Abstract
Hippocampal mossy fibers, which are the axons of dentate granule cells, form powerful excitatory synapses onto the proximal dendrites of CA3 pyramidal cells. It has long been known that high-affinity binding sites for kainate, a glutamate receptor agonist, are present on mossy fibers. Here we summarize recent experiments on the role of these presynaptic kainate receptors (KARs). Application of kainate has a direct effect on the amplitude of the extracellularly recorded fiber volley, with an enhancement by low concentrations and a depression by high concentrations. These effects are mediated by KARs, because they persist in the presence of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-selective antagonist GYKI 53655, but are blocked by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/KAR antagonist 6-cyano-7-nitroquinoxaline-2,3-dione and the KAR antagonist SYM2081. The effects on the fiber volley are most likely caused by a depolarization of the fibers via the known ionotropic actions of KARs, because application of potassium mimics the effects. In addition to these effects on fiber excitability, low concentrations of kainate enhance transmitter release, whereas high concentrations depress transmitter release. Importantly, the synaptic release of glutamate from mossy fibers also activates these presynaptic KARs, causing an enhancement of the fiber volley and a facilitation of release that lasts for many seconds. This positive feedback contributes to the dramatic frequency facilitation that is characteristic of mossy fiber synapses. It will be interesting to determine how widespread facilitatory presynaptic KARs are at other synapses in the central nervous system.
With the notable exception of γ-aminobutyric acid type A (GABAA) receptors and spinal presynaptic inhibition (1, 2), ionotropic neurotransmitter receptors are generally believed to be located postsynaptically. Although virtually all synaptic terminals in the central nervous system express neurotransmitter receptors, these are of the metabotropic type (3, 4). However, recent evidence suggests that presynaptic ionotropic receptors may be more widespread than previously thought (5). In particular, a number of papers indicate that activation of the kainate subtype of glutamate receptor can depress the release of glutamate (6, 7) and GABA (8–10). Although the exact location of these kainate receptors (KARs) and the mechanism by which they inhibit release is somewhat controversial, evidence for the existence of presynaptic KARs has been available for some time. Here we review studies on the role of presynaptic KARs, focusing on hippocampal mossy fiber synapses where these receptors have been most thoroughly studied.
Early Studies on the Localization of KARs
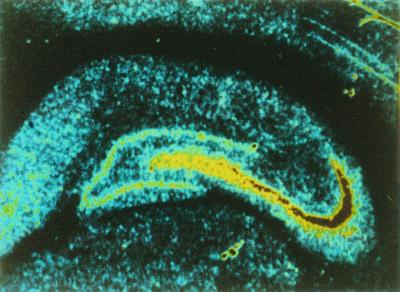
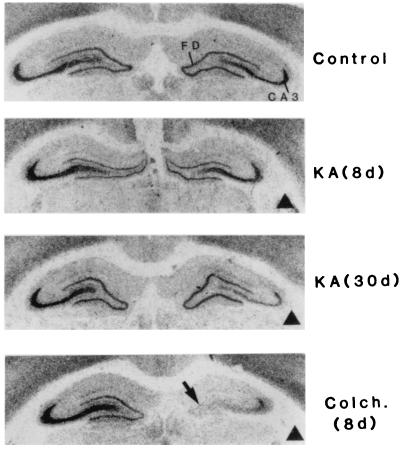
In a curious historical twist that foreshadowed developments to come, one of the most important early studies suggesting the existence of KARs distinct from α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) also demonstrated their axonal localization (11). It was shown that application of the AMPAR/KAR agonist kainate to isolated spinal dorsal root fibers selectively depressed the C-fiber component of the compound action potential and this was interpreted as a result of a depolarization of the C fibers. Importantly, AMPA had no effect in these experiments, clearly establishing that the action of kainate was independent of AMPARs. Autoradiographic studies also suggested the existence of a distinct class of KARs and their presence on axons. Monaghan and Cotman (12) demonstrated the presence of high-affinity kainate binding that was restricted to stratum lucidum in the hippocampus, the mossy fiber termination zone (Fig. 1). Evidence that this binding was present on the mossy fibers was presented by Ben-Ari and colleagues (13), who found that the selective destruction of CA3 pyramidal cells with kainate treatment had little immediate effect on the kainate binding, whereas colchicine-induced destruction of the granule cells, which give rise to the mossy fibers, led to a rapid loss of the binding (Fig. 2).
