Abstract
Objectives
To develop and validate an outcome measure for assessing fears in patients with rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA).
Methods
Fears were identified in a qualitative study, and reformulated as assertions with which participants could rate their agreement (on a 0–10 numeric rating scale). A cross-sectional validation study was performed including patients diagnosed with RA or axSpA. Redundant items (correlation >0.65) were excluded. Internal consistency (Cronbach’s α) and factorial structure (principal component analysis) were assessed. Patients were classified into fear levels (cluster analysis). Associations between patient variables and fear levels were evaluated using multiple logistic regression.
Results
672 patients were included in the validation study (432 RA, 240 axSpA); most had moderate disease activity and were prescribed biologics. The final questionnaire included 10 questions with high internal consistency (α: 0.89) and a single dimension. Mean scores (±SD) were 51.2 (±25.4) in RA and 60.5 (±22.9) in axSpA. Groups of patients with high (17.2%), moderate (41.1%) and low (41.7%) fear scores were identified. High fear scores were associated with high Arthritis Helplessness Index scores (OR 6.85, 95% CI (3.95 to 11.87)); high Hospital Anxiety and Depression Scale anxiety (OR 5.80, 95% CI (1.19 to 4.22)) and depression (OR 2.37, 95% CI (1.29 to 4.37)) scores; low education level (OR 3.48, 95% CI (1.37 to 8.83)); and high perceived disease activity (OR 2.36, 95% CI (1.10 to 5.04)).
Conclusions
Overall, 17.2% of patients had high fear scores, although disease was often well controlled. High fear scores were associated with psychological distress. This questionnaire could be useful both in routine practice and clinical trials.
Keywords: rheumatoid arthritis, ankylosing spondylitis, patient perspective
Introduction
Chronic inflammatory rheumatic diseases (CIRDs) such as rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) have a major impact on quality of life.1 They interfere with many aspects of daily functioning, including recreational activities, work, family life and relationships.2 These aspects of disease burden are frequently underestimated or unrecognised by the patient’s family and friends, as well as the treating physician.2 3 In addition, these diseases may be associated with considerable psychological distress, including anxiety or depression.4–6 Several studies, including a recent qualitative study in France,7 have shown that patients with CIRDs have specific fears about how their disease will progress, limitations in daily activities, being a burden on others and treatment.2 7–9 Although these aspects are important to patients, they are currently difficult to assess due to the lack of a specific evaluation tool.
Although several patient-reported outcome (PRO) measures assess emotional status or anxiety levels,10–13 many of these are generic and none, to our knowledge, specifically assess fears.14 A questionnaire focusing on CIRD-related fears would potentially be useful both in the context of everyday care (eg, to help understand patients’ motivations and reluctance towards treatments) and in clinical trials, since such fears may have an impact on the efficacy of a study drug.15 Current recommendations on PRO development and validation include grounding PROs in the patient perspective, and performing adequate psychometric validation of all such measures.16–20
The objectives of the present study were to develop a PRO for fear assessment in patients with RA or axSpA, and to perform a preliminary psychometric validation of the resulting instrument.
Methods
This study was part of a larger programme of research on patient perceptions in chronic progressive rheumatic diseases. The programme was supervised by a steering committee (the authors of this manuscript), composed of rheumatologists, psychologists, methodologists and representatives of the scientific staff of the Arthritis Fondation Courtin and of UCB Pharma, who jointly funded the programme.
Development of a preliminary questionnaire
In a previously published qualitative study,7 25 patients with RA and 25 with axSpA participated in semistructured interviews about their perceptions of the diseases. Interviews were transcribed verbatim, and the data extracted inductively from the interview transcripts. Fears about the future course of the disease, the impact of disease and its treatment were frequently expressed, and appeared to be shared in common between patients with axSpA and those with RA.
In the present study, all fears that were expressed by >5% of patients in the qualitative study were used. Non-redundant statements were then rephrased as assertions over two working sessions involving members of the Steering Committee and a patient research partner (a member of the EULAR PARE (People with Arthritis and Rheumatism) programme). The agreement with each item was assessed on a scale of 0–10 (‘totally disagree’ to ‘totally agree’). The questions were then tested in a sample of 10 patients with RA and 10 with axSpA for linguistic validation, and cognitive debriefing was performed during individual face-to-face interviews with trained interviewers. This preliminary questionnaire contained 23 items related to fears.
Validation study
This was a prospective, cross-sectional study in patients with RA or axSpA in France. Participants were recruited by hospital and community rheumatologists between July 2014 and October 2015.
