Abstract
Objectives
To assess if the Juvenile Arthritis Disease Activity Score (JADAS71) could be used to correctly identify patients with juvenile idiopathic arthritis (JIA) in need of antitumour necrosis factor therapy (anti-TNF) therapy 3 and 6 months after start of methotrexate (MTX).
Methods
Monocentric retrospective cohort study from 2011 to 2015 analysing all patients with oligoarticular JIA (OJIA) (n=39) and polyarticular course JIA (PJIA) (n=74) first starting MTX. Three and 6 months after MTX start, clinical and laboratory features and the 2011 American College of Rheumatology (ACR) JIA treatment recommendations (ACR clinical practice guideline (ACR-CPG)) were compared between groups starting and not starting anti-TNF therapy. The sensitivity and specificity of the ACR-CPG, JADAS71 and the clinical JADAS to identify non-responders after 12 months were calculated.
Results
Physicians escalated patients with significantly higher physician global assessment, clinical JADAS (cJADAS) and patient Visual Analogue Scale (VAS). The decision not to escalate was correct in 70%–75% as shown by MTX response. The implementation of the ACR-CPG would increase the current anti-TNF use from 12% to 65%. The use of (c)JADAS in identifying patients in need of anti-TNF therapy outperformed the ACR-CPG with a much higher sensitivity, specificity and accuracy. The cJADAS threshold for treatment escalation at month 3 and 6 was >5 and >3 for OJIA and >7 and >4 for PJIA, respectively. The performance of the cJADAS decreased when the patient VAS contribution to the total score was restricted and overall did not improve by adding the erythrocyte sedimentation rate.
Conclusions
The cJADAS identifies patients in need of anti-TNF and is a user-friendly tool ready to be used for treat to target in JIA. The patient VAS is a critical item in the cJADAS for the decision to escalate to anti-TNF.
Keywords: juvenile idiopathic arthritis, treatment, anti-TNF, disease activity, patient perspective
Background
Juvenile idiopathic arthritis (JIA) is with an incidence in Europe of about 16–150/100 000/year, the most common chronic rheumatic disease in children.1 JIA is defined as arthritis with no apparent cause lasting more than 6 weeks with disease onset prior to age 16.2 In the last 10 years, the availability of new potent medications such as biologicals have led to a dramatic improvement in the treatment of JIA.3 However, it is not fully established which patients are really in need of biologicals or when to start them, generally resulting in less than 20% chance of receiving a biological within 5 years after diagnosis in a recent inception cohort.4
To provide guidance and promote beneficial outcomes, the well-cited ‘American College of Rheumatology (ACR) recommendations on the treatment of JIA’ were published in 2011, proposing criteria for escalation of therapy for patients with persistent oligoarticular JIA (OJIA) and polyarticular course JIA (PJIA), 3 and 6 months after the start of methotrexate (MTX).5 A systematic critical appraisal considered this ACR clinical practice guideline (ACR-CPG)5 to be of high quality, but it scored very poorly in applicability (8%) because it did not clearly state the costs and resources needed in order to implement the CPG, nor the criteria for assessing its impact.6 Moreover, with multiple definitions for prognostic features and algorithms for different subtypes of JIA, the ACR-CPG is hard to memorise and probably too complicated for implementation in daily clinical practice.5 Nonetheless, the proposed order to step up to various synthetic and biological disease-modifying antirheumatic drugs for different subtypes of JIA in the ACR-CPG is roughly followed by most paediatric rheumatologists in developed countries.
Therefore, it is appropriate to look for alternative guidance or instruments for the escalation of therapy in JIA. The Juvenile Arthritis Disease Activity Score (JADAS) was recently developed for creating better consistency in disease activity evaluation across physicians and for allowing patients to better understand the meaning of disease activity by providing a single score number.7 The JADAS is constructed around four elements: the active joint count (AJC), physician global assessment (PGA), parent/patient Visual Analogue Scale (VAS) of well-being and the erythrocyte sedimentation rate (ESR). It was found to be a valid instrument and is applicable in standard clinical care, observational studies and clinical trials.7 A 3-element variant without ESR is called clinical JADAS (cJADAS) and does not require waiting for the ESR results.8
The aim of this study was to assess if the JADAS or cJADAS could be used to correctly identify patients with JIA in need of antitumour necrosis factor therapy (anti-TNF) therapy 3 and 6 months after start of MTX. Furthermore, we investigated which factors currently drive the decision to escalate to anti-TNF.
Methodology
The study is a monocentric retrospective cohort study. We built a research data platform with which we extracted pseudonymised data from our electronic medical records. This study did not fall under the scope of the Medical Research Involving Human Subjects Act. This study was conducted according to good CPGs and the Declaration of Helsinki.
We included all patients with OJIA and PJIA in our centre that started MTX for the first time for active JIA from April 2011 to December 2015.
Inclusion criteria (all required)
diagnosis of OJIA (persistent and extended), polyarticular (rheumatoid factor (RF)+ and RF−), psoriatic or undifferentiated JIA as defined by the International League of Associations for Rheumatology criteria2;
biological naive patients with JIA;
first start of MTX;
indication for initiation of MTX is active arthritis;
aged 0–18 years at start of the medication;
at least 12 months follow-up after the start of the treatment in our centre.
Exclusion criterion
start of a biological before the minimum observation of 6 times of MTX administration (ie, <35 days).