Figure 1.
Distribution of kainate binding sites in the hippocampus. Binding site density is color-coded with high to low densities represented by red-yellow-blue. The autoradiography was carried out with [3H]kainate and shows a high labeling density localized to the stratum lucidum, the termination zone for mossy fibers. [Reprinted with permission from ref. 12 (Copyright 1982, Elsevier Science).]
Figure 2.
Effects of selective neuronal lesions on the high-affinity kainate binding in the hippocampus. Shown are the distribution of kainate binding in (perfused) controls, kainate (KA)- or colchcine (Colch.)-treated cases. Dark triangles indicate the side ipsilateral to injection. In perfused controls, the kainate labeling is confined to the supragranular layer of the fascia dentata (FD) and the stratum lucidum of the CA3 region. Note the progressive loss of labeling from the stratum lucidum after kainate and the extensive and rapid loss after colchicine. d, Survival delay in days. [Reprinted with permission from ref. 13 (Copyright 1987, Elsevier Science).]
More recently, molecular biology has allowed a more definitive characterization of KAR genes, which are encoded in two related groups (GluR5–7 and KA1–2) distinct from AMPAR genes (GluR1–4). Granule cells are now recognized to strongly express GluR6, GluR7, KA1, and KA2 (14); however, as subunit-specific antibodies are still unavailable, it remains unclear which subunit or combination of subunits is targeted to stratum lucidum to generate the observed high-affinity binding.
KARs Directly Depolarize Mossy Fibers
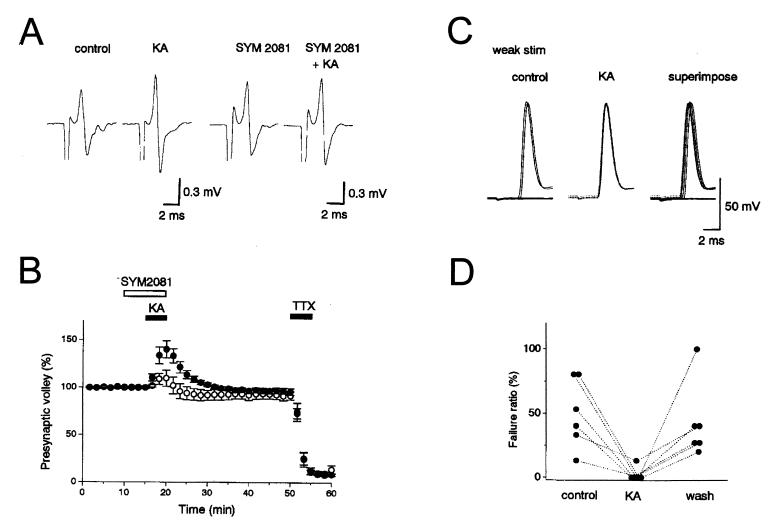
Application of low concentrations of kainate (50 nM-500 nM) increase the amplitude of the extracellularly recorded compound action potential, the mossy fiber volley (Fig. 3 A and B, see also Fig. 5B) (15, 16). With higher concentrations the increase is quickly followed by a decrease in the amplitude of the fiber volley. These effects are mediated by KARs because they are observed in the presence of GYKI 53655, an AMPAR-selective antagonist, but are blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an AMPAR/KAR antagonist and SYM2081, a KAR antagonist. These changes were not accompanied by a rise in extracellular K+ that could account for the effects (16). Moreover, blockade of Ca2+ channels or removal of extracellular Ca2+ had no effect on the kainate-mediated change in the fiber volley, indicating that the effect was not secondary to the Ca-dependent release of an intervening modulatory substance (15, 16). Thus kainate appears to be acting directly on KARs of high affinity that are present on mossy fibers. The action of kainate is most likely caused by the well-established ionotropic action of KARs because both the increase and decrease in fiber volley amplitude are mimicked by the application of elevated potassium (16). Furthermore, the effects on the fiber volley are associated with an increase in the excitability of the mossy fibers, so that stimuli that were just at threshold for activating antidromic spikes in single granule cells became suprathreshold during the application of kainate (Fig. 3 C and D) (15, 16). The increase in the fiber volley may occur as a result of spike broadening in the individual fibers, as well as an increase in the number of activated fibers. The decrease is presumably caused by sodium channel inactivation. Interestingly, low concentrations of kainate, which had no effect on the membrane properties of either the granule cells or CA3 pyramidal cells, still increased the fiber volley. Thus the presynaptic receptors appear to be of higher affinity than those expressed on the CA3 pyramidal cells (17, 18), and the granule cells must preferentially target the high-affinity receptors to their axons.
