Abstract
With the recent rediscovery of brown fat in adult humans, our outlook on adipose tissue biology has undergone a paradigm shift. While we attempt to identify, recruit, and activate classic brown fat stores in humans, identification of beige fat has also raised the possibility of browning our white fat stores. Whether such transformation of human white fat depots can be achieved to enhance the whole body oxidative potential remains to be seen. Evidence to date, however, largely points toward a major oxidative role only for classic brown fat depots, at least in rodents. White fat stores seem to provide the main fuel for sustaining thermogenesis via lipolysis. Interestingly, molecular markers consistent with both classic brown and beige fat identity can be observed in human supraclavicular depot, thereby complicating the discussion on beige fat in humans. Here, we review the recent advances made in our understanding of brown and beige fat in humans and mice. We further provide an overview of their plausible physiological relevance to whole body energy metabolism.
Keywords: brown fat, beige fat, thermogenesis, sympathetic nervous system
INTRODUCTION TO BROWN FAT THERMOGENESIS
Thermogenesis is etymologically derived from ancient Greek “thermos” and “genesis,” which literally means heat generation. Although thermogenesis per se has multiple components, such as diet-induced thermogenesis, exercise-associated thermogenesis, nonexercise activity thermogenesis, shivering thermogenesis, and nonshivering thermogenesis (NST), the term is often used to represent the adaptive phenomenon of NST that evolved to facilitate the survival of species under cold temperature conditions (24). Essentially, taking place in the brown adipose tissue (BAT), NST is contingent on the accelerated substrate oxidative rate led to by mitochondrial uncoupling protein 1 (UCP1), a key molecule that uncouples fuel oxidation from ATP synthesis, resulting in the conversion of stored energy (essentially lipids) into heat (107). Indeed, it is the energy-expending potential of the thermogenic phenomenon that makes it an attractive target for combating the obesity and metabolic syndrome crisis of the 21st century.
Using in vitro approaches, it has been shown that, at the cellular level, the thermogenic process is initiated on the stimulation of the adrenergic receptors (AR) (in particular β3-AR in rodents) that activate a signaling cascade downstream of adenyl cyclase involving cAMP-PKA-p38 MAPK (25). Activation of this pathway results in the phosphorylation of intracellular lipases, including adipose tissue triglyceride lipase (ATGL), hormone sensitive lipase, and monoglyceride lipase (87). Lipolysis of intracellular lipid pools releases free fatty acids (FFAs) that serve two functions: release the purine nucleotide-binding-mediated inhibition of UCP1 (50), and provide fuel for thermogenesis by undergoing β-oxidation (61) (Fig. 1). Besides the intracellular lipid pool, active BAT can also utilize circulating glucose, fatty acids that can be free, i.e., nonesterified FFAs (NEFA), or derived from lipoprotein lipase action on triglyceride (TG)-rich lipoproteins (TRLs), for fueling thermogenesis (6, 23, 70, 78) (Fig. 1). However, as severe thermogenic defects are observed in ATGL-deficient mice (1), intracellular lipid stores are considered to be critical for the thermogenic process in BAT. It is also suggested that glucose and NEFA extracted from circulation by active BAT are utilized for replenishing the intracellular TG stores rather than being combusted directly (23, 72, 81). Blocking lipogenesis in BAT has been shown to result in impaired adaptive thermogenesis (36).
Fig. 1.
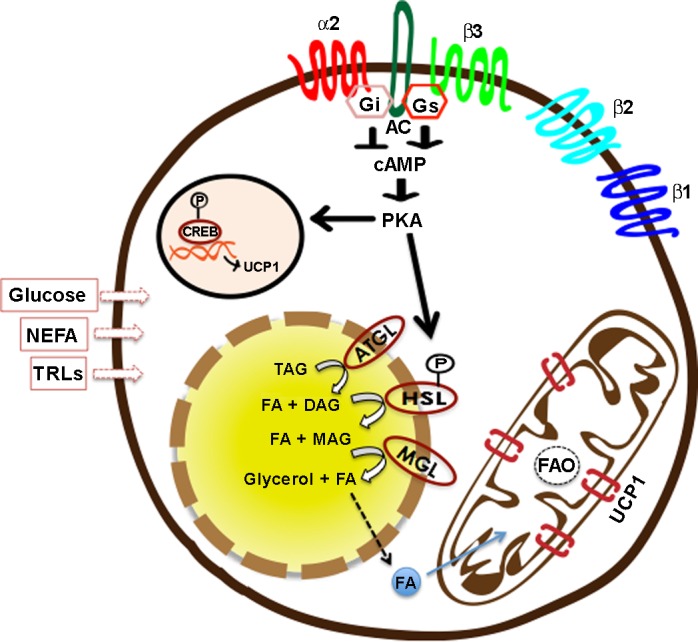
Cellular events underlying UCP1-mediated thermogenesis. Adipocytes can express various adrenergic receptor (AR) subtypes (84). Whereas β-ARs (β1/β2/β3) are coupled with Gs, leading to activation of adenylyl cyclase (AC), α2-ARs coupled to Gi are inhibitory in nature. On stimulation of β-ARs, activation of Gs-AC-cAMP-PKA pathway leads to activation of UCP1 transcription on one hand, and phosphorylation of hormone-sensitive lipase (HSL) and other lipid droplet-associated proteins on the other (25). Sequential hydrolysis of triglycerides (TAG) into diacylglycerol (DAG), monoacylglycerol (MAG), and glycerol, as well as fatty acids (FAs), is achieved by enzymatic activities of adipose triglyceride lipase (ATGL), HSL, and monoglyceride lipase (MGL) (87). FAs released from intracellular lipid stores are taken up by mitochondria, where they undergo β-oxidation, and their stored energy is dissipated via UCP1 that serve to uncouple oxidative phosphorylation from ATP synthesis (23, 61). Besides intracellular lipids, brown adipocytes can also utilize circulating glucose, nonesterified free fatty acids (NEFA), and TAG-rich lipoproteins (TRLs) for fueling thermogenesis (105). Among β-ARs, β1-ARs are often associated with an increase in proliferation and differentiation of brown adipocytes (91), whereas β3-ARs associate with thermogenic activation (121). CREB, cAMP response element binding protein.
Once activated, heat production in BAT can be maintained as long as the thermogenic stimuli and fuel remain present. Rodents are known to increase their food intake three- to fourfold while attempting to maintain their body weight during chronic cold exposure. In cold-adapted rats, up to 60% of the adrenergically enhanced metabolic activity is attributable to BAT thermogenesis, pointing toward a massive thermogenic capacity of the brown adipocyte (53). This has, in turn, been associated with clearance of 50% of ingested TGs and 75% of ingested glucose, reflecting on the untapped potential of NST in burning energy substrates and, therefore, in fighting certain metabolic abnormalities (6, 105).
Although it has always been known to be present (67, 71) and to be involved in thermoregulation during neonatal stages (88), BAT in adult humans and its contribution to energy homeostasis received little to no attention for a very long time, since it was believed that BAT undergoes involution during the early years of human life (104). However, a series of important observations (40, 41, 104) that were followed by imaging studies published in 2009 changed this paradigm, by reporting its presence {identified as [18F]fluoro-deoxy-glucose (18F-FDG) uptake in specific areas using positron emission tomography/computed tomography (PET/CT)} in adult humans exhibiting a spectrum of age and other metabolic conditions (44, 134, 158, 165). These specific areas of metabolic activity have so far been located around supraclavicular, cervical, mediastinal, suprarenal, and perirenal regions of the human body (31, 48, 123, 158). In addition, BAT presence and thermogenic activity have been associated with lower body mass index (BMI) (158), increased insulin sensitivity (33), and improved markers of metabolic health (30, 34) in various studies, thereby highlighting its physiological relevance and underscoring the need to understand human BAT further. Here, we provide an overview of the current understanding of BAT, its subtypes, and their regulation by central and peripheral mechanisms. In addition, we have reviewed the physiological relevance of BAT as well as key issues related to the detection and utility of human BAT.
CLASSIC BAT VS. RECRUITABLE BEIGE FAT
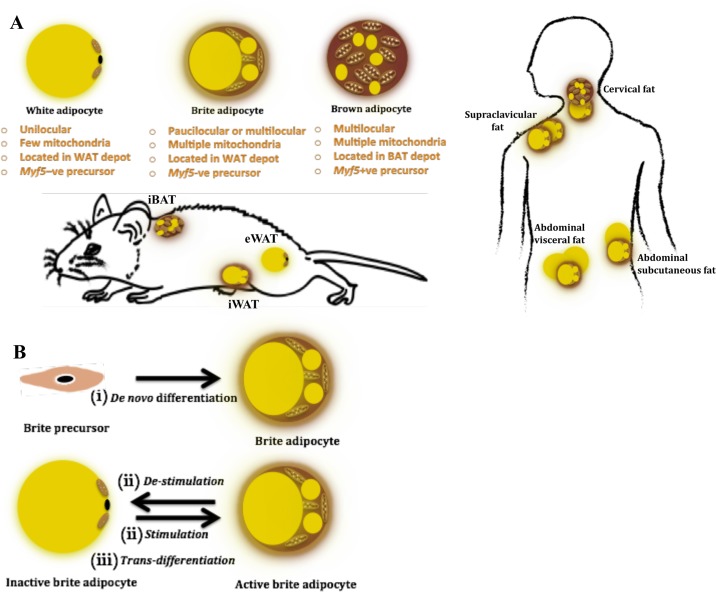
Recent classification of BAT as the “classic BAT” and the “inducible” or “recruitable” beige fat is based on the observations made in rodents, where classic BAT is recognized as the discrete fat depots located in the interscapular, cervical, axillary, and perirenal areas that are exclusively composed of classic brown adipocytes (143). In contrast, the inducible BAT represents the white adipose tissues (WAT) that possess the ability of undergoing browning, that is, of harboring pockets of brown-like adipocytes expressing UCP1 among white adipocytes on stimulation (43, 69, 116, 180). These cells have been referred to as brite (acronym for “brown in white”), beige, or recruitable adipocytes. It is important to note that, although most WAT depots have been shown to undergo browning, the propensity of browning varies considerably among various fat depots, thereby somewhat justifying the present nomenclature. For instance, inguinal WAT (iWAT) undergoes browning to a much greater extent than what is observed for gonadal fat, e.g., epidydmal WAT (eWAT) in rodents (110, 166); hence it has almost become dogmatic to identify iWAT as the beige fat depot, whereas eWAT is looked on as more of a true white fat depot (129, 138, 166, 169) (Fig. 2).
