Abstract
The heme oxygenase (HO) and nitric oxide (NO) synthase (NOS) systems display notable similarities as well as differences. HO and NOS are both oxidative enzymes using NADPH as an electron donor. The constitutive forms of the enzyme are differentially activated, with calcium entry stimulating NOS by binding to calmodulin, whereas calcium entry activates protein kinase C to phosphorylate and activate HO2. Although both NO and carbon monoxide (CO) stimulate soluble guanylyl cyclase to form cGMP, NO also S-nitrosylates selected protein targets. Both involve constitutive and inducible biosynthetic enzymes. However, functions of the inducible forms are virtual opposites. Macrophage-inducible NOS generates NO to kill other cells, whereas HO1 generates bilirubin to exert antioxidant cytoprotective effects and also provides cytoprotection by facilitating iron extrusion from cells. The neuronal form of HO, HO2, is also cytoprotective. Normally, neural NO in the brain seems to exert some sort of behavioral inhibition. However, excess release of NO in response to glutamate's N-methyl-d-aspartate receptor activation leads to stroke damage. On the other hand, massive neuronal firing during a stroke presumably activates HO2, leading to neuroprotective actions of bilirubin. Loss of this neuroprotection after HO inhibition by mutant forms of amyloid precursor protein may mediate neurotoxicity in Familial Alzheimer's Disease. NO and CO both appear to be neurotransmitters in the brain and peripheral autonomic nervous system. They also are physiologic endothelial-derived relaxing factors for blood vessels. In the gastrointestinal pathway, NO and CO appear to function as coneurotransmitters, both stimulating soluble guanylyl cyclase to cause smooth muscle relaxation.
Although nitric oxide (NO) is one of the most recently discovered neurotransmitters, it has been ascribed more neural functions than almost any other. Subsequently, carbon monoxide (CO) has been identified as a putative neurotransmitter. Heme oxygenase (HO), which forms CO, also gives rise to biliverdin, which is very rapidly reduced to bilirubin, as well as to iron. Recent studies reveal important biologic roles for all three HO products. Similarities between HO and NO synthase (NOS) systems abound along with notable discrepancies (Table 1).
Table 1.
Contrasting properties of neuronal NO synthase and HO2
| nNOS | HO2 | |
|---|---|---|
| Biosynthesis | Catalyzes a mixed oxidation of arginine to form the diatomic gas radical, NO. | Catalyzes a mixed oxidation of heme to form the inert diatomic gas, CO. |
| Gene isoforms | iNOS, inducible nNOS, eNOS, constitutive | HO1, inducible HO2, constitutive |
| Subcellular localization | Dendrites, axons, endoplasmic reticulum, and cytosol | Exclusively on the endoplasmic reticulum |
| Activation | Calcium/calmodulin | Protein kinase C |
| Target of gaseous messenger | Soluble guanyl cyclase | Soluble guanyl cyclase |
| Can directly alter protein function by S-nitrosylation. | Other targets? | |
| Role in blood vessels | Originally discovered as endothelial-derived relaxation factor | Like eNOS and nNOS, HO2 is present in both the endothelium and the surrounding adventitial neurons. |
| Role in gastrointestinal tract | Nonadrenergic, noncholinergic neurotransmitter, most prominently in the pylorus | Nonadrenergic, noncholinergic neurotransmitter, most prominently in the internal anal sphincter |
| Role in urogenital tract | nNOS+ neurons innervate the corpus cavernosum. NOS inhibitors prevent penile erections. | HO2+ neurons innervate the bulbospongiosus muscles. Ejaculation is reduced in HO2−/− mice. |
| Behavior | nNOS−/− mice are aggressive and inappropriately mount females, regardless of estrus stage. | ? |
| Neurotoxicity | Activation of nNOS augments toxicity by generation of a free radical. | Activation of HO2 protects against toxicity by quenching free radicals. |
Biosynthesis.
NO is formed by NOS oxidizing arginine to NO with the stoichiometric formation of citrulline. Three distinct genes code for the three forms of NOS: inducible NOS, whose induced synthesis enables macrophages to form the NO that kills tumor cells and bacteria; endothelial NOS (eNOS), which produces the NO that relaxes blood vessels; and neuronal NOS (nNOS), which will be our particular focus (1). Most neurotransmitters are stored in synaptic vesicles, with only a small proportion of storage pools released with each nerve stimulation. By contrast, because NO is a membrane-permeable molecule, it cannot be stored. Thus, NO release occurs after nNOS activation, implying that nNOS activation is highly regulated. Indeed, nNOS is more tightly regulated than any other neurotransmitter-forming enzyme. For instance, most oxidative enzymes use a single electron donor, whereas nNOS utilizes NADPH, Familial Alzheimer's Disease (FAD), FMN, and tetrahydrobiopterin, as well as heme, with appropriate binding sites for these cofactors evident with the first cloning of nNOS (2). Structurally, nNOS is a fusion of an NO-synthesizing moiety and cytochrome P450 reductase, the electron donor for many oxidative enzymes. HO resembles NOS in that there are distinct inducible and noninducible enzymes. The first identified of these enzymes, HO1, is concentrated in the spleen, and its synthesis is markedly induced by heme from aging red blood cells or mitochondrial heme-containing proteins. Multiple cellular stresses induce HO1, which is also thus designated heat-shock protein-32. Just as eNOS and nNOS are constitutive, so HO2, the neuronal form of HO, is also constitutive. Unlike NOS, HO utilizes a separate cytochrome P450 reductase protein to donate electrons for its oxidative cleavage of the heme ring.
Forming a gaseous transmitter de novo with each nerve impulse requires a rapid means of activation. The discovery that nNOS requires calcium–calmodulin established that calcium influx with neuronal depolarization can rapidly activated nNOS (3). A similarly rapid activation of HO2 has not been definitively characterized. However, we have established that phosphorylation of HO2 by protein kinase C as well as phorbol esters that activate protein kinase C lead to augmentation of HO2 catalytic activity as well as enhanced bilirubin staining in brain cultures (4). This activation has not yet been definitively linked to augmentation of CO-mediated neurotransmission.
