Abstract
Prior work has suggested that Golgi tendon organ feedback, via its distributed network linking muscles spanning all joints, could be used by the nervous system to help regulate whole limb mechanics if appropriately organized. We tested this hypothesis by characterizing the patterns of intermuscular force-dependent feedback between the primary extensor muscles spanning the knee, ankle, and toes in decerebrate cat hindlimbs. Intermuscular force feedback was evaluated by stretching tendons of selected muscles in isolation and in pairwise combinations and then measuring the resulting force-dependent intermuscular interactions. The relative inhibitory feedback between extensor muscles was examined, as well as symmetry of the interactions across limbs. Differences in the directional biases of inhibitory feedback were observed across cats, with three patterns identified as points on a spectrum: pattern 1, directional bias of inhibitory feedback onto the ankle extensors and toe flexors; pattern 2, convergence of inhibitory feedback onto ankle extensors and mostly balanced inhibitory feedback between vastus muscle group and flexor hallucis longus, and pattern 3, directional bias of inhibitory feedback onto ankle and knee extensors. The patterns of inhibitory feedback, while different across cats, were symmetric across limbs of individual cats. The variable but structured distribution of force feedback across cat hindlimbs provides preliminary evidence that inhibitory force feedback could be a regulated neural control variable. We propose the directional biases of inhibitory feedback observed experimentally could provide important task-dependent benefits, such as directionally appropriate joint compliance, joint coupling, and compensation for nonuniform inertia.
NEW & NOTEWORTHY Feedback from Golgi tendon organs project widely among extensor motor nuclei in the spinal cord. The distributed nature of force feedback suggests these pathways contribute to the global regulation of limb mechanics. Analysis of this network in individual animals indicates that the strengths of these pathways can be reorganized appropriately for a variety of motor tasks, including level walking, slope walking, and landing.
Keywords: force feedback, Golgi tendon organ, spinal cord, spinal reflex
INTRODUCTION
Proprioceptive feedback from muscle spindles and Golgi tendon organs is known to continuously shape whole limb motor coordination during locomotion (Abelew et al. 2000; af Klint et al. 2010; Maas et al. 2007; Mazzaro et al. 2006; Sinkjaer et al. 1996) and provides a rapid neural mechanism enabling quick corrections to dynamic maneuvers, limb perturbations, and changes in environmental surface (Daley et al. 2007; Duncan and McDonagh 2000; Honeycutt et al. 2012; Prochazka et al. 1977; Zuur et al. 2010). The critical role sensory feedback provides is made clear when diminished or absent. Patients with diminished sensory feedback (e.g., diabetic neuropathy) have increased risk of falls (Lord et al. 1999; MacGilchrist et al. 2010; Menz et al. 2004; Timar et al. 2016), whereas complete loss of sensory feedback results in an inability to walk with rare exception (af Klint et al. 2008; Albin and Albers 1990). Although the importance of sensory feedback is evident and has been extensively studied, identifying the definitive functional role of specific afferent populations remains a challenging research and clinical question, particularly when considered at the level of the whole limb.
The functional role of muscle spindle receptors has been inferred from the relatively localized synaptic contacts made by their afferents into motoneurons and interneurons (Eccles et al. 1957a; Lloyd 1946; Nichols 1999; Nichols and Koffler-Smulevitz 1991; Wilmink and Nichols 2003). Monosynaptic connections onto motoneurons supplying the same muscles leads to an increase in muscular stiffness and, as discovered later, compensation for certain nonlinear properties of muscle (Houk 1972, 1979; Nichols and Houk 1976). These autogenic effects as well as reciprocal inhibition then lead to an increase in joint stiffness (Allum and Mauritz 1984; Sinkjaer et al. 1996; Taylor et al. 1997) and an increase in joint coupling in the case of biarticular muscles. Indeed, disruption of autogenic muscle spindle connectivity for the triceps surae muscles by muscle self-reinnervation (Bullinger et al. 2011; Cope et al. 1994; Lyle et al. 2016) results in the loss of functional ankle stiffness during downslope walking (Abelew et al. 2000; Maas et al. 2007).
It is now well established that force feedback from Golgi tendon organs is more widely distributed among muscles than length feedback from muscle spindles, suggesting that these connections more directly influence whole limb coordination and mechanics (Bonasera and Nichols 1994; Eccles et al. 1957b; Harrison 1985; Harrison et al. 1983; Wilmink and Nichols 2003). The details of this influence would of course critically depend on the sign, strength, and distribution of these intermuscular connections. Force feedback is thought to be primarily inhibitory during static force production or at rest (Bonasera and Nichols 1994; Eccles et al. 1957b; Wilmink and Nichols 2003), but some excitatory pathways are opened during decerebrate locomotion (Conway et al. 1987; Gossard et al. 1994; Guertin et al. 1995; Pearson and Collins 1993). Recent data suggest that excitatory force feedback is primarily autogenic, while intermuscular force feedback remains inhibitory (Nichols and Ross, 2009; Ross and Nichols 2009). Inhibitory feedback from tendon organs is found to connect extensor muscles throughout the limb in anesthetized and decerebrate animals, but the strengths of these pathways are quite variable (Bonasera and Nichols 1994; Eccles et al. 1957b; Wilmink and Nichols 2003). During treadmill stepping in premammillary decerebrate cats, however, force feedback consistently exhibits a directional bias from proximal to more distal muscles (Ross and Nichols 2009). Based on the stiffness regulation concept of Houk (1972), this directional bias in inhibitory feedback would result in the distal joints having the greatest compliance, cushioning the impact of contact with the ground during slow walking.
These data suggest that the strength and distribution of force feedback might vary depending on the demands of the motor task. During landing from a fall, for example, a directional bias toward more proximal muscles might be important to maintain interjoint coordination in the face of inertial disparities among the limb segments (Nichols et al. 2016). The variability that has been observed in the organization of force feedback in decerebrate animals (e.g., inhibition stronger from muscle A to muscle B in a cat and vice versa in another cat) (Bonasera and Nichols 1994; Ross and Nichols 2009; Wilmink and Nichols 2003) suggests that there might indeed be a control system in the brain stem and spinal cord that regulates this organization. Alternatively, this variability could reflect a by-product of the decerebration procedure itself without functional significance for the intact animal. These possibilities are difficult to distinguish based on the available studies (Bonasera and Nichols 1994; Nichols 1999; Wilmink and Nichols 2003), because only a few muscle interactions were evaluated in individual experiments.
We therefore undertook to evaluate the organization of force feedback among a larger number of muscles in individual animals to determine whether patterns would emerge at the level of the limb. We hypothesize task-dependent modulation of force-dependent inhibitory feedback could be a regulated control variable the nervous system uses to help meet task-specific demands. That is, if force-dependent feedback actively influences whole limb coordination, then the strength and distribution of force feedback should be modulated and structured in a way that could influence task-relevant whole limb mechanics. We tested this hypothesis by characterizing the distribution of intermuscular force-dependent feedback between the primary extensor muscles acting at the knee, ankle, and toes in the decerebrate cat within single experiments. The prior observations of interanimal variability in the directional bias of inhibitory feedback between muscle pairs is exploited here to examine whether patterns of force feedback exist within individual cat hindlimbs. Patterns of force feedback such as a consistent directional bias in inhibitory feedback from distal-to-proximal extensor muscles, or vice versa, would support the hypothesis and the possibility of central influence. In contrast, lack of consistency in the directional bias of inhibitory feedback between hindlimb muscles of individual cats would be more indicative of random variability and perhaps an inherent characteristic of the decerebrate state.
METHODS
All procedures were completed in accordance with guidelines from the National Institutes of Health and two approved protocols by the Georgia Institute of Technology Institutional Animal Care and Use Committee. The experiments were performed on eight purpose-bred female cats ranging from 3 to 5 kg. The hypotheses tested in this study are based on results acquired during terminal experiments. In four of the cats, the terminal experiments were completed after the functional consequences of muscle denervation and self-reinnervation [n = 3 vasti; n = 1 flexor hallucis longus (FHL)] were investigated using chronic behavioral measurements during level and slope walking. Details pertaining to the surgical self-reinnervation procedures and recordings have been described previously (Abelew et al. 2000; English 2005; Lyle et al. 2016; Maas et al. 2007). For the purpose of this study, experimental findings were primarily from the nonsurgical limb. However, some comparisons from the reinnervated muscles were included from these four cats to evaluate symmetry of intermuscular feedback across limbs. The comparisons from reinnervated muscles were limited to their actions onto other muscles. This is because muscle self-reinnervation is well known to result in permanent loss of the autogenic stretch reflex in the reinnervated muscle (Bullinger et al. 2011; Cope et al. 1994; Lyle et al. 2016), but efferent motor output recovers and intermuscular force feedback from reinnervated muscles onto other muscles is largely regained (Lyle et al. 2016). The terminal experiments described here were conducted greater than 23 wk after nerve transection and repair providing ample time for complete recovery of motor and intermuscular force feedback (range: 23 to 45 wk).