Fig. 2.
A: characteristics, origin, and location of brown, brite, and white adipocytes in mice and men. Adipocytes can be white, brown, or brite in nature. Location-wise, deep neck biopsies from the cervical region have been reported to express classic brown markers (93, 106), whereas most studies dealing with human supraclavicular fat report a beige fat signature in this depot (46, 74, 140, 142, 175). In addition, evidence exists for abdominal visceral and subcutaneous fat depots to undergo browning in humans (54, 144). In mice, interscapular brown adipose tissue (BAT) represents the most commonly studied classic BAT depot, whereas, based on the extent of browning, inguinal white adipose tissue (iWAT) often represents a beige fat depot, and epidydmal white adipose tissue (eWAT) is considered to be a true WAT depot (129, 138, 166, 169). B: proposed mechanisms of brite adipocyte origin. Brite adipocytes have been proposed to originate either from i) specific precursors via de novo differentiation (171); ii) mature white-looking brite adipocytes upon stimulation (129); or iii) from mature white adipocytes via transdifferentiation (37).
Phenotypic Features
The anatomic distinction of classic BAT vs. inducible beige fat is also retained at the adipocyte level, where a classic brown adipocyte is recognized with its signature phenotype of being multilocular and carrying numerous mitochondria, along with exhibiting significant expression of UCP1 (23, 125) (Fig. 2). In contrast, the phenotype of an UCP1-expressing cell in WAT can vary significantly based on its state of stimulation (37, 175). Under nonstimulated conditions, brite adipocytes can exhibit a unilocular or paucilocular phenotype with few mitochondria and low UCP1 expression, whereas, under stimulated conditions, brite adipocytes resemble classic brown adipocytes with their signature phenotype and significant expression of UCP1 (37, 175) (Fig. 2). Owing to this phenotypic variability, two predominant schools of thought exist regarding the origin of brite adipocytes. While some suggest that these adipocytes are derived from de novo adipogenesis of specific precursors, others propose that mature white adipocytes are capable of undergoing transdifferentiation into brown adipocytes upon stimulation (39) (Fig. 2). Lineage tracing experiments utilizing different approaches have provided evidence that, on cold exposure or adrenergic stimulation, newly developed brite cells in iWAT depot originate from de novo differentiation (171) or directly emerge from mature adipocytes that likely have a brite signature (91). Evidence also exists for brite cells to revert back to unilocular white-like adipocytes on the removal of thermogenic stimulus (129). It seems plausible as of now that the plasticity of beige fat depot represents the ability of a brite adipocyte to exist as an active or dormant thermogenic adipocyte (68, 76).
Ontogeny and Molecular Profile
Evidence further suggests that these two cell types exhibit different ontogeny. Whereas the classic brown adipocytes are derived from skeletal muscle-type progenitors expressing Myf5 or Pax7 (92, 137), brite adipocytes are Myf5 negative and exhibit a smooth muscle cell-like origin with progenitors marked by Myh11 (94) (Fig. 2). Efforts made in recent years to distinguish these adipocytes have also revealed molecular signatures associated with each cell type, which allow for the identification of a UCP1-expressing adipose depot as being classic brown or beige in nature (46, 74, 93, 169, 175). Assessment of these molecular signatures have further uncovered that, while markers consistent with classic BAT can be identified in the interscapular fat (in infants) and deep neck fat biopsies (46, 74, 93), the majority of cervical, supraclavicular, and mediastinal fat depots in adult humans appear to share their molecular signatures with rodent beige fat (46, 93, 106, 140, 175) (Fig. 2). Global and unbiased molecular analyses of human supraclavicular brown fat at the single-cell resolution also revealed a molecular signature resembling that of rodent beige fat (142). Thus, unlike rodents, which exhibit clear anatomic depot segregation of classic brown and beige fat, UCP1-positive adipocytes expressing classic brown- or brite-specific markers can be located very close to each other within a given anatomic location in humans (46, 106). Developmental origins of each of these adipocytes, however, remain unknown.
Thermogenic Function
Despite the anatomical and molecular dissimilarities, a key factor that binds classic brown and brite adipocytes together is the presence of UCP1. It has been shown that UCP1 maintains its uncoupling and thermogenic ability, irrespective of its location (138). However, the relative contribution of beige fat UCP1 to total thermogenic response has been estimated at one-fifth of the classic BAT depot, owing to its quantitatively lower thermogenic density (i.e., UCP1-dependent oxygen consumption per gram of tissue wet weight measured using an in vitro approach), at least in rodents (138). It has been suggested that UCP1-independent futile cycles, such as TG/FFA cycle (52) and Ca2+ futile cycling (153), can contribute to enhanced energy expenditure in WAT. Of note, creatine-driven substrate futile cycling was recently identified as a significant contributor to enhanced energy expenditure in rodent beige fat during cold exposure (77). Interestingly, genes involved in creatine-driven futile cycling exhibited a compensatory upregulation in UCP1-ablated mice, whereas a reduction in creatine bioavailability was associated with lower core body temperature in these mice (77). Deep neck biopsies of human BAT were also recently shown to exhibit a proteome consistent with utilization of both coupled and uncoupled pathways for enhancing energy expenditure, in particular the observed enrichment of mitochondrial creatine kinase and interactome complex (103). Although the relative relevance of these mechanisms remains to be seen, these observations indicate that beige fat has the potential of contributing to whole body energy expenditure significantly via UCP1- and creatine-dependent futile cycles.
REGULATION OF BAT METABOLISM
Sympathetic Nervous System-mediated Regulation of Brown and Beige Fat
Physiologically, NST is under control of the central nervous system. The central nervous system, via the sympathetic nervous system (SNS), is a strict controller of BAT thermogenic activity by means of governing UCP1 expression and its activity (25), as well as the proliferation and differentiation of brown as well as brite adipocytes (91, 101). Indeed, neural centers involved in thermoregulation have been ascribed a role in the control of BAT thermogenesis. In particular, preoptic area (POA)-dorsomedial hypothalamus (DMH)-raphe pallidus pathway is known to exert central thermoregulatory control over BAT (83, 100, 124, 141). In addition, neural centers involved in the regulation of energy balance have been reported to relay to both classic BAT and WAT depots. Using pseudorabies virus (PRV)-labeled retrograde tract tracing approach, paraventricular hypothalamus (PVH), lateral hypothalamus, DMH, arcuate nucleus, and POA have been identified as relaying to BAT (5, 10). Evidence also supports a role for ventromedial hypothalamus in the SNS outflow to BAT (26). In addition, PVH, DMH, and POA have been reported to relay to WAT (7, 9). Despite an overlap among the neuroanatomical brain regions involved in the modulation of BAT and WAT depots, it is clear that downstream regulation of SNS activity at the level of neuropeptide expression, innervation, and SNS drive varies greatly at the depot level, both between BAT and WAT, as well as among various WAT depots (4, 19, 84). Indeed, the enhanced presence of PRV-labeled neurons in the DMH can be seen when PRV injection was made in iWAT relative to eWAT (4). Additionally, cold exposure has been shown to enhance SNS drive to BAT and iWAT much more strongly than to eWAT, whereas glucoprivation was associated with enhanced SNS nerve activity to iWAT selectively (19). Similarly, AR expression as the downstream mediator of SNS circuitry is known to be variable among various fat depots (84).
It is well established that adrenergic control over BAT and beige fat is mediated via ARs (Fig. 1), specifically β3-AR in the case of rodents; β3-AR-less mice exhibit severe thermogenic defects and lack of beige fat development on cold exposure (2, 75). In addition, β3-AR agonism has been shown to replicate the effects of cold exposure on both brown and beige fat depots (60). Rodent adipocytes are known to express β1-, β2-, and β3-ARs, where β1-ARs are reported to be important for the proliferation of brown adipocytes (91) and β3-ARs, largely expressed by mature adipocytes (22), are primarily responsible for mediating their thermogenic activity (121). Lack of β1-AR is associated with inhibition of BAT hyperplasia associated with cold exposure (91), whereas β3-AR stimulation does not induce BAT hyperplasia, despite increasing its activity, suggesting that both β1- and β3-AR are needed for a full thermogenic response (121). Overexpression of β1-AR has also been associated with browning of iWAT in rodents (145). In addition, α2-ARs, known to inhibit cAMP signaling, are known to be present in rodent adipocytes, however to a much less extent than what is seen for human adipocytes. Peripheral and central (in raphe pallidus) injections of α2-AR agonists were recently shown to inhibit BAT thermogenesis in rats (96), indicating that α2-AR exhibit inhibitory control over BAT thermogenesis. Emerging evidence further suggests that an interplay between the relative expression or affinities of various ARs is not only important for the thermogenic outcome in BAT, but may also influence browning of WAT depots in rodents (131). In contrast to rodents, human adipocytes predominantly express β1- and β2-ARs, which have also been shown to play a role in human BAT activity (115, 148). Although β3-ARs mRNA and protein have been reported in human brown fat (165, 181), and at least one recent report indicates that β3-AR agonist treatment can enhance metabolic activity in human BAT (45), direct evidence of major contribution of β3-AR in the control of thermogenic activity in humans needs further elucidation (85, 135, 168) (Fig. 1).