Intracellular sites of transmitter synthesis distinguish nNOS and HO2. In brain homogenates, half of nNOS is soluble and half is particulate. Electron microscopic studies localize nNOS to a variety of cellular membranes, including dendritic spines and shafts, axon terminals, and the endoplasmic reticulum (5, 6). For transmitter release, nNOS appears to be localized to the plasma membrane, permitting rapid egress of NO, conceivably associated with translocation of cytosolic nNOS to the membrane. There, it is bound to postsynaptic density 95 (PSD95) in intimate association with N-methyl-d-aspartate (NMDA) subtype of glutamate receptors, permitting a direct route of calcium through the NMDA receptor channel to nNOS. The soluble protein CAPON, like PSD95, binds to the PDZ domain of NOS (8). Capon–nNOS complexes are unable to interact with PSD95, indicating that CAPON transports cytosolic nNOS away from membrane-associated calcium sources such as the NMDA receptor.
In contrast to the multiple intracellular localizations of nNOS, HO2 is thought to be localized exclusively to the endoplasmic reticulum (ER) (9). ER membranes fuse with plasma membranes in the vicinity of caveolae, which might be sites where CO could be formed and released extracellularly.
Targets.
The best-established target for NO is soluble guanylyl cyclase (sGC). NO binds to heme at the active site of sGC, altering its conformation and activating the enzyme. To relax smooth muscle, it is thought that cGMP stimulates protein kinase G, which phosphorylates receptors for inositol 1,4,5-trisphosphate, Ca2+-activated K+ channels, and phospholamban (10). CO also activates sGC but is only 1% as potent as NO. However, Koesling and coworkers (11) showed that the drug YC1, which binds to sGC, increases CO potency up to 100-fold, suggesting that in intact tissues, comparable conformational changes in sGC render it sensitive to CO. This sensitization is likely because intestinal cGMP levels are markedly reduced in mice with targeted deletion of HO2 (HO2−/−), establishing that CO must regulate cGMP levels in intact animals (12). Moreover, YC1 augments neurotransmission in intestinal preparations from nNOS−/− but not HO2−/− mice, presumably by increasing the potency of CO formed from HO2 in the nNOS−/− animals (C. Watkins and S.H.S., unpublished work).
NO actions through sGC include blood vessel and intestinal relaxation and NMDA–glutamate-mediated augmentation of cGMP in brain preparations from immature rats (13, 14). In other processes such as apoptosis and synaptic vesicle release, NO is thought not to act via cGMP, as its effects are not mimicked by exogenous cGMP derivatives or blocked by inhibitors of sGC (15–18). As a free radical, NO can readily S-nitrosylate cysteines in a variety of proteins, suggesting another potential physiologic target. The bulk of studies on S-nitrosylation have used NO donors in in vitro experiments (19). In vivo, the cytoplasm contains high concentrations of glutathione and metals that can bind and sequester NO from protein targets. Nonetheless, by using a photolytic chemiluminescence technique, Stamler et al. (20) have obtained evidence that some proteins, such as hemoglobin (21), the ryanodine receptor (22), caspase-3 (16), and albumin (20), are nitrosylated under basal conditions.
To comprehensively examine whether S-nitrosylation occurs physiologically, we developed a simple sensitive technique for monitoring protein S-nitrosylation (23). The technique involves decomposing nitrosothiol bonds to thiols, which are reacted with a sulfhydryl-specific biotinylating reagent. Before this procedure, all free thiols in tissues are first blocked. By using this technique, we showed that a substantial number of proteins are endogenously S-nitrosylated in mouse brain. Most of these proteins lose their S-nitrosylation in nNOS−/− brain, establishing that they are physiologically S-nitrosylated by neural NO. This finding establishes definitively that protein S-nitrosylation is a physiologic target of NO generated by nNOS, presumably in association with NO released as a neurotransmitter.
S-nitrosylation targets for eNOS and inducible NOS have not yet been delineated. The range of the neural NO protein targets for S-nitrosylation indicates a broad sphere of NO influences on neural biology (Tables 1 and 2). It was already known that NO donors inhibit ion flux through NMDA receptors, especially the NR2A subunit. Both NR1 and NR2 subunits are physiologically S-nitrosylated and presumably mediate the regulation of NMDA receptor transmission by endogenous NO. This may reflect a feedback mechanism, because calcium entry through NMDA receptors stimulates NO formation. The cyclic nucleotide-gated HAC channel might be normally activated by NO S-nitrosylation, because HAC channels resemble olfactory cyclic nucleotide-gated channels, which are known to be activated by NO (24).
Table 2.
Physiologic targets of nNOS S-nitrosylation
| Neurofilament H-chain |
| NMDA receptor subunit 2 |
| NMDA receptor subunit 1 |
| Retinoblastoma gene product |
| Glycogen phosphorylase |
| Na/K ATPase α subunit |
| Inducible heat-shock protein 72 |
| β-tubulin |
| Creatine kinase |
| β, γ-actin |
| Glyceraldehyde-3-phosphate dehydrogenase |
| Dexras1 |
Some S-nitrosylated targets of NO might mediate its established role in cell growth and differentiation (25, 26). Thus, the CRMP proteins, one target of S-nitrosylation, influence process formation in neurons (27), which might relate to the impaired dendritic outgrowth of nNOS mice (28). The increased organ size in Drosophila lacking nNOS (29) has been attributed to NO activating the retinoblastoma gene product Rb to cause cell cycle arrest (30). nNOS is enriched in a transiently expressed major corticothalamic pathway (31) and has been speculated to regulate neural development, which might involve its activation of cell cycle arrest activities of Rb. S-nitrosylation of Rb might also participate in the inhibition of cell division elicited by NO in vascular smooth muscle, which prevents hyperplasia of the intimal layer and subsequent atherosclerosis (25).