Participants
All rheumatologists currently practising in France were invited to participate in the study through post and email. Each participating rheumatologist was expected to invite up to 20 consecutive patients with RA or axSpA attending a routine consultation who were aged >18 years, and had a diagnosis of RA according to the ACR/EULAR (American College of Rheumatology/European League Against Rheumatism) classification criteria,21 or of axSpA according to the ASAS (Assessment in Spondyloarthritis International Society) classification criteria.22 Patients with psoriatic arthritis or other CIRDs, and those who were unable to complete a questionnaire, were excluded.
Data collection
Patients were asked to complete the preliminary questionnaire, as well as the patient global assessment of overall disease activity (scored from 0 to 10), the Hospital Anxiety and Depression Scale (HADS),11 the Arthritis Helplessness Index (AHI)23 and, for patients with axSpA, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).24 The patient also provided information on sociodemographic indicators, health insurance coverage, disease duration and family history of rheumatic disease. In parallel, the rheumatologist provided information on the patient’s disease activity, as measured by the 28-item Disease Activity Score calculated with erythrocyte sedimentation rate (DAS28(ESR))25 for RA, and an overall assessment of disease activity scored from 0 to 10. Information on current treatment was also collected.
In order to assess the reproducibility of the questionnaire, 30 randomly selected patients were provided with two questionnaires and invited to complete and return the second one 2 weeks later.
Finalisation and psychometric validation of the Fear Assessment in Inflammatory Rheumatic diseases questionnaire
Finalising the questionnaire
The number of items on the fear dimensions was reduced to avoid redundancy. Interitem Pearson’s correlation coefficients between each pair of items were determined across the entire data set, and pairs presenting an r>0.65 were considered redundant. In such cases, the item considered most clear in wording by the Steering Committee was retained. In addition, items only relevant to a subgroup of patients, such as those relating to pregnancy (only applicable to women of childbearing age) or to professional activity (only applicable to people in work), were eliminated. The finalised questionnaire was translated from French into English through two independent forward and backward translations and reconciliation of the translated texts.26
Preliminary validation
All patients for whom both patient and physician questionnaires had been received were considered. Missing values were replaced according to a Missing at Random hypothesis. When the proportion of missing data was <5%, individual missing items were replaced with the median value of the corresponding variable. When the proportion exceeded 5%, multiple imputation methods based on Markov chains and Monte Carlo simulations were used. Score distribution was assessed using mean±SD and median with IQR scores for each disease population.
The factorial structure of the questionnaire was determined using principal component analysis, and eigenvalues calculated. A confirmatory factor analysis was then performed to determine goodness of fit, restricted to dimensions with eigenvalue >1.27 Internal coherence was assessed with Cronbach’s α coefficient.28 Test–retest stability of the PRO was evaluated by determining the Pearson’s correlation coefficient for total scores between two questionnaires completed at 2 weeks’ interval by 30 respondents. Coefficients >0.70 were considered to represent a strong correlation, and coefficients 0.50–0.70, a moderate correlation. The discriminative validity of the PRO was assessed by evaluating the relationship between the scores and other study variables expected to be related to the PRO score, such as HADS anxiety score, helplessness (AHI score) or disease activity score. Anxiety/depression and helplessness were expected to be moderately to strongly correlated with fears, whereas disease activity was expected to be only moderately correlated.
Identification of patient clusters and characteristics associated with fears
Subgroups of patients were identified according to their fear scores using descending cluster analysis (Ward method29). Optimal thresholds to distinguish between high and low fear score clusters were identified using receiver operating characteristic (ROC) curves based on the Youden index (optimal sensitivity and specificity).30
Univariate, then multivariate logistic regression was used to identify patient variables (including demographic, social and economic characteristics; disease status, and anxiety/depression and helplessness levels) independently associated with the highest compared with the lowest fear score cluster. Variables identified in the univariate analysis (p<0.20) were entered into a backward stepwise multiple logistic regression model.
All statistical analyses were performed using SAS V.9.2.
Ethics
The study was performed in accordance with Good Epidemiological Practice31 and relevant French guidelines for patient surveys. Verbal informed consent was obtained from all participating patients.
Results
Participants
All 1618 rheumatologists in France were contacted: 134 agreed to participate in the study, and 100 enrolled at least 1 patient. Twenty were exclusively community based, 51 exclusively hospital based and the remaining 29 had a mixed practice. A total of 796 patients were enrolled, of whom 672 (84.4%) were retained for analysis (see online supplementary figure 1). Patient characteristics are presented in table 1. Disease was moderately active, and use of biologics exceeded 70% in both the RA and axSpA patient populations.
