Abstract
Berberine and Sanguinarine alkaloids belong to a group of naturally occurring chemical compounds that mostly contain basic nitrogen atoms. This group also includes some related compounds with neutral or weakly acidic properties. Alkaloids are produced by a large number of organisms including bacteria, fungi, plants, and animals. Berberine and Sanguinarine both are isoquinoline derivatives and belong to protoberberine and benzophenanthridines, respectively. Tyrosine or phenylalanine is common precursor for the biosynthesis of both. Sanguinarine [13-methyl (1,3) benzodioxolo(5,6-c)-1,3-dioxolo (4,5) phenanthridinium] is a toxin that kills animal cells through its action on the Na+-K+-ATPase transmembrane protein. Berberine, on the other hand, has been reported to cause cytotoxicity and adversely influence the synthesis of DNA. Several workers have reported varied pharmacological properties of these alkaloids as they exhibit antibacterial, antiasthma, anticancer, anti-inflammatory, and antidiabetic activities. This review article illustrates the toxicological effects of berberine and sanguinarine as well as mechanistic part of berberine and sanguinarine mediated toxicity in different living systems. This manuscript has included the lethal doses (LD50) of berberine and sanguinarine in different animals via different routs of exposure. Also, the effects of these alkaloids on the activities of some key enzymes, cell lines and organ development etc. have been summarized.
Keywords: berberine, sanguinarine, alkaloids, toxicity, pharmacological properties
Introduction
Alkaloids are a group of naturally occurring chemical compounds that mostly contain basic nitrogen atoms (Figure 1). Sometimes alkaloids also include some related chemical compounds with weakly acidic and neutral properties. A large variety of organisms produces (bacteria, fungi, plants, and animals) alkaloids that can be purified by acid-base extraction from crude extracts of these organisms. A number of pharmacological activities such as antimalarial (e.g., quinine), antiasthma (e.g., ephedrine), anticancer (e.g., homoharringtonine), cholinomimetic (e.g., galantamine), vasodilatory (e.g., vincamine), antiarrhythmic (e.g., quinidine), analgesic (e.g., morphine), antibacterial (e.g., chelerythrine), and antihyperglycemic activities (vincristine and vinblastine) of alkaloids has been reported. Berberine and sanguinarine both are isoquinoline derivatives and belong to protoberberines and benzophenanthridienes, respectively. Some chelerythrine isolated from Argemone mexicana alkaloids such as N-demethyloxysanguinarine, pancorine, (+)-argenaxine, (+)-higenamine, (+)-reticuline, angoline, and chelerythrine isolated from A. mexicana and have been reported for their cytotoxic activities against human nasopharyngeal carcinoma (HONE-1) and human gastric cancer (NUGC) cell lines (Chang et al., 2003). Berberamine, berberine, palmatine, columbamine, oxyberberine, isocorydine, lambertinea, and magniflorine have been isolated from different species of berries (Berberies vulgaris, Berberies candidula etc.). These alkaloids are reported to exert anti-cancer, anti-inflammatory, antioxidant, antidiabetic, antibacterial, analgesic and anti-nociceptive, and hepatoprotective effects. Several pharmacological activities of berberine and sanguinarine are similar. The aromatic amino acid such as tyrosine or phenylalanine is a common precursor for the biosynthesis of both. The molecular formula of berberine and sanguinarine are C20H19NO5 and C2H15NO5, respectively (Chao et al., 2013; Yatoo et al., 2018).
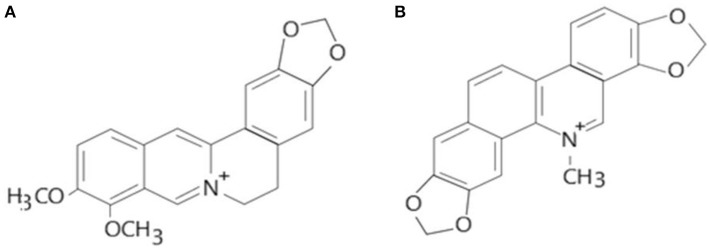
Figure 1.
Molecular structures of berberine (A) and sanguinarine (B) (Source: Hao et al., 2014).
