Abstract
Endocannabinoids, such as 2-arachidonoyl glycerol (2-AG) and anandamide, can elicit long-term depression of both excitatory and inhibitory synapses. This latter effect will result in disinhibition and would therefore be expected to produce an increase in neural circuit output. However, there have been no examples directly linking endocannabinoid-mediated disinhibition to a change in a functional neurobehavioral circuit. The present study uses the well-characterized central nervous system of the medicinal leech, Hirudo verbana, to examine the functional/behavioral relevance of endocannabinoid modulation of an identified afferent synapse. Bath application of 2-AG potentiates synaptic transmission by pressure-sensitive sensory neurons (P cells) as well as the magnitude of the defensive shortening reflex elicited by P-cell stimulation. This potentiation requires activation of TRPV-like channels. Endocannabinoid/TRPV signaling was found to produce sensitization of the shortening reflex elicited by either direct stimulation of nearby nociceptive afferents (N cells) or noxious stimulation applied to skin several segments away. In both cases, heterosynaptic potentiation of P-cell synapses was observed in parallel with an increase in the magnitude of elicited shortening and both synaptic and behavioral effects were blocked by pharmacological inhibition of 2-AG synthesis or TRPV-like channel activation. Serotonin (5-HT) is known to play a critical role in sensitization in Hirudo and other animals, and the 5-HT2 receptor antagonist ritanserin also blocked behavioral sensitization and the accompanying synaptic potentiation. These findings suggest a novel, endocannabinoid-mediated contribution to behavioral sensitization that may interact with known 5-HT-dependent modulatory processes.
NEW & NOTEWORTHY There is considerable interest in the analgesic potential of cannabinoids. However, there is evidence that the cannabinoid system can have both pro- and antinociceptive effects. This study examines how an endogenous cannabinoid transmitter can potentiate nonnociceptive synapses and enhance their capacity to elicit a nocifensive behavioral response.
Keywords: endocannabinoid, leech; nociception; serotonin; synapse; TRPV
INTRODUCTION
Endocannabinoids, such as 2-arachidonoyl glycerol (2-AG) and anandamide, are lipid neurotransmitters that can produce both short-term (seconds) and long-term (tens of minutes to hours) depression of synaptic transmission (Heifets and Castillo 2009). Because endocannabinoids can depress both excitatory and inhibitory synapses, they have the potential to have opposing effects on microcircuit output. Depression of excitatory synapses would decrease circuit output, while depression of inhibitory synapses (disinhibition) would increase circuit output. An example of the functional consequences of these opposing effects can be found in spinal cord afferent circuits, where endocannabinoids have been found to have both antinociceptive effects (due to depression of excitatory synapses) and pronociceptive effects (due to disinhibition) (Christie and Mallet 2009; Kato et al. 2012; Pernía-Andrade et al. 2009). However, endocannabinoid-based modulation is observed throughout the brain, and therefore these bidirectional effects are likely to impact a large range of behavioral processes.
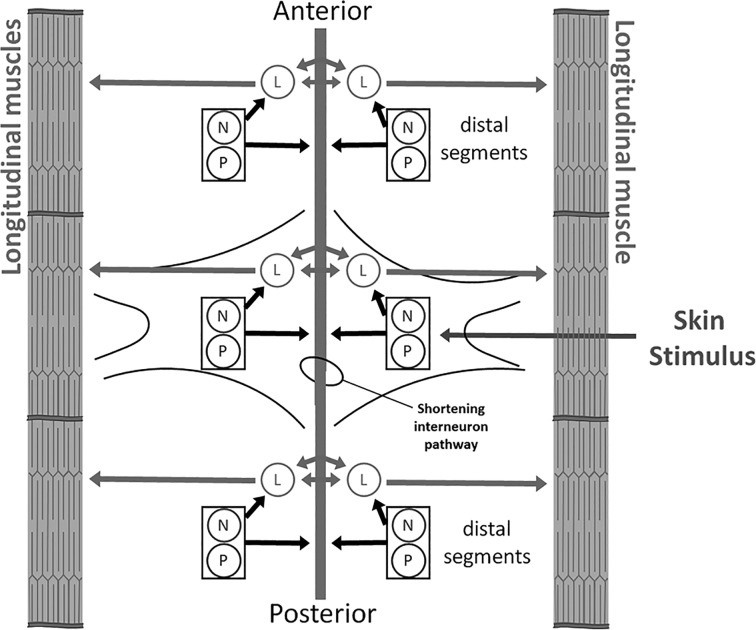
Understanding how the opposing effects of endocannabinoids at the synaptic circuit level are incorporated into behavioral processes, e.g., the patterns of stimuli that engage circuit depression vs. circuit disinhibition and the ultimate behavioral consequences of these opposing effects, is difficult given the complexity of the central nervous system (CNS). However, studies using the nervous system of Hirudo verbana (the medicinal leech) provide an opportunity to link synaptic changes to those at the behavioral level. The Hirudo CNS is especially well characterized in terms of the functional identity, physiological properties, and synaptic connections of many of its neurons (Kristan et al. 2005). In particular, the cutaneous somatosensory neurons located in the CNS have received considerable attention. The Hirudo CNS consists of a linear chain of ganglia, with each possessing three (bilateral) pairs of rapidly adapting touch-sensitive neurons (T cells), two pairs of slow-adapting pressure-sensitive neurons (P cells), and two pairs of nociceptive neurons (N cells) (Nicholls and Baylor 1968). The P and N cells are capable of eliciting a defensive withdrawal behavior, the whole body shortening reflex, that involves the simultaneous contraction of all body segments. During whole body shortening (referred to as simply “shortening” from this point on), the N and P cells activate local motor neurons such as the longitudinal motor neuron (L cell) that innervate the longitudinal muscles required for shortening and an unidentified interneuron pathway that stimulates motor neurons throughout the rest of the CNS (Fig. 1) (Shaw and Kristan 1995, 1999).
Fig. 1.
Summary of the Hirudo whole body shortening circuit. Skin stimulus that activates multiple P-cell receptive fields or a single N-cell receptive field leads to direct activation of local motor neurons that stimulate contractions of the longitudinal muscles. There are multiple motor neurons that innervate these muscles, but only the longitudinal motor neurons (L cells) are shown here. The bidirectional arrow between the L cells represents the electrical coupling between these neurons. The N and P cells also presumably activate an interneuron pathway (indicated by gray bar running down center of figure) that carries the signal to shorten throughout the central nervous system (CNS), leading to coordinated stimulation of motor neurons in every segment. For a more detailed description of the sensory neurons, motor neurons, and interneurons contributing to shortening, see Shaw and Kristan (1995).
The endocannabinoid transmitters 2-AG and anandamide are present in the Hirudo CNS and in other invertebrates where they have been examined (De Petrocellis et al. 1999; Khaliullina et al. 2015; Lehtonen et al. 2008; Matias et al. 2001). In Hirudo, both 2-AG and anandamide have been found to have opposing effects on nociceptive vs. nonnociceptive synapses. Specifically, both 2-AG and anandamide elicit persistent (at least 1 h) depression of N-cell synapses but produce potentiation of P-cell synapses (Higgins et al. 2013; Wang and Burrell 2016; Yuan and Burrell 2010, 2012, 2013a, 2013b). In the case of the N cells, endocannabinoid-mediated long-term depression (eCB-LTD) is mediated by presynaptic TRPV-like channel, similar to eCB-LTD observed in the hippocampus (Edwards 2014; Gibson et al. 2008; Jensen and Edwards 2012). Protostomal invertebrates, such as Hirudo, lack orthologs to the vertebrate CB1 or CB2 receptors (Elphick 2012) but do possess TRPV-like channels. This is significant because in vertebrates 2-AG and anandamide have been shown to mediate a variety of synaptic and behavioral effects via direct activation of TRPV1 (Chávez et al. 2010; Edwards 2014; Gibson et al. 2008; Grueter et al. 2010; Zygmunt et al. 1999, 2013). There is evidence of endocannabinoids and other lipid signaling molecules acting on invertebrate TRPV-like channels, and we have proposed that TRPVs or other transient receptor potential channels may represent the original endocannabinoid receptor (Kahn-Kirby et al. 2004; Leung et al. 2008; Wang and Burrell 2016; Yuan and Burrell 2010). In Hirudo, eCB-LTD of the N synapses can also be elicited by low-frequency stimulation (LFS) of the T cells, a heterosynaptic mechanism that requires 2-AG synthesis in the postsynaptic neuron (L cell) and TRPV activation in the presynaptic N cell (Summers et al. 2014; Yuan and Burrell 2010, 2013a, 2013b). P cells lack TRPV-like channels, but endocannabinoid-mediated potentiation is still TRPV dependent (Higgins et al. 2013; Pastor et al. 1996; Summers et al. 2014; Wang and Burrell 2016). This is because the increase in synaptic transmission is actually thought to be a form of disinhibition in which endocannabinoid-TRPV signaling decreases tonic GABAergic input to the P-cell presynaptic terminals (Higgins et al. 2013; Wang and Burrell 2016). This potentiation can also be elicited heterosynaptically, but in this case through high-frequency stimulation (HFS) of the N cells (Wang and Burrell 2016).