Table 1.
Patient characteristics
| RA (n=432) |
axSpA (n=240) |
Total (n=672) |
|
| Age (years) | n=368 58.3±13.1 |
n=207 47.0±13.2 |
n=575 54.2±14.2 |
| Gender | n=373 | n=208 | n=581 |
| Female | 276 (74.0%) | 94 (45.2%) | 370 (63.7%) |
| Male | 97 (26.0%) | 114 (54.8%) | 211 (36.3%) |
| Professional activity | n=424 | n=237 | n=661 |
| In employment | 162 (38.2%) | 167 (70.5%) | 329 (49.8%) |
| Student | 2 (0.5%) | 1 (0.4%) | 3 (0.5%) |
| Unemployed | 8 (1.9%) | 19 (8.0%) | 27 (4.1%) |
| Retired | 201 (47.4%) | 30 (12.7%) | 231 (34.9%) |
| Other | 51 (12.0%) | 20 (8.4%) | 71 (10.7%) |
| Education level | n=427 | n=238 | n=665 |
| Primary | 77 (18.0%) | 11 (4.6%) | 88 (13.2%) |
| Secondary | 219 (51.3%) | 134 (56.3%) | 353 (53.1%) |
| Tertiary | 131 (30.7%) | 93 (39.1%) | 224 (33.7%) |
| Disease duration (years) | n=358 | n=203 | n=561 |
| 13.1±11.4 | 13.8±10.6 | 13.4±11.1 | |
| Disease activity | n=427 | n=236 | |
| DAS28 | 2.6±1.2 | – | – |
| BASDAI | – | 3.3±2.2 | – |
| Physician global assessment of disease activity (NRS) | n=419 | n=232 | n=651 |
| 2.75±2.12 | 3.44±2.41 | 3.00±2.25 | |
| Patient global assessment of disease activity (NRS) | n=382 | n=216 | n=598 |
| 3.03±2.45 | 4.27±2.61 | 3.48±2.58 | |
| Treatments | n=326 | n=238 | n=564 |
| None | 5 (1.5%) | 7 (2.9%) | 12 (2.1%) |
| Corticosteroids alone | 6 (1.8%) | – | 6 (1.1%) |
| NSAIDs alone | – | 36 (15.1%) | 36 (6.4%) |
| Synthetic DMARDs ± corticosteroids | 61 (18.7%) | – | 61 (10.8%) |
| Synthetic DMARDs ± NSAIDs | – | 15 (6.3%) | 15 (2.7%) |
| Biological DMARDs (alone or in combination) | 252 (77.3%) | 173 (72.7%) | 425 (75.4%) |
| Other | 2 (0.6%) | 7 (2.9%) | 9 (0.7%) |
Data are presented as mean values±SD for continuous variables, and as frequency counts (%) for categorical variables.
axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; DAS28, 28-item Disease Activity Score; DMARD, disease-modifying antirheumatic drug; NRS, numerical rating scale; NSAID, non-steroidal anti-inflammatory drug; RA, rheumatoid arthritis.
annrheumdis-2017-212000supp001.jpg (161.9KB, jpg)
Finalisation of the Fear Assessment in Inflammatory Rheumatic diseases questionnaire
Factorial analysis of the initial 44-item questionnaire (which dealt with both fears and opinions) revealed two highly correlated dimensions related to fears: one to disease outcome, and the other to treatment. After exclusion of redundant items with interitem correlation coefficients >0.65 (see online supplementary table 1), the final scale comprised 10 items (table 2 and online supplementary tables 2 and 3). Each item is scored on a 10-point numerical rating scale ranging from 0 (no fear) to 10 (strong fear). The total score ranges from 0 to 100 and was calculated as the sum of the 10 individual item scores.
Table 2.