Figure 3.
Kainate enhances mossy fiber excitability. (A) Representative traces showing an increase in the presynaptic mossy fiber volley caused by 200 nM kainate, in the presence and absence of SYM2081, which desensitizes KARs. Fiber volleys were recorded in a Ca2+-free solution. (B) The time course of the effects in A. Experiments in the absence (●) and presence (○) of the KAR antagonist SYM2081 are shown. (C) Antidromic spikes are recorded in granule cells in whole-cell current clamp. In control conditions, some stimuli fail to elicit an antidromic spike (Left), whereas in kainate, each stimuli generates a spike (Center). The spikes in kainate are not only more reliable, but have a slightly smaller latency (Right). (D) A summary of the increase in reliability is shown for six cells. [Reprinted with permission from ref. 15 (Copyright 2000, The Physiological Society).]
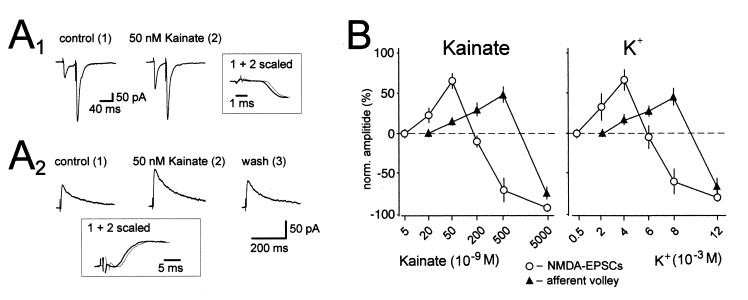
Figure 5.
Bidirectional control of synaptic transmission by kainate and presynaptic membrane potential. (A1) Averaged traces of AMPAR EPSCs recorded at −70 mV holding potential in the presence of picrotoxin (100 μM). Kainate (50 nM) increases the amplitude of the first synaptic current, whereas the second is unchanged, thereby decreasing paired pulse facilitation. Note that the increase is not associated with a change in the rising phase of the EPSC. (A2) Averaged traces of NMDAR-EPSCs recorded at +30 mV holding potential in the presence of the AMPAR antagonist GYKI 53655 (20 μM) and the GABAA receptor antagonist picrotoxin (100 μM) are shown. Kainate (50 nM) reversibly increases the amplitude of the synaptic current. Note that the increase is not associated with a change in kinetics of the EPSC. (B) Concentration dependency of the effects of kainate and K+ additions on NMDAR-EPSCs and afferent volley size. Note that 20 nM kainate and 2 mM K+ significantly increase the amplitude of the NMDAR-EPSC, whereas the fiber volley is not affected. Note also that 500 nM kainate and 8 mM K+ cause an enhancement of the afferent volley, whereas synaptic transmission is strongly suppressed. n ≥ 5 for each experiment. [Reprinted with permission from ref. 22 (Copyright 2001, American Association for the Advancement of Science, www.sciencemag.org).]
