Abstract
Nitric oxide (NO)-mediated vasodilation contributes to the rapid rise in muscle blood flow at exercise onset. This occurs via increased cyclic guanosine monophosphate (cGMP), which is catabolized by phosphodiesterase-5 (PDE-5). Whether PDE-5 limits exercise vasodilation onset kinetics is unknown. We hypothesized the time course of exercise vasodilation would be 1) accelerated during PDE-5 inhibition (sildenafil citrate, SDF) and 2) decelerated during NO synthase inhibition (NG-monomethyl-l-arginine, l-NMMA), and 3) the effect of SDF on vasodilation onset kinetics would be attenuated with concurrent l-NMMA. Data from 29 healthy adults were analyzed. Individuals completed 5 min of moderate-intensity forearm exercise under control conditions and during 1) oral SDF (n = 8), 2) intra-arterial l-NMMA (n = 15), or 3) combined SDF + l-NMMA (n = 6). Forearm blood flow (FBF; Doppler ultrasound of the brachial artery) and mean brachial artery blood pressure (MAP) were measured continuously. Forearm vascular conductance (FVC, FBF ÷ MAP) was curve-fit with a monoexponential model, and vasodilation onset kinetics were assessed by mean response time (MRT, time to achieve 63% of steady state). SDF had no effect on MRT (P = 0.90). NOS inhibition increased MRT (P = 0.01). MRT during SDF+l-NMMA was not different from control exercise (P = 0.76). PDE-5 inhibition alone has no effect on rapid-onset vasodilation. Whereas NOS inhibition decelerates vasodilator kinetics, when combined with SDF, vasodilator kinetics do not differ from control. These data suggest NO-independent activation of cGMP occurs at exercise onset; thus PDE-5 inhibition may improve vasodilation in pathologies where NO bioavailability is impaired.
NEW & NOTEWORTHY We show that when NO bioavailability is reduced, PDE-5 inhibition can restore vasodilation onset kinetics of exercise-mediated vasodilation via NO-independent cGMP pathways. These data suggest PDE-5 inhibition may improve exercise vasodilation onset kinetics in pathologies where NO bioavailability is impaired.
Keywords: cyclic GMP, exercise onset, nitric oxide, sildenafil
INTRODUCTION
The ability to initiate and sustain any given exercise bout is fundamentally affected by the ability of the cardiovascular system to deliver blood, and thus oxygen, to meet the metabolic demand of the active skeletal muscles (27). At the onset of exercise, vasodilation occurs rapidly and increases proportionally to exercise intensity to match blood flow to metabolic demand (36). There are many vasoregulatory mechanisms that contribute to exercise vasodilator onset kinetics (7, 9, 25), and recently, nitric oxide (NO) has been shown to contribute 30–50% of vasodilator onset kinetics to dynamic forearm or leg exercise in healthy humans (5, 6). Along these lines, healthy older adults display slowed kinetics, due to reduced NO signaling (3). NO-mediated vasodilation occurs via increased cyclic guanosine monophosphate (cGMP), which is catabolized by the enzyme phosphodiesterase-5 (PDE-5). Therefore, slowed vasodilation onset kinetics may result from reduced NO signaling, increased cGMP catabolism, or both. Evidence suggests alterations in cGMP metabolism are involved in a number of cardiovascular disease states (10).
Inhibition of PDE-5 (using oral PDE-5 inhibitor sildenafil, SDF) increases blood flow in resting skeletal muscle of healthy and hypertensive individuals (30). SDF has also been shown to improve exercise tolerance (17, 38). In contrast, our group recently showed that SDF does not increase steady-state exercise blood flow in healthy humans (30). The lack of an improvement may be due to the modest role of NO in steady-state vasodilation (~15%) (19). Given NO plays a more important role in the onset kinetics of the exercise vasodilator response (3, 5), we speculate SDF may potentiate the NO-cGMP pathway, accelerate vasodilation onset kinetics, and thus provide new mechanistic insight into observations of improved exercise tolerance with SDF (3, 5, 17, 38). We hypothesized vasodilation onset kinetics (quantified using mean response time, MRT) would be 1) decreased during PDE-5 inhibition (sildenafil citrate, SDF) and 2) increased during NO synthase inhibition (NG-monomethyl-l-arginine, l-NMMA). We further hypothesized any effect of SDF on rapid-onset vasodilation would be attenuated with concurrent l-NMMA, highlighting the importance of the NO-cGMP pathway in vasodilation onset kinetics.
MATERIALS AND METHODS
Approval.
All procedures were approved by the Institutional Review Boards at the Mayo Clinic and University of Wisconsin-Madison, all subjects provided informed, written consent before participation, and all procedures were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Subjects.
Subjects were nonsmokers, were free from pulmonary, cardiovascular, endocrine, or neurological diseases, and were not taking any medications with known cardiovascular effects. Subjects refrained from exercise, alcohol, and caffeine for 24 h before the study. All women were studied during the early follicular phase of their menstrual cycle.
Instrumentation.