Mean scores for each item of the FAIR scale in patients with RA or axSpA
| RA n=432 |
axSpA n=240 |
Total population n=672 |
p Value | |
| Fears related to the progression and consequences of the disease | ||||
| I am afraid of suffering like I did before | 6.4±3.4 | 6.9±3.3 | 6.6±3.4 | 0.082 |
| I am afraid that my disease will progress quickly | 5.0±3.4 | 6.0±3.2 | 5.4±3.4 | <0.001 |
| I am afraid that my spine or some of my bones will fuse together | 4.1±3.6 | 6.4±3.4 | 5.0±3.7 | <0.001 |
| I am afraid I won’t get any help if I lose my autonomy | 4.4±3.7 | 4.9±3.7 | 4.6±3.7 | 0.066 |
| I am afraid I won’t be able to cope with my daily tasks | 6.3±3.2 | 6.9±2.9 | 6.5±3.1 | 0.028 |
| I am afraid I will be considered as a disabled person | 4.9±3.8 | 5.4±3.7 | 5.1±3.7 | 0.118 |
| I am afraid that a time will arrive when no treatment will work for me anymore | 5.2±3.8 | 6.3±3.7 | 5.6±3.8 | <0.001 |
| Fears related to treatment | ||||
| I am afraid of the side effects of treatment for my disease | 6.0±3.2 | 6.3±3.1 | 6.1±3.2 | 0.245 |
| I am afraid that treatments for my disease may cause cancer | 4.1±3.6 | 4.8±3.5 | 4.4±3.6 | 0.013 |
| I am afraid that treatments for my disease will become less effective | 5.7±3.3 | 6.6±2.9 | 6.0±3.2 | 0.001 |
Probability values were determined using the Wilcoxon signed-rank test.
axSpA, axial spondyloarthritis; FAIR, Fear Assessment in Inflammatory Rheumatic diseases; RA, rheumatoid arthritis.
annrheumdis-2017-212000supp004.docx (182.1KB, docx)
Psychometric validation
Internal consistency was high (Cronbach’s α coefficient: 0.89). Principal component analysis identified a single dimension (eigenvalue: 5.1), which accounted for 51.2% of variance in the item scores. Confirmatory factor analysis matching the data to a unidimensional factorial structure revealed a goodness-of-fit index of 0.91. Twenty-eight patients (13 RA and 15 axSpA) provided two questionnaires completed 2 weeks apart. The test–retest correlation coefficient was ≥0.81. Total FAIR (Fear Assessment in Inflammatory Rheumatic diseases) scores were correlated with HADS anxiety (r=0.47; p<0.001) and depression (r=0.40; p<0.001) scores, and with AHI scores (r=0.50; p<0.001) (see online supplementary figure 2).
annrheumdis-2017-212000supp002.jpg (704.5KB, jpg)
Distribution of scores in patients with RA and axSpA
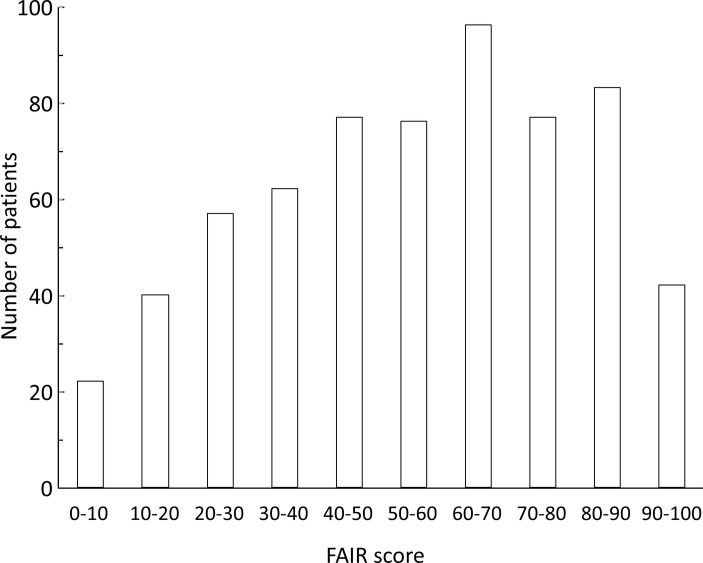
The mean and median FAIR scores were 54.9±24.9 and 57 (IQR: 35–75), respectively. Scores were higher in patients with axSpA (60.5±22.9; 65 (43–79)) than in patients with RA (51.8±25.4; 52 (33–71)). The distribution of PRO scores for the full data set is presented in figure 1. The mean item scores on the FAIR scale are presented in table 2 for the total study population, for patients with RA and for patients with axSpA. Mean fear scores were consistently higher for all items in patients with axSpA compared with those with RA.
Figure 1.
Distribution of FAIR (Fear Assessment in Inflammatory Rheumatic diseases) scores in the full study population. The full study population includes 368 patients with rheumatoid arthritis (RA) and 207 with axial spondyloarthritis (axSpA).
