Abstract
Individuals vary significantly with respect to rate and degree of improvement with motor practice. While the regions that underlie motor learning have been well described, neurophysiological factors underlying differences in response to motor practice are less well understood. The present study examined both resting-state and event-related EEG coherence measures of connectivity as predictors of response to motor practice on a motor sequencing task using the dominant hand. Thirty-two healthy young right-handed participants underwent resting EEG before motor practice. Response to practice was evaluated both across the single session of motor practice and 24 h later at a retention test of short-term motor learning. Behaviorally, the group demonstrated statistically significant gains both in single-session “motor improvement” and across-session “motor learning.” A resting-state measure of whole brain coherence with primary motor cortex (M1) at baseline robustly predicted subsequent motor improvement (validated R2 = 0.55) and motor learning (validated R2 = 0.68) in separate partial least-squares regression models. Specifically, greater M1 coherence with left frontal-premotor cortex (PMC) at baseline was characteristic of individuals likely to demonstrate greater gains in both motor improvement and motor learning. Analysis of event-related coherence with respect to movement found the largest changes occurring in areas implicated in planning and preparation of movement, including PMC and frontal cortices. While event-related coherence provided a stronger prediction of practice-induced motor improvement (validated R2 = 0.73), it did not predict the degree of motor learning (validated R2 = 0.16). These results indicate that connectivity in the resting state is a better predictor of consolidated learning of motor skills.
NEW & NOTEWORTHY Differences in response to motor training have significant societal implications across a lifetime of motor skill practice. By evaluating both resting-state and event-related measures of brain function, our findings highlight interindividual differences in brain connectivity providing unique insights into differences in response to motor training. These findings have wide-ranging implications in settings ranging from advanced professional motor training to rehabilitation after brain injury.
Keywords: EEG, event-related coherence, motor learning, partial least-squares regression, prediction, resting-state connectivity
INTRODUCTION
Acquisition of motor memories is essential to our daily interaction with the world around us. However, the degree to which an individual responds to motor practice and the rate at which new motor skills develop vary significantly (Ackerman 1987; Engel et al. 2014). With the spectrum of motor performance ranging from a professional athlete to an elderly person regaining basic movements after stroke, this variation can have significant functional, social, and economic consequences.
Genetics, brain structure, and brain function have each been shown to contribute to variability in motor skill acquisition and motor learning (Burke Quinlan et al. 2015; McHughen et al. 2010; Tomassini et al. 2011). An individual’s response to a specific form of motor practice is likely to vary according to brain state. For instance, a previous study by our group (Wu et al. 2014) showed that individuals with higher resting-state connectivity between primary motor cortex (M1) and parietal cortex (PAR) before motor practice improved more during practice of a rotor pursuit task. This task had strong visuomotor stimulation and required constant visuomotor integration, given that the visual stimulus was constantly moving (guiding the subject’s next hand movement) and the subject’s hand was also constantly moving. The same study also found that individuals with higher resting-state M1 connectivity with premotor cortex (PMC) showed reduced improvement with practice of the rotor pursuit task. We postulated that while increased connectivity between M1 and PAR represents a favorable network for learning a rotor pursuit task, increased connectivity between M1 and PMC represents an inefficient network for learning a rotor pursuit task. In contrast to learning of a visuomotor integration task such as the rotor pursuit task, learning of a motor sequencing task has been associated with PMC activation (Bischoff-Grethe et al. 2004; Grafton et al. 1994), with increased sequence complexity associated with higher PMC activation (Sadato et al. 1996); studies focused specifically on the encoding of sequence representation emphasize activation in secondary motor areas including PAR (Yokoi et al. 2017). Given that increased resting-state M1-PAR connectivity predisposed individuals to greater performance improvement in the rotor pursuit task, increased resting-state M1-PMC connectivity may predispose individuals to greater performance improvement of a PMC-driven task, such as motor sequencing.
The present study examined how individual differences in brain activity and connectivity predict training-induced improvement on a motor sequence learning task that by design made negligible demands on visuomotor integration. Coherence, a measure of phase and amplitude consistency between EEG signals used to estimate neural connectivity (Murias et al. 2007; Nunez and Srinivasan 2006), was examined as a predictor of both single-session improvements in task performance (“motor improvement”) and across-session improvement in task performance following a 24-h consolidation period (“motor learning”). First, given the known relationship between premotor activity and performance of a motor sequencing task, it was hypothesized that individuals with greater coherence between electrodes over M1 and PMC during baseline resting state would demonstrate greater single-session motor improvement and across-session motor learning. In addition, by characterizing interregional connectivity during movement, event-related coherence between electrodes over M1 and PMC was hypothesized to outperform resting-state measures of connectivity as a predictor of practice-induced motor improvement and motor learning.
Measures of resting-state connectivity can show better correlation with behavior than focal measures of brain function, as they capture interregional interactions between brain areas in functional networks that underlie performance and learning (Baldassarre et al. 2012; Bullmore and Sporns 2009; Supekar et al. 2013; Wu et al. 2014). Event-related measures of brain activation and connectivity have the potential to highlight transitory interregional interactions while performing cognitive and motor tasks (Andrew and Pfurtscheller 1996; Rappelsberger et al. 1994). This study will shed light on whether these transient couplings can better explain interindividual differences in behavior.
MATERIALS AND METHODS
Participants.
Thirty-four healthy young adults, aged 18–30 yr, were recruited and gave written informed consent. Participants had no history of neurological or psychiatric conditions and were right-handed according to the Edinburgh Handedness Scale (Oldfield 1971). To determine the amount of time each participant performed skilled motor functions of the dominant hand in an average week, each participant also completed an informal inventory assessing average time spent performing skilled activities of the hands, including typing, crafting, driving, playing sports, playing instruments, and playing video games. Although it was not a specific exclusion criterion, no participants had a skull defect that could result in an EEG breach rhythm. The study was approved by the University of California, Irvine Institutional Review Board.
Experimental design.
