Abstract
Bone disorders are of significant worry due to their increased prevalence in the median age. Scaffold-based bone tissue engineering holds great promise for the future of osseous defects therapies. Porous composite materials and functional coatings for metallic implants have been introduced in next generation of orthopedic medicine for tissue engineering. While osteoconductive materials such as hydroxyapatite and tricalcium phosphate ceramics as well as some biodegradable polymers are suggested, much interest has recently focused on the use of osteoinductive materials like demineralized bone matrix or bone derivatives. However, physiochemical modifications in terms of porosity, mechanical strength, cell adhesion, biocompatibility, cell proliferation, mineralization and osteogenic differentiation are required. This paper reviews studies on bone tissue engineering from the biomaterial point of view in scaffolding.
Level of evidence: I
Keywords: Bone tissue engineering, Regeneration, Scaffolds
Introduction
The incidence for all fractures among United States white population in 2010 was 4017/100,000 (1). High rates of bone vulnerability to trauma and fractures have attracted extensive researches in the bone tissue regeneration field. Bone has a hierarchical and complex structure that supports its diverse mechanical, biological and chemical functions. The heterogeneous and anisotropic structure of bone is composed of optimized irregular arrangement and orientation of macrostructures (such as cancellous and cortical bone), microstructures (like osteons, and single trabeculae), sub-microstructures (such as lamellae), nano-structures (like fibrillar collagen), and sub-nanostructures (such as minerals, and collagen molecules) (2). These components are architecturally designed to fulfill the functional needs of each particular bone. The mechanical properties of bone are made by its component phases and hierarchical structural organization (3). These properties are defined as compressive and bending strengths as well as the fracture toughness (4). Collagen and hydroxyl-carbonate apatite are the main components of bone with a porosity of 10-30% in the outer layer of the cortical bone and 30-90% in the inner layer of the cancellous bone. Some bones like ribs are more involved in tensile stress, while others, like talus, are under heavy compressive strength.
Any missing piece of bone due to traumas, tumors, avascular necrosis, and/or infections must be replaced with a proper functional alternative. Normally, the healing process starts with an inflammation phase, starting immediately after fracture and lasting up to several days, during which, the blood clot at the fracture cite initiates a stable framework for new bone formation. The clot is later replaced with fibrous and collagenous tissue, the soft callus, which will be hardened weeks after fracture. Bone remodeling will happen during several months after the fracture. Autografts from different bones (fibula, iliac crest, ribs, etc.) are harvested and used for substitution of small missing bones; however, large bone voids are challenging. Tissue engineering has introduced new hopes as combination of cells, scaffolds, and biofactors for bone regeneration. Scaffolds are the masterpiece of bone tissue engineering. A bone scaffold is the 3D matrix that allows and stimulates the attachment and proliferation of osteoinducible cells on its surfaces. The following concerns must be considered in designing bone scaffolds: 1) biocompatibility in terms of cell attachment and proliferation as well as lack of toxicity and inflammatory reactions; 2) biodegradability for programmed safe substitution of the scaffold material with osteoid deposition; 3) mechanical properties to bear weight during the amelioration period; 4) proper architecture in terms of porosity and pore sizes for cell penetration, nutrients and waste transfer, and angiogenesis; 5) sterilibility without loss of bioactivity; and 6) controlled deliverability of bioactive molecules or drugs (5-7).
Probably, seeding cartilage cells onto bone spicules by Green in early 1970 was the first attempt for tissue scaffolding. Since then, seeding cells on properly engineered scaffolds from biocompatible biomaterials was suggested for new tissue formation (8). Bone scaffolds are optimally expected to have both osteoconductive and osteoinductive properties. Osteoconduction is the process whereby the scaffolds provide inward migration of osteoinducible cellular elements such as mesenchymal cells, osteoblasts, and osteoclasts, as well as the supplementary vasculature; whereas, osteoinductivity refers to inducing the differentiation of cells from different lineages into osteogenic cells (9, 10). Various synthetic and natural, biodegradable and non-biodegradable materials have been used in the fabrication of bone scaffolds through different methods (11). Among polymers, ceramics, metals, and composites, each has their specific resorption, surface reactivity, and biocompatibility properties that affect osteoconduction and osteoinduction (12).
Incorporation of growth factors into the scaffold biomaterial can improve osteogenesis and angiogenesis. Fibroblast growth factor (FGFs), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), epidermal growth factor (EPG), transforming growth factor-beta (TGF-β), and bone morphogenic protein (BMP) are among the known growth factors used in scaffold for promoting bone plasticity (9, 13).
The current study has aimed to review the different materials commonly used in fabrication of scaffolds for bone tissue engineering applications. Generally, from the materials point of view, scaffolds for bone tissue engineering can be categorized into four classes: polymeric, ceramic, composite, and metallic scaffolds.
Polymeric scaffolds for bone regeneration
Generally, polymeric materials provide more controllability on physiochemical characteristics of scaffolds such as pore size, porosity, solubility, biocompatibility, enzymatic reactions, and allergic response (14, 15).