An important question of whether a neuroanatomically distinct SNS outflow circuitry exists for the beige fat and brite adipocytes was recently addressed by Bartness and Ryu (8), where they extensively discuss the only study dealing with the issue, reporting a role for DMH-specific neuropeptide Y neurons in the sympathetic outflow to beige (i.e., iWAT and dorso-subcutaneous WAT) but not white fat (eWAT) (29). We know that, even at the maximum level of stimulation of a beige fat depot (for instance iWAT), not all adipocytes acquire a brownlike phenotype, indicating that a mix of pure white and brite adipocytes exists in this depot at all levels of stimulation. The obvious next question thus arises as to whether specific neuroanatomic circuitry relay to brite adipocytes within a beige fat depot. Bartness and Ryu (8) suggest it to be an unlikely scenario based on the general observations that most SNS nerves innervate WAT depots in an en passant manner. Instead, they suggest, and we agree, that a combination of factors, such as differential SNS drive, stimuli-specific effects, propensity of an adipocyte to norepinephrine-mediated stimulation, either due to cell-autonomous factors, or due to AR expression, as well as their sensitivity, would contribute to the commonly observed differences among WAT depots in terms of their browning potential (8).
Sensory Control of Brown and Beige Fat
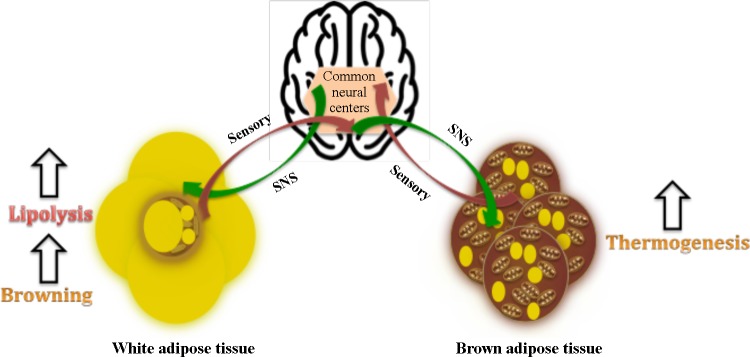
Besides SNS, investigators have looked for both parasympathetic and sensory innervation of BAT and WAT depots. Although partial evidence exists to point toward parasympathetic innervation of BAT and WAT, this idea has been challenged critically (7, 10). However, both BAT and WAT depots (including beige fat depots such as iWAT) are known to exhibit sensory innervation (132, 133, 146, 160). Using the H129 strain of herpes simplex virus and anterograde tract tracing approach, it has been shown that most areas involved in the SNS outflow to BAT and WAT, such as PVH, DMH, and lateral hypothalamus, also connect with the sensory inputs from these fat depots, pointing toward a SNS-sensory feedback loop that exists in both BAT and WAT depots (132, 133) (Fig. 3). It has been postulated that sensory nerves in BAT serve to sense its thermal status, blood flow, or intracellular lipolytic products and hence regulate its thermogenic activity via the SNS loop (10, 133). Direct BAT sensory denervation has been reported to induce BAT atrophy (160). Similarly, sensory nerves in WAT have been proposed to regulate lipolysis via sensing lipolytic products (7, 132). Of note, BAT exhibited relatively lower levels of PRV152 + H129-double-labeled neurons for SNS-sensory inputs than WAT, indicating that WAT is under a stronger sensory control than BAT (133). Nonetheless, a considerable overlap among the neural centers regulating SNS and the sensory circuitry to BAT and WAT also raises the question of whether this circuitry is built for the regulation of both the thermogenic (BAT) and lipolytic (WAT) arms of fat stores that function as part of a so-called “adipose organ” (38), such that a sensory input from WAT alters the thermogenic response in BAT and vice versa. Indeed, a recent study revealed that lipolysis as well as its products, such as FFAs eicosapentanoic acid and arachidonic acid, activate sensory neurons of iWAT, which was further associated with enhanced BAT thermogenesis in Siberian hamsters (56).
Fig. 3.
Sympathetic and sensory control of brown adipose tissue (BAT) and white adipose tissue (WAT) depots. Common neural centers involved in the regulation of energy balance have been proposed to relay sympathetic impulses to, and receive sensory inputs from, BAT and WAT depots (132, 133). While sympathetic nervous system (SNS) sensory feedback loop is proposed to be largely responsible for the regulation of lipolysis in WAT and thermogenesis in BAT, emerging evidence indicates that sensory inputs from WAT can alter thermogenic responses in BAT (56).
It is indeed quite clear that sympathetic outflow to WAT and BAT is concerned with differential physiological output, with SNS being the primary controller of lipolysis in WAT and that of thermogenesis in BAT (Fig. 3). Furthermore, it is also becoming clear that lipolysis and thermogenesis are tightly connected, both at the intracellular and tissue level. Within BAT, intracellular lipolysis is essential for thermogenesis, as its abrogation leads to thermogenic failure (63, 147). Our laboratory has recently shown that blocking intracellular lipolysis leads to a severely blunted metabolic response in the interscapular BAT (iBAT) in rats (81). However, it further seems that thermogenesis in BAT is also dependent on WAT lipolysis at the tissue level, with this communication being under the regulation of SNS sensory loops in both of these depots. Surgical denervation of WAT was recently shown to block increases in BAT temperature during CL316,243 infusion (56). Human studies have also revealed a direct correlation between WAT lipolysis and metabolic activity in BAT (13, 34). Often, we look at this relationship being simply about WAT lipolysis providing fuel for BAT thermogenesis. However, recent work by Garretson et al. (56) suggests that WAT can exert control over BAT beyond thermogenesis, plausibly to regulate circulating NEFA levels. Thus it is likely that any factor or physiological condition that is associated with an upregulation in WAT lipolysis without a need for increasing core body temperature may result in transient BAT activity just to ensure excess NEFA have been take care of to maintain metabolic homeostasis.
Non-SNS-mediated Regulation of Brown and Beige Fat
Besides the direct control exerted by the SNS in the modulation of both brown and beige fat activity, multiple circulating factors falling in the categories of hormones, growth factors, myokines, adipokines, and metabolites have been shown to exert control over the development as well as thermogenic activity of brown and beige fat (164). Interestingly, most of these factors work either by interacting with or modulating the levels of SNS activity or its downstream effector norepinephrine. Thyroid hormones are well known regulators of adrenergic signaling within brown adipocytes as well as of UCP1 gene expression (120). Mice with targeted disruption of type 2 iodothyronine deiodinase in classic brown adipocytes exhibit impaired BAT function, defective adaptive thermogenesis, and hypothermia on cold exposure (36). Thyroid hormones are also known to influence hypothalamic mechanisms to alter SNS activity to BAT, thereby exerting their control over BAT thermogenesis (95). Hyperthyroidism was also shown to be associated with threefold higher enhanced glucose uptake in BAT in men (86). Recently, thyroid hormones were also shown to regulate cold-induced BAT activity in a study involving thyroid carcinoma patients (21).
Atrial and B-type natriuretic peptides (NPs), secreted by the human heart during pressure-related changes such as heart failure, have been shown to induce browning and enhance BAT thermogenesis in rodents (97). Cold exposure was associated with increased levels of circulating NPs, whereas coadministration of ANP and β3-AR was associated with additive effects on browning of white adipocytes, indicating the synergy between SNS and the NP system in regulating beige fat development (97). Enhanced expression of NP receptors, along with an upregulation of browning in gonadal WAT, was also reported recently in a rodent model of Roux-en-Y gastric bypass surgery (108). Central glucagon-like peptide 1 agonism was recently shown to enhance TG-derived fatty acids and glucose uptake by BAT and induce browning of WAT in a rodent study, via an increase in SNS activity to both iBAT and subcutaneous WAT, indicating a plausible role for gut-mediated regulation of BAT and beige fat depots (79). Skeletal muscle is also known to secrete multiple factors during exercise or cold-induced shivering that are known to influence BAT thermogenesis. Irisin, expressed and secreted primarily by skeletal muscle but also by adipocytes, has been shown to induce browning of WAT in rodents by its direct action on adipocytes (16). However, data around its role in human physiology have been questioned and need further exploration (73, 167). Meteorin-like (122) is yet another myokine that has been shown to induce browning of WAT. Its mode of action involves stimulation of eosinophil-mediated type 2 signaling that leads to an accumulation of alternatively activated-type macrophages known to regulate browning of WAT via local release of norepinephrine (122). In addition, multiple other myokines and adipokines, such as myostatin (139), follistatin (17), IL-6 (117), β-aminoisobutyric acid (127), and fibroblast growth factor 21 (51), have been shown to regulate browning of WAT in rodent models. Other circulating factors, such as bone morphogenetic protein (BMP) 4 (119), BMP7 (152) (111), and BMP8b (172), and metabolites, such as lactate (27), ketone bodies (27), and bile acids (49), have been shown to induce browning in rodent studies and in humans (20). A detailed account of various endogenous factors involved in the regulation of BAT and beige biology has been covered by recent reviews (28, 164).