Synaptically Released Glutamate Activates Presynaptic KARs
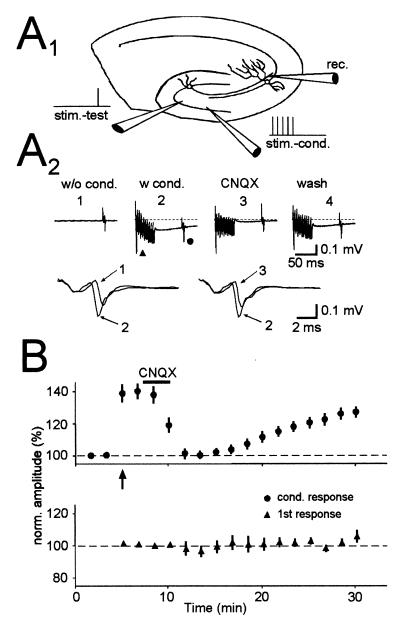
Given that mossy fiber synapses release glutamate, one might expect that synaptically released glutamate also could gain access to these presynaptic autoreceptors. To test for this possibility, two stimulating electrodes were placed in the granule cell layer to activate two independent sets of mossy fibers and an electrode, placed in stratum lucidum, was used to monitor the fiber volley (Fig. 4A1). A brief tetanus to one electrode enhanced the fiber volley evoked by the second stimulating electrode, when the stimulus was delivered 50 ms after the tetanus (Fig. 4 A2 and B) (16). Because this experiment was carried out in the presence of GYKI 53655 and the enhancement in the fiber volley was completely blocked by CNQX, the enhancement is caused by the activation of KARs, suggesting that glutamate can spread heterosynaptically to achieve activation of presynaptic KARs similar to that caused by low doses of kainate.
Figure 4.
Synaptic release of glutamate by brief stimulus trains to mossy fibers causes the heterosynaptic activation of presynaptic KARs. (A1) Schematic drawing of the experimental setup. Two independent sets of mossy fibers were stimulated. The independence was verified by the lack of a refractory period when the two pathways were stimulated at a close interval. One set (stim-cond.) was stimulated repetitively (10 pulses at 200 Hz) to release glutamate, whereas the other set (stim-test) was used to test the effects of synaptically released glutamate. (A2) Traces from a representative experiment are shown. A conditioning train caused a decrease in latency and an increase in amplitude of the test afferent volley as clearly shown in the expanded superimposed traces. All these effects are reversed after a short application of CNQX. (B) Summary graph of six experiments done in the same way as shown in A. (Upper) Responses of the test afferent volley during the experiment (arrow designates start of conditioning). (Lower) The first volley during the conditioning train. [Reprinted with permission from ref. 16 (Copyright 2000, Elsevier Science).]
Kainate Has Biphasic Effects on Mossy Fiber Synaptic Transmission
To examine the possible effects that activation of these presynaptic receptors might have on synaptic transmission, several groups have examined the effects of KAR activation on glutamatergic excitatory postsynaptic currents (EPSCs) evoked by mossy fiber stimulation (15, 16, 19, 20). Application of kainate at concentrations greater than 200 nM caused a large depression in synaptic transmission, an observation in accord with previous results at other excitatory synapses (6, 7, 21). This depression is apparently presynaptic, as it is associated with changes in short-term plasticity and a reduction in the number of quanta released (20).
The subunit composition of the KARs underlying this depression has been considered. The GluR5-selective agonist, (RS)-2-amino-3-(3-hydroxy-5-tbutylisoxazol-4-yl)propanoic acid (ATPA), has been reported to cause a similar depression, and GluR5-selective antagonists block the depression (19). These results suggest that the presynaptic KARs contain GluR5, a surprising result in light of the low expression of this subunit in granule cells (14). However, it has been found that the depressant action of ATPA is accompanied by intense excitation of GABAergic interneurons, which then release GABA (16). Blockade of metabotropic GABAB receptors substantially reduces the depressant action of ATPA on mossy fiber EPSCs, suggesting the depression induced by ATPA is the indirect result of GABA release caused by GluR5-containing KARs on interneurons; in contrast, a depressant action of kainate on mossy fiber EPSCs persists in the presence of GABAB receptor antagonists (16). Moreover, the kainate-induced depression is absent in mice lacking the GluR6 subunit, but not the GluR5 subunit, suggesting that KARs containing GluR6 mediate the depression caused by kainate (20). It therefore seems likely that the presynaptic KARs on dentate granule cells contain GluR6, consistent with expression data, whereas GluR5-containing KARs on interneurons can indirectly depress release at mossy fiber synapses through activation of metabotropic GABAB receptors.