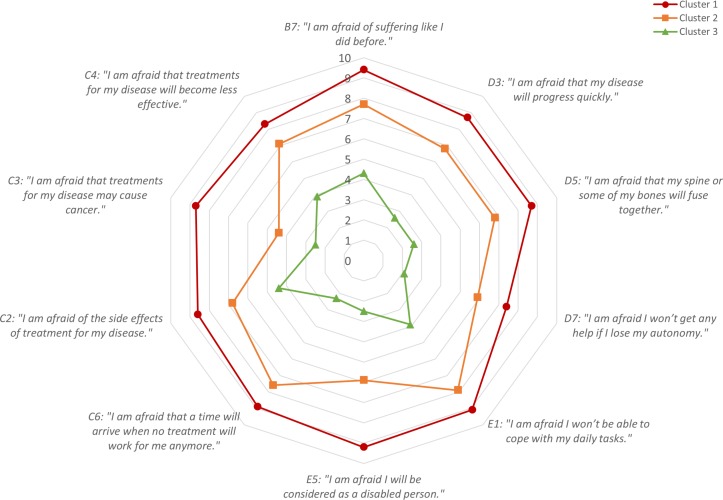
Subgroups of patients
Hierarchical cluster analysis identified three groups of patients characterised by high (cluster 1; n=116; 17.2%; mean score 87.0±7.9), moderate (cluster 2; n=276; 41.1%; mean score 65.8±11.4) and low levels of fear (cluster 3; n=280; 41.7%; mean score 31.1±14.7) (figure 2). These three clusters accounted for 68.3% of the variance in the data set. The most discriminating cut-off threshold to distinguish the high fear cluster from the other two was 77 (sensitivity: 0.90; specificity: 0.91). The most sensitive cut-off threshold to distinguish the low fear cluster from the other two was 51 (sensitivity: 0.92; specificity: 0.93). The area under the ROC curve was >0.97 in both cases (see online supplementary figure 3).
Figure 2.
Distribution of FAIR (Fear Assessment in Inflammatory Rheumatic diseases) scores in patients with rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA). Red: high fear cluster, n=116; orange: moderate fear cluster, n=276; green: low fear cluster, n=280.
annrheumdis-2017-212000supp003.jpg (234.4KB, jpg)
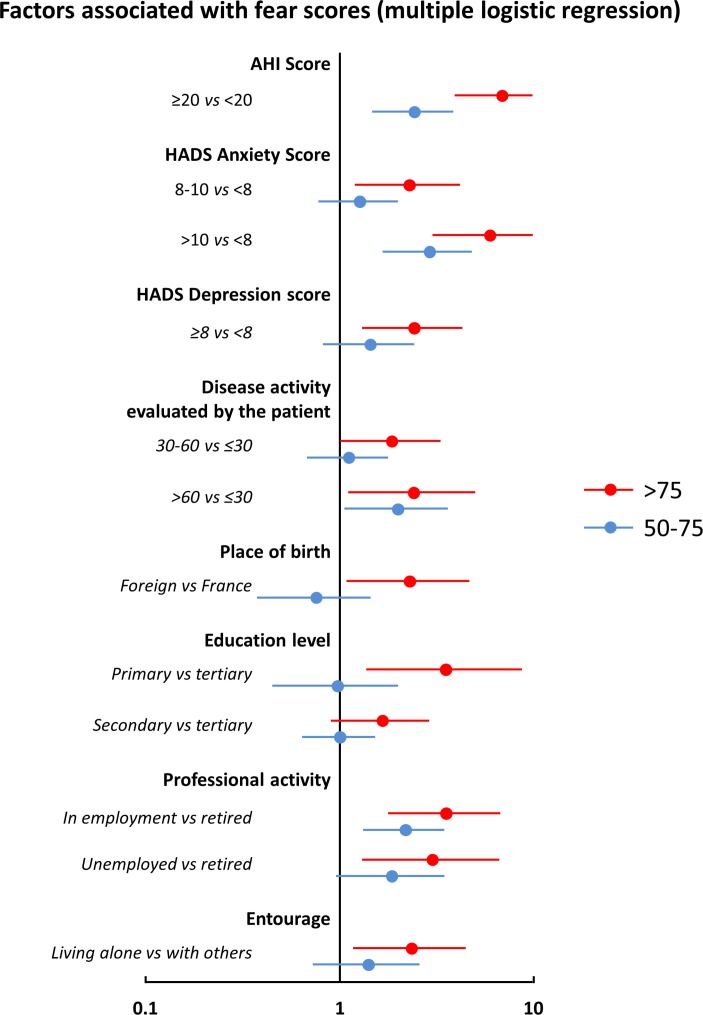
Multiple logistic regression analysis was used to determine patient characteristics independently associated with high fear scores, discriminating between patients in cluster 1 and those in cluster 3 (figure 3). Cluster 1 (high fear scores) was associated with higher global rating of disease activity by the patient, high AHI helplessness scores and high HADS anxiety and depression scores. With respect to sociodemographic variables, low education level, not working and living alone were also associated with higher FAIR score, as was immigrant status. No significant effects of disease type (axSpA vs RA) or age were observed. With respect to the patients in cluster 2 (moderate fear scores), the same variables were identified, although the ORs were lower.