Subjects were supine, and a 20-gauge, 5-cm catheter was placed in the brachial artery of the nondominant arm under aseptic conditions after local anesthesia. A three-port connector was placed in series with a pressure transducer to allow simultaneous administration of study drugs (l-NMMA) and continuous arterial blood pressure monitoring. Brachial artery blood velocity was measured using Doppler ultrasound (Vivid 7 Ultrasound, GE Healthcare, Chalfont St. Giles, UK) (20). All data were recorded continuously on a computer and analyzed offline (PowerLab Chart5, ADInstruments, Colorado Springs, CO). Brachial artery diameter was measured digitally off-line from two-dimensional, longitudinal images. Forearm volume was assessed by water displacement in protocols 1 and 3 and by dual-energy X-ray absorptiometry (DEXA) in protocol 2. Forearm blood flow was calculated as the product of blood velocity and vessel cross-sectional area and is expressed relative to forearm size (ml·dl−1·min−1). To account for changes in blood pressure and to assess vasodilation, forearm vascular conductance (FVC) was calculated (ml·dl−1·min−1·100 mmHg−1).
For each protocol, measurements were made at baseline (rest) and during forearm exercise. After a quiet resting period (10 min), 5 min of submaximal, dynamic handgrip exercise was conducted, where subjects rhythmically squeezed a handgrip ergometer (20 contractions/min) using a load that was 15% of their maximal voluntary contraction (MVC). Subjects were enrolled in three separate protocols, which included a control exercise condition, followed by an experimental condition with addition of 1) oral sildenafil citrate (n = 10), 2) intra-arterial l-NMMA (n = 20), or 3) oral SDF and intra-arterial l-NMMA (n = 10). All subjects’ steady-state exercise data were published previously (30); however, not all subjects were included in this investigation (see Data analysis).
Sildenafil citrate (protocols 1 and 3).
After completion of control exercise, sildenafil citrate (100 mg) was administered orally with a sip of water (13, 24). One hour was allowed for sildenafil citrate to reach peak concentrations and have maximal effects on forearm blood flow (37). Increased vasodilator response to the NO donor, sodium nitroprusside, confirmed effective PDE-5 inhibition (30). Absolute doses of SDF were given as it is commercially available in 100-mg pills and to mimic doses given in other studies examining the effect of sildenafil citrate on hemodynamics (13, 24).
NG-monomethyl-l- arginine (l-NMMA; protocols 2 and 3).
An absolute loading dose (50 mg) of l-NMMA was administered intra-arterially in the instrumented forearm over 5–10 min to locally inhibit NOS. In protocol 2, the loading dose began after 5 min of control handgrip exercise. In protocol 3, the loading dose was administered 1 h after SDF administration. In both protocols, after the completion of the loading dose, an absolute maintenance infusion of l-NMMA began (1 mg/min) and continued for the remainder of the study to match previous studies (11, 12).
Data analysis.
Continuous blood velocity and resting brachial artery diameter measurements were used to calculate blood flow and vascular conductance. During exercise, forearm blood flow and vascular conductance were calculated and averaged over 3 s (each duty cycle; 1-s contraction and 2-s relaxation). These data were then plotted as the absolute change from baseline (ΔFVC = FVCDuty cycle – FVCBaseline) for analysis. The reasons for analyzing the data as a change from baseline are twofold. 1) A stable baseline (equal to 0) controls for natural fluctuation due to HR and respiration and results in a stronger fit (smaller magnitude and equal distribution of residuals). 2) The use of the SDF and l-NMMA alters baseline variables (see Table 2), and thus a change from baseline allows for better comparison between groups (21).
Table 2.
Hemodynamics at rest and during steady state (last 30 s) of exercise
| Control | Drug | P Value | |
|---|---|---|---|
| Baseline forearm vascular conductance, ml·dl−1·min−1·100 mmHg−1 | |||
| SDF | 9.0 ± 2.3 | 13.5 ± 3.1 | 0.005 |
| l-NMMA | 6.5 ± 0.6 | 5.1 ± 0.6 | 0.022 |
| SDF + l-NMMA | 10.6 ± 2.2 | 6.2 ± 1.0 | 0.082 |
| Steady-state forearm vascular conductance, ml·dl−1·min−1·100 mmHg−1 | |||
| SDF | 29.1 ± 2.5 | 34.5 ± 4.8 | 0.008 |
| l-NMMA | 22.6 ± 1.9 | 18.9 ± 1.1 | 0.015 |
| SDF + l-NMMA | 33.7 ± 5.8 | 28.9 ± 6.0 | 0.467 |
| Δ Forearm vascular conductance, ml·dl−1·min−1·100 mmHg−1 | |||
| SDF | 20.1 ± 2.6 | 21.1 ± 3.1 | 0.461 |
| l-NMMA | 16.1 ± 1.9 | 13.8 ± 1.2 | 0.049 |
| SDF + l-NMMA | 23.1 ± 4.1 | 22.7 ± 5.6 | 0.936 |
| Baseline forearm blood flow, ml·dl−1·min−1 | |||
| SDF | 7.8 ± 1.9 | 11.3 ± 2.2 | 0.003 |
| l-NMMA | 6.5 ± 0.7 | 5.0 ± 0.5 | 0.005 |
| SDF + l-NMMA | 10.0 ± 2.1 | 5.9 ± 1.0 | 0.030 |
| Steady-state forearm blood flow, ml·dl−1·min−1 | |||
| SDF | 26.3 ± 3.8 | 30.9 ± 4.3 | 0.004 |
| l-NMMA | 20.8 ± 1.7 | 17.6 ± 0.9 | 0.006 |
| SDF + l-NMMA | 31.7 ± 4.3 | 27.4 ± 4.9 | 0.390 |
| Δ Forearm blood flow, ml·dl−1·min−1 | |||
| SDF | 18.5 ± 2.6 | 19.6 ± 3.0 | 0.200 |
| l-NMMA | 14.3 ± 1.7 | 12.6 ± 0.9 | 0.100 |
| SDF + l-NMMA | 21.1 ± 4.1 | 21.7 ± 4.5 | 0.900 |
Data are reported as means ± SE. The effect of drug assessed by Wilcoxon signed rank test. Boldface values indicate statistical significance, drug vs. control. Data reported previously (30).