Berberine, an isoquinoline alkaloid, belongs to the class of protoberberine alkaloids (Ikuta and Itokawa, 1988). The genus Berberis with more than 500 species belongs to Berberidaceae family (Rounsaville and Ranney, 2010). Berberine is also present in plants of Papaveraceae and Ranunculaceae families. Berberies are an evergreen shrub which possesses yellow, spiny, angled or sulcated bark, oblong, obovate, or elliptic leaves, yellow flowers and red, oblong fruits (Ahrendt, 1961). Berberine is crystal bright yellow in color and present in different parts such as roots, stem, bark, rhizome, fruit and leaves (rarely) of several plant species (mostly in barberry), the Thalictrum rochebrunianum (meadow rue), the Chelidonium majus (celandine), the Hydrastis canadensis (goldenseal), and the Phellodendron amurense (Amur cork tree), etc. Among these berberine are mainly present in a variety of barberry species and goldenseal species which are native to Asia and America, respectively (Manske and Holmes, 1995). Some workers have reported berberine (5.2–7.7%) as a major active component of Rhizoma coptidis (Huang Lian) which is traditional herb of china (Yina et al., 2012). First time in 1988 hypoglycemic effect of berberine has been reported during the treatment of diarrhea in diabetic patients. Since then, berberine as an anti-diabetic agent has been used on large scale and known as folk medicine of China. A number of research workers reported the use of this alkaloid in treatment of various diseases including problems in cardiovascular, endocrine, gastrointestinal, renal and central nervous system (Imanshahidi and Hosseinzadeh, 2008). Recent publications demonstrate the anti-oxidant (Abd El-Wahab et al., 2013), anti-inflammatory (Lin et al., 2013), anti-tumor (Yu et al., 2007), anti-mutagenic (Cernakova et al., 2002), and anti-diabetic (Abd El-Wahab et al., 2013) properties of berberine. The potential antitumor activity of berberine hydrochloride has always been a subject of considerable interest because of the known capability of berberine to bind with nucleic acids. Its ability to bind specifically to oligonucleotides and to stabilize DNA triplexes or G-quadruplexes via telomerase and topoisomerase inhibition accounts for its antiproliferative activity (Tan et al., 2011; Hao et al., 2014). In addition, berberine is reported to induce a significant hormetic dose response, in which the low dose of berberine strongly stimulates the growth of cancer cells, while at high doses it acts as anticancer agents (Bao et al., 2015). Moreover, its extensive occurrence in various plant species and low toxicity suggest that berberine hydrochloride has the potential to become an effective antitumor agent in future.
Sanguinarine [13-methyl (1,3) benzodioxolo (5,6-c)-1,3-dioxolo (4,5) phenanthridinium] derived from the root of Sanguinaria canadensis and other poppy-fumaria species of Papaveraceae family, is the most widely used benzophenanthridine alkaloid (Laster and Lobene, 1990). Sanguinarine is a benzophenanthridine structural homolog of chelerythrine (Pi et al., 2008). A positive moiety is present in the aromatic ring of the molecule. In similarity to berberine, sanguinarine also have antimicrobial, antioxidant and anti-inflammatory properties (Firatli et al., 1994). The cytotoxic and cytostatic effects of sanguinarine on a variety of human cancer cells, including human epidermoid carcinoma, erythroleukemia, prostate cancer, pancreatic carcinoma, colon cancer, breast cancer, lung cancer, promyelocytic leukemia, and bone cancer (Weerasinghe et al., 2001a,b; Matkar et al., 2008; Vrba et al., 2009; Park et al., 2010), have been reported. Sanguinarine exhibits the highest cytotoxicity among benzophenanthridine alkaloids (Slaninová et al., 2001; Vogel et al., 2010).
Sanguinarine is a toxin that kills animal cells through its action on the Na+-K+-ATPase transmembrane protein. The normal physiological functions of Na+-K+-ATPase are to maintain the resting potential and to regulate cellular volume by pumping sodium out of cells and potassium into the cells, both against their concentration gradients. The pumping of Na+-K+ is mediated through active transport, uses energy in the form of ATP and also plays significant role in cell physiology. The Na+-K+-ATPase is known to play key role in regulating mitogen activating protein kinases (MAPK) pathway, reactive oxygen species (ROS) and intracellular calcium by acting as signal transducer. Thus, the sanguinarine mediated toxicity to Na+-K+-ATPase can result into development of abnormal cellular functions (Pitts and Meyerson, 1981; Horisberger and Geering, 2009).
Epidemic dropsy is a disease that results from ingesting sanguinarine. Benzophenanthridine sanguinarine alkaloids involves cell death signaling pathway and apoptosis induction mechanism in cancer cell lines. Induction of apoptosis by sanguinarine targeted through mitochondrial damage, nuclear factor kappa-light-chain enhancer of activated B cells activation, and cell cycle arrest (Malikova et al., 2006b). Sanguinarine has been reported to inhibit microtubule polymerization and benzophenanthridine cytotoxic activity involves intercalation of double-stranded deoxyribonucleotide (DNA) (Lopus and Panda, 2006; Matkar et al., 2008) and induces fragmentation of DNA. Recent studies stated that the cytotoxicity and DNA damaging effect of sanguinarine is more specific to cancer cells than to normal cells (Ahmad et al., 2000; Matkar et al., 2008). Cell death mechanism of sanguinarine particularly involves cytotoxic and apoptotic effects occurring by changing the apoptotic gene expressions in human SH-SY5Y and Kelly neuroblastoma cell lines (Cecen et al., 2014). Toxicity of sanguinarine (other than inhibition of Na+/K+ATPase) have been explained in the form of cell membrane damage by lipid peroxidation by free radicals including ROS and r active nitrogen species (RNS). It also indicates DNA polymarase activity inhibition and accumulation of pyruvate due to increased glycogenolysis (Verma et al., 2001). Sanguinarine inhibits the growth of tumor through different molecular pathways. Sanguinarine also inhibits the proliferation and invasiveness of tumor cells i.e., called as the complex phenomena of tumor angiogenesis. In particular, owing to its pro-apoptotic potential, sanguinarine is a good candidate for the development of new anticancer therapeutics either when used alone or in combination with other chemotherapeutic regimens (Gaziano et al., 2016).