The MTX dosage for JIA in our centre is started at 10–15 mg/m2/week oral and might be increased to 20 mg/m2/week (maximum 30 mg/week). At baseline, 3, 6 and 12 months after the start of MTX clinical and laboratory features, the items for the ACR-CPG criteria were extracted from our electronic medical records. Missing items were not imputed or corrected for. Cases were excluded for specific analysis only if they had missing items that could alter the decision to escalate or not regarding the decision points of the ACR-CPG, JADAS71 and cJADAS71. The interpretation of the ACR-CPG items for the subgroups OJIA and PJIA on both time points 3 and 6 months is shown in the tables in the online supplementary file A. The criteria at the four different decision moments by which the ACR-CPG recommends to escalate to anti-TNF are described below.
annrheumdis-2017-212104supp001.pdf (54.7KB, pdf)
OJIA at 3 months
At least one of the following: radiographically damaged joint OR arthritis of hip/cervical spine OR combination of ankle/wrist arthritis with ESR >100 mm/hour or ESR >13 mm/hour 3 months continuously. On top of that, also at least one of the following: ≥2 active joints OR PGA ≥3/10 OR parent/patient VAS ≥2/10 OR ESR >13 mm/hour (due to JIA) OR C reactive protein (CRP) >10 mg/L (due to JIA).
OJIA at 6 months
At least 3 of the following criteria: ≥2 active joints, ESR >26 mm/hour (due to JIA)/CRP >20 mg/L (due to JIA), PGA ≥7/10, parent/patient VAS ≥4/10.
PJIA at 3 months
Any one of the following: ≥5 active joints OR ESR >13 mm/hour (due to JIA) OR CRP >10 mg/L (due to JIA) OR PGA ≥4/10 OR parent/patient VAS ≥2/10.
PJIA at 6 months
NOT satisfying ALL of the following criteria: AJC=0 AND ESR <13 mm/hour AND CRP <10 mg/L AND PGA=0 AND parent/patient VAS <2.
As in the ACR-CPG all four decision points were again subdivided into 2×2 groups: first, if the ACR-CPG recommended escalation to anti-TNF or not and, second, whether the physician did or did not really escalate to anti-TNF. The ACR-CPG escalation groups and physician escalation groups were compared for every decision point on the respective ACR-CPG items, relevant clinical, laboratory characteristics and composite measures.
The accuracy, sensitivity and specificity of the ACR-CPG, the JADAS-71 and the cJADAS71 to identify non-responders after 12 months (defined as those not meeting the criteria for inactive disease)9 were calculated. Responding patients who started an anti-TNF agent within 12 months were excluded for the analyses of the prognostic test of the ACR-CPG and JADAS based care, since it was impossible to tell if they really would have needed an anti-TNF agent to become a responder. We used median and IQRs and the Mann-Whitney U test for interval and ordinal variables as well as for ESR, which was not normally distributed. A P value less than 0.05 was considered statistically significant. We used SAS Enterprise Guide 7.11 for data collection and IBM SPSS Statistics, V.21.0.0.0 for data analysis.
For further details on methodology, see online supplementary file B.
annrheumdis-2017-212104supp002.pdf (72KB, pdf)
Results
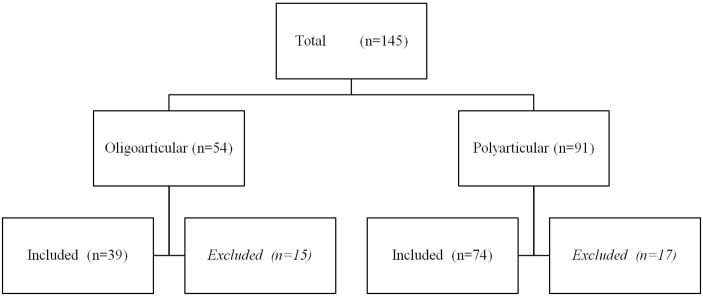
We retrieved the data of 145 patients with JIA for the first time starting with MTX (see figure 1).
Figure 1.
Recruitment of patients.
Fifteen patients were excluded in the OJIA and 17 in the PJIA group, respectively: MTX started for uveitis only (n=9 and n=0, respectively); MTX started in another hospital (n=4 and n=8, respectively) and lost to follow-up (n=2 and n=4, respectively); diagnosis JIA incorrect (n=0 and n=2, respectively); anti-TNF started before MTX effect can be expected (n=0 and n=1, respectively); MTX predominantly started for uveitis (n=0 and n=2, respectively).
The baseline characteristics of both OJIA and PJIA at start of MTX are shown in table 1. Values for RF, HLA-B27, VAS and cJADAS were not available for all cases. Two (PJIA) patients developed uveitis while on MTX; one while on etanercept who was then switched to adalimumab and another at the 12-month visit.
Table 1.
Baseline characteristics for the patients with OJIA and PJIA starting their first MTX
| OJIA | PJIA | |
| Patient characteristics | ||
| N | 39 | 74 |
| Sex, female (%) | 31 (79) | 53 (72) |
| Median age at onset (year) (IQR) | 4.5 (1.9–8.5) | 7.5 (4.3–11.8) |
| Median disease duration (year) (IQR) | 0.8 (0.3–1.9) | 0.5 (0.2–1.1) |
| ANA+ (%) | 16 (41) | 33 (45) |
| Rheumatoid factor+ (%) | 0 (0) | 5 (8) |
| HLA-B27+ (%) | 4 (25) | 6 (16) |
| Subtype of JIA | ||
| Oligoarticular (%) | 39 (100) | 19 (26) |
| Persistent (%) | 38 (97) | |
| Extended (%) | 19 (26) | |
| Polyarticular RF− (%) | 48 (65) | |
| Polyarticular RF+ (%) | 6 (8) | |
| Psoriatic arthritis (%) | 1 (1) | |
| Undifferentiated arthritis (%) | 1 (2.6) | |
| Disease activity at baseline | ||
| Median parent/patient VAS (IQR) | 45 (8-65) | 46 (25-70) |
| Median PGA (IQR) | 20 (15-35) | 30 (20-41) |
| Median number of active joints (IQR) | 2 (1-3) | 6.0 (3.0–9.3) |
| Median cJADAS (IQR) | 8.0 (4.0–10.0) | 14.5 (10.1–20.0) |
| Uveitis present (%) | 0 (0) | 1 (1) |
| Comedication at start MTX | ||
| IA steroids (%) | 6 (15) | 10 (14) |
| Prednisolon (%) | 1 (3) | 9 (12) |
ANA, antinuclear antibody; cJADAS, clinical juvenile arthritis disease activity score; HLA, human leucocyte antigen; IA, intra-articular; JIA, juvenile idiopathic arthritis; MTX, methotrexate; OJIA, oligoarticular JIA; PGA, physician’s global assessment; PJIA, polyarticular course JIA; RF, rheumatoid factor; VAS, Visual Analogue Scale.