To be included in analysis, a minimum of 80% of complete duty cycles (all velocity envelopes were of high quality, and clearly captured within the duty cycle) needed to be obtained in all subjects for each exercise trial. Based on these criteria, data from 11 subjects were excluded [no. of individuals excluded: 2 (protocol 1), 5 (protocol 2), 4 (protocol 3)]. On average, 90% of the duty cycles across exercise trials were used in the model described (protocol 1, 94 ± 1%; protocol 2, 88 ± 1%; protocol 3, 88 ± 2%). Data were then fit using a single-component model [ΔFVC or FBF(t) = G0 + G1(1 − e−(t − TD)/τ)], with G1 representing the amplitude, TD representing time delay, and τ representing the time constant of the response. Thus ΔFVC or FBF(t) is indicative of the time-dependent change in forearm vascular conductance (or forearm blood flow) from baseline (G0). The mean response time (MRT, time taken to reach achieve 63% of the steady-state amplitude) was then calculated as MRT = TD + τ (18, 29).
Statistical analysis.
Subject demographics were compared between protocols using a one-way analysis of variance (ANOVA). Hemodynamic data were compared using a Wilcoxon signed rank test. Data are presented as means ± standard error of the mean (SE). P < 0.05 was considered statistically significant.
RESULTS
Data from 29 subjects were used in the present investigation (protocol 1, n = 8; protocol 2, n = 15; protocol 3, n = 6). Subject demographics did not differ significantly between protocols (see Table 1). As reported previously (30), steady-state exercise vasodilation was attenuated with l-NMMA (P = 0.049) but was otherwise unaffected by SDF alone (P = 0.461) or combined SDF and l-NMMA (P = 0.936) (See Table 2).
Table 1.
Subject demographics
| Protocol 1: SDF Only | Protocol 2: l-NMMA Only | Protocol 3: SDF +l-NMMA | P Value | |
|---|---|---|---|---|
| Sex, M/F | 3/5 | 9/6 | 1/5 | |
| Age, yr | 24.9 ± 1.6 | 25.6 ± 1.8 | 22.3 ± 2.2 | 0.426 |
| Height, cm | 170.6 ± 4.0 | 173.1 ± 2.6 | 173.5 ± 3.7 | 0.826 |
| Weight, kg | 67.8 ± 3.6 | 69.4 ± 2.4 | 65.5 ± 6.0 | 0.464 |
| Body mass index, kg/m2 | 23.2 ± 0.5 | 23.1 ± 0.5 | 21.6 ± 1.3 | 0.293 |
| Forearm volume, ml | 895.0 ± 95.0 | 940.2 ± 58.0 | 849.2 ± 114.6 | 0.686 |
| Maximal voluntary contraction, kg | 35.1 ± 4.7 | 37.9 ± 2.2 | 34.5 ± 4.1 | 0.538 |
Data are reported as means ± SE. Group differences assessed by a one-way ANOVA. No significant differences between protocols (P > 0.05 for all).
An example tracing is shown in Fig. 1 to demonstrate modeling of raw data. Baseline and exercise values for both FBF and FVC are summarized in Table 2. Complete data (means +SE) used in modeling are summarized in Tables 3 and 4.
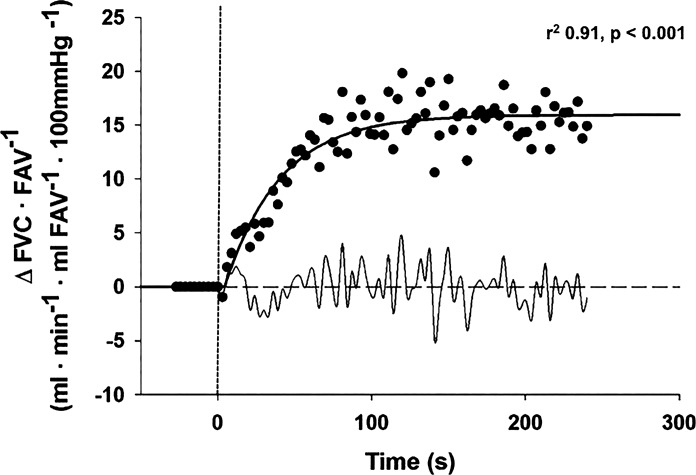
Fig. 1.