Keeping in view the toxicological implications of these two plant-based alkaloids i.e., berberine and sanguinarine, it was considered imperative to update the information on their impacts on the biochemical, cellular and molecular indices in biological systems. The rational for choosing the berberine and sanguinarine (Figure 1) for their toxicological properties are its structural similarity and both are belonging to the isoquinoline group. The structural formulas of berberine and sanguinarine molecules are C20H19NO5 and C20H15NO5, respectively. Usually, isoquinoline alkaloids interact with DNA as intercalators, or they are arranged in a small groove; their external binding with phosphate groups is also possible. The present review article illustrates a recent account of varied aspects of these two alkaloids and their roles in biological systems.
Toxicological effects of berberine
On the basis of the amount of berberine present in any compound, rout of administration and type of organism LD50 value varies. Some data accumulated by Kulkarni et al. (1972), which gives following information. The LD50 value of powdered root Berberis vulgaris which is known as barberry is 2,600 mg/kg in mice on oral administration (Table 1). On orally administration of root extract fraction of B. vulgaris, the LD50 values are 1,280 and 520 mg/kg in rat and mice, respectively (Table 1). In mice the LD50 value of pure berberine on intraperitoneal (IP) and orally administration are 23 and 329 mg/kg, respectively (Table 1). Berberine sulfate isolated from Berberis aristata on intraperitoneal administration in rats have LD50 value equal to 205 mg/kg. However, administration of 50 mg/kg of Berberine sulfate causes diarrhea in 40 % of rats which directly effects the gastrointestinal track (Kulkarni et al., 1972).
Table 1.
LD50 of berberine and sanguinarine (of extract and pure compound) as determined in different animals through different routes of exposure (S.No.1-6 Kulkarni et al., 1972 and S.No.7-9 Becci et al., 1987).
| S.No. | Extract | Animal | Rout | LD50 (mg/kg) |
|---|---|---|---|---|
| 1 | Powdered root Berberis vulgaris | Mice | Oral | 2,600 |
| 2 | Root extract fraction of B. vulgaris | Rat | Oral | 1,280 |
| 3 | Root extract fraction of B. vulgaris | Mice | Oral | 520 |
| S.No. | Pure Compound | Animal | Rout | LD50 (mg/kg) |
| 4 | Pure berberine | Mice | Oral | 329 |
| 5 | Pure berberine | Mice | Intraperitoneal | 23 |
| 6 | Berberine sulfate isolated from Berberisaristata | Rat | Intraperitoneal | 205 |
| 7 | Pure sanguinarine | Rat | Oral (acute) | 1,658 |
| 8 | Pure sanguinarine | Rat | Intra-venous (acute) | 29 |
| 9 | Pure sanguinarine | Rabbit | Dermal (acute) | <200 |
In cats 100 mg/kg (orally) of berberine evokes vomiting in 6–8 h and the same dose for 8–10 days caused death of all animals. In cats 50/100 mg/kg for 10 days oral administration of berberine sulfate caused hemorrhagic inflammatory problems in both small and large intestine. Some mild symptom of low amount of berberine and its compounds poisoning has been seen in dogs. These symptoms are salivation, nausea, diarrhea, emesis, muscular tremor, and sometimes paralysis also appeared in dogs (Lampe, 1992). The sub-acute toxicity of berberine shows gastric ulcers (Kupeli et al., 2002), Freund's complete adjuvant-induced chronic arthritis, liver and kidney enlargement, increase in body weight (up to 30%) (Yesilada and Kupeli, 2002), decreases bilirubin protein binding in adult rats (Ho et al., 2014). Mahmoudi et al. (2016) has been reported some immunotoxic effects of berberine. It is reported that, 10 mg/kg of berberine administration responsible for reduced number of leukocytes, neutrophils, lymphocytes (blood cell count), and spleen weight. Significant decrease generation/differentiation of B- and T-cells and splenic CD19+ B-cells, CD4+ and CD8+ T-cells is also associated with berberine. Totally, 5 mg/kg of berberine is responsible for only influence the proliferation of lymphocytes and delayed-type hypersensitivity response while 10 mg/kg of berberine is responsible for suppressed both cellular and humoral immune functions (Mahmoudi et al., 2016) (Table 2).
