adrenal insufficiency was present in 0%, 30%, 43%, 51%, and 66%, (24, 25, 28, 30). In the study by Ahmet et al (66% rate of adrenal insufficiency), 45% of subjects were using corticosteroids for asthma or rhinitis or both (28). Of 6 patients with adrenal insufficiency recorded by Harel et al, 3 (50%) were using inhaled corticosteroids, compared to only 2/8 (25%) of those without adrenal insufficiency (30). Similarly, Hsu et al found that all five children with diagnosed with adrenal insufficiency by LDST used inhaled steroids for asthma (24). Cumulatively, insufficiency was reported in 6/119 (5.0%) of patients in observational studies that excluded concomitant use of corticosteroids, but 76/227 (33.5%) of patients in studies that did not (Table 2).
Discussion
Topical corticosteroids are a first line treatment for patients with EoE, they improve symptoms such as dysphagia and may decrease stricture severity (7, 8, 14). The theoretical risk of adrenal suppression secondary to swallowed topical corticosteroids has been bolstered by reports of biochemical adrenal insufficiency in patients using topical corticosteroids for EoE (30, 31), although a synthesis of the literature to quantify the problem is lacking. This systematic review found that abnormal cortisol values may be found in a non-trivial minority (16%) of patients taking topical corticosteroids for EoE. One of the more homogeneous subgroups were paediatric patients evaluated by LDST, in whom biochemical adrenal insufficiency was found in up to 18% overall. However, these estimates should be interpreted with caution, as they are driven by uncontrolled observational studies using heterogeneous methods of evaluating the adrenal axis, often without considering baseline adrenal function or concomitant corticosteroid use for other indications.
In the randomised controlled trials, adrenal insufficiency did not occur more frequently with topical corticosteroids than with placebo, albeit following a treatment interval usually 12 weeks or less, nor was a decrease in serum cortisol noted in any of the observational cohorts that measured pre-treatment values. This contrasts with the five uncontrolled, retrospective paediatric studies, which reported prevalence of adrenal insufficiency from 5–65% using the LDST. Accounting for baseline low cortisol values may be particularly critical in EoE, given the generally lower-end HPA axis activity described in allergic and autoimmune conditions (2, 35, 36). It is unclear whether quality of study design, method of assessment of adrenal function, duration of treatment or patient population is the primary explanation for these divergent findings, though each likely contributes. Concomitant use of inhaled, intranasal, or dermal corticosteroids for asthma, rhinitis or dermatitis likely caused higher rates of adrenal insufficiency in 3 observational studies (24, 28, 30).
Greater duration of treatment with topical corticosteroids is a risk factor for adrenal insufficiency in asthma (37), particularly when impairment of growth velocity in childhood is used as a surrogate measure, and assessment for adrenal insufficiency is considered important, although the duration of treatment that warrants investigation is not stated (38–40). Dose is another consideration, with guidelines variably attributing risk to ‘high dose’ fluticasone, or budesonide respectively, and mentioning the risk of adrenal crisis (41). While adrenal effects of topical corticosteroids are likely to be dose-related, the heterogeneity of drugs and delivery used in studies of EoE prohibits a confident assessment of the dose above which the risk of adrenal insufficiency becomes significant. For example, of 7 randomised controlled trials that demonstrated no risk of adrenal insufficiency, 4 used budesonide alone, 2 used fluticasone alone and 1 study compared viscous budesonide to swallowed nebulised budesonide (where there was documented pulmonary deposition) (21). A similar range of medications, doses and formulations were shown in the 5 observational studies that demonstrated a risk of adrenal insufficiency.