PHYSIOLOGICAL RELEVANCE OF BROWN AND BEIGE FAT
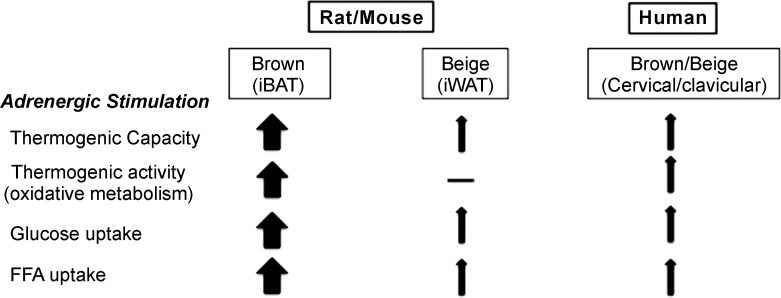
The current interest in BAT biology is essentially underscored by its significant energy-expending capacity that is evident from numerous rodent studies. An increase in BAT metabolic activity has been shown to be associated with reduced body fat, enhanced insulin sensitivity (16), and a cardio-protective lipoprotein profile in rodents (6). In addition, BAT activity was recently shown to be associated with a protective atherosclerosis outcome in a rodent study (11). Besides BAT, physiological benefits of beige fat have also been suggested based on rodent studies (16, 143). However, it is rather difficult to separate the physiological impact of beige fat from BAT in rodents, since most paradigms used for activating beige fat invariably also activate BAT. Few studies using unique approaches, such as unilateral denervation of BAT (136) or use of adiponectin-specific PRDM16 knockout mice (42) that possess dysfunctional beige fat, nonetheless point toward a role for beige fat in regulating whole body metabolic homeostasis. Genetic variability in the beige fat prevalence among rodent models and their propensity to develop obesity is also often cited as an indication for this depot to be playing a significant role in regulating energy balance (62, 176). The role of beige fat in total energy expenditure, however, needs to be better understood, as not every beige depot seems to significantly contribute to total energy expenditure. The archetypical beige depot (i.e., iWAT) in laboratory rodents adapted to cold (thus exhibiting iWAT browning) appears to respond trivially to an acute adrenergic stimulation compared with iBAT (82) (Fig. 4).
Fig. 4.
Physiological relevance of brown and beige fat. Adrenergic agonism (cold exposure) is the strongest known stimulator of brown fat in rodents and humans. Multiple studies covered in the respective section indicate that adrenergically stimulated BAT exhibits significant thermogenic capacity and activity, in association with glucose and FFA uptake. This metabolic activity further contributes to significant increases in energy expenditure in rodents and to a lesser extent in humans. Beige fat, in comparison, has been shown to exhibit dramatic fold-induction in its thermogenic capacity upon adrenergic stimulation relative to its basal state (82). Glucose and FFA uptake also occurs in beige fat; however, this was not associated with any detectable metabolic activity in rodent iWAT, questioning its contribution to whole body energy expenditure (82). In humans, detectable brown fat exhibits glucose and FFA uptake, as well as significant metabolic activity on adrenergic stimulation (113, 154). Human brown fat largely expresses beige markers; however, classic brown fat signatures are also detectable in this depot (46, 74, 93, 106, 142). Note: Arrows are not drawn to reflect on the exact proportional relevance of brown and beige in rodent and human.
Human BAT and Energy Expenditure
Retrospective observations made in multiple studies have reported a negative relationship among BAT presence and BMI, as well as total fat mass in humans (114, 134, 158). Indeed, the tremendous ability of BAT to shift the energy balance equation toward the negative in rodents is a promising utility for human energy metabolism as well, especially in relation to combating obesity and diabetes. As a result, multiple studies have looked at the potential contribution of BAT to whole body energy expenditure. In an historic study, Rothwell and Stock (130) had predicted that 40–50 g of maximally stimulated BAT could contribute to 20% of daily energy expenditure in humans, which, however, seems to be an overestimation. Correcting for allometric differences and likely submaximal state of BAT activity under physiological settings, it was more recently estimated that 50 g of active BAT could contribute to 2.7–5% of basal metabolic rate (157). Since then, a number of controlled cold-exposure studies in acute settings (from 2 to 8 h of cold exposure) have revealed that human BAT is, nonetheless, capable of making significant contributions to the resting energy expenditure (from 13 to 80%) (33, 113, 158, 179).
Despite the initial reports of BAT prevalence to be in the range of 2–7% (31, 114) when assessed in the retrospective settings, BAT prevalence was found to be much higher, ranging from 30 to 100%, in dedicated prospective cold-exposure studies (15). In terms of mass, 0–200 g of BAT can be found in humans across the range of age, BMI, and diabetes (15). Indeed, mass and prevalence of BAT are lower and often below the detection level by 18F-FDG-PET/CT in obese, aged, and diabetic individuals (112, 113, 134, 162). In addition, blood flow to human BAT under stimulated conditions has been estimated to reach 1/50 of what is seen in the case of rodent BAT (121) (Fig. 4). Thus it is clear that our ability to recruit BAT remains an imperative aspect of being able to utilize its thermogenic potential in therapeutic settings eventually. Chronic cold-exposure studies have revealed that BAT in humans can be recruited and stimulated to enhance its thermogenic activity (14, 154). Daily mild (15–16°C for 6 h/day for 10 days) (154) or stronger (10°C for 2 h/day for 4 wk) cold exposure (14) was associated with an increase in BAT volume and NST (154), as well as in its oxidative capacity in healthy subjects (14). Daily mild cold exposure at 17°C for 2 h/day for 6 wk was also seen to enhance metabolic activity in men with low BAT activity, which was further associated with a reduction in their body fat mass (178). Yet another study revealed the thermal plasticity of BAT, where both recruitment and involution of BAT activity could be observed in five healthy individuals in a protocol involving multiple temperature variations (90). In addition, appreciable recruitment of BAT has also been reported in older, metabolically healthy obese individuals upon cold acclimation (65).
Human BAT and Glucose Metabolism
As discussed earlier, besides intracellular TG stores, BAT is known to take up circulating glucose to fuel thermogenesis under stimulated conditions. Thus BAT can play an important role in whole body glucose metabolism (Fig. 4). Cold exposure has been shown to induce significant glucose uptake in BAT (6, 82), whereas BAT transplantation in abdominal visceral adipose tissue has been associated with improved glucose tolerance and insulin sensitivity in rodents (149). In concordance, retrospective human studies have reported lower prevalence of BAT in individuals with diabetes and hyperglycemia (112, 114). Prospective studies have further reported that human BAT exhibits enhanced glucose uptake during acute and chronic cold-exposure settings (14, 113), with glucose uptake being affected by intracellular TG pool of BAT, as opposed to its metabolic activity (14). Insulin has also been shown to enhance glucose uptake in human BAT without the associated increase in blood flow that is generally observed during cold exposure (112). However, yet another study reported enhanced whole body glucose disposal in fasted and insulin-stimulated states during cold exposure (5–8 h) in BAT-positive individuals relative to BAT-negative subjects, indicating that BAT contributes to whole body glucose homeostasis and insulin sensitivity (33). In addition, this study makes the case for direct glucose utilization by BAT for thermogenesis upon prolonged cold exposure (33). A link between insulin resistance and BAT activity, independent of age and obesity, was also recently demonstrated in a study where glucose uptake in BAT was reduced upon cold exposure in healthy subjects made insulin resistant after prolonged fasting (66). Reduced glucose uptake observed in this study was not due to lower glucose availability to BAT but was due to the reduced cellular uptake of glucose by BAT, which was further associated with reduced NST and lower core body temperature in these subjects (66), indicating that BAT, like other tissues, becomes insulin resistant, thereby limiting its ability to execute NST and contribute to thermoregulation. Older overweight men with diabetes were recently reported to exhibit lower BAT volume, reduced glucose uptake per volume, reduced perfusion and whitening of BAT, while maintaining their NEFA uptake and oxidative capacity during acute cold exposure (12).
Human BAT and Lipid Metabolism
Besides glucose, BAT can utilize circulating NEFA as well as fatty acids derived from TRLs to fuel thermogenesis (Fig. 4). In fact, BAT was reported to be the primary site for lipoprotein uptake and TG metabolism during acute extreme (4°C) cold exposure (6), which contributed to the clearance of nearly one-half of ingested TG in a rodent study (105). Although BAT has been shown to exhibit NEFA uptake during cold exposure, this has been estimated to be roughly at 0.25% of total NEFA turnover in humans (113). Instead, cold exposure has been primarily associated with utilization of intracellular TG stores as the radiodensity of BAT increased after 3-h cold exposure (113). Whether prolonged cold exposure changes this equation remains to be seen. However, chronic cold acclimation of subjects (i.e., 10°C for 2 h/day for 4 wk) was associated with a reduction in BAT radiodensity, supporting the concept that BAT serves as a substrate sink for circulating glucose and NEFA that are utilized to restore its intracellular lipid stores for an eventual thermogenic output (12). In this context, WAT lipolysis has been reported to be directly proportional to the oxidative capacity of BAT (12). Although none of the studies have directly investigated TRL uptake by human BAT, a role for BAT in human lipid metabolism can be supported by the observations made in recent studies (33, 35). An increase in the volume and 18F-FDG uptake in BAT after 5–8 h of cold exposure was associated with whole body lipolysis, whole body fat oxidation, and TG-FFA cycling, as well as adipose tissue insulin sensitivity in these studies, indicating that BAT activity may directly or indirectly contribute to lipid mobilization in humans (33, 35). Interestingly, 1 day after cold exposure, circulating levels of TG and very-low-density lipoprotein were found to be lower compared with the levels seen in participants kept at thermoneutral conditions (35). Our laboratory has also reported a positive relationship between UCP1 mRNA levels in human epicardial adipose tissue with circulating high-density lipoprotein-cholesterol levels that would support a role for BAT and beige fat depots in the regulation of whole body lipid metabolism in humans (30).