Synthetic polymers were introduced for their excellent mechanical properties. They consist of aliphatic polyesters such as poly(lactic-acid)(PLA), poly(glycolic-acid)(PGA), and poly(caprolactone)(PCL), and their copolymers which are the most commonly utilized polymers in bone tissue engineering (16-20). They are biocompatible, biodegradable, and can be easily fabricated into different shapes (21). They also can mechanically support demands for a wide range of applications in orthopedics (22). Other synthetic polymers in bone tissue engineering includes poly(methyl methacrylate), poly(e-caprolactone), poly hydroxyl butyrate, polyethylene, polypropylene, polyurethane, poly(-ethylene terephthalate), poly ether ketone, and poly sulfone (23). Although, some synthetic polymers like Poly(propylene fumarate) (PPF) show high compressive strength and a controlled degradation time; however, they lose their strength due to rapid degradation in vivo and created local acidic environment which can make adverse tissue responses (24-26). List of common polymeric scaffolds are presented in Table 1.
Table 1.
Polymeric scaffolds for bone tissue engineering
| Name | Mechanical Properties | Modifications | Advantages | Applications | Toxicity | Chemical structure |
|---|---|---|---|---|---|---|
| SYNTHETIC | ||||||
| Polylactic acid (PLA) (34-36) | +++ | HA incorporation to enhance cell growth | - biocompatible - biodegradable - support cell adhesion |
- bone tissue engineering - sinuses and nasal cavity filler |
- nontoxic - non-inflammatory - FDA approved |
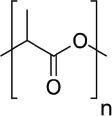 |
| Poly glycolic acid (PGA) (34, 37, 38) | +++ | alkaline hydrolysis for increasing cell replacement and cells biomaterials interaction improvement | - biocompatible - biodegradable - support cell adhesion |
- bone tissue engineering | - nontoxic - non-inflammatory - FDA approved |
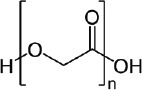 |
| Poly (lactic-co-glicolic acid) (PLGA) (39-44) | + | -HA incorporation for enhancing compressive strength - diamond nanoparticles incorporation for higher mechanical resistance - incorporation of CNTs for higher rate of cell attachment, proliferation, and differentiation |
- biodegradable - support cell adhesion |
- bone tissue engineering | -exhibit immunogenicity and contains pathogenic impurities - FDA approved |
 |
| Poly ɛ-caprolactone (PCL) (45-48) | ++ | - high RGD concentration for increasing osteoblast attachment - CNT addition for mechanical properties, BMSCs proliferation and differentiation enhancement |
- biodegradable | - bone tissue engineering | - deficiency of toxicity - FDA approved |
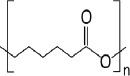 |
| Polyethylene glycol (PEG) (36, 49, 50) | + | RGD peptides for facilitating cell adhesion and spreading | - biocompatible - steering cells into scaffolds - osmotic effects in body |
- bone regeneration - pharmacy - medicine-biology - industrial chemistry - sinuses and nasal cavity filler |
- nontoxic - FDA approved |
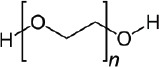 |
| Polybutylene terephthalate (PBT) (47, 51) | ++ | - | - highly biocompatible - biodegradable - impact resistance |
- industry and medicine | - nontoxic - FDA approved |
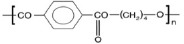 |
| Polyethylene terephthalate (PET) (47, 51) | +++ | - | - highly biocompatible - biodegradable - impact resistance |
- industry and medicine | - nontoxic - FDA approved |
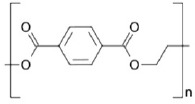 |
| Polyvinyl alcohol (PVA) (52-56) | +++ | - CNT and CNF incorporation for higher concentration of ALP and mineralised matrix | - non-biodegradable - great resistance against organic solvents |
- permanent implants | - little toxic effect in oral consume | 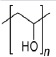 |
| Poly propylene fumarate (PPF) (57, 58) | ++ | linked RGD peptides for osteoblast migration regulation | - biocompatible - suitable physical properties and decomposition rate |
- biomedical engineering - orthopedic applications |
- nontoxic - FDA approved |
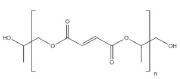 |
| Poly aldehyde guluronate (PAG) (59, 60) | + | -. | - biocompatible | - bone tissue engineering - soft tissue engineering - biomedical applications |
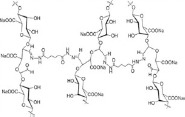 |
|
| polyacrylic acid (PAA) (61, 62) | + | - | - non-biodegradable | - permanent implants | - Non-significant cytotoxic effect - FDA approved |
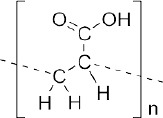 |
| Polyurethane (PUR & PU) (63-65) | + | - | - variable degradablility - injectable |
- soft and firm texture in tissue engineering - bone cement |
- | 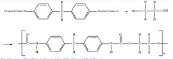 |
| NATURAL | ||||||
| Collagen (type I, type II, type III) (66) | + | - mixing with calcium for mechanical integrity increase - blending with PCL for mechanical improvement |
- biocompatible - degradable |
- tissue engineering - biomedical application |
- nontoxic | 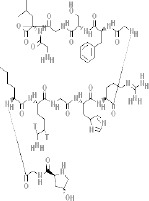 |
| Alginate (67, 68) | + | - addition of HA, calcium phosphate cements, bioglass and other natural and synthetic polymers for upgrading cell adhesion and mechanical properties | - biocompatible - degradable - minimally invasive manner (gel-forming) - ease of chemical modification with adhesion ligands and controlled release of tissue induction factors (e.g., BMP, TGF-β) |
- bone tissue engineering | - nontoxic |  |
| Chitosan (69, 70) | - | - nanocrystalline hydroxyapatite and SWCNT incorporation for mechanically and cytocompatibility enhancement | - | - cartilage and osteochondral tissue engineering | - nontoxic |  |
| Chitin (24) | + | - | - biocompatible - biodegradable |