Excess release of NO, especially in response to NMDA receptor activation, leads to neuronal cell death after stroke and other neurotoxic conditions (32–38). Some S-nitrosylated proteins may mediate these effects. Thus, glyceraldehyde-3-phosphate dehydrogenase and creatine kinase are inhibited by S-nitrosylation (39, 40). Glycogen–phosphorylase activity is stimulated by NO, which would increase tissue levels of glucose-1-phosphate and deplete cellular glycogen (41). S-nitrosylation of the sodium pump, which inhibits its activity, may account for membrane depolarization associated with NO-mediated cell death (42). S-nitrosylation of structural proteins such as actin, tubulin, and neurofilament heavy chain might explain how NO impairs activation of actin filaments (43) and influences destabilization of microtubules and dissolution of actin filaments in cerebellar granule cells (44).
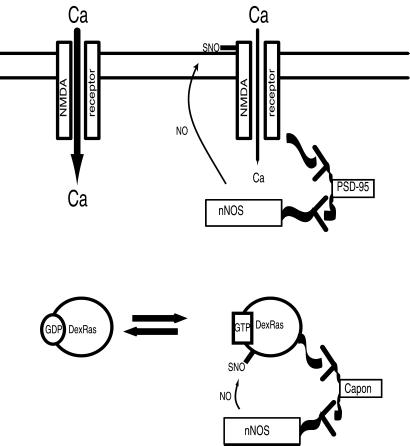
How might neural NO gain access to its targets in the presence of millimolar concentrations of glutathione, which would likely sequester freely diffusing cytosolic NO? One possibility is the targeting of nNOS to its protein targets (Fig. 1). Recently, we found that the nNOS-binding protein CAPON binds in turn to Dexras1, a novel member of the Ras superfamily, which displays guanine nucleotide exchange activity (45). nNOS, CAPON, and Dexras1 occur in a physiologic ternary complex, and Dex-Ras1 is a target for NO-mediated S-nitrosylation. The activity of Dexras1 is physiologically regulated by neural NO, as Dexras1 activation is profoundly reduced in the brains of nNOS−/− mice, whereas activation of other members of the Ras family is unaffected. We speculate that CAPON may convey nNOS to other S-nitrosylation targets. The carboxyl terminus of CAPON binds to a PDZ domain of nNOS, which also binds to PSD95. Conceivably, CAPON and PSD95 compete for binding to nNOS. However, nNOS is a dimer, so that one monomer may bind to PSD95 and the other to CAPON/Dexras1, permitting Dexras1 to be activated by NMDA receptor-gated calcium. NMDA receptor activation is known to influence nuclear events. Such signaling might involve communication via Dexras1 to the nucleus through intracellular signaling events typically initiated by members of the Ras family, such as the mitogen-activated protein kinase system.
Figure 1.
Adaptor proteins confer specificity of S-nitrosylation by targeting nNOS to its effector proteins. nNOS contains a PDZ domain that binds to both PSD95 and Capon. PSD95 binds to the NMDA receptor, allowing nNOS to nitrosylate the channel, decreasing its calcium flux. Capon also binds the PDZ domain of nNOS and targets it to Dexras1, leading to its nitrosylation and activation.
Physiologic Roles
Blood Vessels.
A biologic function for NO was first discovered in mediating endothelial-derived relaxation of blood vessels. eNOS is localized to the endothelial lining of blood vessels. nNOS is contained in a plexus of neuronal fibers in the outer adventitial layer of vessels (46, 47). The extent to which nNOS and eNOS differentially regulate blood vessel relaxation has not been definitively established. Interestingly, HO2 is concentrated both in blood vessel endothelium and adventitial neurons (48), suggesting that HO2 subserves functions that are handled by two NO-generating enzymes, eNOS and nNOS.
Evidence that NO mediates endothelial-derived relaxation includes findings that such relaxation is reduced by treatment with NOS inhibitors. However, in many instances, only partial reversal of relaxation is elicited by these drugs. NO-independent vasorelaxation in certain blood vessels is reversed by HO inhibitors (48). Thus, CO is also an endothelial-derived relaxing factor.
Urogenital Tract.
Our initial immunohistochemical localization of nNOS revealed distribution in many parts of the peripheral autonomic nervous system (46, 47). Intense nNOS staining occurs in the outflow of the pelvic autonomic plexus, especially the cavernous nerve that innervates the penis. Penile erection, elicited by depolarization of the cavernous nerve, is abolished by the selective NOS inhibitor l-nitroarginine methyl ester (l-NAME) and N-methyl-l-arginine but not by N-methyl-d-arginine, which does not inhibit NOS (49). Inhibition of erection is reversed by infusion of arginine. NO has also been shown to relax penile erectile muscle, the corpora cavernosae (50, 51). Thus, NO appears to be a neurotransmitter of the penile innervation. Surprisingly, nNOS−/− mice mate, display penile erections during mounting, and also show penile erection on cavernous nerve stimulation as well as relaxation of corpus cavernosum strips on electrical stimulation (52). All these effects depend on endogenous NO, as they are reversed selectively by l-NAME, suggesting that some form of penile NOS is retained in the nNOS−/− mice. An alternatively spliced form of nNOS, nNOSβ, remains in some neural tissues of nNOS−/− animals, as the exon deleted to construct the knockouts does not occur in nNOSβ (7). In some parts of the brain, such as the pedunculopontine nuclei and the laterodorsal tegmental nuclei, nNOSβ is almost as prominent as the parent nNOSα (53).
nNOS also regulates the urinary tract. Immunohistochemical staining reveals nNOS localized to nerve bundles in the bladder as well as fibers in the urethra (54). A physiologic role for neural NO is evident by the loss in nNOS−/− mice of bladder and urethral relaxation elicited by low-frequency electrical stimulation. These alterations appear to have pathophysiologic relevance. Thus, the bladders of nNOS−/− animals are grossly dilated. The nNOS−/− animals display a greatly augmented frequency of urination compared with wild-type animals and provide an animal model for human urinary incontinence (54). This condition is extremely common in women with detrusor instability, as well as in men with urinary frequency diagnosed as “prostatism” in the absence of bladder outlet obstruction.