Table 2.
Dose dependent effects of berberine and sanguinarine.
| S.No. | Doses | Effects | References |
|---|---|---|---|
| 1 | 50 mg/kg of Berberine sulfate | Affects the gastrointestinal track by inducing diarrhea in rats. | Kulkarni et al., 1972 |
| 2 | 50/100 mg/kg of berberine sulfate for | Causes hemorrhagic inflammatory problems in both small and large intestine after 10 days of exposure in cats. | Lampe, 1992 |
| 3 | 100 mg/kg of berberine | Evokes vomiting (6–8 h) and caused death in cats (8–10 days). | Lampe, 1992 |
| 4 | 10 mg/kg of berberine | Reduces blood cell count (leukocytes, neutrophils, lymphocytes), spleen weight, generation/differentiation of B- and T-cells and splenic CD19+ B-cells, CD4+ and CD8+T-cells (cellular and humoral immune functions). | Mahmoudi et al., 2016 |
| 5 | 5 mg/kg of berberine | Influence the proliferation of lymphocytes and delayed-type hypersensitivity response. | Mahmoudi et al., 2016 |
| 6 | 50, 100 and 150 mg/kg of berberine | Induces liver tissue damages. | Zhou et al., 2008 |
| 7 | 10 mg/kg of sanguinarine | Increase the activity of SGPT and SGOT as well as it was responsible for the hepatotoxicity and drastic loss in microsomal cytochrome P-450 and benzphetamine N-demethylase activity. | Dalvi, 1985 |
| 8 | IC50 value of 0.9 μM of sanguinarine | Decreases the cell viability in human gingival fibroblasts and triggers mouse embryonic stem cell (ESC) apoptosis in a dose-dependent manner. | Malikova et al., 2006a |
| 9 | 0.5–2 μM of sanguinarine | Induces apoptosis and exert negative effect on the mouse embryonic development. | Vrba et al., 2009 |
Sub-chronic toxicity of berberine has reported to damages lung and liver by increasing alanine aminotransferase (ALT) and aspartate aminotransferase (AST), significantly (Ning et al., 2015). In another study on mosquito larvae of Aedes atropatpus, effects of berberine showed chronic toxicity and significantly increased cumulative mortality (Philogene et al., 1984). Another study has revealed that in diabetic rats after 16 weeks of berberine administration at concentrations >50, 100, and 150 mg/kg induces liver tissue damages but these symptoms do not appear in healthy rats (Zhou et al., 2008). Berberine in ApoE-/- mice evokes atherosclerosis after IP treatment for 15 weeks with 5 mg/kg/day (Li et al., 2009). Further, exposure to berberine results into uterine contraction and also may lead to teratogenic effects (the substances responsible for inducing developmental toxicity in an organism from the time of conception till birth) (Table 2).
Experimental studies validated by docking studies has been reported the mode of action (through hydrophobic interactions) and inhibitory effect of berberine against main neurological enzymes namely acetylcholinesterase (AChE), butyryl cholinesterase (BChE), and monoamine oxidase (MAO) (Ji and Shen, 2012). An LD50 of berberine that can inhibit AChE, BChE, MAO-A, and MAO-B are 0.44, 3.44, 126, and 98.2 μM, respectively (Ji and Shen, 2012). It was reported that treatment of 10 and 30 μM of berberine exposure to PC12 cells increased cyto-toxicity that was indicated by increase in apoptotic cell death. The in vitro (5 and 30 mg/kg, i.p. for 21days) and in vivo (10 and 30 μM up to 48 h) studies with berberine against 6-hydroxydopamine (6-OHDA) induced neuro-toxicity in rats and PC-12 cells, respectively, have demonstrated inhibition of dopamine biosynthesis, accompanied by reduced levels of norepinephrine (NE) and dopamine (DA) (Kwon et al., 2010).