Human Beige Fat and Metabolic Benefits
An important question of whether beige fat can be recruited or if it can contribute to whole body energy expenditure in humans leads to an interesting discussion. Knowing that the supraclavicular fat depot that is commonly studied for its metabolic activity using radioactive tracer techniques in humans largely exhibits a molecular profile consistent with rodent beige fat (46, 74, 93), one can predict a significant role for beige fat in human energy metabolism. However, when one looks at this depot as the “BAT” depot of the human body and then addresses the relevance of rodent-like beige fat, one notices that most studies report no evidence for browning or metabolic activity in other subcutaneous fat depots under various cold exposure paradigms (65, 90, 154). A recent study involving burn victims, however, reported significant upregulation in UCP1 as well as in the browning aspect of human subcutaneous fat (144). Browning of subcutaneous fat was also associated with enhanced energy expenditure in this study. However, percent contribution of beige fat to total energy expenditure (7%) was estimated to be comparable to BAT (6.3%), in an individual with 100 g of BAT and 15 kg of WAT (118). It is important to underscore that burning is associated with severe adrenergic stress that leads to hypermetabolism (174), thus it is clear that browning of most subcutaneous fat depots in humans requires a much stronger adrenergic stimulus than what is normally generated with cold exposure. Indeed, browning of visceral adipose tissue has been observed in patients with phaeocytochroma (54). An alternative view on this observation may also be put forward in terms of the hypothesis that neural centers involved in thermoregulation may exert inhibitory control over beige fat thermogenesis when functional bona fide BAT depot (for instance iBAT) is present during a thermogenic stress to avoid hyperthermia. Indeed, in mice, unilateral SNS denervation of iBAT has been shown to induce compensatory upregulation in the induction of beige fat (136). In one of our own recent study utilizing radioactivity tracers, maximum metabolic activity was found to be located in the classic BAT on cold exposure as well as after a β3-adrenergic agonist treatment, indicating that, at least in the presence of classic BAT depot, beige fat exhibits near negligible metabolic activity in rodents (82) (Fig. 4).
SECRETORY RELEVANCE OF BROWN AND BEIGE FAT
In addition to the changes in total body energy expenditure and substrate turnover, metabolic benefits of active BAT and beige fat can also be ascribed to a growing list of molecules that are secreted by these fat depots (163). Some of these molecules act in an autocrine/paracrine role to facilitate either their thermogenic capacity or activity, whereas others act in an inhibitory manner, likely to maintain overall thermogenic homeostasis. For instance, in rodents, BAT is known to secrete nerve growth factor known to enhance its own proliferation by enhancing SNS activity (109), vascular endothelial growth factor known to promote angiogenesis, and perfusion needed for thermogenic substrate flow and heat dissipation (150). Prostaglandins (E2 and I2) have also been shown to induce browning of WAT in an autocrine fashion (55, 161). Besides, BAT can release factors like fatty acids, adenosine (59), nitric oxide (58, 126), and BMPs (99, 111, 172) that act on BAT and beige fat to promote their thermogenic activity. In contrast, factors such as soluble form of LDL receptor (173) and endocannabinoids (80) secreted by BAT under stimulated conditions serve to inhibit BAT thermogenesis.
BAT and beige fat are also known to secrete factors with endocrine features, thereby supporting the idea that these fat depots can exert regulatory control over other organs involved in metabolic homeostasis. Among these, fibroblast growth factor 21, interleukin 6, neuregulin (170), insulin-like growth factor binding protein 2 (18), retinol binding protein 4 (128), and angiopoietin-like protein family member 8 (177) have been reported to target liver, pancreas, and bone, along with the adipose tissues to improve whole body insulin sensitivity (163). In this context, no specific factors exclusively expressed by beige fat over BAT have been identified so far. Similarly, our understanding of the human BAT and beige secretome is at the preliminary stages, as no factor has thus far been identified as being uniquely secreted by either of these depots in humans. Transplantation of human beige adipocytes in murine models has, nonetheless, been shown to improve insulin sensitivity (98), reflecting on the potential of putative “beigo-kines” or “BATo-kines” in improving metabolic homeostasis in humans (163).
DETECTION OF HUMAN BAT: LIMITATIONS AND ISSUES
It is without doubt that much of the progress made in our understanding of human BAT comes from 18F-FDG uptake studies done in both retrospective and prospective settings. However, the technique and its data interpretation are not without limitations. Study of BAT in humans has various facets, including investigation of its presence, volume, and metabolic activity. A variety of factors at the individual level, such as age, sex, metabolic condition, and ethnicity, as well as means used for activation, scanning, and reporting BAT presence and activity contribute to the variability observed in human BAT studies. Apart from technical issues related to volume estimation by PET/CT, brown fat in humans generally is very heterogeneous, being a mixture of white and brown (or beige) adipocytes, making interpretation of volume data difficult. Recently, a consensus statement was released such that common methodological considerations can be utilized to identify a more consistent role for BAT metabolism in humans (32). Furthermore, it is important to note that our referral to the presence (and prevalence) of BAT essentially represents positive 18F-FDG uptake in selected areas. However, we acknowledge that lack of 18F-FDG uptake does not necessarily mean lack of BAT in those individuals. The lack of BAT detectability may obviously arise due to lack of BAT stimulation or detection, owing to multiple factors discussed above.
Notably, 18F-FDG measures glucose uptake per se, which is an indirect measure for the metabolic activity of human BAT. While glucose uptake was recently shown to be directly contributing to distinct mobilizable intracellular lipid pool in rodent primary brown adipocytes (72), such demonstration has not been made in humans. Considering that BAT thermogenesis heavily relies on fatty acids, especially those derived from intracellular lipid stores (14, 113), radioactive tracers that use labeled fatty acids (12, 113) likely represent better tools for assessing BAT activity. In addition, acetate (12, 113) and oxygen tracers (102, 112) that allow for the detection of metabolic activity (i.e., 15O-O2) and perfusion (i.e., 15O-H2O) of BAT, respectively, serve as better tools in assessing human BAT activity.
Considering that 18F-FDG/PET-CT scanning involves exposure to ionizing radiation, thereby limiting its repeated usage, there is increased need for finding better noninvasive, non-radioactive means to study human BAT. Based on various aspects of BAT physiology, such as its magnetic properties, its heat-generating capacity, and high perfusion rates during metabolic activity, methods such as magnetic resonance imaging (159), infrared thermography (89), and near-infrared spectroscopy (102) have been tested for assessing human BAT, respectively. However, these methods come with their own limitations covered in detail by van der Lans et al. (155). Efforts are needed to find suitable techniques that can overcome these limitations while being available for use by the broader research community.
The variability observed in multiple human studies is often, and correctly so, attributed to the length and extent of cold exposure, as well as the methodology and criteria used to interpret 18F-FDG signals. Since 18F-FDG measures glucose uptake and fatty acids are most likely the main energy source, it is difficult to actually translate the 18F-FDG-PET/CT data toward tissue-specific energy expenditure. Second, apart from technical issues related to volume estimation by PET/CT, the brown fat tissue in humans generally is very heterogeneous, being a mixture of white and brown (or beige) adipocytes, making interpretation of volume data difficult.
CONCLUSIONS AND OUTSTANDING QUESTIONS
Recent advances made using imaging studies have clearly established the presence of BAT in humans and have brought its thermogenic potential to the fore once again. Its immense thermogenic potential, coupled with the discovery of brite adipocytes, which happen to be located in white fat depots in rodents, has truly rejuvenated the adipose tissue field, where we now seek to understand the mechanisms involved in their development, proliferation, and thermogenic activity. Whereas brite adipocytes retain their thermogenic potential much like the classic brown adipocytes, an enhanced understanding of their origin is necessary to ascertain whether we focus on converting white adipocytes into brown adipocytes or look for bona fide brite precursors and/or white-looking brite adipocytes. Rodent studies have further demonstrated a clear potential for BAT and beige thermogenesis in ameliorating obesity, diabetes, and atherosclerosis (11, 31, 76). Multiple human studies discussed above have also pointed in the same direction, when we consider the PET-detectable supraclavicular and cervical fat to be the main BAT depot of the body. Once again, whether we focus on recruiting and stimulating this BAT/beige depot or attempt to “brown” additional fat depots, we need to extend our understanding of the local, peripheral, and central control of their thermogenic activity in humans.