Terminal experiment.
After a surgical plane of isoflurane gas anesthesia was achieved, tracheal intubation was performed and 1–3% isoflurane was used thereafter to maintain deep anesthesia. A cannula was inserted into an external jugular vein to administer saline during the experiment. Adequate anesthesia was confirmed during the experiment by absence of withdrawal reflexes. Heart rate, respiratory rate, oxygen saturation, expired carbon dioxide, and core body temperature were monitored during all procedures. A heating pad was used to maintain core body temperature at 37°C.
The head was fixed in a stereotaxic frame, the abdomen was supported by a sling, and the hindlimbs were rigidly fixed. Hindlimb fixation was achieved using threaded rods inserted into the proximal (just distal to the greater trochanter) and distal femur (femoral condyle). The rods were connected by a bar and rigidly fixed to the support frame. A separate threaded rod stabilized the proximal tibia, and the distal tibia was stabilized with an ankle clamp.
The gastrocnemius (G), plantaris (PLANT), FHL, and quadriceps (Q) muscle groups were dissected in both the right and left hindlimbs. Muscles were carefully separated free from adjacent tissues and muscles to minimize mechanical coupling while being careful to preserve their nerve and vascular supply. The tendons of FHL were cut where it merges with the flexor digitorum longus tendon. The PLANT tendons were separated from the G, released from its connection to the calcaneus, and cut distally near its insertion to the flexor digitorum brevis muscle. The G tendons were carefully separated from the soleus at their common insertion onto the calcaneus with small bone chips preserved. The distal tendons of all muscles were attached to custom tendon clamps. The tendon clamps were connected to strain gauge myographs in series with linear motors. The Q was carefully isolated by first releasing the sartorius muscle from its broad distal insertion since the sartorius completely covers the Q. Then, blunt dissection was used to separate the vasti from adjacent tissue along the medial and lateral femur, and the retinaculum was transected proximally (i.e., iliotibial band) and distally around the patella. In addition, blunt dissection was used to separate the vastus muscle group (V) from the rectus femoris (RF). The RF was transected using electrocautery midbelly in all cats. This was done to mitigate the need to stabilize the pelvis. The V insertion was freed by cutting the patellar tendon. A small hole was drilled in the patella, a steel cable (0.9-mm diameter) was threaded through the hole, and the steel cable was connected to a tendon clamp in series with a myograph and linear motor via a pulley system.
A standard precollicular decerebration was completed in seven of eight cats (Silverman et al. 2005). This involved a vertical transection starting at the anterior margin of the superior colliculus. In one cat, a premammillary decerebration was performed, which involved an angled transection just rostral to the superior colliculus preserving the mammillary bodies and subthalamic nucleus (Ross and Nichols 2009; Whelan 1996). For both procedures, all brain matter rostral to the transection was removed. Gelfoam and cotton were placed in the cranium to control bleeding. After the decerebration, isoflurane anesthesia was titrated down over ~5–30 min and withdrawn. At the end of the experiment, the animals were euthanized with either an overdose of concentrated pentobarbital (Euthasol) or potassium chloride solution after reanesthetizing per guidelines from the American Veterinary Medical Association.
Data acquisition.
Intermuscular spinal reflex pathways were examined using a ramp-hold-release muscle stretch protocol. The details of the hardware and software used have been described previously (Lyle et al. 2016; Ross and Nichols 2009; Wilmink and Nichols 2003). In brief, the protocol involved 2-mm muscle stretches with a 50-ms ramp, 100-ms hold, and 50-ms release. The muscle stretches for a given trial were applied in a two-state alternating pattern with a stretch repetition frequency of 0.7 Hz for a duration of 30–40 s (i.e., ~10–15 repetitions per state) (Fig. 1A). In state 1, a single muscle referred to as the recipient was stretched. In state 2, the recipient muscle was stretched simultaneously with another muscle referred to as the donor muscle. Because the focus of this study was to examine intermuscular force feedback pathways, muscle stretch repetitions were applied over a range of background forces by eliciting a crossed extension reflex as in earlier work (Bonasera and Nichols 1994; Lyle et al. 2016; Nichols 1999; Wilmink and Nichols 2003). The crossed extension reflex was elicited by stimulating the tibial nerve just proximal to the medial malleolus with a nerve cuff or hook electrode (0.1-ms pulses, 40 Hz, 20- to 40-s duration). Typically, forces in the contralateral extensor muscles increase rapidly, plateau and slowly decay toward the original 1–3 N background force over a 20- to 40-s time period (Fig. 1A).
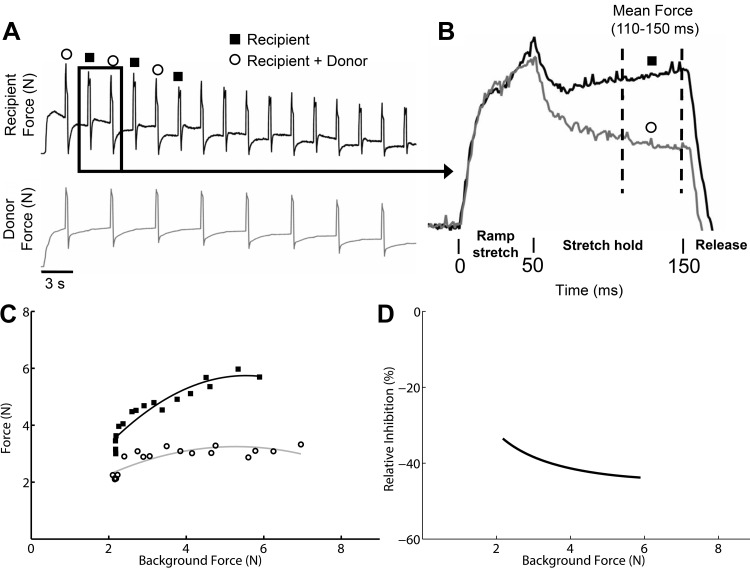
Fig. 1.
Experimental data and analysis [example from Fig. 6A, vastus muscle group (V) onto gastrocnemius (G)]. A: raw force profiles from the recipient and donor muscles collected over a range of background forces during a crossed extension reflex. B: example of 2 of the stretch-evoked force profiles illustrating the reduced force due to inhibitory feedback from the donor muscle. C: shown are the population of force responses from state 1 (solid black squares, recipient stretch only) and state 2 (open circles, recipient plus donor muscle stretch) plotted as a function of background forces and fit with a quadratic polynomial regression line (note that the end of the raw data from A is not shown to save space). D: shown is the difference between the state 1 and state 2 polynomial curves expressed as percent change from the state 1 curve.
Data analysis.
All data were analyzed using custom programs written in MATLAB (MathWorks, Natick, MA). To account for varying background forces and identify the relative force change for each stretch repetition, a baseline force vector was subtracted from the raw force profiles before analyses. The baseline force vectors used to remove the background force offset were determined using linear interpolation from stretch onset to a point 900 ms after stretch onset similar to that previously described (Ross and Nichols 2009). Stretch repetitions that had a sudden force change precluding an accurate baseline force vector determination were eliminated from analysis, as were spontaneous force responses unrelated to muscle stretch or tibial nerve stimulation during a trial.