Comedication for JIA beyond MTX and anti-TNF can be found in table 2. In fact, next to the 15% of patients with OJIA that received intra-articular steroids at the start of MTX, another 44% of the patients with OJIA had already received intra-articular steroids, while the remainder had mostly involvement of wrist, ankle or hip and started with MTX right away. An anti-TNF agent was started in 21% of OJIA and 32% of PJIA in the first year after start of MTX, all minimally 100 days before the 12-month visit. Many data on ACR-CPG items were missing (table 2), but the individual recommendation for escalation by the ACR-CPG could often be deducted despite missing values.
Table 2.
Comedication and unretrievable data at 3 and 6 months for patients with OJIA and PJIA
| OJIA | PJIA | |
| Comedication first 3 months | ||
| IA steroids (%) | 10/39 (26) | 11/74 (15) |
| Systemic steroids (%) | 1/39 (3) | 5/74 (7) |
| Comedication 3–6 months | ||
| IA steroids (%) | 2/36 (6) | 5/74 (7) |
| Systemic steroids (%) | 0/36 (0) | 2/74 (3) |
| Anti-TNF (%) | 3/39 (8) | 13/74 (18) |
| Comedication 6–12 months | ||
| IA steroids (%) | 4/36 (11) | 3/74 (4) |
| Systemic steroids (%) | 0/36 (0) | 2/74 (3) |
| Anti-TNF (%) | 8/39 (21) | 24/74 (32) |
| Unretrievable data at 3 months | ||
| Patient VAS (%) | 13/39 (33) | 23/73 (32) |
| ESR (%) | 5/39 (13) | 10/73 (14) |
| CRP (%) | 7/39 (18) | 16/73 (22) |
| ACR-CPG recommendations (%) | 2/39 (5) | 15/73 (21) |
| Unretrievable data at 6 months | ||
| Patient VAS (%) | 7/36 (19) | 12/61 (20) |
| ESR (%) | 2/36 (6) | 4/61 (7) |
| CRP (%) | 5/36 (14) | 10/61 (16) |
| ACR-CPG recommendations (%) | 1/36 (3) | 5/61 (8) |
Comedication is defined as medication started for juvenile idiopathic arthritis within 30 days before start of MTX and until the end of the observation period. In case of missing items forming part of the decision for the ACR-CPG, it was analysed if it could have altered that decision in any way; only if so, the case was marked missing for such a decision.
ACR-CPG, American College of Rheumatology clinical practice guideline; anti-TNF, anti-tumour necrosis factor therapy; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; IA, intra-articular; OJIA, oligoarticular juvenile idiopathic arthritis; PJIA, polyarticular course juvenile idiopathic arthritis; VAS, Visual Analogue Scale.
Tables 3 and 4 display differences between patients who were recommended to escalate to anti-TNF according to the ACR-CPG and those who were not, as well as between patients who were actually escalated and those who were not at both the 3-month and 6-month visits, for OJIA and PJIA, respectively. For the analysis at 3 months, one patient with PJIA had the follow-up in another centre, and at 6 months, the patients who had already started anti-TNF were excluded.
Table 3.