Regarding the optimal means for assessment of adrenal insufficiency, Endocrine Society Guidelines for primary adrenal insufficiency recommend the high dose (250mcg) ACTH-stimulation test (42). While the LDST may have a higher sensitivity for detection of biochemical adrenal insufficiency, this has not been proven, and the high dose test has more clinical and management implications (43–45). Importantly, there is no evidence-based or definition of tertiary, or glucocorticoid-induced, adrenal insufficiency (47). Often morning cortisol is measured, while interrupting the exogenous glucocorticoid for a period longer than its tissue effect. The intention is to determine underlying endogenous cortisol secretion at or near to its circadian peak. This test has the advantage of relative convenience and low cost, compared to ACTH 1–34 (synacthen®, cosyntropin®) stimulation testing, where blood samples are collected at −1, 30 and in some centres 60 mins. Morning plasma cortisol levels of <34 nmol/L or >340 nmol/L predict subnormal or normal responses to ACTH 1–34 (synacthen®, cosyntropin®) testing, respectively (45), and time-corrected AM cortisol may further reduce the need for ACTH stimulation testing (46). Overall, ACTH stimulation testing, with its known correlation to the less safe insulin hypoglycaemia testing, is considered more definitive than a single morning cortisol assessment (45,46), but most endocrinologists use the morning cortisol test as a rule out test to reduce the need for ACTH stimulation testing. While alternative methods of assessing cortisol levels (e.g. salivary, urine or hair) are attractive as less cumbersome measurements for future studies, these have been inadequately validated for evaluating adrenal insufficiency, and cannot be recommended at present. That none of the observational studies in this review used the full-dose (250 mcg) ACTH stimulation test recommended by Endocrine Society guidelines (42) leaves the clinical implications of the abnormal testing in these studies unclear.
While the randomised controlled trials failed to detect statistically significant changes in adrenal function, they were likely underpowered for these secondary outcomes. They may have suffered from insensitive measures (unstimulated morning cortisol, see discussion above) and inadequate duration of therapy. There is some suggestion within the randomised controlled trial data of a signal for adrenal insufficiency, as cortisol declined somewhat with treatment in three studies, and absolute numbers of low cortisol values were greater in treatment than placebo arms (5, 22, 23). However, Gupta et al failed to show a dose-response trend, the greatest decrease in cortisol occurring in the placebo arm (19). Alexander et al also showed no trend in 24-hour urine cortisol (indeed this was increased) (20), although this is not an accepted measure for diagnosis of adrenal insufficiency (rather the method of diagnosing endogenous Cushing’s disease) (47).
Cases of clinically overt acute adrenal insufficiency (e.g. adrenal crisis) have occurred secondary to non-systemic corticosteroids in non-EoE atopic conditions (41). No cases of adrenal crisis were reported in this review. This may be because patients with mild to moderate secondary adrenal insufficiency will remain asymptomatic if they remain on corticosteroids, unless a major stressor (such as severe infection or major surgery) challenges the hypothalamic pituitary axis (48). However, large studies demonstrating that cortisol testing predicts a need for glucocorticoid supplementation with illness or procedures in glucocorticoid-induced adrenal insufficiency do not exist. Interestingly a recent study of patients taking supraphysiologic doses of prednisolone (>5mg/day) showed that adrenal crises did not develop with renal transplantation when their exogenous glucocorticoid dose was simply maintained (49). Our review has instead estimated the risk of subclinical adrenal insufficiency, defined by lower-than-normal serum cortisol, or by the LDST, and in the context of other steroid use for other allergic diseases. This review, and the available literature, does not address the risk for other adverse effects of exogenous corticosteroids (e.g. metabolic effects, bone and soft tissue damage, or extra-oesophageal infections).
Experience with topical corticosteroids in the gastrointestinal tract is limited (when compared to the respiratory and cutaneous route), and this adds a further layer of uncertainty when contemplating the overall significance of measures of adrenal insufficiency at any given dose. While differences between gastrointestinal and respiratory absorption of topical corticosteroids prohibit complete extrapolation of data, it is worth noting the similarly marked variation across studies that estimate the risk of adrenal insufficiency secondary to inhaled corticosteroids for asthma in children (38, 39, 50). This suggests an intrinsic difficulty in assessing class effects across heterogeneous treatment and measurement characteristics (10, 39). A comparable use of second-generation enteral-targeted corticosteroids is the treatment for ulcerative colitis with budesonide foam enemas, which in a prospective study of 546 patients using budesonide foam enemas (2mg/daily) for proctitis resulted in an abnormal LDST in 16% of treated individuals, compared to 4% of controls at 6 weeks (51). The authors interpreted this finding as demonstrating minimal risk of clinical adrenal insufficiency secondary to topical corticosteroids, in contrast to the view of similar findings in the setting of topical corticosteroids for EoE in children (26, 30). Potential adverse effects must be contextualised by the need for treatment of the underlying disorder given the risks of complications from the disease itself.