Our ability to recruit BAT (14, 65), coupled with observations of preserved BAT oxidative potential, in obese and diabetic individuals seem to be steps in the right direction (12). Reduced blood flow and insulin or catecholamine resistance are proposed to be the plausible factors behind the observations of reduced BAT presence and activity in obese and diabetic individuals (12). Ethnic differences have also been observed in BAT volume and basal resting energy expenditure among humans (3). Focusing on the underlying mechanisms and ways to overcome these limitations may allow us to utilize the full potential of NST in improving the metabolic homeostasis in these populations. Cold exposure has been consistently shown to be effective in improving insulin sensitivity in diabetic individuals (47, 64). It is also known to be the most effective activator of BAT thermogenesis in humans to date (156). A significant role for muscle shivering besides BAT thermogenesis in the beneficial outcomes associated with cold exposure has been implied recently (13, 64). However, most of these studies employ short-term cold exposure protocols. More studies are needed to assess whether long-term cold exposure could shift the relative contribution of BAT and NST in the beneficial outcomes associated with cold exposure toward the heavy side. Indeed, long-term studies are also needed to assess whether activation of BAT thermogenesis is accompanied by an upregulation of compensatory mechanisms to offset enhanced energy expenditure to ascertain the sustainability of BAT-based approaches.
A case has also been made for targeting the browning of visceral fat depots specifically to improve their metabolic function using pharmacological means in humans that may or may not involve enhanced energy expenditure (57). Considering that visceral fat accumulation, cellular hypertrophy, and inflammation are strongly associated with cardiometabolic disease burden (151), enhanced understanding of whether browning can reverse any of these features warrants further investigation in humans. Recent demonstrations that BAT and beige fat secrete factors that are capable of communicating with peripheral organs further point toward their ability to influence whole body metabolic homeostasis (163). An important question of whether their secretome alters during the “active” or “inactive” state or exhibits depot specificity in humans needs to be determined. In conclusion, although it is clear that much work is needed before safe BAT- or beige-based approaches can become a therapeutic reality, thermogenic abilities of BAT and “browned WAT” clearly represent novel and worthy tools to be employed in our arsenal against obesity and metabolic syndrome in humans.
HIGHLIGHTS
Rodent studies point toward the tremendous thermogenic potential of brown fat. Its oxidative capacity is detectable as well as recruitable in most adult humans, including obese and diabetic individuals.
Two types of brown fat are recognizable in humans and rodents, classic brown and beige, which differ with respect to their cellular origins, molecular markers, anatomic location, and extent of inducibility, yet retain similar thermogenic potential.
BAT and beige fat activity is primarily regulated by SNS; however, an increasing number of circulating factors are also being recognized that can influence BAT and beige recruitment and activity, particularly in rodents.
Targeting BAT and/or beige could allow us to improve whole body energy expenditure and/or metabolic homeostasis in humans, although their quantitative contribution still remains to be established.
GRANTS
K. Chechi is the recipient of postdoctoral fellowships from The Fonds de Recherche du Quebec-Santé (2011–2013) and Canadian Institutes of Health Research–Banting Postdoctoral fellowship program (2013–2015). We acknowledge the support of Natural Sciences and Engineering Research Council Discovery Grant RGPIN-2014-06721 to D. Richard and the support of the Dutch Heart Foundation (CVON2014-02 ENERGISE) to W. van Marken Lichtenbelt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.C. drafted manuscript; K.C., W.D.v.M.L., and D.R. approved final version of manuscript; W.D.v.M.L. and D.R. edited and revised manuscript.
REFERENCES
- 1.Ahmadian M, Abbott MJ, Tang T, Hudak CSS, Kim Y, Bruss M, Hellerstein MK, Lee H-Y, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 13: 739–748, 2011. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachman ES, Dhillon H, Zhang C-Y, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 3.Bakker LEH, Boon MR, van der Linden RAD, Arias-Bouda LP, van Klinken JB, Smit F, Verberne HJ, Jukema JW, Tamsma JT, Havekes LM, van Marken Lichtenbelt WD, Jazet IM, Rensen PCN. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol 2: 210–217, 2014. doi: 10.1016/S2213-8587(13)70156-6. [DOI] [PubMed] [Google Scholar]
- 4.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 275: R291–R299, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PLSM, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 7.Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 35: 473–493, 2014. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartness TJ, Ryu V. Neural control of white, beige and brown adipocytes. Int J Obes Suppl 5, Suppl 1: S35–S39, 2015. doi: 10.1038/ijosup.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318: 34–43, 2010. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes 34, Suppl 1: S36–S42, 2010. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berbée JFP, Boon MR, Khedoe PPSJ, Bartelt A, Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C, Jung C, Vazirpanah N, Brouwers LPJ, Gordts PLSM, Esko JD, Hiemstra PS, Havekes LM, Scheja L, Heeren J, Rensen PCN. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6: 6356, 2015. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blondin DP, Labbé SM, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Haman F, Richard D, Carpentier AC. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 64: 2388–2397, 2015. doi: 10.2337/db14-1651. [DOI] [PubMed] [Google Scholar]
- 13.Blondin DP, Labbé SM, Phoenix S, Guérin B, Turcotte EE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol 593: 701–714, 2015. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blondin DP, Labbé SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Carpentier AC, Richard D, Haman F. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab 99: E438–E446, 2014. doi: 10.1210/jc.2013-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blondin DP, Labbé SM, Turcotte EE, Haman F, Richard D, Carpentier AC. A critical appraisal of brown adipose tissue metabolism in humans. Clin Lipidol 10: 259–280, 2015. doi: 10.2217/clp.15.14. [DOI] [Google Scholar]
- 16.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braga M, Reddy ST, Vergnes L, Pervin S, Grijalva V, Stout D, David J, Li X, Tomasian V, Reid CB, Norris KC, Devaskar SU, Reue K, Singh R. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res 55: 375–384, 2014. doi: 10.1194/jlr.M039719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredella MA, Fazeli PK, Lecka-Czernik B, Rosen CJ, Klibanski A. IGFBP-2 is a negative predictor of cold-induced brown fat and bone mineral density in young non-obese women. Bone 53: 336–339, 2013. doi: 10.1016/j.bone.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–5347, 2007. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 20.Broeders EPM, Nascimento EBM, Havekes B, Brans B, Roumans KHM, Tailleux A, Schaart G, Kouach M, Charton J, Deprez B, Bouvy ND, Mottaghy F, Staels B, van Marken Lichtenbelt WD, Schrauwen P. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 22: 418–426, 2015. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Broeders EPM, Vijgen GHEJ, Havekes B, Bouvy ND, Mottaghy FM, Kars M, Schaper NC, Schrauwen P, Brans B, van Marken Lichtenbelt WD. Thyroid hormone activates brown adipose tissue and increases non-shivering thermogenesis—a cohort study in a group of thyroid carcinoma patients. PLoS One 11: e0145049, 2016. doi: 10.1371/journal.pone.0145049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronnikov G, Bengtsson T, Kramarova L, Golozoubova V, Cannon B, Nedergaard J. beta1 to beta3 switch in control of cyclic adenosine monophosphate during brown adipocyte development explains distinct beta-adrenoceptor subtype mediation of proliferation and differentiation. Endocrinology 140: 4185–4197, 1999. doi: 10.1210/endo.140.9.6972. [DOI] [PubMed] [Google Scholar]
- 23.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 24.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214: 242–253, 2011. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 25.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24: 3057–3067, 2004. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao W-H, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 299: R277–R290, 2010. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F, Casteilla L. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63: 3253–3265, 2014. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- 28.Cereijo R, Villarroya J, Villarroya F. Non-sympathetic control of brown adipose tissue. Int J Obes Suppl 5, Suppl 1: S40–S44, 2015. doi: 10.1038/ijosup.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao P-T, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab 13: 573–583, 2011. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chechi K, Blanchard P-G, Mathieu P, Deshaies Y, Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol 167: 2264–2270, 2013. doi: 10.1016/j.ijcard.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol Metab 24: 408–420, 2013. doi: 10.1016/j.tem.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA, Enerbäck S, Kinahan PE, Lichtenbelt W, Lin FI, Sunderland JJ, Virtanen KA, Wahl RL. Brown adipose reporting criteria in imaging studies (BARCIST 1.0): Recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab 24: 210–222, 2016. doi: 10.1016/j.cmet.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbé SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chondronikola M, Volpi E, Børsheim E, Porter C, Saraf MK, Annamalai P, Yfanti C, Chao T, Wong D, Shinoda K, Labbe SM, Hurren NM, Cesani F, Kajimura S, Sidossis LS. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 23: 1200–1206, 2016. doi: 10.1016/j.cmet.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chondronikola M, Volpi E, Børsheim E, Chao T, Porter C, Annamalai P, Yfanti C, Labbe SM, Hurren NM, Malagaris I, Cesani F, Sidossis LS. Brown adipose tissue is linked to a distinct thermoregulatory response to mild cold in people. Front Physiol 7: 129, 2016. doi: 10.3389/fphys.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christoffolete MA, Linardi CCG, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, Bianco AC. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 53: 577–584, 2004. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- 37.Cinti S. Reversible physiological transdifferentiation in the adipose organ. Proc Nutr Soc 68: 340–349, 2009. doi: 10.1017/S0029665109990140. [DOI] [PubMed] [Google Scholar]
- 38.Cinti S. The adipose organ at a glance. Dis Model Mech 5: 588–594, 2012. doi: 10.1242/dmm.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cinti S. UCP1 protein: the molecular hub of adipose organ plasticity. Biochimie 134: 71-76, 2017. doi: 10.1016/j.biochi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 44: 1267–1270, 2003. [PubMed] [Google Scholar]