HO2 also occurs throughout the autonomic nervous system. In the urogenital pathway, its localization and functions are distinct from nNOS. HO2 neuronal staining occurs in the pelvic ganglion and nerve fibers that innervate the bulbospongiosus and related muscles (55), in contrast to nNOS localization in the pelvic plexus and its axonal projections to the corpus cavernosum (56). As in other parts of the body, HO2 occurs in the vascular endothelium and in analogous epithelium in genitourinary structures. HO2 is notably concentrated in the innervation of the bulbospongiosus, which mediates ejaculation. Reflex activity of this muscle is abolished in HO2−/− animals. Moreover, ejaculation during mating is substantially reduced in HO2−/− male mice (55).
Gastrointestinal Pathway.
The closest parallels between nNOS and HO2 occur in the gastrointestinal tract. The stomachs of nNOS−/− animals are grossly distended in association with hypertrophy of the pyloric sphincter, providing an animal model of hypertrophic pyloric stenosis reflecting a loss of a prominent nNOS plexus of nerves in the pyloric sphincter (57). Stomachs of HO2−/− animals are not dilated, as HO2 neurons are not prominent in the pyloric sphincter. In the myenteric plexus of the intestine, on the other hand, HO2 is somewhat more prominent than nNOS. In about 50% of neuronal cells in the plexus, HO2 and nNOS are colocalized, suggesting that they function as cotransmitters. One can readily monitor nonadrenergic, noncholinergic (NANC) transmission in the gut by measuring relaxation after depolarization, a process reflecting the relaxation phase of intestinal peristalsis. NANC relaxation is reduced about 60% in HO2−/− and 40% in nNOS−/− animals (12) and virtually abolished in mice with deletion of both enzymes (58). HO2, but not nNOS, is also localized to interstitial cells of Cajal. Mice lacking these cells display depolarization under basal conditions, suggesting that HO2 activity of these cells helps establish resting membrane potential.
Synaptic inhibitory junction potentials are also reduced in nNOS−/− and HO2−/− muscles, with additive effects in the double knockouts providing definitive evidence that CO and NO are inhibitory neurotransmitters of the enteric nervous system (58). Just how they function as cotransmitters remains to be established. We do not know whether their actions are additive or synergistic. In olfactory tissue, there is evidence that CO may act as a partial agonist of sGC, related to its weaker activity than NO in stimulating the enzyme (59–61). Whether this occurs in the gut remains to be established. HO2 and nNOS physiologically regulate the gastrointestinal tract, as intestinal transit time is accelerated in nNOS−/− and HO2−/− mice (12).
Gastric nNOS may also be important in diabetic gastrointestinal dysfunction, which occurs in up to 75% of patients, reflected by delayed gastric emptying, nausea, vomiting, abdominal pain, and early satiety in genetic and toxin-elicited models of diabetes in mice (62). Gastric emptying and nonadrenergic noncholinergic relaxation of pyloric muscle in diabetic mice are defective, resembling abnormalities in nNOS−/− mice (63). The diabetic mice manifest a pronounced reduction in nNOS mRNA and protein despite intact myenteric neurons. nNOS expression and pyloric function are restored to normal levels by insulin treatment (63). Thus, diabetic abnormalities in gastrointestinal function may reflect an insulin-sensitive loss of nNOS. These findings suggest that the promoter region of pyloric nNOS contains insulin-responsive elements.
Brain and Behavior.
Both nNOS and HO2 occur in multiple neuronal pathways throughout the brain with relatively modest overlap between the two neural systems. The distribution of nNOS neurons differs substantially from the localizations of sGC, suggesting that in those areas enriched in nNOS neurons, but not sGC, NO may be acting by S-nitrosylating the target proteins. It also suggests that cGMP formation in the brain is not attributed primarily to neural NO, fitting with relatively modest decrements in brain cGMP levels in nNOS−/− mice (57). By contrast, localizations of HO2 closely mimic those of sGC (64). Surprisingly, cGMP brain levels are not notably diminished in HO2−/− mice (R. Zakhary and S.H.S., unpublished observations). However, in olfactory neuronal tissue, HO inhibitors profoundly deplete cGMP, whereas NOS inhibitors are inactive (59–61, 64).
Establishing specific neurotransmitter and behavioral functions of substances in the brain is much more difficult than in the periphery. One approach is to evaluate behavior in mutant mice. Superficial examination of nNOS−/− mice reveals grossly normal appearance, locomotor activity, breeding, long-term potentiation, and long-term depression (57, 65, 66). Olfactory sensitivity, strength and agility, and apparent behavioral anxiety are also normal in these animals. Strikingly, nNOS−/− mice display profound increases in aggressive behavior, so much so that they seriously wound or kill their partners if encounters are not terminated (66). They also display excessive and inappropriate mounting behavior when paired with females at various stages of estrus. This suggests that neural NO normally mediates behavioral inhibition.
Treatment of mice with the specific nNOS inhibitor 7-nitroindazole produces augmented aggression in mice similar to that observed in nNOS−/− animals (67). The increased aggressive behavior of nNOS−/− animals is restricted to males, with females displaying no abnormalities in aggressive behavior. These findings fit with the testosterone dependence of the nNOS−/− aggressive behavior (68). Surprisingly, eNOS−/− mice display a notable decrease in aggressive behavior (69).
Whereas balance and coordination in nNOS−/− animals are normal when monitored during the day, mutant mice display balance/coordination deficits when evaluated at night, when mice are normally most active (70). This fits with the extremely high density of nNOS in granule cells of the cerebellum.
Pathophysiology
Neurotoxicity.