This systematic review adds to a body of literature on swallowed topical corticosteroids in EoE. The risk of adrenal insufficiency from short-term topical corticosteroid (<12 weeks) use is negligible. Data estimating the risk of adrenal insufficiency from prolonged topical corticosteroid use is limited to observational studies with numerous methodological shortcomings contributing to a high risk of bias. However, the risk of clinically significant adrenal insufficiency even with extended therapy is still likely to be small, given that symptoms were not described in any study. There remains an unmet need for high-quality, prospective studies to more precisely define the type and magnitude of risk of long-term topical corticosteroid in patients with EoE. These would ideally include patients treated over an extended time period with either topical corticosteroids or alternative methodologies such as elimination diet, proton-pump inhibitor alone, or even dilation alone. Such studies should use a standardised assessment method such as a full-dose ACTH stimulation test, with pre-specification and adjudication of clinically important outcomes rather than only biochemical thresholds for adrenal insufficiency. At present, available data do not support routine assessment of the adrenal axis in patients with EoE on topical corticosteroids. In patients taking prolonged high doses of steroids for multiple conditions (EoE, asthma, atopic dermatitis, allergic rhinitis/sinusitis), more attention to the adrenal axis should be considered, likely in collaboration with colleagues in endocrinology.
Acknowledgments
Funding sources: Dr Hamish Philpott received finding from AusEE, and from the Philip Bushell Foundation to support this project. Drs Dougherty and Reed were supported by NIH award T32 DK007634. Dr Dellon received funding from NIH award R01 DK101856.
Disclosures: Dr Dellon is a consultant for Adare, Alivio, Allakos, Banner, Enumeral, GSK, Cellgene/Receptos, Regeneron, and Shire. Dr Dellon has received research funding from Adare, Meritage, Miraca Life Sciences, Nutricia, Celegene/Receptos, Regeneron, and Shire, and an educational grant from Banner. Dr Philpott has received educational assistance from Shire and Aspen.
Footnotes
Specific author contributions: H Philpott performed literature review and drafted manuscript, MK Dougherty performed literature review and drafted manuscript, C Reed performed literature review and revised the manuscript, M Caldwell, D Kirk, and DJ Torpy contributed to interpretation of the data and revised the manuscript for critical intellectual content, ES Dellon conceptualised the paper, supervised the review and revised the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interest: Complete disclosures of commercial affiliations (above), none of which are perceived as influencing this manuscript.
References
- 1.Philpott H, Nandurkar S, Thien F, Gibson PR, Royce SG. Eosinophilic esophagitis: a clinicopathological review. Pharmacol Ther. 2015;146:12–22. doi: 10.1016/j.pharmthera.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology. 2015;148(6):1143–57. doi: 10.1053/j.gastro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. A prospective open clinical trial of a proton pump inhibitor, elimination diet and/or budesonide for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43(9):985–93. doi: 10.1111/apt.13576. [DOI] [PubMed] [Google Scholar]
- 4.Cotton CC, Eluri S, Wolf WA, Dellon ES. Six-Food Elimination Diet and Topical Steroids are Effective for Eosinophilic Esophagitis: A Meta-Regression. Dig Dis Sci. 2017 doi: 10.1007/s10620-017-4642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I, et al. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology. 2017;152(4):776–86 e5. doi: 10.1053/j.gastro.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Molina-Infante J, Lucendo AJ. Update on topical steroid therapy for eosinophilic esophagitis. Gastroenterol Hepatol. 2015;38(6):388–97. doi: 10.1016/j.gastrohep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Straumann A. The natural history and complications of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18(1):99–118. ix. doi: 10.1016/j.giec.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Kuchen T, Straumann A, Safroneeva E, Romero Y, Bussmann C, Vavricka S, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy. 2014;69(9):1248–54. doi: 10.1111/all.12455. [DOI] [PubMed] [Google Scholar]
- 9.Harel S, Hursh BE, Chan ES, Avinashi V, Panagiotopoulos C. Adrenal insufficiency exists for both swallowed budesonide and fluticasone propionate in the treatment of eosinophilic esophagitis. J Pediatr. 2016;174:281. doi: 10.1016/j.jpeds.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 10.Choi IS, Sim DW, Kim SH, Wui JW. Adrenal insufficiency associated with long-term use of inhaled steroid in asthma. Ann Allergy Asthma Immunol. 2017;118(1):66–72 e1. doi: 10.1016/j.anai.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108(5):679–92. doi: 10.1038/ajg.2013.71. quiz 93. [DOI] [PubMed] [Google Scholar]
- 12.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20 e6. doi: 10.1016/j.jaci.2011.02.040. quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 13.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2013;25(1):47–52 e5. doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 14.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philpott H, Dellon ES. The role of maintenance therapy in eosinophilic esophagitis: who, why, and how? J Gastroenterol. 2017 doi: 10.1007/s00535-017-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.NHLBI. Study Quality Assessment Tools. 2014 Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools.