In further studies, the effect of lower concentrations of kainate have been examined, because concentrations as low as 50 nM have effects on the fiber volley. Low concentrations of kainate actually enhance synaptic transmission, both of the AMPAR EPSC and the N-methyl-d-aspartate (NMDA) EPSC (Fig. 5A), even at concentrations below those affecting the fiber volley (22). This enhancement is caused at least in part by an increase in transmitter release because it is associated with a decrease in paired pulse facilitation and the magnitude of the enhancement is the same for the AMPAR and NMDA receptor (NMDAR) EPSCs. This presynaptic action is blocked by CNQX, indicating the involvement of KARs. A detailed analysis of the dose–response characteristics of the action of kainate indicates that low concentrations of kainate (20 and 50 nM) enhance and high concentrations depress transmitter release (Fig. 5B). Interestingly, at a concentration of 500 nM kainate still enhances the fiber volley but strongly depresses transmission. A virtually identical dose–response biphasic action on the fiber volley and synaptic transmission is seen with elevated potassium (Fig. 5B), strongly suggesting that all of the effects of kainate can be explained by an ionotropic depolarizing action of kainate on the mossy fibers (22).
Presynaptic KARs Contribute to Mossy Fiber Short-Term Plasticity
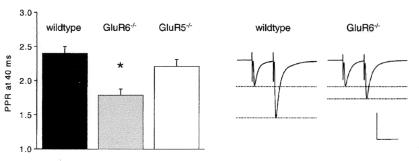
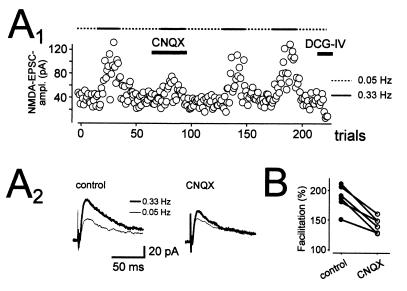
The finding that low concentrations of kainate actually enhance synaptic transmission, and that synaptically released glutamate can activate these presynaptic receptors, raises the possibility that these receptors may normally exert a positive feedback on transmitter release. This possibility was examined in mice lacking GluR6. Mossy fiber synapses undergo a remarkably large facilitation during the second of two stimuli spaced closely together (20–40 ms). Although initial studies found no change in this paired-pulse facilitation in GluR6-deficient mice (23), a subsequent comprehensive analysis showed that this facilitation is dramatically reduced in GluR6-deficient mice (24) (Fig. 6). Similarly, application of CNQX, in the continued presence of GYKI 53655, caused a large reduction in the facilitation seen with brief trains of stimuli at 25 Hz and 100 Hz, without affecting the size of the first EPSC in the tetanus (22). These results indicate that the enhancement is well established within 10–20 ms of synaptic activation. This enhancement is not only rapidly established, but also slow to decay. Increasing the rate of stimulation from 0.05 Hz to 0.33 Hz causes approximately a doubling in the size of the NMDAR EPSC in GYKI 53655. This frequency facilitation is substantially reduced by CNQX (Fig. 7), indicating that KAR-dependent enhancement lasts for seconds. Frequency facilitation is also clearly reduced in the GluR6, but not GluR5, knockout mice (24).
Figure 6.
GluR6-containing KARs contribute to paired-pulse facilitation. The ratio of the second mossy fiber EPSC over the first EPSC are shown, in response to a pair of stimuli given with a 40-ms interpulse interval (Left). This paired-pulse ratio is reduced in mice lacking GluR6, but not GluR5. Representative traces in wild-type and GluR6-deficient mice are shown (Right). Scale bar is 40 ms and 500 pA (wild type) or 675 pA (GluR6−/−). [Reprinted with permission from ref. 24 (Copyright 2001, Elsevier Science).]
Figure 7.
KARs contribute to low-frequency facilitation. (A) Changing the frequency of stimulation from 0.05 Hz to 0.33 Hz results in a facilitation of the NMDAR EPSC, which is depressed by CNQX (10 μM). This is demonstrated in both the trial-by-trial plot (A1) and the example traces below (A2). (B) Graph showing the results from six such experiments. [Reprinted with permission from ref. 22 (Copyright 2001, American Association for the Advancement of Science).]
