Abstract
Background
Pharmacists’ completion of medication reconciliation in the community after hospital discharge is intended to reduce harm due to prescribed or omitted medication and increase healthcare efficiency, but the effectiveness of this approach is not clear. We systematically review the literature to evaluate intervention effectiveness in terms of discrepancy identification and resolution, clinical relevance of resolved discrepancies and healthcare utilisation, including readmission rates, emergency department attendance and primary care workload.
Methods
This is a systematic literature review and meta-analysis of extracted data. Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE, Allied and Complementary Medicine Database (AMED), Education Resources Information Center (ERIC), Scopus, NHS Evidence and the Cochrane databases were searched using a combination of medical subject heading terms and free-text search terms. Controlled studies evaluating pharmacist-led medication reconciliation in the community after hospital discharge were included. Study quality was appraised using the Critical Appraisal Skills Programme. Evidence was assessed through meta-analysis of readmission rates. Discrepancy identification rates, emergency department attendance and primary care workload were assessed narratively.
Results
Fourteen studies were included, comprising five randomised controlled trials, six cohort studies and three pre–post intervention studies. Twelve studies had a moderate or high risk of bias. Increased identification and resolution of discrepancies was demonstrated in the four studies where this was evaluated. Reduction in clinically relevant discrepancies was reported in two studies. Meta-analysis did not demonstrate a significant reduction in readmission rate. There was no consistent evidence of reduction in emergency department attendance or primary care workload.
Conclusions
Pharmacists can identify and resolve discrepancies when completing medication reconciliation after hospital discharge, but patient outcome or care workload improvements were not consistently seen. Future research should examine the clinical relevance of discrepancies and potential benefits on reducing healthcare team workload.
Keywords: medication reconciliation, pharmacists, primary care, transitions in care
Background
There is growing policy interest in improving the safety of transition between different health service locations or settings.1–4 Transitions include admission to hospital from the community, transfers within secondary care and discharge back to the community. Safe transitions often require coordinating care with healthcare professionals in both primary and secondary care and providing patients with accessible information on post-transition care.5 One area where these actions are crucial is in communicating medication information. Harm from prescribed or omitted medications is higher after discharge, and effective medication reconciliation has been promoted as one way to improve safety.1–4 6–8 Multiple definitions of medication reconciliation exist, but all involve defining the list of medications the patient should be taking, altering records to reflect changes and ensuring patients and/or carers are aware of the changes.1–3 9
At the transition from hospital to community, medication reconciliation is necessary for hospital-initiated medication changes to be maintained. The medication taken by patients in the community, and prescribed by their general practitioner or primary care physician (from now on both termed GP), is often changed during hospital admissions.10 On discharge, a document is sent to the patient’s GP, and sometimes their community pharmacist, detailing medication regimen changes implemented during their inpatient stay. Medication reconciliation ensures the list held by the GP or community pharmacist (preadmission medication) is updated to reflect hospital-initiated changes. Following this process, discrepancies that exist between the primary care list of medications and the discharge medication list are either intentional discrepancies (a conscious decision has been made not to implement changes) or unintentional.
From the perspective of the UK, models for providing primary healthcare are changing. As in many sectors of healthcare, the roles of pharmacists (and other health professionals) are being extended.11 Completion of medication reconciliation by community pharmacists (whose traditional role is medication dispensing) and primary care pharmacists (employed by primary care organisations) has been prioritised.12 13 It is assumed this will increase the safety of care after discharge, improve outcomes such as readmission rate and have workload benefits by freeing clinical time for GPs. While in secondary care improvements in patient outcomes of this type of intervention have been reported, effectiveness in the community has not been established.7 A previous systematic review that examined all interventions to improve medication reconciliation in primary care found two studies that evaluated medication reconciliation after hospital discharge by pharmacists.14 These were of low quality and evidence of benefit was not found. A further systematic review evaluated all interventions (including medication reconciliation) undertaken by pharmacists in the community after hospital discharge.15 This showed that pharmacists can identify potential drug-related problems, but the impact on outcomes, such as healthcare utilisation, was inconsistent.
We aimed to focus, in depth, on medication reconciliation performed by community and primary care pharmacists after hospital discharge, by systematically reviewing published studies that compared this process with usual care. The aim was to determine the effectiveness of this intervention on overall discrepancy identification and resolution, the clinical relevance of resolved discrepancies and healthcare utilisation in terms of readmission rates, emergency department attendance and primary care workload.