Typical change in forearm vascular conductance (FVC) relative to forearm volume (FAV) in response to moderate forearm exercise (15% of maximal voluntary contraction). Dashed vertical line indicates exercise onset, solid curve line is the monoexponential fit with correlation coefficient, and light curved line (bottom) is residual profile. Results from representative subject during control exercise trial.
Table 3.
Forearm vascular conductance kinetic parameters to moderate handgrip exercise (15% maximal voluntary contraction)
| Group | G1, ml·dl−1·min−1·100 mmHg−1 | TD, s | Τ, s | MRT, s | r2 |
|---|---|---|---|---|---|
| SDF | |||||
| Control | 20.7 ± 2.5 | 2.5 ± 1.5 | 29.4 ± 7.2 | 31.7 ± 7.0 | 0.81 ± 0.03 |
| Drug | 21.9 ± 3.1 | 4.9 ± 2.0 | 27.6 ± 5.6 | 32.5 ± 3.7 | 0.80 ± 0.05 |
| l-NMMA | |||||
| Control | 15.1 ± 1.6 | 3.3 ± 3.5 | 18.7 ± 5.0 | 22.0 ± 4.8 | 0.73 ± 0.04 |
| Drug | 12.4 ± 0.9 | 3.8 ± 1.8 | 35.0 ± 6.7* | 39.5 ± 5.8* | 0.75 ± 0.03 |
| SDF + l-NMMA | |||||
| Control | 23.8 ± 3.3 | 4.1 ± 2.6 | 40.9 ± 9.6 | 45.1 ± 8.3 | 0.81 ± 0.03 |
| Drug | 23.3 ± 6.0 | 5.0 ± 2.3 | 44.4 ± 12.5 | 49.3 ± 11.3 | 0.83 ± 0.04 |
Data are reported as means ± SE.
P < 0.05, control vs. drug.
Note: G1 drug vs. control in l-NMMA group, P = 0.08.
Table 4.
Forearm blood flow kinetic parameters to moderate handgrip exercise (15% maximal voluntary contraction)
| Group | G1, ml·dl−1·min−1·100 mmHg−1 | TD, s | Τ, s | MRT, s | r2 |
|---|---|---|---|---|---|
| SDF | |||||
| Control | 19.2 ± 2.4 | 2.3 ± 1.5 | 30.4 ± 7.9 | 32.7 ± 7.4 | 0.80 ± 0.03 |
| Drug | 20.5 ± 3.4 | 3.3 ± 1.9 | 31.6 ± 5.3 | 34.9 ± 3.6 | 0.79 ± 0.03 |
| l-NMMA | |||||
| Control | 12.5 ± 0.8 | 1.0 ± 0.41 | 19.3 ± 4.5 | 20.3 ± 4.5 | 0.73 ± 0.04 |
| Drug | 12.4 ± 0.8 | 4.2 ± 3.5 | 35.4 ± 6.8* | 39.6 ± 5.4* | 0.75 ± 0.03 |
| SDF + l-NMMA | |||||
| Control | 22.9 ± 3.2 | 2.8 ± 2.8 | 43.1 ± 10.3 | 45.9 ± 8.7 | 0.80 ± 0.03 |
| Drug | 24.4 ± 8.2 | 5.6 ± 2.8 | 46.5 ± 12.5 | 52.1 ± 10.9 | 0.83 ± 0.04 |
Data are reported as means ± SE.
P < 0.05, control vs. drug.
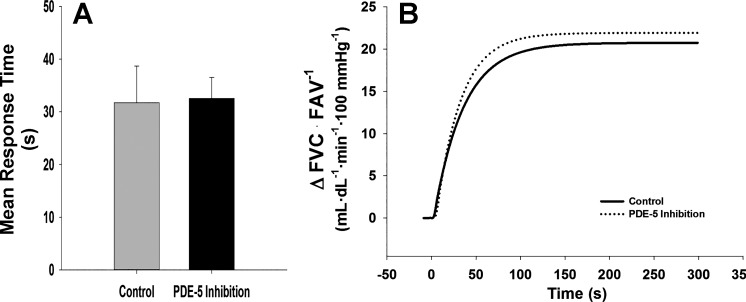
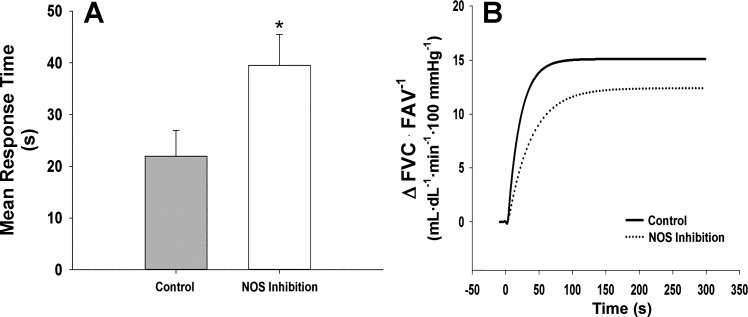
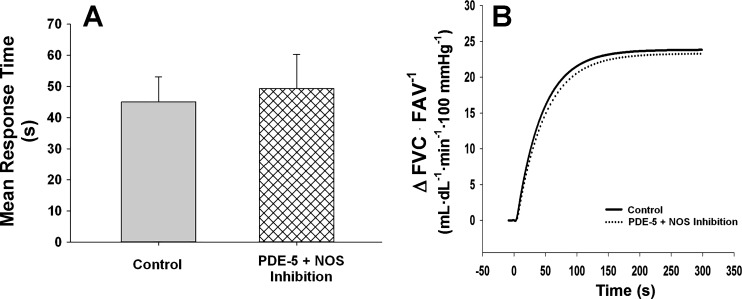
SDF alone had no effect on mean response time (MRT: Control = 31.7 ± 7.0 vs. SDF = 32.5 ± 3.7 s, P = 0.896, Fig. 2). In contrast, l-NMMA increased the time to reach steady-state vasodilation (MRT: Control = 22.0 ± 4.8 vs. l-NMMA = 39.5 ± 5.8 s, P = 0.012, Fig. 3). During combined SDF and l-NMMA, no changes in MRT were observed when compared with control (MRT: Control = 45.1 ± 8.3 vs. SDF + l-NMMA = 49.3 ± 11.3 s, P = 0.761, Fig. 4).
