Abstract
This study tested the hypothesis that intermittent compression of the lower limb would increase blood flow during exercise and postexercise recovery. Data were collected from 12 healthy individuals (8 men) who performed 3 min of standing plantar flexion exercise. The following three conditions were tested: no applied compression (NoComp), compression during the exercise period only (ExComp), and compression during 2 min of standing postexercise recovery. Doppler ultrasound was used to determine superficial femoral artery (SFA) blood flow responses. Mean arterial pressure (MAP) and cardiac stroke volume (SV) were assessed using finger photoplethysmography, with vascular conductance (VC) calculated as VC = SFA flow/MAP. Compared with the NoComp condition, compression resulted in increased MAP during exercise [+3.5 ± 4.1 mmHg (mean ± SD)] but not during postexercise recovery (+1.6 ± 5.9 mmHg). SV increased with compression during both exercise (+4.8 ± 5.1 ml) and recovery (+8.0 ± 6.6 ml) compared with NoComp. There was a greater increase in SFA flow with compression during exercise (+52.1 ± 57.2 ml/min) and during recovery (+58.6 ± 56.7 ml/min). VC immediately following exercise was also significantly greater in the ExComp condition compared with the NoComp condition (+0.57 ± 0.42 ml·min−1·mmHg−1), suggesting the observed increase in blood flow during exercise was in part because of changes in VC. Results from this study support the hypothesis that intermittent compression applied during exercise and recovery from exercise results in increased limb blood flow, potentially contributing to changes in exercise performance and recovery.
NEW & NOTEWORTHY Blood flow to working skeletal muscle is achieved in part through the rhythmic actions of the skeletal muscle pump. This study demonstrated that the application of intermittent pneumatic compression during the diastolic phase of the cardiac cycle, to mimic the mechanical actions of the muscle pump, accentuates muscle blood flow during exercise and elevates blood flow during the postexercise recovery period. Intermittent compression during and after exercise might have implications for exercise performance and recovery.
Keywords: Doppler ultrasound, muscle pump, second heart, vascular conductance
INTRODUCTION
Skeletal muscle contraction acts as a “second heart,” imparting energy to the circulation system to promote the flow of blood back to the heart through a series of one-way valves and thus increase muscle blood flow during exercise (16, 25, 34, 36, 41). On relaxation of the muscle contraction, the veins are relatively empty, creating a transient reduction in venous pressure, increased arterial-venous pressure gradient, and increased arterial inflow (16, 25, 34, 36, 41). Muscle blood flow also increases during exercise as a function of vasodilation, resulting from the simultaneous interactions of multiple neural, chemical, and mechanical mechanisms that are directly related to the metabolic rate (4, 29).
External muscle compression has been used to investigate the muscle pump in the absence of muscle contraction, thus avoiding the confounding influence of the release of vasoactive substances during actual muscle contraction (17, 42). As with skeletal muscle contractions, intermittent compression squeezes the veins and assists the flow of blood back to the heart by promoting venous outflow (24, 27) and arterial inflow (9, 11, 35). Mechanical compression might also promote an increase in vascular conductance through compression-induced vasodilation (5, 22) and shear-induced release of nitric oxide (3). Two previous studies investigated whether intermittent compression applied during exercise might augment skeletal muscle blood flow (17, 31). However, both studies applied compression during muscle contraction when veins were likely collapsed because of increased muscle tension, with the potential of further pressure simply resulting in arterial occlusion and a reduction in muscle blood flow (17, 31). Therefore, the purpose of the current study was to determine if a different pattern of intermittent compression could be used during exercise to enhance muscle blood flow.
A novel intermittent pneumatic compression (IPC) system was used to determine the effects of intermittent compression during exercise and the immediate recovery from exercise. This system applied compression for <300 ms during the local diastolic portion of the cardiac cycle. It was hypothesized that compression applied during periods of muscle relaxation both during exercise and recovery from exercise would result in increased blood flow to the lower limb.
METHODS
Subjects.
Exercise and postexercise recovery hemodynamic responses with and without IPC were assessed in 12 young healthy individuals who participated in the study [8 men, 4 women, age 24.8 ± 3.6 yr (mean ± SD)]. Participants were of varied fitness levels ranging from sedentary to regularly active and were all free of cardiovascular and peripheral vascular disease. All participants signed informed consent forms before participating in the study and were made aware of their right to withdraw from the study at any time without prejudice. Study protocols and procedures were approved by the University of Waterloo, Clinical Research Ethics Committee (ORE#20724) and conformed with the Declaration of Helsinki.
Exercise protocol.
Each exercise session consisted of 2 min of standing rest, 3 min of single-leg plantar flexion exercise, and 2 min of standing recovery. Participants stood on a raised platform, with the left foot slightly ahead of the exercising right leg for balance, and depressed a foot pedal attached to a weighted basket. The weight in the basket was 4–8 kg (6.5 ± 1.3 kg), corresponding to 9.6 ± 1.1% of body mass for each participant. Participants depressed the pedal in time with a metronome set at 75 beats/min in a four-count cycle where the participant depressed the pedal on the first beat, released it on the second beat, and rested for beats three and four. The range of motion of the foot pedal was kept consistent between participants, with each participant being instructed to fully depress and release the pedal smoothly in time with the metronome.
The following three different exercise conditions were tested in this study; control without any IPC (NoComp), exercise with IPC (ExComp), and IPC for 2 min immediately postexercise (RecComp). For the ExComp condition, IPC was applied during the exercise for each cardiac cycle during the rest portion of the exercise duty cycle (foot pedal released) starting after the first depression of the foot pedal. During RecComp, compression was applied for each cardiac cycle during the 2 min of standing recovery with compression started immediately following the last depression of the foot pedal. Each compression condition was repeated two times for a total of six exercise sessions, with the order of testing randomized for each individual participant.
Intermittent pneumatic compressions.
Compression was applied to the lower leg using a custom in-house-built IPC system. The system consisted of a series of five cuffs, each containing an air bladder that inflated independently from the ankle to the knee in a peristaltic “milking” action intended to maximize the removal of blood from the lower limb toward the heart. Each cycle of the cuffs inflating and deflating was completed within 0.3 s, allowing compression to occur within the early diastolic phase of each cardiac cycle.
The sequence of cuff inflation and timing of the compression cycles were controlled electronically using LabVIEW software (LabVIEW; National Instruments, Austin, TX). Compression timing, set to apply compression to the leg during the local diastolic portion of the cardiac cycle, was achieved using the continuous monitoring of the participants’ electrocardiogram (ECG) signals and a calculation of pulse wave transit time determined at the start of testing. Additionally, the positioning of the exercise foot pedal was used as a surrogate for muscle activity with compression only applied when the pedal was fully released, indicating that the participant was not contracting their lower leg muscles.