Methods
The study was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) group guidelines.16 The completed PRISMA checklist is included as an online supplementary file
bmjqs-2017-007087supp001.docx (14.2KB, docx)
Scope of the review
Studies were included that compared community and primary care-based pharmacist-led medication reconciliation with usual practice. We defined medication reconciliation as the reconciliation of preadmission and postadmission lists of medication. Many studies evaluated interventions that included medication reconciliation combined with other actions. Studies where drug-related problems (such as drug interactions) were identified and corrected were included,17 but studies focused on medication review (eg, recommendations to optimise medication regimens) were not.18 Randomised controlled trials (RCTs), cohort studies and pre–post intervention studies were included.
Information sources
We searched the Medline (Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost), EMBASE (Ovid), Allied and Complementary Medicine Database (AMED) (Ovid), Education Resources Information Center (ERIC) (Ovid), NHS Evidence, Cochrane electronic databases and Scopus databases from inception until 1 September 2017. The reference lists of selected studies were hand-searched to identify any additional relevant studies. Citations were imported into RefWorks and all versions of citations lists were kept.19
Search strategy
To identify studies pertaining to our definition of medication reconciliation, a combination of medical subject heading terms and free-text search terms was developed by the review team in collaboration with a knowledge manager, a qualified librarian whose role includes searching and accessing published healthcare evidence. To identify studies describing medication reconciliation, the search terms ‘medication reconciliation’, ‘medicines reconciliation’, ‘medication discrepancy’, ‘medication error’, ‘medication adherence’ and ‘medication counselling’ were combined. Search terms to identify studies at discharge from hospital included ‘discharge’, ‘transition’ and ‘patient transfer’, and terms to identify pharmacists included ‘pharmacist’, ‘pharmacy’ and ‘community pharmacy’. To identify studies set in the community rather than in hospital, several terms were combined, including ‘primary health care’, ‘ambulatory care’, ‘family practice’, ’general practitioner’ and ‘home care services’. No limit was placed on date of publication or language, and the search was adapted for each database. The final search syntax for Medline is available as an online supplementary file.
Eligibility criteria
For inclusion, studies had to fulfil the criteria in table 1. Following removal of duplicates, two reviewers independently screened titles and abstracts of all citations (DM and MR). Full texts of all articles considered to be relevant were obtained and screened by two reviewers independently (DM and MR). Disagreements were resolved by discussion of full article content with the remaining reviewers.
Table 1.
Study inclusion criteria
| Characteristics | Criteria for quantitative studies |
| Population | Patients discharged from hospital to their permanent residence (home, residential unit or nursing home) |
| Intervention of interest | Medicines reconciliation completed by a pharmacist based in the community |
| Comparator | Usual care processes for medication reconciliation |
| Outcome measure | Discrepancy identification Discrepancy categorisation Healthcare usage (readmission, emergency department attendance, GP attendance) Workload/efficiency measures—time to complete medicines reconciliation, effect on number of primary and secondary care appointments needed, and economic outcomes |
| Study design | RCTs, cluster RCTs, quasi-RCTs, cluster quasi-RCTs, controlled pre–post intervention studies, interrupted-time-series, cohort studies (prospective or retrospective), case–control studies, uncontrolled pre–post intervention studies |
| Language | No limitation |
| Publication date | No limitation |
GP, general practitioner or primary care physician; RCTs, randomised controlled trials.
Data extraction
Once the final set of studies was agreed, the lead reviewer (DM) extracted data from all studies. A second data extraction was completed independently by another member of the review team. A template was created to allow collection of data relevant to the study questions. This was piloted with two studies and adapted following discussion of extracted data by the review team. The data extracted comprised details of the authors, publication, funding, aims, study design, inclusion and exclusion criteria, method of allocation to intervention or control group, sample sizes, participant characteristics, setting and details of the intervention, statistical techniques used, outcome data, and reported strengths, weaknesses and conclusions.
Study details were tabulated to codify the study design, type of pharmacists, setting of intervention, number, timing and duration of contacts, and the description of collaboration with other team members. The outcome data that were extracted from each paper were rates of identification and resolution of discrepancies; rates of resolution of clinically relevant discrepancies; and measures of healthcare utilisation (rates of readmission, emergency department attendance, GP attendance and measures of healthcare team member workload).