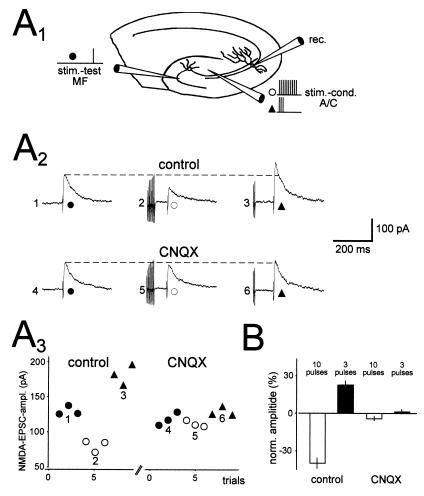
The facilitation is not restricted to the activated synapses, but can spread to neighboring synapses. Brief tetani applied to the neighboring associational/commissural synapses can evoke a heterosynaptic enhancement in synaptic transmission of mossy fiber synapses, an effect that is caused by activation of KARs (Fig. 8) (22). As low concentrations of kainate enhance transmission and higher concentrations depress it, a more robust tetanus might be predicted to achieve stronger activation of these KARs and thereby cause a depression of synaptic transmission, and in fact this has been observed (Fig. 8) (22). Thus, as is the case with bath application of kainate, the synaptic release of glutamate can cause a bidirectional modification of mossy fiber synaptic transmission.
Figure 8.
Synaptic activation of presynaptic KARs can both enhance and depress mossy fiber synaptic transmission. (A1) Schematic drawing of the experimental setup. A set of mossy fibers (stim-test) was stimulated, as was an independent set of associational/commissural fibers (stim-cond). The associational/commissural fibers were stimulated repetitively (3 or 10 pulses at 200 Hz) to release glutamate, whereas the mossy fiber responses were used to test the effects of synaptically released glutamate. (A2) In the presence of GYKI 53655, mossy fiber NMDAR-EPSCs were examined without conditioning (Left), after strong conditioning (10 pulses, Center), and after weak conditioning (three pulses, Right). Strong conditioning depresses the EPSC, whereas weak conditioning enhances it (Upper). These effects are abolished by CNQX (Lower). (A3) The EPSC amplitudes for the experiment in A2 are shown. (B) A summary of three experiments performed as described in A. [Reprinted with permission from ref. 22 (Copyright 2001, American Association for the Advancement of Science).]
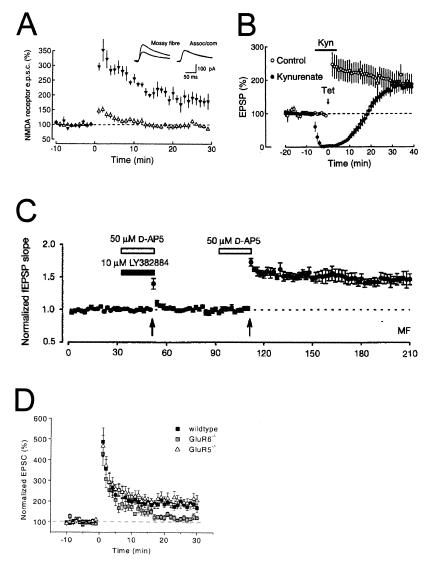
KARs May Be Involved in Mossy Fiber Long-Term Potentiation (LTP)
Mossy fiber synapses undergo an unusual form of activity-dependent LTP that is expressed presynaptically. The induction of mossy fiber LTP is widely agreed to be independent of NMDAR activation, but whether KARs are involved is controversial. Several studies have found that mossy fiber LTP could be elicited even in the presence of AMPAR/KAR antagonists (Fig. 9 A and B) (25–30), which would argue against the involvement of KARs. However, agreement over these results has not been universal (31, 32), and a recently developed antagonist of the GluR5 subunit has been reported to block mossy fiber LTP (Fig. 9C) (33). Deepening the controversy, a subsequent study found that mossy fiber LTP is unimpaired in mice lacking the GluR5 subunit, but is reduced in mice lacking GluR6 (Fig. 9D) (24). A reconciliation of all of these results is presently lacking. However, as GluR6-containing KARs play an important regulatory role in short-term plasticity (see above), it seems plausible that these receptors could influence coupling between the tetanic mossy fiber LTP induction protocol and presynaptic activation. Further experiments to elucidate the possible roles of KARs in mossy fiber LTP will be of interest.