Previous studies using semi-intact preparations have shown that eCB-LTD of N-cell synapses contributes to a decrease in the magnitude of N-elicited shortening following bath application of 2-AG or T-cell LFS (Yuan and Burrell 2013b). However, the effects of 2-AG-mediated potentiation of P synapses on the shortening response are unknown. Addressing this question will contribute to understanding how bidirectional modulation by endocannabinoids functionally contributes to behavioral output. Therefore, in this study we examined whether 2-AG-induced potentiation/disinhibition of synapses affects the defensive withdrawal reflex and whether stimuli that produce sensitization of this behavior utilize 2-AG-dependent modulation.
MATERIALS AND METHODS
H. verbana (3 g) were obtained from commercial suppliers (Niagara Medicinal Leeches, Cheyenne, WY; Leeches USA, Westbury, NY), maintained in artificial pond water (Fifty Fathoms aquarium salt 0.5 g/l H2O), and kept on a 12:12-h light-dark cycle at 18°C. The dissections and recordings were carried out in normal Hirudo saline solution (in mM: 114 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 5 NaOH, and 10 HEPES; pH = 7.4) with 10 mM glucose.
All drugs were prepared from frozen stocks (dissolved in DMSO), and the final concentrations were dissolved in Hirudo saline solution and made just before each experiment. Dimethyl sulfoxide (DMSO) and Orlistat (tetrahydrolipstatin or THL) were obtained from Sigma-Aldrich (St. Louis, MO). SB 366791 (SB), 2-AG, and ritanserin (RIT) were purchased from Tocris (Ellisville, MO). Vehicle control experiments were carried out in saline that contained equivalent levels of DMSO (0.01%).
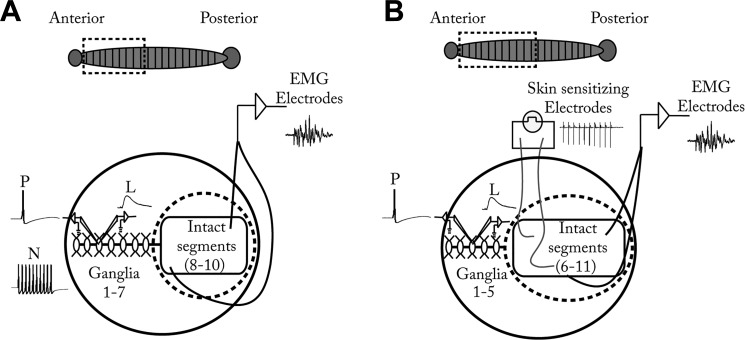
The semi-intact preparations in this study have been described in detail previously (Yuan and Burrell 2013b). Briefly, the preparations consisted of a dissected portion of the CNS in which intracellular recordings were made and an intact portion in which behavior was monitored (Fig. 2). The Hirudo CNS consists of a chain of ganglia linked by a connective nerve (Muller et al. 1981). Similar to the arrangement of a spinal cord, each segmental ganglion has the same complement of sensory neurons, motor neurons, and interneurons (notable exceptions are the head and tail ganglia, which were not used in this study). Individual cells were identified on the basis of their size, position, and electrophysiological properties. Each animal was anesthetized by immersion in ice-cold saline and dissected in frozen Sylgard-filled dishes containing ice-cold glucose (10 mM) saline. During the dissection, the leech was pinned ventral side up, and an incision was made along the midline from segments 1 to 7, with the head ganglion removed to minimize response variability (Shaw and Kristan 1995, 1997). The chain of ganglia from segments 1–7 was dissected from the skin but remained connected to segments 8–10, which were left intact and could still shorten (Fig. 2A). The semi-intact preparation was pinned into a 3-mm Sylgard-lined dish. Teflon-coated silver wires (0.008 in.; A-M Systems, Carlsborg, WA) were bared at the tips and inserted into the longitudinal muscle layer just beneath the skin in the intact portion of the preparation. These wires were connected to a differential amplifier (model 3600; A-M Systems, Everett, WA) and used to make electromyogram (EMG) recordings that provided a measure of the magnitude of the shortening reflex (Lewis and Kristan 1998; Yuan and Burrell 2013b). EMG activity was quantified by first transforming the signal so that the upward and downward peaks were all in the same direction and then measuring the area under the curve for the 2-s period beginning at the point where the P-cell stimulus train started with Clampfit analysis software (Molecular Devices, Sunnyvale, CA). This approach has been shown to accurately reflect the magnitude of the shortening reflex in a previous study (Yuan and Burrell 2013b). A ring-shaped Sylgard barrier was affixed to the bottom of the dish with petroleum jelly to isolate the intact portion from the rest of the bath so that drugs would only be applied to the CNS portion of the preparation.
Fig. 2.
Semi-intact preparations used in this study. A: the ventral nerve cord from segments 1–7 was dissected and pinned onto a Sylgard dish but remained attached to the intact portion of the preparation (segments 8–10), which was loosely pinned to permit shortening. Electromyogram (EMG) electrodes were inserted under the skin to record the shortening response. Dashed line represents a Sylgard ring placed around the intact portion of the preparation to ensure that drugs were exclusively applied to the CNS portion of the preparation. Petroleum jelly was used to secure the ring to the bottom of the dish. In segment 3, intracellular electrodes were used to impale the nociceptive neuron (N) to deliver HFS to induce sensitization and to record synaptic transmission between the P cell and L motor neuron (L). B: for experiments in which sensitization was elicited with noxious cutaneous stimulation, the semi-intact preparation setup was the same with the following changes: The CNS portion of the preparation included ganglia from segments 1–5, and the intact portion of the preparation consisted of segments 6–11 to accommodate both EMG recording electrodes and a second pair of Teflon-coated silver wire electrodes to deliver sensitizing stimuli in segments 6 and 7. Inset at top: drawing of an intact leech, with dashed box representing the portion of the animal used in the semi-intact preparation.
For experiments in which the cutaneous sensitizing stimuli were delivered, the preparations were set up as before. An additional pair of Teflon-coated silver wires (0.0035 in.; A-M Systems) was implanted in the skin between segments 6 and 7 and bared at the point of contact with the skin to stimulate discrete areas of the leech body wall (Fig. 2B). Before the start of the experiment, the magnitude of a single 1-ms electric shock sufficient to elicit whole body shortening was determined. A test shock was delivered at 1 V and then increased in 1-V increments at 2-min intervals until a level was reached that produced robust shortening (for all experiments the stimulus voltage was between 2 and 5 V). This electrical stimulation has been previously shown to activate T, P, and N cells (Burrell and Sahley 1998; Kristan et al. 1982; Shaw and Kristan 1995). Once the threshold was determined, the preparation was allowed to rest for 30 min. For the actual sensitization experiments, the stimuli used to elicit sensitization consisted of two trains of electric shocks (1-s train duration, 10 spikes/train at 10 Hz, 2-min intertrain interval) at the voltage level established before the start of the experiment. This protocol has been shown to produce robust sensitization (Burrell and Sahley 2005).
In these same semi-intact preparations, current-clamp (bridge balanced) intracellular recordings were made with sharp glass microelectrodes (~25–40 MΩ) fabricated from borosilicate glass capillary tubes (1.0-mm OD, 0.75-mm ID; FHC, Bowdoinham, ME) with a horizontal puller (Sutter Instruments P-97; Novato, CA) and were filled with 2 M potassium acetate. Manual micropositioners (model 1480; Siskiyou, Grants Pass, OR) were used to impale individual neurons in ganglion 3 or 4. Current pulses were delivered to electrodes with a multichannel programmable stimulator (STG 1004; Multi-Channel Systems; Reutlingen, Germany), and the signal was recorded with a bridge amplifier (BA-1S; NPI, Tamm, Germany) and digitally converted for analysis (Axoscope; Molecular Devices).
Synaptic transmission by the P cell onto the L motor neuron was recorded intracellularly as previously described (Wang and Burrell 2016). The whole body shortening reflex was elicited by intracellularly stimulating a single P cell with a train of 20 action potentials at 25 Hz (Fig. 3Aiv).