Toxicological effects of sanguinarine
The short-term toxicity of sanguinarine, a benzophenanthridine alkaloid, and two other alkaloids of S. canadensis L. extracts have been reported. The acute oral LD50 in rats were reported to be about 1,658 mg/kg of sanguinarine. When given through intra-venous route, the acute LD50 in rats was observed to be 29 mg/kg of sanguinarine (Table 1). However, the acute dermal LD50 of sanguinarine in rabbits was found to be greater than 200 mg/kg (Becci et al., 1987) (Table 1). Occurrences of epidemic dropsy in the tropics have been examined for its hepatotoxic potential in rats which was due to administration of the alkaloid sanguinarine. In some studies, it was found that a single IP dose of about 10 mg/kg of sanguinarine was responsible for increase in the activity of serum glutamic pyruvic transaminase (SGPT) and serum glutamic-oxaloacetic transaminase (SGOT) substantially as well as it was responsible for the drastic loss in microsomal cytochrome P-450 and benzphetamine N-demethylase activity. Furthermore, in the same study, significant decrease in body and liver weights and slightly enlargement in livers with fibrinous material and peritoneal edema were recorded in the treated rats. Progressive degeneration of cells and necrosis has been examined by microscopy in the liver tissue. Further, all these changes substantiating that sanguinarine is a potential hepatotoxic alkaloid (Dalvi, 1985). Moreover, depending on the dosage sanguinarine and its derivative dihydrosanguinarine were found to induce HL60 cell death through apoptotic or necrotic processes (Vrba et al., 2009). In addition, sanguinarine exhibits antiproliferative and pro-apoptotic properties on normal and cancer cells (Slunská et al., 2010).
Some workers have reported the effect of sanguinarine on cell viability. According to this study, IC50 value of 0.9 μM of sanguinarine decreased the cell viability in human gingival fibroblasts (Malikova et al., 2006a) determined by using the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] MTT assay after 4 h of exposure (Vrba et al., 2009) (Table 2). Another study revealed that with an IC50 value of 0.95 μM, sanguinarine triggered mouse embryonic stem cell (ESC) apoptosis in a dose-dependent manner. The IC50 value of sanguinarine was determined by using the MTT assay after 24 h exposure to the material. On the basis of above study, the cytotoxic effects of 0.5–2 μM sanguinarine on pre- and post-implantation embryonic development was determined. Sanguinarine in dose dependent manner caused apoptosis in mouse blastocysts as determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. These workers have also discovered primary occurrence of sanguinarine mediated cellular loss and apoptosis in the inner cell mass (ICM) by dual differential staining. They have demonstrated in their in vivo and in vitro studies that sanguinarine at 0.5–2 μM may induce apoptosis and exert negative effect on the mouse embryonic development (Chan, 2011). The workers have concluded that sanguinarine even at physiological doses can adversely influence both the pre-implantation and post-implantation embryonic development in rats (Vrba et al., 2009) (Table 2).
Cytotoxicity or antiproliferative properties of sanguinarine in normal or cancer cell lines, such as rat hepatocytes (Choy et al., 2008), human gingival fibroblasts (Malikova et al., 2006b), human osteosarcoma cells (Park et al., 2010), and human promyelocytic leukemia HL-60 cells (Vrba et al., 2009) have been reported by several investigators. A number of alkaloids isolated and evaluated from A. mexicana for cytotoxic activity have been evaluated. The N-demethyloxysanguinarine was reported to cause nasopharyngeal carcinoma (HONE-1) to human and human gastric cancer (NUGC) cell lines (Chang et al., 2003). In another study, Uddin et al. (2011) revealed the cytotoxic activity against healthy mouse fibroblasts (NIH3T3) and three human cancer-cell lines (AGS, HT-29, and MDA-MB-435S) of methanolic extract of A. mexicana leaves by using the MTT assay. One of the cancer cell lines such as MDAMB-435S exhibited more cytotoxic effects of extracts from A. mexicana leaves (IC50 1.82 mg/ml) (Brahmachari et al., 2013).
Conclusion
Berberine and sanguinarine are traditionally used alkaloids with multispectrum pharmacodynamic properties. On the basis of extensive literature survey, berberine, and sanguinarine have been reported to cause toxicity in different living system. In molecular structure of berberine and sanguinarine, both contains a positive moiety which interacts with a number of nucleophilic and anionic moieties of many biomolecules that distort their structure and further resulted in to altered function of biomolecules. Instead of antitumor activity of berberine, it has potential to treat diabetes mellitus. They have been implicated in the occurrence of dropsy. The toxicity of pure compound is greater than the toxicity of plant extract or plant extract fractions. The sub-acute concentrations of berberine lead to altered liver function, gastric troubles, hepato and hematotoxicity, hemorrhagic inflammatory consequences, damage to immune cells and induced apoptosis. The in vivo and in vitro studies have reported that sanguinarine may induce apoptosis and adversely influence the embryonic development (both in the pre-implantation and post-implantation conditions) of mouse. Sanguinarine toxicity is also reflected in terms of increased SGPT and SGOT activities and reduced microsomal cytochrome P-450 and benzphetamine N-demethylase activities. On the other hand, the cytotoxic properties of both of these alkaloids reveal the use of these alkaloids in treatment of cancer. Berberine treatment may improve insulin resistance, promote insulin secretion, inhibit gluconeogenesis in liver, stimulate glycolysis in peripheral tissue cells, modulate gut microbiota, reduce intestinal absorption of glucose, and perturb lipid metabolism. However, more work is required to assess their anticancer potential under different environmental and clinical conditions to ascertain this possibility.