Figure 9.
Evidence for and against the involvement of KARs in mossy fiber LTP. (A) Mossy fiber NMDAR EPSCs are recorded at >+30 mV in the presence of 10 μM CNQX. Tetanization at time = 0 induces mossy fiber LTP (▴), but does not induce LTP at neighboring associational/commissural synapses (▵). [Reprinted with permission from ref. 27 (Copyright 1995, MacMillan Magazines Ltd., www.nature.com).] (B) Mossy fiber field EPSPs are measured before and after tetanic stimulation in the absence (○) or presence (●) of 10–20 mM of the nonselective ionotropic glutamate receptor antagonist kynurenate (n = 5 each). Kynurenate has no effect on mossy fiber LTP, even though it blocks the field EPSP. [Reprinted with permission from ref. 26 (Copyright 1994, Elsevier Science).] (C) Mossy fiber field EPSPs are measured before and after tetanization (arrows). The first tetanus is given in the presence of the GluR5-specific antagonist LY382884 and the NMDAR antagonist AP-5 and does not induce mossy fiber LTP. A second tetanus without LY382884, however, does induce mossy fiber LTP. [Reprinted with permission from ref. 33 (Copyright 1999, MacMillan Magazines, Ltd., www.nature.com).] (D) Mossy fiber EPSCs are recorded in slices from wild-type, GluR5-deficient, and GluR6-deficient mice. Tetanization at time = 0 induces robust mossy fiber LTP in wild-type and GluR5-deficient mice, but only weak mossy fiber LTP in GluR6-deficient mice. [Reprinted with permission from ref. 24 (Copyright 2001, Elsevier Science).]
Conclusions
The hippocampal mossy fiber pathway has proved to be an ideal system for studying the properties of presynaptic ionotropic neurotransmitter receptors. In particular, based on autoradiographic anatomical evidence (12, 13), it is well accepted that the kainate subtype of ionotropic glutamate receptor is present on mossy fibers. It has been shown that kainate, acting directly on these KARs, affects the extracellularly recorded fiber volley in a manner consistent with a depolarization of the fibers (15, 16). Importantly, these presynaptic receptors can be activated by the synaptic release of glutamate, not only from mossy fiber synapses, but also from the neighboring associational/commissural synapses (15). Activation of these presynaptic KARs has complex effects on synaptic transmission, which appears to depend on the degree to which the receptors are activated. Early studies reported a depression in mossy fiber synaptic responses when these receptors were activated by the application of agonists (15, 16, 19, 20). Further studies revealed that more modest activation of these receptors actually enhances synaptic transmission (22) and that this effect contributes importantly to the paired pulse facilitation and frequency facilitation, two prominent features of mossy fiber synapses (22, 24). It remains unclear whether or not KARs are involved in mossy fiber LTP.
A number of questions remain unanswered. Where are the presynaptic receptors located? Are they localized at the synapse or are they distributed throughout the length of the axon, as appears to be the case for the spinal primary afferent C fibers (11)? What is the mechanism by which the presynaptic receptors control transmitter release? Can it be explained entirely by the ionotropic action of these receptors? If so, what advantages does an ionotropic action have over the more direct and better characterized metabotropic neurotransmitter action? How might the depolarization of the terminal modify transmitter release or aid in the induction of long-term plasticity? It has been postulated that the enhancement of mossy fiber transmission observed with modest receptor activation may be caused by inactivation of repolarizing potassium channels secondary to small levels of depolarization, whereas the depression may occur as a consequence of a large depolarization and the inactivation of sodium channels (22). The ability to record directly from mossy fiber boutons (34) now makes it possible to directly examine the mechanisms underlying the action of these presynaptic KARs.
Acknowledgments
D.S. is supported by a grant from the Deutsche Forschungsgemeinschaft (Emmy-Noether-Program). J.M. is supported by a Wellcome Trust Traveling Fellowship. M.F. is supported by a fellowship from the National Institutes of Health (F32 EY13473–01). R.A.N. is supported by grants from the National Institutes of Health and the Bristol-Meyer Squibb Co. R.A.N. is a member of the Keck Center for Integrative Neuroscience and the Conte Center for Neuroscience Research.
