Abstract
Neuronal networks in the turtle spinal cord have considerable computational complexity even in the absence of connections with supraspinal structures. These networks contain central pattern generators (CPGs) for each of several behaviors, including three forms of scratch, two forms of swim, and one form of flexion reflex. Each behavior is activated by a specific set of cutaneous or electrical stimuli. The process of selection among behaviors within the spinal cord has multisecond memories of specific motor patterns. Some spinal cord interneurons are partially shared among several CPGs, whereas other interneurons are active during only one type of behavior. Partial sharing is a proposed mechanism that contributes to the ability of the spinal cord to generate motor pattern blends with characteristics of multiple behaviors. Variations of motor patterns, termed deletions, assist in characterization of the organization of the pattern-generating components of CPGs. Single-neuron recordings during both normal and deletion motor patterns provide support for a CPG organizational structure with unit burst generators (UBGs) whose members serve a direction of a specific degree of freedom of the hindlimb, e.g., the hip-flexor UBG, the hip-extensor UBG, the knee-flexor UBG, the knee-extensor UBG, etc. The classic half-center hypothesis that includes all the hindlimb flexors in a single flexor half-center and all the hindlimb extensors in a single extensor half-center lacks the organizational complexity to account for the motor patterns produced by turtle spinal CPGs. Thus the turtle spinal cord is a valuable model system for studies of mechanisms responsible for selection and generation of motor behaviors.
NEW & NOTEWORTHY The concept of the central pattern generator (CPG) is a major tenet in motor neuroethology that has influenced the design and interpretations of experiments for over a half century. This review concentrates on the turtle spinal cord and describes studies from the 1970s to the present responsible for key developments in understanding the CPG mechanisms responsible for the selection and production of coordinated motor patterns during turtle hindlimb motor behaviors.
Keywords: central pattern generator, fictive motor patterns, forms of a task, motor rhythms, neuronal networks
INTRODUCTION
A central pattern generator (CPG) is a neuronal network that generates patterns of motor activity in the absence of movement-related sensory feedback. The foci of this review are the turtle spinal cord CPGs that produce the hindlimb motor patterns observed during three forms of scratching, two forms of swimming, and one form of hindlimb flexion reflex. The motor patterns for each of these behaviors are produced in an adult turtle spinal cord without connections to supraspinal structures and without movement-related sensory inputs. Each scratch form is elicited by gentle tactile stimulation of specific sites on the body surface. Each swim form is elicited by focal constant-frequency electrical stimulation of axons in specific locations of the white matter of the dorsolateral funiculus. Hindlimb flexion reflex is elicited by a gentle mechanical tap of the dorsum of the foot. Experiments use combinations of specific stimuli and single-neuron recordings during each of these behaviors and during deletion variations. This work provides insights into the modularity of CPG network structures and reveals partial sharing of elements of these CPGs.
This review describes the influences of major developments in CPG research in different model systems (Bucher et al. 2001; Delcomyn 1980; Diaz-Ríos et al. 2017; Goulding 2009; Grillner 1975, 1981; Grillner et al. 1986, 2008; Grillner and El Manira 2015; Grillner and Robertson 2017; Grossmann et al. 2010; Guertin and Steuer 2009; Harris-Warrick and Ramirez 2017; Herman et al. 1976; Hooper and Buschges 2017; Ijspeert et al. 2016; Kiehn 2006, 2011, 2016; Le Gal et al. 2017; Marder and Bucher 2001; Marder and Calabrese 1996; McLean and Dougherty 2015; Orlovsky et al. 1999; Pfluger 2017; Rossignol et al. 2006; Stein 1984, 1999; Stein et al. 1997; Wetzel and Stuart 1976; Wilson 1972; Ziskind-Conhaim and Hochman 2017) on the work in our laboratory and the laboratories of others on a specific model system, the spinal cord CPGs that control hindlimb behaviors in the turtle (Berkowitz 2010, 2015; Berkowitz et al. 2010; Berkowitz and Hao 2011; Currie and Gonsalves 1999; Guzulaitis et al. 2014, 2016; Hao and Berkowitz 2017; Stein 1989, 2005, 2008, 2010; Stein et al. 1986b, 2016).
TURTLE SHELL, PERIPHERAL NERVES, SPINAL CORD, AND LIMBS
The morphology and physiology of red-eared turtles, Trachemys scripta elegans, offer technical advantages for studies of the spinal cord control of limb behaviors (Bojanus 1819; Wyneken et al. 2008).
Turtle Shell, Vertebrae, and Spinal Segments
Ten postcervical and presacral vertebrae (“dorsal” vertebrae) are fused to the carapace, the dorsal parts of the shell (Mortin and Stein 1990; Moustakas-Verho et al. 2017; Zangerl 1969). Spinal cord segments termed the D1-D10 segments located in these vertebrae were studied in vivo following removal of dorsal aspects of the vertebrae located just under the midline of the carapace. Many experiments studied turtles with a complete transection of the spinal cord between the second (D2) and third (D3) postcervical spinal segments (Stein 2005). The spinal cord segments D3-D10, sacral 1 (S1), and sacral 2 (S2) posterior to the complete transection were studied without interactions with supraspinal structures in these turtles and were capable of producing a variety of motor behaviors. This review will describe the sensory inputs to these segments, the neuronal processing in these segments, and the motor pattern output from these segments.
Cutaneous Sensory Neurons
The outer surfaces of turtle shell are innervated by cutaneous afferent neurons that are activated by gentle mechanical stimulation (Currie and Stein 1990). The stereotyped markings on the turtle shell are a technical advantage: they provide visual guides for the experimenter's placement of a mechanical stimulus at the same site multiple times in one individual turtle and at corresponding sites in other turtles (Mortin and Stein 1990). Shell markings also assist in the determination of the dermatome map that describes which segmental dorsal root carries primary afferent action potentials into the spinal cord from each specific location on the cutaneous surface (Currie and Stein 1990; Mortin and Stein 1990).
Propriospinal Interneurons
Propriospinal interneurons have their cell body in the spinal cord and an axon that innervates other spinal cord neurons (Berkowitz and Stein 1994c; Berkowitz 2004; Nissen et al. 2008). Single-unit recordings from axons of descending propriospinal interneurons were obtained in the white matter at the posterior cut surface of the spinal cord (Berkowitz and Stein 1994a, 1994b; Currie and Stein 1990; Stein and Daniels-McQueen 2002, 2003; Stein et al. 2016).
Hindlimb Motor Neurons and Muscles
Turtle hindlimb musculature has been well described (Mayerl et al. 2017; Walker 1973). Muscles that have received special attention in studies (Bakker and Crowe 1982; Robertson et al. 1985; Stein and Daniels-McQueen 2003) are 1) the hip flexor, also called hip protractor, VP-HP, puboischiofemoralis internus, pars anteroventralis; 2) the hip extensor, also called hip retractor, HR-KF, flexor cruris, pars flexor tibialis internus; 3) the monoarticular knee extensor, FT-KE, triceps femoris, pars femorotibialis; 4) the biarticular knee extensor and hip adductor, AM-KE, triceps femoris, pars ambiens; 5) the biarticular knee extensor, hip abductor, and hip flexor, IT-KE, triceps femoris, pars iliotibialis; and 6) the knee flexor, ILFIB, iliofibularis. Cell bodies of hindlimb motor neurons are located in the ventral horns of the five spinal cord segments that comprise the hindlimb (lumbosacral) enlargement (D8, D9, D10, S1, and S2; Ruigrok and Crowe 1984).
Resistance to Anoxia
Turtles dive and hibernate. Their nervous systems are remarkably resistant to anoxia (Hounsgaard and Nicholson 1990; Lutz and Milton 2004). This physiological trait offers important advantages, especially in in vitro studies of spinal cord bathed in physiological saline (Keifer and Stein 1983).
CPGS FOR SCRATCHING IN THE SPINAL CORD
Natural Tactile Stimulation of the Body Surface Evokes Scratching in a Vertebrate with a Complete Transection of the Spinal Cord
Sherrington (1906a, 1906b) pioneered work with the task of scratching as a model system (Stein 1983) in studies with a dog with complete transection of the spinal cord. He emphasized the abilities of the spinal cord to 1) recognize the specific site on the body surface that has received the cutaneous stimulation and 2) generate the appropriate sequences of motor activities that direct the hindlimb to reach toward and rub against the stimulated site. He noted that the hindlimb movements are site specific: the hindlimb movements used to scratch a site on the dog’s back are very different from the hindlimb movements used to rub a site on the belly. He transected the dorsal roots that served a hindlimb while leaving the dorsal roots serving the scratch receptive field for that limb intact. He demonstrated an excellent scratch reflex in the deafferented hindlimb in response to stimulation in the scratch receptive field. This established that there was a scratch CPG in the dog spinal cord. Deafferentation by transection of sensory nerves from the moving body part is a classic technique to demonstrate that a motor pattern can be produced by a CPG in the absence of movement-related feedback.
Deliagina, Orlovsky, and colleagues studied the motor pattern for hindlimb scratching in cat (Arshavsky et al. 1986; Orlovsky et al. 1999). In a preparation with a moving hindlimb, they stimulated a site in the scratch receptive field and used electromyographic (EMG) recordings to characterize the pattern of muscle activities during actual scratching (Deliagina et al. 1975). In a spinal cat deefferented with neuromuscular blockade via a nicotinic acetylcholine receptor antagonist, they stimulated a site in a scratch receptive field and used electroneurographic (ENG) recordings to characterize the fictive scratch motor pattern in peripheral nerves innervating specific muscles (Deliagina et al. 1981). The term “fictive motor pattern” was used to describe motor neuron activities in an immobilized preparation in which there were no muscle activities and no actual movements. The fictive scratch ENG activation pattern in the deefferented preparation was an excellent and robust replica of the actual scratch EMG pattern in the preparation with movement-related feedback (Deliagina et al. 1981). Deefferentation is an alternate technique to demonstrate the existence of a CPG. In an extensive series of elegant experiments, they used single-neuron recordings to establish major characteristics of the cat scratch CPG (Arshavsky et al. 1986). Their work with scratch reflex using deefferentation via neuromuscular blockade helped promulgate the importance of this technique as a method to demonstrate the existence of a CPG (see also Grillner 1975, 1981). The work of Deliagina, Orlovsky, and their colleagues on scratch in cat was an important influence on the decision in our laboratory to explore scratch in turtle as a model system.
Natural Tactile Stimulation of the Midbody Region Evokes Rostral Scratching in a Turtle with a Complete Transection of the Spinal Cord
Valk-Fai and Crowe (1978) introduced the turtle spinal cord as a model system for studies of the task of scratching with their demonstration of rostral scratching in a turtle with a complete transection of the spinal cord posterior to the forelimb enlargement. Gentle mechanical cutaneous stimulation of a site in the midbody region of the shell elicited rhythmic scratching during which the dorsum of the hindfoot reached toward and rubbed against the stimulated site. At the time we became aware of this study, we were developing an in vivo spinal turtle preparation with neuromuscular blockade. During one of our experiments in the late 1970s, we gently rubbed against a site on the midbody shell bridge while recording from motor neuron axons in peripheral nerves. The robust activation of motor neuron activities with outstanding rhythmicity was a major influence on us, and turtle scratching became the major focus of our laboratory.
There is a CPG for rostral scratch in the turtle spinal cord.
Stein and Grossman (1980) and Bakker and Crowe (1982) characterized the EMG motor patterns for rostral scratch in a spinal turtle whose hindlimb reached toward and rubbed rhythmically against a stimulated site in the midbody shell bridge that connects the dorsal carapace to the ventral plastron. The set of sites that evoke a rostral scratch when stimulated is collectively termed the rostral scratch receptive field (Mortin et al. 1985).
Three techniques were used to demonstrate that there was a CPG for rostral scratch in the turtle spinal cord: 1) EMG recordings following deafferentation via complete transection of the dorsal roots serving the hindlimb (Stein and Grossman 1980), 2) ENG recordings following deefferentation via neuromuscular blockade (Robertson et al. 1985; Stein and Grossman 1980), and 3) ENG recordings in an in vitro preparation of turtle shell, spinal cord, and nerves with all muscles removed (Keifer and Stein 1983). EMG recordings during rostral scratch included rhythmic alternation between hip-flexor and hip-extensor activity and monoarticular knee- extensor activity during the latter portion of hip-flexor activity (Earhart and Stein 2000b; Robertson et al. 1985). ENG recordings from the nerves (see traces below “Shell bridge: rostral scratch” in Fig. 1) innervating each of these muscles demonstrated the corresponding pattern of motor neuron activity in response to stimulation of a site in the rostral scratch receptive field (Currie and Stein 1989; Keifer and Stein 1983; Robertson et al. 1985).
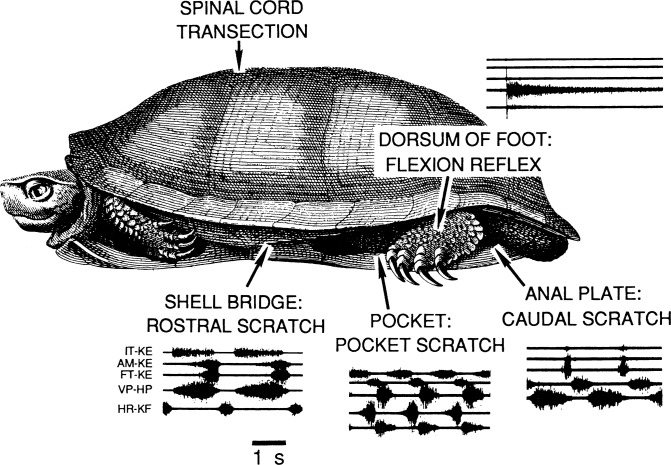
Fig. 1.
Sketch of turtle illustrating location of the complete transection of the spinal cord between the D2 and D3 spinal segments. A turtle with a complete transection of the spinal cord is termed a spinal turtle. Also shown are the locations on the body surface that, when stimulated, activate the fictive motor patterns of rostral scratch, pocket scratch, caudal scratch, and flexion reflex. ENG patterns recorded during each fictive motor behavior from 5 hindlimb motor nerves: IT-KE (top trace) and AM-KE (second trace) are biarticular knee extensors, FT-KE (third trace) is the monoarticular knee extensor, VP-HP (fourth trace) is the hip flexor, and HR-KF (bottom trace) is the hip extensor. A shared feature of all the scratch patterns is rhythmic alternation between hip-flexor and hip-extensor ENGs during all 3 forms of scratch. A distinct feature of each scratch pattern is the specific timing of the monoarticular knee extensor in the cycle of hip-motor activity. A tap to the dorsum of the foot activates multisecond hip-flexor ENG activity. [Turtle sketch adapted from Bojanus (1819). Reprinted from Currie and Stein (1989); used with permission of and copyright 1989 by the Society for Neuroscience.]