- 18.Miehlke S, Hruz P, Vieth M, Bussmann C, von Arnim U, Bajbouj M, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2016;65(3):390–9. doi: 10.1136/gutjnl-2014-308815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13(1):66–76 e3. doi: 10.1016/j.cgh.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Alexander JA, Jung KW, Arora AS, Enders F, Katzka DA, Kephardt GM, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10(7):742–9 e1. doi: 10.1016/j.cgh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Dellon ES, Sheikh A, Speck O, Woodward K, Whitlow AB, Hores JM, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143(2):321–4 e1. doi: 10.1053/j.gastro.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014;147(2):324–33 e5. doi: 10.1053/j.gastro.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139(2):418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Hsu S, Wood C, Pan Z, Rahat H, Zeitler P, Fleischer D, et al. Adrenal Insufficiency in Pediatric Eosinophilic Esophagitis Patients Treated with Swallowed Topical Steroids. Pediatr Allergy Immunol Pulmonol. 2017;30(3):135–40. doi: 10.1089/ped.2017.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose P, Nebesio TD, Hon EC, Gupta SK. Adrenal Insufficiency in Children with Eosinophilic Esophagitis Treated with Topical Steroids. Gastroenterology. 2017;152(5):S436. doi: 10.1097/MPG.0000000000002537. [DOI] [PubMed] [Google Scholar]
- 26.Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, Putnam PE, Backeljauw PF. Adrenal Insufficiency after Chronic Swallowed Glucocorticoid Therapy for Eosinophilic Esophagitis. J Pediatr. 2016;170:240–5. doi: 10.1016/j.jpeds.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Andreae DA, Hanna MG, Magid MS, Malerba S, Andreae MH, Bagiella E, et al. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children With Eosinophilic Esophagitis. Am J Gastroenterol. 2016;111(8):1187–97. doi: 10.1038/ajg.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmet A, Benchimol EI, Goldbloom EB, Barkey JL. Adrenal suppression in children treated with swallowed fluticasone and oral viscous budesonide for eosinophilic esophagitis. Allergy Asthma Clin Immunol. 2016;12:49. doi: 10.1186/s13223-016-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliva S, Rossetti D, Papoff P, Tiberti A, Rossi P, Isoldi S, et al. A New Formulation of Oral Viscous Budesonide in Treating Paediatric Eosinophilic Oesophagitis: A Pilot Study. J Pediatr Gastroenterol Nutr. 2017;64(2):218–24. doi: 10.1097/MPG.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 30.Harel S, Hursh BE, Chan ES, Avinashi V, Panagiotopoulos C. Adrenal Suppression in Children Treated With Oral Viscous Budesonide for Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2015;61(2):190–3. doi: 10.1097/MPG.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 31.Philla KQ, Min SB, Hefner JN, Howard RS, Reinhardt BJ, Nazareno LG, et al. Swallowed glucocorticoid therapy for eosinophilic esophagitis in children does not suppress adrenal function. J Pediatr Endocrinol Metab. 2015;28(9–10):1101–6. doi: 10.1515/jpem-2014-0260. [DOI] [PubMed] [Google Scholar]
- 32.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102(10):2271–9. doi: 10.1111/j.1572-0241.2007.01379.x. quiz 80. [DOI] [PubMed] [Google Scholar]
- 33.Krishna SG, Kakati BR, Olden KW, Brown DK. Treatment of eosinophilic esophagitis: is oral viscous budesonide superior to swallowed fluticasone spray? Gastroenterol Hepatol (N Y) 2011;7(1):55–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. The American journal of gastroenterology. 2007;102(10):2271. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 35.Harbuz MS, Chover-Gonzalez AJ, Jessop DS. Hypothalamo-pituitary-adrenal axis and chronic immune activation. Ann N Y Acad Sci. 2003;992:99–106. doi: 10.1111/j.1749-6632.2003.tb03141.x. [DOI] [PubMed] [Google Scholar]