Interface pressure between the compression cuffs and the lower leg was determined using four pressure sensors located under the two top and two bottom compression cuffs on the lateral surface of the leg. The pressure sensors consisted of a Picopress bladder (Microlab Electronica) connected to an Omega PX390 pressure transducer (Omega Engineering, Laval, Quebec, Canada). At the start of testing, the compression system was adjusted to apply an approximate pressure of 70 mmHg. Pressure data were recorded at 500 Hz (LabVIEW software) for later analysis. Throughout testing, the interface pressure was visually monitored to ensure a consistent magnitude throughout all the testing sessions.
Cardiovascular variables.
A standard three-lead ECG (Pilot 9200; Colin Medical Instruments, San Antonio, TX) was continuously monitored and recorded for the triggering of the IPC system and the calculation of heart rate (HR). Finger photoplethysmography was used for the continuous measurement of arterial blood pressure (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). Pressure measures were height adjusted to heart level and calibrated using the built-in return-to-flow calibration. The arterial pressure waveform was also used to determine Modelflow estimates of cardiac stoke volume (SV).
Blood velocity was measured in the superficial femoral artery (SFA) using a 4-MHz Doppler probe (WAKIe; Atys Medical, Soucieu en Jarrest, France) that was held in place throughout the testing using an elastic bandage. Echo Doppler ultrasound (Mindray M5; Shenzen Mindray Bio-medical Electronics, Shenzen, China) was used for the measurement of popliteal venous and arterial blood velocity. Echo Doppler was also used to image the SFA at the start of testing for the measurement of vessel diameter, and throughout testing for measurements of popliteal artery diameter.
Data analysis.
Arterial pressure, SV, ECG, and SFA instantaneous peak velocity were recorded at 1,000 Hz (Powerlab, LabChart, version 7.3.7; ADInstruments, Colorado Springs, CO). The interval between R-waves on the ECG was used to calculate HR. Cardiac output (CO) was calculated as the product of HR and SV, and mean arterial pressure (MAP) was determined as the average of the recorded arterial blood pressure for each cardiac cycle. The diameter of the SFA was measured using arterial wall-tracking software (MAUI; Hedgehog Medical, Waterloo, Ontario, Canada) that provided a measurement for each frame of two 10-s video clips recorded at 24 frames/s at the start of testing. SFA diameter was calculated as the mean of all the measurements from the two videos (~16 cardiac cycles). The diameter of the SFA was assumed to remain constant throughout the protocol; previous work has shown that the diameter of the popliteal artery remains constant throughout exercise (2, 43) and with the application of compression. At rest, the end of exercise, and the end of recovery, manual caliper measures of popliteal arterial diameter were made to verify that there was no significant effect of compression on vessel dimensions in this study. Instantaneous peak SFA velocity, determined as the outer envelope of the recorded Doppler spectrum, was then multiplied by SFA cross-sectional area for the calculation of blood flow. A calculation of vascular conductance (VC) was made as VC = SFA flow/MAP, where SFA flow is SFA blood flow and MAP is mean arterial pressure at the level of the heart. All above variables were extracted as beat-by-beat means and linearly interpolated to 1-s time points for analysis.
At rest and during exercise, video clips of popliteal arterial and venous blood velocity were recorded. Videos were used for a qualitative analysis of Doppler velocity profiles during exercise with and without the application of compression. Blood velocity was also quantified using custom software (designed by the Vision and Image Processing group at the University of Waterloo) that determined the outer envelope of the recorded Doppler spectrum. Each compression condition was repeated two times to allow for the collection of both popliteal arterial and venous velocity. For all other variables, the two trials of each compression condition were averaged to provide a single measurement for each participant.
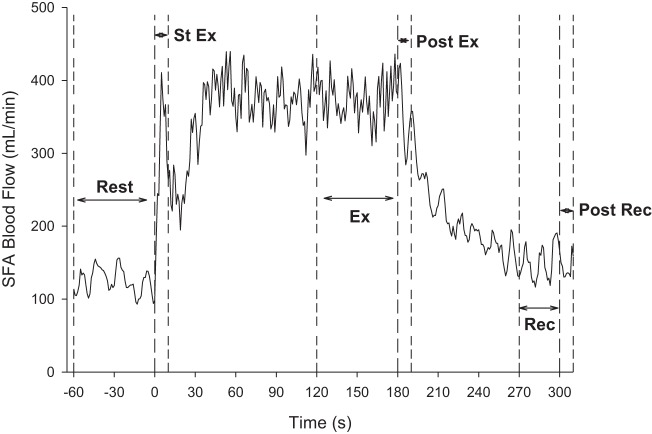
The response to exercise and recovery from exercise were assessed using the time points depicted in Fig. 1. Resting values (Rest: mean of the 60 s before the start of exercise) were compared with the start (St Ex: mean of the first 10 s of exercise) and end (Ex: mean of the last 60 s of exercise) of exercise to determine the responses to exercise with and without compression. Exercise recovery was characterized by examining values immediately following exercise (Post Ex: mean of the first 10 s of recovery) and at the end of the 2 min of recovery (Rec: mean of the last 30 s of the 2-min recovery period). Because of potential confounding influences of compression on venous pressure and the calculation of VC, VC was only assessed at rest for all three conditions, at the start of recovery immediately following exercise (Post Ex) for the ExComp and NoComp conditions, and for the first 10 s following the 2 min of postexercise recovery (Post Rec) for the RecComp and NoComp conditions.
Fig. 1.
Superficial femoral artery (SFA) blood flow response, shown as the 1-s averages of the beat-by-beat means of one subject, to the exercise protocol with the time points selected for analysis indicated. Responses to the exercise protocol were determined by comparing a mean of the 60 s before exercise (Rest) with means of the initial 10 s of exercise (St Ex), the last 60 s of exercise (Ex), the first 10 s following exercise (Post Ex), and the last 30 s of the 2-min recovery period (Rec). Additional 10-s means were determined after 2 min of recovery, when the recovery compressions were stopped (Post Rec), for the no applied compression (NoComp) and compression during 2 min of standing postexercise recovery (RecComp) conditions only.
Popliteal arterial and venous velocity responses to exercise were determined for the NoComp and ExComp conditions by comparing 30-s averages taken at rest with averages of the velocities recorded over the last minute of exercise. Velocities recorded during exercise were further assessed to determine averages for each muscle contraction and relaxation. For this assessment, movement of the foot pedal was used to indicate muscle contraction, with pedal depression indicating muscle contraction and the time when the foot pedal was in a neutral position indicating muscle relaxation. Values for muscle contraction and relaxation were averaged over the last minute of exercise for analysis.
Statistical analysis.
Responses to exercise and recovery were assessed using a two-way repeated-measures ANOVA (SigmaPlot 12.5; Systat Software, San Jose, CA) with the main effects of exercise time (Rest, St Ex, Ex, Post Ex, and Rec) and compression (ExComp, NoComp, RecComp). Variations on this ANOVA were used to assess popliteal venous and arterial velocity responses to exercise, differences in blood flow and velocity with muscle contraction, and VC responses Post Ex and Post Rec with respect to Rest. In the case of significant main effects, Tukey post hoc analysis was used to evaluate pairwise comparisons. For all tests, significance was set at P < 0.05. Unless otherwise stated, all data are expressed as means ± SD for all 12 subjects.