The turtle rostral scratch motor pattern is an example of a “mixed synergy” (Stein and Smith 1997): the monoarticular knee extensor is active during the latter portion of hip-flexor activity. In a rostral scratch, the flexor-to-extensor transition at the knee is phase shifted so that it occurs before the flexor-to-extensor transition at the hip. Knee-flexor EMGs during actual rostral scratch were first described by Bakker and Crowe (1982). Many ENG studies during fictive rostral scratch described the flexor-extensor transition at the hip and the timing of monoarticular knee-extensor activation (Berkowitz and Stein 1994a; Currie and Stein 1989; Robertson et al. 1985; Robertson and Stein 1988; Stein et al. 1995, 1998; Stein and Daniels-McQueen 2002). For many years, however, knee-flexor ENGs were not obtained because of the technical difficulties of dissecting the very short nerve to the knee-flexor muscle. In the early 2000s, we mastered intramuscular dissection of the fascicles of the knee-flexor nerve (Stein and Daniels-McQueen 2003, 2004) to obtain knee-flexor ENGs during fictive rostral scratch. Recordings of both knee-flexor and knee-extensor ENGs during fictive rostral scratch allowed us to measure the timing of the flexor-extensor transition at the knee and demonstrate that this transition at the knee is phase-shifted with respect to the flexor-extensor transition at the hip. We also established that the timing of the end-phase of the knee-flexor ENG burst is correlated with the timing of the start-phase of the knee-extensor ENG burst during fictive rostral scratch. A later section describes our use of start-phase timing of knee-extensor ENGs to assist in the identification of candidate knee-related interneurons (Stein and Daniels-McQueen 2003; Stein et al. 2016).
Three Forms of Turtle Hindlimb Scratching
Studies of turtle scratch CPGs advanced with our discovery that there were three forms of the hindlimb scratch in a turtle with a complete transection of the spinal cord: rostral, pocket, and caudal (Mortin et al. 1985; Robertson et al. 1985; Stein et al. 1986b). Each form uses a distinct part of the hindlimb to rub against a site on the body surface that receives gentle mechanical cutaneous stimulation. The biomechanics of the hindlimb in relation to the body structure are major factors that influence which form the turtle hindlimb uses to rub against a stimulated site on the body surface. The dorsum of the foot is used to reach anteriorly toward sites in the rostral scratch receptive field. The arrow labeled “shell bridge” in Fig. 1 points to a site in the rostral scratch receptive field. The structure of the hindlimb and body prevents any portion of the foot being used to rub against sites in the pocket scratch receptive field. Instead, the sides of the knee and thigh are used to rub against stimulated sites in the pocket region adjacent to the proximal portion of the hindlimb. The arrow labeled “pocket” in Fig. 1 points to a site in the pocket scratch receptive field. The heel of the foot is used to rub against sites in the caudal scratch receptive field. The arrow labeled “anal plate” in Fig. 1 points to a site in the caudal scratch receptive field. It is remarkable that the neuronal circuitry in the turtle spinal cord in the absence of supraspinal influences can select the appropriate set of movements and motor patterns to direct the hindlimb to rub successfully against a site that receives a cutaneous stimulus. Similar abilities were revealed during the wiping reflex in the spinal frog (Berkinblit et al. 1989; Fukson et al. 1980; Giszter et al. 1989) and during grooming behavior in the locust (Berkowitz and Laurent 1996).
The set of sites on the body surface that evoke only one form of scratch is the receptive field for that form (Mortin et al. 1985; Mortin and Stein 1990). There are three receptive fields for the turtle hindlimb scratch: rostral, pocket, and caudal. In addition, there is a rostral-pocket transition zone located in a narrow zone between the rostral scratch receptive field and the pocket scratch receptive field. Stimulation of a single site in the rostral-pocket transition zone can evoke a number of different motor behaviors: 1) a rostral scratch or 2) a pocket scratch, or 3) a blend of rostral and pocket scratches. Blends are either 1) switches in which a cycle of one form is followed by a cycle of the other form or 2) hybrids in which rubs of both forms occur within each of several successive cycles. There is also a pocket-caudal transition zone located between the pocket scratch receptive field and the caudal scratch receptive field. The production of naturally occurring blends in response to stimulation of a single site reveals a major complexity in motor pattern selection in the turtle spinal cord: not only can neuronal networks in the spinal cord select a particular form of behavior, but these networks can also combine several forms into a complex coordinated blended behavior (Robertson et al. 1985; Stein et al. 1986b). A later section describes simultaneous stimulation of a site in one scratch receptive field along with another site in a different scratch receptive field that also evokes blends of two motor patterns (Stein et al. 1986a).
Shared features of motor patterns and movements during each of the three forms of turtle hindlimb scratching.
A key feature of the movements for all three forms of scratch (Field and Stein 1997a; Mortin et al. 1985) is rhythmic alternation between hip flexion and hip extension. EMG recordings during all three forms of scratch exhibit rhythmic alternation between hip-flexor muscle activation and hip-extensor muscle activation (Earhart and Stein 2000b; Robertson et al. 1985). In a spinal turtle with neuromuscular blockade, ENG recordings during all three forms of fictive scratch (Fig. 1) reveal rhythmic alternation between hip-flexor motor nerve activation and hip-extensor motor nerve activation (Robertson et al. 1985).
Distinct features of motor patterns and movements during each of the three forms of turtle hindlimb scratching.
A key characteristic of each scratch form is the extension of the knee joint that occurs during the rub against the stimulated site (Field and Stein 1997a; Mortin et al. 1985). What is different for each form of scratch is the timing of the rub and the timing of knee extension in the rhythmic cycle of hip-flexion and hip-extension movements. These distinct features are a consequence of the biomechanics. To rub against a site in the rostral scratch receptive field, the hip is flexed and the femur is in an anterior position while the knee extends. For rubs against sites in the pocket scratch receptive field, the hip is extending and the femur is moving posteriorly while the knee extends. For rubs against sites in the caudal scratch receptive field, the hip is already extended and the femur is in a posterior position when the knee extends.
EMG recordings from the monoarticular knee-extensor muscle show that its activity occurs in a distinct phase of each form’s cycle (Earhart and Stein 2000b; Robertson et al. 1985). During a rostral scratch, the monoarticular knee extensor is active during the latter portion of hip-flexor muscle activation. During a pocket scratch, the monoarticular knee extensor is active during hip-extensor muscle activation. During a caudal scratch, the monoarticular knee extensor is active following hip-extensor muscle activation. For stimulation in the receptive field for each form of scratch, the patterns of ENG recordings from the peripheral nerves that innervate each of these muscles in a spinal turtle with neuromuscular blockade (Fig. 1) are excellent replicas of the patterns of EMG recordings in the animal with moving limbs (Berkowitz 2002; Currie and Stein 1989; Robertson et al. 1985). These results establish that the motor pattern for each of the three forms of scratch is centrally patterned within the turtle spinal cord; that is, there is a CPG for each form of scratch. Additional experiments with single-neuron recordings described in later sections establish that the CPG for each scratch form shares many neurons with the CPGs for each of the other scratch forms (Berkowitz and Hao 2011).
Electrical Stimulation of Cutaneous Afferents in Scratch Receptive Fields Reveals Multisecond Processing of Scratch-Form Motor Pattern Selection
Sherrington (1906a, 1906b) pioneered electrical stimulation of cutaneous afferents in scratch receptive fields with his use of the “electric flea” in a dog with complete spinal transection. He adjusted the amplitude of an electric stimulus so that 1) a single test pulse activated cutaneous afferents but was subthreshold for evoking a scratch, and 2) a pair of test pulses separated by as much as 1.6 s could evoke a hindlimb scratch directed at the site of the electrodes. This revealed temporal summation of spinal cord cutaneous processing. He also used two electric fleas separated by a distance to reveal spatial summation. He proposed that synaptic processing in the spinal cord was a mechanism that contributed to both temporal and spatial summation.
Multisecond processing of cutaneous information in the turtle spinal cord.
Electrical stimulation of the turtle shell in the rostral scratch receptive field (Crowe and Linnartz 1985; Currie and Stein 1990, 1992; Stein et al. 1995) or of a specific cutaneous nerve that innervates the pocket scratch receptive field (Currie and Stein 1988; Currie and Lee 1996a; Guzulaitis et al. 2012, 2013) was used to evoke rostral or pocket fictive scratch, respectively. The amplitude of each stimulus pulse was adjusted so that 3-Hz stimulation evoked a robust fictive scratch with its form determined by the receptive field of the stimulated afferents. When a single test pulse with that amplitude was delivered in a rested preparation, there was usually no motor response: this indicated that the sensory neuron volley evoked by the single test pulse was subthreshold for activating a scratch in a rested preparation. When test pulses were delivered with interpulse intervals of up to 5 s, a scratch motor pattern was evoked following each successive pulse after the first. This demonstrates multisecond summation of cutaneous information in the turtle spinal cord. Single-unit recordings revealed a class of long-afterdischarge cutaneous interneurons that may contribute to multisecond processing (Currie and Stein 1990). Blockade of NMDA receptors attenuated temporal summation of long-afterdischarge interneuron excitability as well as scratch excitability (Currie and Lee 1996a; Currie and Stein 1992). These results demonstrate that NMDA receptors play a key role in the temporal processing of sensorimotor information in turtle spinal cord (Daw et al. 1993).
Multisecond after-excitability of spinal motor neurons following a scratch episode.
Alaburda and Hounsgaard (2003) used intracellular recordings from hindlimb motor neurons to examine excitability following an episode of pocket scratch. In a rested preparation, they adjusted the amplitude of a test depolarizing current pulse to activate a single action potential in the motor neuron. When the test pulse was delivered a number of seconds following a scratch episode, the test pulse evoked several action potentials. The postscratch responses to the test pulse were attenuated by the application of a metabotropic glutamate receptor 1 (mGluR1) antagonist as well by the application of an L-type calcium channel antagonist. These experiments support the concept that there is intrinsic neuromodulation (Katz and Frost 1996) in the turtle spinal CPGs in which glutamate released by excitatory interneurons activates a metabotropic glutamatergic receptor in motor neurons that in turn facilitate L-type calcium channels in these motor neurons (Alaburda and Hounsgaard 2003).
Multisecond after-excitability of spinal interneurons in motor pattern selection networks.
After all the motor neuron activity of a scratch episode ends, there are multisecond excitability changes of spinal networks that are motor pattern specific. The state of the spinal networks can be probed with the use of a test pulse to the ventral-posterior pocket (VPP) cutaneous nerve. This nerve contains sensory neurons that innervate a portion of the pocket scratch receptive field (Fig. 1 of Currie and Stein 1988). Even though a single test pulse to the VPP nerve in a rested preparation activates no motor output, delivery of a single test pulse to the VPP nerve within a few seconds after the end of pocket scratch motor activity activates another cycle of pocket scratch; this demonstrates multisecond memory of cutaneous processing for motor pattern selection. When the single test pulse to the VPP nerve is delivered just after a rostral scratch, no motor response is observed; this demonstrates that the multisecond cutaneous memory in the spinal cord has motor pattern specificity. Future experiments are needed to reveal the full neuronal mechanisms responsible for the multisecond memories of scratch motor pattern selection.
CPGS FOR LOCOMOTION IN THE SPINAL CORD
Focal Electrical Stimulation Can Evoke Locomotor Behaviors
Sherrington pioneered the use of focal electrical stimulation of the spinal cord to evoke the hindlimb stepping movements in a spinal cat (Roaf and Sherrington 1910). Shik, Severin, and Orlovsky (Orlovsky et al. 1999; Shik et al. 1966) demonstrated that focal electrical stimulation of a site in the midbrain could evoke overground locomotion in a decerebrate cat. By varying the intensity of the stimulation, they could evoke each of several forms of locomotion: walk, trot, or gallop. Wiersma and Ikeda (1964) used electrical stimulation to activate specific command interneurons and evoke crayfish swimmeret locomotor movements (reviewed in Mulloney and Smarandache 2010; Mulloney and Smarandache-Wellmann 2012). These results were major influences on the direction of studies in our laboratory when we began our studies of turtle swimming in the early 1970s.
Focal Electrical Stimulation of the Turtle Spinal Cord Can Evoke Swimming Behaviors
Constant-frequency electrical stimulation of axons in specific white matter sites in the turtle dorsolateral funiculus evoked swimming movements of limbs (Lennard and Stein 1977; Lennard 1985; Stein 1978). Our initial focus was on forward swimming in a hindlimb activated by stimulation of the contralateral dorsolateral funiculus (cDLF) in the midbody spinal cord (Lennard and Stein 1977). Our studies described intact turtles as well as turtles with a complete transection of the midbody spinal cord. We characterized the forward-swimming form in a hindlimb by limb movements and EMG activities of selected muscles of the hip and knee. The powerstroke of the forward swim occurred during hip extension. During the forward-swim powerstroke, the foot was held vertically with webbing extended while the foot moved caudally. We speculated that the cDLF contains axons that activate swimming during voluntary swimming in intact turtles. Focal lesion studies of Samara and Currie (2008) provided experimental support for that speculation.
Juranek and Currie (2000) established that there is a CPG for forward swimming in the turtle spinal cord by recording hindlimb ENGs in response to cDLF stimulation in a spinal turtle deefferented by neuromuscular blockade. These ENGs were excellent replicas of the EMGs recorded during actual movements of the hindlimb.
Lennard and Stein (1977) noted briefly that the hindlimb ipsilateral to some stimulation sites exhibited backpaddling, whereas the contralateral hindlimb produced forward swimming. Backpaddling has been characterized in intact turtles during turning swimming behaviors: backpaddling of an ipsilateral hindlimb occurred during forward swimming of the contralateral hindlimb (Earhart and Stein 2000b; Field and Stein 1997a, 1997b; Welch and Currie 2014). The powerstroke of the backpaddle occurred during hip flexion. During the backpaddle powerstroke, the foot was held vertically with webbing extended while the foot moved rostrally. Currie (2007) established that there is a CPG for backpaddling by recording hindlimb ENGs in an immobilized turtle in response to stimulation of sites in the ipsilateral dorsolateral funiculus (iDLF).
Comparison of swim motor patterns with scratch motor patterns.
During the two forms of swimming and the three forms of scratching, there was rhythmic alternation between hip-flexor motor activity and hip-extensor motor activity (Earhart and Stein 2000b; Hao et al. 2014; Juranek and Currie 2000). The timing of monoarticular knee-extensor activity in the hip cycle during forward swim was different from its timing during backpaddling. Thus the timing of knee activity in the hip-activity cycle was used to distinguish between the two forms of swim as it was used to distinguish between the three forms of scratch. Interestingly, for both the forward swim and the rostral scratch, the onset of monoarticular knee-extensor motor activity occurred in a similar phase of the cycle near the middle of hip-flexor motor activity. Other features, such as the intensities of motor activity, were used to discriminate between forward swim and rostral scratch (Earhart and Stein 2000b). In particular, the hip-extensor activity that occurs during the powerstroke of the forward swim is much higher in amplitude than the hip-extensor activity that occurs during the returnstroke of rostral scratch. In addition, the hip-flexor activity that occurs during the powerstroke of rostral scratch is much higher in amplitude than the hip-flexor activity that occurs during the returnstroke of forward swim.
