Abstract
We tested the hypothesis that carotid chemoreceptors tune breathing through parallel circuit paths that target distinct elements of an inspiratory neuron chain in the ventral respiratory column (VRC). Microelectrode arrays were used to monitor neuronal spike trains simultaneously in the VRC, peri-nucleus tractus solitarius (p-NTS)-medial medulla, the dorsal parafacial region of the lateral tegmental field (FTL-pF), and medullary raphe nuclei together with phrenic nerve activity during selective stimulation of carotid chemoreceptors or transient hypoxia in 19 decerebrate, neuromuscularly blocked, and artificially ventilated cats. Of 994 neurons tested, 56% had a significant change in firing rate. A total of 33,422 cell pairs were evaluated for signs of functional interaction; 63% of chemoresponsive neurons were elements of at least one pair with correlational signatures indicative of paucisynaptic relationships. We detected evidence for postinspiratory neuron inhibition of rostral VRC I-Driver (pre-Bötzinger) neurons, an interaction predicted to modulate breathing frequency, and for reciprocal excitation between chemoresponsive p-NTS neurons and more downstream VRC inspiratory neurons for control of breathing depth. Chemoresponsive pericolumnar tonic expiratory neurons, proposed to amplify inspiratory drive by disinhibition, were correlationally linked to afferent and efferent “chains” of chemoresponsive neurons extending to all monitored regions. The chains included coordinated clusters of chemoresponsive FTL-pF neurons with functional links to widespread medullary sites involved in the control of breathing. The results support long-standing concepts on brain stem network architecture and a circuit model for peripheral chemoreceptor modulation of breathing with multiple circuit loops and chains tuned by tegmental field neurons with quasi-periodic discharge patterns.
NEW & NOTEWORTHY We tested the long-standing hypothesis that carotid chemoreceptors tune the frequency and depth of breathing through parallel circuit operations targeting the ventral respiratory column. Responses to stimulation of the chemoreceptors and identified functional connectivity support differential tuning of inspiratory neuron burst duration and firing rate and a model of brain stem network architecture incorporating tonic expiratory “hub” neurons regulated by convergent neuronal chains and loops through rostral lateral tegmental field neurons with quasi-periodic discharge patterns.
Keywords: brain stem, breathing, carotid chemoreceptors, lateral tegmental field, network
INTRODUCTION
Peripheral chemoreceptors of the carotid body detect changes in arterial O2 and CO2-pH and tune ventilation (Kumar and Prabhakar 2012) by adjusting the frequency and depth of breathing. When driven by hypoxia during hypocapnic apnea, as may occur during sleep-disordered breathing, these chemoreceptors can evoke the reemergence of respiratory rhythmogenesis (Lovering et al. 2012; Nuding et al. 2015). Breathing may be switched on transiently or it may persist under the prior hypocapnic drive conditions due to induced long-term facilitation, a “respiratory memory” (Barnett et al. 2017; Fuller and Mitchell 2016; Millhorn et al. 1980; Morris et al. 1996b, 2001b). Increasingly, peripheral chemoreceptors are considered as therapeutic targets for the management and treatment of various disorders, including heart failure and hypertension (e.g., Guyenet 2014; Honda et al. 1976; Lugliani et al. 1971; Marcus et al. 2014; McBryde et al. 2013; Narkiewicz et al. 2016; Prabhakar et al. 2015; Toledo et al. 2016; Wade et al. 1970), and for rehabilitation following spinal cord injury (Fuller and Mitchell 2016; Navarrete-Opazo et al. 2015).
Carotid chemoreceptors operate on breathing and other autonomic functions (De Burgh Daly 1997; Paintal 1976; Paton 2016) via “chemoresponsive” neurons in the nucleus of the solitary tract (NTS) and ventrolateral medulla (Claps and Torrealba 1988; Davies and Edwards 1973, 1975; De Burgh Daly and Scott 1962; Donoghue et al. 1984; Finley and Katz 1992; Kline et al. 2010; Lipski et al. 1976, 1977; Paton et al. 2001; Wilson and Teppema 2016; Zoccal et al. 2014). Some chemoresponsive NTS neurons receive convergent baroreceptor and pharyngesophageal receptor influences, suggesting a role for chemoreceptor circuits in the coordination of breathing with swallowing, coughing, and other behaviors for airway protection, which also involve circuits in the medial medulla (Bolser et al. 2015; Paton et al. 1999). However, the links to “downstream” circuits of the ventral respiratory column (VRC), and chemoresponsive elements of the raphe-pontomedullary network in which it is embedded, remain incompletely understood (Guyenet 2014; Kline et al. 2010; Koshiya and Guyenet 1996; Li et al. 1999b; Miura and Reis 1972; Morris et al. 1996a, 1996b, 2001a; Nuding et al. 2009b, 2015; Segers et al. 2015; Song et al. 2011; Takakura et al. 2006).
Thirty years ago, Segers et al. (1987) identified a group of preinspiratory neurons just caudal to the Bötzinger complex with correlational signatures of excitatory actions and proposed that this cluster included neurons that drove a downstream chain of VRC inspiratory neurons, a model that has since received considerable support from many complementary experimental approaches (Schwarzacher et al. 1995; Smith et al. 1991; Lindsey et al. 2012; Wu et al. 2017). Subsequently, Morris et al. (1996a) observed that some of the rostral neurons respond to selective stimulation of the carotid chemoreceptors with a shortened burst duration, but with little change in firing rate. In contrast, more caudal inspiratory neurons excited by the rostral neurons respond with a much larger rate increase. These results and associated computational model network simulations (Balis et al. 1994) motivated consideration of the hypothesis that there are distinct parallel circuit mechanisms for peripheral chemoreceptor tuning of breathing frequency and tidal volume (Morris et al. 1996a, 2001a). The first objective of this study was to test this long-standing hypothesis.
A second related goal was to test further the hypothesis that carotid chemoreceptors also tune the drive to breathe via a disinhibitory microcircuit modulated by an efference copy of inspiratory drive. In this model, the efference copy drives inhibitory inspiratory neurons that inhibit pericolumnar tonic VRC expiratory (t-E) neurons. In turn, these t-E neurons inhibit, via recurrent and feedforward inhibition, elements of the excitatory inspiratory neuron chain (Segers et al. 2015). Prior work had provided evidence for a push-pull tuning of these t-E neurons by baroreceptor-modulated raphe circuits (Lindsey et al. 1998), and in revisiting this model we sought to test the specific hypothesis that other circuit paths modulated by carotid chemoreceptors also target the t-E neurons.
A third objective was to extend preliminary observations on functional associations among hypoxia-modulated parafacial-lateral tegmental field neurons and the VRC acquired during a prior study (Ott et al. 2012). The tegmental field has been implicated in the control of breathing by both anatomical and physiological data but remains an underexplored region of the brain stem (e.g., Orer et al. 2006; Smith et al. 1989).
We employed electrode arrays to monitor neurons at multiple brain stem sites simultaneously. Spike trains were screened for correlational signatures of paucisynaptic functional connectivity and altered firing rates during selective stimulation of carotid chemoreceptors or transient systemic hypoxia. The results suggest a model for peripheral chemoreceptor modulation of breathing via multipath routing through a network of circuit loops and chains tuned by tegmental field neurons with quasi-periodic discharge patterns. A preliminary account of some of the results has been reported (Lindsey et al. 2016).
METHODS
All experiments were performed according to protocols approved by the University of South Florida’s Institutional Animal Care and Use Committee with strict adherence to all American Association for Accreditation of Laboratory Animal Care International, National Institutes of Health and National Research Council, and USDA guidelines. Data were obtained from 19 adult cats (2.8 – 6.7 kg; 9 females, 10 males) initially anesthetized with isoflurane mixed with air (3–5%) or with ketamine hydrochloride (5.5 mg/kg im); all were maintained with 0.5–3.0% isoflurane until decerebration (Kirsten and St. John 1978). Animals were artificially ventilated through a tracheal cannula with a respirator, which was triggered by the onset of integrated phrenic nerve activity during some recordings in cats with intact vagi. Immediately before decerebration, an anesthetic assessment was performed (Nuding et al. 2009b) and cats were neuromuscularly blocked (pancuronium bromide, initial bolus 0.1 mg/kg followed by 0.2 mg·kg−1·h−1 iv; or vecuronium bromide, initial bolus 0.1 mg/kg followed by 0.2 mg·kg−1·h−1 iv). Arterial blood pressure, end-tidal CO2, and tracheal pressure were monitored continuously; arterial Po2, Pco2, and pH were measured periodically and maintained within control limits. Some cats were bilaterally vagotomized to remove vagal afferent feedback from pulmonary stretch receptors and to permit comparisons with prior work. Except as noted, a concentric catheter was inserted into the right external carotid artery and advanced to a point immediately caudal to the carotid sinus for subsequent stimulation of carotid chemoreceptors (Arita et al. 1988; Li et al. 1999b). Heparinized saline was slowly infused from the outer catheter to prevent clotting within the inner catheter. At the end of each experiment, animals were euthanized [Beuthanasia (0.97 mg/kg; Merck Animal Health) or sodium pentobarbital (28 mg/kg) followed by a saturated solution of KCl in water].
Descriptions of most of the other methods used have been published (Nuding et al. 2015; Segers et al. 2015). Briefly, spike trains were recorded with electrode arrays at multiple brain stem sites along with signals from phrenic and vagus nerves, arterial blood pressure, end-tidal CO2, and tracheal pressure. Signals from single neurons were isolated using one of two interactive spike sorting software packages (Datawave Technologies, O’Connor et al. 2005). Coordinates of recording sites were mapped into the three-dimensional space of a computer-based brain stem atlas derived from The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates (Berman 1968) with permission of the University of Wisconsin Press.
Stimulation of peripheral chemoreceptors and evaluation of neuronal response.
In cats 1–18, carotid chemoreceptors were stimulated by 1 ml of CO2-saturated saline injected over a period of 30 s into the right carotid sinus; a minimum of three stimulus trials were performed during each recording. In two of these cats and one other (animals 16, 17, and 19), transient intervals of hypoxia were produced by ventilation with a gas mixture of 5% O2 and 95% N2 for 20–40 s.
Changes in firing rates of single neurons during effective stimuli were identified over the entire respiratory cycle as well as during just the inspiratory and expiratory phases using a bootstrap-based statistical method (Nuding et al. 2009b) with the false discovery rate (FDR) controlled to be less than 5% (Benjamini and Hochberg 1995). For each stimulus trial, the first, peak, and maximum contiguous sum of deviations of rate per respiratory cycle in the response window from the control mean, calculated from immediately preceding cycles, were used as measures of a response. The use of local control cycles was intended to mitigate the potential consequences of any nonstationarities in firing probabilities and synaptic efficacies over the course of the recordings (e.g., Morris et al. 1996a, 1996b, 2001b), including those due to other experimental perturbations (e.g., transient hypoxia, selective stimulation of central chemoreceptors, and electrical stimulation of the superior laryngeal nerve), the results of which will be reported elsewhere. Significant deviations, averaged over all trials, were classified into one of several response categories: increase (↑), decrease (↓), biphasic [increase-decrease (↑↓) or decrease-increase (↓↑)], no change (→), or a significant change in the depth of respiratory modulation, i.e., the ratio of the maximum to the minimum mean firing rate or “rate ratio” (↕).
Standard firing rate histograms and phase-normalized respiratory cycle-triggered histograms (CTHs) were calculated using activity recorded during a control period; neurons were classified as respiratory modulated if either of two complementary statistical tests rejected the null hypothesis (P < 0.05) (Morris et al. 1996a; Netick and Orem 1981; Orem and Netick 1982). Description of respiratory modulation was based on the CTHs (Cohen 1968). Neurons were classified as inspiratory (I), expiratory (E), or phase-spanning (IE or EI) according to the interval of greatest average activity. Respiratory modulated neuronal activity was additionally described as phasic (P) if the average firing probability was essentially zero during any part of the CTH or tonic (T) otherwise, and as decrementing (Dec) or augmenting (Aug) if the average peak firing rate occurred during the first or second half of the phase, respectively. Cells without a preferred phase of maximum activity were designated nonrespiratory modulated (NRM).
A complementary approach to identify respiratory, cardiac, or other rhythms present in the spike trains was also used. Joint time-frequency representations were generated using an implementation of the S-transform (Stockwell et al. 1996) as described in Nuding et al. (2015). Briefly, the frequency range used to generate S-transforms for a spike train extended at least one order of magnitude below and above the control respiratory frequency range as measured from the phrenic nerve signal. The S-transform is displayed as a heat map with luminance proportional to the displayed quantity, i.e., the proportion of power in that frequency at that time; the higher the S-transform magnitude value, the brighter the color of that point. Frequency is displayed as a log scale.
Correlation analyses.
To identify functional connectivity within and among the brain stem regions studied, cross-correlation histograms (CCHs) were calculated for all pairs of simultaneously monitored neurons. Significant peak or trough features were identified with Monte Carlo tests using surrogate spike trains (Pauluis and Baker 2000) with gamma-distributed interspike intervals; FDR < 0.05. The shape parameter of the gamma distribution was estimated from the data (Miura et al. 2006). A detectability index (equal to the maximum amplitude of feature departure from background activity divided by the standard deviation of the correlogram noise) greater than 3.0 indicated a significant correlogram feature (Aertsen and Gerstein 1985; Melssen and Epping 1987).
In some instances, subsets of spike times in a train were selected according to durations of preceding and following interspike intervals and used to generate a new time series. For example, spike doublets could be selected or the first spike in each operationally defined “burst” in a train could be written to a file and used subsequently as a marker event to parse data into phase segments for correlational analyses. Correlation feature maps for groups of simultaneously monitored neurons were generated automatically by database queries and software based on the open source graph visualization tool Graphviz (Gansner and North 2000; Segers et al. 2008). The maps included directed graphs representing chains of inferred retrograde and anterograde connections between cells, where each connection corresponds to a cross-correlation between the spike trains of the two cells with an offset peak or trough feature. As an aid in the evaluation of some cross-correlogram features, network simulations were performed using a previously described modeling approach and software package (Rybak et al. 2008). Significant features in spike-triggered averages were identified (method adapted from Poliakov and Schieber 1998) using a two-sided Wilcoxon signed-rank test with Bonferroni correction; P < 0.05 (for details, see Ott et al. 2012).
Gravity analysis.
Cross-correlation analysis is an averaging procedure and as such may obscure more dynamic aspects of neuronal interactions. Gravitational clustering algorithms permit assessment of transient functional associations in groups or assemblies of neurons (Arata et al. 2000; Gerstein 2010; Lindsey and Gerstein 2006). Briefly, each neuron is represented as a particle in n-space. The particle has a time-varying charge that is a filtered version of the corresponding neuron’s spike train. Forces between and movements of the particles reflect neuronal interactions.
Repeated patterns of synchronous spiking were identified by comparing pairwise aggregation velocities of particles over successive plotted time steps in the gravity calculation (Lindsey et al. 1997). Gravity particle condensation rates portrayed in each column of a two-dimensional matrix (in which each row corresponds to a cell pair and each column to a time step) served in turn as a template that was compared with all other columns for a match. Velocities of at least two particle pairs had to be represented in a column for it to be used as a template; pairs that did not aggregate were excluded. Velocity values less than 3.25% of the maximum velocity detected in the data set were nulled to filter out small fluctuations in aggregation velocity.
A set of match criteria based on velocity thresholds was used in each pattern search and tested for significance. More than 99% of the locations of nonnulled elements in the template column had to match with corresponding locations of nonnulled velocities in the target column; there could be no extra nonnulled velocities in the target column, and target velocities for more than 75% of the matched pairs had to be within 25% of the corresponding template velocity values.