RESULTS
All 12 participants complete all exercise trials. Analysis of the recorded interface pressures (Table 1) confirmed that the magnitude of applied pressure was similar between trials. The IPC system used a consistent inflation time for each compression cuff. Therefore, because of the differing sizes of the leg cuffs, a pressure gradient was developed such that the applied pressure was slightly greater at the ankle (S4) compared with the knee (S1).
Table 1.
Magnitude of applied pressure with IPC
| ExComp 1 | ExComp 2 | RecComp 1 | RecComp 2 | |
|---|---|---|---|---|
| S1 | 70.0 ± 10.3 | 67.8 ± 9.5 | 66.9 ± 9.2 | 68.2 ± 8.7 |
| S2 | 71.6 ± 13.3 | 70.2 ± 11.2 | 70.0 ± 11.5 | 68.8 ± 10.1 |
| S3 | 70.1 ± 12.6 | 69.6 ± 13.3 | 73.2 ± 16.3 | 72.0 ± 16.0 |
| S4 | 72.6 ± 12.0 | 78.3 ± 9.9 | 77.8 ± 10.6 | 78.2 ± 12.0 |
| AVG | 71.1 ± 11.8 | 71.2 ± 11.4 | 71.7 ± 12.4 | 71.6 ± 12.2 |
Values (mean ± SD) show the measures of the peak interface pressures between the leg and the top cuffs closest to the knee (S1, S2) and the bottom two cuffs by the ankle (S3, S4). Because of the design of the intermittent pneumatic compression (IPC) system, applied pressure was slightly greater at the ankle compared with the knee. Across all measurement sessions, there was no difference in the average (AVG) pressure applied to the leg. ExComp, compression during the exercise period only; RecComp, compression during 2 min of standing postexercise recovery.
Values at rest, with exercise, and recovery from exercise for HR, MAP, SV, and CO are presented in Table 2. Analysis found a significant interaction between exercise time and compression for HR [F(8,88) = 11.794, P < 0.001], MAP [F(8,88) = 5.421, P < 0.001], and SV [F(8,88) = 7.383, P < 0.001] and a significant main effect of exercise time for CO [F(4,44) = 23.518, P < 0.001]. The foot pedal exercise without compression (NoComp and RecComp) resulted in an initial increase in MAP for the first 10 s of exercise (St Ex) that decreased back to resting values for the duration of the exercise (Ex). Exercise also resulted in an increase in SV that was sustained throughout exercise with either no change (NoComp) or slight reductions in HR (RecComp). With the application of compression during exercise (ExComp), MAP was increased with exercise, accompanied by a greater increase in SV and reduction in HR. These changes were still evident immediately following exercise (Post Ex) but quickly recovered since there were no differences from Rest for the NoComp condition by the end of the 2 min of exercise recovery (Rec). In contrast, the application of compression during this recovery period (RecComp) served to increase SV and reduce HR with no changes in MAP. For all three conditions, CO was increased with exercise and returned to resting values by the end of the recovery period.
Table 2.
Central hemodynamic values at time points throughout the exercise protocol for all three compression conditions
| Rest | St Ex | Ex | Post Ex | Rec | |
|---|---|---|---|---|---|
| HR, beats/min | |||||
| NoComp | 84 ± 12 | 82 ± 12 | 80 ± 11 | 83 ± 11 | 83 ± 13 |
| ExComp | 83 ± 11 | 81 ± 11 | 78 ± 10*† | 79 ± 10*† | 84 ± 13 |
| RecComp | 86 ± 14 | 84 ± 13 | 81 ± 12* | 80 ± 10*† | 78 ± 12*† |
| MAP, mmHg | |||||
| NoComp | 95.7 ± 8.1 | 102.4 ± 8.2* | 99.2 ± 7.7 | 94.9 ± 8.5 | 94.2 ± 8.3 |
| ExComp | 96.6 ± 5.9 | 103.8 ± 8.4* | 102.7 ± 6.0*† | 98.2 ± 6.9† | 95.9 ± 7.1 |
| RecComp | 93.8 ± 6.4 | 98.8 ± 8.0*† | 97.3 ± 5.6 | 97.3 ± 5.5 | 95.8 ± 5.3 |
| SV, ml | |||||
| NoComp | 66.3 ± 14.2 | 75.8 ± 13.8* | 73.1 ± 13.7* | 72.1 ± 13.8* | 66.0 ± 14.9 |
| ExComp | 68.4 ± 14.1 | 77.3 ± 14.0* | 77.9 ± 14.9*† | 77.1 ± 13.8*† | 65.9 ± 14.1 |
| RecComp | 66.1 ± 13.8 | 75.8 ± 13.2* | 74.3 ± 14.7* | 76.4 ± 15.2* | 74.0 ± 16.7*† |
| CO, l/min | |||||
| NoComp | 5.54 ± 1.43 | 6.23 ± 1.61# | 5.89 ± 1.45# | 6.02 ± 1.57# | 5.45 ± 1.48 |
| ExComp | 5.66 ± 1.47 | 6.23 ± 1.47# | 6.05 ± 1.45# | 6.13 ± 1.43# | 5.48 ± 1.46 |
| RecComp | 5.59 ± 1.31 | 6.30 ± 1.41# | 6.00 ± 1.32# | 6.07 ± 1.32# | 5.74 ± 1.49 |
Values (mean ± SD) show the measures of heart rate (HR), mean arterial pressure (MAP), stroke volume (SV), and cardiac output (CO) at rest (Rest), the first 10 s of exercise (St Ex), the last 60 s of exercise (Ex), the first 10 s after exercise (Post Ex), and the final 30 s of the 2-min recovery period (Rec). Significant interaction effects were found for HR, MAP, and SV as follows:
Values that are different from the respective rest value.
Values that are different from the corresponding value in the no applied compression (NoComp) condition.
A significant main effect of exercise was found for CO with values that were different from rest.
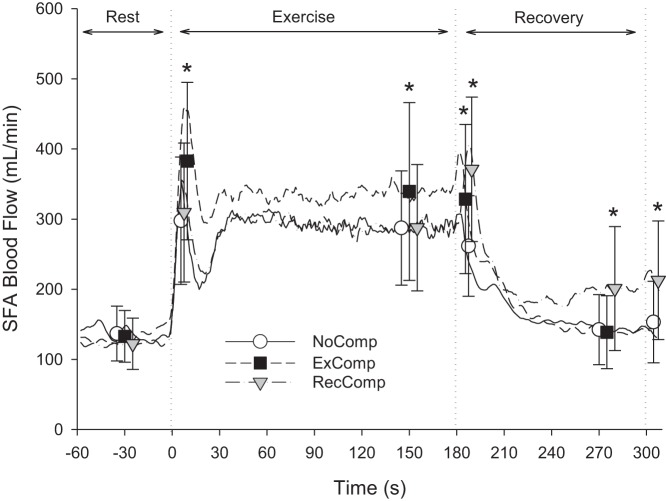
One participant was excluded from the SFA blood flow analysis because of poor signal quality. Assessment of the remaining 11 participants found a significant interaction effect for SFA blood flow [F(8,80) = 8.328, P < 0.001]. With the onset of exercise, the SFA blood flow response was triphasic with an initial overshoot that was greater in the ExComp condition, followed by a reduction and a second increase to a steady-state value by ~45 s of exercise (Fig. 2). For the last minute of exercise, blood flow was not different between the NoComp and RecComp conditions (287.5 ± 81.4 and 287.8 ± 90.0 ml/min, respectively), but was increased in the ExComp condition (339.9 ± 126.7 ml/min).