Abbreviations
- GABA
γ-aminobutyric acid
- KAR
kainate receptor
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- EPSC
excitatory postsynaptic current
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- LTP
long-term potentiation
Footnotes
This paper results from the Inaugural Arthur M. Sackler Colloquium of the National Academy of Sciences, “Neural Signaling,” held February 15–17, 2001, at the National Academy of Sciences in Washington, DC.
References
- 1.Eccles J C. The Physiology of Synapses. Berlin: Springer; 1964. [Google Scholar]
- 2.Nicoll R A, Alger B E. Int Rev Neurobiol. 1979;21:217–258. doi: 10.1016/s0074-7742(08)60639-x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson S M, Capogna M, Scanziani M. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 4.Wu L G, Saggau P. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 5.MacDermott A B, Role L W, Siegelbaum S A. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 6.Chittajallu R, Vignes M, Dev K K, Barnes J M, Collingridge G L, Henley J M. Nature (London) 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya H, Ozawa S. J Physiol (London) 1998;509:833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke V R J, Ballyk B A, Hoo K H, Mandelzys A, Pellizzari A, Bath C P, Thomas J, Sharpe F F, Davies C H, Ornstein P L, et al. Nature (London) 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- 9.Lerma J. Neuron. 1997;19:1155–1158. doi: 10.1016/s0896-6273(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 10.Frerking M, Nicoll R A. Curr Opin Neurobiol. 2000;10:342–351. doi: 10.1016/s0959-4388(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal S G, Evans R H. Br J Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monaghan D T, Cotman C W. Brain Res. 1982;252:91–100. doi: 10.1016/0006-8993(82)90981-7. [DOI] [PubMed] [Google Scholar]
- 13.Represa A, Tremblay E, Ben-Ari Y. Neuroscience. 1987;20:739–748. doi: 10.1016/0306-4522(87)90237-5. [DOI] [PubMed] [Google Scholar]
- 14.Wisden W, Seeburg P H. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamiya H, Ozawa S. J Physiol (London) 2000;523:653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz D, Frerking M, Nicoll R A. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 17.Castillo P E, Malenka R C, Nicoll R A. Nature (London) 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- 18.Vignes M, Collingridge G L. Nature (London) 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- 19.Vignes M, Clarke V R, Parry M J, Bleakman D, Lodge D, Ornstein P L, Collingridge G L. Neuropharmacology. 1998;37:1269–1277. doi: 10.1016/s0028-3908(98)00148-8. [DOI] [PubMed] [Google Scholar]
- 20.Contractor A, Swanson G T, Sailer A, O'Gorman S, Heinemann S F. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerchner G A, Wilding T J, Zhou M, Huettner J E. J Neurosci. 2001;21:59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz D, Mellor J, Nicoll R A. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- 23.Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo P E, Bureau I, Maron C, Gage F H, Mann J R, Bettler B, et al. Nature (London) 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 24.Contractor A, Swanson G, Heinemann S F. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 25.Ito I, Sugiyama H. NeuroReport. 1991;2:333–336. doi: 10.1097/00001756-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Castillo P E, Weisskopf M G, Nicoll R A. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 27.Weisskopf M, Nicoll R A. Nature (London) 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- 28.Tong G, Malenka R C, Nicoll R A. Neuron. 1996;16:1147–1157. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- 29.Yeckel M F, Kapur A, Johnston D. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellor J, Nicoll R A. Nat Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- 31.Nicoll R A, Mellor J, Frerking M, Schmitz D. Nature (London) 2000;406:957. doi: 10.1038/35023075. [DOI] [PubMed] [Google Scholar]
- 32.Bortolotto Z A, Clarke V R J, Delany C M, Vignes M, Collingridge G L. Nature (London) 2000;406:957. [Google Scholar]
- 33.Bortolotto Z, Clarke V R, Delany C M, Parry M C, Smolders I, Vignes M, Ho K H, Miu P, Brinton B T, Fantaske R, et al. Nature (London) 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- 34.Geiger J R P, Jonas P. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]