A Monte Carlo significance test compared the frequency of occurrence of each detected pattern with the number of repetitions found in each of 1,000 surrogate data sets. Each surrogate data set was produced by first randomly rotating the rows of the centered original data set. The rotated data were premultiplied by the transpose of the eigenvector matrix of the sample covariance matrix of the rotated data to get uncorrelated data, which was then scaled to make the variances the same as the eigenvalues of the original data. The scaled, uncorrelated data were then premultiplied by the eigenvectors of the original data and uncentered. The resulting surrogate data rows had the same means, variances, and covariances as those of the original spike data. Values in each column of a matching set that occurred more frequently than in any of the surrogate trials were displayed as a set of vectors with a common origin. The length of the vector indicated the aggregation velocity of the corresponding particles in that pair; the direction of each vector in a plane identified the neuron pair represented. These graphs are called spark plots because of the transient appearance of the vectors in animations of successive particle aggregation steps through time.
Joint pericycle-triggered histograms.
To identify variations in effective connectivity between some pairs of neurons as a function of the time within the respiratory cycle, we used the joint pericycle-triggered histogram (JPCTH) matrix adapted from the joint peristimulus time histogram (JPSTH) (Aertsen et al. 1989; Gerstein 1999). Each histogram was triggered by alignment events marking the onset of inspiratory or expiratory phases. The matrix is a two-dimensional array of bins, each with a color-coded value of the sample Pearson product-moment correlation coefficient (PCC; Pearson 1896) calculated from delayed coincident spike events over all cycles in that time bin. Each time point in the bin, in each cycle, is an x,y pair for the PCC calculation. The JPCTH is displayed together with cycle-triggered histograms (CTHs) plotted along the x- and y-axes to show the average firing rates of the represented neurons.
A rotated bin grid was used to calculate the bin values for three other related displays directly from the underlying data points, rather than from the JPCTH matrix bin values (Gerstein 1999). The scoop histogram (SH) shows PCC values for time-locked near-coincident firings tallied in diagonal bins and corresponds to the peristimulus time coincidence histogram of the JPSTH. The CCH estimates the time average of the near-coincident firing as a function of relative delay and is constructed by tallying the weighted averages of bin PCC values along paradiagonal bins. The PCC in each CCH bin was computed by first calculating a PCC for each bin of the rotated JPCTH matrix. Next, the bins for a particular lag range were averaged (weighted by the number of points) to generate the value for the corresponding CCH bin. The delayed correlation matrix (DCM) facilitates visualization of spike correlation dynamics over two distinct time scales (Gerstein 1999) by providing the ability to set a (finer) time resolution along the lag (relative delay) y-axis and a time-in-cycle x-axis bin width different than the other histograms. The time of the horizontal coordinate of the DCM is the midpoint between the x and y spikes, making the DCM bins equivalent to a rigidly rotated version of the JPCTH grid.
A dithered surrogate Monte Carlo significance test was used (Louis et al. 2010a). The dither shifted the spikes for each neuron in each respiratory cycle by a randomly chosen amount uniformly distributed between limits set by the user (e.g., ±100 ms); all spikes of a particular neuron were shifted by the same amount. The spikes in each cycle were shifted by a different amount than in other cycles. A cycle was dropped if the worst-case dither could cause a spike sequence from the previous retained cycle to be repeated in this cycle. The FDR (Benjamini and Hochberg 1995) was controlled by generating surrogates either until the ratio of expected false positives to positives was less than or equal to 0.05 or until there were no more positives. The four histograms (JPCTH, SH, CCH, and DCM) were tested for significance separately. To keep the overall FDR below 0.05 while separating the significances, the histograms were ordered CCH→SH→DCM→JPCTH and positives in later histograms were not counted unless all the earlier histograms had at least one positive, and the individual FDR thresholds were reduced from 0.05 to 0.0476.
The amount of correlation between spike trains may vary over the course of the cycle. To assess whether such modulation was significant, a PCC “modulation test” was used to determine whether PCC values were larger in one half of the SH than in the other. This test measured the magnitude of the Fourier component of the Discrete Fourier Transform of the SH bin values (PCCs) at the lowest nonzero frequency (one cycle of this frequency is the duration of the SH) and used the phase of the Fourier component to identify the boundaries between the two halves. The null hypothesis was that the observed bin values were in a random order chosen uniformly from all possible permutations of the values and, therefore, if the PCC values were higher in one half of the cycle, it was due to chance. Each time a pair of spike train surrogates was generated for the histograms, a permutation was generated for this magnitude test. The observed test statistic was considered significant if no sample test statistic reached the observed test statistic before the FDR was achieved for the SH (if the cross-correlation histogram also had significant bins).
RESULTS
This work was part of a series of studies on chemoreceptor circuits of the brain stem respiratory network. Complementary results on the central chemoreceptor-evoked responses of neurons in one animal (animal 19) have been reported (Ott et al. 2012), as noted subsequently. We employed electrode arrays to monitor neurons at multiple sites simultaneously in the VRC and, depending on other experimental objectives, in one or more of the following regions: 1) the peri-NTS region and medial medullary reticular formation extending out from the NTS (p-NTS-MM), 2) the lateral tegmental field (FTL)-dorsal parafacial region (FTL-pF), and 3) the medullary raphe nuclei and adjacent reticular formation (raphe-mRF). Table 1 gives, for each recording, a summary of the numbers of neurons recorded in each brain region, number of carotid chemoreceptor stimulus trials, and the presence or absence of an intact vagus nerve.
Table 1.
Recordings included in this study
| Number of Cells in Each Location |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recording Sites |
||||||||||
| Animal | Rec. | Length, min | No. of Trials | Vagus Nerve | p-NTS-MM (right) | FTL-pF (right) | Raphe-mRF (midline) | VRC (right) | VRC (left) | Total |
| 1 | 1* | 184.9 | 5 | intact | 18 | 9 | 16 | 34 | 77 | |
| 2 | 2* | 153.6 | 4 | intact | 26 | 11 | 16 | 24 | 77 | |
| 3 | 3* | 208.0 | 5 | intact | 7 | 11 | 14 | 33 | 65 | |
| 4 | 4* | 167.7 | 5 | intact | 16 | 13 | 13 | 15 | 57 | |
| 5 | 5 | 210.7 | 5 | intact | 9 | 16 | 13 | 27 | 65 | |
| 6 | 6 | 159.2 | 5 | intact | 6 | 12 | 9 | 27 | 54 | |
| 7 | 7 | 203.6 | 5 | intact | 4 | 3 | 13 | 17 | 37 | |
| 8 | 8 | 121.6 | 5 | intact | 13 | 8 | 11 | 18 | 50 | |
| 9 | 9 | 108.0 | 7 | intact | 16 | 12 | 13 | 17 | 58 | |
| 9 | 10 | 120.0 | 5 | cut | 8 | 12 | 10 | 9 | 39 | |
| 10 | 11 | 174.0 | 7 | cut | 10 | 22 | 18 | 33 | 83 | |
| 11 | 12* | 57.0 | 5 | cut | 7 | 15 | 29 | 51 | ||
| 12 | 13 | 147.0 | 5 | cut | 7 | 1 | 25 | 33 | ||
| 13 | 14* | 142.4 | 5 | cut | 4 | 23 | 27 | |||
| 14 | 15 | 166.1 | 5 | cut | 19 | 37 | 56 | |||
| 15 | 16 | 186.0 | 5 | cut | 34 | 59 | 93 | |||
| 16 | 17 | 47.2 | 3 | cut | 24 | 25 | 49 | |||
| 16 | 18† | 58.1 | 3 | cut | 20 | 17 | 37 | |||
| 17 | 19 | 98.7 | 5 | cut | 23 | 18 | 41 | |||
| 17 | 20† | 115.5 | 5 | cut | 24 | 33 | 57 | |||
| 18 | 21 | 142.6 | 5 | cut | 4 | 1 | 6 | 20 | 31 | |
| Total | 151 | 150 | 290 | 526 | 20 | 1,137 | ||||
| 19 | ‡* | 229.0 | cut | 21 | 20 | |||||
Numbers of trials evaluated, coordinate ranges of and numbers of cells recorded within each area, and vagal state for each recording. Stimulation of peripheral chemoreceptors was via close injection of CO2-saturated saline.
Data from these recordings are shown in figures.
Carotid chemoreceptors were additionally stimulated by administration of a gas mixture of 5% O2/95% N2 with resultant transient hypoxia.
Carotid chemoreceptors were stimulated only by transient hypoxia in this animal; results of pairwise analyses are not included in subsequent tables. Coordinate ranges are with respect to obex; positive values are rostral to or to the right of obex. p-NTS-MM, the peri-nucleus tractus solitarius (NTS) region and medial medullary reticular formation extending out from the NTS; FTL-pF, lateral tegmental field-dorsal parafacial region; Raphe-mRF, medullary raphe nuclei and adjacent reticular formation; VRC, ventral respiratory column. Totals shown in bold italics are counts from recordings 1–21.
Neurons responsive to carotid chemoreceptor stimulation are widely distributed in the brain stem.
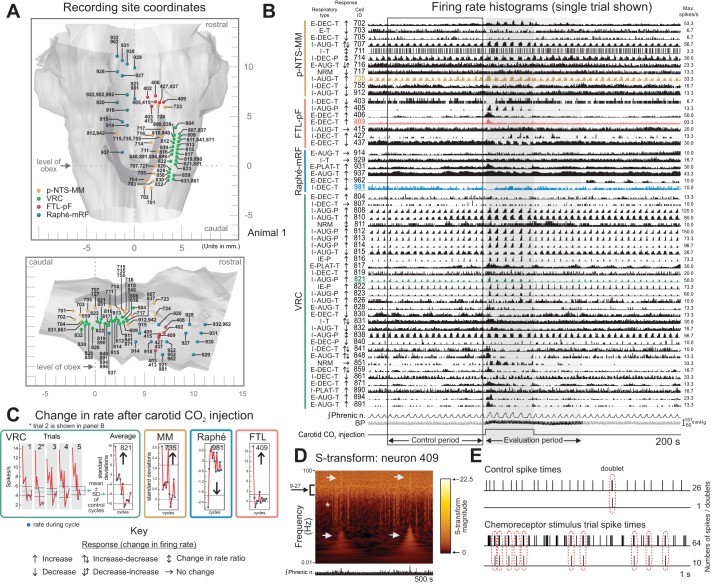
Stereotaxic coordinates of the recording sites in one animal are shown in dorsal and sagittal views of a brain stem atlas (Fig. 1A). Most of the simultaneously monitored neurons had a respiratory-related phase with a higher average firing rate and profile (e.g., augmenting or decrementing) as indicated by the “Respiratory type” label to the left of each individually scaled firing rate histogram (Fig. 1B). Slower rate oscillations that fluctuated in intensity were also present in some neurons (e.g., VRC cell 817). The arrows to the left of the traces indicate the presence and direction of a significant change in average or peak firing rates during respiratory cycles in evaluation periods relative to corresponding rates in preceding control intervals. For example, the green highlighted rate trace for VRC neuron 821 (Fig. 1B) shows enhanced activity during the trial represented, confirmed by analysis of firing rates represented in the “by-cycle” rate plots for all five trials (Fig. 1C, left). Average rate changes in successive respiratory cycles over all trials are also shown for this neuron and for three other highlighted neurons, monitored in the medial medulla, raphe, and FTL, respectively (Fig. 1C, right). A time-frequency representation of the spike train record from FTL neuron 409 (Fig. 1D) revealed several features, including intermittent vertical “streaks” (asterisk) arising from a broad band of increased S-transform magnitude values (left bracket) associated with periods of low- and high-frequency spiking during two stimulus trials (arrows). The upper streaks indicative of high-frequency activity reflected increased numbers of spike doublets and bursts during the trials (Fig. 1E).
Fig. 1.
Recording sites, firing rate histograms, and associated displays of spike train data from animal 1 during selective stimulation of carotid chemoreceptors. A: dorsal (top) and sagittal (bottom) views of color-coded spheres marking recording sites mapped in the coordinate space of a cat brain stem atlas (derived from Berman 1968; by permission of the University of Wisconsin Press). Gold: peri-nucleus tractus solitarius-medial medulla (p-NTS-MM; MM); green: ventral respiratory column (VRC); red: lateral tegmental field dorsal to parafacial region (FTL-pF; FTL); blue: midline raphe-medial reticular formation (raphe-mRF). B: peristimulus firing rate histograms for neurons recorded in parallel at sites in A together with integrated phrenic nerve activity, arterial blood pressure, and carotid stimulus injection marker (trial 2 of 5 is shown). Trial control and response evaluation periods are denoted by a box and gray shading, respectively. For this and subsequent firing rate histograms, each neuron’s location, respiratory-modulated discharge pattern (i.e., I, E, IE, NRM; see methods), response to carotid chemoreceptor stimulation (arrows; see Key), and ID code are to the left of its histogram. BP, blood pressure; Max, maximum; Phrenic n., phrenic nerve. C: by-cycle rate measures of responses to carotid chemoreceptor stimulation for neurons represented in highlighted traces in B; mean ± SD of rates during control cycles immediately preceding stimulus presentation are shown as horizontal solid and dashed lines, respectively, for each trial and average; individual trials and average shown for VRC neuron 821; averages of 5 trials shown for other neurons. D: S-transform for FTL-pF neuron 409 for a 500-s spike train record including 2 stimulus trials; integrated phrenic activity is shown below. The S-transform is displayed as a heat map with a luminance proportional to the S-transform magnitude, represented by the colored scale; the higher the value, the brighter the color of that point. Each S-transform is scaled individually from 0 to its maximum magnitude value; frequency is represented by a log scale. A constant relative time resolution of 4 cycles and a frequency resolution equal to f/4 were used for this and subsequent S-transforms. E: 1-s records showing spike times and occurrences of spike doublets during control and chemoreceptor stimulus intervals. Doublets were defined as pairs of sequential spikes with an interspike interval ≤ 4 ms and no spike within the following 10 ms.
Table 2 is a summary of the numbers of neurons responsive to selective stimulation of carotid chemoreceptors arranged by brain region and control respiratory discharge pattern. Overall, 994 of 1,137 neurons in 18 animals were tested; 561 neurons responded with a change in firing rate, the majority with an increase in activity. Responses during transient intervals of hypoxia in two of these animals and in one other are considered in a subsequent section of the Results.
Table 2.
Evaluation of neuronal firing rates following stimulation of carotid chemoreceptors in animals 1–18 by injection of CO2-saturated saline into the right carotid sinus
| Respiratory Pattern |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Location | Response | I | E | IE | EI | NRM | Totals | No. Cells Responsive | No. Cells Tested | % Responsive | |
| p-NTS-MM | INC | ↑ | 18 | 21 | 15 | 54 | 83 | 128 | 65% | ||
| DEC | ↓ | 5 | 10 | 4 | 19 | ||||||
| RR | ↕ | 5 | 5 | 10 | |||||||
| NC | → | 17 | 12 | 1 | 15 | 45 | |||||
| FTL-pF | INC | ↑ | 15 | 22 | 14 | 51 | 75 | 137 | 55% | ||
| DEC | ↓ | 6 | 6 | 3 | 3 | 18 | |||||
| RR | ↕ | 1 | 4 | 1 | 6 | ||||||
| NC | → | 12 | 26 | 24 | 62 | ||||||
| Raphe | INC | ↑ | 14 | 22 | 22 | 58 | 82 | 238 | 34% | ||
| DEC | ↓ | 5 | 6 | 6 | 17 | ||||||
| RR | ↕ | 2 | 2 | 3 | 7 | ||||||
| NC | → | 26 | 34 | 4 | 92 | 156 | |||||
| VRC | INC | ↑ | 126 | 68 | 4 | 24 | 222 | 321 | 491 | 65% | |
| DEC | ↓ | 31 | 30 | 11 | 72 | ||||||
| RR | ↕ | 13 | 10 | 1 | 3 | 27 | |||||
| NC | → | 57 | 45 | 1 | 67 | 170 | |||||
| No. cells responsive | 241 | 201 | 7 | 1 | 111 | 561 | |||||
| No. cells tested | 353 | 318 | 12 | 2 | 309 | 994 | |||||
| % Responsive | 68% | 63% | 58% | 50% | 36% | 56% | |||||
Of 1,137 recorded neurons, 994 neurons were assessed for changes in firing rate; 561 cells exhibited a change in rate. Changes in firing rate, or lack thereof, are categorized as ↑ INC, increase; ↓ DEC, decrease; ↕ RR, change in depth of respiratory modulation; → NC, no change in firing rate. Biphasic responses are collapsed to their initial response in this table.