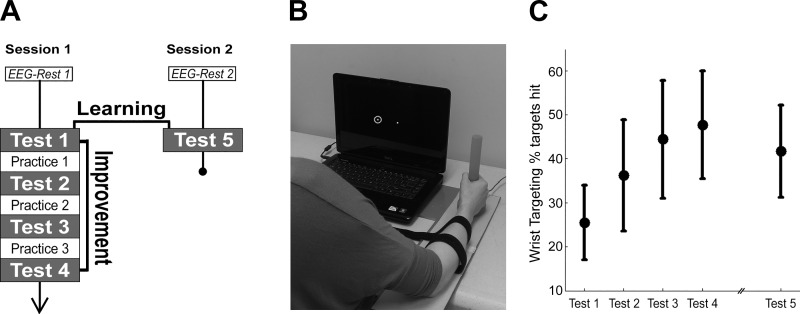
The experiment took place across two sessions separated by 24 h (Fig. 1A). Participants sat in a chair facing a computer screen on a tabletop. At both sessions, 3 min of awake, resting-state EEG was acquired before any description or practice of the motor sequence learning task was provided. Session 1 included assessment of wrist range of motion followed by four Test blocks with three interleaved Practice blocks on the motor sequence learning task. Session 2 included a single Test block to assess retention of learning without further training followed by a questionnaire to evaluate degree of explicit awareness of the motor sequence for each participant.
Fig. 1.
Experimental setup. A: experiment timeline spanning 24 h. B: rotary joystick with presentation laptop. White dot indicates cursor. Dot with halo is one of the targets on the horizontal axis; the halo turned green to indicate trial success and red to indicate trial failure. C: motor improvement (Test 4 − Test 1, mean ± SD) was significant across session 1. Motor learning (Test 5 − Test 1, mean ± SD) was also significant across the 24 h from session 1 to session 2.
For all experimental procedures, participants sat in a standard chair with both feet flat on the floor, were instructed to stare straight ahead at the computer screen, and were instructed to minimize both eyeblinks and body movements outside of the right arm.
Motor sequence learning task.
The motor sequence learning task was used to train and assess single-session motor improvement and across-session short-term motor learning over 24 h. A variation of the serial reaction time task in which individuals learn a sequence of finger button pushes (Moisello et al. 2009; Pascual-Leone et al. 1999), the present task trained a participant to produce a series of precision wrist extension and flexion movements with a custom-rotational joystick (Fig. 1B; single-turn potentiometer, Vishay Intertechnology, Malvern, PA). All participants performed the task with the dominant right hand. Wrist flexion resulted in the cursor moving left on the screen, and wrist extension resulted in the cursor moving right on the screen. To maximize consistency of movements across participants, two soft straps were applied to the participants’ right forearm to limit elbow movement. To standardize task difficulty, cursor movements were normalized to each participant’s active range of motion at the wrist.
Training and testing of the task comprised a series of target trials. The starting position of the task was the center of the screen. At the start of each trial, a target circle occurred at time 0 ms. For each trial, the green target circle (20 pixels) with a yellow halo (50 pixels) appeared in one of seven positions lying along the center horizontal axis of the screen (Fig. 1B). Participants were instructed to move the wrist at onset of the target circle so that the white cursor (10 pixels) overlapped with the target circle. Participants were permitted to overshoot the target as many times as necessary to achieve the target location. To account for overshooting errors, a trial was scored as a success only if the cursor was held within the yellow target halo for a minimum of 500 ms; each trial was 1,425 ms in duration. To achieve trial success, the maximum time to hit the target location was 925 ms after target onset. There were no pauses between trials. Feedback regarding trial success was provided in real time, with the target halo flashing green to indicate success and red for nonsuccess.
Each Test block and each Practice block consisted of 84 targets separated into three subblocks of 28 targets, with subblocks delineated by 10-s breaks. Verbal and written instructions were provided to the participant immediately before the beginning of each block to indicate Test blocks vs. Practice blocks. Otherwise, there were no differences in content between Test blocks and Practice blocks. All participants completed the same predefined sequences of targets at all Practice and Test blocks. A 19-target sequence was inserted into each 24-target subblock. Five additional targets were included both before and after the sequence to obscure the beginning of the 19-target sequence. Given that there were three subblocks in each of the four Test blocks and the three Practice blocks, the 19-target sequence was presented a total of 21 times during session 1 and three times during session 2. All participants completed the same predefined sequences of targets at all Practice and Test blocks. The target sequences were designed so that there were no target runs (i.e., each target from left to right, or right to left, consecutively). Single-session motor improvement was assessed at the end of session 1 (Test 4). Across-session motor learning was assessed at the end of session 2 (Test 5).
Performance was defined to be the percentage of successful trials of the embedded sequence trials (% targets hit). Single-session motor improvement was defined as the absolute change in % targets hit from Test 1 to Test 4. Across-session motor learning was defined as the absolute change in % targets hit from Test 1 to Test 5.
EEG acquisition.
EEG was collected with a 256-lead Hydrocel net [Electrical Geodesics (EGI), Eugene, OR] for 3 min. During all EEG recordings, participants were instructed to sit as still as possible and direct their gaze at the computer screen. The data were acquired with a Net Amp 300 amplifier (EGI) and Net Station 4.5.3 software (EGI). During recording, EEG signals were referenced to Cz with no band-pass filter applied. In postprocessing, EEG data were rereferenced to the average across all channels, an approach that minimizes common reference effects (Nunez and Srinivasan 2006). For the 3-min resting-state recording, participants were instructed to direct their gaze to the center of a fixation cross provided on the computer screen. For EEG recordings during motor sequence training, participants were instructed to direct their gaze to the cursor to minimize eye movement artifact.
EEG preprocessing.
EEG data were exported to MATLAB (7.8.0; MathWorks, Natick, MA) for preprocessing. Because of the increased degree of muscle artifact content in EEG electrodes recording from cheek and neck areas, 62 electrodes were excluded from further analysis across all participants; the remaining 194 electrodes were used in the subsequent analyses.