The dependent variable was the background subtracted recipient muscle forces in response to the ramp-hold-release stretches (Fig. 1B). The average muscle force for a period 110–150 ms after stretch onset (i.e., last 40 ms of hold phase) was used to test hypotheses in this study. The force values during this period have previously been used to evaluate differences in intermuscular effects from a donor muscle onto recipient muscle (Lyle et al. 2016; Nichols 1999). Intermuscular reflex pathways were assessed over a range of background forces by stimulating the contralateral tibial nerve. This protocol resulted in a population of force responses for state 1 and state 2. The populations of force responses for each state were plotted separately as a function of background forces (Fig. 1C). Background force values used in the regression plots were the muscle force means for the 15-ms period before stretch onset for each stretch repetition. The force responses were then fit with least squares quadratic polynomial curves. A regression model was used to evaluate whether the state 1 and state 2 force responses could be fit with the same curve or were statistically different warranting separate polynomial curve fit lines (Kutner et al. 2005; Lyle et al. 2016; Ross and Nichols 2009). This was done by performing an F-test using full and reduced regression models. The dependent variable was the stretch-evoked force responses. The full model predictor variables included a grouping variable (state 1 and state 2), background force, background force squared, background force × grouping variable crossed term, and background force squared × grouping variable crossed term. The reduced model lacked the grouping variable terms. The F-test evaluated the null hypothesis that the population of forces from state 1 and state 2 are the same and should be fit with a single polynomial fit line. In contrast, a P value < 0.05 from the F-test indicates a significant separation of the force populations suggesting intermuscular spinal pathways from the donor muscle influenced recipient motor output. In addition to testing for significant separation with the F-test, the relative magnitude of any difference between the polynomial curve fit vectors from states 1 and 2 were expressed as a percent difference [(state 2 curve fit vector – state 1 curve fit vector)/state 1 curve fit vector *100] (Fig. 1D). The polynomial curve fit difference was only computed across background force ranges spanned by both states. The difference between state 1 and state 2 was expressed as a relative percent to facilitate comparisons across muscle pairs and cats. The maximum intermuscular inhibitory value from each muscle pair interaction was used for the purpose of this study to represent the greatest intermuscular inhibitory potential. The inhibitory values for all muscle pair interactions were examined for patterns at the level of the limb for each cat using schematic figures (see results for description and Fig. 2).
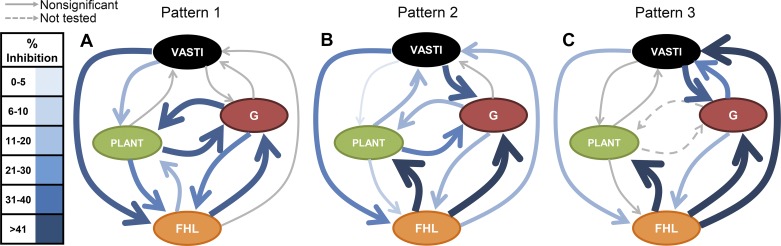
Fig. 2.
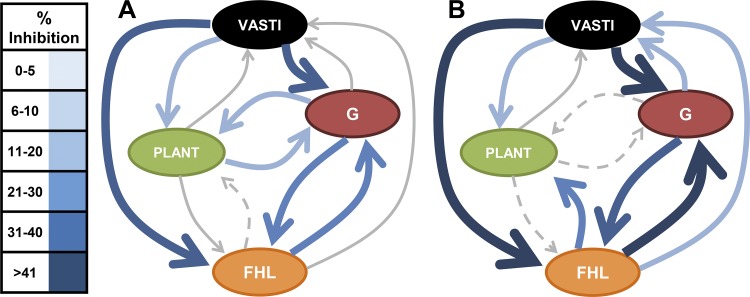
Schematic examples of inhibitory feedback between muscles representing 3 patterns along a spectrum observed across cats. A, pattern 1: directional bias of inhibitory feedback that is mostly projecting onto the ankle extensors and toe flexors (cat 1). B, pattern 2: convergence of inhibitory feedback onto ankle extensors and mostly balanced inhibitory feedback between vasti and flexor hallucis longus (FHL; cat 5). C, pattern 3: directional bias of inhibitory feedback that is mostly projecting onto ankle and knee extensors (cat 8). The size and color intensity (see legend) of arrows represent level of significant inhibitory feedback. Gray solid arrow, nonsignificant interaction; gray dashed arrow, interaction not evaluated. PLANT, plantaris; G, gastrocnemius.
Intermuscular sensory feedback between a donor and recipient muscle as evaluated here relies on change in force as the dependent variable. Thus, it is important to confirm changes in the recipient muscle forces during state 2 are due to neural rather than a mechanical influence from the donor muscle. Mechanical coupling could arise due to insufficient separation of muscles and/or poor fixation of the bony segments. Because neural contributions have a latency of 15–20 ms (Nichols 1999; Wilmink and Nichols 2003), stretch-induced mechanical effects from the donor muscle were assessed for each trial by comparing recipient muscle forces between state 1 and state 2 at 10 ms after stretch onset. In addition, mechanical artifacts can be identified by observing whether recipient muscle background force changes before 15 ms when stretching the donor muscle by itself. There were no significant mechanical artifacts warranting exclusion of data.
RESULTS
The purpose of this study was to evaluate whether and to what extent Golgi tendon organ (GTO) feedback between muscle pairs throughout the limb exhibit organized patterns. This was accomplished by evaluating the strengths of inhibitory feedback between extensor muscles using schematic figures (see Fig. 2). There were clear differences in the directional biases of inhibitory feedback across cats that appears to represent a spectrum of inhibitory feedback patterns with two extremes. We have chosen to classify each cat into three patterns as distinct points in the range to illustrate key discriminative features of potential functional relevance: pattern 1, directional bias of inhibitory feedback onto the ankle extensors and toe flexors (Fig. 2A); pattern 2, convergence of inhibitory feedback onto ankle extensors and mostly balanced inhibitory feedback between V and FHL (Fig. 2B); and pattern 3, directional bias of inhibitory feedback onto ankle and knee extensors (Fig. 2C).
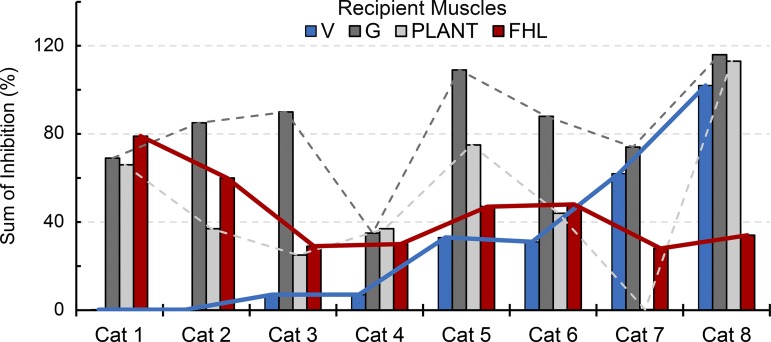
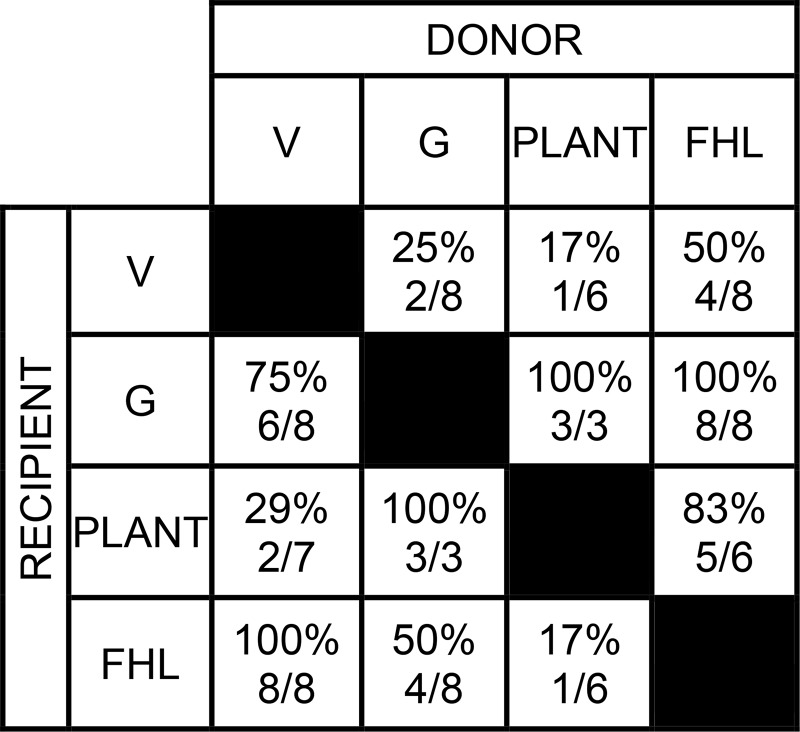
Shown in Table 1 are the number of intermuscular interactions (and muscle pairs) recorded for each cat, as well as the inhibitory pattern classification reflecting the strength and directional biases of inhibitory feedback between muscles. Of the eight cats, two cats were clearly classified as pattern 1 and two cats as pattern 3, while four cats were intermediate and best classified as pattern 2 with some features of 1 or 3. The patterns at the level of the limb are further illustrated in terms of the net inhibition from all donor muscles onto recipient muscles for all cats in Fig. 3. The sum of inhibitory feedback for each recipient muscle is shown as bar plots and ordered according to the pattern descriptions from left (pattern 1) to right (pattern 3) as in Table 1. Although inhibitory feedback from several donor muscles onto a recipient muscle is unlikely a simple sum (Harrison et al. 1983; Wilmink and Nichols 2003), these data support and extend the pattern descriptions above.