Risk of bias
The quality of each study and risk of bias were assessed independently by the two reviewers who performed the data extraction using the relevant Critical Appraisal Skills Programme (CASP) tools.20 These checklists facilitate a systematic approach to considering the presence or absence of certain elements within the study that may cause bias. Following completion of the CASP tool, the two reviewers discussed their findings for each study and graded the risk of bias as low, moderate or high. For example, one section asks: ‘Were controls recruited in an acceptable way?’ Selection bias may be introduced if participants are not randomised but could select allocation to the intervention or control group. Studies that recruited control groups in this manner would be deemed to have a higher risk of bias.
Data synthesis and analysis
Studies were grouped into RCTs, case–control studies and pre–post intervention studies. Other than for readmission rate, meta-analysis of outcome data could not be performed due to lack of data, heterogeneity of data and method of reporting outcome. To synthesise discrepancy rate resolution and healthcare utilisation data, outcomes were compared narratively with the appraised risk of bias of each study defined by the weight given to findings.
Meta-analysis of readmission data was performed by calculating the Mantel-Haenszel risk ratio (RR) and 95% CIs. As interventions in the included studies varied, it was thought that there would not be one ‘true’ effect size; therefore, a random-effects model was used within the Cochrane Review Manager (RevMan) V.5.3 software to synthesise results by constructing a forest plot.21 For studies that reported outcomes over different durations, the longest follow-up period for which all data were presented was used for analysis. Statistical heterogeneity was assessed by calculating τ2, χ2, I2 and P values. Publication bias was evaluated by construction and inspection of a funnel plot.
Results
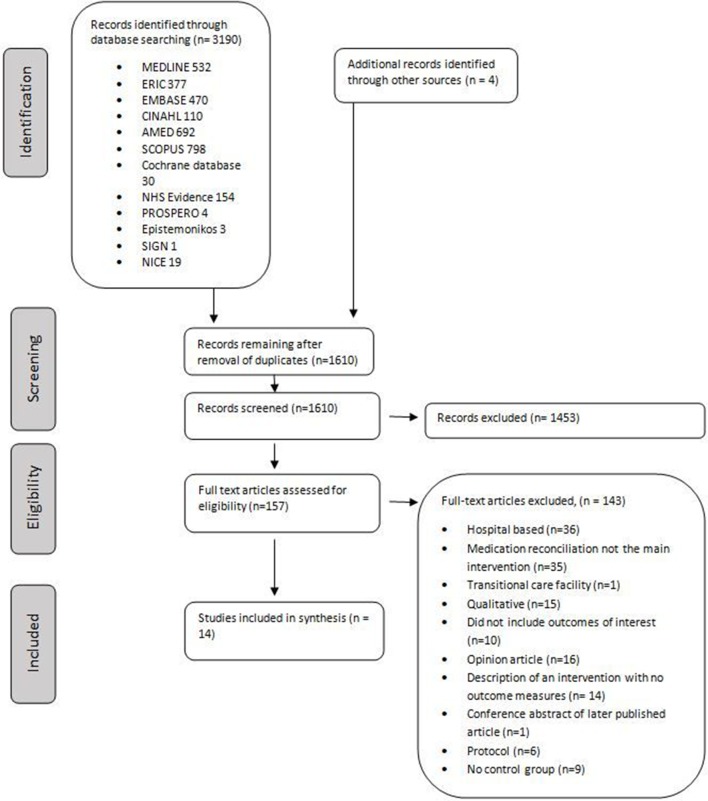
The electronic database search identified 3220 citations, with four more identified from the reference lists of included studies. After removal of duplicates, 1610 citations remained. Following title and abstract review, 157 publications underwent full-text review. Fourteen studies met the inclusion criteria (figure 1).
Figure 1.
PRISMA flow diagram of selection of eligible studies. NICE, National Institute for Health and Care Excellence; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; AMED, Allied and Complementary Medicine Database; ERIC, Education Resources Information Center; CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Characteristics of included studies
Five included studies were RCTs, six were cohort studies, two were pre–post intervention studies and one was a quality improvement (QI) project that presented a run chart detailing pre–post intervention data (table 2). Two studies17 22 were deemed to have a low risk of bias. Although they were not blinded, both studies were RCTs and described robust randomisation techniques to intervention or control group that otherwise received similar care. All significant results were presented and treatment effects were presented in a precise manner. Eight studies were deemed to be of moderate risk of bias.23–30 RCTs in this group had less robust randomisation,24 25 had low numbers25 and were unable to account for all patients who entered the study (one24 reported a large dropout rate, and another25 had several patients who were unable to be reached by telephone for follow-up). Cohort studies in this group had robust methods to select controls and presented relevant data in a precise manner.26–29 Four studies had a high risk of bias.31–34 These studies had less robust methods for assigning patients to intervention or control groups,31 32 or did not present all information on group allocation.33 34
Table 2.