Figure 3.
Variables independently associated with a high or moderate FAIR (Fear Assessment in Inflammatory Rheumatic diseases) score versus a low score in patients with rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA). High scores indicate those >75 (shown in red); moderate scores, 50–75 (shown in blue); and low scores, <50. Data are presented as ORs (95% CIs). AHI, Arthritis Helplessness Index; HADS, Hospital Anxiety and Depression Scale.
Discussion
This large national survey of patients with RA or axSpA generated two principal results. First, almost one-fifth (17.2%) of evaluated patients had high fear scores, despite both diseases being typically well managed, and these scores were associated with psychological distress. Thus, the fears identified in this study may reflect psychological distress, and need to be addressed even in patients who have moderate to low disease activity. Second, we have developed the FAIR questionnaire: a disease-specific, psychometrically validated PRO to measure disease and treatment-related fears in patients with RA or axSpA. This instrument demonstrated acceptable psychometric properties: notably unidimensionality, high internal coherence, good discriminant validity and adequate test–retest stability. The FAIR is short (10 items), simple to score and may be a useful tool both in routine practice and clinical trials.
The strengths of this study include the size of the study population, the high level of patient involvement in the development of the questionnaire and the psychometric validation16–20 of this instrument in line with the recommended guidelines. Limitations include a potential cultural bias, since the items were derived from a qualitative survey of patients in France, and potential redundancy with existing disease-specific PROs for CIRDs.12 32–34 These aspects will need to be evaluated in future studies. Although some questions within the questionnaire may seem redundant, statistical tests were used to remove truly redundant questions, and all questions underwent validation with patients.
In this study, it was possible to classify patients according to their level of fear using the FAIR score. Fear scores did not appear to be related to objective disease activity scores (DAS28(ESR) or BASDAI), although patients with high perceived disease activity (>6) were more frequently classified in the high fear cluster. In contrast, a strong association was observed between FAIR scores and scores on the AHI (≥20) or HADS (≥10 for anxiety and ≥8 for depression), all of which are non-specific markers of psychological distress.
Patients with RA commonly present a higher level of psychological distress compared with the general population.35 36 In agreement with this, we observed a robust association between fears and non-specific measures of psychological distress, such as the AHI, the HADS anxiety score and, to a lesser extent, the HADS depression score. Moreover, the fears expressed by our patients are likely to represent specific expressions of psychological distress in inflammatory rheumatic diseases. This would suggest that the FAIR questionnaire could be employed to measure psychological distress in a disease-specific way in patients with RA or axSpA. To this end, it might be beneficial to compare the FAIR questionnaire with existing generic scales, such as the mental component score of the SF-36 or SF-12 (36-Item and 12-Item Short Form Health Survey),10 or the anxiety and depression items of the Arthritis Impact Measurement Scales,12 in future studies. The FAIR instrument will also need to be tested in independent populations to verify its robustness and psychometric validity.
An association, although less marked, was also observed between FAIR scores and disease activity as rated by the patient. Four sociodemographic variables were also associated with high fear scores, namely low education level, living alone, being born outside France and either being in or seeking employment. Low education levels may be associated with lower access to, or more limited understanding of, information about the disease; this may also be the case for immigrants. Patients living alone may lack adequate social support for coping with stressful situations, and patients in employment or seeking employment may be particularly worried about the impact of their disease on their future career and income. On the other hand, age, gender, diagnosis (RA or axSpA) and treatment were not independently associated with high fear scores. Previous studies have identified female gender, lack of social support and a lower educational level as being associated with anxiety and depression (or both) in patients with RA.37–39
The FAIR questionnaire may be a useful PRO in several contexts. First, it may be helpful for physicians taking care of patients with RA and axSpA to evaluate the levels of fear and psychological distress in their patients, in order to provide an appropriate level of psychological support and to initiate a physician–patient dialogue to dispel unwarranted fears and facilitate adaptive coping. In clinical research, the questionnaire may be useful for investigating differences in psychological distress between patient groups, and to provide a basis for explaining such differences. Finally, the FAIR could be included in clinical trial protocols to measure the impact of specific interventions on psychological distress; however, this would first require an assessment of the instrument’s sensitivity to change. In this context, a disease-specific PRO might be more sensitive than a non-specific tool such as the HADS.