Future studies of turtle hindlimb locomotor behaviors.
Red-eared turtles are semiaquatic and produce a wide variety of locomotor behaviors (Blob et al. 2008; Earhart and Stein 2000b; Mayerl et al. 2017; Rivera and Blob 2010; Rivera et al. 2006; Walker 1979; Welch and Currie 2014; Zug 1971). They locomote gracefully in water during forward swims, turning swims, and backward swims. They move adequately on land during terrestrial overground locomotion (walking) in the forward direction as well as during turning stepping. They locomote nicely in water on the bottom of lakes and streams during bottom walking that combines features of hindlimb movements during terrestrial walking with a thrust at the end of hindlimb retraction similar to the powerstroke of forward swimming. Future studies are required to characterize the EMG motor patterns during the naturally occurring hybrid behavior of bottom walking. Additional challenges for future studies of turtle locomotion are to examine different forms of walking up and down slopes, similar to studies in cat (Smith and Carlson-Kuhta 1995; Stein and Smith 1997; Zernicke and Smith, 2011).
EMG motor patterns for terrestrial forward locomotion (walking) share some features with EMG motor patterns for the three forms of scratch and the two forms of swimming, including rhythmic alternation between hip-flexor and hip-extensor activities (Earhart and Stein 2000b; Mayerl et al. 2017). The timing of the monoarticular knee extensor during walking is particularly interesting in that it fires in two distinct bursts during the step cycle, once during hip flexion and a second time during hip extension. Whereas electrical stimulation of the DLF of the spinal cord has been utilized to generate the motor patterns for both actual and fictive aquatic locomotion (forward swimming and backpaddling), a turtle preparation suitable for studying other forms of fictive locomotion has not yet been developed. Future work is required to demonstrate a CPG for terrestrial walking and bottom walking in the turtle.
CPG FOR HINDLIMB FLEXION REFLEX
The spinal turtle generates a brisk flexion reflex in response to a gentle pinch to the toes or tap to the dorsum of the foot. We observed hindlimb flexion-reflex movements and hip-flexor EMGs in the spinal turtle over many years in our laboratory but did not include these data in our publications. In contrast, there are a number of reports of ENG recordings from hip-flexor motor neurons during fictive flexion reflex (see traces above “Dorsum of foot: flexion reflex” in Fig. 1; Berkowitz 2007; Currie and Lee 1996b; Currie and Stein 1989; Elson and Berkowitz 2016; Johnson et al. 2015, 2017; Robertson and Stein 1988; Stein et al. 1982; Stein and Schild 1989). The term CPG is usually applied to rhythmic behaviors, but it can apply equally well to the neuronal network driving the coordinated motor output for a ballistic behavior such as the hindlimb withdrawal triggered by a brief stimulus that evokes flexion reflex.
Hip-flexor ENGs provide an excellent assay of the fictive flexion-reflex motor pattern. There is a brief high-amplitude burst of activity in the hip flexors just following the tactile stimulus to the limb that is followed by a longer lasting burst of hip-flexor ENG activity that gradually decrements in amplitude over the time course of several seconds (Fig. 1). Application of 2-amino-5-phosphonovaleric acid (APV), an NMDA receptor antagonist, to the anterior segments of the hindlimb enlargement attenuates the multisecond response of the hip-flexor ENG (Stein and Schild 1989). This demonstrates that NMDA receptor processing contributes to the multisecond time course of hip-flexor activity during flexion reflex. Application of nifedipine, an L-type calcium channel antagonist, to the hindlimb enlargement also attenuates the multisecond response of the hip-flexor ENG during flexion reflex (Johnson et al. 2015, 2017). This demonstrates that L-type calcium channels also contribute to the multisecond time course of hip-flexor activity during the flexion reflex. Application of a trio of stimuli that evoke flexion reflexes at multisecond intervals produces windup, an increased amplitude of response to each successive stimulus. Windup is also attenuated by nifedipine (Johnson et al. 2015, 2017), demonstrating the contribution of L-type calcium channels to windup, as well.
FEATURES OF NETWORK ORGANIZATION OF CPGS
Features of CPG Network Organization
The CPG for one task form may share neuronal elements with the CPG for another task form.
Some physiological studies with single-unit interneuronal recordings have focused on a single form of a task; e.g., what are the properties of interneurons in a network that produce the motor patterns that direct an appendage to perform forward walking? From behavioral grounds, it is well understood that limbs perform several forms of overground locomotion, e.g., backward as well as forward overground locomotion, as well as other tasks such as scratching, swimming, and withdrawal. The work of Deliagina, Orlovsky, and colleagues (Berkinblit et al. 1978a, 1978b) with cat spinal-cord single-unit interneuronal recordings demonstrated individual spinal interneurons active during fictive scratch. Some of these interneurons were also active during slow oscillations that resembled fictive step. They suggested that the CPG for cat scratch may share neuronal elements with the CPG for cat step (see also Trejo et al. 2015). Once we began to study the several forms of turtle scratch, we realized that it would be very interesting to obtain single-unit recordings of turtle interneurons during each of several scratch forms (Berkowitz and Stein 1994a, 1994b).
Multifunctional CPGs.
Grillner elaborated on the notion of a CPG shared for several forms with the “unit-burst generator (UBG)” hypothesis (see Fig. 31 of Grillner 1981). He proposed that there is a UBG for each direction of each degree of freedom of the cat hindlimb, e.g., a hip-flexor UBG, a hip-extensor UBG, a knee-flexor UBG, a knee-extensor UBG, etc. He suggested that in the UBG for each degree of freedom, there are excitatory interneurons that synaptically excite the neurons for that degree of freedom and inhibitory interneurons that synaptically inhibit the neurons in the antagonist UBG for that degree of freedom. Thus the UBG hypothesis includes reciprocal inhibitory connections between agonist and antagonist UBGs at each degree of freedom. The UBG hypothesis suggests that 1) interneurons are shared and used for multiple forms of behavior, e.g., activation during cat forward step as well as during cat backward step, and 2) there are variable synaptic connections between UBGs at different degrees of freedom so that the relative timing of one UBG during the cycle of another UBG can be adjusted according to the needs of the task form. Grillner’s UBG hypothesis implies 1) individual CPG interneurons may be active during more than one task form, and 2) there is a modular organization within the CPG such that units in each UBG will be active during one distinct fraction of the rhythmic cycle and quiescent during another distinct fraction of the rhythmic cycle.
The basis of rhythmicity in the UBG hypothesis arises from the rhythmogenic capabilities of individual UBGs as well as from rhythmicity due to the connections between different UBGs, e.g., reciprocal inhibition at each degree of freedom (Grillner 1981). An alternate hypothesis about the organization of the CPG network controlling hindlimb stepping is the two-layer hypothesis of McCrea and Rybak (2008), which postulates that rhythmicity originates in a rhythm-generator layer that drives modules in a pattern-generator layer with organizational features similar to Grillner’s UBGs. Perret and Cabelguen (1980) and Burke et al. (2001) have also presented versions of a two-layer hypotheses of the organization of the cat-stepping CPG. A point of view that is open to discussion is the extent to which the modular organization of the pattern-generator layer of the McCrea and Rybak hypothesis shares characteristics with the modular organization of the Grillner UBG hypothesis.
Intracellular recordings from motor neurons provide insights into CPG organization.
The vertebrate motor neuron has long served as a model cell for studies of neuronal integration (Brownstone and Stuart 2011; Hounsgaard 2017; Johnson and Heckman 2014; Stuart and Brownstone 2011). Synaptic potentials in turtle motor neurons during activation of CPG networks have been of special interest in the laboratories of Jorn Hounsgaard and collaborators (Alaburda and Hounsgaard 2003; Alaburda et al. 2005; Berg et al. 2007; Grigonis and Alaburda 2017; Guzulaitis et al. 2012; Guzulaitis et al. 2013, 2014; Guzulaitis and Hounsgaard 2015; Guzulaitis et al. 2016; Guzulaitis and Hounsgaard 2017; Petersen et al. 2014), as well as in the laboratory of Ari Berkowitz (Hao and Berkowitz 2017) and in our own laboratory (Robertson and Stein 1988; Stein et al. 1982).
Neuromodulation of CPGs.
Another major influence on thinking about the organization of CPGs that developed in the 1980s and has flourished since is the concept of neuromodulation of a CPG (Harris-Warrick and Marder 1991; Katz and Frost 1996; Marder and Calabrese 1996; Marder 2012): multiple motor patterns can be produced by a single anatomical network under the influence of different neuromodulators. This concept gathered major support from experiments with the crustacean stomatogastric nervous system and with many other model systems. Neuromodulatory mechanisms serve important roles in vertebrate CPGs (Diaz-Ríos et al. 2017; Miles and Sillar 2011). Intrinsic neuromodulation (Katz and Frost 1996) has been described for the turtle scratch CPG (Alaburda and Hounsgaard 2003).
Features of Turtle Scratch CPG Network Organization
Turtle motor neurons are active during several forms of scratch.
ENG recordings from motor nerves innervating individual hip-flexor, hip-extensor, and knee-extensor muscles during scratch CPG activation establish that each of these motor pools is active during each of the three forms of scratch (Robertson et al. 1985). Intracellular recordings from the cell bodies of individual motor neurons further show that specific members of each of these motor pools are active during each of the three forms of scratch (Robertson and Stein 1988). Future work is needed to determine if most or all of the motor pools of the hindlimb are active during each of the three forms of scratch.
Synaptic inhibition rhythmically alternates with synaptic excitation in turtle motor neurons during each form of scratch.
A fundamental feature of the pattern of synaptic activation of turtle motor neurons during each of the three forms of scratch is rhythmic alternation between synaptic excitation and synaptic inhibition (see Fig. 8 of Robertson and Stein 1988; Stein et al. 1982; Stein 2010). Robertson and Stein (1988) used current injections and injections of chloride ions to manipulate the chloride equilibrium potential relative to the resting potential and to determine the timing of chloride-dependent synaptic inhibition in motor neurons.
Guzulaitis and Hounsgaard (2015, 2017) used voltage-clamp techniques while recording from turtle hip-flexor motor neurons during fictive pocket scratch. Their work provided additional evidence supporting rhythmic alternation between synaptic excitation and synaptic inhibition in turtle motor neurons during fictive scratch. They also characterized the considerable activation of intrinsic conductances in motor neurons that occurred during the voltage increases associated with synaptic excitation. Earlier work by Berg et al. (2007) did not consider these intrinsic conductances and asserted that the major peak of synaptic inhibition occurred during the major peak of synaptic excitation (see also Petersen et al. 2014 and Vich et al. 2017). The recent work of Guzulaitis and Hounsgaard (2015, 2017) does not support this assertion of Berg et al. (2007), however. Instead, Guzulaitis and Hounsgaard (2015, 2017) support the earlier conclusion of Robertson and Stein (1988) that synaptic excitation rhythmically alternated with synaptic inhibition in turtle motor neurons during fictive scratch. Geertsen et al. (2011) provide strong support for a similar conclusion for cat motor neurons during fictive scratch. Motor neuron synaptic drive with rhythmic alternation between synaptic excitation and synaptic inhibition in turtle scratch CPGs is a characteristic of many other spinal cord CPGs (Grillner and Jessell 2009; Kishore et al. 2014; Stein 2010).
For some type-identified motor neurons during some phases of fictive scratch, there is coactivation of synaptic excitation and synaptic inhibition.
Robertson and Stein (1988) found distinct phases of the scratch cycle for certain type-identified motor neurons during specific forms of scratch in which there was some coactivation of synaptic excitation and synaptic inhibition. For example, during fictive rostral scratch in both hip-flexor and in monoarticular knee-extensor motor neurons just before each motor neuron’s burst of action potentials, there is concurrent synaptic inhibition and synaptic excitation. Guzulaitis and Hounsgaard (2015, 2017) also found some coactivation of synaptic inhibition during a portion of the synaptic excitation in hip-flexor motor neurons during fictive pocket scratch. Further work with other type-identified motor neurons during each of the three forms of fictive scratch with voltage-clamp techniques will be very important in establishing a full characterization of the relative timings of excitatory and inhibitory synaptic drives in motor neurons during turtle scratch motor rhythms.
Turtle spinal interneurons are active during several forms of scratch and are broadly tuned to a region of the body surface.
Many individual spinal interneurons that fire during fictive scratch are active during more than one scratch form (Berkowitz 2001a, 2001b, 2002, 2005, 2008, 2010, 2015; Berkowitz et al. 2006, 2010; Berkowitz and Hao 2011; Berkowitz and Stein 1994a, 1994b; Mui et al. 2012). Individual interneurons are broadly tuned: they are maximally activated by stimulation of a site in one form’s scratch receptive field, active to a lesser extent at neighboring sites in that receptive field, and active to a still lesser extent in adjacent scratch receptive fields for other forms. This supports the concept that the CPG for one form of scratch shares interneurons with the CPG for another form of scratch.
Population coding via broadly tuned interneurons is a potential contributing mechanism for turtle scratch motor pattern selection (Berkowitz 2001b, 2015; Berkowitz and Stein 1994b; Snyder and Rubin 2015). According to this hypothesis, each broadly tuned interneuron with maximal firing in one form’s receptive field has output connections that favor the hip-knee timing characteristic of that form. Thus the appropriate hip-knee synergy for a given form is activated by the collective output connections of the interneurons whose firing is broadly tuned in the receptive field of that form. The population-coding hypothesis is compatible with the modular UBG hypothesis: elements in each specific hip-related UBG may have a set of broadly tuned interneurons; each member of that set may have output connections that support the hip-knee timing associated with that interneuron’s input characteristics.
Grillner's UBG hypothesis can be utilized to describe the CPGs for the three forms of turtle scratch.
A number of observations support the utilization of Grillner's UBG hypothesis for the turtle spinal cord CPGs controlling the three forms of turtle scratch (Robertson et al. 1985; Stein 2008). The UBG hypothesis is consistent with a number of shared features among the three forms of scratch: 1) rhythmic alternation between hip flexors and hip extensors, 2) alternation between synaptic excitation and synaptic inhibition in motor neurons for three forms of scratch, and 3) interneurons are active and shared for several forms of scratch. The UBG hypothesis with modifiable interactions between hip UBGs and knee UBGs provides a potential basis for such distinct features as the specific timing of monoarticular knee-extensor activity in the hip-activity cycle.