Fig. 2.
Mean response time and mean fit for on-transient vasodilator kinetics to exercise (15% of maximal voluntary contraction) under control conditions and following PDE-5 inhibition with oral sildenafil citrate (SDF). Data are reported as means ± SE. PDE-5 inhibition alone did not alter time to steady-state vasodilation with SDF alone (A and B: MRT, P = 0.896).
Fig. 3.
Mean response time and mean fit for on-transient vasodilator kinetics to exercise (15% of maximal voluntary contraction) under control conditions and following NOS inhibition with intra-arterial l-NMMA. Data are reported as means ± SE. Following NOS inhibition with l-NMMA, time increased to reach steady-state vasodilation (A and B: P = 0.012). *P < 0.05 vs. Control.
Fig. 4.
Mean response time and mean fit for on-transient vasodilator kinetics to exercise (15% of maximal voluntary contraction) under control conditions and following combined PDE-5 (SDF) and NOS (l-NMMA) inhibition. Data are reported as means ± SE. Combined l-NMMA and SDF had no effect on the time to reach steady-state vasodilation (A and B: MRT: P = 0.761) when compared with control.
DISCUSSION
In contrast to our hypothesis, the present data demonstrate that PDE-5 inhibition does not accelerate vasodilation onset kinetics. Moreover, onset kinetics did not differ from control exercise during combined NOS and PDE-5 inhibition. These findings indicate that although PDE-5 activity does not limit vasodilation onset kinetics during moderate exercise, inhibition of PDE-5 preserves onset kinetics under conditions of reduced NO bioavailability. These findings suggest PDE-5 inhibitors may improve vasodilation onset kinetics of vasodilation in patient populations with impaired NO signaling.
Recent evidence indicates NO plays a greater relative contribution to rapid vasodilation and the vasodilation kinetics of the vascular response to exercise [30–50% (3, 5)] than to steady-state exercise hyperemia [~15% (19)]. Data from the present investigation confirm the work from Casey et al. (3), depicting a dramatic increase in the time course of vasodilation following inhibition of NOS (l-NMMA) in young healthy humans (Fig. 3, A and B). This increased MRT mirrors slowed vasodilation onset kinetics observed in older adults during moderate-intensity handgrip exercise (3) implying loss of NO signaling in clinical conditions contributes to impaired vasodilation onset kinetics. One potential solution to restoring vasodilation onset kinetics may be to bypass dysfunctional NO signaling by increasing downstream levels of cGMP.
Interestingly, and contrary to our hypothesis, PDE-5 inhibition did not improve the time course of vasodilation during handgripping exercise (Fig. 2). These data indicate that potentiation of the cGMP pathway does not improve vasodilatory responses [kinetic or steady state (30)] to moderate exercise in young healthy adults when there are no limitations to NO bioavailability. We propose two possible explanations for this finding: 1) PDE-5 activity does not limit NO-mediated vasodilation at exercise onset or steady-state vasodilation, or 2) enhancing cGMP pathway during exercise onset does not exceed the contributions of other rapid vasodilator mechanisms (7, 9).
Given PDE-5 inhibition alone had no effect on vasodilation onset kinetics (Fig. 2), one might expect combined PDE-5 and NOS inhibition would increase MRT similarly to that observed during NOS inhibition alone (Figs. 3). In contrast, onset vasodilator kinetics did not differ from control during PDE-5 + NOS inhibition (Fig. 4). These data indicate PDE-5 inhibition can enhance NO-independent pathways that activate cGMP, and restore exercise vasodilation when NO signaling is compromised. Such a mechanism might explain observations of improved blood flow and exercise responses following treatment with SDF in humans with conditions that have been observed to have lower NO bioavailability (40). For example, oral SDF has been shown to improve vasodilation during knee extensor exercise in older adults (33) and recovery from handgrip exercise in hypertensive patients (1), and to improve exercise tolerance in persons with pulmonary hypertension (38).