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Gabrielle von Krause (patient research partner; Paris, France) for developing the wording and content of the questionnaire; Susanne Wiegratz (UCB Pharma GmbH, Monheim am Rhein, Germany) for publication coordination; and Sam Fraser, PhD (Costello Medical Consulting Ltd, Cambridge, UK) for editorial assistance in preparing this manuscript for publication, based on the authors’ input and direction.
Footnotes
Handling editor: Tore K Kvien
Contributors: All authors were involved in drafting and in final approval of the publication, and revising it critically for important intellectual content.
Funding: UCB Pharma and Fondation Arthritis (France).
Competing interests: LG, PC, AS, CH, CD, ST, FB: no competing interests.
GC and J-MJ are employees of UCB Pharma; TdC was an employee of UCB Pharma at the time of the study; VS and FR-M are employees of Fondation Arthritis.
Ethics approval: The Ethics Committee of the St Antoine Hospital, Paris (session of 7 October 2014), the National Advisory Committee on Medical Research Information (CCTIRS) and the French national data protection agency (CNIL).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:123–30. 10.1016/j.semarthrit.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 2. Pouchot J, Le Parc JM, Queffelec L, et al. Perceptions in 7700 patients with rheumatoid arthritis compared to their families and physicians. Joint Bone Spine 2007;74:622–6. 10.1016/j.jbspin.2006.11.024 [DOI] [PubMed] [Google Scholar]
- 3. Riemsma RP, Taal E, Rasker JJ. Perceptions about perceived functional disabilities and pain of people with rheumatoid arthritis: differences between patients and their spouses and correlates with well-being. Arthritis Care Res 2000;13:255–61. [DOI] [PubMed] [Google Scholar]
- 4. Bacconnier L, Rincheval N, Flipo RM, et al. Psychological distress over time in early rheumatoid arthritis: results from a longitudinal study in an early arthritis cohort. Rheumatology 2015;54:520–7. 10.1093/rheumatology/keu371 [DOI] [PubMed] [Google Scholar]
- 5. Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep 2008;10:258–64. 10.1007/s11920-008-0042-1 [DOI] [PubMed] [Google Scholar]
- 6. Lok EY, Mok CC, Cheng CW, et al. Prevalence and determinants of psychiatric disorders in patients with rheumatoid arthritis. Psychosomatics 2010;51:338–338.e8. 10.1176/appi.psy.51.4.338 [DOI] [PubMed] [Google Scholar]
- 7. Berenbaum F, Chauvin P, Hudry C, et al. Fears and beliefs in rheumatoid arthritis and spondyloarthritis: a qualitative study. PLoS One 2014;9:e114350 10.1371/journal.pone.0114350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamilton-West KE, Quine L. Living with ankylosing spondylitis: the patient’s perspective. J Health Psychol 2009;14:820–30. 10.1177/1359105309341394 [DOI] [PubMed] [Google Scholar]
- 9. Lütze U, Archenholtz B. The impact of arthritis on daily life with the patient perspective in focus. Scand J Caring Sci 2007;21:64–70. 10.1111/j.1471-6712.2007.00443.x [DOI] [PubMed] [Google Scholar]
- 10. Ware JE, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care 1995;33:AS264–79. [PubMed] [Google Scholar]
- 11. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 12. Meenan RF, Gertman PM, Mason JH. Measuring health status in arthritis. The arthritis impact measurement scales. Arthritis Rheum 1980;23:146–52. [DOI] [PubMed] [Google Scholar]
- 13. Pincus T, Swearingen C, Wolfe F. Toward a multidimensional health assessment questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum 1999;42:2220–30. [DOI] [PubMed] [Google Scholar]
- 14. Kilic L, Erden A, Bingham CO, et al. The reporting of patient-reported outcomes in studies of patients with rheumatoid arthritis: a systematic review of 250 articles. J Rheumatol 2016;43:1300–5. 10.3899/jrheum.151177 [DOI] [PubMed] [Google Scholar]
- 15. Stolwijk C, Castillo-Ortiz JD, Gignac M, et al. Importance of contextual factors when measuring work outcome in ankylosing spondylitis: a systematic review by the OMERACT worker productivity group. Arthritis Care Res 2015;67:1316–27. 10.1002/acr.22573 [DOI] [PubMed] [Google Scholar]