Differences (in the rows) between patients who were recommended to escalate to anti-TNF according to the ACR-CPG and those who were not, as well as between patients who were actually escalated and those who were not (in the columns) at both the 3-month and 6-month visits, for patients with OJIA
| OJIA | ACR-CPG recommends anti-TNF | P value | Physician started anti-TNF | P value | ||
| No | Yes | No | Yes | |||
| 3 months, N (%) | 30 (77) | 7 (18) | 36 (92) | 3 (8) | ||
| ACR escalation criteria | ||||||
| One of these: | ||||||
| ≥2 joints | 8 (27) | 4 (57) | 10 (27.8) | 2 (67) | ||
| ESR/CRP >ULN | 6 (20) | 4 (57) | 10 (27.8) | 1 (33) | ||
| PGA ≥3 | 1 (3) | 3 (43) | 2 (5.6) | 2 (67) | ||
| Patient VAS ≥2 | 9 (30) | 5 (71) | 12 (33.3) | 2 (67) | ||
| AND one of these: | ||||||
| Hip/cervical spine | 0 (0) | 1 (14) | 1 (2.8) | 1 (33) | ||
| Damaged joint ≥1 | 0 (0) | 2 (29) | 2 (5.6) | 0 (0) | ||
| Ankle/wrist+>ULN 6 months/ESR >100 | 1 (3) | 4 (57) | 5 (13.9) | 1 (33) | ||
| ACR and physician correspond | 29 (97) | 2 (29) | 31 (86) | 2 (67) | ||
| AJC (median, IQR) | 1.0 (0.0–2.0) | 2.0 (1.0–2.5) | 0.02 | 1.0 (0.0–2.0) | 2.0 (1.5–2.5) | 0.12 |
| ΔAJC (median, IQR) | 1.0 (1.0–2.0) | 0 (0–0) | 0.006 | 1.0 (0.5–2.0) | 0 (0–0) | 0.02 |
| ESR in mm/hour (median, IQR) | 9.0 (5.0–14.0) | 17.0 (4.5–25.5) | 0.39 | 10.0 (5.0–16.5) | 5.0 (4.5–45.5) | 0.95 |
| PGA (median, IQR) | 0.9 (0.0–1.5) | 2.5 (2.1–3.3) | 0.002 | 1.1 (0.0–2.0) | 3.5 (2.8–3.8) | 0.02 |
| Patient VAS (median, IQR) | 1.6 (0.4–3.6) | 2.4 (2.2–4.2) | 0.17 | 1.9 (0.6–3.0) | 5.0 (4.2–5.7) | 0.07 |
| cJADAS (median, IQR) | 3.5 (0.9–5.5) | 7.5 (7.4–8.7) | 0.02 | 4.3 (1.5–7.0) | 9.2 (8.7–9.7) | 0.03 |
| ΔcJADAS (median, IQR) | 3.7 (1.8–5.3) | 2.4 (0.4–4.8) | 0.46 | 3.7 (1.7–5.3) | 1.7 (0.4–2.9) | 0.30 |
| 6 months, N (%) | 34 (94) | 1 (3) | 32 (89) | 4 (11) | ||
| ACR escalation criteria | ||||||
| Three of these: | ||||||
| ≥2 joints | 6 (18) | 1 (100) | 7 (22) | 1 (25) | ||
| ESR/CRP >2× ULN | 0 (0) | 1 (100) | 1 (3) | 0 (0) | ||
| PGA ≥7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Patient VAS ≥4 | 9 (26) | 1 (100) | 7 (22) | 3 (75) | ||
| ACR and physician correspond | 30 (88) | 0 (0) | 31 (97) | 0 (0) | ||
| AJC (median, IQR) | 0.0 (0.0–1.0) | 3 | 0.10 | 0.0 (0.0–1.0) | 1.0 (1.0–1.5) | 0.14 |
| ESR in mm/hour (median, IQR) | 7.0 (5.0–9.0) | 65 | 0.09 | 7.0 (5.0–9.0) | 8.0 (4.0–12.0) | 0.89 |
| PGA (median, IQR) | 0.1 (0.0–2.0) | 3.0 | 0.07 | 0.0 (0.0–1.3) | 2.8 (2.3–3.5) | 0.003 |
| Patient VAS (median, IQR) | 1.7 (0.5–4.5) | 8.2 | 0.13 | 1.2 (0.4–4.1) | 5.3 (4.0–7.1) | 0.02 |
| cJADAS (median, IQR) | 3.3 (0.8–6.3) | 14.2 | 0.10 | 3.2 (0.6–5.3) | 9.5 (8.7–10.6) | 0.005 |
Values are numbers (percentages), except where indicated otherwise. Percentages are based on the column totals for 3 and 6 months, respectively. Decreases (Δ) of AJC and cJADAS at the 3-month visit compared with baseline visit. Damaged joint had to be proven radiographically. Without correction for missing data, the ACR-CPG recommended escalation to anti-TNF in 18% of patients with OJIA at 3 months, while only 8% were actually escalated by the physician. At 6 months, only patients not yet escalated (n=36) were analysed. At 6 months, ACR-CPG recommended escalation to anti-TNF in only 3% vs 11% who were actually escalated. Because escalation was recommended in only one patient at 6 months, the items and P values are in italics.
Δ (delta), difference compared with baseline; ACR, American College of Rheumatology; ACR-CPG, ACR clinical practice guideline; AJC, active joint count; cJADAS, clinical Juvenile Arthritis Disease Activity Score; anti-TNF, antitumour necrosis factor therapy; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; OJIA, persistent oligoarticular juvenile idiopathic arthritis; PGA, physician global assessment; ULN, upper limit of normal; VAS, Visual Analogue Scale.
Table 4.