Abundant evidence exists for a role of excess glutamate release mediating neural damage in stroke. After cerebral ischemia, extracellular levels of glutamate increase about 50-fold to neurotoxic levels, as drugs blocking glutamate receptors, especially NMDA receptors, provide major protection against stroke damage. Because NMDA receptor activation stimulates nNOS activity, it was reasonable to propose that NO mediates NMDA neurotoxicity. NMDA-elicited neurotoxicity in cerebral cortical cultures is markedly diminished in brain cultures from nNOS−/− mice (32) and by NOS inhibitors (71). Stroke damage is substantially reduced in nNOS−/− mice (38) and after treatment with NOS inhibitors (33–37). Although there have been suggestions that certain ionic forms of NO are neuroprotective (72), in general, excess NO is neurotoxic, particularly after its combination with superoxide to provide peroxynitrite that rapidly degrades to the very toxic hydroxyl free radical (73).
Whereas NO is highly reactive, CO is relatively inert. Recent evidence favors a neuroprotective role for HO2. Biliverdin formed from HO2 is rapidly reduced to bilirubin because of the high levels of biliverdin reductase in most tissues, so that biliverdin does not typically accumulate to detectable levels. Ames and coworkers noted years ago that bilirubin has antioxidant properties (74, 75). Neurotoxicity in brain cultures is markedly augmented in HO2−/− mice (4). Augmented neurotoxicity is associated with a selective increase in apoptotic death and is rescued by HO2 transfection (76). HO2−/− animals also display greatly increased neural damage after middle cerebral artery occlusion (77). This damage does not reflect systemic morbidity associated with gene knockout, as the HO2−/− animals appear robustly healthy. Moreover, HO1−/− mice, which are notably debilitated and die when 3–4 months old, do not display augmented stroke damage.
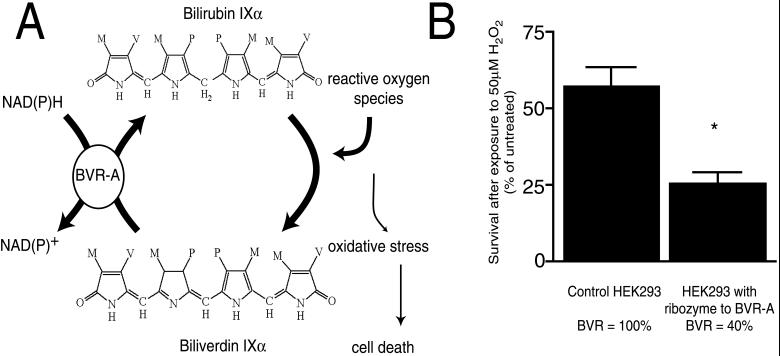
Bilirubin appears to be the product of HO2, whose loss in mutant mice leads to neurotoxicity. Thus, bilirubin itself is markedly neuroprotective (4). The neuroprotective effects of bilirubin occur in concentrations as low as 10 nM, corresponding to the low endogenous levels of bilirubin in the brain, thousands-fold lower than neurotoxic levels of bilirubin that occur during kernicterous. The neuroprotective potency of bilirubin is perplexing, as 10 nM bilirubin prevents neurotoxicity elicited by 10,000 times higher concentrations of hydrogen peroxide. Recent observations indicate that bilirubin exerts its neuroprotective effects by redox cycling (unpublished work). Each molecule of bilirubin that acts as an antioxidant is thereby itself oxidized to biliverdin. The high tissue levels of biliverdin reductase immediately reduce the biliverdin back to bilirubin. Evidence for this catalytic cycle includes the increased vulnerability to oxidative stress of cell lines designed to express less biliverdin reductase (Fig. 2). Evidence for a physiologic neuroprotectant role of HO2 comes from the neuroprotectant actions of phorbol esters, which stimulate protein kinase C activity. Phorbol esters cause stimulation of HO2 activity and bilirubin accumulation, fitting with findings that protein kinase C phosphorylation of HO2 augments its activity (4). Neuroprotective effects of phorbol esters are abolished in HO2−/− brain cultures.
Figure 2.
(A) Redox cycling of bilirubin may scavenge reactive oxygen species, protecting cells from oxidative stress. Cultured neurons are protected from micromolar amounts of hydrogen peroxide by nanomolar amounts of bilirubin. Bilirubin, a potent antioxidant, reacts with peroxyl radicals to form biliverdin. Biliverdin reductase A (BVR-A) reduces biliverdin to bilirubin by using either NADH or NADPH as an electron donor (M, methyl; V, vinyl; P, propionate). (B) Cell lines deficient in BVR are more susceptible to oxidative stress. A HEK293 cell line was generated that expresses a ribozyme targeted to BVR-A. BVR activity was 40% of a control cell line expressing a null ribozyme (targeted to luciferase). Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay after exposure to 50 μM H2O2 for 20 h (*, P < 0.001).
The neuroprotective actions of bilirubin may be relevant to neurotoxicity in forms of Alzheimer's disease (Table 3). Yeast two-hybrid and other studies establish that HO2 and HO1 bind to amyloid precursor proteins (APP), which are processed to the amyloid β peptides that occur in the amyloid plaques of Alzheimer's disease (78). Certain forms of FAD are caused by single point mutations in APP. Various forms of FAD involve different mutations and are designated “Swedish,” “Dutch,” and “London” mutants. Swedish, Dutch, and London mutants all bind much more tightly to HO1 and HO2 than wild-type APP. This binding results in modestly decreased HO activity with wild-type APP but pronounced declines with the FAD mutants. The inhibition is physiologically relevant, as cerebral cortical cultures of “Swedish” mutant mice display negligible bilirubin staining with levels only about one-fifth those of wild-type values after phorbol ester stimulation. Neurotoxicity in Swedish cultures is considerably greater than in wild-type cultures. Whereas neurotoxicity is worsened in wild-type brain cultures after treatment with HO inhibitors, no worsening occurs in the Swedish cultures, whose HO is already evidently inhibited to a maximal extension. Thus, inhibition of neuroprotective bilirubin formation by mutant APPs may contribute to neurotoxicity in FAD patients.
Table 3.