Table 1.
Cat subject characteristics and classification of intermuscular force feedback patterns for individual cat hindlimbs and the frequency of biases in inhibitory feedback between individual muscle pairs
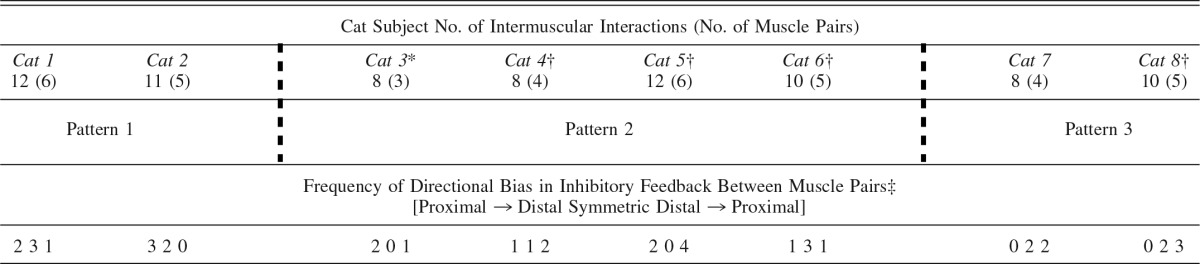
For cats 2 and 3, the number of intermuscular interactions and muscle pairs do not correspond because force feedback was only evaluated in one direction for a muscle pair. See text and Fig. 2 for description of patterns.
*Premammillary decerebration;
†reinnervation of the flexor hallucis longus muscle (cat 3) or vasti muscles on nontested limb;
‡directional inhibitory bias for a muscle pair was defined as relative inhibitory feedback being larger by ≥10% in one direction.
Fig. 3.
Sum of inhibitory feedback onto recipient muscles across cats. The left-to-right listing of cat subjects corresponds with the inhibitory pattern as described in the text and Table 1. Red and blue lines denote the inverse relation between inhibitory feedback onto FHL and V muscles, respectively (cat 1, pattern 1, less inhibitory feedback onto V and strong onto FHL and ankle extensors; cat 8, pattern 3, less inhibitory feedback onto FHL and strong onto V and ankle extensors).
Several features characteristic of the patterns of inhibitory feedback across cats can be seen from Figs. 2 and 3. First, common to all cats was a general tendency for convergence of inhibitory feedback from FHL and/or V onto the ankle extensors, particularly G (Figs. 2 and 3). In fact, the sum of inhibitory feedback converging onto the G was 83 ± 25% on average across cats (range: 35–116%). Second, a defining feature of the patterns is a shift in the net inhibitory feedback onto the V and FHL, respectively. For example, it can be seen that the inhibitory feedback evoked from the V muscles onto FHL and Plant steadily decreases from Fig. 2, A to C, whereas the inhibitory feedback evoked from FHL steadily increases. Similarly, the inhibitory feedback onto V and FHL exhibit an inverse relation at each end of the pattern spectrum (see blue and red lines, Fig. 3). Lastly, the magnitude of inhibitory feedback clearly illustrates the capacity for GTO feedback to influence motor behavior, particularly when considering input from several donor muscles concurrently even if not purely additive. Indeed, the net inhibition onto both the ankle and toe muscles exceeded 50% for the pattern 1 classification while the net inhibitory feedback onto the ankle and knee exceeded 50% in pattern 3. The directional bias of inhibitory feedback characteristic of pattern 1 could function to increase compliance of the ankle and metatarsophalangeal joints (Fig. 2A), whereas pattern 3 could increase compliance of the ankle and knee (Fig. 2C). The apparent spectrum of inhibitory feedback and tendency for a directional bias of inhibitory feedback within cat hindlimbs implies central control of intermuscular force feedback, despite the many physiological factors that could impart random variability.
A similar approach to evaluating patterns at the level of the limb was completed by focusing on the directional bias of inhibitory feedback between individual muscle pairs in the limb. A directional bias of inhibition between a given muscle pair was operationally defined as the inhibitory magnitude being larger by 10% or more in one direction, resulting in three categories (i.e., proximal to distal, symmetric, distal to proximal). A summary of the directional biases for each muscle pair by cat is listed in Table 1. As expected, a net directional bias in proximal-to-distal and distal-to-proximal muscle pairs is present for patterns 1 and 3, respectively. Consistent with the observations described above, inhibitory feedback was not biased in the same direction for all muscle pairs in the hindlimb for any cat (Table 1).
Trends in intermuscular inhibitory feedback for individual muscle pairs across cats.
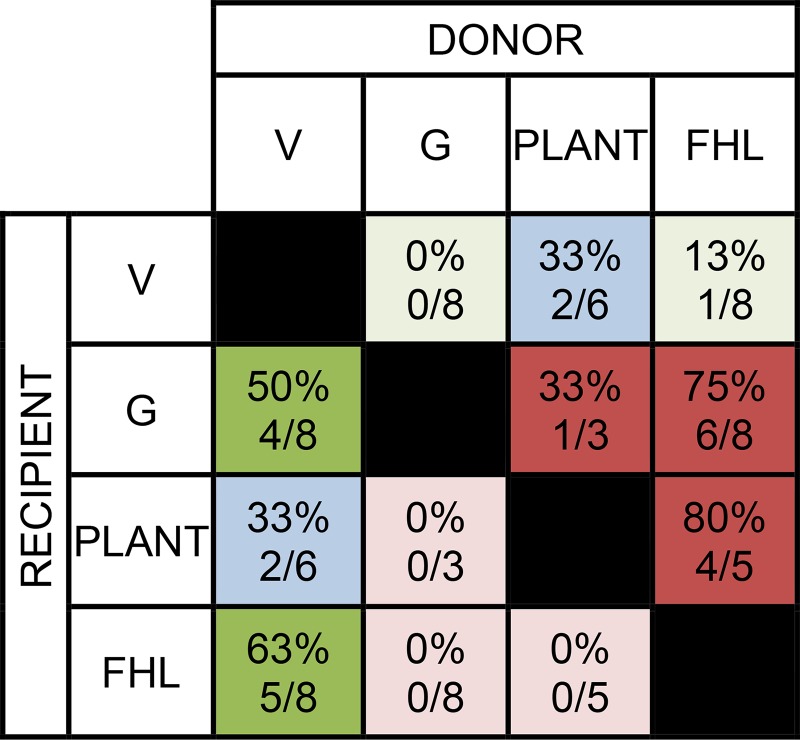
In addition to patterns of inhibition within individual cat hindlimbs, a more global analysis at the level of muscle pairs across cats was performed to provide further insight regarding general trends in the distribution of inhibitory feedback for the sample. This was accomplished by identifying the frequency of directional biases in inhibitory feedback for individual muscle pairs across cats. A directional bias of inhibition between a given muscle pair was operationally defined as above (inhibitory magnitude larger by 10% or more in one direction). Some muscle pairs exhibited a directional bias of inhibitory feedback more frequently across cats (Fig. 4). For example, a directional bias of inhibition from FHL onto G and FHL onto PLANT was observed in ≥75% of cats. Frequencies of intermuscular interactions with inhibitory strength ≥15% were also determined. Inhibitory strength of 15% was used to reflect a magnitude of likely behavioral relevance. This criterion was met by 50% or more of the cats for 8 of 12 intermuscular interactions (Fig. 5). Similar to above, these data show a common finding of inhibitory feedback converging onto the G from the V and FHL muscle groups.