- biotechnology and medical application | - nontoxic |  |
Natural polymeric scaffolds are composed of extracellular biomaterials in 3 classes: 1) proteins (collagen, gelatin, fibrinogen, elastin, keratin, silk, …); 2) polysaccharides (glycosaminoglycans, cellulose, amylose, dextran, chitin, …); and 3) polynucleotides (DNA, RNA) (27-29). Extracellular matrix (ECM)-based scaffolds have been suggested as most similar ones to the original tissue (30, 31). They have also shown osteoinductive properties. This group of natural scaffolds could be cell-derived (cells are used to generate new bone tissue or seeded onto a supporting matrix) or tissue-derived (bone tissue is directly used) (32-34). In contrast with autogenous ECM-based scaffolds, allogenous and xenogenous constructs should be devitalized or decellularized to avoid host immune response. Although, autogenous scaffolds have minimum immunological rejections; high histocompatibility; high osteoconductive, osteoinductive, and osteogenic properties; however, their application has been limited due to the need for additional surgery, donor site morbidity, and lack of availability. Allogeneic and xenogenic scaffolds have osteoconductive and osteoinductive effects with no need for additional surgery and donor site morbidity; however, they are limited due to the risk of disease transmission and immunogenicity. Availability is the main problem with the allogenous ECM-based scaffolds. Although xenogenous scaffolds are abundant, they are limited due to DNA or mutation transfer (9, 35, 36). Strong human immune response to the residual cellular components of xenogeneic grafts is the main cause of transplant rejection. Transplantation of xenografts triggers inflammatory, immune, and coagulatory responses. Osteoblastic differentiation of human mesenchymal stem cells has been reported with porous bovine cartilage matrix derived scaffolds (37). Although, natural polymers have shown a great biocompatibility and controlled biodegradation; poor mechanical properties is the major concern with them as bone scaffolds (38, 39). The mechanical properties, biodegradability, and consistency from batch to batch are hardly controllable in naturally derived biomaterials. These biopolymers fail to provide sufficient architectural support and protection for the osteogenic cells. Also, immunogenic reactions and pathogen transmission due to the impure content in natural biopolymers may also happen (40).
Ceramic scaffolds
Bone tissue consists of about 70% of hydroxyapatite (HA) and 30% of collagen by weight (41). Bioceramics almost mimic bone tissue and provide a higher osteoblasts adherence and proliferation compared to other materials (12, 42). Calcium phosphate ceramics (CPCs) have been greatly studied for bone tissue repair as tunable bioactive materials (43). Their physiochemical properties result in osteoconduction and osteoinduction. Hydroxyapatite, tricalcium phosphate (TCP), and their combination as biphasic and amorphous calcium phosphates (Biphasic calcium phosphate (BCPs and ACPs) are common types of CPCs used in bone tissue engineering (26, 44). Recent studies have shown that modification of the mechanical strength, dissolution rates, and biocompatibility of the scaffold can be done through addition of calcium phosphate (45). Doping β-TCP scaffolds with SiO2 (0.5%) and ZnO (0.25%) has been shown to upgrade the compressive strength to 2.5-fold and increase cell viability up to 92% (46). Solubility and surface topography are the most significant factors that influence cell behavior. Therefore, designing CPCs with suitable physical and chemical properties, and osteoinductive potential may improve their bioactivity in vivo (44).
Although the mechanical strength of ceramics is superior compared to polymers, it is still inferior to natural bones especially in terms of tensile and torsion strength. Also, HA has a great compressive (500-1000 MPa) and bending strength (115-200 MPa) in comparison with cortical human bone (100-230 and 50-150 MPa respectively); however, its fracture toughness (1 MPa m0.5) is much less (2-12 MPa m0.5) (4).
Composed structures as optimized scaffolds
Recently, bioactive composite materials have been suggested to combine the advantages of two or more different materials (metallic, ceramic, and polymeric materials) (23). Composite materials improve the scaffold properties and allow controlled degradation for tissue engineering applications (47, 48). Excellent mechanical properties and osteoconductivity have made polymer/ceramic composites as promising materials for bone tissue engineering (49, 50). Composites of main natural bone bioceramics including CP, HA, and TCP with Poly(L-lactic acid) (PLLA), collagen, gelatin, and chitosan have been greatly used as scaffolding materials for bone repair studies (11, 51-54). Reinforcement of high density polyethylene (HDPE) and Poly(l-lactide-co-glycolide acid) (PLGA) with HA has introduced the structures that mimic and match bone properties as well as matrices for bone mineralization and cell differentiation (23). Calcium phosphate (CP)-polymer composites combine mechanical integrity and bioactivity together (26). Collagen/bioglass nanocomposites have shown early mineralization and upgraded ALP expression (55). Simple calcium phosphate coating method on metals, glasses, inorganic ceramics and organic polymers (such as PLGA, PS, PP, and silicone), collagens, and silk fibers can improve biocompatibility or enhance the bioreactivity for orthopedic applications (11, 56). Mechanical reinforcement of these composite scaffolds has not yet matched the bone tissue demands in vivo.