Description of study and intervention characteristics including collaboration between pharmacist and GP of included studies
| Study | Country | Study design | Risk of bias | Authors extracting data and assessing bias | Characteristics and number of participants | Setting | Contacts (n) | Timing of contacts | Length of follow-up observation | Collaboration with healthcare team |
| Nazareth et al 22 | UK | RCT | Low | DM, PB | Patients discharged from elderly care wards Intervention=181 Control=181 |
Home visit by community pharmacist | 1 or 2 | 7–14 days | 3 and 6 months | Liaise with GPs |
| Holland et al 17 | UK | RCT | Low | DM, JM | Age >80 on two or more medicines Intervention=429 Control=400 |
Home visit | 2 | 14 and 60 days | 6 months | Send report to GP |
| Ho et al 23 | USA | RCT | Moderate | DM, MR | Admitted to one of 4 Veteran Affairs hospital with acute coronary syndrome Exclude if used non-Veteran Affairs pharmacy Intervention=122 Control=119 |
Primary care clinic | 2 | 7–10 days—visit 30 days phone call |
12 months | Send report to GP |
| Duggan et al 24 | UK | RCT | Moderate | DM, GM | Age 16–79 recruited by ward pharmacist Intervention=237 Control=264 |
Community pharmacy | 0 | N/A | N/A | Not clear |
| Hawes et al 25 | USA | RCT | Moderate | DM, AR | Year 1: long-term condition or more than 3 admissions, or 8 or more medication Year 2: 8 or more medications Intervention=24 Control=37 |
Primary care clinic | 1 | 3 days | 30 days | Seen prior to GP appointment |
| Shcherbakova and Tereso26 | USA | Cohort | Moderate | DM, JM | Patients enrolled in health plan 180 days before admission Intervention=156 Control=89 |
Home visit | 1 | 8 days | 30 days | Contact GP to authorise changes |
| Kilcup et al 27 | USA | Cohort | Moderate | DM, AR | Patients considered high risk for readmission Intervention=243 Control=251 |
Home visit | 1 | 3–7 days | 30 days | Send report to GP |
| Setter et al 28 | USA | Cohort | Moderate | DM, GM | Age >50 transitioning from acute to home care with long-term condition Intervention=110 Control=110 |
Home visit | 1 | Not clear | 60 days | Work with community nurses and send report to GP |
| Tedesco et al 30 | USA | Cohort | Moderate | DM, JM | Age >65 Intervention=34 Control=43 |
Primary care clinic | 1 or 2 phone calls and follow-up face-to-face review if needed | Phone call within 3 days, face-to-face 7–14 days | 30 days | Discussed with GP |
| Polinski et al 31 | USA | Cohort | High | DM, JM | Considered high or moderate risk of readmission Intervention=131 Control=131 |
By telephone or in patient home | Mean number contacts 5; details not fully reported | 3 days | 30 days | Contacted GP to arrange appointments and report medication changes and health concerns |
| Zeitouni et al 33 | USA | Cohort | High | DM, GM | Identified as high risk of readmission Intervention=72 Control=24 |
Telephone | 1 | 2 days | 30 days | Arranged appointment with GP |
| Boockvar et al 29 | USA | Pre/post intervention | Moderate | DM, GM | Nursing home residents Intervention=87 Control=81 |
Nursing home | 1 | 1 day | 60 days | Send report to GP who responds to each request |
| Gray et al 32 | UK | Pre/post intervention | High | DM, MR | Discharged from elderly care wards Intervention=41 Control=45 |
GP practice | None | None | N/A | Email, send note or discuss with GP if needed |
| Vuong et al 34 | Canada | QI project—pre/post intervention | High | DM, JM | Nursing home residents Intervention=monthly sample of 10 patients |
Nursing home | 1 | 2 days before nursing home admission | 90 days | Three-way telephone call— pharmacist, nurse and GP |
GP, general practitioner or primary care physician; N/A, not applicable; QI, quality improvement; RCT, randomised controlled trials.