For each experiment, pretest recordings of the P-to-L excitatory postsynaptic potentials (EPSPs) and shortening response were conducted. EPSPs were recorded every 10 s, and the peak EPSP amplitude was calculated by averaging ~5–10 recordings for both the pre- and posttests. For EMG recordings of the shortening reflex, the average of three responses elicited with a 2-min intertrial interval was calculated for both the pre- and posttests. In the first set of experiments, the effects of 2-AG on EPSP amplitude and the shortening reflex were tested by applying 100 µM 2-AG to the exposed CNS portion of the preparation for 15 min followed by washout with normal saline and then posttest recordings of the EPSP and shortening reflex 60 min later. In the second set of experiments, the effects of N-cell HFS (20 trains, 10 action potentials/train at 25 Hz with 10-s intertrain interval) were examined. The stimulated N cell was in the same segment as and ipsilateral to the P cell used to elicit shortening and P-to-L synaptic responses. The soma of the L motor neuron was located contralateral to the N and P cells. L cells actually stimulate muscles contralateral to where their soma are located and receive synaptic input on both sides of the ganglion (Muller and McMahan 1976; Nicholls and Purves 1970; Shaw and Kristan 1995), owing to the fact that they function to produce symmetrical muscle contractions. Recordings of the P-to-L EPSPs and P cell-elicited shortening were made before HFS (pretest) and 60 min after HFS ended (posttest). In the third set of experiments, sensitizing stimuli (cutaneous electric shocks) were applied to the intact portion of the preparation, similar to earlier experiments on sensitization of the Hirudo shortening reflex (Burrell et al. 2001; Burrell and Sahley 2005). Again, recordings of the P-to-L EPSPs and P cell-elicited shortening were made before sensitizing stimuli (pretest) and 60 min after sensitizing stimuli ended (posttest). For experiments that incorporated pretreatment with SB, THL, or RIT, pretest recordings of the P-to-L EPSPs were made after 15-min bath application of SB, THL, or RIT. After coapplication of drug treatment and either the HFS or the sensitizing stimuli, drug application was continued for an additional 15 min. Control experiments utilized the same pretreatment drug treatment duration. All treatment groups were tested in parallel with a vehicle control group, either 0.01% DMSO in the case of 2-AG or SB, THL, or RIT in the case of pretreatment experiments.
Synaptic recording experiments were also conducted in isolated ganglia as described in previous studies (Wang and Burrell 2016; Yuan and Burrell 2013a). Briefly, animals were dissected to remove an individual ganglion under ice-cold saline. The ganglion was then placed in a recording microchamber. Intracellular recordings were conducted in the P cell and one of its postsynaptic target neurons, either the L motor neuron or the anterior pagoda (AP) cell. The tissue was pretreated with 10 µM RIT, during which time pretest measurements of the P-elicited EPSP and the postsynaptic input resistance (IR) were measured. Next, 10 µM RIT + 100 µM 2-AG was bath-applied for 15 min. The RIT + 2-AG solution was washed out for 60 min, and then posttest recordings of the P-elicited EPSP and postsynaptic IR were carried out. The effects of RIT + 2-AG on the P-to-L and P-to-AP synapses were compared to tissue treated with the vehicle (0.01% DMSO in saline).
Statistics.
EPSP amplitude measurements of the pre- and posttest recordings were normalized and are presented as means ± SE. Statistical analyses using t-test or a one-way analysis of variance (ANOVA) were performed to determine main effects, with Newman-Keuls post hoc tests to confirm the ANOVA results. The analyses were carried out with SigmaPlot. All significances were determined at an α level of at least P < 0.05. Sample size refers to the number of animals tested.
RESULTS
Effects of 2-AG on nonnociceptive synapses and behavior require activation of TRPV-like receptor.
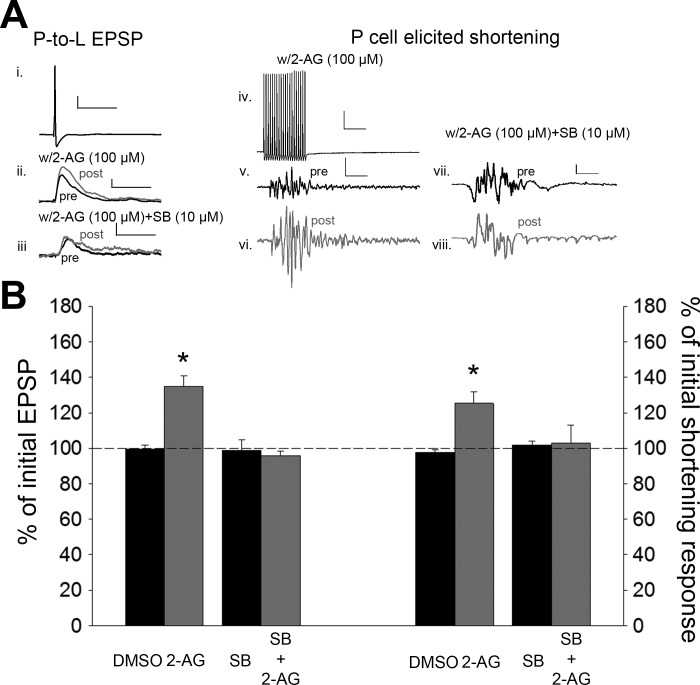
Previous Hirudo studies have shown that 2-AG potentiates nonnociceptive P-cell synapses and that this potentiation could be prevented by pretreatment of the TRPV receptor antagonist SB (Higgins et al. 2013; Wang and Burrell 2016). Here we examined whether 2-AG/TRPV-mediated potentiation of P-cell synapses could alter the P-elicited shortening response. 2-AG (100 µM; 15 min) was bath-applied selectively to the CNS portion of the preparation (ganglia from segments 1–7; Fig. 2A) and then washed out with normal saline for 60 min. In the 2-AG-treated preparations, the P-to-L synapses were significantly enhanced during posttest compared with the pretest recordings [Fig. 3; 1-way ANOVA, F(3,19) = 16.52, P < 0.001; post hoc 2-AG vs. DMSO, P < 0.001], consistent with previous findings. This synaptic change was accompanied by an increase in P cell-elicited shortening reflex [Fig. 3A; 1-way ANOVA, F(3,19) = 4.22, P < 0.05; post hoc 2-AG vs. DMSO, P < 0.05]. To determine whether TRPV-like channels are required for 2-AG-induced increases in shortening, experiments were carried out in which SB (10 µM) was preapplied for 15 min and then coapplied with 2-AG for 15 min. Both SB and 2-AG were washed out for 60 min, and then posttest recordings were carried out. No potentiation was observed in the SB + 2-AG-treated P-cell synapses, again consistent with previous experiments (Fig. 3; post hoc 2-AG vs. SB + 2-AG, P < 0.001). In the same SB + 2-AG-treated preparations, no change in the P-elicited shortening reflex was observed (Fig. 3; post hoc 2-AG vs. SB + 2-AG, P < 0.05). No change in synaptic transmission or behavior was observed in the vehicle control group (0.01% DMSO) or in preparations in which SB was bath-applied alone. No changes in the postsynaptic (L cell) IR were observed in the DMSO control (t = −0.04, P > 0.05), 2-AG (t = −1.11, P > 0.05), SB alone (t = −0.76, P > 0.05), or SB + 2-AG (t = 0.23, P > 0.05) groups. These results indicate that 2-AG facilitates whole body shortening in a TRPV-dependent manner with its potentiation/disinhibition of the P-to-L synapses.
Fig. 3.
2-Arachidonoyl glycerol (2-AG) potentiates the nonnociceptive P-cell synapses and P cell-elicited shortening. A: sample recording of P-cell action potential (i; scale bar: 20.0 mV, 50 ms) and the EPSPs elicited before and after bath application of 2-AG (ii; scale bar: 2 mV, 50 ms) or 2-AG + SB (iii; scale bar same as ii) and sample recording of the train of P-cell action potentials used to elicit shortening (iv; scale bar: 20.0 mV, 50 ms) and the shortening responses following treatment with either 2-AG (v and vi; scale bar: 2 mV, 50 ms) or 2-AG + SB (vii and viii; scale bar: 2.0 mV, 50 ms). Black and gray traces are pre- and posttest recordings, respectively. B: average ± SE changes in EPSP amplitude (left) and magnitude of the shortening response (right) (in both cases, y-axis represents % of pretest amplitude) following vehicle (DMSO), 2-AG, or SB + 2-AG (left). *Statistically significant difference compared with controls based on a 1-way ANOVA with Student-Newman-Keuls post hoc test (see results). n = 5 for all treatment and control groups.
Stimulation of a single nociceptive neuron potentiated both shortening behavior and nonnociceptive synapses.
Endocannabinoid-mediated potentiation of the P-cell synapses can also be produced via certain patterns of afferent activation. Specifically, HFS of a single N cell (20 trains with 10 spikes at 25 Hz with 10-s intertrain interval) potentiates P-cell synapses in experiments using isolated ganglia, and this potentiation requires 2-AG synthesis and TRPV-like channel activation (Wang and Burrell 2016). In those earlier experiments, HFS of the N cell was chosen because it was thought that it would mimic the effects of a noxious sensitizing stimuli. Therefore, it is appropriate to examine whether this HFS of a single N cell does in fact produce behavioral sensitization and if that sensitization involves endocannabinoid signaling.