Functional connectivity among chemoresponsive neurons.
Cross-correlation analysis of the spike train data set represented in Fig. 1 identified numerous peaks and troughs offset with respect to the origin, features commonly interpreted as signatures of functional excitation and inhibition (see discussion). One pair composed of p-NTS-MM neuron 735 and VRC cell 821 had plateau-like and augmenting inspiratory discharge profiles, respectively (CTHs, Fig. 2A, left). Corresponding autocorrelograms indicated an absence of spike “doublets” or periodicities in the discharge of either neuron (Fig. 2A, right). Their cross-correlogram had dual peak features (Fig. 2B), one on each side of the origin, consistent with reciprocal excitatory actions. The two correlogram peaks, shown in a higher temporal resolution correlogram (Fig. 2C, left), were similar to features from a pair of model integrate-and-fire neurons with reciprocal excitatory interactions (Fig. 2C, right).
Fig. 2.
Spike train correlations for neurons represented in Fig. 1 support distributed functional connectivity among chemoresponsive neurons. A: respiratory cycle-triggered histograms for p-NTS-MM neuron 735 and VRC neuron 821 (overlaid on the phrenic nerve record) and their auto-correlograms. B: cross-correlogram triggered by spikes of neuron 735 for target neuron 821 with peaks on each side of the origin; significant bins (indicated by red blocks) have values exceeding confidence limits (green lines) defined by surrogate spike trains with a p-value threshold adjusted for multiple comparisons and a false discovery rate (FDR) set to 0.05 (see methods). C: left, higher temporal resolution cross-correlogram for pair 735–821; right, schematic of simulated reciprocal excitatory synaptic interactions between model neurons A and B, and a cross-correlogram from spike trains generated by the represented interaction in an integrate-and-fire style model network (methods). Each model neuron was influenced by 30 independent fiber inputs, each with 4 (neuron A, weight 0.004) or 5 (neuron B, weight 0.005) excitatory synaptic terminals (2.0 ms time constant, 115 mV equilibrium potential). The firing probability for each fiber was 0.05 at each simulation time step (0.5 ms). The excitatory connection from A to B had 6 terminals with a time constant of 1.5 ms, synaptic weights of 0.009, and a conduction time range of 2.0 to 2.5 ms. The excitatory connection from B to A had 5 terminals with a time constant of 3.0 ms, synaptic weights of 0.0045, and a conduction time range of 3.0 to 3.5 ms. D: illustration of two 0.5-s windows (i and ii), defined by the time of the first spike from neuron 821 in each respiratory cycle, used to parse spike times in early and later segments of successive inspiratory phases into separate files for cross-correlation analysis. E: positive-lag peak detected in cross-correlograms triggered by spikes in neuron 821 for target 735 during the first (i, top) but not the second (ii, bottom) segments of inspiratory activity. Note that the trigger and target neurons for these cross-correlation histograms (CCHs) have been switched relative to the CCH shown in B. F: cross-correlograms for the indicated pairs, labeled to facilitate their description and corresponding representation in the associated correlation feature map in G. In these and subsequent correlograms, the minimum (min.) and maximum firing rates are indicated. The detectability index (DI) and bin width (BW) for each cross-correlogram are as follows: 1: 8.5, 1.5; 2: 5.2, 1.5; 3: 4.2, 1.5; 4: 4.3, 2.5; 5: 4.5, 1.5; 6: 5.7, 1.5; 7: 6.8, 0.5; 8: 8.0, 5.5; 9: 5.3, 1.5; 10: 3.6, 0.5; 11: 10.8, 5.5; 12: 8.9, 10.5; 13: 16.0, 5.5; 14: 10.7, 2.5; 15: 4.6, 7.5; 16: 5.1, 5.5. Number of spikes for each cell: 406: 48,785; 409: 220,368; 707: 146,367; 716: 95,128; 735: 85,243; 804: 12,388; 812: 182,596; 813: 79,088; 814: 34,711; 816: 17,154; 819: 50,335; 821: 35,940; 848: 15,574; 851: 1,671; 890: 57,739; 931: 32,566; 981: 25,758. G: offset features present in cross-correlograms shown in F are summarized in the correlation feature map. Neurons (large circles) are grouped by brain stem location and labeled by ID code, respiratory modulation (color; see Key), and response to carotid chemoreceptor stimulation. Small white and black circles indicate the presence of an offset peak or trough feature, respectively.
The spike trains from this pair were segmented by phase. The first spike in each inspiratory burst of neuron 821 was used to parse spikes from that neuron and cell 735 into two time series representing concatenated 500-ms blocks of data from early (i) and late (ii) intervals of successive respiratory cycles (Fig. 2D). Corresponding cross-correlograms from each series, generated using spikes from cell 821 as trigger events, had peaks to the left of the origin consistent with neuron 735 driving 821 (Fig. 2E). However, a peak with a positive lag suggestive of a reciprocal excitatory action of 821 was present only in the correlogram from early in the inspiratory phase (Fig. 2E, top). The narrow peak in the averaged phrenic nerve signal triggered by spikes in neuron 821 was consistent with that neuron also having a premotor function (Fig. 2G, a, arrow). These and other similar correlation feature sets documented subsequently provide evidence for recurrent positive feedback loops as amplifiers of chemoreceptor-evoked excitation of caudal VRC inspiratory neurons and support one aspect of the model of Morris et al. (1996a; see discussion).
Additional functional associations identified in this group of neurons (Fig. 2F) are summarized in a correlation feature map (Fig. 2G). Neuron 812 was correlated with both p-NTS-MM neuron 735 (Fig. 2F, 2) and VRC cell 821 (Fig. 2F, 3). The dual peak correlogram feature for the latter pair suggests the involvement of neuron 821 in multiple recurrent excitatory loops. Cell 813 had a similar offset peak with p-NTS-MM target neuron 707 (Fig. 2F, 4), and was also correlated with 821 (Fig. 2F, 5). There was an increase in spike firing probability in IE phase-spanning neuron 816 following spikes in 812 (Fig. 2F, 6), and a decrease in 812 after spikes in inspiratory neuron 814 (Fig. 2F, 7). In addition to offset peaks in correlograms with VRC triggers and p-NTS-MM targets, we identified a transient decline in firing probability in p-NTS-MM t-E neuron 716 after spikes in VRC t-E cell 804 (Fig. 2F, 8). Spikes in p-NTS-MM neuron 735 were also associated with an increased firing probability in FTL-pF neuron 406 (Fig. 2F, 9), which was also a putative target of neuron 409 (Fig. 2F, 10).
Fig. 3.
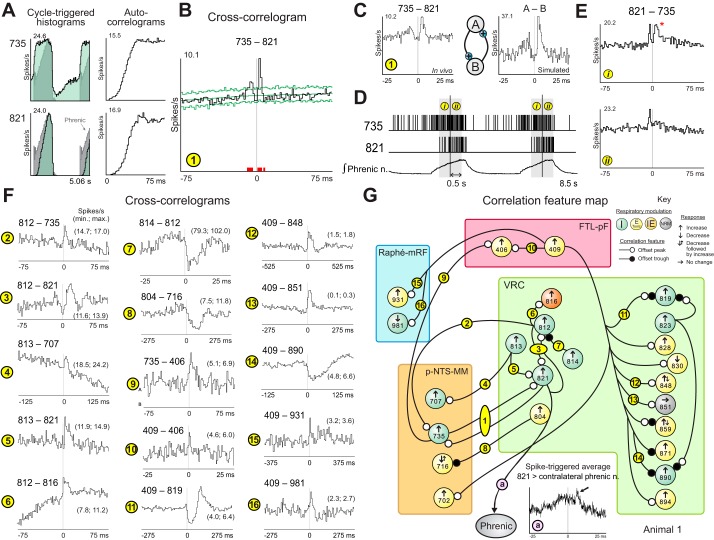
Recording sites and examples of neuron correlations involving VRC inspiratory neurons. A: coordinates of recording sites of all 1,137 recorded neurons mapped in a brain stem atlas (derived from Berman 1968; by permission of the University of Wisconsin Press) and represented as spheres, color-coded to show the regions explored; left: dorsal view; right: sagittal view from which recording sites within the VRC have been omitted to better allow visualization of sites within p-NTS-MM and raphe-mRF. Locations of neurons represented in subsequent panels of this figure and in Fig. 4 (left) are labeled. An., animal; BötC, Bötzinger complex; RTN, retrotrapezoid nucleus. B: respiratory cycle-triggered histograms overlaid on the phrenic nerve record and corresponding auto-correlograms for rostral inspiratory neuron 806, caudal VRC inspiratory neuron 828, and caudal decrementing expiratory (postinspiratory) neuron 830 monitored simultaneously in animal 13. C: firing rate histograms of the neurons during a carotid chemoreceptor stimulus trial together with integrated efferent phrenic nerve activity, a plot of inspiratory phase durations relative to the mean of a comparable number of prior control cycle durations (± 2 SD), and arterial blood pressure. D, 1: cross-correlograms for neuron pair 806–828; high-resolution BW = 0.1 ms; low-resolution BW = 0.5 ms; DI = 19.3. D, 2: correlograms for pair 830–806; DI = 9.3. Numbers of spikes: 806: 440,568; 828: 171,551; 830: 80,128. E: correlation feature map for the trio of neurons shown in D with respiratory modulation and rate changes in response to carotid chemoreceptor stimulation (Key). F: different lags to peak in averages of contralateral phrenic nerve signal triggered by spikes in neurons 806 and 828. ADU, analog-to-digital units G, H: cross-correlogram for pair 824–713 from animal 4 (DI = 5.1; BW = 1.5 ms) and corresponding correlation feature map. I and J: cross-correlogram for pair 416–836 from animal 3 (DI = 5.5; BW = 0.1 ms) and corresponding correlation feature map. Numbers of spikes: 824: 45,742; 713: 79,883; 416: 104,558; 836: 27,753. See text for other details.
Fig. 4.
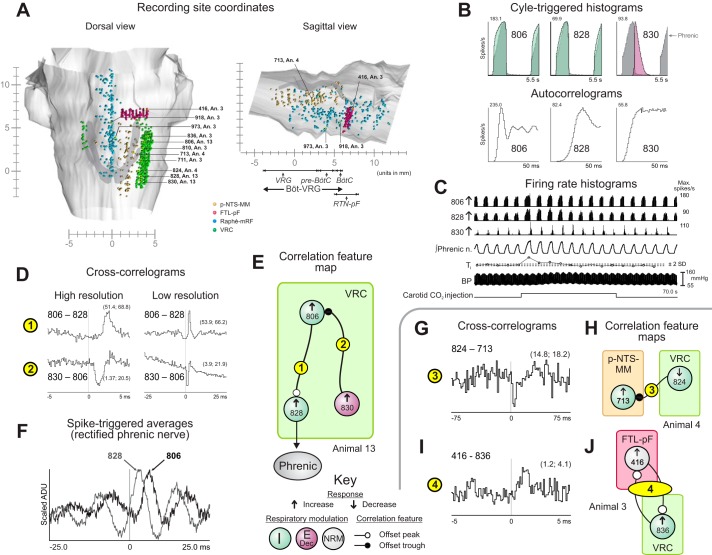
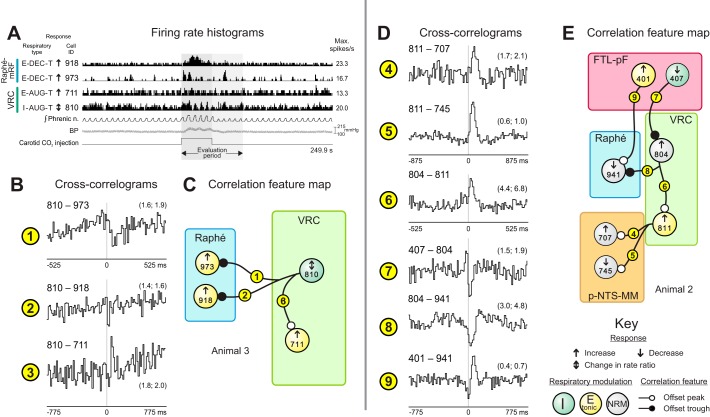
Evidence for distributed functional connectivity of VRC neurons correlationally linked to chemoresponsive raphe neurons. A: firing rate histograms of raphe and VRC neurons during a carotid chemoreceptor stimulation trial in animal 3 together with phrenic nerve, blood pressure, and stimulus marker traces. B1–3, C: cross-correlograms and corresponding feature map for pairs in animal 3. DI and BW (in ms) for each cross-correlogram are as follows: 1: 3.2, 10.5; 2: 3.5, 15.5; 3: 3.8, 15.5. Numbers of spikes: 711: 24,591; 810: 102,201; 918: 22,399; 973: 22,621. D4–9, E: cross-correlograms and corresponding feature map for pairs in animal 2; 4: 3.8, 17.5; 5: 8.4, 17.5; 6: 4.9, 10.5; 7: 3.3, 15.5; 8: 4.5, 15.5; 9: 4.6, 15.5. Numbers of spikes: 401: 49,927; 407: 55,227; 707: 18,629; 745: 1,608; 804: 7,280; 811: 56,238; 941: 19,278. See text for other details.
Fig. 5.
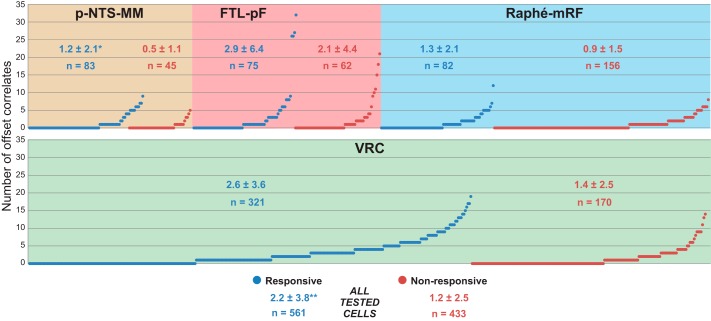
Plot shows number of offset correlates for each of 994 neurons tested for response to carotid CO2 injection, grouped by region and number of correlates. Mean (± SD) of number of offset correlates and number of neurons in each group are shown within each region. *Chemoresponsive cells in the p-NTS-MM (P = 0.00001) and VRC (P = 0.04) had more offset correlates than did nonresponsive cells in each region. **On the whole, chemoresponsive cells had more offset correlates than did nonresponsive neurons (P = 0.0000003).
Fig. 6.