Sample sizes ranged from 61 patients25 to 829.17 Interventions varied by the patient group targeted, the setting within which it was completed, and the timing and number of contacts. Most studies targeted those considered at higher risk of readmission either through age17 22 30 32 or presence of a long-term condition.25 28 Five studies evaluated medication reconciliation undertaken by the pharmacist in the patient’s home,17 22 26–28 whereas in three studies medication reconciliation was performed with the patient at a primary care clinic appointment.23 25 30 In one study, medication reconciliation was completed by telephone,33 and in another, reconciliation was performed either at a home visit for those with high risk of medication-related problems or by telephone for those with moderate risk.31 Two were set in nursing homes29 34 and one in a community pharmacy.24 In two studies medication reconciliation was completed in the absence of the patient.24 32
In seven studies patients were contacted once,25–29 33 34 in two studies twice,17 23 and in three studies the number of contacts varied dependent on patient preference and perceived need by pharmacists.22 30 31 Medication reconciliation was completed 2 days before hospital discharge to the nursing home in one study.34 Six studies contacted the patient within the first week of discharge25 27 29–31 33 and four in the second week.17 22 23 26 In seven studies, pharmacists discussed outcomes of medication reconciliation with other team members such as the GP or nursing staff,22 25 26 30–33 whereas in four a written report was produced for other clinical staff.17 23 27 28
Effectiveness of identification, resolution and clinical relevance of discrepancies
The identification and resolution of discrepancies by pharmacists completing medication reconciliation was compared with usual care in four studies.24 25 28 32 In all four studies, rates of identification and resolution were greater in the intervention group (table 3).
Table 3.
Identification, resolution and clinical relevance of discrepancies and reported healthcare utilisation
| Study design | Study | Risk of bias | Discrepancy resolution | Clinical relevance of discrepancies | Healthcare utilisation |
| RCT | Nazareth et al 22 | Low | Not evaluated | Not evaluated | No statistically significant effect on readmission rate or GP attendance at 3 and 6 months Hospital readmission at 3 months: Intervention=64/164 (39.0%) Control=69/176 (39.2%) Hospital readmission between 3 and 6 months: Intervention=38/136 (27.9%) Control=43/151 (28.4%) Mean pharmacist time per visit: Journey time 17 min, visit time 38 min, admin time 32 min (total 1 hour 27 min) |
| Holland et al 17 | Low | Not evaluated | Not evaluated | Increased readmission rate at 6 months by 30% Total number admission over 6 months: Intervention=234/429 (54.5%) Control=178/426 (41.8%) Increased need for GP home visit by 43% Intervention=204 visits Control=125 visits Rate ratio 1.41, P=0.002 |
|
| Ho et al 23 | Moderate | Not evaluated | Not evaluated | No statistically significant reduction in readmission rate for revascularisation or for myocardial infarction at 12 months Intervention=22/122(18.0%) Control=26/119 (21.8%) Mean pharmacist time 3 hours 51 min |
|
| Duggan et al 24 | Moderate | Remaining unintentional discrepancy rate (per drug prescribed): Control=700/1328 (52.7%) Intervention=454/1408 (32.2%) |
Consensus panel judged to have possible adverse effects: Intervention=51/1408 (3.6%) Control=83/1328 (6.3%) Definite adverse effect: Intervention=23/1408 (1.6%) Control 41/1328 (3.1%) Absolute risk reduction 5.3% Number Needed to Treat=19 |
Not evaluated | |
| Hawes et al 25 | Moderate | Increased discrepancy resolution rate per patient: Intervention=6/12 (50%) Control=2/21 (9.5%) |
Type of discrepancy reported not clinical relevance | Reduced readmission rate at 30 days Intervention=0/24 (0%) Control=12/37 (40.5%) Reduced emergency department attendance at 30 days Intervention=0/24 (0%) Control=11/37 (29.7%) |
|
| Cohort | Shcherbakova et al 26 | Moderate | Pharmacist identified 301 medication-related problems in 156 patients=mean 1.93 per patient No figures reported for control group |
Type of discrepancy reported not clinical relevance | No statistically significant effect on readmission rate at 30 days Intervention=16/156 (10.3%) Control=6/89 (6.7%) No statistically significant difference in emergency department attendance at 30 days Intervention=34/156 (21.8%) Control=13/89 (14.6%) |
| Kilcup et al 27 | Moderate | Pharmacist resolved discrepancies present in >80% of patients (exact figures not given). Data on control group not measured and reported |