The proliferation and differentiation rate of human mesenchymal stem cells on Fe foam coated with calcium-phosphate have shown to be higher than on uncoated samples (57). However, although, the coating enhances bioactivity, it inhibits the degradation of Fe foams (58). Addition of phosphorus increases the compressive yield which is comparable to the typical bone. Fe alloys have shown faster in vitro degradation compared to the pure form (59). Making porous structure from all biodegradable metals affects mechanical and degradation properties of the construct, the cell regeneration, and degradation product transport in the structure (60). Metallic scaffolds gridded with carbon and Ta deposits have shown high biocompatibility in animal experiments. Trabecular networks have shown appropriate bone growth and high stability; therefore, they can be used in orthopedic implants and instruments (61-65). Incorporation of Cobalt (Co) in meso-porous bioglass scaffolds have been shown to induce hypoxia that increased bone marrow-derived stem cell proliferation, differentiation, and bone-related gene expression (66).
Metallic scaffolds in bone tissue engineering
Iron (Fe) and magnesium (Mg) based metals such as Mg-RE (rare earth) alloys, Mg-Ca, pure Fe, Fe-Mn alloys, and Fe foam have been used for bone scaffold (57, 67-70).
Fe has a 211GPa elastic modulus, higher than Mg (41GPa) and its alloys (44GPa) and 316L stainless steel (190GPa) (71). However, inflammatory response and systemic toxicity have been observed with in vivo implantation of Fe stents in descending aorta of rabbits (70).
Magnesium (Mg) and its alloys are other metals that are used in bone tissue engineering. Bio-resorbabililty, high biodegradability, suitable mechanical properties, non-inflammatory responses, and bone cells activation support have been counted as its characteristics (72-74). Mg-based implants have shown superior increase in bone area in comparison with PLA. Their corrosion layer also has been observed to contain calcium phosphates (72). Porous Mg has better degradation behavior (slower hydrogen evolution) and slower decrement of compressive yield strength in simulated body fluid (SBF) immersion tests (75). Mg and its alloys have a wide range of elongation (from 3% to 21.8%) and tensile strength (from 86.8 to 280MPa). Its elastic modulus (41–45GPa) is closer to that of the bone compared to other metals (76). Very quick pure Mg corrosion produces hydrogen gas at a high rate that is too to be handled with by the host tissue (72). Addition of 0.4–4wt% REs, and other trace elements such as Cd and Al, has shown to decelerate the corrosion rate of alloyed Mg (77). Also porosity and pore size modifications can adjust its stiffness and strength range to that of bone; however, higher porosity decreases the corrosion resistance of Mg. Cerium, neodymium, calcium, and praseodymium are used in orthopedic applications with Mg alloys (69, 78). High corrosion and toxins of Mg has limited the application of this metal in medicine (79). Early stages of in vivo biocompatibility studies of Mg scaffolds have recently been started (80).
Titanium (Ti) porous scaffolds have also been studied as bone replacement materials (81). These elements are not biodegradable and do not integrate with biomolecules. Surface modifications has been suggested to improve Ti bioactivity (82). Ti and its alloy particles have shown inhibition of bone-cell proliferation and reduction in bone formation markers (83). Oxidization (TiO2), surface of modification, and combination of chrome-cobalt (Cr-Co) alloys and stainless steel with titanium alloys can improve its biocompatibility. Titanium-aluminum-vanadium alloys (ASTM F1472, ASTM f136, ASTM F110) possess better mechanical properties compared to pure titanium and can be used in joint implants. Non-toxic alloys of beta titanium like Nb, Ta, and Zr are also offered (84). Biocompatibility has been increased in the 2nd generation of titanium alloys like Ti-15 Mo-5Zr-3Al, Ti-15Zr-4Nb-2Ta-0.2Pd, Ti-12Mo-6Zr-2Fe, and Ti-29Nb-13Ta-4.6Zr. Titanium and titanium alloy trabecular networks are used in spine surgery (85). Incorporation of TGF-β and BMP has shown to improve the osteoinductivity of titanium and its alloys (13, 86). Porous titanium and its alloys can be used in permanent implants due to their good mechanical properties (87-89). Nitinol (NiTi), a metal alloy of titanium with nickel, has shown high biocompatibility and significant plasticity for bone scaffolding; it has been also used for nail manufacturing and spine separator in scoliosis treatment (90-92). Nitinol-coating of stainless steel surfaces results in higher biocompatibility. The use of NiTi alloys has been banned in America and Europe due to allergic response and toxicity problems of Ni ions (93).