In Grillner’s UBG hypothesis, there is postulated reciprocal inhibition between the flexor UBG and the extensor UBG at each degree of freedom. This point of view contrasts with the classical half-center organization of spinal CPGs that there is 1) a flexor half-center that controls the activities of all the flexors of the hindlimb, 2) an extensor half-center that controls the activities of all the extensors of the hindlimb, and 3) reciprocal inhibition between the flexor half-center and the extensor half-center (Brown 1911, 1914; Jankowska et al. 1967; Lundberg 1981). The classical half-center hypothesis lacks the organizational complexity to account for the motor patterns for each of the three forms of turtle scratch. In the classical half-center hypothesis, reciprocal inhibitory connections between the flexor and extensor half-centers are the basis of rhythmicity. The classical half-center hypothesis also lacks the complexity to account for the hip-extensor deletions during rostral scratch described in a later section. During this variation, hip flexors are rhythmic even when hip extensors are quiescent.
Turtle hip-related spinal interneurons can be classified according to their overlap with hip-flexor motor activity during normal rostral scratches.
We categorized the start-phases and end-phases of bursts of activity of descending propriospinal interneurons recorded extracellularly during each cycle of normal rostral scratch (Stein and Daniels-McQueen 2002). We proposed that 1) interneurons whose bursts had large overlap with the hip flexor motor neuron burst were candidate hip-flexor interneurons and candidate members of the hip-flexor UBG and 2) interneurons whose bursts had little or no overlap with the hip-flexor motor neuron burst were candidate hip-extensor interneurons and candidate members of the hip-extensor UBG. We also observed other interneurons with intermediate levels of overlap with hip-flexor motor activity during fictive rostral scratch.
Turtle knee-related spinal interneurons with intermediate overlap with hip-flexor activity can be classified according to the correlations of their start- or end-phases with the start-phases of knee-extensor motor activity during normal fictive rostral scratch.
The transition from knee-flexor to knee-extensor activity occurred near the middle of hip-flexor activity during normal fictive rostral scratch (Stein and Daniels-McQueen 2003, 2004; Stein et al. 2016). Some interneurons with intermediate levels of overlap with hip-flexor motor activity had their activity start-phases correlated with and near the start-phases of knee extensor activities. These interneurons were termed ON-units and were candidate members of the knee-extensor UBG module. Other interneurons with intermediate levels of overlap with hip-flexor motor activity had their activity end-phases correlated with and near the start-phases of knee-extensor activities. These interneurons were termed OFF-units and were candidate members of the knee-flexor UBG module.
The UBG hypothesis can be used to predict the firing patterns of candidate members of specific UBGs during motor-pattern variations termed deletions.
The UBG hypothesis modified for turtle scratch was initially developed on the basis of the characteristics of neuronal activities during normal cycles of scratch. In the next section, we summarize work with motor pattern variations, termed deletions. We use the UBG hypothesis to generate predictions about the activities of candidate interneurons in specific modules during each type of deletion.
DELETIONS ARE MOTOR PATTERN VARIATIONS THAT REVEAL MODULAR ASPECTS OF CPG ORGANIZATION
Motor pattern deletions are variations in which a normally occurring burst of activity in one or more motor pools is absent. Naturally occurring deletions have been found in studies of mammalian CPGs (Lafreniere-Roula and McCrea 2005; Zhong et al. 2012). In studies of mammalian fictive motor patterns, some deletions were resetting, that is, when they occurred, the motor rhythm timing was shifted; other deletions were nonresetting, that is, when they occurred, the motor rhythm timing was not shifted. The occurrence of nonresetting deletions in mammals provides support for two-layer modular models of CPG organization (McCrea and Rybak 2008; Zhong et al. 2012). The pattern-generating modules of the McCrea-Rybak hypothesis share some characteristics with Grillner’s UBG modules. To date, it has not been possible to categorize deletions of turtle rostral scratch motor patterns as either resetting or nonresetting. Thus deletion data cannot yet be used in studies of turtle CPGs to discriminate between ideas about Grillner’s UBG modules vs. McCrea-Rybak’s modules in the pattern-generator layer. Future experiments are now needed to obtain multiple examples of a long sequence of normal fictive rostral scratching that is interrupted by a spontaneous hip-extensor deletion. Examination of whether each deletion does or does not result in a reset of the rostral scratch rhythm may assist in determining whether or not there is a rhythm-generator layer that is separate from a pattern-generator layer in turtle CPGs.
Variations in Rostral-Scratch Motor Patterns: Hip-Extensor Deletions
Stein and Grossman (1980; Stein et al. 1982) noted a variation of the rostral scratch termed a “B-phase deletion.” Robertson and Stein (1988) termed this variation an “HR-KF deletion.” This variation is now called a “hip-extensor deletion” (Stein 2008; Stein and Daniels-McQueen 2002, 2003, 2004; Stein et al. 1995, 1998, 2016).
In a cycle of normal rostral scratch (Fig. 2A; Robertson et al. 1985; Stein and Daniels-McQueen 2002, 2003, 2004), there are five important characteristics: 1) there is rhythmic alternation between hip-flexor motor activity and hip-flexor motor quiescence; 2) hip-extensor motor activity occurs during hip-flexor quiescence; 3) there is rhythmic alternation between monoarticular knee-extensor motor activity and quiescence; 4) knee-flexor motor activity occurs during monoarticular knee-extensor quiescence; and 5) monoarticular knee-extensor activity occurs during the latter portion of hip-flexor activity. In cycles of hip-extensor deletion rostral scratch (marked with a diamond in Fig. 2B; Robertson and Stein 1988; Stein and Daniels-McQueen 2002, 2004), the first two characteristics are different: 1) several rhythmic bursts of hip-flexor motor activity occur with no intervening hip-flexor quiescence; and 2) there is no hip-extensor motor activity. Characteristics 3–5 of normal rostral scratch are also characteristics of hip-extensor deletion rostral scratch. Thus, during a hip-extensor deletion rostral scratch, there is rhythmic alternation between knee-flexor and knee-extensor motor activities even though the hip extensors are quiet during rhythmic activation of hip-flexor motor activity.
Fig. 2.
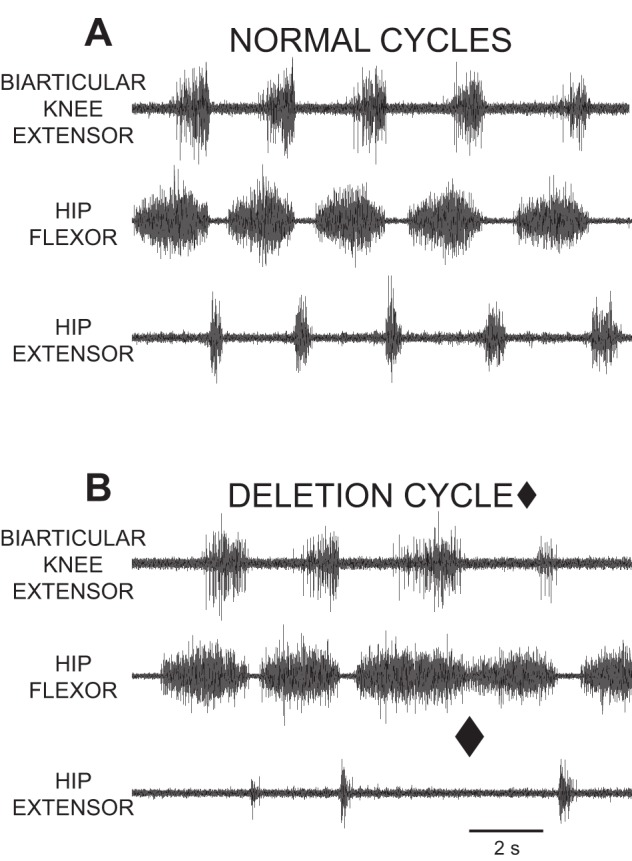
ENG recordings from motor nerves during fictive rostral scratch in the spinal turtle. In A and B, the biarticular knee-extensor (AM-KE) recording is the top trace, the hip-flexor (VP-HP) recording is the middle trace, and the hip-extensor (HR-KF) recording is the bottom trace. A: normal fictive rostral scratch with rhythmic alternation between hip-flexor activity and quiescence. Hip-extensor activity occurs during hip-flexor quiescence. Knee-extensor activity occurs during the latter portion of hip-flexor activity. B: one cycle of a hip-extensor deletion with the end of the cycle marked with a filled diamond. At the filled diamond, there is no hip-extensor activity, and no quiescent period between the end of the hip-flexor burst and the start of the next hip-flexor burst. The other cycles in B are examples of normal rostral scratch. [Reprinted from Stein and Daniels-McQueen (2002); used with permission of and copyright 2002 by the Society for Neuroscience.]
The UBG hypothesis provides a framework to make predictions about neuronal activities during hip-extensor deletion rostral cycles (Stein 2008, 2010). According to the UBG hypothesis: during a normal rostral scratch, there is rhythmic alternation between the hip-flexor UBG module and the hip-extensor UBG module; during a hip-extensor deletion rostral scratch, there is rhythmic activation of the hip-flexor UBG module and a lack of activity in neurons in the hip-extensor UBG module. Thus the hip-flexor UBG module can be rhythmogenic even in the absence of rhythmic inhibitory inputs from the hip-extensor UBG. Since there is rhythmic alternation between knee-extensor and knee-flexor activity during both normal rostral scratches and hip-extensor deletion rostral scratches (Stein and Daniels-McQueen 2003, 2004), the UBG hypothesis predicts rhythmic alternation between the knee extensor UBG and the knee flexor UBG for both normal and hip-extensor deletion rostral scratch.
Synaptic potentials in hip motor neurons during hip-extensor deletion rostral scratch.
Hip-extensor motor neurons receive synaptic excitation and hip-flexor motor neurons receive synaptic inhibition during the hip-extensor phase of a normal rostral-scratch cycle. During a hip-extensor deletion rostral scratch (marked with triangles in Fig. 3), 1) there is no synaptic excitation and no action potentials in hip-extensor motor neurons (Fig. 3B) and 2) the prominent synaptic inhibition of hip-flexor motor neurons is absent (Fig. 3A; Robertson and Stein 1988). These observations are consistent with these UBG hypotheses: 1) during normal rostral scratch, the hip-extensor UBG contains excitatory interneurons that produce excitatory postsynaptic potentials (EPSPs) in hip-extensor motor neurons and inhibitory interneurons that produce inhibitory postsynaptic potentials (IPSPs) in hip-flexor motor neurons; and 2) during hip-extensor deletion rostral scratch, these interneurons in the hip-extensor UBG are quiet and therefore do not produce postsynaptic potentials in hip motor neurons.
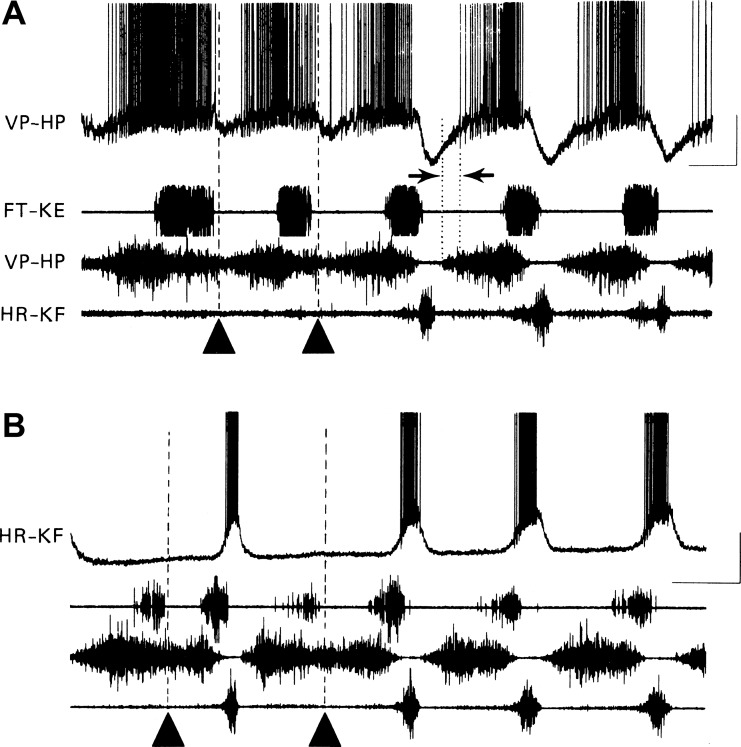
Fig. 3.
Voltage traces recorded intracellularly from hip-flexor (VP-HP) motor neuron (top trace in A) and hip-extensor (HR-KF) motor neuron (top trace in B) during normal fictive rostral scratch and during hip-extensor deletion fictive rostral scratch. In A and B, ENG recordings are from the monoarticular knee-extensor nerve (FT-KE; second trace), hip-flexor nerve (VP-HP; third trace), and hip-extensor nerve (HR-KF; bottom trace). A: hip-extensor motor neuron activity is deleted in first 2 cycles (marked with filled triangles), and the hip-flexor motor neuron intracellular recording shows corresponding deletion of hyperpolarization normally associated with hip-extensor activity (compare with last 3 cycles that show hip-extensor nerve activity). B: hip-extensor nerve activity is deleted in the first and third cycles (marked with filled triangles) and is associated with a complete absence of depolarization in the hip-extensor motor neuron intracellular recording (compare with cycles with hip-extensor nerve activity). Calibrations: 1 s, 20 mV. [Reprinted from Robertson and Stein (1988); used with permission of and copyright 1988 by The Physiological Society and John Wiley and Sons.]
Single-unit interneuronal recordings during hip-extensor deletion rostral scratch.
Stein and Daniels-McQueen (2002) recorded from descending propriospinal interneurons whose activity bursts had little or no overlap with hip-flexor motor activity during normal fictive rostral scratch (Fig. 4). We classified these as hip-extensor interneurons and as candidate members of the hip-extensor UBG. These hip-extensor interneurons are mainly quiet during hip-extensor deletion rostral scratching (marked with a diamond in Fig. 4A) consistent with the prediction that interneurons in the hip-extensor UBG are quiet during hip-extensor deletion rostral scratch.
Fig. 4.