Preprocessing steps have been previously described by our group (Wu et al. 2014, 2015, 2016), and the toolbox with relevant documentation is publicly available at https://github.com/mdnunez/artscreenEEG. In sum, preprocessed EEG time series first were submitted to a second-order, 50-Hz low-pass Butterworth filter before being segmented into nonoverlapping 1-s epochs. The data were then mean detrended before undergoing a visual inspection. Epochs demonstrating contamination by nonphysiological sources, such as from environmental electrical pulses, showing very high-amplitude square-wave morphology occurring across all channels were removed from subsequent analyses. Remaining epochs underwent an Infomax independent component analysis decomposition with the EEGLAB toolbox (Delorme and Makeig 2004). Amplitude topography, frequency spectra, and time series were inspected for each independent component analysis component to identify eyeblinks, eye movements, and heart rhythms, which were removed. The remaining data were transformed back to channel space. Epochs were again visually inspected to ensure absence of extrabrain artifacts.
Coherence.
Spectral analysis was performed by submitting the EEG time series to a discrete fast Fourier transform with the MATLAB fft function and normalizing by epoch length. The frequency resolution was 1 Hz. No windowing function was used. Spectral coherence was calculated from the cross-correlation of the Fourier coefficients. Coherence is a unitless value that ranges from 0 to 1. For a given pair of signals, coherence values near 1 represent signals in which the phase and amplitude are highly related at a given frequency. In contrast, coherence values near 0 represent two signals in which phase and amplitude are not related at a given frequency.
For the present study, the primary frequency band of interest was high beta (20–30 Hz), as it is a range associated with function of the cortical motor system, particularly during learning of a sensorimotor task (Classen et al. 1998; Roopun et al. 2006). Furthermore, given the central role of M1 in motor learning (Muellbacher et al. 2002; Sanes 2003), the primary coherence measure of interest was mean beta coherence with a seed region approximately overlying left M1; the left M1 seed was defined as C3 and the six electrodes that immediately surround C3 (Wu et al. 2014, 2015). In the 10-20 EEG system (Oostenveld and Praamstra 2001), the de facto standard for clinical EEG, C3 is the electrode that approximates the location of the precentral gyrus. Also, studies show that EEG signal recorded from C3 largely reflects neural activity from M1 (Homan et al. 1987).
Event-related coherence.
EEG data recorded during Test blocks were segmented according to visual onset of the target. Each epoch started at 250 ms before target onset and concluded at 1,250 ms after target onset. Preprocessing steps for EEG data recorded during Test blocks were the same as the preprocessing steps for EEG data recorded during rest. To maximize temporal resolution of coherence calculations of EEG data recorded during Test blocks, data underwent a Morlet wavelet transform and coherence was calculated from the cross-correlation of the transform coefficients. Similar to EEG data acquired at rest, the primary metric assessed was mean beta coherence with a seed region overlying left M1. Baseline for each epoch was defined to be 250 ms to 149 ms before target onset. For each electrode, a statistically significant change was defined as >2 SD difference in mean coherence with left M1 compared with baseline. Although segmentation was defined with respect to target onset, to facilitate interpretation of results event-related results are presented with respect to mean movement onset across all subjects (mean reaction times across all session 1 trials: 225.0 ± 4.4 ms, mean ± SD).
Partial least-square analysis.
Brain-behavior relationships were established with partial least-squares (PLS) regression analyses (N-way toolbox, Andersson and Bro 2000). PLS is gaining traction in the analyses of neuroimaging studies, as multiple comparisons and a high degree of multicollinearity can reduce statistical power (McIntosh et al. 1996; McIntosh and Lobaugh 2004).
The present PLS analyses are in line with previously published methods from our group (Krishnan et al. 2013; Wu et al. 2014, 2015, 2016). Overall, the primary objective of a PLS model is to maximize the degree to which variance in the dependent variable is represented in a minimal number of components of the independent variable. More specifically, the PLS analyses are based on the optimization of a least-squares fit of a partial correlation matrix between the independent and dependent variables of interest. For the present study, the independent variables were the EEG measures of brain, including both resting and event-related measures of coherence, and the dependent variables were motor improvement at session 1 and motor learning at session 2. As a preprocessing step, data were mean-centered and underwent a direct orthogonal signal correction to remove the component of the independent variable (EEG coherence) that is maximally orthogonal to the behavioral data (Westerhuis et al. 2001). This step allows for more efficient PLS models with fewer components. Using the variables of interest, the PLS algorithm generates a series of models in which successively more components are included until all variance in the dependent variable is represented. The value of the PLS model for improving prediction strength, then, is dimensional reduction. Where EEG coherence represents 256 individual predictors to examine, each PLS component represents a cumulative predictor in which each EEG coherence is attributed a regression coefficient. For comparison of prediction strength across models, all PLS models in the present study included only the top two components. In addition, cross-validation of each model was performed with a leave-one-out and predict approach, which is an established method for assessing generalization of a prediction model to independent data (Huang et al. 2011; Kang et al. 2016). An arbitrary threshold was used to identify regions where coherence was most strongly related to behavioral status: for each PLS model, correlation coefficients were compared to an arbitrary threshold at |ri| > 0.7 × rmax, where ri is the correlation coefficients at the ith electrode and rmax is the largest |ri| value across all electrodes. Significant electrode clusters for each PLS model were defined as any group of contiguous electrodes that exceeded the threshold.
Statistical analyses.
Single-session motor improvement and across-session motor learning were assessed with a two-tailed paired t-test. Bivariate brain-behavior linear regression analyses were two-tailed with statistical significance set at P < 0.05. Statistical significance of event-related changes in coherence was examined with a paired t-test. Parametric statistical methods were used, as all measures were normally distributed. All statistical tests were performed with the MATLAB 7.8.0 statistical package.
RESULTS
The 34 participants who were recruited completed all study protocols. Data from two participants were excluded from analyses because of excessive muscle artifact in the EEG recording. For the remaining 32 participants, sex was 14 M/18 F and age was 19.4 ± 1.6 yr (mean ± SD).
Behavioral data.