APP inhibition of HO augments neurotoxicity
| APP coimmunoprecipitates with both HO1 and HO2. |
| Endogenous APP colocalizes with HO2 in primary cultured rat neurons. |
| Transfection of APP reduces HO1 and HO2 activity in cell extracts. |
| In HEK293 cells, transfection of APP decreases protection from oxidative stress conferred by overexpression of HO1 or HO2. |
| FAD mutants of APP are more potent inhibitors of HO2 activity than wild-type APP and completely abolish protection conferred by HO2. |
| Unlike wild-type neurons, neurons from transgenic mice overexpressing FAD mutant APP do not produce significant bilirubin in response to phorbol esters. |
| Phorbol esters, which activate HO2, confer protection from oxidative stress to wild-type neuron cultures but not FAD mutant transgenic cultures. |
The “physiologic” jaundice of the newborn has long been a puzzle, as serum bilirubin levels in a major proportion of newborns hover close to levels that could cause brain damage (79). Bilirubin accounts for the bulk of plasma antioxidant activity (74, 75, 80). Preterm infants with elevated plasma bilirubin levels suffer from less oxidative-stress injuries (81). Elevated bilirubin levels also correlate with less retinopathy of prematurity (82). Thus, physiologic jaundice may reflect neuroprotection by bilirubin.
HO1 and Cytoprotection.
Whereas HO2−/− mice thrive, HO1−/− mice typically die by 40 weeks of age with a massive accumulation of iron in various tissues, especially the liver, despite low serum levels of iron (83). This suggests that HO1 might have something to do with iron efflux from tissues into the circulation. Transfecting HO1 into cells markedly augments iron efflux, whereas iron efflux is substantially reduced in fibroblasts from HO1−/− mice (Table 4) (84). Iron can be markedly toxic, so that stimulation of iron efflux may be cytoprotective, and HO1−/− cells might be more susceptible to stressful stimuli than wild-type cells. Exposure to stresses such as serum deprivation, staurosporine, or etoposide, in concentrations that do not affect wild-type cells, causes apoptotic death in HO1−/− cells (84). Transfection of HO1 into the cells protects against stress-induced cell death. Interestingly, the apoptotic cell death associated with HO1 deletion is reminiscent of the apoptotic cell death of neural tissue in HO2−/− brain (76). HO1 prevents cell death by augmenting the efflux of iron, because iron chelators protect HO1−/− fibroblasts.
Table 4.
HO1 mediates cellular iron extrusion
| Apoptosis after serum deprivation | 55Fe uptake | 55Fe release | |
|---|---|---|---|
| HO1 overexpression (stable transfection in HEK293) | Decreased | Decreased | Increased |
| Genetic deletion (fibroblast cell line from HO1−/−) | Increased (reversed by iron chelators: desferoxamine or apotransferrin.) | Increased | Decreased |
HO1 cannot itself pump iron out of cells, as it lacks properties of conventional iron pumps. Presumably iron is extruded by a pump resembling the P-type ATPases that cause calcium and copper extrusion. We discovered a specific iron ATPase reflected by ATP-dependent iron transport into tissue microsomal preparations with considerable selectivity for iron and negligible activity toward calcium or other cations (85). Its tissue distribution closely resembles HO1 with highest concentrations in the spleen. The pump is inducible with greatly increased activity in tissues of HO1−/− animals that display the most iron overload. In the spleen where red blood cells are degraded, pump activity is reduced in HO1−/− animals, consistent with their pronounced anemia. Iron pump activity is stimulated by iron itself with 500% increases in macrophage cell lines grown in media with high levels of iron.
A close association of HO1 and the iron pump makes good teleologic sense. HO1 generates highly toxic iron, which must be rapidly exported from the cell, presumably by the iron pump we have identified. A physical association of HO1 and the iron pump is conceivable. Recently, a protein associated with iron extrusion from cells has been cloned (86–88). It lacks classic motifs of ion transporters but might associate with another as-yet-unidentified protein to comprise the iron pump.
Acknowledgments
This work was supported by U.S. Public Health Service Grant DA-000266 (S.H.S.), Research Scientist Award DA-00074 (S.H.S.), and fellowship DA-05900 (D.E.B.).
Abbreviations
- APP
amyloid precursor protein
- FAD
Familial Alzheimer's Disease
- HO
heme oxygenase
- l-NAME
l-nitroarginine methyl ester
- NMDA
N-methyl-d-aspartate
- NOS
NO synthase
- eNOS
endothelial NOS
- nNOS
neuronal NOS
- sGC
soluble guanylyl cyclase
- PSD95
postsynaptic density 95
Footnotes
This paper was presented at the Inaugural Arthur M. Sackler Colloquium of the National Academy of Sciences, “Neural Signaling,” held February 15–17, 2001, at the National Academy of Sciences in Washington, DC.