Tantalum (Ta) is widely used in bone tissue engineering and knee replacement surgeries. The similar elasticity of Ta to bone can decrease the imposed stress levels (61).
Metal implants are light-weight, strong, biocompatible, and osteoconductive; but they may inhibit of bone formation markers, stimulation of bone loss or resorption, show poor osseointegration with the surrounding bone due to the stiffness difference, and release toxic ions by corrosion which may cause inflammatory responses (9, 12, 36). Metal scaffolds are usually unrecognizable by biological factors too. They act more as permanent implants than scaffolds.
Although, an optimal scaffold for bone tissue engineering is still a question, none of the studied materials alone has fulfilled the bone scaffold requirements. Polymers are great for designing controllable biodegradability beside osteoconductivity; however, they are weak in mechanical resistance. Ceramics have better mechanical strength and are osteoinductive; however, they are vulnerable to fracture. Hence, recent researches have been shifted towards composite materials with incorporation of biomolecules. Proper integration between ceramic particles and polymeric matrix is necessary for the improvement of mechanical performance (89). Modification of scaffold chemistry, cells seeding, and growth factors like TGF-β, BMP, and Vascular endothelial growth factor (VEGF) can improve osteoinductivity and angiogenesis (25, 55, 90, 91). Beside, scaffold pore size and porosity can control the rate and efficiency of delivery.
According to the conventional definition, scaffolds are meant to be biodegradable. Metals have introduced as mechanically strong materials, but, they are non-biodegradable. Therefore, none of suggested materials could be perfect to be used for bone scaffolds unless the definition boarders are trespassed. Binary combinations of polymer/ceramic, polymer/metal, or metal/ceramic composite materials have been reported as mechanically strong scaffolds; however, they have not matched the original bone tissue yet.
Considering the low mechanical properties of polymeric, ceramic, and composite biomaterials as well as lack of biocompatibility of metals, an optimal scaffold for bone tissue engineering applications can only be a well-orchestrated multiphasic construct composed of all biocompatible materials. The core of such an optimal structure can be composed of a ceramic-coated biocompatible metal in order to compensate the mechanical properties. The next phase might be an osteoinductive composite loaded with proper growth factors. Surface modifications can be done by biomolecules like collage and/or gelatin. Aligned porosity with adjusted pore sizes that allow angiogenesis must also be considered. As these scaffolds with metallic cores trespass the regular definition of degradability, they will be the next generation of scaffold/prosthesis complexes, the “ScaTheses”. The scaffold part will play its role and degrade in a time manner, while, the metallic portion will stay much longer in the body, without interrupting the bone physical integrity and hence function.
The authors declare no conflict of interest regarding this manuscript.
References
- 1.Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ., 3rd Trends in Fracture incidence: a population-based study over 20 years. J Bone Miner Res. 2014;29(3):581–9. doi: 10.1002/jbmr.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20(2):92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 3.Weiner S, Traub W. Bone structure: from angstroms to microns. FASEB J. 1992;6(3):879–85. [PubMed] [Google Scholar]
- 4.Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24(13):2161–75. doi: 10.1016/s0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–91. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 7.Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25(6):1539–60. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 8.Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10(3):569–76. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D. Orthopaedic applications of bone graft & graft substitutes: a review. Indian J Med Res. 2010;132(1):15–30. [PubMed] [Google Scholar]
- 10.Perry CR. Bone repair techniques, bone graft, and bone graft substitutes. Clin Orthop Relat Res. 1999;360(10):71–86. doi: 10.1097/00003086-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Dhandayuthapani B, Yoshida Y, Meakawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polymer Sci. 2011;2011(19):290602. [Google Scholar]
- 12.Stevens B, Yang Y, Mohandas A, Stucker B, Nguyen KT. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater. 2008;85(2):573–82. doi: 10.1002/jbm.b.30962. [DOI] [PubMed] [Google Scholar]
- 13.Alam S, Ueki K, Marukawa K, Ohara T, Hase T, Takazakura D, et al. Expression of bone morphogenetic protein 2 and fibroblast growth factor 2 during bone regeneration using different implant materials as an onlay bone graft in rabbit mandibles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(1):16–26. doi: 10.1016/j.tripleo.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs JR, Nasseri BA, Vacanti JP. Tissue engineering: a 21st century solution to surgical reconstruction. Ann Thorac Surg. 2001;72(2):577–91. doi: 10.1016/s0003-4975(01)02820-x. [DOI] [PubMed] [Google Scholar]