Fig. 2.
Superficial femoral artery exercise and recovery blood flow responses. Blood flow averaged across all participants (n = 11) for the NoComp, compression during the exercise period only (ExComp), and RecComp conditions are smoothed for presentation using a 5-s moving average. Symbols represent the points used for statistical analysis. Vertical dotted lines indicate the start of exercise at time 0 s, the end of exercise at 180 s, and the end of the applied compression during recovery at 300 s. Analysis showed significant interaction between compression condition and time. *Values that are statistically different from the NoComp condition.
Following exercise (Fig. 2), the elevated SFA blood flow persisted in ExComp (323.7 ± 106.5 ml/min) for the first 10 s (Post Ex) compared with NoComp (261.7 ± 71.7 ml/min). After 2 min of standing recovery, blood flow in ExComp and NoComp had returned to resting levels. In contrast, the application of compression at the end of exercise served to increase blood flow in the RecComp condition Post Ex (371.2 ± 102.9 ml/min) and maintain blood flow above resting levels to the end of the 2 min of recovery (Rec, 201.1 ± 88.4 ml/min).
Analysis of SFA flow for the first 10 s after the completion of 2 min of compression during the postexercise recovery period (Post Rec, Fig. 2) also found a significant interaction effect [F(1,9) = 21.174, P < 0.001]. Post Rec, SFA blood flow in the NoComp condition (153.4 ± 58.1 ml/min) was not different from Rest (136.2 ± 41.0 ml/min), whereas SFA blood flow Post Rec in the RecComp condition (212.9 ± 84.6 ml/min) was elevated with respect to Rest (123.9 ± 38.2 ml/min) and the NoComp condition.
SFA VC, assessed at Rest and Post Ex for NoComp and ExComp, showed a significant interaction [F(1,10) = 43.53, P < 0.001], with VC increased Post Ex compared with Rest in both conditions and a larger increase in the ExComp condition (Fig. 3). Analysis of VC in the NoComp and RecComp conditions for Rest and Post Rec also had a significant interaction effect [F(1,9) = 23.96, P < 0.001], with RecComp elevated above Rest and NoComp, which was not different from Rest (Fig. 3).
Fig. 3.
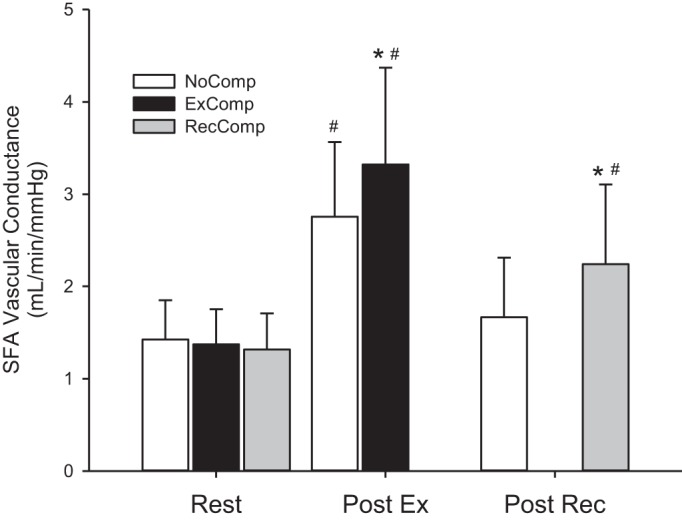
SFA vascular conductance values (mean ± SD) for the NoComp, ExComp, and RecComp conditions at rest, immediately following exercise (n = 11), and immediately following the cessation of compression during the postexercise recovery period (n = 10). #Values that are different from rest. *Significant difference from the NoComp condition.
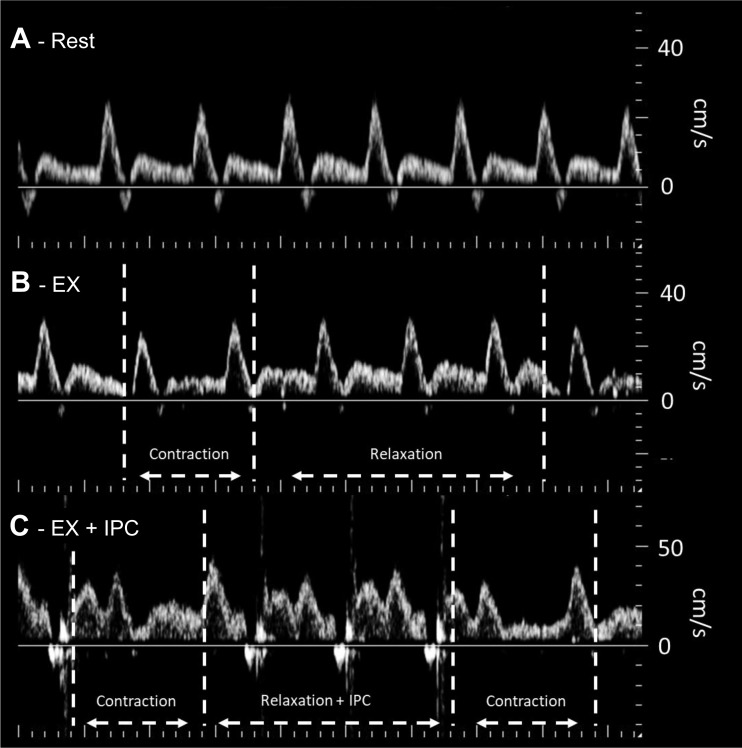
Qualitative assessment of popliteal venous and arterial blood velocity profiles indicated changes in the pattern of response with the application of compression and muscle contraction during exercise. In comparison with arterial velocity at rest (Fig. 4A), popliteal arterial velocity was elevated with exercise in the NoComp (Fig. 4B) and ExComp (Fig. 4C) conditions with slight reductions at the start of each muscle contraction. Additionally, small flow reversals can be seen with the application of compression in the ExComp tracing.
Fig. 4.
Example of the Doppler tracings acquired from the popliteal artery at rest (A), during exercise without compression (B), and during exercise with compression (C). Compared with rest, blood velocity was elevated in both exercise conditions. Muscle contraction during exercise resulted in slight reductions in the Doppler trace, where the application of compression during the relaxation phase is clearly visible as brief periods of reverse velocity followed by an increase in diastolic flow. Note: contrast has been slightly increased to allow for better visualization of changes in the velocity profile.