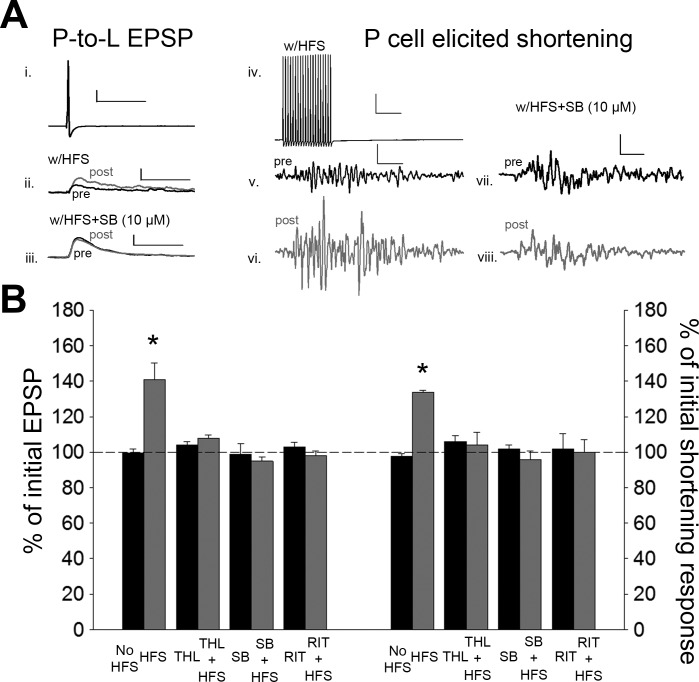
After pretest recordings of P-cell synaptic transmission and P-elicited shortening, HFS of a single N cell was carried out in the same ganglion where the P cell used to elicit shortening was located. Posttest recordings were carried out 60 min after the end of the HFS. Consistent with previous observation in isolated ganglia, N-cell HFS induced a significant increase in the P-to-L synaptic transmission [Fig. 4; 1-way ANOVA, F(7,39) = 11.32; P < 0.001; post hoc HFS vs. no stimulation (No Stim), P < 0.001]. In parallel with the observed synaptic potentiation, a significant increase in the magnitude of the shortening response was also observed [Fig. 4; 1-way ANOVA, F(7,39) = 5.40; P < 0.001; post hoc HFS vs. No Stim, P < 0.001]. No synaptic or behavioral changes were observed in control experiments with no HFS. No changes were observed in the postsynaptic IR in either the HFS group (t = 0.06, P > 0.05) or the no stimulus control group (t = 0.08, P > 0.05).
Fig. 4.
N-cell HFS elicits potentiation of the P-cell synapses and P-elicited shortening that are both 2-AG/TRPV dependent. A: sample recording of P-cell action potential (i; scale bar: 20.0 mV, 50 ms) and the EPSPs elicited before and after N-cell HFS (ii; scale bar: 2 mV, 50 ms) or HFS + SB (iii; scale bar same as ii) and sample recording of the train of P-cell action potentials used to elicit shortening (iv; scale bar: 20.0 mV, 50 ms) and the shortening responses following N-cell HFS (v and vi; scale bar: 2 mV, 50 ms) or N-cell HFS + SB (vii and viii; scale bar: 2.0 mV, 50 ms). Black and gray traces are pre- and posttest recordings, respectively. B: average ± SE changes in EPSP amplitude (left) and magnitude of the shortening response (right) in the no-stimulation control (No HFS), HFS, THL with no stimulation (THL), THL + HFS, SB with no stimulation (SB), SB + HFS, ritanserin with no stimulation (RIT), and RIT + HFS groups. *Statistically significant difference compared with controls based on a 1-way ANOVA with Student-Newman-Keuls post hoc test (see results). n = 5 for all treatment and control groups.
Next, we examined whether inhibition of 2-AG synthesis or TRPV-like channels would prevent HFS-mediated sensitization of the shortening behavior. THL is an inhibitor of diacylglycerol lipase which is primarily responsible for 2-AG synthesis (Lee et al. 1995). THL has been shown to block activity-dependent endocannabinoid modulation of Hirudo synapses (Wang and Burrell 2016; Yuan and Burrell 2013b). In semi-intact preparations in which N-cell HFS was carried out in the presence of THL (10 µM), no potentiation of the P-cell synapse and no sensitization of the shortening reflex were observed (Fig. 4; synapses, post hoc HFS vs. HFS + THL, P < 0.001; behavior, post hoc HFS vs. HFS + THL, P < 0.001). No synaptic or behavioral changes were observed in vehicle control experiments (0.01% DMSO) or in experiments in which THL alone was applied. To test the involvement of TRPV-like channels, N-cell HFS was delivered in the presence of SB. Again, no potentiation of the P-cell synapse and no sensitization of the shortening reflex were observed in HFS + SB preparations (Fig. 4; synapses, post hoc HFS vs. HFS + SB, P < 0.001; behavior, post hoc HFS vs. HFS + SB, P < 0.001). No synaptic or behavioral changes were observed in experiments in which SB alone was applied. Furthermore, no changes were observed in postsynaptic IR in the THL (t = −0.33, P > 0.05), HFS + THL (t = 0.07, P > 0.05), or HFS + SB (t = −0.48, P > 0.05) group. Taken together, these results demonstrated that N-cell HFS produced potentiation of both P-cell synaptic transmission and the P-elicited shortening response and that both effects were 2-AG/TRPV dependent.
Cutaneous noxious stimuli at a secondary site elicit endocannabinoid-mediated synaptic potentiation and behavioral sensitization.
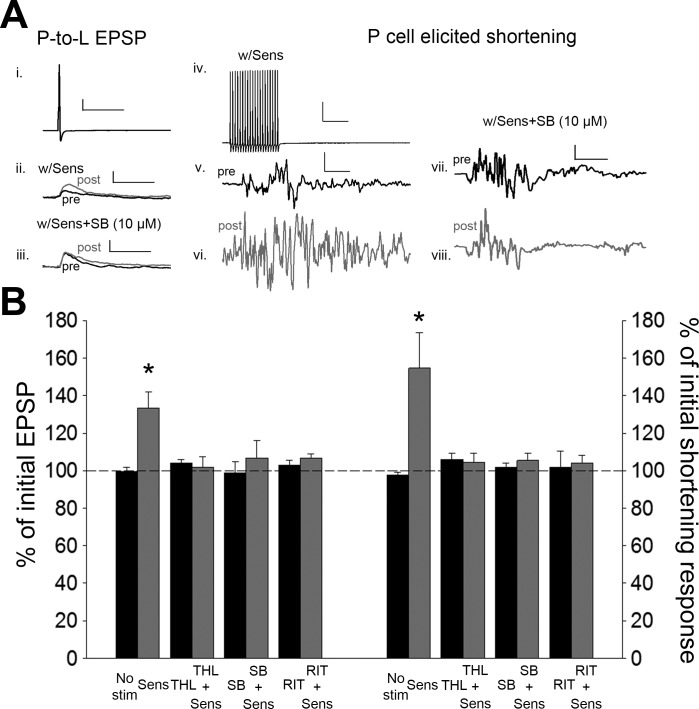
In the previous set of experiments, synaptic potentiation and behavioral sensitization were produced by intracellular stimulation of a single nociceptor. Next we wished to examine whether a more realistic noxious stimulus could produce the same effects in an endocannabinoid-dependent manner. Electric shocks applied to the skin can activate N, P, and T cells and can produce behavioral sensitization even when applied at some distance from the test stimuli used to elicit the behavior being sensitized. Such stimuli are known to produce sensitization of the Hirudo local bending and whole body shortening reflex (Burrell et al. 2001; Burrell and Sahley 2005; Ehrlich et al. 1992; Lockery and Kristan 1991). Here, a sensitizing stimulus in the form of a train of noxious electric shocks was applied to segments 6 and 7 skin and was sufficiently far away from the test stimulus (in segment 3) that there was no overlap in the sensory cells activated (see Fig. 2B).
As stated in materials and methods, after pretest recordings of P-cell synaptic transmission and P-elicited shortening, sensitizing stimuli were delivered to skin and then posttest recordings were carried out 60 min later. Sensitizing stimuli produced potentiation of the P-cell synapses [Fig. 5; 1-way ANOVA, F(7,39) = 4.00; P < 0.005; post hoc sensitizing stimuli (Sens) vs. No Stim, P < 0.005] as well as an increase in P-elicited body shortening [Fig. 5; 1-way ANOVA, F(7,39) = 5.32; P < 0.005; post hoc Sens vs. No Stim, P < 0.005]. No synaptic or behavioral changes were observed in control experiments in which no sensitizing stimuli were applied.
Fig. 5.
Noxious electrical stimulation applied to the skin (sensitizing stimuli) elicits potentiation of the P-cell synapse and P-elicited shortening that is 2-AG/TRPV dependent. A: sample recording of P-cell action potential (i; scale bar: 20.0 mV, 50 ms) and the EPSPs elicited before and after application of sensitizing stimuli (Sens) (ii; scale bar: 2 mV, 50 ms) or Sens + SB (iii; scale bar same as ii) and sample recording of the train of P-cell action potentials used to elicit shortening (iv; scale bar: 20.0 mV, 50 ms) and the shortening responses following Sens (v and vi; scale bar: 2 mV, 50 ms) or Sens + SB (vii and viii; scale bar: 2.0 mV, 50 ms). Black and gray traces are pre- and posttest recordings, respectively. B: average ± SE changes in EPSP amplitude (left) and magnitude of the shortening response (right) in the no sensitization control (No stim), sensitized (Sens), THL with no stimulation (THL), THL + Sens, SB with no stimulation (SB), SB + Sens, ritanserin with no stimulation (RIT), and RIT + Sens groups. *Statistically significant difference compared with controls based on a 1-way ANOVA with Student-Newman-Keuls post hoc test (see results). n = 5 for all treatment and control groups.