Author contributions
NS: Wrote the review article prepared and assembled the figure and table; BS: Critically organized and revised the manuscript by incorporating significant reports.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
NS is grateful to the University Grant Commission (UGC), New Delhi, India for providing financial assistance in the form of a Research Fellowship. The authors also acknowledge the support received from DST-FIST and UGC-SAP, New Delhi India to the Department.
References
- Abd El-Wahab A. E., Ghareeb D. A., Sarhan E. E., Abu Serie M. M., El Demellawy M. A. (2013). In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, antiacetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 13:218. 10.1186/1472-6882-13-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N., Gupta S., Husain M. M., Heiskanen K. M., Mukhtar H. (2000). Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin. Cancer Res. 6, 1524–1528. [PubMed] [Google Scholar]
- Ahrendt L. W. A. (1961). Berberis and Mahonia: a taxonomic revision. Bot. J. Linn. Soc. 57, 1–410. [Google Scholar]
- Bao J., Huang B., Zou L., Chen S., Zhang C., Zhang Y., et al. (2015). Hormetic effect of berberine attenuates the anticancer activity of chemotherapeutic agent. PLoS ONE 10:e0139298 10.1371/journal.pone.0139298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becci P. J., Schwartz H., Barnes H. H., Southard G. L. (1987). Short-term toxicity studies of sanguinarine and of two alkaloid extracts of Sanguinaria canadensis L. J. Toxicol. Environ. Health 20, 199–208. 10.1080/15287398709530972 [DOI] [PubMed] [Google Scholar]
- Brahmachari G., Gorai D., Roy R. (2013). Argemone mexicana: chemical and pharmacological aspects. Braz. J. Pharmacogn. 23, 559–575. 10.1590/S0102-695X2013005000021 [DOI] [Google Scholar]
- Cecen E., Altu Z., Ercetin P., Aktas S., Olgun N. (2014). Promoting effects of sanguinarine on apoptotic gene expression in human neuroblastoma cells. Asian Pac. J. Cancer Prev. 15, 9445–9451. 10.7314/APJCP.2014.15.21.9445 [DOI] [PubMed] [Google Scholar]
- Cernakova M., Kost'alova D., Kettmann V., Plodova M., Toth J., Drimal J. (2002). Potential antimutagenic activity of berberine, a constituent of Mahonia aquifolium. BMC Complement. Altern. Med. 2:2. 10.1186/1472-6882-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. H. (2011). Embryonic toxicity of sanguinarine through apoptotic processes in mouse blastocysts. Toxicol. Lett. 205, 285–292. 10.1016/j.toxlet.2011.06.018 [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Chang F. R., Khalil A. T., Hsieh P. W., Wu Y. C. (2003). Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z. Naturforsch C. 58, 521–526. 10.1515/znc-2003-7-813 [DOI] [PubMed] [Google Scholar]
- Chao J., Liao J. W., Peng W. H., Lee M. S., Pao L. H., Cheng H. Y. (2013). Antioxidant, analgesic, anti-inflammatory, and hepatoprotective effects of the ethanol extract of mahonia oiwakensis stem. Int. J. Mol. Sci. 14, 2928–2945. 10.3390/ijms14022928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy C. S., Cheah K. P., Chiou H. Y., Li J. S., Liu Y. H., Yong S. F., et al. (2008). Induction of hepatotoxicity by sanguinarine is associated with oxidation of protein thiols and disturbance of mitochondrial respiration. J. Appl. Toxicol. 28, 945–956. 10.1002/jat.1360 [DOI] [PubMed] [Google Scholar]
- Dalvi R. R. (1985). Sanguinarine: its potential, as a liver toxic alkaloid present in the seeds of Argemone mexicana. Experientia 41, 77–78. 10.1007/BF02005884 [DOI] [PubMed] [Google Scholar]
- Firatli E., Unal T., Onan U., Sandalli P. (1994). Antioxidative activities of some chemotherapeutics. A possible mechanism in reducing gingival inflammation. J. Clin. Periodontol. 21, 680–683. 10.1111/j.1600-051X.1994.tb00786.x [DOI] [PubMed] [Google Scholar]
- Gaziano R., Moroni G., Buè C., Miele M. T., Paola Vallebona S., Pica F. (2016). Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J. Gastrointest. Oncol. 15, 30–39. 10.4251/wjgo.v8.i1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao R., Liu Y., Zhong R. (2014). Application of electrospray ionization mass spectrometry for the evaluation of alkaloids binding to G-quadruplex of HIV-1 integrase inhibitors. Anal. Methods 6, 1059–1066. 10.1039/c3ay41494a [DOI] [Google Scholar]