- 15.Meyer U, Wiesmann HP. Bone and cartilage engineering. New York: Springer Science & Business Media; 2006. [Google Scholar]
- 16.Ma PX. Scaffolds for tissue fabrication. Mater Today. 2004;7(5):30–40. [Google Scholar]
- 17.Kretlow JD, Mikos AG. Mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13(5):927–38. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 18.Moradi A, Dalilottojari A, Pingguan-Murphy B, Djordjevic I. Fabrication and characterization of elastomeric scaffolds comprised of a citric acid-based polyester/hydroxyapatite microcomposite. Mater Design. 2013;50:446–50. [Google Scholar]
- 19.Ali Akbari Ghavimi S, Ebrahimzadeh MH, Solati-Hashjin M, Osman A, Azuan N. Polycaprolactone/starch composite: Fabrication, structure, properties, and applications. J Biomed Mater Res A. 2015;103(7):2482–98. doi: 10.1002/jbm.a.35371. [DOI] [PubMed] [Google Scholar]
- 20.Ghavimi SA, Ebrahimzadeh MH, Shokrgozar MA, Solati-Hashjin M, Osman NA. Effect of starch content on the biodegradation of polycaprolactone/starch composite for fabricating in situ pore-forming scaffolds. Polymer Test. 2015;43(2):94–102. [Google Scholar]
- 21.Ishaug SL, Yaszemski MJ, Bizios R, Mikos AG. Osteoblast function on synthetic biodegradable polymers. J Biomed Mater Res. 1994;28(12):1445–53. doi: 10.1002/jbm.820281210. [DOI] [PubMed] [Google Scholar]
- 22.Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14(7):726–37. doi: 10.1016/s0749-8063(98)70099-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24(13):2133–51. doi: 10.1016/s0142-9612(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, Li J, Runge MB, Dadsetan M, Chen Q, Lu L, et al. Cross-linking characteristics and mechanical properties of an injectable biomaterial composed of polypropylene fumarate and polycaprolactone co-polymer. J Biomater Sci Polym. (Ed) 2011;22(4-6):489–504. doi: 10.1163/092050610X487765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung HY, Lau KT, Lu TP, Hui D. A critical review on polymer-based bio-engineered materials for scaffold development. Composites B Eng. 2007;38(3):291–300. [Google Scholar]
- 26.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short AR, Koralla D, Deshmukh A, Wissel B, Stocker B, Calhoun M, et al. Hydrogels that allow and facilitate bone repair, remodeling, and regeneration. J Mater Chem B. 2015;3(40):7818–30. doi: 10.1039/C5TB01043H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials science: an introduction to materials in medicine. Massachusetts: Academic Press; 2004. [Google Scholar]
- 29.Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59(4):339–59. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Moradi A. Development of bovine cartilage extracellular matrix as a potential scaffold for chondrogenic induction of human dermal fibroblasts [Doctoral Dissertation] Kuala Lumpur, Malaysia: University of Malaya; 2015. [Google Scholar]
- 31.Moradi A, Ataollahi F, Sayar K, Pramanik S, Chong PP, Khalil AA, et al. Chondrogenic potential of physically treated bovine cartilage matrix derived porous scaffolds on human dermal fibroblast cells. J Biomed Mater Res A. 2016;104(1):245–56. doi: 10.1002/jbm.a.35561. [DOI] [PubMed] [Google Scholar]
- 32.Pei M, Li J, Shoukry M, Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater. 2011;22(333):343. doi: 10.22203/ecm.v022a25. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q, Peng J, Lu SB, Guo QY, Zhao B, Zhang L, et al. Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin Med J. 2011;124(23):3930–8. [PubMed] [Google Scholar]
- 34.Koob S, Torio-Padron N, Stark GB, Hannig C, Stankovic Z, Finkenzeller G. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A. 2010;17(3-4):311–21. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- 35.Cowley SP, Anderson LD. Hernias through donor sites for iliac-bone grafts. J Bone Joint Surg Am. 1983;65(7):1023–5. [PubMed] [Google Scholar]
- 36.Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007;19(4):513–21. doi: 10.1016/j.coms.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Fuentes N, Reynoso-Ducoing O, Rodríguez-Hernández A, Ambrosio-Hernández JR, Piña-Barba MC, Zepeda-Rodríguez A, et al. Isolation of human mesenchymal stem cells and their cultivation on the porous bone matrix. J Vis Exp. 2015;9(96):e51999. doi: 10.3791/51999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17(2):205–13. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 39.Yarlagadda PK, Chandrasekharan M, Shyan JY. Recent advances and current developments in tissue scaffolding. Biomed Mater Eng. 2005;15(3):159–77. [PubMed] [Google Scholar]
- 40.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32(3):477–86. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 41.Biltz RM, Pellegrino ED. The chemical anatomy of bone: I. A comparative study of bone composition in sixteen vertebrates. J Bone Joint Surg Am. 1969;51(3):456–66. [PubMed] [Google Scholar]