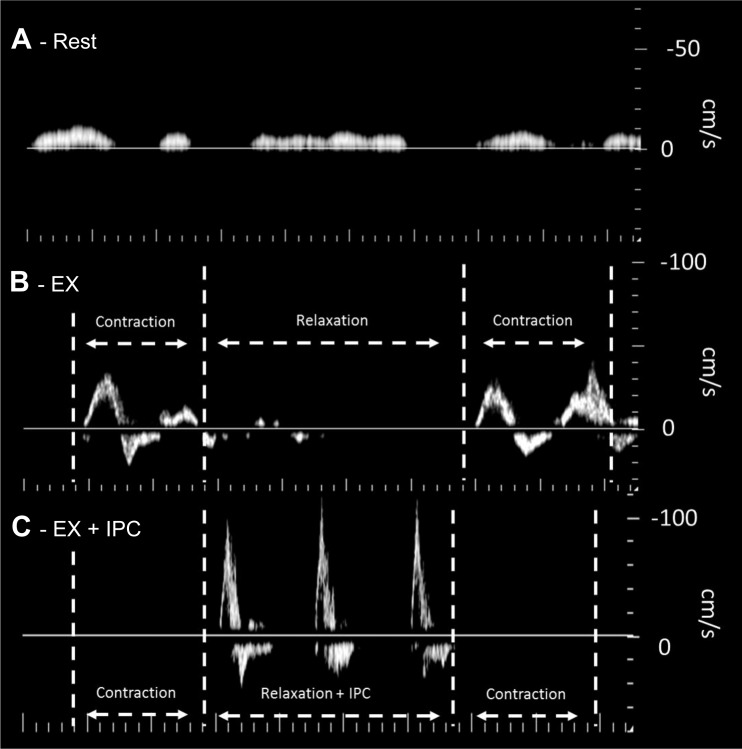
Popliteal venous blood velocity was low and intermittent at rest (Fig. 5A) with very different profiles during exercise for NoComp (Fig. 5B) and ExComp (Fig. 5C). In the NoComp condition, each muscle contraction resulted in an increase in venous velocity with little to no venous velocity being noted during the relaxation phase. In contrast, during the ExComp condition, compression applied during the relaxation phase increased venous velocity with no venous velocity evident during the following muscle contraction.
Fig. 5.
Example of the Doppler tracings acquired from the popliteal vein at rest (A), during exercise without compression (B), and during exercise with compression (C). Compared with rest, both exercise conditions showed distinctly different profiles. Where blood velocity was low and intermittent at rest, muscle contraction during exercise produced increases in venous velocity with little signal during the relaxation phase. In contrast, with compression during exercise, venous blood velocity was elevated with each applied compression during the relaxation phase, with no subsequent increase in venous velocity with muscle contraction. Note: contrast has been slightly increased to allow for better visualization of changes in the velocity profile.
Quantification of popliteal arterial flow and venous velocity at rest and with exercise (n = 8) supported the qualitative assessments. Similar to the SFA blood flow results, a significant interaction was found for popliteal arterial flow [F(1,7) = 8.129, P = 0.025]. Compared with rest (155.2 ± 52.5 and 153.2 ± 62.6 ml/min for NoComp and ExComp, respectively), arterial flow was elevated with exercise in the NoComp condition (350.5 ± 128.5 ml/min), with a larger increase in the ExComp condition (419.4 ± 125.8 ml/min). No effects of exercise [F(2,14) = 3.078, P = 0.078] or compression [F(2,14) = 0.106, P = 0.901] were seen for measures of popliteal arterial diameter, suggesting that the observed changes in flow were primarily the result of changes in arterial velocity. For venous velocity, only a significant main effect of exercise was found [F(1,7) = 24.777, P = 0.002], indicating an increase in venous velocity with exercise in both compression conditions.
Popliteal arterial and venous velocity during exercise was further assessed to determine average values during each muscle contraction and relaxation. Significant interaction effects were found between muscle contraction and compression conditions for popliteal arterial velocity [F(1,7) = 8.539, P = 0.022] and venous velocity [F(1,7) = 29.166, P = 0.001]. Average arterial velocity (Fig. 6A) during muscle contraction in the NoComp condition was lower (14.85 ± 3.64 cm/s) than during muscle relaxation (17.59 ± 5.41 cm/s). In contrast, in the ExComp condition, there was no difference between contraction (19.49 ± 3.91 cm/s) and relaxation (19.82 ± 4.37 cm/s). Comparing between conditions, velocity in the ExComp condition was greater than NoComp during muscle contraction, but not relaxation. Consistent with the qualitative analysis, popliteal venous velocity (Fig. 6B) in the NoComp condition was elevated during muscle contraction (14.06 ± 6.42 cm/s) compared with muscle relaxation (2.19 ± 2.12 cm/s), whereas velocity was elevated during muscle relaxation (10.18 ± 5.85 cm/s) compared with muscle contraction (1.86 ± 1.46 cm/s) in the ExComp condition.
Fig. 6.
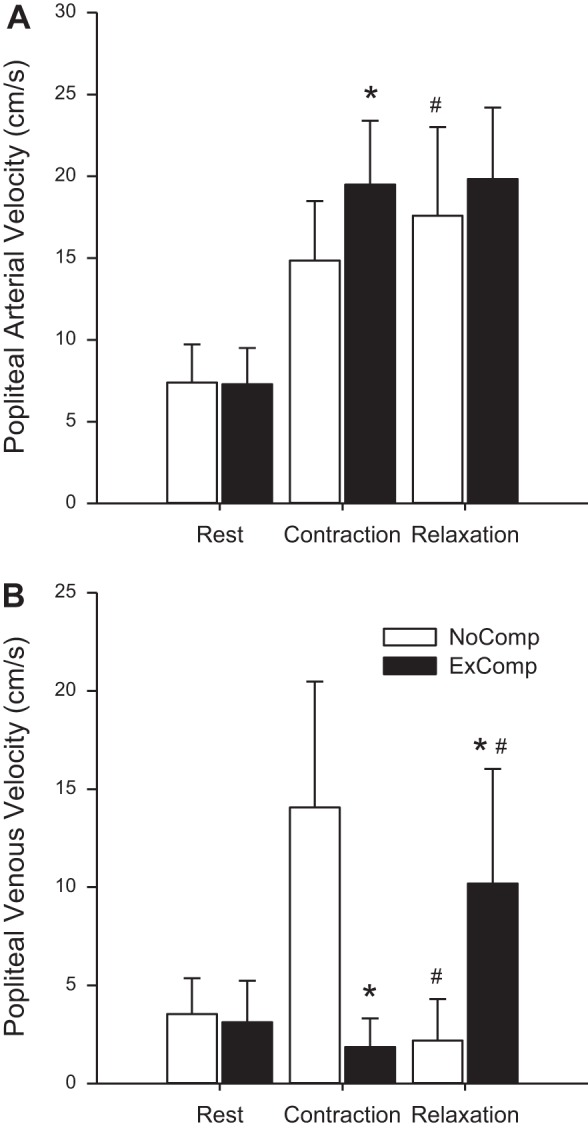
Popliteal arterial (A) and venous (B) blood velocity (mean ± SD) at rest and during exercise periods of muscle contraction and relaxation for the NoComp and ExComp conditions. Resting values were not included in the analysis but are presented to illustrated the differences in blood velocity during exercise. Significant interactions between muscle contraction and compression were found for both arterial and venous velocity. *ExComp velocity values during muscle contraction and relaxation that are different from the respective velocities in the NoComp condition. #Relaxation velocities that are different from the respective values during muscle contraction.