As noted above, SDF alone had no effect on the hemodynamic response to the onset of dynamic exercise except in the presence of NOS inhibition. These data would suggest SDF-mediated improvements in vascular responses during exercise onset could be mediated via NO-independent signaling. In contrast to NO signaling via soluble guanylyl cyclase, natriuretic peptides (e.g., atrial, brain, or C-type natriuretic peptide) can be stimulated by exercise (22, 32, 39) and increased cGMP via particulate guanylyl cyclase in the plasma membrane can cause vascular smooth muscle relaxation independent of NO (16, 34, 35). Both of the aforementioned pathways are regulated in part by PDE-5 (16, 34, 35), and thus inhibition of PDE-5 may result in accumulation of cGMP from NO and non-NO (i.e., natriuretic peptide) sources. For example, NO inhibits natriuretic peptide-mediated vasodilation (28), such that, NOS inhibition (l-NMMA) might increase the contribution of natriuretic peptides to exercise-mediated vasodilation. This speculation requires new studies to directly assess the role of natriuretic peptides in exercise vascular signaling.
Implications.
Current therapies aimed at enhancing NO bioavailability have not always been as successful in restoring the rapid vascular response to exercise. Thus our data offer alternative NO-independent mechanism(s) that may restore impaired exercise dilation onset kinetics. Ascorbic acid infusion (known to increase NO bioavailability) improves the steady-state but not the rapid hyperemic response to handgrip exercise in healthy older adults (26). Similarly, dietary nitrate can significantly augment steady-state vasodilation during hypoxic forearm exercise in aging humans (4). However, the effectiveness of nitrate supplementation has been challenged (23). Therefore, mechanisms of impaired vasodilation appear to differ between onset vs. steady-state exercise, making it important to determine contributing mechanism responses to both in a variety of patient populations, to develop targeted therapies to improve exercise tolerance and adherence in conditions with limited NO bioavailability. These new data suggest increasing cGMP levels act as a successful alternative mechanism to achieve normal vasodilation onset kinetics of vasodilation.
Experimental considerations.
The current study investigated a sample of healthy adults studied under tight experimental control, including the use of pharmacological inhibitors to explore vascular control mechanisms. However, there are some limitations to consider. First, sildenafil citrate may cause increases in muscle sympathetic nerve activity (MSNA) and plasma norepinephrine (14). Enhanced vasoconstriction due to an increase in MSNA could partially mask any increase in exercise hyperemia as a result of sildenafil citrate. This should mainly affect baseline flow and not the hyperemic response, since MSNA has a minimal impact on rapid onset vasodilation (2). Second, our results are specific to the moderate (15% MVC) exercise workload studied. Casey and colleagues (2013) have shown increased contribution of NOS to vasodilation onset kinetics as exercise intensity increases. Therefore, we would anticipate that our data might underestimate the impact of PDE-5 inhibition at higher exercise intensities. Third, our experiment included three research cohorts. Whereas a single cohort that experienced every experimental condition would have been ideal, pharmacokinetics, subject safety, and subject burden prohibited our ability to employ such a design. Importantly, each individual served as their own control within study days (completing both a control and intervention trial). Similar study designs have been employed by other groups using a comparable research model in humans to lower subject burden and decrease risk (8, 31). Although studying three separate cohorts increases the potential for interindividual variability in control values, it is important to note reported values fall within the range of values published previously (3, 15, 18, 29). Therefore, we propose studying separate cohorts does not significantly limit conclusions regarding the effect of SDF on exercise hyperemia. Fourth, protocol 3 contained mostly female subjects; however, a post hoc analysis found no differences in MRT between men and women under control conditions (P = 0.14). Finally, the positive effect of SDF + l-NMMA observed on the vasodilation onset kinetics in the present investigation implies that by decreasing MRT or tau, SDF may allow for faster or better matching of oxygen supply to demand via better blood flow distribution. However, our methods of bulk muscle flow do not allow greater insight into flow distribution and how that distribution would impact muscle fatigue; thus additional studies will be necessary.
Conclusions.
In conclusion, our results indicate inhibiting PDE-5 during moderate dynamic handgrip exercise does not improve vasodilation onset kinetics in young healthy adults. Whereas NOS inhibition limits the time course (MRT) of the vasodilator response, combined inhibition of PDE-5 and NOS maintains the response at control levels. These data suggest PDE-5 inhibition does not potentiate the NO-cGMP pathway during moderate exercise. However, when NO bioavailability is reduced, PDE-5 inhibition can restore exercise-mediated vascular responses via NO-independent cGMP pathways. Together our data suggest PDE-5 inhibition may improve exercise vasodilation onset kinetics in pathologies where NO bioavailability is limited.