References
- 1.Griffith O W, Stuehr D J. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 2.Bredt D S, Hwang P M, Glatt C E, Lowenstein C, Reed R R, Snyder S H. Nature (London) 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 3.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dore S, Takahashi M, Ferris C D, Hester L D, Guastella D, Snyder S H. Proc Natl Acad Sci USA. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothe F, Canzler U, Wolf G. Neuroscience. 1998;83:259–269. doi: 10.1016/s0306-4522(97)00373-4. [DOI] [PubMed] [Google Scholar]
- 6.Aoki C, Fenstemaker S, Lubin M, Go C G. Brain Res. 1993;620:97–113. doi: 10.1016/0006-8993(93)90275-r. [DOI] [PubMed] [Google Scholar]
- 7.Brenman J E, Chao D S, Gee S H, McGee A W, Craven S E, Santillano D R, Wu Z, Huang F, Xia H, Peters M F, et al. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 8.Jaffrey S R, Snowman A M, Eliasson M J, Cohen N A, Snyder S H. Neuron. 1998;20:115–124. doi: 10.1016/s0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 9.Maines M D. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 10.Lohmann S M, Vaandrager A B, Smolenski A, Walter U, De Jonge H R. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 11.Friebe A, Schultz G, Koesling D. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- 12.Zakhary R, Poss K D, Jaffrey S R, Ferris C D, Tonegawa S, Snyder S H. Proc Natl Acad Sci USA. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garthwaite J, Garthwaite G, Palmer R M, Moncada S. Eur J Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- 14.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannick J B, Asano K, Izumi K, Kieff E, Stamler J S. Cell. 1994;79:1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 16.Mannick J B, Hausladen A, Liu L, Hess D T, Zeng M, Miao Q X, Kane L S, Gow A J, Stamler J S. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 17.Meffert M K, Premack B A, Schulman H. Neuron. 1994;12:1235–1244. doi: 10.1016/0896-6273(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 18.Meffert M K, Calakos N C, Scheller R H, Schulman H. Neuron. 1996;16:1229–1236. doi: 10.1016/s0896-6273(00)80149-x. [DOI] [PubMed] [Google Scholar]
- 19.Hess D T, Matsumoto A, Nudelman R, Stamler J S. Nat Cell Biol. 2001;3:E46–E49. doi: 10.1038/35055152. [DOI] [PubMed] [Google Scholar]
- 20.Stamler J S, Simon D I, Osborne J A, Mullins M E, Jaraki O, Michel T, Singel D J, Loscalzo J. Proc Natl Acad Sci USA. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia L, Bonaventura C, Bonaventura J, Stamler J S. Nature (London) 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Eu J P, Meissner G, Stamler J S. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 23.Jaffrey S R, Erdjument-Bromage H, Ferris C D, Tempst P, Snyder S H. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 24.Broillet M C, Firestein S. Neuron. 1996;16:377–385. doi: 10.1016/s0896-6273(00)80055-0. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Forstermann U. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Enikolopov G, Banerji J, Kuzin B. Cell Death Differ. 1999;6:956–963. doi: 10.1038/sj.cdd.4400577. [DOI] [PubMed] [Google Scholar]
- 27.Quinn C C, Gray G E, Hockfield S. J Neurobiol. 1999;41:158–164. [PubMed] [Google Scholar]
- 28.Inglis F M, Furia F, Zuckerman K E, Strittmatter S M, Kalb R G. J Neurosci. 1998;18:10493–10501. doi: 10.1523/JNEUROSCI.18-24-10493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuzin B, Roberts I, Peunova N, Enikolopov G. Cell. 1996;87:639–649. doi: 10.1016/s0092-8674(00)81384-7. [DOI] [PubMed] [Google Scholar]
- 30.Kuzin B, Regulski M, Stasiv Y, Scheinker V, Tully T, Enikolopov G. Curr Biol. 2000;10:459–462. doi: 10.1016/s0960-9822(00)00443-7. [DOI] [PubMed] [Google Scholar]
- 31.Bredt D S, Snyder S H. Neuron. 1994;13:301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 32.Dawson V L, Kizushi V M, Huang P L, Snyder S H, Dawson T M. J Neurosci. 1996;16:2479–2487. doi: 10.1523/JNEUROSCI.16-08-02479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowicki J P, Duval D, Poignet H, Scatton B. Eur J Pharmacol. 1991;204:339–340. doi: 10.1016/0014-2999(91)90862-k. [DOI] [PubMed] [Google Scholar]
- 34.Nagafuji T, Matsui T, Koide T, Asano T. Neurosci Lett. 1992;147:159–162. doi: 10.1016/0304-3940(92)90584-t. [DOI] [PubMed] [Google Scholar]
- 35.Buisson A, Plotkine M, Boulu R G. Br J Pharmacol. 1992;106:766–767. doi: 10.1111/j.1476-5381.1992.tb14410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trifiletti R R. Eur J Pharmacol. 1992;218:197–198. doi: 10.1016/0014-2999(92)90168-4. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa T, Kirsch J R, Koehler R C, Bredt D S, Snyder S H, Traystman R J. Stroke. 1993;24:1717–1724. doi: 10.1161/01.str.24.11.1717. [DOI] [PubMed] [Google Scholar]
- 38.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Snyder S H. Proc Natl Acad Sci USA. 1992;89:9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolosker H, Panizzutti R, Engelender S. FEBS Lett. 1996;392:274–276. doi: 10.1016/0014-5793(96)00829-0. [DOI] [PubMed] [Google Scholar]
- 41.Borgs M, Bollen M, Keppens S, Yap S H, Stalmans W, Vanstapel F. Hepatology. 1996;23:1564–1571. doi: 10.1002/hep.510230637. [DOI] [PubMed] [Google Scholar]
- 42.Sato T, Kamata Y, Irifune M, Nishikawa T. J Neurochem. 1997;68:1312–1318. doi: 10.1046/j.1471-4159.1997.68031312.x. [DOI] [PubMed] [Google Scholar]
- 43.Andrade F H, Reid M B, Allen D G, Westerblad H. J Physiol. 1998;509:577–586. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonfoco E, Leist M, Zhivotovsky B, Orrenius S, Lipton S A, Nicotera P. J Neurochem. 1996;67:2484–2493. doi: 10.1046/j.1471-4159.1996.67062484.x. [DOI] [PubMed] [Google Scholar]