Differences (in the rows) between patients who were recommended to escalate to anti-TNF according to the ACR-CPG and those who were not, as well as between patients who were actually escalated and those who were not (in the columns) at both the 3-month and 6-month visits, for patients with PJIA
| PJIA | ACR-CPG recommends anti-TNF | P value | Physician started anti-TNF | P value | ||
| No | Yes | No | Yes | |||
| 3 months, N (%) | 14 (19) | 44 (59) | 60 (81) | 13 (18) | ||
| ACR escalation criteria | ||||||
| One of these: | ||||||
| ≥5 joints | 0 (0) | 23 (52) | 14 (23) | 10 (77) | ||
| ESR/CRP >ULN | 0 (0) | 15 (34) | 9 (15) | 6 (46) | ||
| PGA ≥4 | 0 (0) | 11 (25) | 6 (10) | 5 (38) | ||
| Patient VAS ≥2 | 0 (0) | 29 (66) | 20 (33) | 9 (69) | ||
| ACR and physician correspond | 14 (100) | 13 (30) | 14 (23) | 13 (100) | ||
| AJC (median, IQR) | 1.0 (0.0–2.0) | 5.0 (2.5–6.5) | <0.0005 | 2.0 (1.0–4.0) | 7.0 (6.0–8.0) | <0.0005 |
| ΔAJC (median, IQR) | 5.0 (3.0–9.0) | 2.0 (−0.5–4.0) | 0.002 | 3.0 (1.0–5.0) | 0.0 (−3.0–1.0) | 0.001 |
| ESR in mm/hour (median, IQR) | 7.0 (6.0–14.0) | 16.0 (7.0–28.0) | 0.10 | 11.0 (5.0–20.0) | 20.0 (11.0–31.0) | 0.045 |
| PGA (median, IQR) | 0.7 (0.2–1.5) | 2.6 (1.5–3.8) | <0.0005 | 1.5 (0.5–2.5) | 3.5 (3.0–4.5) | <0.0005 |
| Patient VAS (median, IQR) | 0.7 (0.1–1.4) | 4.4 (2.1–5.9) | <0.0005 | 1.9 (1.0–4.5) | 5.5 (4.3–6.0) | 0.005 |
| cJADAS (median, IQR) | 2.8 (1.8–4.5) | 10.4 (8.2–15.0) | <0.0005 | 6.7 (3.2–8.9) | 15.0 (14.0–19.0) | <0.0005 |
| ΔcJADAS (median, IQR) | 9.7 (6.5–14.2) | 4.2 (0.9–8.9) | 0.003 | 6.6 (2.2–11.3) | 2.1 (−1.2–4.4) | 0.026 |
| 6 months, N (%) | 13 (21) | 43 (70) | 53 (72) | 8 (13) | ||
| ACR escalation criteria | ||||||
| One of these: | ||||||
| ≥1 joint | 0 (0) | 39 (91) | 31 (58) | 8 (100) | ||
| ESR/CRP >ULN | 0 (0) | 16 (37) | 12 (23) | 4 (50) | ||
| PGA >0 | 0 (0) | 39 (91) | 31 (58) | 8 (100) | ||
| Patient VAS ≥2 | 0 (0) | 18 (42) | 11 (21) | 7 (88) | ||
| ACR and physician correspond | 13 (100) | 8 (19) | 13 (25) | 8 (100) | ||
| AJC (median, IQR) | 0 (0.0–0.0) | 2.0 (1.0–3.5) | <0.0005 | 1.0 (0.0–2.0) | 6.0 (3.5–7.5) | <0.0005 |
| ESR in mm/hour (median, IQR) | 6.0 (3.0–15.0) | 8.0 (5.0–15.0) | 0.52 | 7.0 (3.0–14.0) | 13.0 (5.5–16.0) | 0.50 |
| PGA (median, IQR) | 0 (0.0–0.0) | 1.5 (0.9–2.5) | <0.0005 | 0.7 (0.0–1.5) | 3.3 (2.4–4.7) | <0.0005 |
| Patient VAS (median, IQR) | 0.3 (0.0–0.8) | 1.8 (0.5–4.0) | 0.002 | 0.7 (0.2–2.0) | 4.0 (3.2–5.2) | 0.002 |
| cJADAS (median, IQR) | 0.3 (0.0–0.8) | 6.7 (2.9–10.0) | <0.0005 | 2.8 (0.8–6.8) | 12.4 (9.6–14.7) | <0.0005 |
Values are numbers (percentages), except where indicated otherwise. Percentages are based on the column totals for 3 and 6 months, respectively. Decreases (Δ) of AJC and cJADAS at the 3-month visit compared with baseline visit. Without correction for missing data, the ACR-CPG recommended escalation to anti-TNF in 59% of all patients with PJIA at 3 months, while only 18% were actually escalated by the physician. At 6 months, only patients not yet escalated (n=61) were analysed. The ACR-CPG recommended escalation to anti-TNF at 6 months in 70% vs only 13% who were actually escalated.
Δ (delta), difference compared with baseline; ACR, American College of Rheumatology; ACR-CPG, ACR clinical practice guideline; AJC, active joint count; anti-TNF, antitumour necrosis factor therapy; cJADAS, clinical Juvenile Arthritis Disease Activity Score; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; PJIA, polyarticular course juvenile idiopathic arthritis; PGA, physician global assessment; ULN, upper limit of normal; VAS Visual Analogue Scale.
OJIA at 3 months MTX
As displayed in table 3, the ACR-CPG recommended escalation to anti-TNF in 18% of all patients with OJIA at 3 months (equal to 19% of patients with available data). The most frequently met criterion among the ACR-CPG was the patient VAS ≥2/10, but with removal of the VAS, still 71.4% of the recommended patients would have received the same advice. Of the second necessary ACR items, wrist/ankle involvement with prolonged increased ESR (defined as >6 months) was most prevalent with 57%. Conversely, markedly increased ESR (defined as >100 mm/hour) was never found. Significant differences between those recommended to escalate or not were found for some items: AJC, decrease of AJC compared with baseline (delta AJC), PGA and the cJADAS, but not for others: ESR, patient VAS, decrease of cJADAS compared with baseline (delta cJADAS).
Only 8% were really escalated by the physician. In 29% of the cases when ACR-CPG recommended escalation, the physician decided to do so, while anti-TNF was not started in 97% of cases when the ACR did not recommend to. The physician’s decision significantly favoured escalation in patients with less reduction of AJC, higher PGA and cJADAS.
OJIA at 6 months MTX
For OJIA at the 6-month visit, the ACR-CPG recommended only one patient to escalate to anti-TNF (table 3). The criterion of PGA ≥7/10 was never met, thus necessitating all other three criteria to be present. The criteria of VAS ≥4/10 and ≥2 joints were present in 26% and 18%, respectively, but just one patient had an ESR/CRP more than twice the upper limit of normal. This was the sole patient recommended to be escalated.