Degree of training-induced motor improvement, defined as total increase in % targets hit from Test 1 (25.4 ± 8.5%, mean ± SD) to Test 4 (47.4 ± 11.8%), was statistically significant [Fig. 1C; t(31) = 15.4, P = 4.5 × 10−16, 2-tailed paired t-test]. Motor learning was defined as total increase in % targets hit from Test 1 to Test 5 (41.7 ± 10.5%) and was also statistically significant [Fig. 1C, t(31) = −9.57, P = 9.0 × 10−11, 2-tailed paired t-test]. There was also a decrease in reaction time from Test 1 (227.4 ± 23.2 ms, mean ± SD) to Test 4 (210.3 ± 23.0 ms) that was statistically significant [t(31) = −4.05, P = 3.2 × 10−4]; the decrease from Test 1 to Test 5 (196.6 ± 24.0 ms) was also statistically significant [t(31) = −6.89, P = 1.00 × 10−7]. In the questionnaire following the retention test at session 2, 19 of 32 participants reported awareness of a sequence in target presentation. However, none of the participants reproduced more than four sequential targets correctly.
Behavioral and demographic measures did not predict motor improvement or motor learning.
Baseline behavioral and demographic measures were weak predictors of single-session motor improvement and across-session motor learning. Baseline performance on the motor sequence task (% targets hit at Test 1, r = 0.09, P = 0.65, n = 32), age (r = 0.19, P = 0.29, n = 32), handedness score (r = −0.23, P = 0.20, n = 32), and scores on the motor skill inventory (r = 0.08, P = 0.67, n = 32) each did not predict single-session motor improvement. Similarly, baseline performance (r = −0.3, P = 0.09, n = 32), age (r = −0.05, P = 0.8, n = 32), handedness (r = −0.3, P = 0.07, n = 32), and motor skills inventory scores (r = 0.1, P = 0.5, n = 32) each did not predict across-session motor learning.
Resting-state EEG coherence at baseline predicts both single-session motor improvement and across-session motor learning.
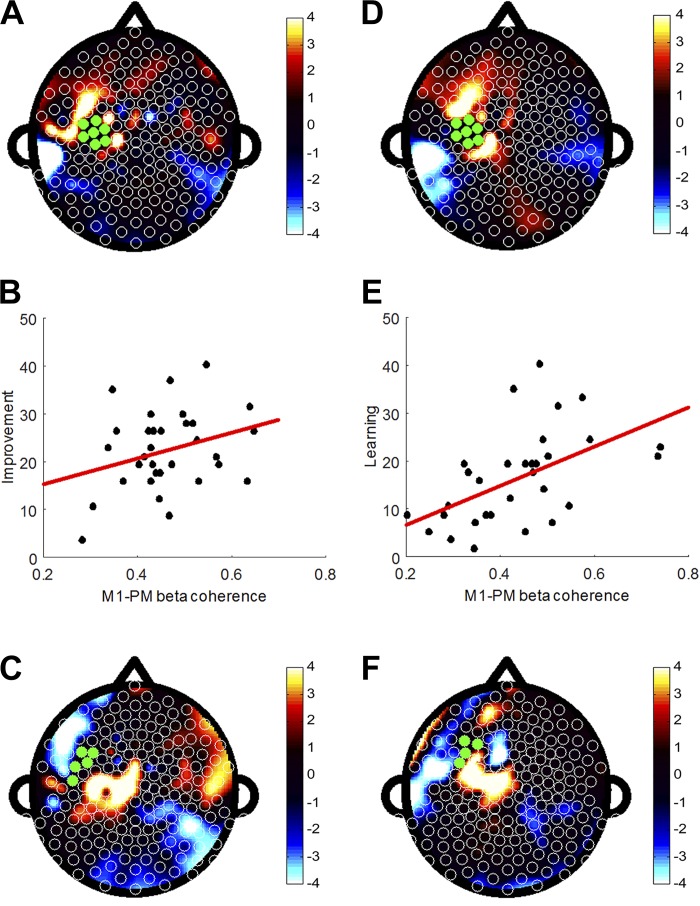
Whole brain beta coherence with left M1 at rest, before any motor training, predicted subsequent single-session motor improvement in a PLS model (Fig. 2A; fitted R2 = 0.58) and retained good predictive strength when cross-validated (validated R2 = 0.55). Electrodes that made the largest contribution to this model were clustered in a region overlying left PMC, where regression coefficients were positive, suggesting that individuals with relatively higher left M1-PMC beta coherence at rest could potentially be more likely to demonstrate greater single-session motor improvement (Fig. 2B; r = 0.30, P = 0.09, n = 32); however, this association was not statistically significant when the M1-PMC coherence averaged over all channel pairs was tested separately in a univariate regression model.
Fig. 2.
Resting-state coherence between electrodes overlying left M1 and the rest of the scalp predicts single-session motor improvement and across-session motor learning. A: topographic plot of regression coefficients from the PLS model using high beta (20–30 Hz) coherence with left M1 to predict single-session motor improvement (fitted R2 = 0.58, validated R2 = 0.55). B: coherence between left M1 and left frontal-premotor (PMC) regions that were most significant in the PLS model showed a trend toward positive correlation with motor improvement (r = 0.30, P = 0.09). C: topographic plot of regression coefficients from the PLS model using high beta coherence with left PMC to predict single-session motor improvement (fitted R2 = 0.76, validated R2 = 0.68). D: topographic plot of regression coefficients from PLS model using high beta coherence with left M1 to predict across-session motor learning (fitted R2 = 0.89, validated R2 = 0.68). E: coherence between left M1 and left PMC regions that were most significant in the PLS model was positively correlated with motor learning over a 24-h retention test (r = 0.52, P = 0.002). F: topographic plot of regression coefficients from PLS model using high beta coherence with left PMC to predict across-session motor learning (fitted R2 = 0.68, validated R2 = 0.67).
Because of the proximity of the PMC electrodes with the M1 seed, it is possible that M1-PMC coherence could reflect increased drive from a common source underlying M1 and PMC. To explore this, a subsequent analysis examined resting-state whole brain beta coherence using the left PMC region defined in Fig. 2A as the seed region (Fig. 2C; fitted R2 = 0.76, validated R2 = 0.68). Significant electrodes were clustered in regions overlying left M1 and left dorsal prefrontal cortex. It should be noted that while both models in Fig. 2A and Fig. 2C emphasize the M1-PM connection to contribute significantly to the overall prediction model, the distribution of significant electrodes does not lie along a gradient. Thus it is unlikely that M1-PMC coherence in the present context reflects increased drive from a common source.