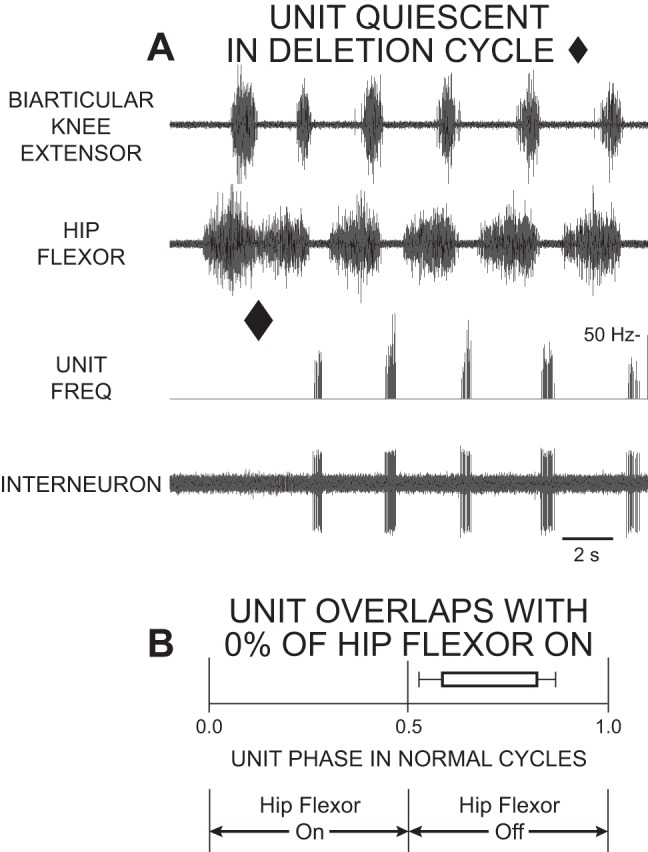
Extracellular single-unit recording of hip-extensor interneuron with 0% overlap with the hip-flexor burst was active in a burst during hip-flexor quiescence of normal fictive rostral scratch and was quiet during fictive rostral scratch with a hip-extensor deletion. A: ENG recording of the biarticular knee-extensor motor nerve (AM-KE; top trace), ENG recording of the hip-flexor motor nerve (VP-HP; second trace); instantaneous frequency of unit (third trace); and interneuron unit activity (bottom trace). The first cycle is an example of rostral scratch with a hip-extensor deletion (end of cycle marked with filled diamond). The other cycles are examples of normal rostral scratch. B: start and end of bar represent mean start-phase and mean end-phase, respectively, of unit firing during normal rostral scratch. [Reprinted from Stein and Daniels-McQueen (2002); used with permission of and copyright 2002 by the Society for Neuroscience.]
We also recorded from interneurons whose activity bursts had large overlaps with hip-flexor motor activity during normal fictive rostral scratch (Stein and Daniels-McQueen 2002). We classified these as hip-flexor interneurons and as candidate members of the hip-flexor UBG. These hip-flexor interneurons are rhythmically active for most or all of the hip-flexor burst in a hip-extensor deletion rostral scratch. This rhythmic activity of hip-flexor interneurons during hip-extensor deletions is consistent with the hypothesis that the hip-flexor UBG is rhythmogenic even in the absence of hip-extensor UBG activity.
We also recorded from interneurons with intermediate overlap with the hip-flexor ENG burst during fictive rostral scratch (Stein and Daniels-McQueen 2002, 2003; Stein et al. 2016). These interneurons are candidate members of knee UBGs. The start-phases of some intermediate-overlap interneurons are positively correlated with and near the start-phases of knee-extensor motor activity. Since these interneurons turn on at about the same time as knee-extensor motor activities, these interneurons are termed ON-units and are candidate members of the knee-extensor UBG. The end-phases of other intermediate-overlap interneurons are positively correlated with and near the start-phases of knee-extensor motor activity. Since these interneurons turn off at about the same time as knee-flexor motor activities, these interneurons are termed OFF-units and are candidate members of the knee-flexor UBG. Both ON-units and OFF-units continue to fire in bursts during hip-extensor deletions. The rhythmic alternation between bursts of ON-units and bursts of OFF-units during hip-extensor deletions is consistent with the hypothesis that there is rhythmic alternation of knee-flexor and knee-extensor UBGs during hip-extensor deletions. Rhythmic alternation between the knee-extensor UBG and the knee-flexor UBG during normal rostral scratch and during hip-extensor deletion rostral scratch differs from the hypothesized behavior of these UBGs during the knee-related deletions that are described in the following section.
Variations in Rostral-Scratch Motor Patterns: Knee-Related Deletions
Stein and Daniels-McQueen (2004) described two types of knee-related deletions during rostral scratch: knee-flexor deletions and knee-extensor deletions. During a knee-flexor deletion rostral scratch, there is no knee-flexor motor activity and no quiescence between successive knee-extensor bursts. This is consistent with the hypothesis that the knee-extensor UBG is rhythmogenic in the absence of knee-flexor UBG activity. During a knee-extensor deletion rostral scratch, there is no knee-extensor activity and no quiescence between successive knee-flexor bursts. This is consistent with the hypothesis that the knee-flexor UBG is rhythmogenic in the absence of knee-extensor UBG activity.
Single-unit interneuronal recordings during knee-related rostral scratch deletions.
OFF-units are candidate members of the knee-flexor UBG. OFF-units are mainly quiet during knee-flexor deletion rostral scratching (Stein et al. 2016). ON-units are candidate members of the knee-extensor UBG. ON-units are mainly quiet during knee-extensor deletion rostral scratching (Stein et al. 2016). Thus units whose timing is related to the knee-flexor to knee-extensor transition have properties during knee-related rostral-scratch deletions consistent with the predictions of the UBG organization of the rostral scratch CPG.
LEFT HINDLIMB RHYTHMS ARE USUALLY OUT OF PHASE WITH RIGHT HINDLIMB RHYTHMS
Turtle left and right hindlimbs usually move out of phase with each other during symmetric rhythmic behaviors in which both limbs are performing the same task, e.g., forward swimming or forward walking in turtles with an intact nervous system (Earhart and Stein 2000b; Field and Stein 1997b; Welch and Currie 2014). Flexion at one hip usually alternates with flexion of the contralateral hip. Interestingly, this out-of-phase coordination pattern at the hip also occurs during asymmetric behaviors such as turning swimming, in which one hindlimb performs a forward swim and the other hindlimb performs a backpaddle. During a turning swim, however, left and right knee-extension movements are nearly in phase with each other.
Left-right out-of-phase coordination at the hip can also be observed in low-spinal turtles during bilateral rostral scratching and bilateral pocket scratching (Field and Stein 1997b). These bilateral motor behaviors are evoked by simultaneous stimulation of mirror-image sites in left and right scratch receptive fields. Left-right coordination of hip movements during bilateral caudal scratching can be either out of phase or in phase. Simultaneous stimulation of an ipsilateral site in a rostral scratch receptive field and a contralateral site in a pocket scratch receptive field produces mixed-form bilateral scratch behaviors in which the ipsilateral hindlimb performs a rostral scratch and the contralateral hindlimb produces a pocket scratch (Field and Stein 1997b). Interestingly, during this mixed-form bilateral scratch behavior, there is left-right out-of-phase coordination at the hip even though coordination at the knee is nearly in phase.
Left-right out-of-phase coupling of hip motor bursts is also observed during bilateral fictive rostral scratching in response to bilateral mirror-image stimulation of rostral scratch receptive fields (Currie and Gonsalves 1997, 1999; Stein et al. 1995). Similar coupling is also observed during bilateral pocket scratching (Guzulaitis et al. 2014). Thus out-of-phase interlimb coordination is a property of turtle rostral and pocket scratch CPGs.
The observation that several categories of same-form bilateral behaviors and several categories of mixed-form bilateral behaviors display out-of-phase coordination at the hip is consistent with the hypothesis that left-right coupling arises, in part, due to reciprocal inhibition between left and right hip-flexor UBGs (Currie and Gonsalves 1999; Stein et al. 1995). An assertion consistent with these data is that each hip UBG serves as a rhythm generator for its hindlimb. Further support for this assertion is described below with descriptions of the phase shifts in fictive scratch and swim motor rhythms by fictive flexion reflex (Currie and Stein 1989; Elson and Berkowitz 2016).
ANATOMICAL LOCATIONS OF CPG NETWORKS
Cutaneous afferents enter the spinal cord via dorsal roots in specific segments. The dermatome map for the turtle shell was described by Mortin and Stein (1990) for segments D3–D10, S1, and S2. The turtle dermatome map is used in combination with the scratch receptive field map (Mortin et al. 1985) to characterize the input segments for cutaneous sensory afferents that innervate each of the scratch receptive fields. Afferents in the rostral scratch receptive field enter the spinal cord in the D3–D6 segments; afferents in the pocket scratch receptive field enter the spinal cord in the D6–D8 segments; afferents in the caudal-scratch receptive field enter the spinal cord in the S2 segment and several segments just caudal to S2. The segmental locations of cell bodies of pools of turtle motor neurons in segments D8–D10, S1, and S2 were described by Ruigrok and Crowe (1984). The axon of each motor neuron exits the spinal cord in ventral roots in the same segment that its cell body is located.
The technique of successive complete transections of the spinal cord can be used to determine the contributions of each of the spinal segments posterior to the initial transection at the D2–D3 segmental border (Currie and Lee 1996a; Currie and Gonsalves 1999; Hao et al. 2014; Mortin and Stein 1989). The one-transection preparation is termed a D3-end preparation. The posterior two segments of the hindlimb enlargement, S1 and S2, can be removed (D3–D10 preparation), and both rostral and pocket motor patterns can still be produced. When additional segments are removed to produce a D3–D8 preparation, rostral motor patterns are still evoked, but a higher percentage of the cycles exhibit the hip-extensor deletion rostral scratch compared with the D3-end preparation. This suggests that when segments D9, D10, S1, and S2 are removed, portions of the hip-extensor UBG for the rostral scratch may also be removed. These segments contain hip-extensor motor neuron cell bodies (Ruigrok and Crowe 1984) and may also contain some of the interneuronal members of the hip-extensor UBG.
A fictive swim is activated by cDLF stimulation in the D3-end preparation (Hao et al. 2014; Juranek and Currie 2000). During a fictive swim in this preparation, there is rhythmic alternation between hip-flexor activity and hip-flexor quiescence. When a fictive swim is activated in the D3–D8 preparation, this rhythmic alternation is maintained in contrast to the hip-extensor deletions observed during rostral scratch in this preparation. Hao et al. (2014) suggest that this result may, in part, be due to the different intensities of hip-extensor activity in forward swim compared with rostral scratch.
In addition, the anterior three segments of the hindlimb enlargement, D8–D10, are sufficient to produce a rhythmic pocket scratch motor pattern (Mortin and Stein 1989). The D8 segment, when isolated from all other spinal segments, is sufficient to generate a rhythmic motor pattern (Mortin and Stein 1989). Currie and Lee (1996a) demonstrated pocket scratch characteristics in the motor rhythm produced by the isolated D8 segment. The rhythmogenic capacities of anterior segments of the hindlimb enlargement have also been described for cat (Deliagina et al. 1983), chick (Ho and O’Donovan 1993), and rat (Antri et al. 2011; Kiehn and Kjaerulff 1998; Kjaerulff and Kiehn 1996).
Contralateral Circuitry Contributions to the Production of the Normal Pattern of Rostral Scratch
Cutaneous stimulation of a site in a rostral-scratch receptive field evokes a rostral scratch motor pattern in hindlimb nerves ipsilateral to the stimulated site (Robertson et al. 1985). There is some rhythmic motor output in contralateral motor nerves, most prominently in the hip-extensor ENG (Berkowitz and Stein 1994a; Currie and Gonsalves 1997, 1999; Currie and Stein 1989; Stein et al. 1995; 1998). The contralateral hip-extensor burst alternates with the ipsilateral hip-extensor burst in a mainly out-of-phase coordination pattern. This suggests that contralateral CPG networks are activated in response to stimulation of an ipsilateral site in a rostral scratch receptive field.
Effects of removal of contralateral circuitry.
Stein et al. (1998) removed the contralateral half of the spinal cord at the border of the D6 and D7 segments. Following this contralateral hemisection, ipsilateral rostral scratch stimulation generated mainly hip-extensor deletion rostral scratch in ipsilateral ENGs. A similar result occurred following complete removal of the left halves of segments D7–D10, S1, and S2 (Stein et al. 1995). This indicates that contralateral circuitry assists in the activation of ipsilateral hip-extensor activity in response to stimulation of a site in the ipsilateral rostral scratch receptive field. In both cases of contralateral circuitry removal, stimulation of a site in the contralateral rostral scratch receptive field activated rhythmic ipsilateral hip-extensor motor activity. Cutaneous afferents from the rostral scratch receptive field enter the spinal cord in the D3–D6 segments (Mortin and Stein 1990); therefore, contralateral rostral scratch receptive field stimulation activates interneurons whose axons cross over to the ipsilateral spinal cord anterior to the D7 segment. In the preparation with contralateral hemisection at the D6–D7 border, bilateral stimulation of sites in left and right rostral scratch receptive fields evoked a reconstructed normal pattern of rostral scratch with rhythmic alternation between hip-flexor and hip-extensor activities (Stein et al. 1998). The interpretation of this result with the UBG hypothesis is that, following the contralateral hemisection, 1) ipsilateral stimulation strongly activates the ipsilateral hip-flexor UBG, but the hip-extensor UBG receives little or no activation; 2) contralateral stimulation strongly activates the ipsilateral hip-extensor UBG, but the ipsilateral hip-flexor UBG receives little or no activation; and 3) bilateral stimulation activates both the hip-flexor and the hip-extensor UBGs, and the reciprocal inhibition between these modules assists in the production of ipsilateral hip-flexor activities that rhythmically alternate with ipsilateral hip-extensor activities. Future work with single-unit recordings in this preparation may provide very interesting tests of the application of the UBG hypothesis to studies of turtle rostral scratch CPGs.
Effects of removal of posterior segments of the hindlimb enlargement.
In a D3–D9 or a D3–D8 preparation with the posterior three to four segments of the hindlimb enlargement removed, unilateral stimulation of a site in the rostral scratch receptive field mainly results in hip-extensor deletion rostral scratch in ipsilateral hindlimb nerves (Currie and Gonsalves 1999; Hao et al. 2014). Bilateral stimulation in rostral scratch receptive fields in these preparations results in rostral scratch motor patterns with the characteristics of normal rostral scratch with rhythmic alternation between hip-flexor activation and hip-flexor quiescence (Currie and Gonsalves 1999). Left hip-flexor activity rhythmically alternates with right hip-flexor activity. The UBG interpretation is that reciprocal inhibition between left and right hip-flexor UBGs is now able to generate hip-flexor activation that alternates with hip-flexor quiescence (Currie and Gonsalves 1999; Stein et al. 1995).
Contributions of Segments Anterior to the Hindlimb Enlargement to Scratch Rhythmicity
Currie and Gonsalves (1997) examined the contributions of left-right circuitry to scratch rhythmogenesis using recordings from two populations of respiratory motor neurons in the D6 and D7 segments. These motor neurons are rhythmically active during fictive rostral scratch in a D3-end preparation. The transverse motor pool is mainly active during hip-flexor activity and alternates activity with the oblique motor pool that is mainly active during hip-flexor quiescence. Hao et al. (2014) observed similar firing patterns during fictive forward swim activated by cDLF stimulation in the D3-end preparation.