Fig. 4.
Frequency of directional biases in inhibitory feedback for individual muscle pairs across the sample (i.e. an inhibitory bias was present when the relative inhibition was larger by ≥10% in 1 direction). The donor/recipient muscle pairs shaded dark green indicate proximal-to-distal inhibitory bias, while blue indicates symmetry and red distal-to-proximal gradient.
Fig. 5.
Frequency of intermuscular interactions with inhibitory feedback that reduced the recipient stretch-evoked force response by ≥ 15%. The cat numbers shown for V → Plant and FHL ↔ Plant in this table is different than in Fig. 4 because the latter required that both directions were evaluated in the same cat.
Inhibitory force feedback tends to be symmetric for muscle pairs across limbs.
As noted in the previous section, the strength and directional bias of intermuscular inhibition varies across cats. The directional trends observed in inhibitory feedback within hindlimbs of individual cats provide evidence against random variability that could result from the decerebrate preparation. The symmetry of inhibitory magnitudes for muscle pairs between limbs was used to further evaluate for consistency in the patterns of intermuscular interactions, and to test whether the observed patterns might result from a global control system rather than either a more localized control system or the effects of surgical preparation. Symmetry of inhibitory magnitude for a given muscle pair between limbs was reduced to a frequency with four ordinal categories (difference ≤5, 10, 15, and ≥15%).
Six cats with four or more intermuscular interactions were available for evaluating interlimb symmetry. As shown from a schematic example (Fig. 6, cat 2), the magnitude of intermuscular inhibitory feedback was similar across limbs, as was the directional bias of inhibitory feedback between muscle pairs. The symmetry of intermuscular inhibition within individual cats for the muscle interactions available for comparison is shown in Table 2. Most muscle pairs compared across limbs within individual cats can be seen to differ by ≤10%. Across cats, the magnitudes of interlimb inhibitory feedback were within 10% for 22 of 36 intermuscular interactions evaluated (61%, Table 2). In contrast, there were 9 of 36 intermuscular interactions evaluated with differences that exceeded 15%. Importantly, the presence of interlimb differences >10% did not influence the whole limb inhibitory feedback pattern, as can be seen for cat 2 in Fig. 6 and Table 2. And although a definitive reason cannot be confirmed, the most parsimonious explanation for asymmetry in some intermuscular interactions relates to trial conditions (e.g., differences in background force response magnitudes across limbs for donor and/or recipient muscles). An illustrative example can be offered by looking more closely at four intermuscular interactions with data from at least five of six cats (Table 3). The intermuscular interactions from V onto FHL and FHL onto G were particularly similar with five of six and four of five cats exhibiting interlimb differences less than 5%. However, the inhibitory feedback from V onto G exhibited less symmetry across cats. For this interaction, the two cats with interlimb differences less than 5% had background forces for the recipient and donor muscles on both sides during the crossed extension reflex that were within 6 N. Cats with larger interlimb differences had higher background force ranges on the side with larger inhibition, or no change in the recipient force requiring the donor muscle background force to be used to fit the regression line. The background force of the donor and recipient muscles can influence the relative inhibition during trials (Bonasera and Nichols 1994), thus the observed asymmetry in inhibitory feedback was likely due, at least in part, to the differences in trial conditions. Nonetheless, the relative symmetry of intermuscular inhibitory feedback across limbs is remarkable given the many factors that cannot be directly controlled.
Fig. 6.
Shown is a schematic illustrating a similar pattern of inhibitory feedback between the left (A) and right (B) limbs from cat 2. The magnitudes of inhibitory feedback were similar, as was the general directional bias of inhibitory feedback from proximal-to-distal muscles. The size and color intensity (see legend) of arrows represent level of significant inhibitory feedback. Gray solid arrow, nonsignificant interaction; gray dashed arrow, interaction not evaluated.
Table 2.
Symmetry of intermuscular inhibition across limbs
| Cat Subject (No. of Interlimb Interactions) | ||||||
|---|---|---|---|---|---|---|
| % Interlimb Difference | Cat 2 (8) | Cat 4 (4) | Cat 5 (6) | Cat 6 (5) | Cat 7 (6) | Cat 8 (7) |
| Frequency No. | ||||||
| ≤5 % | 3 | 0 | 3 | 2 | 4 | 3 |
| 6–10 % | 1 | 3 | 0 | 1 | 0 | 2 |
| 11–15 % | 1 | 0 | 3 | 0 | 0 | 1 |
| >15 % | 3 | 1 | 0 | 2 | 2 | 1 |
| Relative Frequency % | ||||||
| <10% | 50% | 75% | 50% | 60% | 67% | 71% |
Table 3.
Symmetry of intermuscular inhibition for 4 muscle interactions evaluated in 5 or more cats
| Muscle Interactions, no. of cats | ||||
|---|---|---|---|---|
| % Interlimb Difference | V→G (n = 6) | V→FHL (n = 6) | G→FHL (n = 5) | FHL→G (n = 5) |
| ≤5 % | 2 | 5 | 2 | 4 |
| 6–10 % | 0 | 1 | 2 | 0 |
| 11–15 % | 2 | 0 | 1 | 0 |
| >15 % | 2 | 0 | 0 | 1 |
| Relative Frequency % | ||||
| <10% | 33% | 100% | 80% | 80% |
DISCUSSION
Prior work has suggested that Golgi tendon organ (GTO) feedback, via its distributed network linking muscles spanning all joints, could be used by the nervous system to help regulate whole limb mechanics if appropriately organized (Harrison et al. 1983; Nichols et al. 1999, 2016). In this study, we tested this hypothesis by examining the strength and distribution of intermuscular force feedback between the major extensor muscles spanning the knee, ankle, and toes in individual cats. The primary finding was that intermuscular force feedback, rather than being random or fixed, exhibited a spectrum of inhibitory feedback patterns organized at the level of the limb across cats. At the ends of the spectrum, a directional bias of inhibitory feedback was either onto the ankle and knee extensors, or onto the ankle extensors and toe flexors. Moreover, the intermuscular inhibition between any given muscle pair was often substantial, suggesting a strong capability to influence limb mechanics. These data provide preliminary support for the hypothesis that intermuscular force feedback could be a regulated control variable used by the nervous system to influence whole limb mechanics. In the following discussion, we expand on this possibility, and potential functional implications of directional biases of inhibitory feedback.
Directional bias of intermuscular force feedback as a potential neural control variable.
The functional role of proprioceptive feedback is principally determined by how the sensory network is organized and its capacity for central regulatory modulation. It is well known that muscle spindle feedback is modulated within and across tasks (Leukel et al. 2008; Makihara et al. 2012; Simonsen and Dyhre-Poulsen 1999; Sinkjaer et al. 1996), indicating a central influence that appears to organize proprioceptive feedback to help meet task demands. Current evidence, albeit conflicting, supports state-dependent modulation of GTO feedback. When in a quiescent anesthetized or decerebrate state as in this study, intermuscular force feedback from extensor muscles has an inhibitory influence onto other extensor muscles (Eccles et al. 1957b; Harrison et al. 1983; Wilmink and Nichols 2003). In one study where intramuscular stimulation was used to elicit force feedback, widespread excitatory feedback was observed (Pratt 1995). This approach is currently being reevaluated and validated to check whether the stimulation caused mechanical perturbation of the sensory receptors or direct activation of Ia and Ib afferents (Lyle et al. 2015). During locomotion in the decerebrate state, some studies utilizing nerve stimulation suggest that intermuscular force feedback becomes excitatory between some muscle pairs (Conway et al. 1987; Gossard et al. 1994; Guertin et al. 1995; Pearson and Collins 1993). In another study using the mechanographic method described here, autogenic excitation and persistence of intermuscular inhibition were observed (Ross and Nichols 2009). In fact, Ross and Nichols (2009) found that the intermuscular inhibition between muscle pairs, when considered collectively across the cats studied, exhibited a proximal-to-distal bias of inhibition during spontaneous decerebrate stepping similar to pattern 1 in this study. Some inhibitory interactions decreased while others remained the same or increased compared with the quiescent standing posture, resulting in a redistribution of inhibition toward the proximal-to-distal bias rather than a wholesale reduction. These data suggest that the locomotor state induces a more fixed pattern of intermuscular inhibition at least for slow walking, in contrast to the several patterns observed in this study. Some evidence also exists for state-dependent modulation of intermuscular force feedback in humans. Despite methodological challenges limiting study of intermuscular force feedback from the medial gastrocnemius onto soleus, it has been observed that inhibitory feedback at rest becomes less inhibitory or slightly excitatory during locomotion (Faist et al. 2006; Stephens and Yang 1996). These data could be consistent with a generalized decrease, or alternatively, a redistribution of inhibition. These possibilities can only be resolved by evaluating the intermuscular feedback among a larger set of muscles.