- 16. European medicines agency. Committee for medicinal products for human use (CHMP): reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. London: EMA, 2005. [Google Scholar]
- 17. US department of health and human services and food and drug administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring: FDA, 2009. [Google Scholar]
- 18. Kirwan JR, Bartlett SJ, Beaton DE, et al. Updating the OMERACT filter: implications for patient-reported outcomes. J Rheumatol 2014;41:1011–5. 10.3899/jrheum.131312 [DOI] [PubMed] [Google Scholar]
- 19. Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539–49. 10.1007/s11136-010-9606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res 2013;22:1889–905. 10.1007/s11136-012-0344-y [DOI] [PubMed] [Google Scholar]
- 21. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 22. Zeidler H, Amor B. The assessment in spondyloarthritis international society (ASAS) classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general: the spondyloarthritis concept in progress. Ann Rheum Dis 2011;70:1–3. 10.1136/ard.2010.135889 [DOI] [PubMed] [Google Scholar]
- 23. Nicassio PM, Wallston KA, Callahan LF, et al. The measurement of helplessness in rheumatoid arthritis. The development of the arthritis helplessness index. J Rheumatol 1985;12:462–7. [PubMed] [Google Scholar]
- 24. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 25. Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 26. Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health 2005;8:94–104. 10.1111/j.1524-4733.2005.04054.x [DOI] [PubMed] [Google Scholar]
- 27. Jöreskog KG. A general approach to confirmatory maximum likelihood factor analysis. Psychometrika 1969;34:183–202. 10.1007/BF02289343 [DOI] [Google Scholar]
- 28. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16:297–334. 10.1007/BF02310555 [DOI] [Google Scholar]
- 29. Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc 1963;58:236–44. 10.1080/01621459.1963.10500845 [DOI] [Google Scholar]
- 30. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 31. Council for International Organizations of Medical Sciences. International ethical guidelines for epidemiological studies. Geneva: CIOMS, 2008. [Google Scholar]
- 32. Cleanthous S, Isenberg DA, Newman SP, et al. Patient uncertainty questionnaire-rheumatology (PUQ-R): development and validation of a new patient-reported outcome instrument for systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) in a mixed methods study. Health Qual Life Outcomes 2016;14:33 10.1186/s12955-016-0432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman S, Fitzpatrick R, Lamb R, et al. Patterns of coping in rheumatoid arthritis. Psychol Health 1990;4:187–200. 10.1080/08870449008400389 [DOI] [Google Scholar]
- 34. Gossec L, Dougados M, Rincheval N, et al. Elaboration of the preliminary rheumatoid arthritis impact of disease (RAID) score: a EULAR initiative. Ann Rheum Dis 2009;68:1680–5. 10.1136/ard.2008.100271 [DOI] [PubMed] [Google Scholar]
- 35. Dickens C, McGowan L, Clark-Carter D, et al. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med 2002;64:52–60. 10.1097/00006842-200201000-00008 [DOI] [PubMed] [Google Scholar]
- 36. Pincus T, Griffith J, Pearce S, et al. Prevalence of self-reported depression in patients with rheumatoid arthritis. Br J Rheumatol 1996;35:879–83. 10.1093/rheumatology/35.9.879 [DOI] [PubMed] [Google Scholar]
- 37. Zyrianova Y, Kelly BD, Gallagher C, et al. Depression and anxiety in rheumatoid arthritis: the role of perceived social support. Ir J Med Sci 2006;175:32–6. 10.1007/BF03167946 [DOI] [PubMed] [Google Scholar]
- 38. Evers AW, Kraaimaat FW, Geenen R, et al. Longterm predictors of anxiety and depressed mood in early rheumatoid arthritis: a 3 and 5 year followup. J Rheumatol 2002;29:2327–36. [PubMed] [Google Scholar]
- 39. Dirik G, Karanci AN. Psychological distress in rheumatoid arthritis patients: an evaluation within the conservation of resources theory. Psychol Health 2010;25:617–32. 10.1080/08870440902721818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2017-212000supp001.jpg (161.9KB, jpg)
annrheumdis-2017-212000supp004.docx (182.1KB, docx)
annrheumdis-2017-212000supp002.jpg (704.5KB, jpg)
annrheumdis-2017-212000supp003.jpg (234.4KB, jpg)