Type of discrepancy reported not clinical relevance | Reduction of readmission at 7 days and 14 days but not statistically significant at 30 days 30-day readmission rate: Intervention=28/243 (11.5%) Control=34/251 (13.5%) (P=0.29) |
|
| Setter et al 28 | Moderate | Increased resolution rate: Intervention=154/220 (70%) Control=139/231 (60%) |
Discrepancies classified as patient or system factors and not by clinical relevance | Reduced number of days admitted to hospital per patient in intervention group Intervention=0.4±1.2 Control=1.1±4.2 Reduced planned physician visits: Intervention=2.9±1.5 Control=3.5±2.7 Reduced unplanned physician visit: Intervention=0.2±0.6 Control=0.4±1.0 |
|
| Tedesco et al 30 | Moderate | Not evaluated | Not evaluated | Readmission 30 days Intervention=5/34 (14.7%) Control=12/45 (26.7%) P=0.27 |
|
| Polinski et al 31 | High | Discrepancy rate not reported | State 88 of 131 (67%) of medication reconciliation an omission of a prehospital medication or an identified gap based on clinical guidelines was identified Drug–drug interactions present in 21 of 131 (16%) of cases—no comment on severity |
Reduced 30 day readmission rate Intervention group=16/131 (12.2%) Control group=29/131 (22.1%) Risk ratio (95% CI)=0.5 (0.29 to 0.88) |
|
| Zeitouni et al 33 | High | Not reported | Not reported | Reduction in readmission at 1 month: Intervention=27% Control=45% |
|
| Pre/post intervention studies | Boockvar et al 29 | Moderate | Found 696 discrepancies following 259 discharges=2.69 per patient (not measured in preintervention phase) | Calculated a drug discrepancy risk index; where this was raised, two reviewers reviewed notes to determine if possible discrepancy related adverse drug event: Postintervention=1/43 (2.3%) Preintervention=10/69 (14.5%) |
No figures reported but state no difference in readmission rate Physician responded to discrepancies: Awareness of discrepancy=429/598 (71.7%) Intention to review=41/598 (6.9%) Intention to adjust regimen=49/598 (8.2%) Intention to increase monitoring=23/598 (3.8%) |
| Gray et al 32 | High | Increased resolution rate Intervention=33 plans implemented out of 41 (80.5%) Control=23 plans implemented out of 45 (51%) |
Examples of discrepancy listed but not quantified | Not evaluated | |
| Vuong et al 34 | High | No preintervention data presented Mean discrepancy rate of 2 per medication reconciliation reported postintervention |
No preintervention data presented Mean number of clinical concerns per medication reconciliation postintervention=6 |
90-day readmission and emergency department attendance rate—no difference preintervention and postintervention; remained at median of 13% for each cohort Freed up 3 hours of nursing time and 1 hour physician time Consulted with pharmacist for 2 hours |
GP, general practitioner or primary care physician.
Two studies compared the clinical relevance of resolved discrepancies between intervention and control groups and suggested that there was the potential for fewer adverse drug events after pharmacists had completed medication reconciliation (table 3).24 29 Seven studies described the type of discrepancy found when pharmacists perform medication reconciliation (such as drug–drug interaction identified) but did not describe the clinical relevance.25–28 31 32 34
Healthcare utilisation
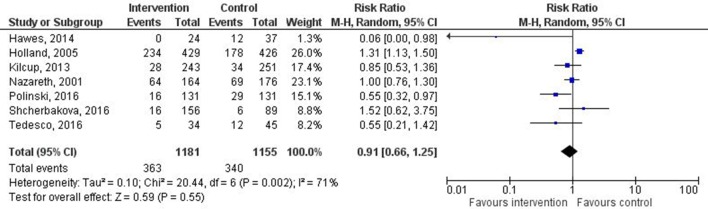
Healthcare utilisation was reported in 12 of the included studies. The different outcome measures reported included readmission rate at 1, 3, 6 and 12 months, emergency department attendance, and additional GP and secondary care consultations (table 3). Three studies reported a statistically significant reduction in readmission rate,25 31 33 whereas one reported an increase in readmission rate.17 Data from seven studies were included for meta-analysis. One study was excluded as only admissions related to myocardial infarction or coronary revascularisation were included,23 another as the number of days hospitalised (rather than readmission rate) was reported28 and three more were excluded as they did not report numbers of patients readmitted.29 33 34 One of these33 reported a reduced readmission rate, whereas the others29 34 reported no change. Two studies reported readmission rates over different time scales.22 27 In one study the longer time scale was used.27 The shorter time frame was used in the second study as the composite readmission rate over the longer time frame was not clear. The pooled RR across all included studies (total number of patients=2336) was 0.91 (95% CI 0.66 to 1.25), indicating no clear effect on readmission rate (figure 2). There was a high degree of statistical heterogeneity. As few studies were included, I2 is the most suitable statistic for assessing the impact of heterogeneity. An I2 value of 71% and P=0.002 were calculated, indicating high heterogeneity.35
Figure 2.