To determine whether sensitization-induced synaptic and behavioral changes were 2-AG/TRPV dependent, experiments were conducted in which the sensitizing stimuli were delivered in the presence of THL or SB. Sensitization-induced potentiation of P-cell synapses and increases in the P-elicited shortening reflex were absent in preparations treated with THL during delivery of the sensitizing stimuli (Fig. 5; synapses, post hoc Sens vs. Sens + THL, P < 0.005; behavior, post hoc Sens vs. Sens + THL, P < 0.005). Identical results were observed when SB was applied during delivery of the sensitizing stimuli (Fig. 5; synapses, post hoc Sens vs. Sens + SB, P < 0.005; behavior, post hoc Sens vs. Sens + SB, P < 0.005). No synaptic or behavioral changes were observed in preparations treated with the vehicle or when THL or SB was applied without the sensitizing stimuli. In addition, no changes were observed in postsynaptic IR in the Sens (t = −0.46, P > 0.05), Sens + THL (t = −0.20, P > 0.05), or Sens + SB (t = −1.10, P > 0.05) groups. These results show that stimuli well established as producing sensitization of the shortening reflex also produced potentiation of P-cell synaptic transmission and that both behavioral and synaptic changes were 2-AG dependent and required activation of TRPV-like channels.
Role of serotonin signaling during sensitization-induced synaptic and behavioral effects.
Serotonin (5-HT) is known to play a critical role in sensitization of the Hirudo shortening reflex (Burrell and Sahley 2005; Ehrlich et al. 1992). In addition, experiments in isolated ganglia have shown that inhibition of 5-HT receptors prevents endocannabinoid-mediated synaptic modulation. Specifically, it was found that RIT, an inhibitor of the mammalian 5-HT2 receptor that has been successfully used in invertebrates such as Aplysia and Helix (Cohen et al. 2003; Kiss et al. 2003), blocked the activity-dependent synaptic plasticity in Hirudo that was found to be endocannabinoid dependent (Li and Burrell 2010; Yuan and Burrell 2012). Here similar experiments were conducted to determine whether RIT (10 µM) could affect the behavioral sensitization and synaptic potentiation. In experiments in which RIT was applied to semi-intact preparations during N-cell HFS, both potentiation of the P-cell synapse and increases in the P-elicited shortening reflex appeared to be blocked (Fig. 4B; synapses, post hoc HFS vs. HFS + RIT, P < 0.001; behavior, post hoc HFS vs. HFS + RIT, P < 0.001). Control experiments in which RIT was applied without N-cell HFS exhibited no synaptic or behavioral changes. Also, no changes in postsynaptic IR were observed with either HFS + RIT (t = −0.06, P > 0.05) or RIT alone (t = −0.48, P > 0.05).
Identical results were observed when RIT was applied during the delivery of cutaneous sensitizing stimuli. In RIT + sensitization preparations, no potentiation of P-cell synaptic transmission or increase in the magnitude of the shortening reflex was observed (Fig. 5B; synapses, Sens vs. Sens + RIT, P < 0.005; behavior, post hoc Sens vs. Sens + RIT, P < 0.005). Again, no changes in postsynaptic IR were observed in the Sens + RIT group (t = −0.69, P > 0.05). Together, these results suggest involvement of 5-HT-mediated signaling, specifically a 5-HT2-like receptor, in eCB-modulation at the synaptic and behavioral levels.
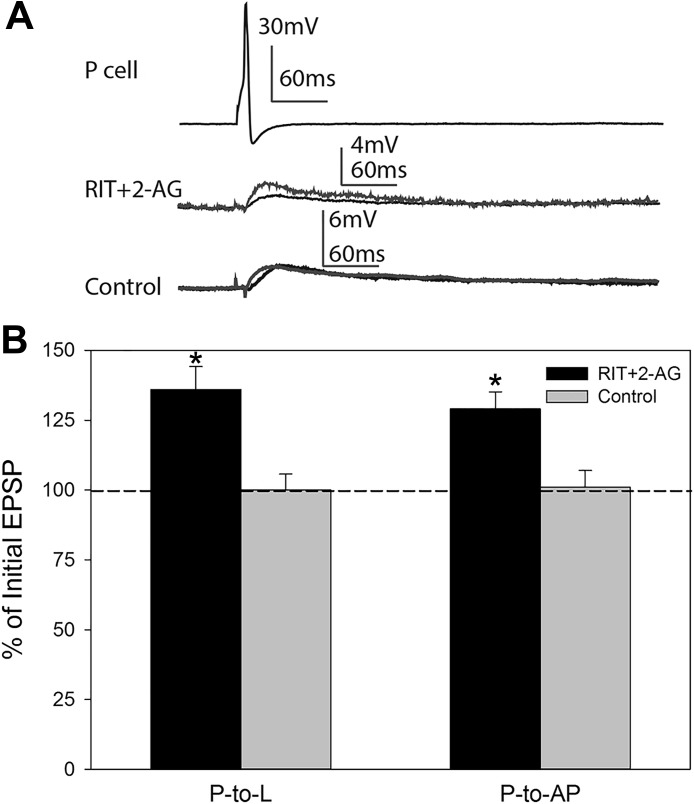
It is unclear whether 5-HT receptor activation is upstream or downstream of 2-AG synthesis. That is, does afferent activity stimulate 5-HT release that then elicits 2-AG synthesis or the other way around? One way to address this question is to bath-apply 2-AG in the presence of RIT. If the 2-AG-mediated synaptic potentiation requires downstream 5-HT receptor activation, then RIT should prevent the effects of bath-applied 2-AG. To address this question, isolated ganglia were pretreated with 10 µM RIT as in previous studies and then treated with 100 µM 2-AG for 15 min. In separate experiments, P-to-L and P-to-AP synapses were tested before 2-AG bath application and 60 min after 2-AG washout. Potentiation of P synapses was still observed in RIT + 2-AG-treated ganglia (Fig. 6); a 36 ± 8.5% increase in the P-to-L EPSP compared with a 0.0 ± 5.7% change in control synapses (t = 3.52, P ≤ 0.05) and a 29 ± 6.1% increase in the P-to-AP EPSP compared with 1 ± 5.2% increase in control synapses (t = 3.53, P ≤ 0.05). No change in postsynaptic IR was observed in either the L motor neurons (t = 0.39, P > 0.05) or the AP cell (t = 1.41, P > 0.05) during these experiments. These results indicate that the potentiating effects of 2-AG on P synapses are not 5-HT dependent and suggest that the 5-HT receptor activation is upstream of 2-AG synthesis.
Fig. 6.
Application of 5-HT receptor inhibitor does not affect 2-AG-mediated potentiation of P synapses. A: sample traces of P-elicited EPSP in L motor neuron in ganglia pretreated with ritanserin and then 2-AG (RIT+2-AG) vs. vehicle control experiments. B: average ± SE changes in EPSP amplitude in P-to-L and P-to-AP synapses in RIT+2-AG- and vehicle control-treated groups. *Statistically significant difference based on unpaired t-test (see results). n = 3 for RIT+2-AG treated P-to-L and P-to-AP synapses, respectively, n = 3 for P-to-L control synapses, and n = 4 for P-to-AP control synapses.
DISCUSSION
The endocannabinoid 2-AG has already been shown to potentiate the P-cell synapse through activation of a TRPV-like channel (Higgins et al. 2013; Wang and Burrell 2016). P cells lack TRPV-like channels, and 2-AG-induced potentiation is thought to be due to depression of tonic GABAergic input since treatments that interfere with GABAergic inhibition block this increase in synaptic transmission (Higgins et al. 2013; Pastor et al. 1996; Summers et al. 2014; Wang and Burrell 2016). In addition, endocannabinoid-induced potentiation of these synapses can also be produced by HFS of the N cells that requires 2-AG synthesis and TRPV-like channel function (Wang and Burrell 2016). In this study, we extended these synaptic findings to the functional/behavioral level by showing that 2-AG enhances the magnitude of the shortening reflex elicited by stimulation of a single P cell in a semi-intact preparation in parallel with P-cell synaptic potentiation. Furthermore, two related stimulation paradigms that produced synaptic potentiation and behavioral sensitization were both shown to be 2-AG/TRPV dependent. The first was the same N-cell HFS described above, and the second was noxious stimuli applied to the skin, which is well established as producing sensitization of the shortening reflex (Boulis and Sahley 1988; Burrell et al. 2001; Burrell and Sahley 2005; Ehrlich et al. 1992). Both of these stimuli involve strong activation of the N cells, but they differ in that N-cell HFS was applied in the segment in which the P cell used to elicit shortening was located (segment 3) and obviously involved activation of the N cell alone, whereas the noxious skin stimulation was delivered several segments away (in segments 6 and 7) from where the test stimulus was applied and activated N, P, and T cells. This suggests that sensitizing stimuli are capable of eliciting 2-AG synthesis and release throughout the Hirudo CNS. Figure 7 summarizes the proposed model for how noxious stimuli produce 2-AG synthesis that leads to disinhibition of the P-cell synapses that is hypothesized to contribute to sensitization. 2-AG synthesis and release as a result of noxious stimuli is inferred based on the THL experiments, and the identity of the neuron(s) responsible for 2-AG synthesis and release is unknown. However, one potential candidate is the S interneuron, which is critical for the induction of sensitization, receives input from all three mechanoafferents, and has been implicated in other forms of 2-AG-mediated neuromodulation (Baccus et al. 2000; Li and Burrell 2011; Modney et al. 1997; Muller and Scott 1981; Sahley et al. 1994).