- 41.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 44: 170–176, 2003. [PubMed] [Google Scholar]
- 42.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JPG, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156: 304–316, 2014. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 103: 931–942, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21: 33–38, 2015. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, Chacko AT, Deschamps LN, Herder LM, Truchan N, Glasgow AL, Holman AR, Gavrila A, Hasselgren P-O, Mori MA, Molla M, Tseng Y-H. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19: 635–639, 2013. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daanen HAM, Van Marken Lichtenbelt WD. Human whole body cold adaptation. Temperature (Austin) 3: 104–118, 2016. doi: 10.1080/23328940.2015.1135688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enerbäck S. Human brown adipose tissue. Cell Metab 11: 248–252, 2010. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 21: 159–165, 2015. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151: 400–413, 2012. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. Biochim Biophys Acta 1831: 986–1003, 2013. doi: 10.1016/j.bbalip.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Foster DO. Quantitative contribution of brown adipose tissue thermogenesis to overall metabolism. Can J Biochem Cell Biol 62: 618–622, 1984. doi: 10.1139/o84-082. [DOI] [PubMed] [Google Scholar]
- 54.Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, Guerrieri M, Cinti S. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta 1831: 950–959, 2013. doi: 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 55.García-Alonso V, Clària J. Prostaglandin E2 signals white-to-brown adipogenic differentiation. Adipocyte 3: 290–296, 2014. doi: 10.4161/adip.29993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab 5: 626–634, 2016. doi: 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov 15: 405–424, 2016. doi: 10.1038/nrd.2016.31. [DOI] [PubMed] [Google Scholar]
- 58.Giordano A, Tonello C, Bulbarelli A, Cozzi V, Cinti S, Carruba MO, Nisoli E. Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett 514: 135–140, 2002. doi: 10.1016/S0014-5793(02)02245-7. [DOI] [PubMed] [Google Scholar]
- 59.Gnad T, Scheibler S, von Kügelgen I, Scheele C, Kilić A, Glöde A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, El-Tayeb A, Kranz M, Deuther-Conrad W, Brust P, Lidell ME, Betz MJ, Enerbäck S, Schrader J, Yegutkin GG, Müller CE, Pfeifer A. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516: 395–399, 2014. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 60.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of β3-adrenergic receptor activation. Am J Physiol Endocrinol Metab 289: E608–E616, 2005. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 61.Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest 102: 1724–1731, 1998. doi: 10.1172/JCI4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 102: 412–420, 1998. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312: 734–737, 2006. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 64.Hanssen MJW, Hoeks J, Brans B, van der Lans AAJJ, Schaart G, van den Driessche JJ, Jörgensen JA, Boekschoten MV, Hesselink MKC, Havekes B, Kersten S, Mottaghy FM, van Marken Lichtenbelt WD, Schrauwen P. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21: 863–865, 2015. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 65.Hanssen MJW, van der Lans AAJJ, Brans B, Hoeks J, Jardon KMC, Schaart G, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 65: 1179–1189, 2016. doi: 10.2337/db15-1372. [DOI] [PubMed] [Google Scholar]
- 66.Hanssen MJW, Wierts R, Hoeks J, Gemmink A, Brans B, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Glucose uptake in human brown adipose tissue is impaired upon fasting-induced insulin resistance. Diabetologia 58: 586–595, 2015. doi: 10.1007/s00125-014-3465-8. [DOI] [PubMed] [Google Scholar]
- 67.Heaton JM. The distribution of brown adipose tissue in the human. J Anat 112: 35–39, 1972. [PMC free article] [PubMed] [Google Scholar]
- 68.Hepler C, Gupta RK. The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment. Mol Cell Endocrinol 445: 95-108, 2017. doi: 10.1016/j.mce.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Hoeke G, Kooijman S, Boon MR, Rensen PCN, Berbée JFP. Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ Res 118: 173–182, 2016. doi: 10.1161/CIRCRESAHA.115.306647. [DOI] [PubMed] [Google Scholar]
- 71.Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol 46: 339–345, 1981. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- 72.Irshad Z, Dimitri F, Christian M, Zammit VA. Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J Lipid Res 58: 15–30, 2017. doi: 10.1194/jlr.M068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irving BA, Still CD, Argyropoulos G. Does IRISIN have a BRITE future as a therapeutic agent in humans? Curr Obes Rep 3: 235–241, 2014. doi: 10.1007/s13679-014-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, Pedersen BK, Møller K, Scheele C. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 17: 798–805, 2013. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Jimenez M, Barbatelli G, Allevi R, Cinti S, Seydoux J, Giacobino J-P, Muzzin P, Preitner F. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur J Biochem 270: 699–705, 2003. doi: 10.1046/j.1432-1033.2003.03422.x. [DOI] [PubMed] [Google Scholar]
- 76.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22: 546–559, 2015. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi SP, Spiegelman BM. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163: 643–655, 2015. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khedoe PPSJ, Hoeke G, Kooijman S, Dijk W, Buijs JT, Kersten S, Havekes LM, Hiemstra PS, Berbée JFP, Boon MR, Rensen PCN. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J Lipid Res 56: 51–59, 2015. doi: 10.1194/jlr.M052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kooijman S, Wang Y, Parlevliet ET, Boon MR, Edelschaap D, Snaterse G, Pijl H, Romijn JA, Rensen PCN. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 58: 2637–2646, 2015. doi: 10.1007/s00125-015-3727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krott LM, Piscitelli F, Heine M, Borrino S, Scheja L, Silvestri C, Heeren J, Di Marzo V. Endocannabinoid regulation in white and brown adipose tissue following thermogenic activation. J Lipid Res 57: 464–473, 2016. doi: 10.1194/jlr.M065227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Labbé SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, Richard D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29: 2046–2058, 2015. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- 82.Labbé SM, Caron A, Chechi K, Laplante M, Lecomte R, Richard D. Metabolic activity of brown, “beige,” and white adipose tissues in response to chronic adrenergic stimulation in male mice. Am J Physiol Endocrinol Metab 311: E260–E268, 2016. doi: 10.1152/ajpendo.00545.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labbé SM, Caron A, Lanfray D, Monge-Rofarello B, Bartness TJ, Richard D. Hypothalamic control of brown adipose tissue thermogenesis. Front Syst Neurosci 9: 150, 2015. doi: 10.3389/fnsys.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res 34: 1057–1091, 1993. [PubMed] [Google Scholar]
- 85.Lafontan M. Advances in adipose tissue metabolism. Int J Obes 32, Suppl 7: S39–S51, 2008. doi: 10.1038/ijo.2008.237. [DOI] [PubMed] [Google Scholar]
- 86.Lahesmaa M, Orava J, Schalin-Jäntti C, Soinio M, Hannukainen JC, Noponen T, Kirjavainen A, Iida H, Kudomi N, Enerbäck S, Virtanen KA, Nuutila P. Hyperthyroidism increases brown fat metabolism in humans. J Clin Endocrinol Metab 99: E28–E35, 2014. doi: 10.1210/jc.2013-2312. [DOI] [PubMed] [Google Scholar]
- 87.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 50: 14–27, 2011. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lean MEJ. Brown adipose tissue in humans. Proc Nutr Soc 48: 243–256, 1989. doi: 10.1079/PNS19890036. [DOI] [PubMed] [Google Scholar]
- 89.Lee P, Ho KKY, Greenfield JR. Hot fat in a cool man: infrared thermography and brown adipose tissue. Diabetes Obes Metab 13: 92–93, 2011. [DOI] [PubMed] [Google Scholar]
- 90.Lee Y-H, Mottillo EP, Granneman JG. Adipose tissue plasticity from WAT to BAT and in between. Biochim Biophys Acta 1842: 358–369, 2014. doi: 10.1016/j.bbadis.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee Y-H, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 29: 286–299, 2015. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lepper C, Fan C-M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48: 424–436, 2010. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, Virtanen KA, Beuschlein F, Persson A, Borga M, Enerbäck S. Evidence for two types of brown adipose tissue in humans. Nat Med 19: 631–634, 2013. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 94.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ Jr, Rosen ED, Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab 19: 810–820, 2014. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.López M, Varela L, Vázquez MJ, Rodríguez-Cuenca S, González CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martínez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Diéguez C, Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 16: 1001–1008, 2010. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Madden CJ, Tupone D, Cano G, Morrison SF. α2 Adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci 33: 2017–2028, 2013. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122: 1022–1036, 2012. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, Noh H-L, Kim JK, Cooper MP, Fitzgibbons T, Brehm MA, Corvera S. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22: 312–318, 2016. doi: 10.1038/nm.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Modica S, Straub LG, Balaz M, Sun W, Varga L, Stefanicka P, Profant M, Simon E, Neubauer H, Ukropcova B, Ukropec J, Wolfrum C. Bmp4 promotes a brown to white-like adipocyte shift. Cell Reports 16: 2243–2258, 2016. doi: 10.1016/j.celrep.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 100.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19: 741–756, 2014. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat 214: 171–178, 2009. doi: 10.1111/j.1469-7580.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 54: 523–531, 2013. doi: 10.2967/jnumed.112.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Müller S, Balaz M, Stefanicka P, Varga L, Amri E-Z, Ukropec J, Wollscheid B, Wolfrum C. Proteomic analysis of human brown adipose tissue reveals utilization of coupled and uncoupled energy expenditure pathways. Sci Rep 6: 30030, 2016. doi: 10.1038/srep30030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 105.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab 13: 238–240, 2011. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 106.Nedergaard J, Cannon B. How brown is brown fat? It depends where you look. Nat Med 19: 540–541, 2013. doi: 10.1038/nm.3187. [DOI] [PubMed] [Google Scholar]