In the D3–D7 preparation, ipsilateral transverse motor activity is weakly modulated or tonically active in response to ipsilateral stimulation in a rostral scratch receptive field (Currie and Gonsalves 1997). Interestingly, bilateral stimulation in this preparation produces excellent rhythmic alternation between ipsilateral transverse and ipsilateral oblique ENGs and between ipsilateral transverse and contralateral transverse ENGs. This supports the concept that a bilateral UBG organization with reciprocal inhibition between mirror-image modules contributes to CPG rhythmicity in segments anterior to the hindlimb enlargement.
Hao et al. (2014) also examined ipsilateral transverse and oblique motor activity in the D3–D7 preparation. They found weakly modulated ipsilateral transverse motor activity in response to stimulation in either the ipsilateral rostral scratch or ipsilateral pocket scratch receptive fields with no activation of ipsilateral oblique motor activity. Interestingly, cDLF stimulation activated rhythmic ipsilateral oblique motor activity with no activation of ipsilateral transverse motor activity. These differences in intensity of activation of respiratory motor neurons during rostral scratch vs. forward swim motor patterns in the D3–D7 preparation parallel differences in intensities of hip-flexor and hip-extensor activation during those behaviors in the D3-end preparation (Earhart and Stein 2000a).
Guzulaitis et al. (2014) examined the contribution of the midbody D4 segment to scratch rhythmicity in a D4–S2 preparation. They recorded intracellularly from D4 interneurons during fictive pocket scratch activated by stimulation in the D6–D7 dermatomes. These interneurons were rhythmically activated during scratch episodes at specific phases of the cycle. Some of these were descending propriospinal interneurons, based on anatomical identifications of descending axons. They examined scratch rhythmicity in D3–D10 preparations compared with D6–D10 preparations and found that removal of midbody segments resulted in a lower frequency scratch rhythm. Thus the D3–D5 midbody segments contribute to scratch rhythmicity. See also Langlet et al. (2005) for contributions in cat to hindlimb locomotion by spinal segments anterior to segments that contain hindlimb motor neurons.
There are cutaneous interneurons in the D4 segment, but there are no motor neurons due to the lack of musculature in that part of the turtle body. Currie and Stein (1990) characterized descending propriospinal interneurons in a D4 segment isolated from the rest of the nervous system. These interneurons were activated by cutaneous simulation in the D4 dermatome, but their activation was not rhythmic. In the D4–S2 preparation, it is likely that ascending propriospinal interneurons from more posterior segments are driving the rhythmic activation of D4 interneurons (Guzulaitis et al. 2014) and that some of these D4 interneurons may, in turn, have axons that project back to more posterior segments. Similar long-loop oscillatory pathways have been characterized during scratch in the cat (Arshavsky et al. 1986).
FLEXION REFLEX RESETS SCRATCH AND SWIM RHYTHMS
Fictive flexion reflex can be activated by a brief tap or electrical stimulus applied to the dorsum of the turtle foot (Currie and Stein 1989; Elson and Berkowitz 2016; Robertson and Stein 1988; Stein et al. 1982). The brief stimulus activates EPSPs and action potentials in hip-flexor motor neurons and IPSPs in knee-extensor motor neurons. Flexion reflex can interrupt and reset the rhythm of an ongoing scratch (Currie and Stein 1989) or swim (Elson and Berkowitz 2016). This behavior is an example of a switch type of blend. These results are similar to the resets of the stepping and the scratching rhythm by cutaneous stimulation in cats (Duysens et al. 2013; Frigon and Gossard 2010).
When ipsilateral flexion reflex is activated during hip-flexor activity of scratch for each of the three forms of scratch, this produces a phase delay in the onset of the next hip-flexor burst. When ipsilateral flexion reflex is activated during hip-extensor activity of scratch or swim, this produces a phase advance in the onset of the next hip-flexor burst. Note that each form of scratch has a distinct timing of knee activity in the cycle of hip activity, and the type of phase shift depends on the timing of the flexion-reflex stimulus in the hip cycle and not on the timing of the flexion-reflex stimulus in the knee cycle. These results are consistent with the concept that hip UBGs serve as the rhythm generator for turtle scratch and swim CPGs. A prior section described additional support for this concept based on bilateral same-form and mixed-form out-of-phase interlimb coordination at the hip. Additional experiments are required to determine if turtle hip UBGs also serve as rhythm generators for turtle CPGs or whether a separate rhythm generator module drives the turtle hip UBGs as described in the hypothesis of McCrea and Rybak (2008).
BLENDS OF MOTOR PATTERNS: HYBRIDS AND SWITCHES
A prior section described one-site stimulation in a scratch transition zone that can generate motor pattern blends (Mortin et al. 1985; Robertson et al. 1985). Blends can be switches in which the scratch form in one cycle is smoothly followed by a different scratch form in the next cycle. Blends can also be hybrids in which characteristics of two different scratch forms occur in each of several successive cycles. Transition zone stimulation demonstrates that blends are a naturally occurring property of scratch CPGs.
A variety of techniques using the application of two types of stimulation demonstrate that the production of blends is a fundamental property of the set of CPGs that control turtle hindlimb motor behaviors. A hypothesis consistent with these results is that there is shared circuitry for forward swim, flexion reflex, and three forms of scratch. A prior section has already described broadly tuned interneurons during each of the forms of the scratch; a later section extends that description to interneurons active during forward swim, flexion reflex, and scratch.
Blends of Several Scratch Forms Elicited by Stimulation of Two Sites, Each in a Different Scratch Receptive Field
Stein et al. (1986a) elicited blends of rostral and caudal scratch forms during actual scratch and during fictive scratch with simultaneous two-site stimulation, with one site in the rostral scratch receptive field and the other site in the caudal scratch receptive field. Some blends were switches in which a cycle with one form was followed smoothly by a cycle with the other form. Other blends were hybrids in which each of several successive cycles had two rubs, one against the rostral site and the other against the caudal site. There were two bursts of monoarticular knee-extensor activity in each hybrid cycle, each burst associated with one of the rubs.
Blends of Swim and Scratch Elicited by Stimulation of cDLF and a Site in a Scratch Receptive Field
Earhart and Stein (2000a) demonstrated rostral scratch and forward swim hybrids during actual limb movements in a low-spinal turtle. We applied cDLF stimulation to activate forward swim at the same time as mechanical stimulation in the rostral scratch receptive field. The resulting hybrid behavior included individual cycles containing both a rub against the stimulated rostral site during hip flexion and a swim powerstroke during hip extension. Juranek and Currie (2000) demonstrated switches between fictive forward swim and fictive rostral scratch with several combinations of cDLF and rostral scratch receptive field stimulation.
Hao et al. (2011) extended this work with cDLF stimulation and scratch receptive field stimulation to produce switches and hybrids. In addition, they demonstrated interactions in which there were increases in cycle frequency when stimuli were combined as well as interactions between stimuli that, when delivered alone, were subthreshold for generating a rhythm.
Hao and Berkowitz (2017) presented additional tests of the hypothesis that shared components of rhythm generation for locomotion and scratching exist before motor neurons. They utilized intracellular recordings from motor neurons during cDLF stimulation and during scratch receptive field stimulation, as well as combined stimulation of the cDLF and a scratch receptive site to produce blended motor patterns. They adjusted the cDLF stimulation to generate a swim rhythm with a different cycle frequency than the scratch rhythm. If the swim CPG and scratch CPG were not well integrated before the motor neuron level, they expected to find evidence for several frequencies of synaptic drive in motor neurons during combined stimulation. Instead, they found a single cycle frequency with combined stimulation and no evidence for separate oscillations of motor neuron synaptic potentials from separate rhythm generators. This supports the concept that the swim-rhythm generator may be largely shared with the scratch-rhythm generator or that the two rhythm generators are integrated into a single rhythm by strong interactions among rhythm-generating interneurons.
Work with blends is consistent with the hypothesis that there is some sharing among CPGs for the three forms of scratch, forward swim, and flexion reflex. The next section describes the partial sharing of interneurons among the scratch, swim, and flexion-reflex CPGs.
PARTIAL SHARING OF CPG NETWORKS AMONG SCRATCH, SWIM, AND FLEXION-REFLEX CIRCUITRY
Berkowitz and collaborators established that there are interneurons that are active during each of several fictive behaviors. It is likely that many of these interneurons are members of the CPGs for these behaviors. This supports the concept that there is partial sharing of these interneurons among the several CPGs in the turtle spinal cord (Berkowitz and Hao 2011). Our initial study focused on single-unit extracellular interneuronal recordings during rostral and pocket scratch (Berkowitz and Stein 1994a, 1994b). His work expanded with extracellular recordings during the three forms of scratch (Berkowitz 2001a, 2001b). He established that each interneuron was broadly tuned, that is, it fired maximally at a site in a scratch receptive field, fired with lower frequencies at nearby sites in the same scratch receptive field, and fired with still lower frequencies at sites in other scratch receptive fields.
He further used extracellular interneuronal recordings to compare the firing properties of single units during forward swim and flexion reflex as well as during the three forms of scratch (Berkowitz 2002). Some interneurons were rhythmically activated during both scratch and swim. Some of these scratch-swim interneurons were also activated during flexion reflex. Still other interneurons were scratch specialized and were inhibited during forward swim. Some of the scratch-specialized interneurons were also activated during flexion reflex.
Berkowitz and colleagues expanded their work with intracellular recording and dye filling of interneurons (Berkowitz 2005, 2007, 2008, 2010; Berkowitz et al. 2006; Holmes and Berkowitz 2014). They characterized interneurons activated during flexion reflex that were inhibited during scratch and forward swim. They described T (transverse) neurons, a morphologically and physiologically identifiable interneuron type with prominent dendrites in the transverse direction, that fired during the three forms of scratch and during forward swim. They also recorded from a heterogeneous population, termed non-T neurons; some of these neurons were scratch specialized and were inhibited during forward swim. A major characteristic of these interneuronal recordings during scratch and forward swim was rhythmic alternation between synaptic excitation and synaptic inhibition.
SUMMARY
Neuronal networks in the turtle spinal cord serve as model systems for studies of the central pattern generator mechanisms responsible for the selection and production of motor behaviors. The motor patterns for these behaviors can be produced in the absence of movement-related sensory inputs and without connections to supraspinal structures. Single-neuron recordings during normal behaviors, deletion behaviors, and blends of behaviors support hypotheses of organizational structures consisting of a modular network whose interconnections can be adjusted to select an appropriate motor pattern. Additional predictions of these hypotheses await future experimental tests in turtles.
GRANTS
Work in the P. S. G. Stein laboratory described in this review was supported by grants from the National Science Foundation (1972–1979 and 1989–1992) and the National Institutes of Health (1979–1989 and 1992–2011). Washington University supported the writing of this review.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
P.S.S. drafted manuscript; P.S.S. edited and revised manuscript; P.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Drs. Ari Berkowitz and Keith Hengen for helpful comments on an earlier version of the manuscript. I thank all my colleagues who have contributed to our understandings of turtle spinal cord CPGs. I thank the National Science Foundation and the National Institutes of Health for support of our experimental program.