Resting-state whole brain beta coherence at baseline also predicted subsequent across-session motor learning in a separate PLS model (Fig. 2D; fitted R2 = 0.89) that remained robust in cross-validation (validated R2 = 0.68). A cluster of significant electrodes was again identified overlying left PMC, such that individuals who demonstrated relatively higher left M1-PMC coherence before motor training were significantly more likely to demonstrate greater across-session motor learning (Fig. 2E; r = 0.52, P = 0.002, n = 32). As above, a PLS model of resting-state whole brain beta coherence with left PMC also predicted subsequent across-session motor learning (Fig. 2F; fitted R2 = 0.68, validated R2 = 0.67); significant electrodes, including C3, were clustered in a region overlying left M1.
Event-related changes in EEG coherence evolve across performance of a motor sequence learning task.
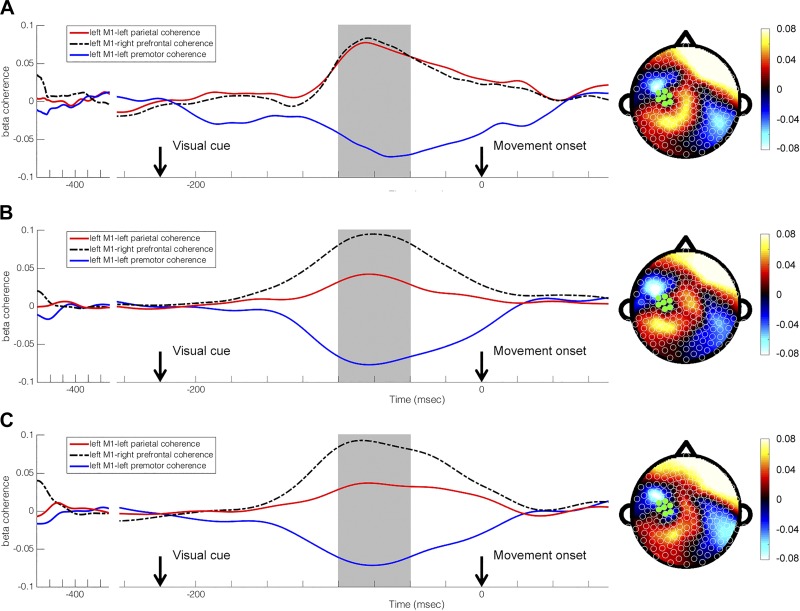
Performance of the sequence learning task caused similar modulation in whole brain beta coherence with left M1 during participants’ initial encounter with the task (Test 1), at the end of a single session of training (Test 4), and after a 24-h consolidation period (Test 5). During Test 1 (Fig. 3A), electrodes where statistically significant changes in event-related beta coherence with left M1 occurred were in clusters overlying left PMC, left PAR (t-ratio = 9.45, P < 0.0001), and right prefrontal cortex (PF, t-ratio = 9.82, P < 0.0001). For Test 4 (Fig. 3B), electrodes where statistically significant changes in event-related left M1 coherence occurred were in clusters overlying left PMC (t-ratio = −7.13, P < 0.0001), left PAR (t-ratio = 6.86, P < 0.0001), and right PF (t-ratio = 9.75, P < 0.0001) regions. Similarly, Test 5 (Fig. 3C) found statistically significant changes in event-related left M1 coherence to also occur at left PMC (t-ratio = −8.08, P < 0.0001), left PAR (t-ratio = 5.54, P < 0.0001), and right PF (t-ratio = 8.05, P < 0.0001).
Fig. 3.
Subject-averaged evolution of event-related high beta (20–30 Hz) coherence with left M1 during trial performance represented as topographic maps with corresponding line graphs at Test 1 (A), Test 4 (B), and Test 5 (C). Time is displayed relative to movement onset. Baseline is defined to be −475 ms to −375 ms relative to movement onset. Visual cue and movement onset are indicated by black downward arrows. Timing of selected topographic maps in relation to line graphs is indicated by gray blocks.
Training and consolidation of practice showed differences in the timing of changes in left M1 coherence with specific regions of the brain. Specifically, left M1-right PF coherence was found to peak later at Test 1 compared with Test 4 (Test 1: −62.0 ± 39.4 ms, Test 4: −83.4 ± 49.6 ms, mean ± SD, t-ratio = −2.51, P = 0.017). Timing of changes in left M1 coherence with left PAR also demonstrated a difference from Test 1 to Test 4 (Test 1: −75.5 ± 61.0 ms, Test 4: −106.0 ± 57.5 ms, t-ratio = −2.32, P = 0.027) and from Test 4 to Test 5 (Test 5: −79.9 ± 60.2 ms, t-ratio = 2.04, P < 0.05). No changes in timing were noted for left M1-left PMC coherence.
Event-related EEG coherence predicts single-session motor improvement better than across-session motor learning.