The physician escalated 11% (n=4) patients to anti-TNF at the 6-month visit. None of the patients recommended to escalate by the ACR-CPG were indeed escalated by the physician, while anti-TNF was indeed not started in 88% of cases where the ACR-CPG did not recommend to. The physician’s decision significantly favoured escalation in patients with higher PGA, cJADAS and higher patient VAS.
PJIA at 3 months MTX
As displayed in table 4, the ACR-CPG recommended 59% of all patients with PJIA at 3 months to be escalated to anti-TNF (equal to 76% of patients with available data). The most frequently met ACR-CPG criterion was the patient VAS ≥2/10 in 66% of all patients (81% for the patients who had a VAS available). If the VAS would have been removed as criterion, only 59% of the patients recommended to escalate would have received the same advice. Significant differences between those recommended to escalate and not were found in some items incorporated in the ACR-CPG itself (AJC, PGA, VAS and the combined cJADAS). Also, significantly lower reductions in delta AJC and delta cJADAS were seen in patients recommended to escalate.
The physician escalated just 18% of the patients, all in accordance with the ACR-CPG. When the ACR-CPG recommended to escalate, in only 29% the physician indeed did so, while anti-TNF was not started in 100% if the ACR-CPG did not recommend to. The physician’s decision significantly favoured escalation in patients with higher 3-month AJC, ESR, PGA, VAS and cJADAS as well as less reduction of AJC and cJADAS compared with baseline.
PJIA at 6 months MTX
At 6 months, the ACR-CPG recommended 70% of all patients with PJIA to be escalated to anti-TNF (equal to 77% with available data; see table 4). The most frequently met ACR-CPG criterion was ≥1 active joint with a corresponding PGA >0 in 91% of patients. A patient VAS ≥2/10 was found in 42% of all patients (50% of patients with available VAS), but the VAS as single reason for escalation was only found in 45%. If the VAS would have been removed, still 93% of these patients would have been recommended to escalate. There were significant differences in the ACR-CPG incorporated items AJC, PGA, VAS and combined score of cJADAS between those recommended to escalate and not.
The physician escalated 13% of the patients, all in accordance with ACR-CPG. In only 19% when the ACR-CPG recommended to escalate the physician decided similarly, while in 100%, anti-TNF was indeed not started when the ACR did not recommend it. The physician’s decision favoured escalation in patients with higher 6-month AJC, PGA, VAS and cJADAS.
Prediction of failure at 12 months
Treatment failures (not reaching the criteria for inactive disease at 12 months)9 for OJIA were 26% in the biological naive and 25% in patients using anti-TNF. Of the five patients who did not receive anti-TNF at 3 months despite their ACR-CPG recommendation, two were escalated at 6 months and the remaining three responded to MTX. The two patients with OJIA with worsening of their cJADAS compared with baseline were not escalated to anti-TNF but received it after all at 6 and 8 months. The physician’s decision not to escalate at 3 and 6 months appeared to be right at 12 months in 75.% and 72% of patients with OJIA, respectively (table 5).
Table 5.
Prognostic tests for predicting failure to respond after start methotrexate according to ACR recommendations and cJADAS scores in patients with oligoarticular and polyarticular juvenile idiopathic arthritis
| 3 months | Oligoarticular patients | Polyarticular patients | ||||||
| Rule | Accuracy (%) | Sensitivity (%) | Specificity (%) | Sum (%) | Accuracy (%) | Sensitivity (%) | Specificity (%) | Sum (%) |
| ACR escalation | 62.5 | 10.0 | 86.4 | 96.4 | 57.1 | 86.7 | 44.4 | 131.1 |
| cJADAS >4 | 20.0 | 71.4 | 55.6 | 127.0 | 47.8 | 81.3 | 30.0 | 111.3 |
| cJADAS >5 | 70.8 | 71.4 | 70.6 | 142.0 | 58.1 | 81.3 | 44.4 | 125.7 |
| cJADAS >6 | 68.2 | 33.3 | 81.3 | 114.6 | 64.3 | 81.3 | 53.8 | 135.1 |
| cJADAS >7 | 72.7 | 33.3 | 87.5 | 120.8 | 75.6 | 81.3 | 72.0 | 153.3 |
| cJADAS >8 | 77.3 | 33.3 | 93.8 | 127.1 | 75.0 | 73.3 | 76.0 | 149.3 |
| Correctly not escalated | 75.0 | 70.5 | ||||||
| 6 months | Accuracy (%) | Oligoarticular patients | Accuracy (%) | Polyarticular patients | ||||
| Rule | Sensitivity (%) | Specificity (%) | Sum (%) | Sensitivity (%) | Specificity (%) | Sum (%) | ||
| ACR escalation | 67.7 | 0 | 95.5 | 95.5 | 46.9 | 77.8 | 29.0 | 106.8 |
| cJADAS >2 | 62.1 | 100 | 50.0 | 150.0 | 51.1 | 76.5 | 36.7 | 113.2 |
| cJADAS >3 | 67.9 | 100 | 57.1 | 157.1 | 66.7 | 70.6 | 64.3 | 134.9 |
| cJADAS >4 | 70.4 | 66.7 | 71.4 | 138.1 | 75.6 | 70.6 | 78.6 | 149.2 |
| cJADAS >5 | 74.1 | 66.7 | 76.2 | 142.9 | 72.1 | 56.3 | 81.5 | 137.8 |
| Correctly not escalated | 71.9 | 71.7 | ||||||
The cJADAS as prognostic tests outperformed the ACR recommendations as shown by accuracy, sensitivity and specificity and the sum of the latter two. At 3 months, we were aiming at a high specificity (avoiding overtreatment) and the best performing cut-off values were >5 and >7 for OJIA and PJIA, respectively. At 6 months, we were aiming at a high sensitivity (avoiding undertreatment) and the best performing cut-off values at 6 months were >3 and >4 for OJIA and PJIA, respectively. The percentages of correct physician decisions when not escalated (percentage of patients not escalated at that decision point who at 12 months indeed appeared to be a responder on MTX) are displayed in the row of ‘correctly not escalated’.