Fig. 7.
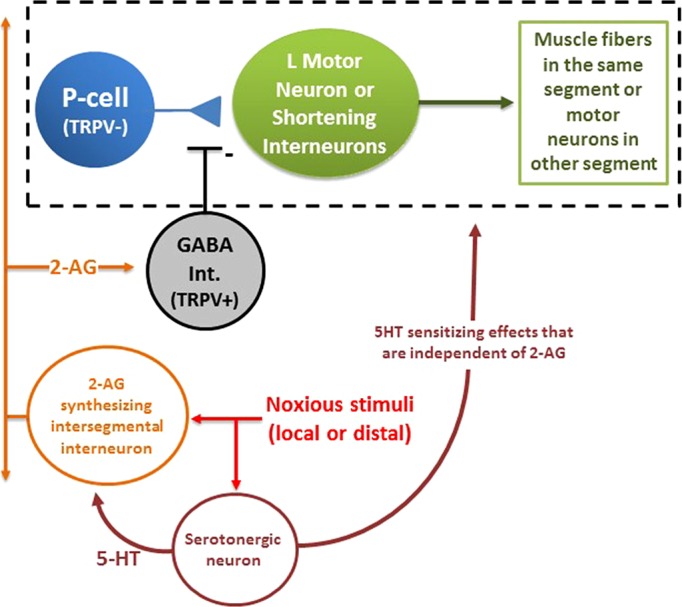
Simplified model of how endocannabinoid-mediated disinhibition enhances behavioral responses elicited by the nonnociceptive P cells. Elements of the whole body shortening circuit (see Fig. 1) have been consolidated so that the modulatory elements can be more easily presented. Noxious stimuli that can be either in close proximity to the P cell eliciting shortening (see N-cell HFS data in Fig. 4) or some distance away (see sensitizing stimuli data in Fig. 5) activate both serotonergic neurons, possibly Retzius cells, throughout the CNS (Crisp and Burrell 2008) and neuron(s) that synthesize 2-AG. It is hypothesized that the combination of the afferent stimulation and 5-HT from the serotonergic neurons stimulates 2-AG synthesis and release. The identity of this 2-AG synthesizing neuron associated with sensitization is unknown, but its influence would appear to extend throughout the CNS. It is possible that each ganglion has a 2-AG cell that receives intersegmental afferent input, similar to the serotonergic Retzius cells (Szczupak and Kristan 1995). Alternatively, the 2-AG cell itself may be an intersegmental interneuron that extends through the entire CNS. The released 2-AG depresses GABAergic transmission, and this in turn disinhibits the P-cell synapse, increasing the signaling by the P cell to both the local motor neurons involved in shortening and the interneuron that carries the signal to shorten to the rest of the CNS. 5-HT also contributes to sensitization through effects on the shortening neural circuit that are 2-AG independent.
An additional discovery was that activation of a 5-HT2-like receptor was also required for behavioral sensitization and synaptic potentiation. It has long been known that 5-HT is required for behavioral sensitization in Hirudo (Ehrlich et al. 1992; Burrell and Sahley 2005; Lockery and Kristan 1991), but this is the first time that 5-HT-dependent synaptic facilitation in Hirudo has been linked with behavioral sensitization. Activation of 5-HT2 receptors have been shown to stimulate 2-AG synthesis, most likely because these receptors activate the PLC/inositol trisphosphate/diacylglycerol pathway that often contributes to this endocannabinoid synthesis (Best and Regehr 2008; Connelly and Baggott 2009; Heifets and Castillo 2009; Parrish and Nichols 2006). Previous experiments in Hirudo have found that the 5-HT2 antagonist RIT blocks 2-AG-mediated synaptic depression elicited by LFS of afferents (Li and Burrell 2010; Yuan and Burrell 2012). In the present study, RIT pretreatment did not block synaptic potentiation elicited by subsequent bath application of 2-AG, indicating that the effects of 2-AG do not depend on the downstream activation of 5-HT2-like receptors. Although not conclusive, these findings support the idea that 5-HT2-like receptors may play a role in stimulating 2-AG synthesis. It is should also be noted, however, that 5-HT contributes to sensitization through other, parallel mechanisms that have nothing to do with 2-AG/TRPV signaling. For example, sensitization also produced increases in excitability in the S interneuron that are mediated by 5-HT through activation of a receptor coupled to the cAMP/PKA pathway (Burrell and Sahley 2005).
5-HT is a well-known contributor to sensitization in other animals, most prominently in the well-studied examples of sensitization of the gill and siphon withdrawal behaviors in Aplysia (Glanzman 2006; Hawkins et al. 2006). An interesting implication of our findings is that 5-HT may mediate the synaptic facilitation associated with sensitization in these experiments, at least in part, through endocannabinoid-dependent mechanisms since 5-HT receptor activation is hypothesized to be upstream of endocannabinoid synthesis. There is evidence in the Aplysia literature for disinhibition to have a role in behavioral sensitization as a result of serotonergic modulation (Trudeau and Castellucci 1993) that could potentially be mediated by endocannabinoids. This does not diminish the role of the other mechanisms by which 5-HT enhances synaptic transmission or excitability during sensitization but suggests an additional, endocannabinoid-mediated component of this form of neuroplasticity.
These findings are especially relevant to understanding the potential pro- and antinociceptive effects of the endocannabinoid system. In vertebrates, there are distinct sensory neurons for detecting nonnociceptive (primarily by Aβ fibers) vs. nociceptive (by Aδ and C fibers) stimuli and the pathways for these nociceptive and nonnociceptive afferents are usually anatomically and functionally segregated within the CNS (Willis and Westlund 1997). However, nonnociceptive afferents do have access to the nociceptive circuitry in the spinal cord through polysynaptic connections, and this access is controlled by local inhibitory interneurons that act as a gate to permit or block input to the secondary afferents. Consequently, disinhibition can allow these nonnociceptive afferents to have access to the nociceptive circuitry that they do not normally have, thereby contributing to allodynia, a form of sensitization in which nonpainful stimuli are perceived as painful (Lu et al. 2013; Petitjean et al. 2015; Torsney and MacDermott 2006).
Invertebrates also utilize distinct sensory neurons for detecting and communicating nociceptive vs. nonnociceptive stimuli (Im and Galko 2012; Smith and Lewin 2009). In Hirudo the N and P cells often converge to have synaptic input on the same neurons, such as the L motor neurons, that contribute to whole body shortening (Nicholls and Purves 1970), but these inputs lack the distinction seen in vertebrates, where nociceptive inputs are monosynaptic and nonnociceptive inputs are polysynaptic. In all the cases that have been studied to date, the P and N synapses either are both a mix of mono- and polysynaptic inputs or are both polysynaptic (Higgins et al. 2013; Nicholls and Purves 1970; Velázquez-Ulloa et al. 2003). Nevertheless, in the case of the shortening reflex the N and P cells differ in terms of their ability to elicit this behavior. A train of 10 action potentials at 10 Hz in a single N cell can elicit robust shortening, but for the P-elicited shortening either multiple P cells must be simultaneously activated or, as shown in this study, a single P cell must be stimulated to a much greater extent (Shaw and Kristan 1995; Yuan and Burrell 2013b). Similar to the role of inhibitory neurons in regulating Aβ input to nociceptive circuits, GABAergic inhibition can regulate the gain of P-cell synapses and their input to withdrawal behavior circuits (Baca et al. 2008; Wang et al. 2015). Therefore, while the neuroanatomical details may differ between the Hirudo ganglion and the vertebrate spinal cord, there appears to be a shared mechanism by which pressure-sensitive afferent signaling is enhanced through disinhibition to increase responses to nonnociceptive stimuli.
What makes these details relevant is that the Hirudo CNS provides a system to address the question of how endocannabinoids can have both pro- and antinociceptive effects (Carey et al. 2016; Christie and Mallet 2009; Pernía-Andrade et al. 2009). The antinociceptive effects of endocannabinoids are likely due primarily to depression of glutamatergic synapses (Gregg et al. 2012; Hohmann et al. 2005; Kato et al. 2012; Kinsey et al. 2009; Liang et al. 2004; Morisset and Urban 2001). The pronociceptive effects of endocannabinoids are likely due to depression of inhibitory synaptic signaling leading to disinhibition (Pernía-Andrade et al. 2009). Collectively, our studies in Hirudo provided support for this model. On the antinociceptive side, endocannabinoids depress N-cell synaptic transmission and also depress the magnitude of the shortening response elicited by N-cell activation (Yuan and Burrell 2010, 2013b). These effects are mediated by the activation of presynaptic TRPV-like channels that reduce neurotransmitter release via calcineurin- and translation-dependent mechanisms (Yuan and Burrell 2012, 2013a). On the pronociceptive side, endocannabinoids potentiate P-cell synaptic transmission (Higgins et al. 2013; Wang and Burrell 2016) and also sensitize the shortening response elicited by P-cell stimulation (present data). These findings suggest a mechanism in which noxious stimuli or injury stimulates the production of endocannabinoids that, in turn, leads to disinhibition of nonnociceptive afferent signaling and behavioral sensitization. In Hirudo pharmacological evidence suggests that endocannabinoids mediate this effect through activation of a TRPV-like channel, while CB1 receptors have been implicated in this pronociceptive effect in mammals (Pernía-Andrade et al. 2009). However, TRPV1 channels have also been found to mediate a form of allodynia that involves disinhibition within the spinal nociceptive circuitry in mice, although what activates TRPV1 to produce allodynia is not known (Kim et al. 2012).