An important finding of this study was that the inhibitory magnitude varied, but patterns in the organization of intermuscular force feedback were observed and could be generally classified as three patterns along a spectrum for this sample of quiescent decerebrate cats. Differences in the strength and directional biases of inhibitory feedback between muscle pairs across decerebrate cats is in agreement with earlier work (Bonasera and Nichols 1994; Wilmink and Nichols 2003). Because prior studies only evaluated a small number of muscle pairs in a given experiment, it was unclear whether the differences across cats represented random variability or modulatory potential. We favor the latter for two reasons. First, despite individual differences across cats, there was a surprising tendency for directional biases of inhibition within individual cat hindlimbs. The distinct trends in the directional bias of inhibitory feedback supports central modulatory potential, because factors outside experimental control (e.g., response to decerebration and peripheral dissection) are more likely to result in random patterns. Second, the patterns of inhibitory feedback were often symmetric across limbs, suggesting that, even in the quiescent decerebrate cat, the observations are best explained by bilateral descending regulation of as yet unknown origin rather than limb-specific differences such as altered blood flow or disruption of muscles due to the dissection. Importantly, the central states of decerebrate cats are unknown, so the regulatory control inferred here presupposes that remaining brain stem tissue influences the force feedback network. Individual differences in the physiological response to decerebration (e.g., extent of brain stem dieback and thus varied descending monoaminergic and brain stem nuclei inputs) is the most parsimonious explanation for the spectrum of inhibitory patterns observed. Taken together, we interpret the observed patterns of inhibitory feedback as evidence in favor of a diverse network the nervous system could use to influence task-dependent whole limb behavior.
The directional trends in inhibitory feedback suggest the potential for central regulatory control, but additional explanations could contribute to the findings in this study. The directional bias in inhibitory feedback was found to be largely influenced by the relative inhibitory strength projecting from the V and FHL, respectively. An alternative explanation for an inhibitory bias could be due to differences in the relative excitability of the motoneuron pools of proximal and distal muscles. This is relevant because the ability to observe intermuscular inhibitory effects requires the recipient muscle to have a robust autogenic excitatory response (Sherrington 1932). The central excitability cannot be directly controlled during experiments, but we mitigated this concern by examining intermuscular interactions during a crossed extension reflex. The crossed extension reflex resulted in motoneuron pools of the extensor muscles exceeding threshold (i.e., increase background force) in almost all of the interactions reported here. As such, the experimental conditions created the best possible opportunity to observe inhibitory interactions across the muscles evaluated. Another consideration is that the tibial nerve stimulation used to evoke the crossed extension reflex could itself influence the organization of inhibitory feedback. However, this is unlikely because the directional bias of inhibitory feedback, if present, is typically very similar when evaluated during a steady background force (i.e., without contralateral tibial nerve stimulation) so long as the recipient muscle has a robust stretch reflex. Lastly, it is possible that the inhibitory feedback patterns in this study could have been influenced by the experimental procedures such as the extensive dissection in the periphery. Despite similar surgical procedures for each cat, it is possible that cutaneous and nociceptive feedback, for example, could have acted to bias the organization to impose the different patterns (see next paragraph).
Potential descending systems influencing intermuscular force feedback.
The actions of GTO feedback are mediated by a complex network of premotor interneurons that receive a convergence of descending and sensory inputs (e.g., cutaneous, muscle spindle; see Jankowska and Edgley 2010). As such, modulation of GTO feedback could be achieved via pre- or postsynaptic influence onto the premotor interneurons and/or via presynaptic influence at the motoneuron synaptic contact. Although the definitive pathways responsible for central control of force feedback remain unknown, current evidence indicates several possibilities. First, it is known that complete spinal transection (Eccles and Lundberg 1959), as well as acute and chronic lateral hemisection (Niazi 2015), results in an amplification of inhibitory feedback, most profoundly from FHL onto ankle extensor muscles. These findings appear to be independent of the additional plasticity well described for the stretch reflex and motoneurons (ElBasiouny et al. 2010). Preliminary evidence further suggests that dorsal hemisection alone, in contrast, does not affect intermuscular force feedback (Niazi 2015); thus descending pathways in the ventral cord such as vestibulospinal and reticulospinal are of particular interest since they both can reset locomotor rhythm in fictive cats (Rossignol and Frigon 2011). Recent work has suggested that the vestibulospinal pathways, carrying converging signals from receptors in muscles of the neck and otolith organs, can influence the organization of force feedback (Gottschall and Nichols 2007, 2011; Nichols et al. 2014). Evidence also supports modulatory potential from reticulospinal projections. Leblond et al. (2000) showed that electrically evoked inputs from reticulospinal descending tracts in the medial longitudinal fasciculus converge onto extensor group I pathways. Future work is warranted to identify and characterize how descending systems influence the strength and distribution of intermuscular force feedback.
Potential task-specific benefits afforded by a directional bias in intermuscular force feedback.
Animals and humans have a remarkable ability to rapidly adapt limb mechanics to accommodate different tasks and environmental surfaces (Daley et al. 2007; Dickinson et al. 2000; Ferris and Farley 1997; Krutky et al. 2010). Requisite for success, therefore, is the ability to variably tune whole limb stiffness. While the details of how this is accomplished remains unknown and likely involves passive and active musculotendon contributions (Griffiths 1991; Konow et al. 2012; Prochazka et al. 1977; Zuur et al. 2010), proprioceptive circuitry from muscle spindles and GTOs—given their rapid feedback—likely have an important role in regulating limb mechanics across motor tasks (Cronin et al. 2011; Duncan and McDonagh 2000; Maas et al. 2007; Prochazka et al. 1977; Zuur et al. 2010). We propose a bias in the widespread distribution of inhibitory feedback could provide several important task-dependent benefits.
One benefit of a directional inhibitory bias could be to promote energy absorption via joint-specific compliance. When landing from a jump, for example, the muscles crossing the ankle, knee, and hip act as brakes to decelerate the body. A distal-to-proximal bias of inhibition could function to promote energy absorption by the larger proximal muscles at the knee and hip, as well as compensate for the nonuniform segment inertias. In support of this general idea, Prochazka et al. (1977) observed a brief decrease in activation of the lateral gastrocnemius for a period shortly after foot contact in cats landing from heights ranging from 30 to 50 cm. The decreased lateral gastrocnemius activation was speculated to result from inhibitory force feedback from the toe flexor FHL. We propose that activities such as stair and curb descent could benefit from similar distal-to-proximal gradients of inhibition (according to pattern 3). In contrast, recent evidence suggests other tasks such as walking may benefit from a proximal-to-distal gradient of inhibitory feedback (pattern 1: Ross and Nichols 2009). Inhibitory feedback organized to help increase distal joint compliance (balanced or proximal to distal) could be useful to facilitate forward progression of the shank and also to quickly adapt foot contact to uneven or varied terrain. It has also been proposed that a neuromechanical gradient regulates limb mechanics during perturbed running in which the ankle extensors and digit flexors likely rely on higher load dependent feedback gains than more proximal muscles (Daley et al. 2007). It is therefore consistent with our hypothesis that the relative stiffness of the distal joints is modulated according to the demands of the motor task.