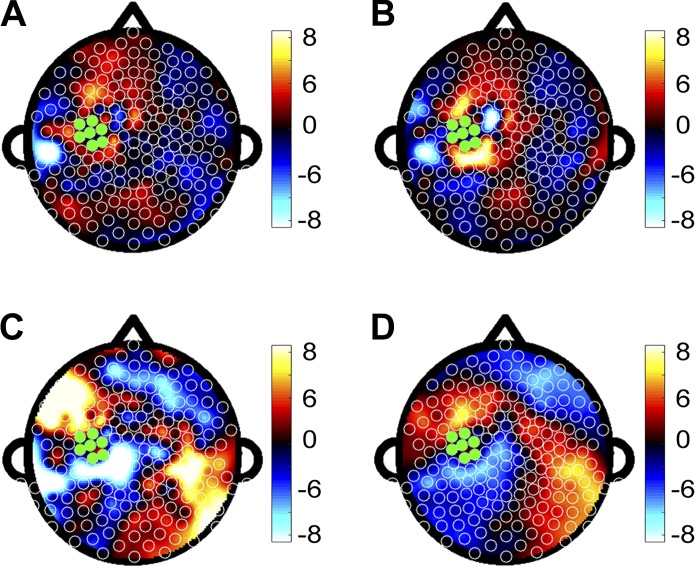
As a corollary to the resting-state analyses, event-related EEG coherence was collapsed across the duration of the epoch and showed that mean M1 coherence was not predictive of either subsequent motor improvement (Fig. 4A; fitted R2 = 0.77, validated R2 < 0.01) or subsequent motor learning (Fig. 4B; fitted R2 = 0.98, validated R2 < 0.01). In comparison, mean M1 coherence at specific subintervals, such as the period from −100 ms to −50 ms before movement onset, when change in M1 coherence was maximal, was predictive of subsequent motor improvement (Fig. 4C; fitted R2 = 0.86, validated R2 = 0.73). Significant electrodes were clustered in regions overlying left dorsal prefrontal cortex (dPF), left lateral parietal cortex (latPAR), and left sensory cortex (S1). Mean M1 coherence with dPF was positively correlated with motor improvement (r = 0.46, P = 0.008), while M1-latPAR coherence (P > 0.05) and M1-S1 coherence (P > 0.05) did not correlate. In contrast, mean M1 coherence at the subinterval from −100 ms to −50 ms before movement onset showed limited prediction of subsequent motor learning (Fig. 4D; fitted R2 = 0.98, validated R2 = 0.16).
Fig. 4.
A and B: topographic plots of regression coefficients from separate PLS models in which event-related EEG coherence was collapsed across the duration of the epoch and used to predict single-session motor improvement (fitted R2 = 0.77, validated R2 < 0.01) (A) and across-session motor learning (fitted R2 = 0.98, validated R2 < 0.01) (B). C and D: topographic plots of regression coefficients from separate PLS models in which event-related EEG coherence was collapsed across the interval from −100 ms to −50 ms before movement onset and used to predict single-session motor improvement (fitted R2 = 0.86, validated R2 = 0.73) (C) and across-session motor learning (R2 = 0.98, validated R2 = 0.16) (D).
DISCUSSION
Individuals demonstrate significant variability with respect to the rate and degree of motor learning with practice. Although the neural structures that contribute to motor learning are well described, the neurophysiology that determines the degree of interindividual differences in response to practice is less well defined. The present study examined both resting-state and event-related EEG coherence as predictors of practice-induced motor improvement across a single session and motor learning across a 24-h period. Resting-state EEG connectivity of the motor network was found to predict a significant fraction of variance in practice-induced motor improvement across a single session and motor learning across a 24-h consolidation period. The present results highlight connectivity between M1 and PMC as a key determinant of interindividual differences in response to practice of the motor sequencing task. In addition, we found that EEG coherence during the period of maximum event-related modulation (−100 to −50 ms before movement) provided stronger prediction of motor improvement but did not predict motor learning. These results provide insights useful for understanding the neurophysiology of interindividual differences in response to training of a new motor skill.
The present study extends results from a previous study examining resting-state EEG connectivity as a predictor of subsequent motor improvement on a skilled motor task (Wu et al. 2014). The present results demonstrate again that EEG coherence measures of connectivity are useful for predicting degree of practice-induced motor improvement, and do so here in an independent and larger cohort. A key new finding is that EEG measures also predicted degree of short-term motor learning over a 24-h period, a timescale that is a more robust measure of neural changes underlying motor learning (Kantak and Winstein 2012).
The PLS models emphasize M1-PMC connectivity as a key predictor of practice-induced motor improvement and motor learning for the present motor sequencing task. In our previous study (Wu et al. 2014) using a rotor pursuit task, increased M1-PMC connectivity was negatively correlated with subsequent practice-induced motor improvement. Those results suggested that increased PMC connectivity indicated an inefficient motor system for acquisition of a visuomotor integration task that emphasized parietal cortex activation (Cavaco et al. 2015; Wu et al. 2014). It was therefore postulated that individuals with higher M1-PMC connectivity would demonstrate greater practice-induced gains on a motor task that had greater emphasis on PMC activation, such as a motor sequencing task (Bischoff-Grethe et al. 2004; Honda et al. 1998; Jenkins et al. 1994). By demonstrating that increased M1-PMC connectivity predicts greater practice-induced gains in single-session motor improvement as well as across-session motor learning, the present study demonstrates that increased resting connectivity between M1 and PMC, key motor regions for learning of a motor sequencing task, predisposes individuals to greater improvement with practice of a motor sequencing task. This M1-PMC connectivity is interpreted as reflecting the interaction between these two regions, with PMC components more related to encoding of sequence representation and M1 components more related to encoding the individual movements that were executed (Yokoi et al. 2017). Together with the results from the preceding study, the present results show that the degree of connectivity between specific motor regions can be used to predict an individual’s response to a specific form of motor practice (Wu et al. 2014).
Event-related connectivity provides four-dimensional characterization of brain function during task performance. As such, event-related measures of brain function may provide unique insights into intersubject variability in motor performance (Pfurtscheller and Andrew 1999). In line with previous literature, PMC, frontal cortices, and parietal cortex are implicated in processes responsible for motor planning and visuomotor integration (Gerloff et al. 1998; Scheeringa et al. 2009). Furthermore, these same structures were found to demonstrate the greatest variation across movement-related measures of coherence in the present study. From Test 1 (Fig. 3A) to Test 5 (Fig. 3C), there was a notable shift across performance of a trial in terms of event-related M1-PMC, M1-PAR, and M1-PF coherences. In the setting of motor practice of a sequence learning task, this evolution may reflect practice-induced reorganization of cortical motor networks from PAR-driven sensorimotor processing in response to visual cues to PMC-driven motor preparatory processes with behavioral improvement of a motor sequence (Grafton et al. 1995). Practice-induced reduction of M1-PAR coherence 75 ms before movement may demonstrate simplification of the motor network underlying performance of the motor sequencing task, a known mechanism of motor learning (Deeny et al. 2003).