DISCUSSION
During physical activity, muscle blood flow increases to meet the metabolic demand of the working muscle through the coordinated interactions of vasodilation and the skeletal muscle pump. The purpose of the current study was to determine if intermittent compression could be used during exercise to augment the actions of the muscle pump. To test this, we employed a novel system that applied intermittent compression to the lower leg only during muscle relaxation and only during the local diastolic phase of the cardiac cycle. The results confirmed the hypothesis and showed that appropriately timed application of compression during exercise increases arterial blood flow to the working muscle. Additionally, application of compression during recovery from exercise also increased muscle blood flow with potential implications for exercise recovery.
To our knowledge, the current study is the first to have a primary objective of determining if intermittent compression can be used as a method to augment the actions of the muscle pump during exercise. Two studies have previously used intermittent compression during exercise; however, in both cases the compression profiles were timed to coincide with muscle contraction independent of cardiac cycle (17, 31). González-Alonso et al. (17) applied intermittent compression at 200 mmHg to the thigh during passive and low-intensity knee extension exercise only during periods of muscle contraction. Whereas compression during the passive exercise did enhance muscle blood flow, the results demonstrated that additional pressure application during voluntary contraction did not augment the muscle pump. The study of Nishiyasu et al. (31) was primarily focused on the occlusive effects of intermittent compression applied to the thighs during cycling exercise. They applied compression at a set magnitude (150 mmHg), rate (60 compressions/min) initiated with the start of muscle contraction, and with the duration of the compressions progressively increasing (300, 600, 900, or 1,000 ms, the latter being static compression). Tissue oxygenation, as a reflection of blood flow and oxygen delivery, increased slightly at 50 W cycling with 300 ms compression but was reduced at 900 or 1,000 ms compression, showing that there was insufficient time for muscle blood flow.
The pattern of compression used in the current study was chosen to maximize blood flow responses by applying compression to the lower leg during the diastolic portion of the cardiac cycle, when forward flow is lowest (see Fig. 4), and during periods of muscle relaxation when intramuscular pressure would be lowest. Additionally, where other systems in the literature applied an estimated pressure of 150–200 mmHg (9, 12, 17, 31), the compression system used for this study applied a much lower pressure of 71.4 ± 11.8 mmHg. It was believed that this timing and pressure would have the greatest effect on limb hemodynamics, since compression would be applied in a manner that would not impede arterial inflow and would improve venous outflow.
During physical activity, the muscle pump serves to promote circulation by increasing venous outflow and, thereby, arterial inflow (19, 25, 34, 41). During the contraction phase of exercise, intramuscular pressure is increased, squeezing the venous vessels, promoting venous outflow (25, 41), and compressing arterioles, effectively reducing arterial inflow. During the relaxation phase, arterial inflow is increased, promoting venous refilling. Therefore, during rhythmic exercise, there is a cyclic variation in local hemodynamics related to the timing of muscle contraction and relaxation. This effect was seen in the current study both qualitatively (Figs. 4 and 5) and quantitatively, since muscle contraction produced slight reductions in arterial velocity (Fig. 6A) and consistent increases in venous velocity (Fig. 6B). However, the application of compression during muscle relaxation altered this pattern. In contrast to the NoComp condition, popliteal arterial velocity was not different between muscle contraction and relaxation (Fig. 6A), and application of compression during exercise increased popliteal venous velocity during muscle relaxation and significantly reduced the increase in venous velocity resulting from muscle contraction (Fig. 6B).
Lower limb venous pressure with walking or calf exercise, commonly assessed in ankle or dorsal foot veins, consistently shows a rapid reduction with the onset of exercise (23, 30, 38, 39). Variations in the pressure recording can also be identified that correspond to rhythmic muscle contraction (23, 30). In the current study, compression was applied between muscle contractions in a time where an increase in venous pressure, indicating venous refilling, would normally be noted. In effect, the application of compression during muscle relaxation serves to increase the pumping frequency of the second heart, consisting of both applied intermittent compression and muscle contraction. Increased muscle contraction frequency has been shown to improve the actions of the muscle pump (41), and a study by Kügler et al. (23) has demonstrated a greater reduction in venous pressure with increased walking cadence. Therefore, the application of compression during the relaxation phase of the exercise potentially acted to prevent venous refilling, thereby maintaining a lower venous pressure throughout the exercise and helping to maintain arterial flow with each depression of the foot pedal.
Intermittent compression of limbs in a dependent position, as in the current study, effectively increases the arteriovenous pressure gradient (42a), but compression with limbs elevated (10, 26) confirmed that increased VC, resulting from myogenic (5, 22, 25) or biochemical mechanisms (3), also contributes to the observed increase in arterial inflow. The current study measured VC immediately after stopping exercise and/or compression to avoid the confounding effects of changes in venous pressure (36). We demonstrated that external compression further enhanced VC immediately after stopping exercise (Post Ex) and elevated VC during the postexercise recovery phase (Post Rec). Enhanced VC during exercise suggests that myogenic mechanisms had not achieved their full effect during the plantar flexor exercise, allowing for an increase with the application of compression, but other mechanisms for this response cannot be excluded.
The single-leg plantar flexion exercise was sufficient to increase MAP, SV, and CO. Application of intermittent external compression to the calf muscle augmented MAP and SV during the exercise. Increased MAP in ExComp appeared to have stimulated the arterial baroreflex, since HR was reduced and CO was not different compared with the NoComp condition. The external compression affected arterial inflow during the cardiac cycle, as seen in the brief reverse blood flow with pressure application (Fig. 4C), and might have elevated total vascular resistance to increase MAP. Previous research with intermittent compression applied at rest also found reduced HR (15, 31, 40) and increased SV (1), suggesting the possibility of increased venous return because of the application of compression; however, further study is needed to better quantify the effects of intermittent compression on cardiac function.
Functional consequences of the observed increase in blood flow during exercise and postexercise recovery remain to be determined. For a healthy population, blood flow to the working muscle is generally not a limiting factor for exercise performance at low and moderate intensities. It would then follow that the application of intermittent compression during exercise would be beneficial in different disease states where blood flow to the working muscle is limited (12, 32) or as a method of reducing edema resulting from exercise-induced vasculitis (14, 21, 33). In clinical settings, IPC use has been shown to act as a prophylaxis against the development of deep vein thrombosis (7), improve wound healing and limb salvage (8, 20), and improve walking ability in intermittent claudication patients (12). However, to date, no studies have been conducted to examine the use of IPC during exercise in these populations.