Similar to regulating joint compliance, intermuscular force feedback could also function to promote joint coupling during motor tasks. A common behavioral observation is temporal coupling of ankle and knee joint flexion during the loading phase of many locomotor behaviors (Goslow et al. 1973; Lay et al. 2006; Nuesch et al. 2017), and coupling of ankle, knee, and hip joint flexion during more dynamic tasks such as landing and stair descent (Devita and Skelly 1992; Goslow et al. 1973; Protopapadaki et al. 2007). A consistent finding in this study, regardless of the directional inhibitory bias, was convergence of inhibitory feedback from V and FHL onto the gastrocnemius muscles. The convergence of feedback onto G, taken together with the directional bias largely dictated by either the V or FHL muscles, could provide a rapid context-dependent mechanism to enable temporal coupling of toes-ankle or ankle-knee flexion. Coupling between ankle and knee is also promoted by the biarticular gastrocnemius and multiarticular plantaris muscles. However, the coupling is asymmetric due to the fixed 3:1 ratio of ankle-to-knee moment arms (Lan and Crago 1992). Inhibitory feedback to these muscles could be used to provide more flexible coupling between the two joints.
In addition to differences in the organization of inhibitory feedback, the magnitude of inhibition varied between given muscle pairs within and across cats in this study, suggesting the possibility that both the distribution and strength could be controlled by the nervous system. Indeed, it has been shown that the strength of inhibition can be modulated by signals from the brain stem (Nichols et al. 2014). It should be noted that prior reports have suggested that the magnitude of inhibitory feedback in the decerebrate cat is suppressed (Eccles and Lundberg 1959). It was clearly observed here, and in earlier work (Bonasera and Nichols 1994; Lyle et al. 2016; Nichols 1999; Wilmink and Nichols 2003), that intermuscular inhibitory feedback can be quite powerful and thus able to influence mechanical output from muscles spanning all joints. And while the methods used here evaluated inhibitory feedback between single muscle pairs, the real world perspective is intermuscular force feedback exchanged between many muscles simultaneously with convergence (Harrison et al. 1983; Wilmink and Nichols 2003). It is perhaps most informative, therefore, to view the net effect of inhibitory feedback from all potential donor muscles collectively, even if not a simple additive sum, to appreciate their full potential (see Fig. 3).
Central control of the strength of force feedback could also function to “normalize” the relative effects of muscles with differing force generating capacity. For example, one possibility is that larger muscles have a disproportionate GTO force-dependent effect onto other muscles compared with smaller muscles (e.g., V vs FHL). If this were the case, it would be expected that a fixed directional bias of inhibition would be observed in this study; however, distal-to-proximal inhibitory gradients were found just as often as the converse. In addition, inhibitory feedback from the FHL onto G was often of similar or greater magnitude when compared with the V muscle group. These data suggest the possibility of a central and/or peripheral mechanism for scaling inhibitory feedback magnitude across muscles of the hindlimb.
Conclusion.
In summary, the primary finding was that intermuscular force feedback between the primary extensor muscles was organized with directional biases of inhibitory feedback that varied across individual cat hindlimbs. The inhibitory feedback, despite exhibiting different patterns across cats, was organized and symmetric across limbs within cats, suggesting the potential to purposefully influence whole limb mechanics. We propose that intermuscular force feedback could function to provide neural linkages that help coordinate task-dependent limb behavior. The potential functional role for force feedback suggested by this study remains to be evaluated in intact and freely moving cats.
GRANTS
This study was supported by F32NS080393 to M. A. Lyle and HD32571 to T. R. Nichols.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.L. and T.R.N. conceived and designed research; M.A.L. and T.R.N. performed experiments; M.A.L. and T.R.N. analyzed data; M.A.L. and T.R.N. interpreted results of experiments; M.A.L. prepared figures; M.A.L. drafted manuscript; M.A.L. and T.R.N. edited and revised manuscript; M.A.L. and T.R.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Boris Prilutsky, Alexander Klishko, and Ricky Mehta for providing the cats with quadriceps reinnervation surgeries and Chris Tuthill and Elma Kajtaz for help with collecting data.
REFERENCES
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84: 2709–2714, 2000. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- af Klint R, Mazzaro N, Nielsen JB, Sinkjaer T, Grey MJ. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103: 2747–2756, 2010. doi: 10.1152/jn.00547.2009. [DOI] [PubMed] [Google Scholar]
- af Klint R, Nielsen JB, Cole J, Sinkjaer T, Grey MJ. Within-step modulation of leg muscle activity by afferent feedback in human walking. J Physiol 586: 4643–4648, 2008. doi: 10.1113/jphysiol.2008.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Albers JW. Long-term follow-up of pyridoxine-induced acute sensory neuropathy-neuronopathy. Neurology 40: 1319, 1990. doi: 10.1212/WNL.40.8.1319. [DOI] [PubMed] [Google Scholar]
- Allum JH, Mauritz KH. Compensation for intrinsic muscle stiffness by short-latency reflexes in human triceps surae muscles. J Neurophysiol 52: 797–818, 1984. doi: 10.1152/jn.1984.52.5.797. [DOI] [PubMed] [Google Scholar]
- Bonasera SJ, Nichols TR. Mechanical actions of heterogenic reflexes linking long toe flexors with ankle and knee extensors of the cat hindlimb. J Neurophysiol 71: 1096–1110, 1994. doi: 10.1152/jn.1994.71.3.1096. [DOI] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol 106: 2471–2485, 2011. doi: 10.1152/jn.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 71: 817–820, 1994. doi: 10.1152/jn.1994.71.2.817. [DOI] [PubMed] [Google Scholar]
- Cronin NJ, Carty CP, Barrett RS. Triceps surae short latency stretch reflexes contribute to ankle stiffness regulation during human running. PLoS One 6: e23917, 2011. doi: 10.1371/journal.pone.0023917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley MA, Felix G, Biewener AA. Running stability is enhanced by a proximo-distal gradient in joint neuromechanical control. J Exp Biol 210: 383–394, 2007. doi: 10.1242/jeb.02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devita P, Skelly WA. Effect of landing stiffness on joint kinetics and energetics in the lower extremity. Med Sci Sports Exerc 24: 108–115, 1992. doi: 10.1249/00005768-199201000-00018. [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Farley CT, Full RJ, Koehl MA, Kram R, Lehman S. How animals move: an integrative view. Science 288: 100–106, 2000. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- Duncan A, McDonagh MJ. Stretch reflex distinguished from pre-programmed muscle activations following landing impacts in man. J Physiol 526: 457–468, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol 137: 22–50, 1957a. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol 138: 227–252, 1957b. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol 147: 565–584, 1959. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElBasiouny SM, Schuster JE, Heckman CJ. Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophysiol 121: 1669–1679, 2010. doi: 10.1016/j.clinph.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol 490: 427–441, 2005. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- Faist M, Hoefer C, Hodapp M, Dietz V, Berger W, Duysens J. In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res 1076: 87–92, 2006. doi: 10.1016/j.brainres.2005.12.069. [DOI] [PubMed] [Google Scholar]
- Ferris DP, Farley CT. Interaction of leg stiffness and surfaces stiffness during human hopping. J Appl Physiol (1985) 82: 15–22, 1997. doi: 10.1152/jappl.1997.82.1.15. [DOI] [PubMed] [Google Scholar]
- Goslow GE Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–41, 1973. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Gottschall JS, Nichols TR. Head pitch affects muscle activity in the decerebrate cat hindlimb during walking. Exp Brain Res 182: 131–135, 2007. doi: 10.1007/s00221-007-1084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall JS, Nichols TR. Neuromuscular strategies for the transitions between level and hill surfaces during walking. Philos Trans R Soc Lond B Biol Sci 366: 1565–1579, 2011. doi: 10.1098/rstb.2010.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RI. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol 436: 219–236, 1991. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 487: 197–209, 1995. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. An interneuronal system contributing to the coordination of the cat hind limb. In: Coordination of Motor Behaviour, edited by Bush BMH, Clarac F. Cambridge, UK: Cambridge University Press, 1985, p. 163–179. [Google Scholar]