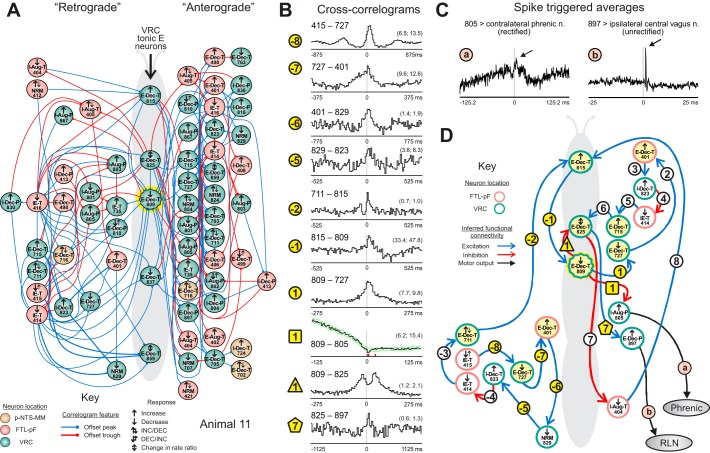
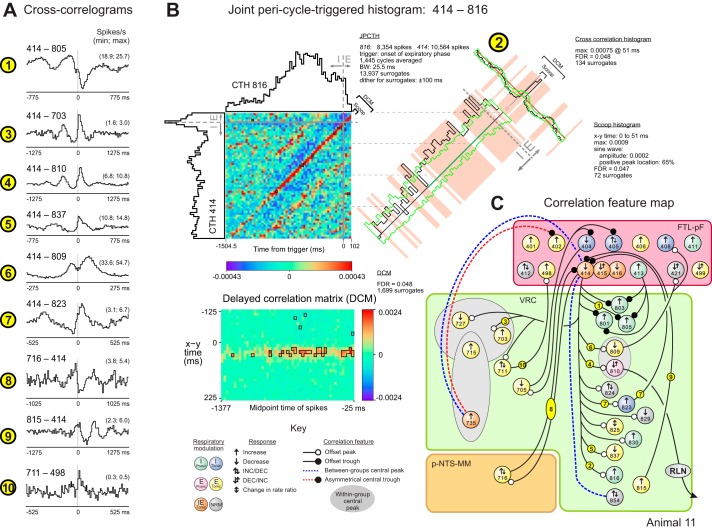
Directed graph from cross-correlogram features supports extended connectivity of VRC tonic expiratory neurons. A: directed graphs automatically generated by database queries represent in compact form the inferred “retrograde” and “anterograde” chains of neurons projecting to and from chemoresponsive t-E neurons (center) in animal 11. These graphs are called “moth plots” because of their wing-shaped appearance. B: cross-correlograms with features supporting the moth plot; −8: 415–727 (DI = 10.6, BW = 17.5); −7: 727–401 (4.6, 7.5); −6: 401–829 (3.3, 15.5); −5: 829–823 (3.7, 10.5); −2: 711–815 (6.2, 10.5); −1: 815–809 (5.5, 10.5); 1: 809–727 (6.5, 5.5); 1square: 809–805 (5.8, 2.5; red blocks indicate bins with values exceeding confidence limits (green lines) defined by surrogate spike trains); 1triangle: 809–825 (10.8, 5.5); 7pentagon: 825–897 (4.1, 22.5). Numbers of spikes: 401: 35,778; 415: 16,751; 711: 61,804; 727: 28,062; 805: 81,975; 809: 135,950; 815: 2,366; 823: 16,621, 825: 5,259; 829: 5,365; 897: 2,740. C: spike triggered averages of efferent activity recorded in phrenic and central vagus nerves. Spike-triggered averaging results were consistent with cell 805 being a phrenic premotor neuron and cell 897 being a recurrent laryngeal nerve (RLN) motor neuron. D: example of one set of chains centered around t-E neuron 809. Links within the chain are indicated by circles and numbered according to their position within the retrograde (negative numbers) or anterograde (positive numbers) chains and are colored yellow if the corresponding CCH is shown in B; additional links of interest are marked with a different symbol.
Fig. 7.
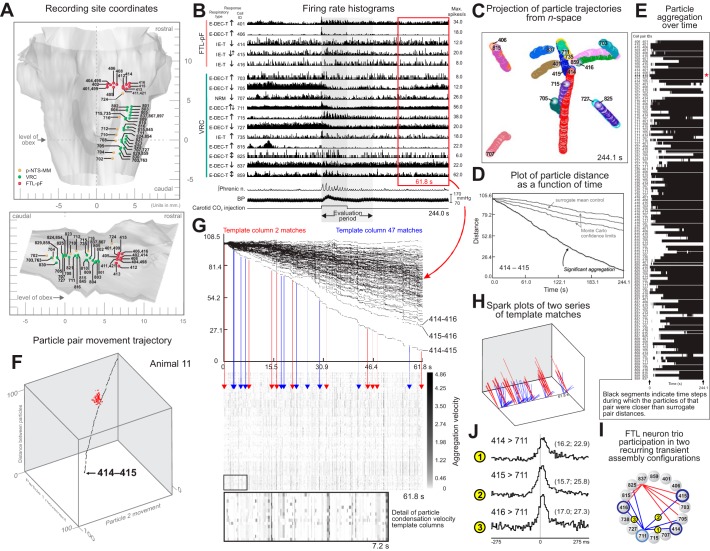
Recurring transient circuit configurations detected with gravitational clustering analysis of parallel spike trains. A: recording sites in animal 11. Images derived from cat brain stem atlas (Berman 1968), by permission of the University of Wisconsin Press. B: firing rate histograms of 16 neurons during a carotid chemoreceptor stimulation trial together with phrenic nerve, blood pressure, and stimulus marker traces. C: projection of gravity particle trajectories from n-space during the interval represented in B; acceptor charges backward, effector charges forward; charge decay time constants were 5.5 ms. D: particle pairwise distance as a function of time (PDFT) plot shows that the distance between particles representing neurons 414 and 415 was less than that of all particle pairs for 1,000 corresponding surrogate spike trains (Pauluis and Baker 2000) with gamma-distributed interspike intervals (Miura et al. 2006). Monte-Carlo significance confidence limits were defined by the minimum and maximum distances between particles for the corresponding surrogates at each plotted time step, yielding a “cone” shown with mean distance of surrogate particle pairs. E: black bands indicate time steps during which particles of the indicated pair were closer than all corresponding surrogate pair distances; red star indicates pair 414–415 from D. F: 3-dimensional trajectory path of a point representing the distance between particles for neurons 414 and 415 and the distance each particle in the pair moved from its original location in the n-space over time, together with a “cloud” of red trajectory end points for 100 of the 1,000 pairs generated from surrogate spike trains. G: top: PDFT plots for all particle pairs during a 61.8-s poststimulus interval (red box in B); same charge decay parameters as in C–E; different particle mobility coefficient. Arrows (red, blue) indicate 2 series of time steps and particle pair condensation velocity columns, each set matching a particular prior template column (bottom; see methods for match criteria); red arrows indicate template column 2 and its matches; columns matching column 47 are identified by blue arrows. H: spark plot representations of 2 significant recurring patterns composed of 6 pairs (red, 9 repetitions) and 7 pairs (blue, 10 repetitions), respectively; FDR = 0.01. I: connected pairs of labeled circles representing the spark patterns show that neurons 414, 415, and 416 were common elements in both circuit configurations; labels indicate corresponding cross-correlograms in J. J: peaks in cross-correlograms triggered by spikes in neurons 414, 415, and 416, each with the same target cell (711). DI and BW for each correlogram: 1: 7.2, 5.5; 2: 5.3, 5.5; 3: 9.6, 5.5. Numbers of spikes: 414: 15,631; 415: 16,751; 416: 5,158; 711: 61,804.
Fig. 8.
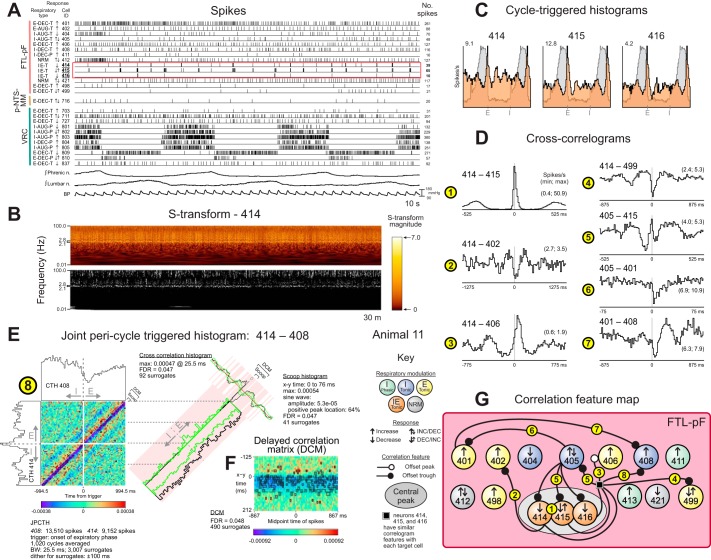
Quasi-periodic fluctuations in firing rate and evidence for functional connectivity in a cluster of parafacial FTL neurons. A: spike times for a subset of recorded neurons together with integrated phrenic and lumbar nerve efferent activities and arterial blood pressure. Neurons had a variety of firing rates and patterns. The red rectangle highlights the coordinated quasi-periodic discharge patterns of neurons 414, 415, and 416. B: top: S-transform time-frequency representation of a 30-min control spike train sample from FTL-pF neuron 414. Bottom: corresponding plot of significant activity defined by points in the S-transform whose magnitude was great enough to cross a P value threshold (0.01), the null hypothesis being that the observed rate in the window at each time frequency point was generated by a Bernoulli process with a probability chosen at random from a uniform distribution conditioned on the observed rate in the window. For this data set, the FDR for each highlighted point in the display was 0.08. C: respiratory cycle-triggered histograms for FTL-pF neurons 414, 415, and 416 overlaid on averaged phrenic nerve activity. D: cross-correlograms for FTL-pF neuron pairs. DI and BW for each correlogram: 1: 48.2, 10.5; 2: 3.2, 25.5; 3: 9.1, 15.5; 4: 8.5, 17.5; 5: 6.1, 10.5; 6: 8.5, 1.5; 7: 5.4, 17.5. Numbers of spikes: 401: 35,778; 402: 10,773; 405: 54,517; 408: 23,316; 414: 15,631; 415: 16,751; 499: 15,528. E and F: joint pericycle-triggered histogram (JPCTH) triggered by the onset of the expiratory phase for FTL-pF neuron pair 414–408 together with corresponding scoop (green baseline indicates cycle half with significantly increased correlation), cross-correlogram, and delayed correlation matrix displays (significant bins in this and the JPCTH are highlighted by black boxes in this and subsequent figures); BW = 25.5 for all plots; see methods and text for details. G: correlation feature map for FTL-pF neurons in animal 11.
Fig. 9.
A: cross-correlograms for neuron pairs in animal 11. DI and BW for correlograms: 1: 4.4, 15.5; 3: 7.8, 25.5; 4: 5.4, 25.5; 5: 4.8, 15.5; 6: 10.6, 5.5; 7: 6.7, 10.5; 8: 3.4, 20.5; 9: 5.9, 25.5; 10: 5.4, 10.5. Numbers of spikes: 414: 15,631; 498: 1,245; 703: 7,140; 711: 1,804; 716: 7,018; 805: 81,975; 809: 135,950; 810: 26,793; 816: 9,402; 823: 16,621; 837: 41,446. B: JPCTH triggered by the onset of the expiratory phase for neuron pair 414–816 with corresponding scoop (green baseline indicates cycle half with significantly increased correlation), cross-correlogram, and delayed correlation matrix; 2: DI = 22.9, BW = 25.5 ms, 414: 10,564 and 816: 8,354 spikes; see text for details. C: correlation feature map with summary of correlational evidence for functional connectivity between FTL-pF neurons and target cells of the p-NTS-MM and VRC.
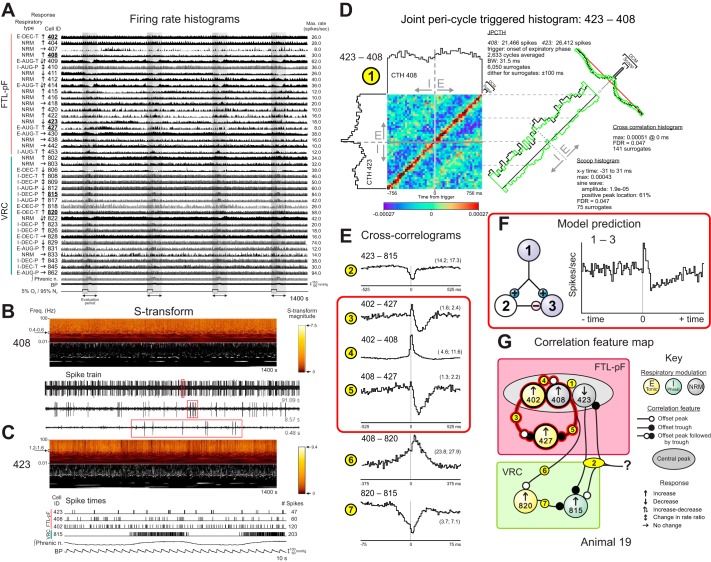
Fig. 10.
Firing rates of FTL and VRC neurons monitored during transient hypoxia and correlational evidence for functional connectivity. A: firing rate histograms of FTL and VRC neurons with integrated phrenic nerve activity and blood pressure during 4 intervals of ventilation with a 5% O2/95% N2 gas mixture. B: top: S-transform of spike train of FTL neuron 408 and (middle) recapitulation of data showing significant magnitudes in some frequency bands as described for Fig. 8. The FDR for each highlighted point in the display was 0.1. Bottom: sample of spike train data showing interspike intervals of neuron 408 at 3 different temporal resolutions (red rectangles). C: top: S-transform of spike train of FTL neuron 423 and (middle) recapitulation of data showing significant magnitudes in some frequency bands. The FDR for each highlighted point in the display was 0.1. Bottom: segment of recording showing times of spike occurrences in 3 FTL neurons and a VRC cell, elements of a correlational assembly documented subsequently, together with integrated phrenic nerve activity and blood pressure; note quasi-periodic bursting of cell 423 and the inspiratory phase bursting of VRC neuron 815. D: JPCTH triggered by the onset of the expiratory phase for FTL neuron pair 423–408 with corresponding scoop (there was no significant difference in the amount of correlation during one half of the cycle vs. the other) and cross-correlogram displays documents spike synchrony during the respiratory cycle; DI = 43.6; BW = 31.5 ms; see text for details. E: cross-correlograms highlighted by red rectangle can be interpreted as excitation of target cell 427 by trigger neuron 402 and a parallel feedforward inhibitory relationship between the 2 neurons via cell 408. DI and BW for each correlogram are as follows: 2: 6.0, 10.5; 3: 9.3, 10.5; 4: 40.1, 10.5; 5: 7.2, 10.5; 6: 5.8, 7.5; 7:17.9, 1.5. Numbers of spikes: 402: 162,947; 408: 71,133; 423: 91,783; 427: 28,459; 815: 243,607; 820: 353,650. F: schematic of a simulated 3-neuron circuit with excitation and parallel delayed feedforward inhibition of a common target and a cross-correlogram from spike trains generated by model neurons 1 and 3. Simulation parameters: Neuron 1 was influenced by 30 independent fiber inputs, each with 4 excitatory synaptic terminals (synaptic weight of 0.004, 2.0 ms time constant, 115 mV equilibrium potential). The firing probability for each fiber was 0.05 at each simulation time step (0.5 ms). The excitatory connection from 1 to 3 had 4 terminals with a time constant of 1.5 ms, an equilibrium potential of 115.0 mV, synaptic weight of 0.015, and a 2.0 ms conduction time. The excitatory connection from 1 to 2 had 4 terminals with a time constant of 1.5 ms, an equilibrium potential of 115.0 mV, synaptic weight of 0.08, and a 5.0 ms conduction time. The inhibitory connection from 2 to 3 had 4 terminals with a time constant of 40.0 ms, an equilibrium potential of −25 mV, synaptic weight of 0.048, and a conduction time range of 8.0 to 9.0 ms. G: correlation feature map for the represented neurons.