Forest plot of intervention effects on the proportion of patients with all-cause readmission. Diamond represents pooled estimate of relative risk calculated using Mantel-Haenszel (M-H) random effects model and 95% CIs. Squares represent study weighting, and horizontal bars represent 95% CI.
Emergency department attendance rate was measured in three studies.25 26 34 No difference was observed between intervention and control groups in two studies,26 34 whereas in one a large reduction was found and this was a small RCT with a moderate risk of bias.
One study17 reported an increase in GP visits of 43% in the intervention group, while another reported no significant difference in GP attendance.22 Two studies reported that pharmacist completing medication reconciliation had the potential to free up clinical time for other healthcare team members. One reported that 2 hours of pharmacist time freed 3 hours of nursing time and 1 hour of physician time,34 and the other stated that planned and unplanned physician visits were reduced.28 Three studies reported the mean time taken to complete medication reconciliation by pharmacist. This varied from 1 hour 27 min to 3 hours 51 min per patient.
Discussion
The literature was systematically reviewed to evaluate the effectiveness of pharmacist-led medication reconciliation performed in the community after hospital discharge. Pharmacists were more effective at identifying and resolving discrepancies compared with the usual care process. Meta-analysis did not demonstrate a statically significant reduction in readmission rates, and the effect on emergency department attendance and workload of other healthcare team members was rarely measured and no consistent evidence of related benefit was found.
Comparison with previous literature
Previous systematic reviews also reported the ability of pharmacists to effectively identify and resolve discrepancies in community14 and hospital settings.8 36 37 The clinical relevance of reduced discrepancy resolution has been questioned in studies set in the community as many discrepancies remained after interventions14 and the effect on patient outcomes was not consistent.15 Several of our included studies derived their taxonomies of discrepancies empirically, which did not aid evaluation of clinical relevance.25–28 31 32 34 The lowest mean time to complete medication reconciliation reported in our included studies was 1 hour 27 min.22 The time taken in usual care processes was never accurately reported. Having more time to perform this task may be the reason why more discrepancies are identified.
Unlike our study, a recent systematic review and meta-analysis of pharmacist-led medication reconciliation in hospital performed at care transitions demonstrated a reduction in healthcare use after discharge.7 One possible explanation is that Mekonnen et al included studies with multiple intervention components, including patient education, follow-up telephone call, home visit, medication review, enhanced communication with primary care and the use of strategies to enhance adherence. Interventions in our systematic review included some of these components but excluded those describing a medication review and, as medication reconciliation was performed in the community, infrequently involved interventions to improve primary/secondary care communication. This may reflect the problem of varying definitions of medication reconciliation. The WHO defines medication reconciliation as ‘The formal process in which healthcare professionals partner with patients to ensure accurate and complete medication information transfer at interfaces of care’.1 Such a definition may legitimately include all the aspects of interventions included by Mekonnen et al. The Joint Commission definition of ‘The process of comparing a patient’s medication orders to all of the medications that the patient has been taking’ is more precise and may not include such diverse activities.3 It may be that these additional components are important to influence health outcomes; however, recent systematic reviews of pharmacist-completed medication reviews in various settings have failed to show a benefit to patient outcomes.38 39
It is reported that roughly half of all discharge communications have been found to contain unintended medications.40 Performing an accurate medication reconciliation using such a list is unlikely to improve patient outcomes as unintended medications will continue to be prescribed.17 However, even when medication is reconciled before discharge and patients followed up by pharmacists to improve adherence, clinically important medication errors and harm due to medication are not reduced.40
Implication for future policy and research
The lack of effect on patient outcomes raises the question of what role the pharmacists should play postdischarge. Patients are at a high risk of harm due to medication following discharge, and the involvement of pharmacists seems a logical step to reduce the risk of harm.41 Despite this, there is a paucity of high-quality studies investigating pharmacist-led medication reconciliation postdischarge, and the few that do exist do not provide conclusive evidence of benefit. At present, pharmacist-completed medication reconciliation postdischarge cannot be promoted to reduce harm and improve health outcome. Future research must do more than evaluate process measures such as discrepancy rate detection, and focus on evaluating the clinical relevance of resolved discrepancies such as potential or actual adverse drug events. This may be more resource-intensive as clinical review of notes is required to make judgements on clinical relevance.24 29 40 In addition, the development of an agreed taxonomy of discrepancies would be beneficial to aid process evaluation of such interventions and understanding of discrepancy relevance and why they occur.9
The lack of improvement in patient outcomes may be less important to policymakers and front-line clinical teams if reduction in workload pressures improves performance in other areas of primary care such as face-to-face clinical care or administrative tasks such as laboratory test results handling. High levels of workload are perceived as a major safety concern in UK general practice, and one of the main policy drivers of pharmacist role development is to free clinical and administrative time for GPs.42 The effect of pharmacist-led medication reconciliation on these related systems has not been studied previously and further research is clearly needed.