- 107.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504: 82–106, 2001. doi: 10.1016/S0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 108.Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, Aguirre V, Gupta RK, Clegg DJ. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab 4: 427–436, 2015. doi: 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Néchad M, Ruka E, Thibault J. Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp Biochem Physiol Comp Physiol 107: 381–388, 1994. doi: 10.1016/0300-9629(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 110.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15: 395–404, 2012. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Okla M, Ha JH, Temel RE, Chung S. BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids 50: 111–120, 2015. doi: 10.1007/s11745-014-3981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279, 2011. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 113.Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122: 545–552, 2012. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96: 192–199, 2011. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 115.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 32: 351–357, 2007. doi: 10.1097/01.rlu.0000259570.69163.04. [DOI] [PubMed] [Google Scholar]
- 116.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20: 433–447, 2014. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 118.Porter C, Chondronikola M, Sidossis LS. The therapeutic potential of brown adipocytes in humans. Front Endocrinol (Lausanne) 6: 156, 2015. doi: 10.3389/fendo.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qian S-W, Tang Y, Li X, Liu Y, Zhang Y-Y, Huang H-Y, Xue R-D, Yu H-Y, Guo L, Gao H-D, Liu Y, Sun X, Li Y-M, Jia W-P, Tang Q-Q. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA 110: E798–E807, 2013. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rabelo R, Schifman A, Rubio A, Sheng X, Silva JE. Delineation of thyroid hormone-responsive sequences within a critical enhancer in the rat uncoupling protein gene. Endocrinology 136: 1003–1013, 1995. doi: 10.1210/endo.136.3.7867554. [DOI] [PubMed] [Google Scholar]
- 121.Ramseyer VD, Granneman JG. Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 5: 119–129, 2016. doi: 10.1080/21623945.2016.1145846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157: 1279–1291, 2014. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Richard D, Picard F. Brown fat biology and thermogenesis. Front Biosci (Landmark Ed) 16: 1233–1260, 2011. doi: 10.2741/3786. [DOI] [PubMed] [Google Scholar]
- 124.Richard D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat Rev Endocrinol 11: 489–501, 2015. doi: 10.1038/nrendo.2015.103. [DOI] [PubMed] [Google Scholar]
- 125.Ricquier D. Neonatal brown adipose tissue, UCP1 and the novel uncoupling proteins. Biochem Soc Trans 26: 120–123, 1998. doi: 10.1042/bst0260120. [DOI] [PubMed] [Google Scholar]
- 126.Roberts LD, Ashmore T, Kotwica AO, Murfitt SA, Fernandez BO, Feelisch M, Murray AJ, Griffin JL. Inorganic nitrate promotes the browning of white adipose tissue through the nitrate-nitrite-nitric oxide pathway. Diabetes 64: 471–484, 2015. doi: 10.2337/db14-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee Y-K, Palma MJ, Calhoun S, Georgiadi A, Chen M-H, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19: 96–108, 2014. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosell M, Hondares E, Iwamoto S, Gonzalez FJ, Wabitsch M, Staels B, Olmos Y, Monsalve M, Giralt M, Iglesias R, Villarroya F. Peroxisome proliferator-activated receptors-α and -γ, and cAMP-mediated pathways, control retinol-binding protein-4 gene expression in brown adipose tissue. Endocrinology 153: 1162–1173, 2012. doi: 10.1210/en.2011-1367. [DOI] [PubMed] [Google Scholar]
- 129.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15: 659–667, 2013. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 130.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 131.Ruohonen ST, Savontaus E, Norlund W, Ailanen L, Röyttä M, Baur N, Joos T, Scheinin M. Increased energy expenditure, white adipose tissue browning, and resistance to central obesity and type 2 diabetes in mice lacking alpha2-adrenoceptors. FASEB J 30: 1293.7–1293.7, 2016. [Google Scholar]
- 132.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol 306: R886–R900, 2014. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ryu V, Garretson JT, Liu Y, Vaughan CH, Bartness TJ. Brown adipose tissue has sympathetic-sensory feedback circuits. J Neurosci 35: 2181–2190, 2015. doi: 10.1523/JNEUROSCI.3306-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schiffelers SLH, Blaak EE, Saris WHM, van Baak MA. In vivo β3-adrenergic stimulation of human thermogenesis and lipid use. Clin Pharmacol Ther 67: 558–566, 2000. doi: 10.1067/mcp.2000.106794. [DOI] [PubMed] [Google Scholar]
- 136.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng Y-H. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495: 379–383, 2013. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967, 2008. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shabalina IG, Petrovic N, de Jong JMA, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports 5: 1196–1203, 2013. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 139.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J 27: 1981–1989, 2013. doi: 10.1096/fj.12-225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7: e49452, 2012. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 3: 3, 2012. doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shinoda K, Luijten IHN, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng Y-H, Nedergaard J, Sidossis LS, Kajimura S. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 21: 389–394, 2015. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125: 478–486, 2015. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sidossis LS, Porter C, Saraf MK, Børsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 22: 219–227, 2015. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soloveva V, Graves RA, Rasenick MM, Spiegelman BM, Ross SR. Transgenic mice overexpressing the β 1-adrenergic receptor in adipose tissue are resistant to obesity. Mol Endocrinol 11: 27–38, 1997. doi: 10.1210/mend.11.1.9870. [DOI] [PubMed] [Google Scholar]
- 146.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R501–R511, 2009. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Souza SC, Christoffolete MA, Ribeiro MO, Miyoshi H, Strissel KJ, Stancheva ZS, Rogers NH, D’Eon TM, Perfield JW II, Imachi H, Obin MS, Bianco AC, Greenberg AS. Perilipin regulates the thermogenic actions of norepinephrine in brown adipose tissue. J Lipid Res 48: 1273–1279, 2007. doi: 10.1194/jlr.M700047-JLR200. [DOI] [PubMed] [Google Scholar]
- 148.Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 34: 1018–1022, 2007. doi: 10.1007/s00259-006-0318-9. [DOI] [PubMed] [Google Scholar]
- 149.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng Y-H, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sung H-K, Doh K-O, Son JE, Park JG, Bae Y, Choi S, Nelson SML, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 17: 61–72, 2013. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 151.Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev 93: 359–404, 2013. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 152.Tseng Y-H, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454: 1000–1004, 2008. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem 281: 31894–31908, 2006. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
- 154.van der Lans AAJJ, Hoeks J, Brans B, Vijgen GHEJ, Visser MGW, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 123: 3395–3403, 2013. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van der Lans AAJJ, Wierts R, Vosselman MJ, Schrauwen P, Brans B, van Marken Lichtenbelt WD. Cold-activated brown adipose tissue in human adults: methodological issues. Am J Physiol Regul Integr Comp Physiol 307: R103–R113, 2014. doi: 10.1152/ajpregu.00021.2014. [DOI] [PubMed] [Google Scholar]
- 156.Lichtenbelt W, Kingma B, van der Lans A, Schellen L. Cold exposure–an approach to increasing energy expenditure in humans. Trends Endocrinol Metab 25: 165–167, 2014. doi: 10.1016/j.tem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 157.van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol 301: R285–R296, 2011. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- 158.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 159.van Rooijen BD, van der Lans AAJJ, Brans B, Wildberger JE, Mottaghy FM, Schrauwen P, Backes WH, van Marken Lichtenbelt WD. Imaging cold-activated brown adipose tissue using dynamic T2*-weighted magnetic resonance imaging and 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography. Invest Radiol 48: 708–714, 2013. doi: 10.1097/RLI.0b013e31829363b8. [DOI] [PubMed] [Google Scholar]
- 160.Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol 302: R1049–R1058, 2012. doi: 10.1152/ajpregu.00640.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nüsing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328: 1158–1161, 2010. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 162.Vijgen GHEJ, Bouvy ND, Teule GJJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One 6: e17247, 2011. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13: 26–35, 2017. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 164.Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab 17: 638–643, 2013. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 165.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto N-J, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 166.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res 53: 619–629, 2012. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EBM, van der Lans AAJJ, Broeders EPM, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes 39: 1696–1702, 2015. doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- 168.Vosselman MJ, van der Lans AAJJ, Brans B, Wierts R, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD. Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes 61: 3106–3113, 2012. doi: 10.2337/db12-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 302: E19–E31, 2012. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 170.Wang G-X, Zhao X-Y, Meng Z-X, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Blüher M, Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 20: 1436–1443, 2014. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19: 1338–1344, 2013. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, Morgan D, Csikasz RI, Gallego R, Rodríguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, López M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149: 871–885, 2012. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Whittle AJ, Jiang M, Peirce V, Relat J, Virtue S, Ebinuma H, Fukamachi I, Yamaguchi T, Takahashi M, Murano T, Tatsuno I, Takeuchi M, Nakaseko C, Jin W, Jin Z, Campbell M, Schneider WJ, Vidal-Puig A, Bujo H. Soluble LR11/SorLA represses thermogenesis in adipose tissue and correlates with BMI in humans. Nat Commun 6: 8951, 2015. doi: 10.1038/ncomms9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg 36: 583–596, 2009. doi: 10.1016/j.cps.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 48: 41–51, 2007. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 177.Yi P, Park J-S, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell 153: 747–758, 2013. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123: 3404–3408, 2013. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 19: 1755–1760, 2011. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 180.Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 167: 10–14, 1984. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 181.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23: 3113–3120, 2009. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]