To the best of our knowledge, the present study is also the first to directly compare resting-state and event-related measures of brain function as predictors of practice-induced motor learning. While coherence averaged over the entire task interval did not predict degree of subsequent practice-induced motor improvement and motor learning, the specific interval where change in event-related coherence with M1 was maximal (−100 ms to −50 ms before movement onset) strongly predicted subsequent practice-induced motor improvement with robust prediction strength (validated R2 = 0.73). Event-related coherence just before execution was more effective at predicting motor improvement than the resting-state coherence (validated R2 = 0.55). In contrast, event-related coherence during the interval when event-related coherence with M1 was maximal showed limited prediction of practice-induced motor learning (validated R2 = 0.16), while resting-state coherence provided strong prediction of motor learning (validated R2 = 0.68). This result suggests that the strength of connectivity at rest has a stronger influence on consolidation of motor learning that takes place during the 24-h period between training and testing in our experiment.
The present study has some limitations to note. First, we acknowledge that scalp EEG has limited spatial resolution. As a result, the relationship between signal recorded at a specific electrode and neural activity produced by a specific brain structure is imperfect. The present study limits this concern by using a high-density electrode system that has been shown to provide significantly improved spatial resolution compared with traditional 10-20 systems (Luu et al. 2001; Petrov et al. 2014; Ryynänen et al. 2004). Second, the present study design examines prediction of motor improvement across a single training session and prediction of motor learning across a 24-h consolidation period. As a result, the degree to which the present results extend to prediction of motor improvement across multiple training sessions and prediction of motor learning across weeks and months is unknown. A third limitation of the present study relates to capture of joystick movements during performance of the motor sequencing task. While onset of visual stimulus was recorded within the EEG record, joystick positional data were recorded separately. As a result, there was a ±4.93-ms margin of error for all reaction time calculations. Therefore, the present event-related analyses have been performed with respect to visual stimulus onset. Additional studies with improved methods for determining reaction time with the present apparatus are needed to determine the degree to which variability in reaction time both across subjects and across trials affects the present results.
The neurophysiology that underlies interindividual responses to motor practice is still incompletely understood. The present findings emphasize the utility of resting-state measures of connectivity to understand these differences. The connection between M1 and PMC is highlighted as a key predictor of degree of practice-induced motor improvement and motor learning on a motor sequencing task. In addition to prediction of response to motor training, future studies may also examine the degree to which baseline measures of resting-state connectivity can be leveraged to predict the rate of learning and response to different training schedules or the total hours of training needed for an individual to achieve performance plateau. Such studies may be of particular utility in several areas of high societal impact, including selection of individuals for highly specialized motor skill training (Deeny et al. 2003), stratification of patients on the basis of neural function to maximize effects of intervention (Cramer 2010), and prediction of individual response to treatment across a spectrum of neuropsychiatric disorders (Sanz-Arigita et al. 2010; Siegle et al. 2006; Zhu et al. 2008).
GRANTS
Support for this research was provided by the National Institute of Neurological Disorders and Stroke (Grant K24 HD-074722) and the Stanley Behrens Public Impact Fellowship.
DISCLOSURES
S. C. Cramer has served as a consultant for Dart Neuroscience, MicroTransponder, and Toyama. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W., S.C.C., and R.S. conceived and designed research; J.W. and F.K. performed experiments; J.W., F.K., S.C.C., and R.S. analyzed data; J.W., F.K., S.C.C., and R.S. interpreted results of experiments; J.W., F.K., and R.S. prepared figures; J.W., F.K., S.C.C., and R.S. drafted manuscript; J.W., S.C.C., and R.S. edited and revised manuscript; J.W., F.K., S.C.C., and R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank V. Le and A. Kaur for technical assistance.
REFERENCES
- Ackerman PL. Individual differences in skill learning: an integration of psychometric and information processing perspectives. Psychol Bull 102: 3–27, 1987. doi: 10.1037/0033-2909.102.1.3. [DOI] [Google Scholar]
- Andersson CA, Bro R. The N-way Toolbox for MATLAB. Chemom Intell Lab Syst 52: 1–4, 2000. doi: 10.1016/S0169-7439(00)00071-X. [DOI] [Google Scholar]
- Andrew C, Pfurtscheller G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol 98: 144–148, 1996. doi: 10.1016/0013-4694(95)00228-6. [DOI] [PubMed] [Google Scholar]
- Baldassarre A, Lewis CM, Committeri G, Snyder AZ, Romani GL, Corbetta M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci USA 109: 3516–3521, 2012. (Erratum. Proc Natl Acad Sci USA 113: E6723, 2016). doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. J Cogn Neurosci 16: 127–138, 2004. doi: 10.1162/089892904322755610. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198, 2009. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, Shahbaba B, Cramer SC. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol 77: 132–145, 2015. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Chen KH, Teixeira-Pinto A, Damasio H. Parietal damage impairs learning of a visuomotor tracking skill. Neuropsychologia 79: 106–112, 2015. doi: 10.1016/j.neuropsychologia.2015.10.038. [DOI] [PubMed] [Google Scholar]
- Classen J, Gerloff C, Honda M, Hallett M. Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. J Neurophysiol 79: 1567–1573, 1998. doi: 10.1152/jn.1998.79.3.1567. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Stratifying patients with stroke in trials that target brain repair. Stroke 41, Suppl: S114–S116, 2010. doi: 10.1161/STROKEAHA.110.595165. [DOI] [PubMed] [Google Scholar]