REFERENCES
- Alaburda A, Hounsgaard J. Metabotropic modulation of motoneurons by scratch-like spinal network activity. J Neurosci 23: 8625–8629, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaburda A, Russo R, MacAulay N, Hounsgaard J. Periodic high-conductance states in spinal neurons during scratch-like network activity in adult turtles. J Neurosci 25: 6316–6321, 2005. doi: 10.1523/JNEUROSCI.0843-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antri M, Mellen N, Cazalets JR. Functional organization of locomotor interneurons in the ventral lumbar spinal cord of the newborn rat. PLoS One 6: e20529, 2011. doi: 10.1371/journal.pone.0020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN. Cerebellum and Rhythmical Movements. Berlin: Springer, 1986. [Google Scholar]
- Bakker JG, Crowe A. Multicyclic scratch reflex movements in the terrapin Pseudemys scripta elegans. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 145: 477–484, 1982. doi: 10.1007/BF00612813. [DOI] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- Berkinblit MB, Deliagina TG, Feldman AG, Gelfand IM, Orlovsky GN. Generation of scratching. I. Activity of spinal interneurons during scratching. J Neurophysiol 41: 1040–1057, 1978a. doi: 10.1152/jn.1978.41.4.1040. [DOI] [PubMed] [Google Scholar]
- Berkinblit MB, Deliagina TG, Feldman AG, Gelfand IM, Orlovsky GN. Generation of scratching. II. Nonregular regimes of generation. J Neurophysiol 41: 1058–1069, 1978b. doi: 10.1152/jn.1978.41.4.1058. [DOI] [PubMed] [Google Scholar]
- Berkinblit MB, Feldman AG, Fukson OI. Wiping reflex in the frog: movement patterns, receptive fields, and blends. In: Visuomotor Coordination, edited by Ewert JP and Arbib MA. New York: Plenum, 1989, p. 615–629. [Google Scholar]
- Berkowitz A. Broadly tuned spinal neurons for each form of fictive scratching in spinal turtles. J Neurophysiol 86: 1017–1025, 2001a. doi: 10.1152/jn.2001.86.2.1017. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Rhythmicity of spinal neurons activated during each form of fictive scratching in spinal turtles. J Neurophysiol 86: 1026–1036, 2001b. doi: 10.1152/jn.2001.86.2.1026. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Both shared and specialized spinal circuitry for scratching and swimming in turtles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 225–234, 2002. doi: 10.1007/s00359-002-0297-7. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Propriospinal projections to the ventral horn of the rostral and caudal hindlimb enlargement in turtles. Brain Res 1014: 164–176, 2004. doi: 10.1016/j.brainres.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Physiology and morphology indicate that individual spinal interneurons contribute to diverse limb movements. J Neurophysiol 94: 4455–4470, 2005. doi: 10.1152/jn.00229.2005. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Spinal interneurons that are selectively activated during fictive flexion reflex. J Neurosci 27: 4634–4641, 2007. doi: 10.1523/JNEUROSCI.5602-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A. Physiology and morphology of shared and specialized spinal interneurons for locomotion and scratching. J Neurophysiol 99: 2887–2901, 2008. doi: 10.1152/jn.90235.2008. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Multifunctional and specialized spinal interneurons for turtle limb movements. Ann N Y Acad Sci 1198: 119–132, 2010. doi: 10.1111/j.1749-6632.2009.05428.x. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Control of locomotion and scratching in turtles. In: Encyclopedia of Computational Neuroscience, edited by Jaeger D and Jung R. Berlin: Springer, 2015, p. 834–845. [Google Scholar]
- Berkowitz A, Hao ZZ. Partly shared spinal cord networks for locomotion and scratching. Integr Comp Biol 51: 890–902, 2011. doi: 10.1093/icb/icr041. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Laurent G. Local control of leg movements and motor patterns during grooming in locusts. J Neurosci 16: 8067–8078, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Front Behav Neurosci 4: 36, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Stein PS. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: broad tuning to regions of the body surface. J Neurosci 14: 5089–5104, 1994a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Stein PS. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: phase analyses. J Neurosci 14: 5105–5119, 1994b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Stein PS. Descending propriospinal axons in the hindlimb enlargement of the red-eared turtle: cells of origin and funicular courses. J Comp Neurol 346: 321–336, 1994c. doi: 10.1002/cne.903460302. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Yosten GL, Ballard RM. Somato-dendritic morphology predicts physiology for neurons that contribute to several kinds of limb movements. J Neurophysiol 95: 2821–2831, 2006. doi: 10.1152/jn.01246.2005. [DOI] [PubMed] [Google Scholar]
- Blob RW, Rivera AR, Westneat MW. Hindlimb function in turtle locomotion: limb movements and muscular activation across taxa, environment, and ontogeny. In: Biology of Turtles, edited by Wyneken J, Godfrey MH, and Bels V. Boca Raton, FL: CRC, 2008, p. 139–162. [Google Scholar]
- Bojanus LH. Anatome Testudinis Europaeae. Vilnius, Lithuania: Joseph Zawadzki, 1819, vol. I Biodiversity Heritage Library. doi: 10.5962/bhl.title.3878. [DOI] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond Biol 84: 308–319, 1911. [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol 48: 18–46, 1914. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Stuart DG. Whither motoneurons? Brain Res 1409: 93–103, 2011. doi: 10.1016/j.brainres.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Bucher D, Haspel G, Golowasch J, Nadim F. Central pattern generators. In: eLS. Chichester, UK: Wiley, 2001. doi: 10.1002/9780470015902.a0000032.pub2. [DOI] [Google Scholar]
- Burke RE, Degtyarenko AM, Simon ES. Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J Neurophysiol 86: 447–462, 2001. doi: 10.1152/jn.2001.86.1.447. [DOI] [PubMed] [Google Scholar]
- Crowe A, Linnartz P. Studies on the excitability of the central program generator in the spinal cord of the terrapin Pseudemys scripta elegans. Comp Biochem Physiol A 81: 905–909, 1985. doi: 10.1016/0300-9629(85)90928-4. [DOI] [PubMed] [Google Scholar]
- Currie SN. Interaction between right and left locomotor networks in the turtle spinal cord. Program No. 924.12 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007. [Google Scholar]
- Currie SN, Gonsalves GG. Right-left interactions between rostral scratch networks generate rhythmicity in the preenlargement spinal cord of the turtle. J Neurophysiol 78: 3479–3483, 1997. doi: 10.1152/jn.1997.78.6.3479. [DOI] [PubMed] [Google Scholar]
- Currie SN, Gonsalves GG. Reciprocal interactions in the turtle hindlimb enlargement contribute to scratch rhythmogenesis. J Neurophysiol 81: 2977–2987, 1999. doi: 10.1152/jn.1999.81.6.2977. [DOI] [PubMed] [Google Scholar]
- Currie SN, Lee S. Sensory-evoked pocket scratch motor patterns in the in vitro turtle spinal cord: reduction of excitability by an N-methyl-d-aspartate antagonist. J Neurophysiol 76: 81–92, 1996a. doi: 10.1152/jn.1996.76.1.81. [DOI] [PubMed] [Google Scholar]
- Currie SN, Lee S. Glycinergic inhibition in the turtle spinal cord regulates the intensity and pattern of fictive flexion reflex motor output. Neurosci Lett 205: 75–78, 1996b. doi: 10.1016/0304-3940(96)12387-9. [DOI] [PubMed] [Google Scholar]
- Currie SN, Stein PS. Electrical activation of the pocket scratch central pattern generator in the turtle. J Neurophysiol 60: 2122–2137, 1988. doi: 10.1152/jn.1988.60.6.2122. [DOI] [PubMed] [Google Scholar]
- Currie SN, Stein PS. Interruptions of fictive scratch motor rhythms by activation of cutaneous flexion reflex afferents in the turtle. J Neurosci 9: 488–496, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SN, Stein PS. Cutaneous stimulation evokes long-lasting excitation of spinal interneurons in the turtle. J Neurophysiol 64: 1134–1148, 1990. doi: 10.1152/jn.1990.64.4.1134. [DOI] [PubMed] [Google Scholar]
- Currie SN, Stein PS. Glutamate antagonists applied to midbody spinal cord segments reduce the excitability of the fictive rostral scratch reflex in the turtle. Brain Res 581: 91–100, 1992. doi: 10.1016/0006-8993(92)90347-C. [DOI] [PubMed] [Google Scholar]
- Daw NW, Stein PS, Fox K. The role of NMDA receptors in information processing. Annu Rev Neurosci 16: 207–222, 1993. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science 210: 492–498, 1980. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Pavlova GA. The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res 53: 81–90, 1983. doi: 10.1007/BF00239400. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Perret C. Efferent activity during fictitious scratch reflex in the cat. J Neurophysiol 45: 595–604, 1981. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Feldman AG, Gelfand IM, Orlovsky GN. On the role of central program and afferent inflow in the control of scratching movements in the cat. Brain Res 100: 297–313, 1975. doi: 10.1016/0006-8993(75)90484-9. [DOI] [PubMed] [Google Scholar]
- Diaz-Ríos M, Guertin PA, Rivera-Oliver M. Neuromodulation of spinal locomotor networks in rodents. Curr Pharm Des 23: 1741–1752, 2017. doi: 10.2174/1381612823666170124111729. [DOI] [PubMed] [Google Scholar]
- Duysens J, De Groote F, Jonkers I. The flexion synergy, mother of all synergies and father of new models of gait. Front Comput Neurosci 7: 14, 2013. doi: 10.3389/fncom.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earhart GM, Stein PS. Scratch-swim hybrids in the spinal turtle: blending of rostral scratch and forward swim. J Neurophysiol 83: 156–165, 2000a. doi: 10.1152/jn.2000.83.1.156. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Stein PS. Step, swim, and scratch motor patterns in the turtle. J Neurophysiol 84: 2181–2190, 2000b. . [DOI] [PubMed] [Google Scholar]
- Elson MS, Berkowitz A. Flexion reflex can interrupt and reset the swimming rhythm. J Neurosci 36: 2819–2826, 2016. doi: 10.1523/JNEUROSCI.3587-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field EC, Stein PS. Spinal cord coordination of hindlimb movements in the turtle: intralimb temporal relationships during scratching and swimming. J Neurophysiol 78: 1394–1403, 1997a. doi: 10.1152/jn.1997.78.3.1394. [DOI] [PubMed] [Google Scholar]
- Field EC, Stein PS. Spinal cord coordination of hindlimb movements in the turtle: interlimb temporal relationships during bilateral scratching and swimming. J Neurophysiol 78: 1404–1413, 1997b. doi: 10.1152/jn.1997.78.3.1404. [DOI] [PubMed] [Google Scholar]
- Frigon A, Gossard JP. Evidence for specialized rhythm-generating mechanisms in the adult mammalian spinal cord. J Neurosci 30: 7061–7071, 2010. doi: 10.1523/JNEUROSCI.0450-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukson OI, Berkinblit MB, Feldman AG. The spinal frog takes into account the scheme of its body during the wiping reflex. Science 209: 1261–1263, 1980. doi: 10.1126/science.7403886. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, Stecina K, Meehan CF, Nielsen JB, Hultborn H. Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. J Physiol 589: 119–134, 2011. doi: 10.1113/jphysiol.2010.199125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, McIntyre J, Bizzi E. Kinematic strategies and sensorimotor transformations in the wiping movements of frogs. J Neurophysiol 62: 750–767, 1989. doi: 10.1152/jn.1989.62.3.750. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigonis R, Alaburda A. Spike threshold dynamics in spinal motoneurons during scratching and swimming. J Physiol 595: 5843–5855, 2017. doi: 10.1113/JP274434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev 55: 247–304, 1975. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Grillner S.Control of locomotion in bipeds, tetrapods, and fish. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc., 1981, sect. 1, vol. 2, p. 1179–1236. [Google Scholar]
- Grillner S, El Manira A. The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol 31: 244–249, 2015. doi: 10.1016/j.conb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Grillner S, El Manira A, Kiehn O, Rossignol S, Stein PS. Networks in motion. Brain Res Brain Res Rev 57: 1–270, 2008. doi: 10.1016/j.brainresrev.2007.11.005. [DOI] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19: 572–586, 2009. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Robertson B. Selection of action—a vertebrate perspective. In: Neurobiology of Motor Control: Fundamental Concepts and New Directions, edited by Hooper S and Buschges A. Hoboken, NJ: Wiley, 2017, p. 181–194. [Google Scholar]
- Grillner S, Stein PS, Stuart DG, Forssberg H, Herman RM (editors). Neurobiology of Vertebrate Locomotion. London: Macmillan, 1986. [Google Scholar]
- Grossmann KS, Giraudin A, Britz O, Zhang J, Goulding M. Genetic dissection of rhythmic motor networks in mice. Prog Brain Res 187: 19–37, 2010. doi: 10.1016/B978-0-444-53613-6.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin PA, Steuer I. Key central pattern generators of the spinal cord. J Neurosci Res 87: 2399–2405, 2009. doi: 10.1002/jnr.22067. [DOI] [PubMed] [Google Scholar]
- Guzulaitis R, Alaburda A, Hounsgaard J. Increased activity of pre-motor network does not change the excitability of motoneurons during protracted scratch initiation. J Physiol 591: 1851–1858, 2013. doi: 10.1113/jphysiol.2012.246025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzulaitis R, Alaburda A, Hounsgaard J. Dense distributed processing in a hindlimb scratch motor network. J Neurosci 34: 10756–10764, 2014. doi: 10.1523/JNEUROSCI.1079-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzulaitis R, Hounsgaard J. The high conductance state in motoneurons during rhythmic motor behaviour is composed of synaptic and intrinsic conductance. Program No. 420.04 2015 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2015. [Google Scholar]
- Guzulaitis R, Hounsgaard J. Synaptic excitation in spinal motoneurons alternates with synaptic inhibition and is balanced by outward rectification during rhythmic motor network activity. J Neurosci 37: 9239–9248, 2017. doi: 10.1523/JNEUROSCI.0800-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzulaitis R, Hounsgaard J, Alaburda A. Inhibition of motoneurons during the cutaneous silent period in the spinal cord of the turtle. Exp Brain Res 220: 23–28, 2012. doi: 10.1007/s00221-012-3111-y. [DOI] [PubMed] [Google Scholar]
- Guzulaitis R, Hounsgaard J, Alaburda A. Irregular firing and high-conductance states in spinal motoneurons during scratching and swimming. J Neurosci 36: 5799–5807, 2016. doi: 10.1523/JNEUROSCI.0320-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao ZZ, Berkowitz A. Shared components of rhythm generation for locomotion and scratching exist prior to motoneurons. Front Neural Circuits 11: 54, 2017. doi: 10.3389/fncir.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao ZZ, Meier ML, Berkowitz A. Rostral spinal cord segments are sufficient to generate a rhythm for both locomotion and scratching but affect their hip extensor phases differently. J Neurophysiol 112: 147–155, 2014. doi: 10.1152/jn.00119.2014. [DOI] [PubMed] [Google Scholar]
- Hao ZZ, Spardy LE, Nguyen EB, Rubin JE, Berkowitz A. Strong interactions between spinal cord networks for locomotion and scratching. J Neurophysiol 106: 1766–1781, 2011. doi: 10.1152/jn.00460.2011. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R, Ramirez JM. Neural networks for the generation of rhythmic motor behaviors. In: Neurobiology of Motor Control: Fundamental Concepts and New Directions, edited by Hooper S and Buschges A. Hoboken, NJ: Wiley, 2017, p. 225–262. [Google Scholar]
- Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci 14: 39–57, 1991. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- Herman RM, Grillner S, Stein PS, Stuart DG (editors). Neural Control of Locomotion. New York: Plenum, 1976. [Google Scholar]
- Ho S, O’Donovan MJ. Regionalization and intersegmental coordination of rhythm-generating networks in the spinal cord of the chick embryo. J Neurosci 13: 1354–1371, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JR, Berkowitz A. Dendritic orientation and branching distinguish a class of multifunctional turtle spinal interneurons. Front Neural Circuits 8: 136, 2014. doi: 10.3389/fncir.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper S, Buschges A (editors). Neurobiology of Motor Control: Fundamental Concepts and New Directions. Hoboken, NJ: Wiley, 2017. [Google Scholar]
- Hounsgaard J. Motor neurons. Compr Physiol 7: 463–484, 2017. doi: 10.1002/cphy.c160025. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Nicholson C. The isolated turtle brain and the physiology of neuronal circuits. In: Preparations of Vertebrate Central Nervous System In Vitro, edited by Jahnsen H. New York: Wiley, 1990, p. 155–181. [Google Scholar]