- 36.Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134(5):1084–92 e1. doi: 10.1016/j.jaci.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broersen LHA, Pereira AM, Jørgensen JOL, Dekkers OM. Adrenal Insufficiency in Corticosteroids Use: Systematic Review and Meta-Analysis. The Journal of Clinical Endocrinology & Metabolism. 2015;100(6):2171–80. doi: 10.1210/jc.2015-1218. [DOI] [PubMed] [Google Scholar]
- 38.Bacharier LB, Raissy HH, Wilson L, McWilliams B, Strunk RC, Kelly HW. Long-term effect of budesonide on hypothalamic-pituitary-adrenal axis function in children with mild to moderate asthma. Pediatrics. 2004;113(6):1693–9. doi: 10.1542/peds.113.6.1693. [DOI] [PubMed] [Google Scholar]
- 39.Smith RW, Downey K, Gordon M, Hudak A, Meeder R, Barker S, et al. Prevalence of hypothalamic-pituitary-adrenal axis suppression in children treated for asthma with inhaled corticosteroid. Paediatr Child Health. 2012;17(5):e34–9. doi: 10.1093/pch/17.5.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulliver T, Morton R, Eid N. Inhaled corticosteroids in children with asthma: pharmacologic determinants of safety and efficacy and other clinical considerations. Paediatr Drugs. 2007;9(3):185–94. doi: 10.2165/00148581-200709030-00007. [DOI] [PubMed] [Google Scholar]
- 41.Todd GR, Acerini CL, Buck JJ, Murphy NP, Ross-Russell R, Warner JT, et al. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur Respir J. 2002;19(6):1207–9. doi: 10.1183/09031936.02.00274402. [DOI] [PubMed] [Google Scholar]
- 42.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2016;101(2):364–89. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, et al. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93(11):4245–53. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 44.Tordjman K, Jaffe A, Trostanetsky Y, Greenman Y, Limor R, Stern N. Low-dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo-pituitary-adrenal axis function: sensitivity, specificity and accuracy in comparison with the high-dose (250 microgram) test. Clin Endocrinol (Oxf) 2000;52(5):633–40. doi: 10.1046/j.1365-2265.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 45.Woods CP, Argese N, Chapman M, Boot C, Webster R, Dabhi V, et al. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur J Endocrinol. 2015;173(5):633–42. doi: 10.1530/EJE-15-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown S, Hadlow N, Badshah I, Henley D. A time-adjusted cortisol cut-off can reduce referral rate for Synacthen stimulation test whilst maintaining diagnostic performance. Clin Endocrinol (Oxf) 2017;87(5):418–24. doi: 10.1111/cen.13405. [DOI] [PubMed] [Google Scholar]
- 47.Fleseriu M, Hamrahian AH, Hoffman AR, Kelly DF, Katznelson L. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY DISEASE STATE CLINICAL REVIEW: DIAGNOSIS OF RECURRENCE IN CUSHING DISEASE. Endocrine Practice. 2016;22(12):1436–48. doi: 10.4158/EP161512.DSCR. [DOI] [PubMed] [Google Scholar]
- 48.Rushworth RL TD. Management of glucocorticoid induced adrenal insufficiency: An epidemiological study and review. Endocrine Practice. 2017 (In Press) [Google Scholar]
- 49.Bromberg JS, Baliga P, Cofer JB, Rajagopalan PR, Friedman RJ. Stress steroids are not required for patients receiving a renal allograft and undergoing operation. J Am Coll Surg. 1995;180(5):532–6. [PubMed] [Google Scholar]
- 50.Broide J, Soferman R, Kivity S, Golander A, Dickstein G, Spirer Z, et al. Low-dose adrenocorticotropin test reveals impaired adrenal function in patients taking inhaled corticosteroids. J Clin Endocrinol Metab. 1995;80(4):1243–6. doi: 10.1210/jcem.80.4.7714095. [DOI] [PubMed] [Google Scholar]
- 51.Sandborn WJ, Bosworth B, Zakko S, Gordon GL, Clemmons DR, Golden PL, et al. Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis. Gastroenterology. 2015;148(4):740–50 e2. doi: 10.1053/j.gastro.2015.01.037. [DOI] [PubMed] [Google Scholar]