- Deeny SP, Hillman CH, Janelle CM, Hatfield BD. Cortico-cortical communication and superior performance in skilled marksmen : an EEG coherence analysis. J Sport Exerc Psychol 25: 188–204, 2003. doi: 10.1123/jsep.25.2.188. [DOI] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Engel A, Hijmans BS, Cerliani L, Bangert M, Nanetti L, Keller PE, Keysers C. Inter-individual differences in audio-motor learning of piano melodies and white matter fiber tract architecture. Hum Brain Mapp 35: 2483–2497, 2014. doi: 10.1002/hbm.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Richard J, Hadley J, Schulman AE, Honda M, Hallett M. Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121: 1513–1531, 1998. doi: 10.1093/brain/121.8.1513. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci 7: 497–510, 1995. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Tyszka M. Functional imaging of procedural motor learning: Relating cerebral blood flow with individual subject performance. Hum Brain Mapp 1: 221–234, 1994. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P. Cerebral location of international 10-20 system electrode placement. Electroencephalogr Clin Neurophysiol 66: 376–382, 1987. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibáñez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain 121: 2159–2173, 1998. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Huang X, Qin G, Fang Y. Optimal combinations of diagnostic tests based on AUC. Biometrics 67: 568–576, 2011. doi: 10.1111/j.1541-0420.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Frackowiak RS, Passingham FE. Motor sequence learning : a study with positron emission tomography. J Neurosci 14: 3775–3790, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Liu A, Tian L. Linear combination methods to improve diagnostic/prognostic accuracy on future observations. Stat Methods Med Res 25: 1359–1380, 2016. doi: 10.1177/0962280213481053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak SS, Winstein CJ. Learning-performance distinction and memory processes for motor skills: a focused review and perspective. Behav Brain Res 228: 219–231, 2012. doi: 10.1016/j.bbr.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Krishnan L, Kang A, Sperling G, Srinivasan R. Neural strategies for selective attention distinguish fast-action video game players. Brain Topogr 26: 83–97, 2013. doi: 10.1007/s10548-012-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Englander R, Lockfeld A, Lutsep H, Oken B. Localizing acute stroke-related EEG changes: assessing the effects of spatial undersampling. J Clin Neurophysiol 18: 302–317, 2001. doi: 10.1097/00004691-200107000-00002. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, Cramer SC. BDNF Val66Met polymorphism influences motor system function in the human brain. Cereb Cortex 20: 1254–1262, 2010. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3: 143–157, 1996. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage 23, Suppl 1: S250–S263, 2004. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Moisello C, Crupi D, Tunik E, Quartarone A, Bove M, Tononi G, Ghilardi MF. The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task. Exp Brain Res 194: 143–155, 2009. doi: 10.1007/s00221-008-1681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature 415: 640–644, 2002. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Murias M, Swanson JM, Srinivasan R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cereb Cortex 17: 1788–1799, 2007. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain (2nd ed.). New York: Oxford Univ. Press, 2006. doi: 10.1093/acprof:oso/9780195050387.001.0001 [DOI] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 112: 713–719, 2001. doi: 10.1016/S1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tarazona F, Keenan J, Tormos JM, Hamilton R, Catala MD. Transcranial magnetic stimulation and neuroplasticity. Neuropsychologia 37: 207–217, 1999. doi: 10.1016/S0028-3932(98)00095-5. [DOI] [PubMed] [Google Scholar]
- Petrov Y, Nador J, Hughes C, Tran S, Yavuzcetin O, Sridhar S. Ultra-dense EEG sampling results in two-fold increase of functional brain information. Neuroimage 90: 140–145, 2014. doi: 10.1016/j.neuroimage.2013.12.041. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Andrew C. Event-related changes of band power and coherence: methodology and interpretation. J Clin Neurophysiol 16: 512–519, 1999. doi: 10.1097/00004691-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Rappelsberger P, Pfurtscheller G, Filz O. Calculation of event-related coherence—a new method to study short-lasting coupling between brain areas. Brain Topogr 7: 121–127, 1994. doi: 10.1007/BF01186770. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Middleton SJ, Cunningham MO, LeBeau FE, Bibbig A, Whittington MA, Traub RD. A beta2-frequency (20-30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc Natl Acad Sci USA 103: 15646–15650, 2006. doi: 10.1073/pnas.0607443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryynänen OR, Hyttinen JA, Laarne PH, Malmivuo JA. Effect of electrode density and measurement noise on the spatial resolution of cortical potential distribution. IEEE Trans Biomed Eng 51: 1547–1554, 2004. doi: 10.1109/TBME.2004.828036. [DOI] [PubMed] [Google Scholar]
- Sadato N, Campbell G, Ibáñez V, Deiber M, Hallett M. Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16: 2691–2700, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN. Neocortical mechanisms in motor learning. Curr Opin Neurobiol 13: 225–231, 2003. doi: 10.1016/S0959-4388(03)00046-1. [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, Rombouts SA, Maris E, Barkhof F, Scheltens P, Stam CJ. Loss of “small-world” networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One 5: e13788, 2010. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MC. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage 44: 1224–1238, 2009. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry 163: 735–738, 2006. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-Lee M, Fuchs L, Menon V. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci USA 110: 8230–8235, 2013. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Kincses ZT, Bosnell R, Douaud G, Pozzilli C, Matthews PM, Johansen-Berg H. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp 32: 494–508, 2011. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhuis JA, de Jong S, Smilde AK. Direct orthogonal signal correction. Chemom Intell Lab Syst 56: 13–25, 2001. doi: 10.1016/S0169-7439(01)00102-2. [DOI] [Google Scholar]
- Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, Augsburger R, See J, Le VH, Srinivasan R, Cramer SC. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain 138: 2359–2369, 2015. doi: 10.1093/brain/awv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Srinivasan R, Burke Quinlan E, Solodkin A, Small SL, Cramer SC. Utility of EEG measures of brain function in patients with acute stroke. J Neurophysiol 115: 2399–2405, 2016. doi: 10.1152/jn.00978.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Srinivasan R, Kaur A, Cramer SC. Resting-state cortical connectivity predicts motor skill acquisition. Neuroimage 91: 84–90, 2014. doi: 10.1016/j.neuroimage.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi A, Arbuckle SA, Diedrichsen J. Does human primary motor cortex represent sequences of finger movements? (Preprint) bioRxiv 157438, 2017. doi: 10.1101/157438. [DOI]
- Zhu CZ, Zang YF, Cao QJ, Yan CG, He Y, Jiang TZ, Sui MQ, Wang YF. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage 40: 110–120, 2008. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]