- Ijspeert AJ, Bicanski AJ, Knuesel J, Cabelguen JM. Motor pattern generation. In: From Neuron to Cognition via Computational Neuroscience, edited by Arbib MA and Bonaiuto JJ. Cambridge, MA: MIT Press, 2016, p. 251–284. [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand 70: 369–388, 1967. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Tran SM, Siegrist EA, Elson MS, Berkowitz A. Innocuous flexion reflex shows windup, mediated partly by L-type calcium channels. Program No. 157.05 2015 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2015. [Google Scholar]
- Johnson KP, Tran SM, Siegrist EA, Paidimarri KB, Elson MS, Berkowitz A. Turtle flexion reflex motor patterns show windup, mediated partly by L-type calcium channels. Front Neural Circuits 11: 83, 2017. doi: 10.3389/fncir.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Heckman CJ. Gain control mechanisms in spinal motoneurons. Front Neural Circuits 8: 81, 2014. doi: 10.3389/fncir.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Currie SN. Electrically evoked fictive swimming in the low-spinal immobilized turtle. J Neurophysiol 83: 146–155, 2000. doi: 10.1152/jn.2000.83.1.146. [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci 19: 54–61, 1996. doi: 10.1016/0166-2236(96)89621-4. [DOI] [PubMed] [Google Scholar]
- Keifer J, Stein PS. In vitro motor program for the rostral scratch reflex generated by the turtle spinal cord. Brain Res 266: 148–151, 1983. doi: 10.1016/0006-8993(83)91318-5. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21: 100–109, 2011. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17: 224–238, 2016. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci 860: 110–129, 1998. doi: 10.1111/j.1749-6632.1998.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Kishore S, Bagnall MW, McLean DL. Systematic shifts in the balance of excitation and inhibition coordinate the activity of axial motor pools at different speeds of locomotion. J Neurosci 34: 14046–14054, 2014. doi: 10.1523/JNEUROSCI.0514-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol 94: 1120–1132, 2005. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Langlet C, Leblond H, Rossignol S. Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J Neurophysiol 93: 2474–2488, 2005. doi: 10.1152/jn.00909.2004. [DOI] [PubMed] [Google Scholar]
- Le Gal JP, Dubuc R, Smarandache-Wellmann C. Coordination of rhythmic movements. In: Neurobiology of Motor Control: Fundamental Concepts and New Directions, edited by Hooper S and Buschges A. Hoboken, NJ: Wiley, 2017, p. 305–340. [Google Scholar]
- Lennard PR. Afferent perturbations during “monopodal” swimming movements in the turtle: phase-dependent cutaneous modulation and proprioceptive resetting of the locomotor rhythm. J Neurosci 5: 1434–1445, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard PR, Stein PS. Swimming movements elicited by electrical stimulation of turtle spinal cord. I. Low-spinal and intact preparations. J Neurophysiol 40: 768–778, 1977. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Half-centres revisited. In: Advances in Physiological Sciences, edited by Szentagothai J, Palkovits M, and Hamori J. New York: Pergamon, 1981, vol. 1, p. 155–167. [Google Scholar]
- Lutz PL, Milton SL. Negotiating brain anoxia survival in the turtle. J Exp Biol 207: 3141–3147, 2004. doi: 10.1242/jeb.01056. [DOI] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–R996, 2001. doi: 10.1016/S0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996. [DOI] [PubMed] [Google Scholar]
- Mayerl CJ, Pruett JE, Summerlin MN, Rivera ARV, Blob RW. Hindlimb muscle function in turtles: is novel skeletal design correlated with novel muscle function? J Exp Biol 220: 2554–2562, 2017. doi: 10.1242/jeb.157792. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Brain Res Rev 57: 134–146, 2008. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Dougherty KJ. Peeling back the layers of locomotor control in the spinal cord. Curr Opin Neurobiol 33: 63–70, 2015. doi: 10.1016/j.conb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Sillar KT. Neuromodulation of vertebrate locomotor control networks. Physiology (Bethesda) 26: 393–411, 2011. doi: 10.1152/physiol.00013.2011. [DOI] [PubMed] [Google Scholar]
- Mortin LI, Keifer J, Stein PS. Three forms of the scratch reflex in the spinal turtle: movement analyses. J Neurophysiol 53: 1501–1516, 1985. doi: 10.1152/jn.1985.53.6.1501. [DOI] [PubMed] [Google Scholar]
- Mortin LI, Stein PS. Spinal cord segments containing key elements of the central pattern generators for three forms of scratch reflex in the turtle. J Neurosci 9: 2285–2296, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortin LI, Stein PS. Cutaneous dermatomes for initiation of three forms of the scratch reflex in the spinal turtle. J Comp Neurol 295: 515–529, 1990. doi: 10.1002/cne.902950402. [DOI] [PubMed] [Google Scholar]
- Moustakas-Verho JE, Cebra-Thomas J, Gilbert SF. Patterning of the turtle shell. Curr Opin Genet Dev 45: 124–131, 2017. doi: 10.1016/j.gde.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Mui JW, Willis KL, Hao ZZ, Berkowitz A. Distributions of active spinal cord neurons during swimming and scratching motor patterns. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 198: 877–889, 2012. doi: 10.1007/s00359-012-0758-6. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Smarandache C. Fifty years of CPGs: two neuroethological papers that shaped the course of neuroscience. Front Behav Neurosci 4: 45, 2010. doi: 10.3389/fnbeh.2010.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloney B, Smarandache-Wellmann C. Neurobiology of the crustacean swimmeret system. Prog Neurobiol 96: 242–267, 2012. doi: 10.1016/j.pneurobio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen UV, Moldovan M, Hounsgaard J, Glover JC. Organization of projection-specific interneurons in the spinal cord of the red-eared turtle. Brain Behav Evol 72: 179–191, 2008. doi: 10.1159/000157355. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion from Mollusc to Man. New York: Oxford University Press, 1999. [Google Scholar]
- Perret C, Cabelguen JM. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res 187: 333–352, 1980. doi: 10.1016/0006-8993(80)90207-3. [DOI] [PubMed] [Google Scholar]
- Petersen PC, Vestergaard M, Jensen KH, Berg RW. Premotor spinal network with balanced excitation and inhibition during motor patterns has high resilience to structural division. J Neurosci 34: 2774–2784, 2014. doi: 10.1523/JNEUROSCI.3349-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger HJ. Motor pattern selection and initiation in invertebrates with an emphasis on insects. In: Neurobiology of Motor Control: Fundamental Concepts and New Directions, edited by Hooper S and Buschges A. Hoboken, NJ: Wiley, 2017, p. 195–224. [Google Scholar]
- Rivera AR, Blob RW. Forelimb kinematics and motor patterns of the slider turtle (Trachemys scripta) during swimming and walking: shared and novel strategies for meeting locomotor demands of water and land. J Exp Biol 213: 3515–3526, 2010. doi: 10.1242/jeb.047167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera G, Rivera AR, Dougherty EE, Blob RW. Aquatic turning performance of painted turtles (Chrysemys picta) and functional consequences of a rigid body design. J Exp Biol 209: 4203–4213, 2006. doi: 10.1242/jeb.02488. [DOI] [PubMed] [Google Scholar]
- Roaf HE, Sherrington CS. Further remarks on the mammalian spinal preparation. Q J Exp Physiol 3: 209–211, 1910. doi: 10.1113/expphysiol.1910.sp000064. [DOI] [Google Scholar]
- Robertson GA, Mortin LI, Keifer J, Stein PS. Three forms of the scratch reflex in the spinal turtle: central generation of motor patterns. J Neurophysiol 53: 1517–1534, 1985. doi: 10.1152/jn.1985.53.6.1517. [DOI] [PubMed] [Google Scholar]
- Robertson GA, Stein PS. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J Physiol 404: 101–128, 1988. doi: 10.1113/jphysiol.1988.sp017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJH, Crowe A. The organization of motoneurons in the turtle lumbar spinal cord. J Comp Neurol 228: 24–37, 1984. doi: 10.1002/cne.902280105. [DOI] [PubMed] [Google Scholar]
- Samara RF, Currie SN. Location of spinal cord pathways that control hindlimb movement amplitude and interlimb coordination during voluntary swimming in turtles. J Neurophysiol 99: 1953–1968, 2008. doi: 10.1152/jn.01087.2007. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Observations on the scratch-reflex in the spinal dog. J Physiol 34: 1–50, 1906a. doi: 10.1113/jphysiol.1906.sp001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale University Press, 1906b. [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the midbrain. Biophysics (Oxf) 11: 756–765, 1966. [Google Scholar]
- Smith JL, Carlson-Kuhta P. Unexpected motor patterns for hindlimb muscles during slope walking in the cat. J Neurophysiol 74: 2211–2215, 1995. [DOI] [PubMed] [Google Scholar]
- Snyder AC, Rubin JE. Conditions for multi-functionality in a rhythm generating network inspired by turtle scratching. J Math Neurosci 5: 15, 2015. doi: 10.1186/s13408-015-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS. Swimming movements elicited by electrical stimulation of the turtle spinal cord: the high spinal preparation. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 124: 203–210, 1978. doi: 10.1007/BF00657052. [DOI] [Google Scholar]
- Stein PS. The vertebrate scratch reflex. Symp Soc Exp Biol 37: 383–403, 1983. [PubMed] [Google Scholar]
- Stein PS. Central pattern generators in the spinal cord. In: Handbook of the Spinal Cord. Anatomy and Physiology, edited by Davidoff RA. New York: Marcel Dekker, 1984, vols. 2 and 3, p. 647–672. [Google Scholar]
- Stein PS. Spinal cord circuits for motor pattern selection in the turtle. Ann N Y Acad Sci 563: 1–10, 1989. doi: 10.1111/j.1749-6632.1989.tb42186.x. [DOI] [PubMed] [Google Scholar]
- Stein PS. Central pattern generators and interphyletic awareness. Prog Brain Res 123: 259–271, 1999. doi: 10.1016/S0079-6123(08)62862-9. [DOI] [PubMed] [Google Scholar]
- Stein PS. Neuronal control of turtle hindlimb motor rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 213–229, 2005. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- Stein PS. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Brain Res Rev 57: 118–124, 2008. doi: 10.1016/j.brainresrev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS. Alternation of agonists and antagonists during turtle hindlimb motor rhythms. Ann N Y Acad Sci 1198: 105–118, 2010. doi: 10.1111/j.1749-6632.2010.05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Camp AW, Robertson GA, Mortin LI. Blends of rostral and caudal scratch reflex motor patterns elicited by simultaneous stimulation of two sites in the spinal turtle. J Neurosci 6: 2259–2266, 1986a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J Neurosci 22: 6800–6809, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Timing of knee-related spinal neurons during fictive rostral scratching in the turtle. J Neurophysiol 90: 3585–3593, 2003. doi: 10.1152/jn.00762.2003. [DOI] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Variations in motor patterns during fictive rostral scratching in the turtle: knee-related deletions. J Neurophysiol 91: 2380–2384, 2004. doi: 10.1152/jn.01184.2003. [DOI] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S, Lai J, Liu Z, Corman TS. Modular organization of the multipartite central pattern generator for turtle rostral scratch: knee-related interneurons during deletions. J Neurophysiol 115: 3130–3139, 2016. doi: 10.1152/jn.00871.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Grillner S, Selverston AI, Stuart DG (editors). Neurons, Networks, and Motor Behavior. Cambridge, MA: MIT Press, 1997. [Google Scholar]
- Stein PS, Grossman ML. Central program for scratch reflex in turtle. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 140: 287–294, 1980. doi: 10.1007/BF00606269. [DOI] [Google Scholar]
- Stein PS, McCullough ML, Currie SN. Reconstruction of flexor/extensor alternation during fictive rostral scratching by two-site stimulation in the spinal turtle with a transverse spinal hemisection. J Neurosci 18: 467–479, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Mortin LI, Robertson GA. The forms of a task and their blends. In: Neurobiology of Vertebrate Locomotion, edited by Grillner S, Stein PS, Stuart DG, Forssberg H, and Herman RM. London: Macmillan, 1986b, p. 201–216. [Google Scholar]
- Stein PS, Robertson GA, Keifer J, Grossman ML, Berenbeim JA, Lennard PR. Motor neuron synaptic potentials during fictive scratch reflex in turtle. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 146: 401–409, 1982. doi: 10.1007/BF00612709. [DOI] [Google Scholar]
- Stein PS, Schild CP. N-methyl-d-aspartate antagonist applied to the spinal cord hindlimb enlargement reduces the amplitude of flexion reflex in the turtle. Brain Res 479: 379–383, 1989. doi: 10.1016/0006-8993(89)91645-4. [DOI] [PubMed] [Google Scholar]
- Stein PS, Smith JL. Neural and biomechanical control strategies for different forms of vertebrate hindlimb motor tasks. In: Neurons, Networks, and Motor Behavior, edited by Stein PS, Grillner S, Selverston AI, and Stuart DG. Cambridge, MA: MIT Press, 1997, p. 61–73. [Google Scholar]
- Stein PS, Victor JC, Field EC, Currie SN. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci 15: 4343–4355, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart DG, Brownstone RM. The beginning of intracellular recording in spinal neurons: facts, reflections, and speculations. Brain Res 1409: 62–92, 2011. doi: 10.1016/j.brainres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo A, Tapia JA, De la Torre Valdovinos B, Huidobro N, Flores G, Flores-Hernandez J, Flores A, Manjarrez E. Transition of pattern generation: the phenomenon of post-scratching locomotion. Neuroscience 288: 156–166, 2015. doi: 10.1016/j.neuroscience.2014.12.038. [DOI] [PubMed] [Google Scholar]
- Valk-Fai T, Crowe A. Analyses of reflex movements in the hind limbs of the terrapin Pseudemys scripta elegans. J Comp Physiol 125: 351–357, 1978. doi: 10.1007/BF00656870. [DOI] [PubMed] [Google Scholar]
- Vich C, Berg RW, Guillamon A, Ditlevsen S. Estimation of synaptic conductances in presence of nonlinear effects caused by subthreshold ionic currents. Front Comput Neurosci 11: 69, 2017. doi: 10.3389/fncom.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WF. The locomotor apparatus of testudines. In: Biology of the Reptilia, edited by Gans C and Parsons TS. New York: Academic, 1973, vol. 4, p. 1–100. [Google Scholar]
- Walker WF. Locomotion. In: Turtles, Perspectives and Research, edited by Harless M and Morlock H. New York: Wiley, 1979, p. 435–454. [Google Scholar]
- Welch DB, Currie SN. Sensory-evoked turning locomotion in red-eared turtles: kinematic analysis and electromyography. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200: 641–656, 2014. doi: 10.1007/s00359-014-0908-0. [DOI] [PubMed] [Google Scholar]
- Wetzel MC, Stuart DG. Ensemble characteristics of cat locomotion and its neural control. Prog Neurobiol 7: 1–98, 1976. doi: 10.1016/0301-0082(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Wiersma CAG, Ikeda K. Interneurons commanding swimmeret movements in the crayfish, Procambarus clarkii (Girard). Comp Biochem Physiol 12: 509–525, 1964. doi: 10.1016/0010-406X(64)90153-7. [DOI] [PubMed] [Google Scholar]
- Wilson DM. Genetic and sensory mechanisms for locomotion and orientation in animals. Am Sci 60: 358–365, 1972. [PubMed] [Google Scholar]
- Wyneken J, Godfrey MH, Bels V (editors). Biology of Turtles. Boca Raton, FL: CRC, 2008. [Google Scholar]
- Zangerl R. The turtle shell. In: Biology of the Reptilia, edited by Gans C. New York: Academic, 1969, vol. 1, p. 311–339. [Google Scholar]
- Zernicke RF, Smith JL. Biomechanical insights into neural control of movement. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 2011, p. 293–330. [Google Scholar]
- Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol 590: 4735–4759, 2012. doi: 10.1113/jphysiol.2012.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Hochman S. Diversity of molecularly defined spinal interneurons engaged in mammalian locomotor pattern generation. J Neurophysiol 118: 2956–2974, 2017. doi: 10.1152/jn.00322.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug GR. Buoyancy, Locomotion, Morphology of the Pelvic Girdle and Hindlimb, and Systematics of Cryptodiran Turtles. Miscellaneous Publications of the Museum of Zoology, University of Michigan, No. 42 Ann Arbor, MI: Museum of Zoology, University of Michigan, 1971. [Google Scholar]