- Ho C. E., Goh Y. L., Zhang C. (2014). From prejudice to evidence: the case of rhizomacoptidis in singapore. Evid. Based Complement Alternat. Med. 871720:25 10.1155/2014/871720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger J. D., Geering K. (2009). Brain Na, K-ATPase, in Encyclopedia of Neuroscience, Vol. 1 (Elsevier Ltd; ). [Google Scholar]
- Ikuta A., Itokawa H. (1988). Berberine: production through plant (Thalictrum spp.) cell cultures, in Medicinal and Aromatic Plants I. Biotechnology in Agriculture and Forestry, Vol 4, ed Bajaj Y. P. S. (Berlin; Heidelberg: Springer; ), 282–293. [Google Scholar]
- Imanshahidi M., Hosseinzadeh H. (2008). Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 22, 999–1012. 10.1002/ptr.2399 [DOI] [PubMed] [Google Scholar]
- Ji H. F., Shen L. (2012). Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer's disease. Sci. World J. 2012:823201. 10.1100/2012/823201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S. K., Dandiya P. C., Varandani N. L. (1972). Pharmacological investigations of berberine sulphate. Jpn. J. Pharmacol. 22, 11–16. 10.1254/jjp.22.11 [DOI] [PubMed] [Google Scholar]
- Kupeli E., Kosar M., Yesilada E., Husnu K., Baser C. (2002). A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of turkishberberis species. Life Sci. 72, 645–657. 10.1016/S0024-3205(02)02200-2 [DOI] [PubMed] [Google Scholar]
- Kwon I. H., Choi H. S., Shin K. S., Lee B. K., Lee C. K., Hwang B. Y., et al. (2010). Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson's disease. Neurosci. Lett. 486, 29–33. 10.1016/j.neulet.2010.09.038 [DOI] [PubMed] [Google Scholar]
- Lampe D. (1992). Waste watch. Nat. Civic. Rev. 81, 192–194. 10.1002/ncr.4100810215 [DOI] [Google Scholar]
- Laster L. L., Lobene R. R. (1990). New perspectives on sanguinaria clinicals: individual toothpaste and oral rise testing. J. Can. Dent. Assoc. 56, 19–30. [PubMed] [Google Scholar]
- Li K., Yao W., Zheng X., Liao K. (2009). Berberine promotes the development of atherosclerosis a d foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 19, 1006–1017. 10.1038/cr.2009.76 [DOI] [PubMed] [Google Scholar]
- Lin K., Liu S., Shen Y., Li Q. (2013). Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation 36, 1079–1086. 10.1007/s10753-013-9640-0 [DOI] [PubMed] [Google Scholar]
- Lopus M., Panda D. (2006). The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. A possible mechanism for its antiproliferative activity. FEBS J. 273, 2139–2150. 10.1111/j.1742-4658.2006.05227.x [DOI] [PubMed] [Google Scholar]
- Mahmoudi M., ZamaniTaghizadeh Rabe S., Balali-Mood M., Karimi G., Memar B., Rahnama M., et al. (2016). Immunotoxicity induced in mice by subacute exposure to berberine. J. Immunotoxicol. 13, 255–262. 10.3109/1547691X.2015.1058306 [DOI] [PubMed] [Google Scholar]
- Malikova J., Zdarilova A., Hlobilkova A. (2006a). Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 150, 5–12. 10.5507/bp.2006.001 [DOI] [PubMed] [Google Scholar]
- Malikova J., Zdarilova A., Hlobilkova A., Ulrichova J. (2006b) The effect of chelerythrine on cell growth, apoptosis, cell cycle in human normal cancer cells in comparison with sanguinarine. Cell Biol. Toxicol. 22, 439–453. 10.1007/s10565-006-0109-x [DOI] [PubMed] [Google Scholar]
- Manske R. H. F., Holmes H. L. (1995). The Alkaliods: Chemistry and Physiology. New York, NY: Academic Press. [Google Scholar]
- Matkar S. S., Wrischnik L. A., Hellmann-Blumberg U. (2008). Sanguinarine causes DNA damage and p53-independent cell death in human colon cancer cell lines. Chem. Biol. Interact. 172, 63–71. 10.1016/j.cbi.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Ning N., Wang Y. Z., Zou Z. Y., Zhang D. Z., Wang D. Z., Li X. G. (2015). Pharmacological and safety evaluation of fibrous root of rhizomacoptidis. Environ. Toxicol. Pharmacol. 39, 53–69. 10.1016/j.etap.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Park H., Bergeron E., Senta H., Guillemette K., Beauvais S., Blouin R., et al. (2010). Sanguinarine induces apoptosis of human osteosarcoma cells through the extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 399, 446–451. 10.1016/j.bbrc.2010.07.114 [DOI] [PubMed] [Google Scholar]