During recovery from exercise, intermittent compression has been suggested to improve blood lactate clearance (18, 28). In the current study, the application of compression immediately following exercise (RecComp condition) resulted in a rapid increase in arterial blood flow that was sustained throughout the 2-min postexercise recovery period. Further study is required to determine how this increase in blood flow affects exercise recovery and subsequent exercise performance.
Limitations.
The IPC system was configured during the study setup to apply pressure to the lower leg at ~70 mmHg. Analysis of the pressure measurements during testing found some variability in applied pressure, likely because of factors including cuff movement with respect to the pressure sensors, resulting in uneven loading of the pressure sensors, and differences in the curvature of the leg between participants and with muscle contraction. With the limitations of the pressure sensors used in the current study, it is unclear how much of the variations in interface pressure measurements between subjects reflect actual differences in applied pressure.
For the assessment of leg blood flow, the assumption was made that the SFA and popliteal arteries would not change in dimension with experimental protocol. This assumption was supported by previous work that did not find a difference in popliteal artery diameter with similar intensities of exercise (2, 43) and by pilot testing that did not find a change with the application of compression. In the current study, manual measurements of popliteal artery diameter were not different with exercise or the application of compression. Comparison of the popliteal artery velocity measurements during exercise suggests that an ~10% difference in vessel diameter would be required to equate blood flow with and without the application of compression. Although there is some error in the manual measurement of vessel dimension, it is highly unlikely that a 10% change would be missed using this method.
The exercise in the current study was very low intensity (~10% body mass) with a slow rate of muscle contraction (~18 contractions/min) conducted in a stationary position to facilitate ultrasound measurements. Therefore, it is not known if the intermittent compressions would have a similar effect during different activities, such as walking. It is possible that, because of the low exercise intensity, the muscle pump was not working at peak capability, and the leg veins were not completely emptied of blood with each muscle contraction, enabling the compressions to mobilize this residual venous pooling and increase arterial inflow. Future investigations with appropriate sensors to quantify muscle blood flow are required to determine if external compressions can enhance flow during walking activities.
Central circulatory dynamics were assessed using pulse contour analysis of the finger arterial pulse wave. Although widely used in research studies, there are conditions under which the algorithm does not track changes in CO (13, 37). Future studies should consider an independent method to assess central circulation responses to intermittent compression.
In summary, this study demonstrated the ability of intermittent compression applied to the lower leg to enhance the actions of the muscle pump by increasing blood flow to the working muscle during plantar flexion exercise and postexercise recovery. Compression was applied in a manner to have the greatest effect on limb hemodynamics, since the purpose of the current study was to determine if intermittent compression could be used to enhance muscle blood flow during exercise. Therefore, compression was only applied during the local diastolic phase of the cardiac cycle and only during periods of muscle relaxation. This pattern of compression served to alter the hemodynamic conditions such that there was an increase in venous velocity during relaxation and a better maintenance of arterial inflow during each depression of the foot pedal.
GRANTS
This work was supported by a grant from Lockheed Martin with additional support from the Ontario Centres of Excellence (OCE) VIP II program to S. D. Peterson and R. L. Hughson (24529) and the Natural Sciences and Engineering Research Council of Canada to R. L. Hughson (RGPIN-6473).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.Z., C.N.P., R.L.H., and S.D.P. conceived and designed research; K.A.Z. and C.N.P. performed experiments; K.A.Z. and C.N.P. analyzed data; K.A.Z., C.N.P., R.L.H., and S.D.P. interpreted results of experiments; K.A.Z. prepared figures; K.A.Z. drafted manuscript; K.A.Z., C.N.P., R.L.H., and S.D.P. edited and revised manuscript; K.A.Z., C.N.P., R.L.H., and S.D.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ivan Beentjes for designing and constructing the intermittent pneumatic compression system employed in this study. We further thank the Centre for Bioengineering and Biotechnology at the University of Waterloo for initiating and supporting the collaborative work in this study.
REFERENCES
- 1.Bickel A, Shturman A, Grevtzev I, Roguin N, Eitan A. The physiological impact of intermittent sequential pneumatic compression (ISPC) leg sleeves on cardiac activity. Am J Surg 202: 16–22, 2011. doi: 10.1016/j.amjsurg.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Book J, Prince CN, Villar R, Hughson RL, Peterson SD. Investigating the impact of passive external lower limb compression on central and peripheral hemodynamics during exercise. Eur J Appl Physiol 116: 717–727, 2016. doi: 10.1007/s00421-016-3331-0. [DOI] [PubMed] [Google Scholar]
- 3.Chen LE, Liu K, Qi WN, Joneschild E, Tan X, Seaber AV, Stamler JS, Urbaniak JR. Role of nitric oxide in vasodilation in upstream muscle during intermittent pneumatic compression. J Appl Physiol (1985) 92: 559–566, 2002. doi: 10.1152/japplphysiol.00365.2001. [DOI] [PubMed] [Google Scholar]
- 4.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol (1985) 97: 393–403, 2004. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 5.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell CW Jr, Froimson MI, Mont MA, Ritter MA, Trousdale RT, Buehler KC, Spitzer A, Donaldson TK, Padgett DE. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am 92: 527–535, 2010. doi: 10.2106/JBJS.I.00047. [DOI] [PubMed] [Google Scholar]
- 8.Comerota AJ. Intermittent pneumatic compression: physiologic and clinical basis to improve management of venous leg ulcers. J Vasc Surg 53: 1121–1129, 2011. doi: 10.1016/j.jvs.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 9.Delis KT, Labropoulos N, Nicolaides AN, Glenville B, Stansby G. Effect of intermittent pneumatic foot compression on popliteal artery haemodynamics. Eur J Vasc Endovasc Surg 19: 270–277, 2000. doi: 10.1053/ejvs.1999.1028. [DOI] [PubMed] [Google Scholar]
- 10.Delis KT, Azizi ZA, Stevens RJ, Wolfe JH, Nicolaides AN. Optimum intermittent pneumatic compression stimulus for lower-limb venous emptying. Eur J Vasc Endovasc Surg 19: 261–269, 2000. doi: 10.1053/ejvs.1999.1047. [DOI] [PubMed] [Google Scholar]