FTL-pF neuron 409 had other correlational associations with widely distributed chemoresponsive target cells in the VRC and p-NTS-MM (e.g., Fig. 2F, 11–14) and in the midline raphe region (Fig. 2F, 15–16). Features included offset peaks, troughs, and sequential trough-peak combinations, results that, when considered together with diverse target neuron responses, suggest a variety of paucisynaptic interactions and functions.
Fig. 11.
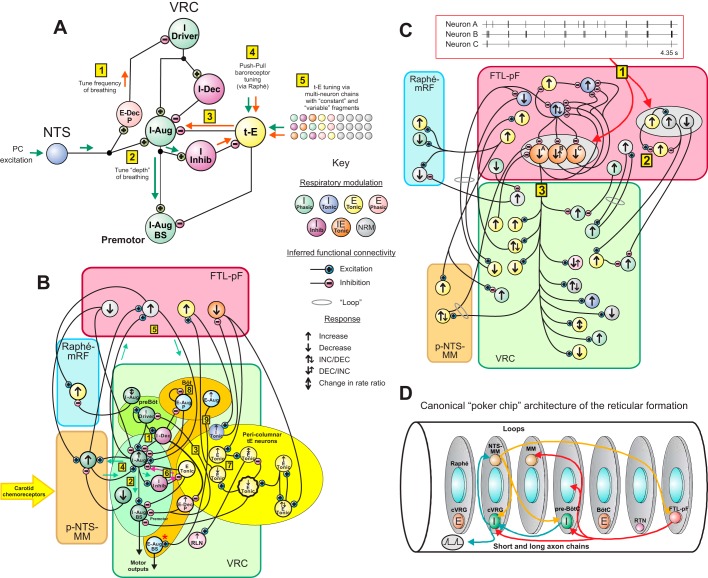
Functional connectivity models and brain stem reticular formation architecture. A: summary of circuit mechanisms for tuning the VRC inspiratory neuron chain to regulate the frequency and depth of breathing, extending the motivating models of Morris et al. (1996a) and Segers et al. (2015). B and C: detailed summaries of inferred chain and loop circuits. D: “poker chip” modular reticular formation circuit architecture derived from concepts proposed by Scheibel and Scheibel (1967) and others; see text.
Table 3 is a summary of correlation features detected among all 33,422 neuron pairs evaluated. Overall, 63% of the neurons that responded to carotid chemoreceptor stimulation were elements of at least one pair with an offset or central correlational signature indicative of an interaction. The coordinates of all recording sites were mapped in a brain stem atlas (Fig. 3A); labels indicate coordinates of some chemoresponsive VRC inspiratory neurons and linked target cells described in the next section.
Table 3.
Correlogram features detected among all 33,422 neuron pairs grouped according to brain stem location and response to carotid chemoreceptor stimulation
| Target Cell Location and Response to Carotid Chemoreceptor Stimulation |
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-NTS-MM |
FTL-pF |
Raphe-mRF |
VRC |
|||||||||||||||||||||||||||||||
| ↑ |
↓ |
↕ |
→ |
↑ |
↓ |
↕ |
→ |
↑ |
↓ |
↕ |
→ |
↑ |
↓ |
↕ |
→ |
|||||||||||||||||||
| Trigger Cell Location and Response | Feature Location | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | |
| p-NTS-MM | ↑ | Offset | * | † | 1 | 2* | 2† | 1 | 1 | 1* | 1 | 1 | † | 2 | 1* | 3 | † | 1 | ||||||||||||||||
| Central | 1 | 1 | 1 | 5 | 1 | 1 | 2 | 12 | 3 | 1 | 1 | 2 | 1 | |||||||||||||||||||||
| Total | 148 | 57 | 60 | 259 | 276 | 69 | 22 | 291 | 183 | 37 | 16 | 355 | 718 | 214 | 70 | 332 | ||||||||||||||||||
| ↓ | Offset | 1 | ‡ | 1§ | 1 | ‡ | § | 1 | ‡ | 1§ | 2 | ‡ | 2§ | 2 | 1 | |||||||||||||||||||
| Central | 2 | 1 | 1 | 2 | 1 | 1 | 1 | |||||||||||||||||||||||||||
| Total | 21 | 18 | 95 | 57 | 32 | 4 | 70 | 52 | 12 | 5 | 142 | 280 | 65 | 36 | 126 | |||||||||||||||||||
| ↕ | Offset | 1 | 1 | |||||||||||||||||||||||||||||||
| Central | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||||||||
| Total | 6 | 76 | 42 | 9 | 4 | 59 | 45 | 8 | 7 | 79 | 127 | 28 | 12 | 39 | ||||||||||||||||||||
| → | Offset | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | |||||||||||||||||||||||
| Central | 1 | 3 | 2 | 5 | 1 | 4 | 3 | 1 | 1 | 3 | 2 | |||||||||||||||||||||||
| Total | 255 | 185 | 56 | 36 | 477 | 172 | 44 | 44 | 643 | 614 | 207 | 92 | 610 | |||||||||||||||||||||
| FTL-pF | ↑ | Offset | 2* | 3 | † | 1 | 3* | 3 | 3 | 3† | 1 | 1 | 2* | 2 | † | 2 | 1 | 17* | 9 | 4 | 3† | 2 | 2 | 2 | ||||||||||
| Central | 6 | 1 | 9 | 10 | 5 | 2 | 1 | 2 | 1 | 4 | 1 | 20 | 14 | 13 | 6 | 7 | 3 | 3 | 1 | |||||||||||||||
| Total | 142 | 81 | 27 | 269 | 165 | 35 | 9 | 362 | 618 | 215 | 64 | 387 | ||||||||||||||||||||||
| ↓ | Offset | 2 | ‡ | § | 2 | 2‡ | 1§ | 1 | ‡ | § | 17 | 7‡ | 14§ | 4 | 4 | 1 | 2 | |||||||||||||||||
| Central | 3 | 2 | 1 | 1 | 18 | 3 | 4 | 1 | 4 | 2 | 2 | |||||||||||||||||||||||
| Total | 17 | 3 | 55 | 38 | 9 | 5 | 84 | 249 | 93 | 41 | 111 | |||||||||||||||||||||||
| ↕ | Offset | 1 | ||||||||||||||||||||||||||||||||
| Central | 1 | 1 | ||||||||||||||||||||||||||||||||
| Total | 2 | 52 | 6 | 3 | 5 | 65 | 45 | 22 | 7 | 70 | ||||||||||||||||||||||||
| → | Offset | 5 | 1 | 3 | 3 | 2 | 2 | 2 | 1 | 6 | 4 | 8 | 1 | 3 | 2 | 12 | 1 | 4 | 2 | 2 | 1 | 1 | 12 | 7 | ||||||||||
| Central | 7 | 1 | 1 | 5 | 3 | 7 | 1 | 2 | 6 | |||||||||||||||||||||||||
| Total | 267 | 185 | 64 | 42 | 681 | 615 | 244 | 96 | 818 | |||||||||||||||||||||||||
| Raphe-mRF | ↑ | Offset | 1* | † | 1 | * | 1† | 1 | 1* | 1 | 1 | 2† | 4 | 3 | 1* | 3 | 2† | 3 | 2 | |||||||||||||||
| Central | 6 | 3 | 18 | 1 | 5 | 1 | 2 | 1 | 6 | 1 | ||||||||||||||||||||||||
| Total | 143 | 82 | 21 | 629 | 815 | 192 | 77 | 703 | ||||||||||||||||||||||||||
| ↓ | Offset | ‡ | § | 1 | ‡ | § | 1 | 1 | ‡ | § | 2 | ‡ | § | 1 | ||||||||||||||||||||
| Central | 4 | 1 | 4 | 1 | 2 | 2 | ||||||||||||||||||||||||||||
| Total | 6 | 7 | 216 | 225 | 64 | 24 | 235 | |||||||||||||||||||||||||||
| ↕ | Offset | |||||||||||||||||||||||||||||||||
| Central | 1 | 1 | 1 | |||||||||||||||||||||||||||||||
| Total | 1 | 82 | 60 | 24 | 12 | 86 | ||||||||||||||||||||||||||||
| → | Offset | 1 | 4 | 1 | 1 | 4 | 1 | 1 | 15 | 10 | 8 | 1 | 5 | 2 | 7 | 3 | ||||||||||||||||||
| Central | 22 | 8 | 14 | 11 | 5 | 19 | 5 | |||||||||||||||||||||||||||
| Total | 1,472 | 2,015 | 599 | 252 | 2,975 | |||||||||||||||||||||||||||||
| VRC | ↑ | Offset | 14* | 6 | 2 | 4† | 2 | 4 | 1 | 6* | 3 | 4 | 9† | 7 | 1 | 3* | 4 | 2 | 3† | 6 | 5 | 74* | 74 | 12 | 19† | 6 | 1 | 19 | 9 | |||||
| Central | 121 | 26 | 44 | 26 | 17 | 7 | 35 | 11 | ||||||||||||||||||||||||||
| Total | 1,524 | 933 | 349 | 2,159 | ||||||||||||||||||||||||||||||
| ↓ | Offset | 3 | 1‡ | § | 1 | 5 | 1‡ | 4§ | 9 | 6 | 4‡ | § | 1 | 2 | 2 | 12 | 17‡ | 4§ | 6 | 1 | 2 | 1 | 3 | |||||||||||
| Central | 9 | 1 | 9 | 3 | 6 | 6 | ||||||||||||||||||||||||||||
| Total | 142 | 114 | 719 | |||||||||||||||||||||||||||||||
| ↕ | Offset | 1 | 1 | 1 | 3 | 6 | 1 | 2 | 1 | 4 | 5 | 1 | 1 | 2 | 3 | 4 | ||||||||||||||||||
| Central | 1 | 4 | 2 | |||||||||||||||||||||||||||||||
| Total | 18 | 319 | ||||||||||||||||||||||||||||||||
| → | Offset | 3 | 3 | 1 | 1 | 2 | 5 | 1 | 2 | 1 | 16 | 4 | 3 | 2 | 1 | 3 | 7 | 3 | 9 | 14 | 5 | 8 | 5 | 1 | 21 | 21 | ||||||||
| Central | 30 | 8 | ||||||||||||||||||||||||||||||||
| Total | 1,662 | |||||||||||||||||||||||||||||||||
Offset (n = 913) and central (n = 768) correlogram features detected among 33,422 neuron pairs grouped according to brain stem location and response to injection of CO2-saturated saline into the carotid artery (arrows; neurons with no response and those not tested for response are grouped together). Trigger (left) and target (top) neurons are organized so that offset features have positive time lags. Numbers of offset and central peaks (P) and troughs (T) are reported; numbers in italics are the total number of pairs composed of neurons with the locations and stimulus responses indicated by the row and column labels. Central features are reported only in the top right portion of the table because the designation of trigger and target neuron is irrelevant for central feature correlations: the central feature will still be present after switching the 2 spike trains. Similarly, total numbers of specific pairings are found only in the top right portion of the table. For example, a total of 718 cell pairs composed of a p-NTS-MM↑ and a VRC↑ neuron were analyzed (see the 2 boldface cell groups). Correlograms calculated for 24 of these pairs contained an offset feature: 4 were suggestive of a p-NTS-MM↑ → VRC↑ influence (1 peak, 3 troughs) and 20 of a VRC↑ → p-NTS-MM↑ effect (14 peaks, 6 troughs). In addition, 15 central features were detected (12 peaks, 3 troughs) for pairs of this type. Symbols indicate the pair types (and numbers of such pairs) whose offset correlogram feature and neuronal responses are simply interpreted as functional actions that promote changes in target cell activity during response to peripheral chemoreceptor stimulation:
excitation (feature, response of trigger and target cell: peak, ↑↑);
inhibition (trough, ↑↓);
disinhibition (trough, ↓↑);
disfacilitation (peak, ↓↓).
Distributed inspiratory neuron connectivity.
A rostral inspiratory neuron (806) was monitored together with caudal VRC inspiratory cell 828 and expiratory (postinspiratory) neuron 830 in animal 13 (Fig. 3B). The peak firing rates of all three neurons increased during carotid chemoreceptor stimulation (Fig. 3C). Cross-correlograms triggered by 806 with target cell 828 had a primary offset peak (Fig. 3D, 1); the lower resolution plot revealed secondary bilateral troughs consistent with the trigger neuron’s autocorrelation, results consistent with an excitatory interaction. When neuron 806 served, in turn, as the target in correlograms triggered by neuron 830, a primary offset trough was detected. The time lag of ~0.5 ms between the trigger neuron spikes and the start of the trough feature was consistent with direct inhibition; the small subsequent peak could reflect a postinhibitory rebound.
This data set supports a second key element of the model of Morris et al. (1996a): this transient decline in firing probability (presumptive inhibition), considered together with the trigger neuron’s phase-timing and increased firing rate, is consistent with chemoreceptor-evoked enhancement of a central “off-switch” function limiting inspiratory phase duration. The feature map represents a serial correlational linkage incorporating the inspiratory neurons and phrenic motor neurons (Fig. 3E), an interpretation supported by the different time lags to peak in the respective spike triggered averages of the contralateral rectified phrenic nerve signal (Fig. 3F).
Other correlograms triggered by chemoresponsive VRC inspiratory neurons had a variety of features indicative of distributed actions with target neurons in the p-NTS-MM and FTL-pF. A VRC inspiratory neuron that responded with a reduced firing rate was correlationally linked via an offset trough feature with a p-NTS-MM inspiratory target neuron with an enhanced firing rate response (Fig. 3G, H). The cross-correlogram for a pair composed of chemoresponsive VRC and FTL-pF neurons had two peaks, asymmetrical in magnitude, each on opposite sides of and temporally offset from the origin (Fig. 3I). Such a feature, as noted previously, is consistent with a recurrent loop mediating reciprocal excitation (Fig. 3J).
Chemoresponsive VRC neurons were also elements of correlational assemblies incorporating linkages with medullary raphe neurons. For example, VRC neuron 810, which exhibited an evoked change in the depth of respiratory rate modulation, was linked with raphe neurons 918 and 973 and VRC t-E cell 711, all of which had increased rate responses (Fig. 4A). Correlation features included two offset troughs suggestive of divergent functional inhibition of the raphe neurons (Fig. 4B, 1–2) together with an offset peak consistent with concurrent excitation of the t-E cell (Fig. 4B, 3 and C).
Tonic expiratory neurons are embedded in extensively linked correlational assemblies.
Prior results supported the hypothesis that carotid chemoreceptors dynamically tune inspiratory drive, in part, via coordinated clusters of inhibitory t-E neurons. However, it is also likely that t-E neurons constitute a heterogeneous class of different subtypes with different functions and a shared attribute of higher rate modulation during the expiratory phase of the respiratory cycle. For example, correlograms triggered by VRC t-E neuron 811 featured offset peaks for p-NTS-MM target neurons 707 and 745, which had opposite rate change responses to chemoreceptor stimulation (Fig. 4D, 4–5 and E), a result consistent with concurrent actions that promoted and limited chemoreceptor-evoked rate changes in the respective targets. Inferred retrograde connectivity of neuron 811 included excitatory drive from NRM neuron 804 (Fig. 4D, 6), which was modulated by the disinhibitory action of FTL-pF cell 407 (Fig. 4D, 7). Neuron 804 also triggered a correlogram with an offset trough with raphe target neuron 941 (Fig. 4D, 8), which had a reduced firing rate in response to carotid chemoreceptor stimulation. A parallel counterbalancing excitatory action on the same target raphe cell was suggested by an offset peak in the correlogram triggered by FTL-pF neuron 401 (Fig. 4D, 9).