- Philogene B. J., Arnason J. T., Towers G. H., Abramowski Z., Campos F., Champagne D., et al. (1984). Berberine: a naturally occurring phototoxic alkaloid. J. Chem. Ecol. 10, 115–123. 10.1007/BF00987648 [DOI] [PubMed] [Google Scholar]
- Pi G., Ren P., Yu J., Shi R., Yuan Z., Wang C. (2008). Separation of sanguinarine and chelerythrine in Macleaya cordata (Willd) R. Br. based on methyl acrylate-co-divinylbenzene macroporous adsorbents. J. Chromatogr. A 1192, 17–24. 10.1016/j.chroma.2008.03.039 [DOI] [PubMed] [Google Scholar]
- Pitts B. J. R., Meyerson L. R. (1981). Inhibition_of_Na-K ATPase_activity_and_ouabain_binding_by_sanguinarine. Drug Dev. Res. 1, 43–49. 10.1111/j.1095-8339.1961.tb00889.x [DOI] [Google Scholar]
- Rounsaville T. J., Ranney T. G. (2010). Ploidy levels and genome sizes of Berberis L. and Mahonia Nutt. species, hybrids, and cultivars. HortScience 45, 1029–1033. [Google Scholar]
- Slaninová I., Táborská E., Bochoráková H., Slanina J. (2001). Interaction of benzo[c]phenanthridine and protoberberine alkaloids with animal and yeast cells. Cell. Biol. Toxicol. 17, 51–63. 10.1023/A:1010907231602 [DOI] [PubMed] [Google Scholar]
- Slunská Z., Gelnarová E., Hammerová J., Táborská E., Slaninová I. (2010). Effect of quaternary benzo[c]phenanthridine alkaloids sanguilutine and chelilutine on normal and cancer cells. Toxicol. in Vitro 24, 697–706. 10.1016/j.tiv.2010.01.012 [DOI] [PubMed] [Google Scholar]
- Tan W., Li Y., Chen M., Wang Y. (2011). Berberine hydrochloride: anticancer activity and nanoparticulate delivery system. Int. J. Nanomed. 6, 1773–1777. 10.2147/IJN.S22683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S. J., Grice D., Tiralongo E. (2011). Cytotoxic effects of Bangladeshi medicinal plant extracts. Evid. Based Comp. Altern. Med. 2011:578092. 10.1093/ecam/nep111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S. K., Dev G., Tyagi A. K., Goomber S., Jain G. V. (2001). Argemone mexicana poisoning: autopsy findings of twocases. Forensic Sci. Inter. 115, 135–141. 10.1016/S0379-0738(00)00322-4 [DOI] [PubMed] [Google Scholar]
- Vogel M., Lawson M., Sippl W., Conrad U., Roos W. (2010). Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J. Biol. Chem. 285, 18397–18406. 10.1074/jbc.M109.088989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrba J., Dolezel P., Vicar J., Ulrichová J. (2009). Cytotoxic activity of sanguinarine and dihydrosanguinarine in human promyelocytic leukemia HL-60 cells. Toxicol. In Vitro 23, 580–588. 10.1016/j.tiv.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Weerasinghe P., Hallock S., Liepins A. (2001a). Bax, Bcl-2, and NF-kappaB expression in sanguinarine induced bimodal celldeath. Exp. Mol. Pathol. 71, 89–98. 10.1006/exmp.2001.2355 [DOI] [PubMed] [Google Scholar]
- Weerasinghe P., Hallock S., Tang S. C., Liepins A. (2001b). Role of Bcl-2 family proteins and caspase-3 in sanguinarine-induced bimodal cell death. Cell Biol. Toxicol. 17, 371–381. 10.1023/A:1013796432521 [DOI] [PubMed] [Google Scholar]
- Yatoo M. I., Gopalakrishnan A., Saxena A., Parray O. R., Tufani N. A., Chakraborty S., et al. (2018). Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders -a review. Drug Discov. 10.2174/1872213X12666180115153635 [DOI] [PubMed] [Google Scholar]
- Yesilada E., Kupeli E. (2002). Berberis crataegina, D.C. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J. Ethnopharmacol. 79, 237–248. 10.1016/S0378-8741(01)00387-7 [DOI] [PubMed] [Google Scholar]
- Yina J., Yeb J., Jia W. (2012). Effects and mechanisms of berberine in diabetes treatment. Acta Pharmaceut. Sin. 2, 327–334. 10.1016/j.apsb.2012.06.003 [DOI] [Google Scholar]
- Yu F. S., Yang J. S., Lin H. J., Yu C. S., Tan T. W., Lin Y. T., et al. (2007). Berberine inhibits WEHI-3 leukemia cells in vivo. In Vivo 21, 407–412. [PubMed] [Google Scholar]
- Zhou J. Y., Zhou S. W., Zhang K. B., Tang J. L., Guang L. X., Ying Y., et al. (2008). Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol. Pharm. Bull. 31, 1169–1176. 10.1248/bpb.31.1169 [DOI] [PubMed] [Google Scholar]