- 42.Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20(23-24):2287–303. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 43.Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed Engl. 2002;41(17):3130–46. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Samavedi S, Whittington AR, Goldstein AS. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 2013;9(9):8037–45. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Drzewiecka K, Krasowski J, Krasowski M, Łapińska B. Mechanical properties of composite material modified with amorphous calcium phosphate. J Achiev Mater Manufact Eng. 2016;74(1):22–8. [Google Scholar]
- 46.Fielding GA, Bandyopadhyay A, Bose S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent Mater. 2012;28(2):113–22. doi: 10.1016/j.dental.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cascone M, Barbani N, Cristallini C, Giusti P, Ciardelli G, Lazzeri L. Bioartificial polymeric materials based on polysaccharides. J Biomater Sci Polym Ed. 2001;12(3):267–81. doi: 10.1163/156856201750180807. [DOI] [PubMed] [Google Scholar]
- 48.Ciardelli G, Chiono V, Vozzi G, Pracella M, Ahluwalia A, Barbani N, et al. Blends of poly-(ε-caprolactone) and polysaccharides in tissue engineering applications. Biomacromolecules. 2005;6(4):1961–76. doi: 10.1021/bm0500805. [DOI] [PubMed] [Google Scholar]
- 49.Kang HG, Kim SY, Lee YM. Novel porous gelatin scaffolds by overrun/particle leaching process for tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2006;79(2):388–97. doi: 10.1002/jbm.b.30553. [DOI] [PubMed] [Google Scholar]
- 50.Roether J, Boccaccini AR, Hench L, Maquet V, Gautier S, Jérôme R. Development and in vitro characterisation of novel bioresorbable and bioactive composite materials based on polylactide foams and Bioglass®for tissue engineering applications. Biomaterials. 2002;23(18):3871–8. doi: 10.1016/s0142-9612(02)00131-x. [DOI] [PubMed] [Google Scholar]
- 51.Woo BH, Kostanski JW, Gebrekidan S, Dani BA, Thanoo B, DeLuca PP. Preparation, characterization and in vivo evaluation of 120-day poly (D, L-lactide) leuprolide microspheres. J Control Release. 2001;75(3):307–15. doi: 10.1016/s0168-3659(01)00403-5. [DOI] [PubMed] [Google Scholar]
- 52.Du C, Cui F, Zhu X, de Groot K. Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J Biomed Mater Res. 1999;44(4):407–15. doi: 10.1002/(sici)1097-4636(19990315)44:4<407::aid-jbm6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 53.Bigi A, Boanini E, Panzavolta S, Roveri N, Rubini K. Bonelike apatite growth on hydroxyapatite–gelatin sponges from simulated body fluid. J Biomed Mater Res. 2002;59(4):709–15. doi: 10.1002/jbm.10045. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Zhang M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J Biomed Mater Res. 2001;55(3):304–12. doi: 10.1002/1097-4636(20010605)55:3<304::aid-jbm1018>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee SS, Tarafder S, Davies NM, Bandyopadhyay A, Bose S. Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of β-TCP ceramics. Acta Biomater. 2010;6(10):4167–74. doi: 10.1016/j.actbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Li F, Feng QL, Cui FZ, Li HD, Schubert H. A simple biomimetic method for calcium phosphate coating. Surf Coat Technol. 2002;154(1):88–93. [Google Scholar]
- 57.Farack J, Wolf-Brandstetter C, Glorius S, Nies B, Standke G, Quadbeck P, et al. The effect of perfusion culture on proliferation and differentiation of human mesenchymal stem cells on biocorrodible bone replacement material. Mater Sci Engin. 2011;176(20):1767–72. [Google Scholar]
- 58.Hermawan H, Dubé D, Mantovani D. Degradable metallic biomaterials: design and development of Fe-Mn alloys for stents. J Biomed Mater Res A. 2010;93(1):1–11. doi: 10.1002/jbm.a.32224. [DOI] [PubMed] [Google Scholar]
- 59.Quadbeck P, Hauser R, Kümmel K, Standke G, Stephani G, Nies B, et al. Iron based cellular metals for degradable synthetic bone replacement. PM2010 World Congress, Florenz, Italy. 2010 [Google Scholar]
- 60.Yusop A, Bakir A, Shaharom NA, Abdul Kadir M, Hermawan H. Porous biodegradable metals for hard tissue scaffolds: a review. Int J Biomater. 2012;2012(1):641430. doi: 10.1155/2012/641430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bobyn J, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81(5):907–14. doi: 10.1302/0301-620x.81b5.9283. [DOI] [PubMed] [Google Scholar]
- 62.Bobyn JD, Toh KK, Hacking SA, Tanzer M, Krygier JJ. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty. 1999;14(3):347–54. doi: 10.1016/s0883-5403(99)90062-1. [DOI] [PubMed] [Google Scholar]
- 63.Adams JE, Zobitz ME, Reach JS, Jr, An KN, Lewallen DG, Steinmann SP. Canine carpal joint fusion: a model for four-corner arthrodesis using a porous tantalum implant. J Hand Surg Am. 2005;30(6):1128–35. doi: 10.1016/j.jhsa.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Meneghini RM, Lewallen DG, Hanssen AD. Use of porous tantalum metaphyseal cones for severe tibial bone loss during revision total knee replacement: surgical technique. J Bone Joint Surg Am. 2009;91(Suppl 2 Pt 1):131–8. doi: 10.2106/JBJS.H.01061. [DOI] [PubMed] [Google Scholar]
- 65.Vehof JW, Spauwen PH, Jansen JA. Bone formation in calcium-phosphate-coated titanium mesh. Biomaterials. 2000;21(19):2003–9. doi: 10.1016/s0142-9612(00)00094-6. [DOI] [PubMed] [Google Scholar]