GRANTS
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) Grants UL1-TR-000427 (UW-Madison) and UL1-TR-000135 (Mayo Clinic). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Financial support was provided by the NIH Grants HL-46493 (M. J. Joyner), HL-078019 (M. J. Joyner), HL-105820 (W. G. Schrage), RR-17520 (T. B. Curry), the American Heart Association (15POST23100020, J. M. Kellawan), as well as the Mayo Clinic Department of Anesthesiology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.T.N., W.G.S., M.J.J., and T.B.C. conceived and designed research; J.M.K., J.K.L., W.T.N., W.G.S., M.J.J., and T.B.C. performed experiments; J.M.K., J.K.L., Z.M.S., and T.B.C. analyzed data; J.M.K., J.K.L., Z.M.S., W.T.N., W.G.S., M.J.J., and T.B.C. interpreted results of experiments; J.M.K. and Z.M.S. prepared figures; J.M.K. and J.K.L. drafted manuscript; J.M.K., J.K.L., W.G.S., M.J.J., and T.B.C. edited and revised manuscript; J.M.K., J.K.L., Z.M.S., W.T.N., W.G.S., M.J.J., and T.B.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We give many thanks to our research participants. Technical and other support was provided by M. Somaraju, C. Johnson, K. Krucker, B. Madery, S. Roberts, B. Walker, and B. Welch (Mayo Clinic), and M. Crain, J. Sebranek, M. Eldridge, B. Walker, J. Harrell, R. Johansson, and G. Peltonen (Univ. of Wisconsin).
REFERENCES
- 1.Attinà TM, Malatino LS, Maxwell SR, Padfield PL, Webb DJ. Phosphodiesterase type 5 inhibition reverses impaired forearm exercise-induced vasodilatation in hypertensive patients. J Hypertens 26: 501–507, 2008. doi: 10.1097/HJH.0b013e3282f382ff. [DOI] [PubMed] [Google Scholar]
- 2.Casey DP, Joyner MJ. Influence of α-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol (1985) 113: 1201–1212, 2012. doi: 10.1152/japplphysiol.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey DP, Ranadive SM, Joyner MJ. Aging is associated with altered vasodilator kinetics in dynamically contracting muscle: role of nitric oxide. J Appl Physiol (1985) 119: 232–241, 2015. doi: 10.1152/japplphysiol.00787.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey DP, Treichler DP, Ganger CT IV, Schneider AC, Ueda K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J Appl Physiol (1985) 118: 178–186, 2015. doi: 10.1152/japplphysiol.00662.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol (1985) 115: 446–455, 2013. doi: 10.1152/japplphysiol.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen PM, Nyberg M, Mortensen SP, Nielsen JJ, Secher NH, Damsgaard R, Hellsten Y, Bangsbo J. Leg oxygen uptake in the initial phase of intense exercise is slowed by a marked reduction in oxygen delivery. Am J Physiol Regul Integr Comp Physiol 305: R313–R321, 2013. doi: 10.1152/ajpregu.00048.2013. [DOI] [PubMed] [Google Scholar]
- 7.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol 589: 3671–3683, 2011. doi: 10.1113/jphysiol.2011.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steady-state exercise hyperemia in humans. Am J Physiol Heart Circ Physiol 307: H782–H791, 2014. doi: 10.1152/ajpheart.00212.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A, Durrant D, Salloum FN, Xi L, Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther 147: 12–21, 2015. doi: 10.1016/j.pharmthera.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- 13.Dishy V, Sofowora G, Harris PA, Kandcer M, Zhan F, Wood AJ, Stein CM. The effect of sildenafil on nitric oxide-mediated vasodilation in healthy men. Clin Pharmacol Ther 70: 270–279, 2001. doi: 10.1067/mcp.2001.117995. [DOI] [PubMed] [Google Scholar]
- 14.Dopp JM, Agapitov AV, Sinkey CA, Haynes WG, Phillips BG. Sildenafil increases sympathetically mediated vascular tone in humans. Am J Hypertens 26: 762–769, 2013. doi: 10.1093/ajh/hpt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faisal A, Dyson KS, Hughson RL. Prolonged ischaemia impairs muscle blood flow and oxygen uptake dynamics during subsequent heavy exercise. J Physiol 588: 3785–3797, 2010. doi: 10.1113/jphysiol.2010.188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feiteiro J, Verde I, Cairrão E. Cyclic guanosine monophosphate compartmentation in human vascular smooth muscle cells. Cell Signal 28: 109–116, 2016. doi: 10.1016/j.cellsig.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Hsu AR, Barnholt KE, Grundmann NK, Lin JH, McCallum SW, Friedlander AL. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol (1985) 100: 2031–2040, 2006. doi: 10.1152/japplphysiol.00806.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hughson RL, Shoemaker JK, Tschakovsky ME, Kowalchuk JM. Dependence of muscle V̇o2 on blood flow dynamics at onset of forearm exercise. J Appl Physiol (1985) 81: 1619–1626, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol (1985) 83: 1785–1796, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Kellawan JM, Johansson RE, Harrell JW, Sebranek JJ, Walker BJ, Eldridge MW, Schrage WG. Exercise vasodilation is greater in women: contributions of nitric oxide synthase and cyclooxygenase. Eur J Appl Physiol 115: 1735–1746, 2015. doi: 10.1007/s00421-015-3160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HL, Kim YN, Lee SK, Seo JB, Chung WY, Shin S, Kim SH, Yoon JH, Kim MA, Zo JH. The impact of acute bouts of exercise on circulating cardiac natriuretic peptide and left ventricular function in untrained healthy young subjects. J Sports Med Phys Fitness 56: 1574–1582, 2016. [PubMed] [Google Scholar]