Together with our earlier studies, these findings also provide insight into how different patterns of activity can elicit different forms of endocannabinoid-mediated neuromodulation. Endocannabinoid synthesis and release is an activity-dependent process (Heifets and Castillo 2009), and there is evidence that different patterns of activity within synaptic circuits can produce either endocannabinoid-dependent or -independent forms of synaptic modulation (Adermark and Lovinger 2009; Li and Burrell 2009; Zhu and Lovinger 2007). Our studies extend this observation to show that different patterns of activity can selectively produce different forms of endocannabinoid-mediated modulation in specific synaptic circuits. From previous studies we have observed that LFS of T cells produces 2-AG-mediated LTD of N-cell synapses without affecting P-cell synapses (Wang and Burrell 2016; Yuan and Burrell 2010, 2013b). Furthermore, it is known that the L motor neuron is the site of 2-AG synthesis responsible for this depression of the N cell (Yuan and Burrell 2013b). On the other hand, from this study and earlier experiments (Wang and Burrell 2016) we have found that HFS of the N cells elicits 2-AG-mediated potentiation of the P-cell synapses, although it is not known what neuron is the source of 2-AG. This HFS does not elicit 2-AG-mediated depression in the N cells and in fact appears to produce NMDA receptor potentiation in N-cell synapses (unpublished data). Although these findings demonstrate that different patterns of activity can elicit different forms of endocannabinoid-mediated plasticity within a neural circuit, questions remain. What factors control which form of modulation is generated? Are there different sources of 2-AG synthesis that are either selectively activated by different afferent inputs or are differentially sensitive to HFS vs. LFS? Do the different forms of endocannabinoid plasticity interact in such a way that activation of one process prevents the activation of the other? For example, stimuli that elicit 2-AG-mediated potentiation in the P cell may prevent the activation of 2-AG-mediated depression of N-cell synapses.
It is critical to understand how neural circuits selectively activate and segregate endocannabinoid modulation of excitatory vs. inhibitory synapses. Depression of excitatory synapses will ultimately result in a decrease in circuit output, while depression of inhibitory synapses will produce an increase in circuit output. This is relevant not only for pain, where, as already discussed, endocannabinoids can have opposing effects, but also for other behavioral processes where endocannabinoids may contribute, such as learning and memory and stress. The utility of endocannabinoid-based therapies may very well depend on how effectively they can target the desired effect at the microcircuit level.
GRANTS
This research was supported by grants from the National Science Foundation (IOS-1051734; B. D. Burrell) and the National Institute of Neurological Disorders and Stroke (R01 NS-092716) and by support from the University of South Dakota Division of Basic Biomedical Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.W. performed experiments; Y.W. analyzed data; Y.W. and B.D.B. interpreted results of experiments; Y.W. and B.D.B. prepared figures; Y.W. and B.D.B. drafted manuscript; B.D.B. conceived and designed research; B.D.B. edited and revised manuscript; B.D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the following, who reviewed this manuscript as part of Y. Wang’s thesis: Drs. Gina Forster, Pasquale Manzera, Yi-Fan Li, and Cliff Summers.
Present address of Y. Wang: Dept. of Neuroscience, Icahn School of Medicine at Mt. Sinai, New York, NY 10029.
REFERENCES
- Adermark L, Lovinger DM. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. J Neurosci 29: 1375–1380, 2009. doi: 10.1523/JNEUROSCI.3842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca SM, Marin-Burgin A, Wagenaar DA, Kristan WB Jr. Widespread inhibition proportional to excitation controls the gain of a leech behavioral circuit. Neuron 57: 276–289, 2008. doi: 10.1016/j.neuron.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccus SA, Burrell BD, Sahley CL, Muller KJ. Action potential reflection and failure at axon branch points cause stepwise changes in EPSPs in a neuron essential for learning. J Neurophysiol 83: 1693–1700, 2000. doi: 10.1152/jn.2000.83.3.1693. [DOI] [PubMed] [Google Scholar]
- Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci 28: 6508–6515, 2008. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulis NM, Sahley CL. A behavioral analysis of habituation and sensitization of shortening in the semi-intact leech. J Neurosci 8: 4621–4627, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Generalization of habituation and intrinsic sensitization in the leech. Learn Mem 5: 405–419, 1998. [PMC free article] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Serotonin depletion does not prevent intrinsic sensitization in the leech. Learn Mem 6: 509–520, 1999. doi: 10.1101/lm.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Serotonin mediates learning-induced potentiation of excitability. J Neurophysiol 94: 4002–4010, 2005. doi: 10.1152/jn.00432.2005. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL, Muller KJ. Non-associative learning and serotonin induce similar bi-directional changes in excitability of a neuron critical for learning in the medicinal leech. J Neurosci 21: 1401–1412, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM, Slivicki RA, Leishman E, Cornett B, Mackie K, Bradshaw H, Hohmann AG. A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase. Mol Pain 12: 1744806916649192, 2016. doi: 10.1177/1744806916649192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci 13: 1511–1518, 2010. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Mallet C. Endocannabinoids can open the pain gate. Sci Signal 2: pe57, 2009. doi: 10.1126/scisignal.288pe57. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Onyike CU, McElroy VL, Lin AH, Abrams TW. Pharmacological characterization of an adenylyl cyclase-coupled 5-HT receptor in Aplysia: comparison with mammalian 5-HT receptors. J Neurophysiol 89: 1440–1455, 2003. doi: 10.1152/jn.01004.2002. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Baggott MJ. Role of endocannabinoids in 5-HT2 receptor-mediated effects. J Neurophysiol 101: 5–7, 2009. doi: 10.1152/jn.91054.2008. [DOI] [PubMed] [Google Scholar]
- Crisp KM, Burrell BD. Cellular and behavioral properties of learning in the leech and other annelids. In: Annelids as Model Systems in the Biological Sciences, edited by Shain D. New York: Wiley, 2008. [Google Scholar]
- De Petrocellis L, Melck D, Bisogno T, Milone A, Di Marzo V. Finding of the endocannabinoid signalling system in Hydra, a very primitive organism: possible role in the feeding response. Neuroscience 92: 377–387, 1999. doi: 10.1016/S0306-4522(98)00749-0. [DOI] [PubMed] [Google Scholar]
- Edwards JG. TRPV1 in the central nervous system: synaptic plasticity, function and pharmacological implications. In: Capsaicin as a Therapeutic Molecule, edited by Abdel-Salam OM. Basel: Springer, 2014, p. 77–104. doi: 10.1007/978-3-0348-0828-6_3. [DOI] [PubMed] [Google Scholar]
- Ehrlich JS, Boulis NM, Karrer T, Sahley CL. Differential effects of serotonin depletion on sensitization and dishabituation in the leech, Hirudo medicinalis. J Neurobiol 23: 270–279, 1992. doi: 10.1002/neu.480230306. [DOI] [PubMed] [Google Scholar]
- Elphick MR. The evolution and comparative neurobiology of endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 367: 3201–3215, 2012. doi: 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57: 746–759, 2008. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL. The cellular mechanisms of learning in Aplysia: of blind men and elephants. Biol Bull 210: 271–279, 2006. doi: 10.2307/4134563. [DOI] [PubMed] [Google Scholar]
- Gregg LC, Jung KM, Spradley JM, Nyilas R, Suplita RL 2d, Zimmer A, Watanabe M, Mackie K, Katona I, Piomelli D, Hohmann AG. Activation of type 5 metabotropic glutamate receptors and diacylglycerol lipase-α initiates 2-arachidonoylglycerol formation and endocannabinoid-mediated analgesia. J Neurosci 32: 9457–9468, 2012. doi: 10.1523/JNEUROSCI.0013-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13: 1519–1525, 2010. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull 210: 174–191, 2006. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol 71: 283–306, 2009. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins A, Yuan S, Wang Y, Burrell BD. Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids. Mol Pain 9: 26, 2013. doi: 10.1186/1744-8069-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature 435: 1108–1112, 2005. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Im SH, Galko MJ. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev Dyn 241: 16–26, 2012. doi: 10.1002/dvdy.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T, Edwards JG. Calcineurin is required for TRPV1-induced long-term depression of hippocampal interneurons. Neurosci Lett 510: 82–87, 2012. doi: 10.1016/j.neulet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 119: 889–900, 2004. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kato A, Punnakkal P, Pernía-Andrade AJ, von Schoultz C, Sharopov S, Nyilas R, Katona I, Zeilhofer HU. Endocannabinoid-dependent plasticity at spinal nociceptor synapses. J Physiol 590: 4717–4733, 2012. doi: 10.1113/jphysiol.2012.234229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliullina H, Bilgin M, Sampaio JL, Shevchenko A, Eaton S. Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc Natl Acad Sci USA 112: 3415–3420, 2015. doi: 10.1073/pnas.1416463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, Na HS, Bae YC, Kim SJ, Kim JS, Jung SJ, Oh SB. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron 74: 640–647, 2012. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330: 902–910, 2009. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Hiripi L, Papp N, Elekes K. Dopamine and serotonin receptors mediating contractions of the snail, Helix pomatia, salivary duct. Neuroscience 116: 775–790, 2003. doi: 10.1016/S0306-4522(02)00754-6. [DOI] [PubMed] [Google Scholar]