Offset peak or trough correlogram features commonly interpreted as signs of functional excitation and inhibition, respectively, were detected in all parallel spike train recordings. Forty-eight percent of all 994 tested neurons, 55% of 561 chemoresponsive cells, and 39% of 433 nonresponsive neurons were correlated with at least one other neuron, which could be any recorded cell, as indicated by an offset correlogram feature. The plots in Fig. 5 show the number of offset correlates, in ascending order, for every tested neuron; the maximum number of offset correlates of a single cell was 32 (FTL-pF neuron 416 in animal 11). On average, the number of offset correlates of chemoresponsive neurons was greater than of nonresponsive cells (2.2 ± 3.8 vs. 1.2 ± 2.5; P = 0.0000003).
As a next step, series of offset correlations were used to generate directed graphs of putative “retrograde” and “anterograde” linkages to and from specific chemoresponsive t-E neurons. Overall, 71 of 110 tested pericolumnar t-E neurons were chemoresponsive; 17 of these 71 cells were putative targets of neuronal circuit “chains” composed of 2 to 15 antecedent correlationally linked elements distributed among all monitored regions. For 26 of 27 responsive VRC t-E pairs with a central feature cross-correlogram, we identified putative shared inputs from 16 neurons located within other sampled domains, predominantly the FTL-pF (n = 14), but also p-NTS-MM and raphe-mRF.
Differences in the numbers of nodes and loops in the respective chains resulted in asymmetric winglike linkage patterns leading to and arising from particular neurons. The automatically generated summary “moth plot” from animal 11 (Fig. 6A) highlights 5 chemoresponsive VRC t-E cells (center) and their associated retrograde and anterograde linkage chains. For example, 564 distinct afferent and 430 distinct efferent chains of 5 to 18 neurons ended or began with cell 809 (see supplemental data; Supplemental Material for this article is available online at the Journal website.). Connectivity inferred from associated correlograms and spike-triggered averages (e.g., Fig. 6, B and C and subsequent figures) included recurrent loops and multiple pathways. Figure 6D focuses on t-E neuron 809 and one set of isolated chains. The retrograde pathway (circled negative numbers) consisted of 8 links beginning with neuron 415; the anterograde chain (positive numbers) “ended” with cell 815, which in turn looped back to 809. Note that a link may be a component of both retro- and anterograde pathways (e.g., 823–414) and a circuit fragment may be part of more than one chain; however, no cell is repeated within a chain, and a chain is counted only if it is not a fragment of another chain.
Coordinated FTL-pF neurons have extended functional connectivity.
Recording sites in animal 11 (Fig. 7A) and firing rate histograms from 16 of the monitored neurons during one peristimulus interval show the diversity of responses to carotid chemoreceptor stimulation, including enhanced or reduced firing rates or sequential combinations of both changes (Fig. 7B). Gravitational clustering analysis of the same spike train data revealed particle trajectories indicative of extensive correlational linkages (Fig. 7C). Because of the loss of information in such projections from n-space to a plane, plots of the distance between each pair of particles as a function of time were also evaluated. Particles representing FTL-pF neurons 414 and 415 aggregated significantly (Fig. 7D), as did all but three of the pairs evaluated under the null hypothesis defined by surrogate spike trains in a Monte Carlo significance test (Fig. 7E).
All particles carried both “acceptor” and “effector” charges so that the gravity calculations were sensitive to firing sequence. Acceptor charge kernels decayed backward and effector charges forward in time. This gravity parameter configuration resulted in a particular particle moving as if it represented a postsynaptic neuron toward a relatively stationary particle representing a presynaptic cell; particles representing neurons with shared inputs would both move toward each other. Three-dimensional plots of trajectories of the interparticle distance and the distance each particle moved from its original location gave another perspective on clustering in the n-space and aided with parsing the types of interactions. The trajectory for pair 414–415, for example, descended toward the floor along a path near the principal diagonal and achieved a final position that was outside the “cloud” of trajectory end points generated from surrogate spike trains (Fig. 7F). Both particles moved about equal distances from their starting positions, a result indicative of synchronous spiking of the two FTL neurons.
Spike data from a 61-s sample of this recording between two stimulus trials (red box in Fig. 7B) were screened with a template-matching method to detect recurring patterns of spike synchrony (Fig. 7G; see methods). Distinct patterns that exceeded the number expected by chance occurred 10 and 11 times, respectively (Fig. 7, G and H). A graph of associations represented by the spark patterns shows that a trio of mutually synchronous FTL neurons (414, 415, and 416) participated in different repeating transient correlational assembly configurations (Fig. 7I). Examples of cross-correlograms from some of the pairs represented are shown in Fig. 7J and subsequently.
Visual inspection of spike times for these FTL-pF neurons revealed synchronous quasi-periodic bursts in each member of this trio (highlighted traces, Fig. 8A). S-transform time-frequency representations of the trio’s spike trains included high-magnitude values in a 2.1- to 2.8-Hz band together with higher frequency components (e.g., Fig. 8B). The trio also had greater firing probabilities at the inspiratory-expiratory phase transition (Fig. 8C). Central peaks were identified in cross-correlograms for each of the three pairs of FTL-pF neurons in the trio (e.g., Fig. 8D, 1). Features in correlograms of other FTL neurons triggered by each member of the trio were indicative of a variety of functional associations (e.g., trigger neuron 414 in Fig. 8D, 2–4). Correlogram features for other neurons in the monitored group included bilateral offset troughs for pair 405–415, suggestive of reciprocal inhibition (Fig. 8D, 5) and a serial inhibitory neuron chain, extending from neuron 405 to 401 to 408 (Fig. 8D, 6–7).
Evidence for convergent inhibition of neuron 408 by other FTL neurons, including 414, 415, 416, and 401, was also detected. The joint pericycle-triggered histogram (JPCTH) documents a reduced firing probability in cell 408 following spikes in 414 (Fig. 8E, 8). The dither surrogate method identified a band of paradiagonal bins with significantly low PCC values relative to the null hypothesis in both the JPCTH and associated delayed correlation matrix (Fig. 8F). Secondary bands with longer positive and negative lags were also found throughout the interval around the inspiratory-expiratory phase transition. Bins exceeding the confidence bands defined by the surrogates were also identified in both the scoop and cross-correlation histograms. Similar features were identified in correlograms triggered by neurons 415 and 416 as indicated in the summary feature map (Fig. 8G).
We detected numerous associations among FTL neurons and VRC targets in the full data set from animal 11. Trigger spikes in neuron 414 were followed by transient declines in the firing probabilities of a rostral cluster of 3 target inspiratory neurons (801, 803, and 805; e.g., Fig. 9A, 1), all of which had increased firing rates during carotid chemoreceptor stimulation. Spikes in FTL neuron 414 were followed by transient increases in the firing probabilities of VRC inspiratory neurons 830 (not shown) and 816 (Fig. 9B, 2). The JPCTH and associated histograms document several aspects of the latter interaction throughout the inspiratory and postinspiratory phases, indicated by the CTHs for neurons 414 and 816 bordering the JPCTH plot (Fig. 9B). Offset peak features were also identified for target cells 703, 810, and 837 (Fig. 9A, 3–5). The correlogram for target neuron 809 had a dual-peak feature (Fig. 9A, 6), while the positive-lag peak and negative-lag trough for target 823 were suggestive of stabilizing interactions of opposite sign between that VRC neuron and the FTL trigger (Fig. 9A, 7), as were both the correlational relationship between neurons 716 and 414 and their opposing firing rate changes in response to carotid chemoreceptor stimulation (Fig. 9A, 8).
Tonic expiratory neuron 815 triggered a multifeature correlogram with FTL neuron 414, with an offset trough predominating (Fig. 9A, 9). Neuron 711 triggered a correlogram with FTL t-E neuron 498 featuring an offset peak (Fig. 9A, 10). These and other previously noted correlogram signatures of interaction are summarized in Fig. 9C. We note that given the coordinated activities of some FTL neurons, their triggered correlations with other target neurons are likely to include contributions of presynaptic synchrony with other observed and unobserved neurons.
Overall, for all recordings in which carotid chemoreceptors were selectively stimulated, we detected FTL-pF neurons with functional interactions extending over 12 mm caudally and with up to 21 targets. Thirty-three FTL-pF neurons (including 19 pairs with central correlogram peaks) had offset correlogram features with 80 distinct chemoresponsive target cells, including 63 outside the FTL-pF. Nineteen of the 33 FTL-pF trigger neurons triggered offset feature correlograms with more than one chemoresponsive target cell, and 39 target neurons had offset features with more than one FTL-pF trigger cell.
Responses to transient hypoxia.
Fifty-six neurons in recordings 18 and 20 were tested for responses to repeated 20–40-s episodes of hypoxia (transient ventilation with 5% O2/95% N2), a physiological perturbation commonly used to evoke carotid chemoreceptor reflex modulation of the respiratory motor pattern. Responses were detected in 22 of 25 VRC cells (16 of the 22 responders were respiratory modulated). Twenty-five of 31 raphe cells responded (19 of the 25 responders were not respiratory modulated). Forty-five neurons were tested for responses to both hypoxia and carotid CO2 injection. Similar responses (or lack thereof) to both challenges were detected in 6 of 20 VRC cells and 4 of 25 raphe neurons.
In an additional recording from animal 19, acquired during a complementary study on central chemoreceptor circuits that motivated the present work (Fig. 5 in Ott et al. 2012), 15 of 21 FTL neurons responded to transient hypoxia; 9 of the 15 were not respiratory modulated. Diverse firing rate modulations were detected during an interval with four hypoxic stimulus trials (Fig. 10A). The S-transform of the spike train from FTL neuron 408 revealed frequencies with significant values, including those in the 0.4–0.6 Hz band (Fig. 10B, top), along with higher frequency doublets and bursts that were visually apparent in the recorded signal (Fig. 10B, bottom). Tegmental field neuron 423, with a decreased firing rate during hypoxia, also exhibited quasi-periodic bursting with a significant band in the frequency range of 1.2–1.6 Hz (Fig. 10C, top), as shown in a record of its spike times along with those of 408, another chemoresponsive FTL neuron (402), and inspiratory VRC cell 815 (Fig. 10C, bottom).
Correlational signatures of association were indicative of both local and distributed interactions among these hypoxia-responsive neurons. The JPCTH for neurons 408 and 423 revealed coordinated synchronous firing in both inspiratory and expiratory phases; both the JPCTH and corresponding cross-correlogram also indicated reduced firing probabilities relative to background rates both preceding and following coordinated spikes (Fig. 10D), features similar to those observed for the synchronous FTL neuron cluster in animal 11.
Other features were consistent with divergent and parallel signaling paths for modulation of VRC inspiratory neuron activity. Neuron 423 discharged asynchronously with VRC cell 815; the asymmetrical correlogram trough was consistent with an inhibitory action of 423. An alternative inference is that the two neurons received unobserved shared influences of opposite sign, a simple model consistent with the opposite rate changes in response to carotid chemoreceptor stimulation (Fig. 10E, 2). The highlighted correlograms from a trio of FTL neurons (Fig. 10E, 3–5) included results for pair 402–427, which featured a peak-trough sequence (Fig. 10E, 3). Prior work (e.g., Mittmann et al. 2005), including results from our simulation library (e.g., Dick et al. 2008; Lindsey et al. 1990), indicated that this feature can be the signature of a microcircuit with an excitatory synaptic action generating the initial peak and an indirect action of an unobserved inhibitory neuron also excited by (or synchronous with) the trigger neuron and acting on the target (Fig. 10F). Correlogram features for pairs 402–408 and 408–427 (Fig. 10E, 4 and 5) were fully consistent with this “indirect inhibition” model (Fig. 10G). Additional correlational linkages (Fig. 10E, 6 and 7) were consistent with a multisynaptic path from 408 to 815 via VRC neuron 820 (Fig. 10G).
DISCUSSION
The present results support our motivating hypothesis and a network model for regulation of the depth and frequency of breathing (Fig. 11A) via differential tuning along the VRC inspiratory neuron chain (Morris et al. 1996a, cf. Fig. 10 therein). The concept of “I-Driver” neurons in a region now labeled the pre-Bötzinger complex, together with downstream VRC inspiratory neuron chains (Schwarzacher et al. 1995; Segers et al. 1987; Smith et al. 1991) and their modulation by inhibition (Pierrefiche et al. 1998), remain core elements of contemporary models of the respiratory network, supported by a variety of physiological, pharmacological, genetic, and optogenetic approaches in different animal models (Alsahafi et al. 2015; Koizumi et al. 2016; Marchenko et al. 2016; Segers et al. 2015; Sherman et al. 2015; Vann et al. 2016).
Responses and correlogram features provide evidence for chemoreceptor-evoked enhancement of decrementing expiratory (postinspiratory) neurons with inhibitory actions on pre-Bötzinger inspiratory neurons, contributing to regulation of inspiratory phase duration (Fig. 11A, 1). Parallel increases in downstream inspiratory neurons enhance drive (Fig. 11A, 2). Spike trains from a computational model circuit with reciprocal excitatory synaptic interactions featured twin peaks on opposite sides of the cross-correlogram origin. Such a correlational signature has been observed previously (Onimaru et al. 1993) and was similar to those for chemoresponsive p-NTS-MM and FTL neurons and their respective VRC inspiratory neuron targets (Fig. 11B, 1–5), supporting the inference that reciprocal excitation between the p-NTS-MM and VRC neurons generate an early I-phase “boost” in response to carotid chemoreceptor stimulation, limited by synaptic depression in the recurrent arm of the loop. Efference copy of inspiratory drive through both upstream and downstream chemoreceptor circuit loops (see also, e.g., Lindsey et al. 1994; Lipski et al. 1994) may also operate during various conditions and disorders and associated therapeutic interventions (e.g., Guyenet 2014; Marcus et al. 2014; McBryde et al. 2013; Narkiewicz et al. 2016; Prabhakar et al. 2015; Toledo et al. 2016).
These gain-modulation mechanisms could operate in parallel with other processes that drive more downstream inspiratory neurons (Li et al. 1999a, 1999b; Lipski et al. 1976; Mifflin 1996; Morris et al. 2001a; Paton et al. 1999). We have previously proposed (Segers et al. 2015) that coordinated clusters of pericolumnar t-E neurons operate as sites of convergence of sensory (e.g., baroreceptor; cf. Lindsey et al. 1998) and motor efference copy influences for tuning inspiratory drive (Fig. 11A, 3–4). The identification of extended “retrograde” and “anterograde” chemoresponsive neuronal chains and loops correlationally linked to t-E neurons (Fig. 11A, 5), including neurons of the parafacial lateral tegmental field (Fig. 11B, 6–7), extends our multipath network model for chemoreceptor modulation of breathing and suggests additional network nodes for peripheral and central chemoreceptor influences (Nuding et al. 2009b; Ott et al. 2011, 2012; Segers et al. 2015; Wilson and Teppema 2016). We note that groups of these inferred chains shared the same “distal” circuit fragments, with distinct sequences of neurons nearer the target neuron. Signaling along groups of such chains could contribute to the generation of different motor patterns for breathing or breathing regulated behaviors such as coughing, sniffing, and vocalization.
Other chemoresponsive circuits.