ACR, American College of Rheumatology; cJADAS, clinical Juvenile Arthritis Disease Activity Score; OJIA, oligoarticular juvenile idiopathic arthritis; PJIA, polyarticular juvenile idiopathic arthritis.
For PJIA, the treatment failures were comparable with OJIA with 28% in the biological naive and 29% of the patients using anti-TNF. Only 19% of the 31 patients not receiving anti-TNF at 3 months despite the ACR-CPG recommendation were escalated still at 6 months and an additional 10% in the months thereafter, while 68% of the remaining 22 patients responded to conventional treatment. Interestingly, 46% with worsening of AJC at 3 months started anti-TNF, while 27% of the rest received it later. Similarly, only 40% of those with worsening cJADAS were escalated to anti-TNF, while 40% received it later and the one who never escalated failed to respond at 12 months. At 6 months, only 9% of the patients with PJIA not receiving anti-TNF despite their ACR-CPG recommendation were escalated before the 12-month visit, while 72% of the other 32 patients responded to conventional treatment. The physician’s decision not to escalate at 3 and 6 months appeared to be the right decision in 71% and 72% of patients with PJIA, respectively (table 5).
The capability of the ACR-CPG as prognostic test at 3 and 6 months to predict MTX failure at 12 months is displayed in table 5. For OJIA, the correct identification of patients in need of anti-TNF (sensitivity) of the ACR-CPG was low at 3 and 6 months (10% and 0% of non-responders were recommended to escalate), while the correct recommendation not to escalate (specificity) was high (86% and 95% of responders not recommended to escalate). For PJIA, at 3 and 6 months, conversely the sensitivity was high (87% and 78%) and the specificity was low (44% and 29%).
The cJADAS had better accuracy, higher sensitivity, specificity and sum scores of the latter two. The cut-off values for cJADAS that best performed to predict failure on MTX and thus the need to escalate to anti-TNF were >5 for OJIA and >7 for PJIA at 3 months. At 6 months, the best cut-off values for cJADAS were >3 for OJIA and >4 for PJIA. There was no considerable benefit of including the ESR (JADAS71), and the prognostic value even decreased considerably when OJIA and PJIA were taken together, when the patient VAS scores had lower relative contribution or if a decrease in cJADAS at 3 months was taken into account (online supplementary file C).
annrheumdis-2017-212104supp003.pdf (85.9KB, pdf)
Discussion
Clearly, in our clinical practice, we were not collecting all data needed to guide us through the complex ACR-CPG decision algorithms, and our decisions were in line with it in only 0%–30%. Our physicians only escalated in 12% of all time points (25/209 decision points for OJIA and PJIA at 3 and 6 months), while the ACR-CPG recommended it in 65% (121/186). The implementation of the ACR-CPG would make us treat with anti-TNF 11% more OJIA at 3 months and almost none at 6 months, while for PJIA, we would need to treat with anti-TNF around 60% more patients at 3 and 6 months since the ACR-CPG recommends to escalate >75% of these patients. All cases responding to anti-TNF do also encompass all the already overtreated patients, but it is impossible to know which ones that would be. However, our physician’s decision not to escalate was correct in 70%–75% of the cases, so the ACR-CPG seems to result in overtreatment with anti-TNF, even in some patients that are regarded as inactive by their physician at that exact moment. The VAS was the most prevalent missing value in our study without any difference between patients with OJIA and PJIA. The ACR-CPG recommendation could mostly be deducted and was only missing in 3%–8% of the decision points. However, for the 3-month visit of PJIA, it was missing in 21% of the cases mostly due to missing VAS. Only a VAS of 2/10 would have sufficed to get an ACR-CPG recommendation to escalate, being already the most frequently met criterion in 81% for patients with available VAS. We therefore probably underestimated the ACR-CPG recommendation to escalate to anti-TNF in now 75% of patients with available data, which would lead to even more overtreatment.
At this moment for JIA, we do not have fixed aims or cut-offs per timeframe, and therefore, it is conceivable that comparable patients would receive anti-TNF at different time points. In rheumatoid arthritis (RA), there is evidence that the strategy is more important than the specific agent used,10 and the 2016 update of the EULAR recommendations for treatment of RA (EULAR-RA) state that treatment should be aimed at reaching a target of sustained remission or low disease activity in every patient and monitoring should be every 1–3 months in active disease.11 Trials also illustrate the value of a frequently quantitative monitored index,10 and therapy should be adjusted if there is no improvement by at most 3 months after the start of treatment or if the target has not been reached by 6 months.11 The quantitative JADAS can be used in the assessment of therapeutic efficacy in clinical trials and in monitoring disease activity in individual patients in standard clinical practice.7 The latter objective has not been evaluated anywhere yet. We showed that both JADAS71 and the cJADAS can be used in clinical practice for predicting treatment failure 12 months after start of MTX. The goal of our decision rule at 3 months was to avoid overtreatment of patients. Therefore, we focused on a cut-off value with the highest specificity, without losing too much sensitivity. Conversely, the goal at 6 months was to avoid undertreatment of patients, in order to induce disease inactivity within 12 months for as many patients as possible. Therefore, we focused on a cut-off point with the highest sensitivity, without losing too much specificity. Consequently, the threshold to start anti-TNF will be lower at 6 months than at 3 months.