- Kristan WB Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol 76: 279–327, 2005. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kristan WB, McGirr SJ, Simpson GV. Behavioural and mechanosensory neurone responses to skin stimulation in leeches. J Exp Biol 96: 143–160, 1982. [Google Scholar]
- Lee MW, Kraemer FB, Severson DL. Characterization of a partially purified diacylglycerol lipase from bovine aorta. Biochim Biophys Acta 1254: 311–318, 1995. doi: 10.1016/0005-2760(94)00193-3. [DOI] [PubMed] [Google Scholar]
- Lehtonen M, Reisner K, Auriola S, Wong G, Callaway JC. Mass-spectrometric identification of anandamide and 2-arachidonoylglycerol in nematodes. Chem Biodivers 5: 2431–2441, 2008. doi: 10.1002/cbdv.200890208. [DOI] [PubMed] [Google Scholar]
- Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 58: 884–896, 2008. doi: 10.1016/j.neuron.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JE, Kristan WB Jr. Quantitative analysis of a directed behavior in the medicinal leech: implications for organizing motor output. J Neurosci 18: 1571–1582, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Burrell BD. Two forms of long-term depression in a polysynaptic pathway in the leech CNS: one NMDA receptor-dependent and the other cannabinoid-dependent. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 831–841, 2009. doi: 10.1007/s00359-009-0462-3. [DOI] [PubMed] [Google Scholar]
- Li Q, Burrell BD. Properties of cannabinoid-dependent long-term depression in the leech. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 196: 841–851, 2010. doi: 10.1007/s00359-010-0566-9. [DOI] [PubMed] [Google Scholar]
- Li Q, Burrell BD. Associative, bidirectional changes in neural signaling utilizing NMDA receptor- and endocannabinoid-dependent mechanisms. Learn Mem 18: 545–553, 2011. doi: 10.1101/lm.2252511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YC, Huang CC, Hsu KS, Takahashi T. Cannabinoid-induced presynaptic inhibition at the primary afferent trigeminal synapse of juvenile rat brainstem slices. J Physiol 555: 85–96, 2004. doi: 10.1113/jphysiol.2003.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockery SR, Kristan WB Jr. Two forms of sensitization of the local bending reflex of the medicinal leech. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 168: 165–177, 1991. doi: 10.1007/BF00218409. [DOI] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123: 4050–4062, 2013. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Bisogno T, Melck D, Vandenbulcke F, Verger-Bocquet M, De Petrocellis L, Sergheraert C, Breton C, Di Marzo V, Salzet M. Evidence for an endocannabinoid system in the central nervous system of the leech Hirudo medicinalis. Brain Res Mol Brain Res 87: 145–159, 2001. doi: 10.1016/S0169-328X(00)00290-4. [DOI] [PubMed] [Google Scholar]
- Modney BK, Sahley CL, Muller KJ. Regeneration of a central synapse restores nonassociative learning. J Neurosci 17: 6478–6482, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol 86: 40–48, 2001. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- Muller KJ, McMahan UJ. The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond B Biol Sci 194: 481–499, 1976. doi: 10.1098/rspb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Muller KJ, Nicholls JG, Stent GS. Neurobiology of the Leech. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1981. [Google Scholar]
- Muller KJ, Scott SA. Transmission at a “direct” electrical connexion mediated by an interneurone in the leech. J Physiol 311: 565–583, 1981. doi: 10.1113/jphysiol.1981.sp013605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JG, Baylor DA. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol 31: 740–756, 1968. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Nicholls JG, Purves D. Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J Physiol 209: 647–667, 1970. doi: 10.1113/jphysiol.1970.sp009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JC, Nichols DE. Serotonin 5-HT2A receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem 99: 1164–1175, 2006. doi: 10.1111/j.1471-4159.2006.04173.x. [DOI] [PubMed] [Google Scholar]
- Pastor J, Soria B, Belmonte C. Properties of the nociceptive neurons of the leech segmental ganglion. J Neurophysiol 75: 2268–2279, 1996. doi: 10.1152/jn.1996.75.6.2268. [DOI] [PubMed] [Google Scholar]
- Pernía-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schüttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325: 760–764, 2009. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep 13: 1246–1257, 2015. doi: 10.1016/j.celrep.2015.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahley CL, Modney BK, Boulis NM, Muller KJ. The S cell: an interneuron essential for sensitization and full dishabituation of leech shortening. J Neurosci 14: 6715–6721, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BK, Kristan WB Jr. The whole-body shortening reflex of the medicinal leech: motor pattern, sensory basis, and interneuronal pathways. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 177: 667–681, 1995. doi: 10.1007/BF00187626. [DOI] [PubMed] [Google Scholar]
- Shaw BK, Kristan WB Jr. The neuronal basis of the behavioral choice between swimming and shortening in the leech: control is not selectively exercised at higher circuit levels. J Neurosci 17: 786–795, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BK, Kristan WB Jr. Relative roles of the S cell network and parallel interneuronal pathways in the whole-body shortening reflex of the medicinal leech. J Neurophysiol 82: 1114–1123, 1999. doi: 10.1152/jn.1999.82.3.1114. [DOI] [PubMed] [Google Scholar]
- Smith ES, Lewin GR. Nociceptors: a phylogenetic view. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 1089–1106, 2009. doi: 10.1007/s00359-009-0482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers T, Holec S, Burrell BD. Physiological and behavioral evidence of a capsaicin-sensitive TRPV-like channel in the medicinal leech. J Exp Biol 217: 4167–4173, 2014. doi: 10.1242/jeb.110049. [DOI] [PubMed] [Google Scholar]
- Szczupak L, Kristan WB Jr. Widespread mechanosensory activation of the serotonergic system of the medicinal leech. J Neurophysiol 74: 2614–2624, 1995. doi: 10.1152/jn.1995.74.6.2614. [DOI] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 26: 1833–1843, 2006. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF. Sensitization of the gill and siphon withdrawal reflex of Aplysia: multiple sites of change in the neuronal network. J Neurophysiol 70: 1210–1220, 1993. doi: 10.1152/jn.1993.70.3.1210. [DOI] [PubMed] [Google Scholar]
- Velázquez-Ulloa N, Blackshaw SE, Szczupak L, Trueta C, García E, De-Miguel FF. Convergence of mechanosensory inputs onto neuromodulatory serotonergic neurons in the leech. J Neurobiol 54: 604–617, 2003. doi: 10.1002/neu.10184. [DOI] [PubMed] [Google Scholar]
- Wang Y, Burrell BD. Differences in chloride gradients allow for three distinct types of synaptic modulation by endocannabinoids. J Neurophysiol 116: 619–628, 2016. doi: 10.1152/jn.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Summers T, Peterson W, Miiller E, Burrell BD. Differential effects of GABA in modulating nociceptive vs. non-nociceptive synapses. Neuroscience 298: 397–409, 2015. doi: 10.1016/j.neuroscience.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14: 2–31, 1997. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor. J Neurophysiol 104: 2766–2777, 2010. doi: 10.1152/jn.00491.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Long-term depression of nociceptive synapses by non-nociceptive afferent activity: role of endocannabinoids, Ca2+, and calcineurin. Brain Res 1460: 1–11, 2012. doi: 10.1016/j.brainres.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Endocannabinoid-dependent long-term depression in a nociceptive synapse requires coordinated presynaptic and postsynaptic transcription and translation. J Neurosci 33: 4349–4358, 2013a. doi: 10.1523/JNEUROSCI.3922-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Burrell BD. Nonnociceptive afferent activity depresses nocifensive behavior and nociceptive synapses via an endocannabinoid-dependent mechanism. J Neurophysiol 110: 2607–2616, 2013b. doi: 10.1152/jn.00170.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol 97: 4386–4389, 2007. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Ermund A, Movahed P, Andersson DA, Simonsen C, Jönsson BA, Blomgren A, Birnir B, Bevan S, Eschalier A, Mallet C, Gomis A, Högestätt ED. Monoacylglycerols activate TRPV1—a link between phospholipase C and TRPV1. PLoS One 8: e81618, 2013. doi: 10.1371/journal.pone.0081618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]