- 11.Delis KT, Husmann MJWW, Cheshire NJ, Nicolaides AN. Effects of intermittent pneumatic compression of the calf and thigh on arterial calf inflow: a study of normals, claudicants, and grafted arteriopaths. Surgery 129: 188–195, 2001. doi: 10.1067/msy.2001.110023. [DOI] [PubMed] [Google Scholar]
- 12.Delis KT, Nicolaides AN, Wolfe JHN, Stansby G. Improving walking ability and ankle brachial pressure indices in symptomatic peripheral vascular disease with intermittent pneumatic foot compression: a prospective controlled study with one-year follow-up. J Vasc Surg 31: 650–661, 2000. doi: 10.1067/mva.2000.103969. [DOI] [PubMed] [Google Scholar]
- 13.Dyson KS, Shoemaker JK, Arbeille P, Hughson RL. Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Exp Physiol 95: 561–568, 2010. doi: 10.1113/expphysiol.2009.050815. [DOI] [PubMed] [Google Scholar]
- 14.Espitia O, Dréno B, Cassagnau E, Didier Q, Quillard T, Nicol C, Le Bouch Y, Planchon B, Pistorius MA. Exercise-induced vasculitis: A review with illustrated cases. Am J Clin Dermatol 17: 635–642, 2016. doi: 10.1007/s40257-016-0218-0. [DOI] [PubMed] [Google Scholar]
- 15.Fanelli G, Zasa M, Baciarello M, Mazzani R, Di Cianni S, Rossi M, Casati A. Systemic hemodynamic effects of sequential pneumatic compression of the lower limbs: a prospective study in healthy volunteers. J Clin Anesth 20: 338–342, 2008. doi: 10.1016/j.jclinane.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Folkow B, Gaskell P, Waaler BA. Blood flow through limb muscles during heavy rhythmic exercise. Acta Physiol Scand 80: 61–72, 1970. doi: 10.1111/j.1748-1716.1970.tb04770.x. [DOI] [PubMed] [Google Scholar]
- 17.González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586: 2405–2417, 2008. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson E, Stetter K, Li R, Thomas A. An intermittent pneumatic compression device reduces blood lactate concentrations more effectively than passive recovery after wingate testing. J Athl Enhanc 2: 2–5, 2013. [Google Scholar]
- 19.Janicki JS, Sheriff DD, Robotham JL, Wise RA. Cardiac output during exercise: contributions of the cardiac, circulatory, and respiratory systems. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am Physiol Sci, p. 649–704, sect. 12, 1996. [Google Scholar]
- 20.Kavros SJ, Delis KT, Turner NS, Voll AE, Liedl DA, Gloviczki P, Rooke TW. Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up. J Vasc Surg 47: 543–549, 2008. doi: 10.1016/j.jvs.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol 46: 11–14, 2005. doi: 10.1111/j.1440-0960.2005.00127.x. [DOI] [PubMed] [Google Scholar]
- 22.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kügler C, Strunk M, Rudofsky G. Venous pressure dynamics of the healthy human leg. Role of muscle activity, joint mobility and anthropometric factors. J Vasc Res 38: 20–29, 2001. doi: 10.1159/000051026. [DOI] [PubMed] [Google Scholar]
- 24.Labropoulos N, Cunningham J, Kang SS, Mansour MA, Baker WH. Optimising the performance of intermittent pneumatic compression devices. Eur J Vasc Endovasc Surg 19: 593–597, 2000. doi: 10.1053/ejvs.2000.1067. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am Physiol Sci, p. 705–769, sect. 12, 1996. [Google Scholar]
- 26.Lurie F, Awaya DJ, Kistner RL, Eklof B. Hemodynamic effect of intermittent pneumatic compression and the position of the body. J Vasc Surg 37: 137–142, 2003. doi: 10.1067/mva.2002.24. [DOI] [PubMed] [Google Scholar]
- 27.Lurie F, Scott V, Yoon H-C, Kistner RL. On the mechanism of action of pneumatic compression devices: Combined magnetic resonance imaging and duplex ultrasound investigation. J Vasc Surg 48: 1000–1006, 2008. doi: 10.1016/j.jvs.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Martin JS, Friedenreich ZD, Borges AR, Roberts MD. Acute effects of peristaltic pneumatic compression on repeated anaerobic exercise performance and blood lactate clearance. J Strength Cond Res 29: 2900–2906, 2015. doi: 10.1519/JSC.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen SP, Saltin B. Regulation of the skeletal muscle blood flow in humans. Exp Physiol 99: 1552–1558, 2014. doi: 10.1113/expphysiol.2014.081620. [DOI] [PubMed] [Google Scholar]
- 30.Nicolaides AN, Zukowski AJ. The value of dynamic venous pressure measurements. World J Surg 10: 919–924, 1986. doi: 10.1007/BF01658640. [DOI] [PubMed] [Google Scholar]
- 31.Nishiyasu T, Maekawa T, Sone R, Tan N, Kondo N. Effects of rhythmic muscle compression on cardiovascular responses and muscle oxygenation at rest and during dynamic exercise. Exp Physiol 91: 103–109, 2006. doi: 10.1113/expphysiol.2005.032052. [DOI] [PubMed] [Google Scholar]
- 32.Ramaswami G, D’Ayala M, Hollier LH, Deutsch R, McElhinney AJ. Rapid foot and calf compression increases walking distance in patients with intermittent claudication: results of a randomized study. J Vasc Surg 41: 794–801, 2005. doi: 10.1016/j.jvs.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 33.Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol 20: 423–427, 2006. doi: 10.1111/j.1468-3083.2006.01504.x. [DOI] [PubMed] [Google Scholar]
- 34.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876-2003): cycles of revision and new vision. J Appl Physiol (1985) 97: 384–392, 2004. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- 35.Sheldon RD, Roseguini BT, Thyfault JP, Crist BD, Laughlin MH, Newcomer SC. Acute impact of intermittent pneumatic leg compression frequency on limb hemodynamics, vascular function, and skeletal muscle gene expression in humans. J Appl Physiol (1985) 112: 2099–2109, 2012. doi: 10.1152/japplphysiol.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol 265: H1227–H1234, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol 300: R486–R491, 2011. doi: 10.1152/ajpregu.00505.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stick C, Hiedl U, Witzleb E. Venous pressure in the saphenous vein near the ankle during changes in posture and exercise at different ambient temperatures. Eur J Appl Physiol Occup Physiol 66: 434–438, 1993. doi: 10.1007/BF00599617. [DOI] [PubMed] [Google Scholar]
- 39.Stick C, Jaeger H, Witzleb E. Measurements of volume changes and venous pressure in the human lower leg during walking and running. J Appl Physiol (1985) 72: 2063–2068, 1992. doi: 10.1152/jappl.1992.72.6.2063. [DOI] [PubMed] [Google Scholar]
- 40.Tochikubo O, Ri S, Kura N. Effects of pulse-synchronized massage with air cuffs on peripheral blood flow and autonomic nervous system. Circ J 70: 1159–1163, 2006. doi: 10.1253/circj.70.1159. [DOI] [PubMed] [Google Scholar]
- 41.Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol (1985) 97: 739–747, 2004. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- 42.Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physioll 271: H1697–H1701, 1996. [DOI] [PubMed] [Google Scholar]
- 42a.van Bemmelen PS, Mattos MA, Faught WE, Mansour MA, Barkmeier LD, Hodgson KJ, Ramsey DE, Sumner DS. Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg 19: 1052–1058, 1994. doi: 10.1016/S0741-5214(94)70217-9. [DOI] [PubMed] [Google Scholar]
- 43.Villar R, Hughson RL. Repeatability of popliteal blood flow and lower limb vascular conductance at rest and exercise during body tilt using Doppler ultrasound. Physiol Meas 34: 291–306, 2013. doi: 10.1088/0967-3334/34/3/291. [DOI] [PubMed] [Google Scholar]