An overarching principle in the EULAR-RA is that ‘the treatment must be based on a shared decision between the patient and the rheumatologist’.11 In our study, it was impossible to retrieve the decisive motivation for physicians to escalate to anti-TNF, but with the number of missing patient VAS values, it is unlikely that each decision was indeed a shared one. However, we showed the importance of the patient VAS well-being scores, since the omission of it in the cJADAS prognostic test resulted in a decreased identification of MTX non-responders. Maybe this can be explained by the fact that the patient and his/her family consider all days and do not just evaluate a hospital visit snapshot. Furthermore, patients take into account all complaints such as morning stiffness and joint pain and not merely the AJC.
The cJADAS incorporates the patient perspective, is very user-friendly and does not need waiting for ESR results before a decision can be made. We therefore believe that the cJADAS can be used for treat-to-target therapy in JIA. The cut-off values for cJADAS that we found for the need to escalate to anti-TNF were >5 for OJIA and >7 for PJIA at 3 months and >3 for OJIA and >4 for PJIA at 6 months. The newest cJADAS71 cut-off values for moderate disease activity found by rating of a large group of international paediatric rheumatologists are >3.4 for OJIA and >5.1 for PJIA.12 These cut-offs are rather close to what we found, although they did not relate their cut-off values to any specific time point in the disease.
Since our study is a single-centre experience and our patient numbers are rather small, larger multicentre studies are needed to validate our findings, as well as to optimise the cut-off values for the cJADAS in order to further decrease the number of patients incorrectly not escalated to anti-TNF and to reduce the number of patients unnecessarily treated with anti-TNF. Furthermore, in large prospective studies, the predictive value of multiple biomarkers in addition to the clinical JADAS should be explored.13
In conclusion, we show here that ACR-CPG was not followed by our physicians for treatment decisions. Comparison between ACR-CPG and actual physician’s decisions showed that more OJIAs were escalated than recommended and less PJIAs over the course of 6 months. cJADAS showed to be a useful tool for guiding treatment decisions as shown by a good predictive value. We believe that the cJADAS can be used for treat-to-target therapy in JIA. Larger multicentre studies are needed to validate our findings.
Acknowledgments
We thank Martine M Ros, Senior Data Manager of the Team Directorate Information Technology of the UMC Utrecht for her cooperation in building our Research Data Platform.
Footnotes
Handling editor: Tore K Kvien
Contributors: JFS, NMW and SdR planned the study. JFS and NMW shaped the electronic medical records in such a way that meaningful data could be extracted. SdR built the research data platform used for the analyses of this study. JFS checked 100% of the data from the research data platform with the source (electronic medical records). JFS, EHPvD and SdR analysed the data. JFS wrote the manuscript. EHPvD, NMW and SdR critically reviewed and edited the manuscript before submission. All authors contributed substantially to discussion of content.
Funding: This study was supported by Pfizer (grant no WI189796) and by the Dutch Arthritis Foundation (Reumafonds) grant RF 901.
Competing interests: None declared.
Ethics approval: Utrecht Medical Ethical Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. 10.1016/S0140-6736(07)60363-8 [DOI] [PubMed] [Google Scholar]
- 2. Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 3. Ruperto N, Martini A. Current medical treatments for juvenile idiopathic arthritis. Front Pharmacol 2011;2:60 10.3389/fphar.2011.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guzman J, Oen K, Tucker LB, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854–60. 10.1136/annrheumdis-2014-205372 [DOI] [PubMed] [Google Scholar]
- 5. Beukelman T, Patkar NM, Saag KG, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res 2011;63:465–82. 10.1002/acr.20460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith CA, Toupin-April K, Jutai JW, et al. A systematic critical appraisal of clinical practice guidelines in Juvenile Idiopathic Arthritis using the appraisal of guidelines for research and evaluation II (AGREE II) instrument. PLoS One 2015;10:1–22. 10.1371/journal.pone.0137180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Consolaro A, Ruperto N, Bazso A, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. 10.1002/art.24516 [DOI] [PubMed] [Google Scholar]
- 8. Consolaro A, Negro G, Chiara Gallo M, et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three-variable juvenile arthritis disease activity score. Arthritis Care Res 2014;66:1703–9. 10.1002/acr.22393 [DOI] [PubMed] [Google Scholar]
- 9. Wallace CA, Ruperto N, Giannini E, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004;31:2290–4. [PubMed] [Google Scholar]
- 10. Pincus T, Castrejon I. Evidence that the strategy is more important than the agent to treat rheumatoid arthritis. Data from clinical trials of combinations of non-biologic DMARDs, with protocol-driven intensification of therapy for tight control or treat-to-target. BullHospJtDis 2013;71(Suppl 1):S33–40. [PubMed] [Google Scholar]
- 11. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritiswith synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017. [DOI] [PubMed] [Google Scholar]
- 12. Consolaro A, Van Dijkhuizen EHP, Espada G, et al. Development of New JADAS and cJADAS cut-offs for disease activity states in oligoarthritis and rf-negative polyarthritis from a large multinational cohort of children with juvenile idiopathic arthritis. Pediatr Rheumatol 2017;12(Suppl 2):64. [Google Scholar]
- 13. Swart JF, de Roock S, Prakken BJ. Understanding inflammation in juvenile idiopathic arthritis: How immune biomarkers guide clinical strategies in the systemic onset subtype. Eur J Immunol 2016;46:2068–77. 10.1002/eji.201546092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2017-212104supp001.pdf (54.7KB, pdf)
annrheumdis-2017-212104supp002.pdf (72KB, pdf)
annrheumdis-2017-212104supp003.pdf (85.9KB, pdf)