- 23.Kim J-K, Moore DJ, Maurer DG, Kim-Shapiro DB, Basu S, Flanagan MP, Skulas-Ray AC, Kris-Etherton P, Proctor DN. Acute dietary nitrate supplementation does not augment submaximal forearm exercise hyperemia in healthy young men. Appl Physiol Nutr Metab 40: 122–128, 2015. doi: 10.1139/apnm-2014-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura M, Higashi Y, Hara K, Noma K, Sasaki S, Nakagawa K, Goto C, Oshima T, Yoshizumi M, Chayama K. PDE5 inhibitor sildenafil citrate augments endothelium-dependent vasodilation in smokers. Hypertension 41: 1106–1110, 2003. doi: 10.1161/01.HYP.0000068202.42431.CC. [DOI] [PubMed] [Google Scholar]
- 25.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 28.Liang CF, Au ALS, Leung SWS, Ng KFJ, Félétou M, Kwan YW, Man RYK, Vanhoutte PM. Endothelium-derived nitric oxide inhibits the relaxation of the porcine coronary artery to natriuretic peptides by desensitizing big conductance calcium-activated potassium channels of vascular smooth muscle. J Pharmacol Exp Ther 334: 223–231, 2010. doi: 10.1124/jpet.110.166652. [DOI] [PubMed] [Google Scholar]
- 29.Limberg JK, Kellawan JM, Harrell JW, Johansson RE, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Exercise-mediated vasodilation in human obesity and metabolic syndrome: effect of acute ascorbic acid infusion. Am J Physiol Heart Circ Physiol 307: H840–H847, 2014. doi: 10.1152/ajpheart.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limberg JK, Malterer KR, Mikhail Kellawan J, Schrage WG, Wilkins BW, Nicholson WT, Eisenach JH, Joyner MJ, Curry TB. Potentiation of the NO-cGMP pathway and blood flow responses during dynamic exercise in healthy humans. Eur J Appl Physiol 117: 237–246, 2017. doi: 10.1007/s00421-016-3523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markwald RR, Kirby BS, Crecelius AR, Carlson RE, Voyles WF, Dinenno FA. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol 589: 1979–1990, 2011. doi: 10.1113/jphysiol.2011.205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikimi T, Morimoto A, Ishikawa K, Saito Y, Kangawa K, Matsuo H, Kitamura K, Takishita S, Matsuoka H. Different secretion patterns of adrenomedullin, brain natriuretic peptide, and atrial natriuretic peptide during exercise in hypertensive and normotensive subjects. Clin Exp Hypertens 19: 503–518, 1997. doi: 10.3109/10641969709084511. [DOI] [PubMed] [Google Scholar]
- 33.Nyberg M, Piil P, Egelund J, Sprague RS, Mortensen SP, Hellsten Y. Potentiation of cGMP signaling increases oxygen delivery and oxidative metabolism in contracting skeletal muscle of older but not young humans. Physiol Rep 3: e12508–e12512, 2015. doi: 10.14814/phy2.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rautureau Y, Gowers I, Wheeler-Jones CPD, Baxter GF. C-type natriuretic peptide regulation of guanosine-3′,5′-cyclic monophosphate production in human endothelial cells. Auton Autacoid Pharmacol 30: 185–192, 2010. doi: 10.1111/j.1474-8673.2009.00449.x. [DOI] [PubMed] [Google Scholar]
- 35.Rose RA, Giles WR. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol 586: 353–366, 2008. doi: 10.1113/jphysiol.2007.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders NR, Pyke KE, Tschakovsky ME. Dynamic response characteristics of local muscle blood flow regulatory mechanisms in human forearm exercise. J Appl Physiol (1985) 98: 1286–1296, 2005. doi: 10.1152/japplphysiol.01118.2004. [DOI] [PubMed] [Google Scholar]
- 37.Schalcher C, Schad K, Brunner-La Rocca HP, Schindler R, Oechslin E, Scharf C, Suetsch G, Bertel O, Kiowski W. Interaction of sildenafil with cAMP-mediated vasodilation in vivo. Hypertension 40: 763–767, 2002. doi: 10.1161/01.HYP.0000036027.71527.3E. [DOI] [PubMed] [Google Scholar]
- 38.Singh TP, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J 151: 851.e1–851.e5, 2006. doi: 10.1016/j.ahj.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Vilela EM, Bettencourt-Silva R, Nunes JPL, Ribeiro VG. BNP and NT-proBNP elevation after running–a systematic review. Acta Cardiol 70: 501–509, 2015. doi: 10.1080/AC.70.5.3110509. [DOI] [PubMed] [Google Scholar]
- 40.Zuo L, Rose BA, Roberts WJ, He F, Banes-Berceli AK. Molecular characterization of reactive oxygen species in systemic and pulmonary hypertension. Am J Hypertens 27: 643–650, 2014. doi: 10.1093/ajh/hpt292. [DOI] [PubMed] [Google Scholar]