Perturbations of peripheral chemoreceptors evoked diverse modulations of firing rate in neurons simultaneously monitored at widely distributed brain stem sites, including the p-NTS-MM, VRC, lateral tegmental field, and medullary raphe nuclei. These concurrent responses, considered together with correlational signatures of interactions, associated feature maps, and prior work (Guyenet 2014; Li et al. 1999a, 1999b; Lindsey et al. 1997, 2012; Morris et al. 1996a, 2001b; Nuding et al. 2009a; Segers et al. 2015), also support other circuits for peripheral chemoreceptor modulation of breathing. For example, we confirmed the responsiveness of Bötzinger complex expiratory neurons to selective stimulation of carotid chemoreceptors (Lipski et al. 1984). These inhibitory neurons are influenced by local and reciprocal interactions (Duffin and van Alphen 1995; Jiang and Lipski 1990; Lindsey et al. 1987, 1989a) and have long axonal projections targeting neurons in the ventral respiratory column (Jiang and Lipski 1990), dorsal respiratory group (Lipski and Merrill 1980; Merrill et al. 1983), and spinal cord (Merrill and Fedorko 1984) (Fig. 11B, 8). Their response and disinhibitory actions at end expiration could enhance inspiratory drive via postinhibitory rebound.
Perhaps less well appreciated are the excitatory actions of a small subset of Bötzinger neurons on expiratory neurons of the caudal VRC, a region that provides drive to spinal circuits (Jiang and Lipski 1990; also see Bongianni et al. 1990). When considered with more recent work and models, this route (Fig. 11B, 9) can be viewed as one component of a parallel push-pull excitatory and disinhibitory control circuit for expiratory drive tuning (de Britto and Moraes 2017; Huckstepp et al. 2016; Ott et al. 2012).
FTL neurons have quasi-periodic bursts, recurring patterns of synchrony, and distributed actions.
Five FTL-pF neurons had coordinated quasi-periodic burst patterns and widely distributed correlational interaction characterized by diverse target cell peak and trough firing probability profiles, indicative of functional excitatory and inhibitory interactions, respectively (Fig. 11C, 1). Evidence for dual-sign interactions such as peak-trough sequences (Fig. 11C, 2), predicted from model simulations of feedforward inhibitory circuits operating in parallel with direct excitation, were also detected and supported experimentally by multiple correlations among neurons in the same recording, suggesting a neural substrate for generation of the detected spike timing relationships.
The quasi-periodic bursts and evidence for wide spread actions (Fig. 11C, 3) support a “beacon-like broadcast” mechanism for gain control and tunable filtering of sensory or internally generated signals in the respiratory network. Oscillator circuits control many types of rhythmic activity. The current data set contained neurons that displayed several types of oscillatory behavior, some well known and described, such as cardiac, breathing and Mayer-related oscillations (Morris et al. 2010). CTHs for one group indicated that intraburst spike frequencies were modulated over the course of the respiratory cycle, with greater firing probabilities around the inspiratory-expiratory phase transition. The periodic discharge patterns may also mediate regular transmitter or neuromodulator “dosing” of circuit elements to generate particular respiratory network states, configurations, or loop gains. Amplitude, frequency, and phase fluctuations of oscillatory networks have been implicated in parallel processing of information (e.g., Arata et al. 2000; Palmigiano et al. 2017; Segers et al. 2015; van Aerde et al. 2009).
Consideration of methods and approach.
Cross-correlation and other complementary approaches were used to identify statistical signs of dependencies between spike trains of neurons recorded simultaneously under the same conditions; advantages and limitations of many aspects of the analytical methods have been considered (Grün and Rotter 2010; Nuding et al. 2015; Ott et al. 2012; Segers et al. 2008). The JPCTH incorporated a dithered surrogate for Monte Carlo significance testing, which followed nonstationarities in the data, preserved the autocorrelation of the spike train, and allowed any number of spikes per bin (see methods, Louis et al. 2010b), thereby enhancing the original implementation (Aertsen et al. 1989). The sensitivity of the gravitational clustering approach, its efficacy under conditions of nonstationary activity, and various enhancements (see methods; Gerstein 2010) also facilitated assessment of functional connectivity and its dynamics.
We note several caveats with respect to the experimental approach. 1) Some brain stem regions with neurons known to be modulated by stimulation of carotid chemoreceptors were not monitored in this study, including the retrotrapezoid nucleus (Basting et al. 2016; Takakura et al. 2006), pons (Dutschmann and Dick 2012), and downstream autonomic circuits (Guyenet 2014). 2) Transient systemic hypoxia, which served as a positive control in some experiments, may have direct central actions in addition to activation of the peripheral chemoreceptors (Rajani et al. 2017; Wilson and Teppema 2016). Furthermore, our transient challenges also modulated blood pressure, which could have secondarily influenced neuronal activity patterns through altered baroreceptor feedback. 3) Some experiments were conducted in bilaterally vagotomized animals to remove the influence of pulmonary stretch receptors on the respiratory motor pattern, a procedure commonly used in both in vivo and in vitro studies of brain stem mechanisms that control breathing. Vagotomy alters the respiratory modulated discharge patterns of some brain stem neurons relative to vagal intact animals (Dick et al. 2008; Morris et al. 2010) and may affect a lung-volume dependent modulation of chemoreceptor drive (Takakura et al. 2007).
Network architecture: neuronal chains and loops.
The results support the canonical framework of reticular formation organization proposed some years ago by Scheibel and Scheibel (1961, 1966): a network architecture based on the observation of a “rostro-caudal compression” of reticular neuron dendrites resembling “. . . horizontally oriented stacks of poker chips” (Scheibel and Scheibel 1967). This result inspired the concept of modular brain stem circuits linked together by short axon chains and long axons of large reticular neurons with an “enormously diverse topography on which to exert their effects . . .,” and “. . . a circuit scheme so richly redundant that convincing arguments could be advanced for almost any conceivable loop or chain” (Scheibel and Scheibel 1967). Subsequent computational models have built on this framework (Humphries et al. 2006; Kilmer et al. 1969; MacGregor 1972).
Contemporary models of the brain stem respiratory network have such a modular architecture, which is readily apparent when its organization is evaluated using a variety of experimental approaches (Lindsey et al. 2012). Neurons within each brain region explored in this work are connected by local circuit interactions (Dean et al. 2001; Lindsey et al. 1989b, 1992a, 1992b, 1992c; Ott et al. 2011, 2012; Rekling et al. 2000; Smith et al. 2007). The present results provide experimental support for multipath routing of peripheral chemoreceptor influences in and among these modules via functional circuit loops and chains such as those envisioned by the Scheibels (Fig. 11D). The results also suggest that future experiments aimed at parsing the roles of these circuit chains and loops in the generation of breathing related behaviors (Bolser et al. 2015) and vocalizations (Subramanian et al. 2016) may be fruitful.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant NS19814.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.F.M., S.C.N., L.S.S., M.M.O., T.E.T.-C., D.C.B., and B.G.L. conceived and designed research; K.F.M., S.C.N., L.S.S., K.E.I., J.B.D., M.M.O., P.A.A., K.-K.H., T.E.T.-C., and B.G.L. performed experiments; K.F.M., S.C.N., L.S.S., K.E.I., R.O., M.M.O., P.A.A., D.S., and B.G.L. analyzed data; K.F.M., S.C.N., L.S.S., R.O., M.M.O., and B.G.L. interpreted results of experiments; K.F.M., L.S.S., and B.G.L. drafted manuscript; K.F.M., S.C.N., L.S.S., K.E.I., R.O., J.B.D., M.M.O., P.A.A., D.S., K.-K.H., T.E.T.-C., D.C.B., and B.G.L. edited and revised manuscript; K.F.M., S.C.N., L.S.S., K.E.I., R.O., J.B.D., M.M.O., P.A.A., D.S., K.-K.H., T.E.T.-C., D.C.B., and B.G.L. approved final version of manuscript; S.C.N., L.S.S., and B.G.L. prepared figures.
Supplemental Data
Chains of 5 to 14 neurons culminating in chemoresponsive VRC tonic E cell 809
Chains of 8 to 18 neurons extending from chemoresponsive VRC tonic E cell 809
ACKNOWLEDGMENTS
We thank Margaret Baldwin, Jane Perkins, Rebecca McGowan Fariss, and Kim Ruff Carpintier for excellent surgical skills, and Peter Barnhill, Kathryn Ross, and Andy Ross for technical support.
REFERENCES
- Aertsen AM, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res 340: 341–354, 1985. doi: 10.1016/0006-8993(85)90931-X. [DOI] [PubMed] [Google Scholar]
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J Neurophysiol 61: 900–917, 1989. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Alsahafi Z, Dickson CT, Pagliardini S. Optogenetic excitation of preBötzinger complex neurons potently drives inspiratory activity in vivo. J Physiol 593: 3673–3692, 2015. doi: 10.1113/JP270471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arata A, Hernandez YM, Lindsey BG, Morris KF, Shannon R. Transient configurations of baroresponsive respiratory-related brainstem neuronal assemblies in the cat. J Physiol 525: 509–530, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita H, Kogo N, Ichikawa K. Locations of medullary neurons with non-phasic discharges excited by stimulation of central and/or peripheral chemoreceptors and by activation of nociceptors in cat. Brain Res 442: 1–10, 1988. doi: 10.1016/0006-8993(88)91426-6. [DOI] [PubMed] [Google Scholar]
- Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulations of a ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biol Cybern 70: 311–327, 1994. doi: 10.1007/BF00200329. [DOI] [PubMed] [Google Scholar]
- Barnett WH, Abdala AP, Paton JFR, Rybak IA, Zoccal DB, Molkov YI. Chemoreception and neuroplasticity in respiratory circuits. Exp Neurol 287: 153–164, 2017. doi: 10.1016/j.expneurol.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basting TM, Abe C, Viar KE, Stornetta RL, Guyenet PG. Is plasticity within the retrotrapezoid nucleus responsible for the recovery of the PCO2 set-point after carotid body denervation in rats? J Physiol 594: 3371–3390, 2016. doi: 10.1113/JP272046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. [Google Scholar]
- Berman AL. The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison, WI: University of Wisconsin Press, 1968. [Google Scholar]
- Bolser DC, Pitts TE, Davenport PW, Morris KF. Role of the dorsal medulla in the neurogenesis of airway protection. Pulm Pharmacol Ther 35: 105–110, 2015. doi: 10.1016/j.pupt.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongianni F, Corda M, Fontana G, Pantaleo T. Expiration-related neurons in the caudal ventral respiratory group of the cat: influences of the activation of Bötzinger complex neurons. Brain Res 526: 299–302, 1990. doi: 10.1016/0006-8993(90)91235-9. [DOI] [PubMed] [Google Scholar]
- Claps A, Torrealba F. The carotid body connections: a WGA-HRP study in the cat. Brain Res 455: 123–133, 1988. doi: 10.1016/0006-8993(88)90121-7. [DOI] [PubMed] [Google Scholar]
- Cohen MI. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol 31: 142–165, 1968. doi: 10.1152/jn.1968.31.2.142. [DOI] [PubMed] [Google Scholar]
- Davies RO, Edwards MW Jr. Distribution of carotid body chemoreceptor afferents in the medulla of the cat. Brain Res 64: 451–454, 1973. doi: 10.1016/0006-8993(73)90204-7. [DOI] [PubMed] [Google Scholar]
- Davies RO, Edwards MW Jr. Medullary relay neurons in the carotid-body chemoreceptor pathway of cats. Respir Physiol 24: 69–79, 1975. doi: 10.1016/0034-5687(75)90122-X. [DOI] [PubMed] [Google Scholar]
- de Britto AA, Moraes DJA. Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats. J Physiol 595: 2043–2064, 2017. doi: 10.1113/JP273335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Burgh Daly M. Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration. New York: Oxford University Press, 1997. [Google Scholar]
- De Burgh Daly M, Scott MJ. An analysis of the primary cardiovascular reflex effects of stimulation of the carotid body chemoreceptors in the dog. J Physiol 162: 555–573, 1962. doi: 10.1113/jphysiol.1962.sp006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JB, Kinkade EA, Putnam RW. Cell-cell coupling in CO2/H+-excited neurons in brainstem slices. Respir Physiol 129: 83–100, 2001. doi: 10.1016/S0034-5687(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol 586: 4265–4282, 2008. doi: 10.1113/jphysiol.2008.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol 347: 397–409, 1984. doi: 10.1113/jphysiol.1984.sp015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J, van Alphen J. Cross-correlation of augmenting expiratory neurons of the Bötzinger complex in the cat. Exp Brain Res 103: 251–255, 1995. doi: 10.1007/BF00231711. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol 2: 2443–2469, 2012. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116, 1992. doi: 10.1016/0006-8993(92)90458-L. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Mitchell GS. Respiratory neuroplasticity – Overview, significance and future directions. Exp Neurol 287: 144–152, 2016. doi: 10.1016/j.expneurol.2016.1005.1022. [DOI] [PubMed] [Google Scholar]
- Gansner ER, North SC. An open graph visualization system and its applications to software engineering. Softw Pract Exper 30: 1203–1233, 2000. doi:. [DOI] [Google Scholar]
- Gerstein GL. Correlation based analysis methods for neural ensemble data. In: Methods for Neural Ensemble Recordings, edited by Nicolelis MAL. Boca Raton: CRC, 1999, p. 157–178. [Google Scholar]
- Gerstein GL. Gravitational clustering. In: Analysis of Parallel Spike Trains, edited by Grün S, Rotter S. New York: Springer, 2010, p. 157–172. doi: 10.1007/978-1-4419-5675-0_8. [DOI] [Google Scholar]
- Grün S, Rotter S, editors. Analysis of Parallel Spike Trains. New York: Springer, 2010. doi: 10.1007/978-1-4419-5675-0. [DOI] [Google Scholar]
- Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Watanabe S, Hasegawa S, Myojo S, Takizawa H, Sugita T, Kimura K, Hasegawa T, Kuriyama T, Saito Y. Respiration in man after chronic glomectomy. In: Morphology and Mechanisms of Chemoreceptors: Proceedings of an International Satellite Symposium Held in Srinagar, Kashmir, India, October 9–12, 1974, edited by Paintal AS. Delhi, India: Vallabhbhai Patel Chest Institute, 1976, p. 147–155. [Google Scholar]
- Huckstepp RTR, Henderson LE, Cardoza KP, Feldman JL. Interactions between respiratory oscillators in adult rats. eLife 5: e14203, 2016. doi: 10.7554/eLife.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Gurney K, Prescott TJ. The brainstem reticular formation is a small-world, not scale-free, network. Proc Biol Sci 273: 503–511, 2006. doi: 10.1098/rspb.2005.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res 81: 639–648, 1990. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Kilmer WL, McCulloch WS, Blum J. A model of the vertebrate central command system. Int J Man Mach Stud 1: 279–309, 1969. doi: 10.1016/S0020-7373(69)80025-8. [DOI] [Google Scholar]
- Kirsten EB, St. John WM. A feline decerebration technique with low mortality and long-term homeostasis. J Pharmacol Methods 1: 263–268, 1978. doi: 10.1016/0160-5402(78)90057-8. [DOI] [Google Scholar]
- Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010. doi: 10.1016/j.neuroscience.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Mosher B, Tariq MF, Zhang R, Koshiya N, Smith JC. Voltage-dependent rhythmogenic property of respiratory Pre-Bötzinger complex glutamatergic, Dbx1-derived, and somatostatin-expressing neuron populations revealed by graded optogenetic inhibition. eNeuro 3: 3, 2016. doi: 10.1523/ENEURO.0081-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Regul Integr Comp Physiol 270: R1273–R1278, 1996. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Multimodal medullary neurons and correlational linkages of the respiratory network. J Neurophysiol 82: 188–201, 1999a. doi: 10.1152/jn.1999.82.1.188. [DOI] [PubMed] [Google Scholar]
- Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Responses of simultaneously recorded respiratory-related medullary neurons to stimulation of multiple sensory modalities. J Neurophysiol 82: 176–187, 1999b. doi: 10.1152/jn.1999.82.1.176. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Arata A, Morris KF, Hernandez YM, Shannon R. Medullary raphe neurones and baroreceptor modulation of the respiratory motor pattern in the cat. J Physiol 512: 863–882, 1998. doi: 10.1111/j.1469-7793.1998.863bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BG, Gerstein GL. Two enhancements of the gravity algorithm for multiple spike train analysis. J Neurosci Methods 150: 116–127, 2006. doi: 10.1016/j.jneumeth.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Hernandez YM, Morris KF, Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J Neurophysiol 67: 890–904, 1992a. doi: 10.1152/jn.1992.67.4.890. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol 67: 923–930, 1992b. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Respiratory-related neural assemblies in the brain stem midline. J Neurophysiol 67: 905–922, 1992c. doi: 10.1152/jn.1992.67.4.905. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Morris KF, Shannon R. Multineuron recordings and the analysis of state dependent network configurations in respiratory neuron assemblies. International Joint Conference on Neural Networks 2: 657–661, 1990. doi: 10.1109/IJCNN.1990.137775. [DOI] [Google Scholar]
- Lindsey BG, Morris KF, Shannon R, Gerstein GL. Repeated patterns of distributed synchrony in neuronal assemblies. J Neurophysiol 78: 1714–1719, 1997. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Nuding SC, Segers LS, Iceman KE, O’Connor R, Dean JB, Taylor-Clark T, Alencar P, Shuman D, Horton K, Bolser DC, Morris KF. Carotid chemoreceptors tune breathing via a multipath brain stem network modulated by coordinated groups of tegmental field-parafacial neurons. Joint Meeting of the American Physiological Society and The Physiological Society. Dublin, Ireland, 2016, Abstract PCA276. [Google Scholar]
- Lindsey BG, Rybak IA, Smith JC. Computational models and emergent properties of respiratory neural networks. Compr Physiol 2: 1619–1670, 2012. doi: 10.1002/cphy.c110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Morris KF, Hernandez YM, Saporta S, Shannon R. Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis. J Neurophysiol 72: 1830–1851, 1994. doi: 10.1152/jn.1994.72.4.1830. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol 57: 1101–1117, 1987. doi: 10.1152/jn.1987.57.4.1101. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Shannon R. Discharge patterns of rostrolateral medullary expiratory neurons in the cat: regulation by concurrent network processes. J Neurophysiol 61: 1185–1196, 1989a. doi: 10.1152/jn.1989.61.6.1185. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Shannon R, Gerstein GL. Gravitational representation of simultaneously recorded brainstem respiratory neuron spike trains. Brain Res 483: 373–378, 1989b. doi: 10.1016/0006-8993(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Lipski J, McAllen RM, Spyer KM. The carotid chemoreceptor input to the respiratory neurones of the nucleus of tractus solitarius. J Physiol 269: 797–810, 1977. doi: 10.1113/jphysiol.1977.sp011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, McAllen RM, Trzebski A. Carotid baroreceptor and chemoreceptor inputs onto single medullary neurones. Brain Res 107: 132–136, 1976. doi: 10.1016/0006-8993(76)90101-3. [DOI] [PubMed] [Google Scholar]
- Lipski J, Merrill EG. Electrophysiological demonstration of the projection from expiratory neurones in rostral medulla to contralateral dorsal respiratory group. Brain Res 197: 521–524, 1980. doi: 10.1016/0006-8993(80)91140-3. [DOI] [PubMed] [Google Scholar]
- Lipski J, Trzebski A, Chodobska J, Kruk P. Effects of carotid chemoreceptor excitation on medullary expiratory neurons in cats. Respir Physiol 57: 279–291, 1984. doi: 10.1016/0034-5687(84)90077-X. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640: 171–184, 1994. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Louis S, Borgelt C, Grün S. Generation and selection of surrogate methods for correlation analysis. In: Analysis of Parallel Spike Trains, edited by Grün S, Rotter S. New York: Springer, 2010a, p. 359–382. doi: 10.1007/978-1-4419-5675-0_17. [DOI] [Google Scholar]
- Louis S, Gerstein GL, Grün S, Diesmann M. Surrogate spike train generation through dithering in operational time. Front Comput Neurosci 4: 127, 2010b. doi: 10.3389/fncom.2010.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AT, Fraigne JJ, Dunin-Barkowski WL, Vidruk EH, Orem JM. Tonic and phasic drive to medullary respiratory neurons during periodic breathing. Respir Physiol Neurobiol 181: 286–301, 2012. doi: 10.1016/j.resp.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med 285: 1105–1111, 1971. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- MacGregor RJ. A model for reticular-like networks: ladder nets, recruitment fuses, and sustained responses. Brain Res 41: 345–363, 1972. doi: 10.1016/0006-8993(72)90507-0. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Koizumi H, Mosher B, Koshiya N, Tariq MF, Bezdudnaya TG, Zhang R, Molkov YI, Rybak IA, Smith JC. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibition within Pre-Bötzinger and Bötzinger complexes. eNeuro 3: 3, 2016. doi: 10.1523/ENEURO.0011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus NJ, Del Rio R, Schultz EP, Xia X-H, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592: 391–408, 2014. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJA, Sobotka PA, Paton JFR. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun 4: 2395, 2013. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- Melssen WJ, Epping WJ. Detection and estimation of neural connectivity based on crosscorrelation analysis. Biol Cybern 57: 403–414, 1987. doi: 10.1007/BF00354985. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Bötzinger neurons. J Neurosci 4: 2350–2353, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Lipski J, Kubin L, Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res 263: 43–50, 1983. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol 271: R870–R880, 1996. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Mittmann W, Koch U, Häusser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol 563: 369–378, 2005. doi: 10.1113/jphysiol.2004.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Okada M, Amari S. Estimating spiking irregularities under changing environments. Neural Comput 18: 2359–2386, 2006. doi: 10.1162/neco.2006.18.10.2359. [DOI] [PubMed] [Google Scholar]
- Miura M, Reis DJ. The role of the solitary and paramedian reticular nuclei in mediating cardiovascular reflex responses from carotid baro- and chemoreceptors. J Physiol 223: 525–548, 1972. doi: 10.1113/jphysiol.1972.sp009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Arata A, Shannon R, Lindsey BG. Inspiratory drive and phase duration during carotid chemoreceptor stimulation in the cat: medullary neurone correlations. J Physiol 491: 241–259, 1996a. doi: 10.1113/jphysiol.1996.sp021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Arata A, Shannon R, Lindsey BG. Long-term facilitation of phrenic nerve activity in cats: responses and short time scale correlations of medullary neurones. J Physiol 490: 463–480, 1996b. doi: 10.1113/jphysiol.1996.sp021158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Baekey DM, Nuding SC, Segers LS, Dick TE, Shannon R, Lindsey BG. Integration of cardiorespiratory responses to carotid chemoreceptor stimulation by medullary and pontine neural networks in cats [Abstract] Soc Neurosci Abstr 27: 172.8, 2001a. [Google Scholar]
- Morris KF, Nuding SC, Segers LS, Baekey DM, Shannon R, Lindsey BG, Dick TE. Respiratory and Mayer wave-related discharge patterns of raphé and pontine neurons change with vagotomy. J Appl Physiol (1985) 109: 189–202, 2010. doi: 10.1152/japplphysiol.01324.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol 532: 483–497, 2001b. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Ratcliffe LEK, Hart EC, Briant LJB, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK, Paton JFR. Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl Sci 1: 313–324, 2016. doi: 10.1016/j.jacbts.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266: 1–10, 2015. doi: 10.1016/j.expneurol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netick A, Orem J. Erroneous classification of neuronal activity by the respiratory modulation index. Neurosci Lett 21: 301–306, 1981. doi: 10.1016/0304-3940(81)90221-4. [DOI] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Baekey DM, Dick TE, Solomon IC, Shannon R, Morris KF, Lindsey BG. Pontine-ventral respiratory column interactions through raphe circuits detected using multi-array spike train recordings. J Neurophysiol 101: 2943–2960, 2009a. doi: 10.1152/jn.91305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Iceman KE, O’Connor R, Dean JB, Bolser DC, Baekey DM, Dick TE, Shannon R, Morris KF, Lindsey BG. Functional connectivity in raphé-pontomedullary circuits supports active suppression of breathing during hypocapnic apnea. J Neurophysiol 114: 2162–2186, 2015. doi: 10.1152/jn.00608.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Shannon R, O’Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphé-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci 364: 2501–2516, 2009b. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RE, Barnhill PR, Nuding SC, Morris KF, Lindsey BG. Open source spike sorting software for large multi-electrode systems [Abstract] Society for Neuroscience. Washington, DC, 2005. Program No. 689.3. [Google Scholar]
- Onimaru H, Kashiwagi M, Arata A, Homma I. Possible mutual excitatory couplings between inspiratory neurons in caudal ventrolateral medulla of brainstem-spinal cord preparation isolated from newborn rat. Neurosci Lett 150: 203–206, 1993. doi: 10.1016/0304-3940(93)90536-T. [DOI] [PubMed] [Google Scholar]
- Orem J, Netick A. Characteristics of midbrain respiratory neurons in sleep and wakefulness in the cat. Brain Res 244: 231–241, 1982. doi: 10.1016/0006-8993(82)90082-8. [DOI] [PubMed] [Google Scholar]
- Orer HS, Gebber GL, Barman SM. Medullary lateral tegmental field neurons influence the timing and pattern of phrenic nerve activity in cats. J Appl Physiol (1985) 101: 521–530, 2006. doi: 10.1152/japplphysiol.00059.2006. [DOI] [PubMed] [Google Scholar]
- Ott MM, Nuding SC, Segers LS, Lindsey BG, Morris KF. Ventrolateral medullary functional connectivity and the respiratory and central chemoreceptor-evoked modulation of retrotrapezoid-parafacial neurons. J Neurophysiol 105: 2960–2975, 2011. doi: 10.1152/jn.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott MM, Nuding SC, Segers LS, O’Connor R, Morris KF, Lindsey BG. Central chemoreceptor modulation of breathing via multipath tuning in medullary ventrolateral respiratory column circuits. J Neurophysiol 107: 603–617, 2012. doi: 10.1152/jn.00808.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal A, editor. Morphology and Mechanisms of Chemoreceptors: Proceedings of an International Satellite Symposium Held in Srinagar, Kashmir, India, October 9–12, 1974. Delhi, India: Vallabhbhai Patel Chest Institute, 1976. [Google Scholar]
- Palmigiano A, Geisel T, Wolf F, Battaglia D. Flexible information routing by transient synchrony. Nat Neurosci 20: 1014–1022, 2017. doi: 10.1038/nn.4569. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Appeasing the carotid body after chronic intermittent hypoxia. Hypertension 68: 315–317, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07377. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Deuchars J, Li YW, Kasparov S. Properties of solitary tract neurones responding to peripheral arterial chemoreceptors. Neuroscience 105: 231–248, 2001. doi: 10.1016/S0306-4522(01)00106-3. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Li YW, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience 93: 143–154, 1999. doi: 10.1016/S0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Pauluis Q, Baker SN. An accurate measure of the instantaneous discharge probability, with application to unitary joint-even analysis. Neural Comput 12: 647–669, 2000. doi: 10.1162/089976600300015736. [DOI] [PubMed] [Google Scholar]
- Pearson K. Mathematical contributions to the theory of evolution. III. Regression, heredity, and panmixia. Proc R Soc Lond 187: 253–318, 1896. [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol 509: 245–254, 1998. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov AV, Schieber MH. Multiple fragment statistical analysis of post-spike effects in spike-triggered averages of rectified EMG. J Neurosci Methods 79: 143–150, 1998. doi: 10.1016/S0165-0270(97)00172-6. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng Y-J, Kumar GK, Nanduri J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol 5: 561–577, 2015. doi: 10.1002/cphy.c140039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV, Funk GD. Release of ATP by pre-Bötzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca2+-dependent P2Y1 receptor mechanism. J Physiol 2017. Jul 5 [Epub ahead of print]. doi: 10.1113/JP274727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger complex. J Neurosci 20: RC113 (1–5), 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. On circuit patterns of the brain stem reticular core. Ann N Y Acad Sci 89: 857–865, 1961. doi: 10.1111/j.1749-6632.1961.tb20182.x. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. The organization of the nucleus reticularis thalami: a Golgi study. Brain Res 1: 43–62, 1966. doi: 10.1016/0006-8993(66)90104-1. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Anatomical basis of attention mechanisms in vertebrate brains. In: The Neurosciences, edited by Quarton CC, Melnechuk T, Schmitt FO. New York: Rockefeller Univ. Press, 1967, p. 577–601. [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1749–1769, 2008. doi: 10.1152/jn.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers LS, Nuding SC, Ott MM, Dean JB, Bolser DC, O’Connor R, Morris KF, Lindsey BG. Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network. J Neurophysiol 113: 352–368, 2015. doi: 10.1152/jn.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol 57: 1078–1100, 1987. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci 18: 408–414, 2015. doi: 10.1038/nn.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala APL, Koizumi H, Rybak IA, Paton JFR. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kölliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience 175: 145–153, 2011. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: the S transform. IEEE Trans Signal Process 44: 998–1001, 1996. doi: 10.1109/78.492555. [DOI] [Google Scholar]
- Subramanian HH, Arun M, Silburn PA, Holstege G. Motor organization of positive and negative emotional vocalization in the cat midbrain periaqueductal gray. J Comp Neurol 524: 1540–1557, 2016. doi: 10.1002/cne.23869. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J Neurophysiol 98: 374–381, 2007. doi: 10.1152/jn.00322.2007. [DOI] [PubMed] [Google Scholar]
- Toledo C, Andrade DC, Lucero C, Schultz HD, Marcus N, Retamal M, Madrid C, Del Rio R. Contribution of peripheral and central chemoreceptors to sympatho-excitation in heart failure. J Physiol 595: 43–51, 2016. doi: 10.1113/jp272075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aerde KI, Mann EO, Canto CB, Heistek TS, Linkenkaer-Hansen K, Mulder AB, van der Roest M, Paulsen O, Brussaard AB, Mansvelder HD. Flexible spike timing of layer 5 neurons during dynamic beta oscillation shifts in rat prefrontal cortex. J Physiol 587: 5177–5196, 2009. doi: 10.1113/jphysiol.2009.178384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann NC, Pham FD, Hayes JA, Kottick A, Del Negro CA. Transient suppression of Dbx1 PreBötzinger interneurons disrupts breathing in adult mice. PLoS One 11: e0162418, 2016. doi: 10.1371/journal.pone.0162418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JG, Larson CPJ Jr, Hickey RF, Ehrenfeld WK, Severinghaus JW. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med 282: 823–829, 1970. doi: 10.1056/NEJM197004092821501. [DOI] [PubMed] [Google Scholar]
- Wilson RJA, Teppema LJ. Integration of central and peripheral respiratory chemoreflexes. Compr Physiol 6: 1005–1041, 2016. doi: 10.1002/cphy.c140040. [DOI] [PubMed] [Google Scholar]
- Wu J, Capelli P, Bouvier J, Goulding M, Arber S, Fortin G. A V0 core neuronal circuit for inspiration. Nat Commun 8: 544, 2017. doi: 10.1038/s41467-017-00589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Furuya WI, Bassi M, Colombari DSA, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol 5: 238, 2014. doi: 10.3389/fphys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chains of 5 to 14 neurons culminating in chemoresponsive VRC tonic E cell 809
Chains of 8 to 18 neurons extending from chemoresponsive VRC tonic E cell 809