- 45.Fang M, Jaffrey S R, Sawa A, Ye K, Luo X, Snyder S H. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 46.Bredt D S, Hwang P M, Snyder S H. Nature (London) 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 47.Bredt D S, Glatt C E, Hwang P M, Fotuhi M, Dawson T M, Snyder S H. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 48.Zakhary R, Gaine S P, Dinerman J L, Ruat M, Flavahan N A, Snyder S H. Proc Natl Acad Sci USA. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burnett A L, Lowenstein C J, Bredt D S, Chang T S, Snyder S H. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 50.Bush P A, Aronson W J, Buga G M, Rajfer J, Ignarro L J. J Urol. 1992;147:1650–1655. doi: 10.1016/s0022-5347(17)37671-1. [DOI] [PubMed] [Google Scholar]
- 51.Rajfer J, Aronson W J, Bush P A, Dorey F J, Ignarro L J. N Engl J Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 52.Burnett A L, Nelson R J, Calvin D C, Liu J X, Demas G E, Klein S L, Kriegsfeld L J, Dawson V L, Dawson T M, Snyder S H. Mol Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- 53.Eliasson M J, Blackshaw S, Schell M J, Snyder S H. Proc Natl Acad Sci USA. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burnett A L, Calvin D C, Chamness S L, Liu J X, Nelson R J, Klein S L, Dawson V L, Dawson T M, Snyder S H. Nat Med. 1997;3:571–574. doi: 10.1038/nm0597-571. [DOI] [PubMed] [Google Scholar]
- 55.Burnett A L, Johns D G, Kriegsfeld L J, Klein S L, Calvin D C, Demas G E, Schramm L P, Tonegawa S, Nelson R J, Snyder S H, Poss K D. Nat Med. 1998;4:84–87. doi: 10.1038/nm0198-084. [DOI] [PubMed] [Google Scholar]
- 56.Burnett A L, Tillman S L, Chang T S, Epstein J I, Lowenstein C J, Bredt D S, Snyder S H, Walsh P C. J Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- 57.Huang P L, Dawson T M, Bredt D S, Snyder S H, Fishman M C. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 58.Xue L, Farrugia G, Miller S M, Ferris C D, Snyder S H, Szurszewski J H. Proc Natl Acad Sci USA. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ingi T, Ronnett G V. J Neurosci. 1995;15:8214–8222. doi: 10.1523/JNEUROSCI.15-12-08214.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ingi T, Chiang G, Ronnett G V. J Neurosci. 1996;16:5621–5628. doi: 10.1523/JNEUROSCI.16-18-05621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ingi T, Cheng J, Ronnett G V. Neuron. 1996;16:835–842. doi: 10.1016/s0896-6273(00)80103-8. [DOI] [PubMed] [Google Scholar]
- 62.Mearin F, Camilleri M, Malagelada J R. Gastroenterology. 1986;90:1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 63.Watkins C C, Sawa A, Jaffrey S, Blackshaw S, Barrow R K, Snyder S H, Ferris C D. J Clin Invest. 2000;106:373–384. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 65.O'Dell T J, Huang P L, Dawson T M, Dinerman J L, Snyder S H, Kandel E R, Fishman M C. Science. 1994;265:542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- 66.Nelson R J, Demas G E, Huang P L, Fishman M C, Dawson V L, Dawson T M, Snyder S H. Nature (London) 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 67.Demas G E, Eliasson M J, Dawson T M, Dawson V L, Kriegsfeld L J, Nelson R J, Snyder S H. Mol Med. 1997;3:610–616. [PMC free article] [PubMed] [Google Scholar]
- 68.Kriegsfeld L J, Dawson T M, Dawson V L, Nelson R J, Snyder S H. Brain Res. 1997;769:66–70. doi: 10.1016/s0006-8993(97)00688-4. [DOI] [PubMed] [Google Scholar]
- 69.Demas G E, Kriegsfeld L J, Blackshaw S, Huang P, Gammie S C, Nelson R J, Snyder S H. J Neurosci. 1999;19:RC30. doi: 10.1523/JNEUROSCI.19-19-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kriegsfeld L J, Eliasson M J, Demas G E, Blackshaw S, Dawson T M, Nelson R J, Snyder S H. Neuroscience. 1999;89:311–315. doi: 10.1016/s0306-4522(98)00614-9. [DOI] [PubMed] [Google Scholar]
- 71.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipton S A, Choi Y B, Pan Z H, Lei S Z, Chen H S, Sucher N J, Loscalzo J, Singel D J, Stamler J S. Nature (London) 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 73.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stocker R, Glazer A N, Ames B N. Proc Natl Acad Sci USA. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stocker R, Yamamoto Y, McDonagh A F, Glazer A N, Ames B N. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 76.Dore S, Goto S, Sampei K, Blackshaw S, Hester L D, Ingi T, Sawa A, Traystman R J, Koehler R C, Snyder S H. Neuroscience. 2000;99:587–592. doi: 10.1016/s0306-4522(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 77.Dore S, Sampei K, Goto S, Alkayed N J, Guastella D, Blackshaw S, Gallagher M, Traystman R J, Hurn P D, Koehler R C, Snyder S H. Mol Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi M, Dore S, Ferris C D, Tomita T, Sawa A, Wolosker H, Borchelt D R, Iwatsubo T, Kim S, Thinakaran G, et al. Neuron. 2000;28:461–473. doi: 10.1016/s0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 79.Newman T B, Maisels M J. Pediatrics. 1992;90:132. [PubMed] [Google Scholar]
- 80.Belanger S, Lavoie J C, Chessex P. Biol Neonate. 1997;71:233–238. doi: 10.1159/000244422. [DOI] [PubMed] [Google Scholar]
- 81.Hegyi T, Goldie E, Hiatt M. J Perinatol. 1994;14:296–300. [PubMed] [Google Scholar]
- 82.Heyman E, Ohlsson A, Girschek P. N Engl J Med. 1989;320:256. doi: 10.1056/NEJM198901263200420. [DOI] [PubMed] [Google Scholar]
- 83.Poss K D, Tonegawa S. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferris C D, Jaffrey S R, Sawa A, Takahashi M, Brady S D, Barrow R K, Tysoe S A, Wolosker H, Baranano D E, Dore S, et al. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 85.Baranano D E, Wolosker H, Bae B I, Barrow R K, Snyder S H, Ferris C D. J Biol Chem. 2000;275:15166–15173. doi: 10.1074/jbc.275.20.15166. [DOI] [PubMed] [Google Scholar]
- 86.Abboud S, Haile D J. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 87.McKie A T, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters T J, Farzaneh F, et al. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 88.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt S J, Moynihan J, Paw B H, Drejer A, Barut B, Zapata A, et al. Nature (London) 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]