- 66.Wu C, Zhou Y, Fan W, Han P, Chang J, Yuen J, et al. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33(7):2076–85. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 67.Hermawan H, Alamdari H, Mantovani D, Dube D. Iron-manganese: new class of metallic degradable biomaterials prepared by powder metallurgy. Powder Metallurgy. 2008;51(1):38–45. [Google Scholar]
- 68.Di Mario C, Griffiths H, Goktekin O, Peeters N, Verbist J, Bosiers M, et al. Drug-eluting bioabsorbable magnesium stent. J Interv Cardiol. 2004;17(6):391–5. doi: 10.1111/j.1540-8183.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 69.Li Z, Gu X, Lou S, Zheng Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials. 2008;29(10):1329–44. doi: 10.1016/j.biomaterials.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 70.Peuster M, Wohlsein P, Brügmann M, Ehlerding M, Seidler K, Fink C, et al. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6-18 months after implantation into New Zealand white rabbits. Heart. 2001;86(5):563–9. doi: 10.1136/heart.86.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song G. Control of biodegradation of biocompatable magnesium alloys. Corrosion Sci. 2007;49(4):1696–701. [Google Scholar]
- 72.Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557–63. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 73.Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–34. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology?Heart. 2003; 89(6):651–6. doi: 10.1136/heart.89.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu XN, Zhou WR, Zheng YF, Liu Y, Li YX. Degradation and cytotoxicity of lotus-type porous pure magnesium as potential tissue engineering scaffold material. Mater Lett. 2010;64(17):1871–4. [Google Scholar]
- 76.Gu XN, Zheng YF. A review on magnesium alloys as biodegradable materials. Front Mater Sci China. 2010;4(2):111–5. [Google Scholar]
- 77.Stroganov GB, Savitsky EM, Tikhova NM, Terekhova VF, Volkov MV, Sivash KM, et al. Magnesium-base alloy for use in bone surgery. Washington, DC: Patent and Trademark Office; 1972. [Google Scholar]
- 78.Witte F, Fischer J, Nellesen J, Crostack HA, Kaese V, Pisch A, et al. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27(7):1013–8. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 79.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294(1-2):1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 80.Witte F, Ulrich H, Palm C, Willbold E. Biodegradable magnesium scaffolds: Part II: peri-implant bone remodeling. J Biomed Mater Res. 2007;81(3):757–65. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 81.Balla VK, Bodhak S, Bose S, Bandyopadhyay A. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010;6(8):3349–59. doi: 10.1016/j.actbio.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das K, Balla VK, Bandyopadhyay A, Bose S. Surface modification of laser-processed porous titanium for load-bearing implants. Scripta Mater. 2008;59(8):822–5. [Google Scholar]
- 83.Goodman SB, Ma T, Chiu R, Ramachandran R, Smith RL. Effects of orthopaedic wear particles on osteoprogenitor cells. Biomaterials. 2006;27(36):6096–101. doi: 10.1016/j.biomaterials.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 84.Okazaki Y. A new Ti-15Zr-4Nb-4Ta alloy for medical applications. Curr Opin Solid State Mater Sci. 2001;5(1):45–53. [Google Scholar]
- 85.Zdeblick TA, Phillips FM. Interbody cage devices. Spine. 2003;28(15 Suppl):S2–7. doi: 10.1097/01.BRS.0000076841.93570.78. [DOI] [PubMed] [Google Scholar]
- 86.Jansen JA, Vehof JW, Ruhe PQ, Kroeze-Deutman H, Kuboki Y, Takita H, et al. Growth factor-loaded scaffolds for bone engineering. J Control Release. 2005;101(1-3):127–36. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 87.Crowninshield RD. Mechanical properties of porous metal total hip prostheses. Instr Course Lect. 1985;35(1):144–8. [PubMed] [Google Scholar]
- 88.Faria PE, Carvalho AL, Felipucci DN, Wen C, Sennerby L, Salata LA. Bone formation following implantation of titanium sponge rods into humeral osteotomies in dogs: a histological and histometrical study. Clin Implant Dent Relat Res. 2010;12(1):72–9. doi: 10.1111/j.1708-8208.2008.00132.x. [DOI] [PubMed] [Google Scholar]
- 89.van den Dolder J, Jansen JA. Titanium fiber mesh: a nondegradable scaffold material. London: Engineering of Functional Skeletal Tissues; 2007. pp. 69–80. [Google Scholar]
- 90.Prymak O, Bogdanski D, Köller M, Esenwein SA, Muhr G, Beckmann F, et al. Morphological characterization and in vitro biocompatibility of a porous nickel-titanium alloy. Biomaterials. 2005;26(29):5801–7. doi: 10.1016/j.biomaterials.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 91.Greiner C, Oppenheimer SM, Dunand DC. High strength, low stiffness, porous NiTi with superelastic properties. Acta Biomater. 2005;1(6):705–16. doi: 10.1016/j.actbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Tarniţă D, Tarniţă DN, Bîzdoacă N, Mîndrilă I, Vasilescu M. Properties and medical applications of shape memory alloys. Rom J Morphol Embryol. 2009;50(1):15–21. [PubMed] [Google Scholar]
- 93.Assad M, Chernyshov A, Leroux MA, Rivard CH. A new porous titanium-nickel alloy: part 1. Cytotoxicity and genotoxicity evaluation. Biomed Mater Engin. 2002;12(3):225–37. [PubMed] [Google Scholar]