- Harrison PJ, Jankowska E, Johannisson T. Shared reflex pathways of group I afferents of different cat hind-limb muscles. J Physiol 338: 113–128, 1983. doi: 10.1113/jphysiol.1983.sp014664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Nardelli P, Cope TC, Nichols TR. Muscle spindle responses to horizontal support surface perturbation in the anesthetized cat: insights into the role of autogenic feedback in whole body postural control. J Neurophysiol 108: 1253–1261, 2012. doi: 10.1152/jn.00929.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC. On the significance of various command signals during voluntary control. Brain Res 40: 49–58, 1972. doi: 10.1016/0006-8993(72)90105-9. [DOI] [PubMed] [Google Scholar]
- Houk JC. Regulation of stiffness by skeletomotor reflexes. Annu Rev Physiol 41: 99–114, 1979. doi: 10.1146/annurev.ph.41.030179.000531. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32: 881–893, 2010. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konow N, Azizi E, Roberts TJ. Muscle power attenuation by tendon during energy dissipation. Proc Biol Sci 279: 1108–1113, 2012. doi: 10.1098/rspb.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ. Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm. J Neurophysiol 103: 429–440, 2010. doi: 10.1152/jn.00679.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. New York: McGraw-Hill/Irwin, 2005. [Google Scholar]
- Lan N, Crago PE. A noninvasive technique for in vivo measurement of joint torques of biarticular muscles. J Biomech 25: 1075–1079, 1992. doi: 10.1016/0021-9290(92)90043-Z. [DOI] [PubMed] [Google Scholar]
- Lay AN, Hass CJ, Gregor RJ. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. J Biomech 39: 1621–1628, 2006. doi: 10.1016/j.jbiomech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Leblond H, Menard A, Gossard JP. Bulbospinal control of spinal cord pathways generating locomotor extensor activities in the cat. J Physiol 525: 225–240, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leukel C, Gollhofer A, Keller M, Taube W. Phase- and task-specific modulation of soleus H-reflexes during drop-jumps and landings. Exp Brain Res 190: 71–79, 2008. doi: 10.1007/s00221-008-1450-5. [DOI] [PubMed] [Google Scholar]
- Lloyd DP. Integrative pattern of excitation and inhibition in two-neuron reflex arcs. J Neurophysiol 9: 439–444, 1946. doi: 10.1152/jn.1946.9.6.439. [DOI] [PubMed] [Google Scholar]
- Lord SR, Rogers MW, Howland A, Fitzpatrick R. Lateral stability, sensorimotor function and falls in older people. J Am Geriatr Soc 47: 1077–1081, 1999. doi: 10.1111/j.1532-5415.1999.tb05230.x. [DOI] [PubMed] [Google Scholar]
- Lyle MA, Cloutier A, Nichols TR. Mapping intermuscular force dependent reflex pathways selectively using intramuscular stimulation in the decerebrate cat. Program no. 241.12 Society for Neuroscience Annual Meeting, Chicago, IL, October 17–21, 2015. [Google Scholar]
- Lyle MA, Prilutsky BI, Gregor RJ, Abelew TA, Nichols TR. Self-reinnervated muscles lose autogenic length feedback, but intermuscular feedback can recover functional connectivity. J Neurophysiol 116: 1055–1067, 2016. doi: 10.1152/jn.00335.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Prilutsky BI, Nichols TR, Gregor RJ. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res 181: 377–393, 2007. doi: 10.1007/s00221-007-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGilchrist C, Paul L, Ellis BM, Howe TE, Kennon B, Godwin J. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med 27: 162–168, 2010. doi: 10.1111/j.1464-5491.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- Makihara Y, Segal RL, Wolpaw JR, Thompson AK. H-reflex modulation in the human medial and lateral gastrocnemii during standing and walking. Muscle Nerve 45: 116–125, 2012. doi: 10.1002/mus.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res 173: 713–723, 2006. doi: 10.1007/s00221-006-0451-5. [DOI] [PubMed] [Google Scholar]
- Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil 85: 245–252, 2004. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- Niazi I. Altered Intermuscular Force Feedback After Spinal Cord Injury in Cat (PhD Dissertation) Atlanta, GA: Georgia Institute of Technology, 2015. [Google Scholar]
- Nichols R, Ross KT. The implications of force feedback for the lambda model. Adv Exp Med Biol 629: 663–679, 2009. doi: 10.1007/978-0-387-77064-2_36. [DOI] [PubMed] [Google Scholar]
- Nichols TR. Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. J Neurophysiol 81: 467–478, 1999. doi: 10.1152/jn.1999.81.2.467. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Bunderson NE, Lyle MA. Neural regulation of limb mechanics: insights from the organization of proprioceptive circuits. In: Neuromechanical Modeling of Posture and Locomotion, edited by Prilutsky BI, Edwards DH. New York: Springer, 2016. doi: 10.1007/978-1-4939-3267-2_3. [DOI] [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exerc Sport Sci Rev 27: 255–284, 1999. doi: 10.1249/00003677-199900270-00010. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Gottschall JS, and Tuthill C. The regulation of limb stiffness in the context of locomotor task. In: Progress in Motor Control IX, edited by Levin MF. New York: Springer, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol 39: 119–142, 1976. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Koffler-Smulevitz D. Mechanical analysis of heterogenic inhibition between soleus muscle and the pretibial flexors in the cat. J Neurophysiol 66: 1139–1155, 1991. doi: 10.1152/jn.1991.66.4.1139. [DOI] [PubMed] [Google Scholar]
- Nüesch C, Roos E, Pagenstert G, Mündermann A. Measuring joint kinematics of treadmill walking and running: Comparison between an inertial sensor based system and a camera-based system. J Biomech 57: 32–38, 2017. doi: 10.1016/j.jbiomech.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Pratt CA. Evidence of positive force feedback among hindlimb extensors in the intact standing cat. J Neurophysiol 73: 2578–2583, 1995. doi: 10.1152/jn.1995.73.6.2578. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Schofield P, Westerman RA, Ziccone SP. Reflexes in cat ankle muscles after landing from falls. J Physiol 272: 705–719, 1977. doi: 10.1113/jphysiol.1977.sp012068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopapadaki A, Drechsler WI, Cramp MC, Coutts FJ, Scott OM. Hip, knee, ankle kinematics and kinetics during stair ascent and descent in healthy young individuals. Clin Biomech (Bristol, Avon) 22: 203–210, 2007. doi: 10.1016/j.clinbiomech.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ross KT, Nichols TR. Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat. J Neurophysiol 101: 184–197, 2009. doi: 10.1152/jn.90338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci 34: 413–440, 2011. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Nobel Lecture: Inhibition as a Coordinative Factor, 1932. https://www.nobelprize.org/nobel_prizes/medicine/laureates/1932/sherrington-lecture.html.
- Silverman J, Garnett NL, Giszter SF, Heckman CJ II, Kulpa-Eddy JA, Lemay MA, Perry CK, Pinter M. Decerebrate mammalian preparations: unalleviated or fully alleviated pain? A review and opinion. Contemp Top Lab Anim Sci 44: 34–36, 2005. [PubMed] [Google Scholar]
- Simonsen EB, Dyhre-Poulsen P. Amplitude of the human soleus H reflex during walking and running. J Physiol 515: 929–939, 1999. doi: 10.1111/j.1469-7793.1999.929ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol 76: 1112–1120, 1996. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Stephens MJ, Yang JF. Short latency, non-reciprocal group I inhibition is reduced during the stance phase of walking in humans. Brain Res 743: 24–31, 1996. doi: 10.1016/S0006-8993(96)00977-8. [DOI] [PubMed] [Google Scholar]
- Taylor JS, Friedman RF, Munson JB, Vierck CJ Jr. Stretch hyperreflexia of triceps surae muscles in the conscious cat after dorsolateral spinal lesions. J Neurosci 17: 5004–5015, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timar B, Timar R, Gaiță L, Oancea C, Levai C, Lungeanu D. The impact of diabetic neuropathy on balance and on the risk of falls in patients with Type 2 diabetes mellitus: a cross-sectional study. PLoS One 11: e0154654, 2016. doi: 10.1371/journal.pone.0154654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol 49: 481–515, 1996. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- Wilmink RJ, Nichols TR. Distribution of heterogenic reflexes among the quadriceps and triceps surae muscles of the cat hind limb. J Neurophysiol 90: 2310–2324, 2003. doi: 10.1152/jn.00833.2002. [DOI] [PubMed] [Google Scholar]
- Zuur AT, Lundbye-Jensen J, Leukel C, Taube W, Grey MJ, Gollhofer A, Nielsen JB, Gruber M. Contribution of afferent feedback and descending drive to human hopping. J Physiol 588: 799–807, 2010. doi: 10.1113/jphysiol.2009.182709. [DOI] [PMC free article] [PubMed] [Google Scholar]