If the pharmacist’s role in medication reconciliation postdischarge is to free clinical time, then implementation at scale will require significant financial and personnel resources.43 Future research should determine if these predicted efficiency savings exist and if other healthcare team members, such as pharmacy technicians or existing primary care staff, can perform medication reconciliation equally safely and improve cost-effectiveness.44 This research may identify if certain high-risk groups are more likely to derive benefit from pharmacy input postdischarge and what type of intervention has the most impact on medication safety (eg, reconciliation, review, adherence aids, health literacy aids).
Strengths and limitations
The search strategy included several relevant databases, with no limitation placed on date of publication or language. Broader terms than medication reconciliation were included in the search to incorporate studies reporting medication reconciliation as part of wider interventions. For example, although Holland et al describe their intervention as a medication review, we deemed it to be similar enough to our classification of medication reconciliation to be included. Screening for inclusion, data abstraction and quality appraisal were independently completed by two reviewers to enhance study rigour.
The study has several limitations. Some studies that would have been valuable in answering our questions may have been excluded as their focus of intervention was not on medication reconciliation per se.45 One study evaluated a community liaison pharmacist intervention but was based in hospital and so was excluded.46 Healthcare settings vary and findings from different countries may not be comparable. For example, studies were set in North American primary care services run by large organisations often with links to hospitals that may blur the lines between primary and secondary care.23 33 Others involved home care services that may not be present in other areas.28
The CASP tools used to assess bias and quality are designed for use in RCTs and cohort studies and were adapted to assess the quality of QI projects and pre–post intervention studies. This led to these studies being treated as having a higher risk of bias. Several of the included studies were described as pilot projects25 32 or QI projects,27 33 34 and require more robust evaluation of their findings to determine if they are replicated at scale or in different settings. Included studies were generally of low to moderate quality and susceptible to bias, which means the positive outcomes reported in this systematic review must be treated with caution.
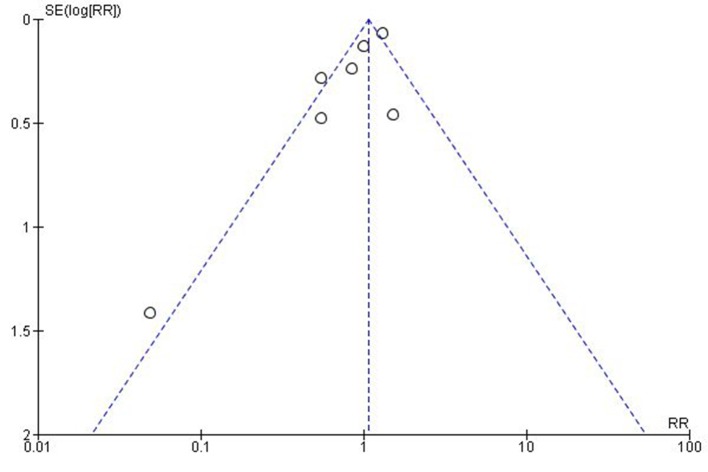
The meta-analysis of data from studies reporting readmission rates was limited to studies that reported similar outcomes; however, this approach may still be open to challenge. A high level of heterogeneity was identified with possible reasons including different study designs, settings, intervention components, outcome definitions and follow-up periods. This means that it is difficult to draw definitive conclusions from the meta-analysis other than to say that there is currently no firm evidence that readmission rate is reduced. Meta-analysis of other outcome measures was not possible due to heterogeneity of reported outcomes. For example, discrepancy identification rates were reported as the number of discrepancies per drug prescribed24; the number of patients in a study who had a discrepancy25; full or partial implementation of the patient plan32; and the number of discrepancies resolved.28 Despite the inclusion of a wide range of study type, publication bias may still influence results as demonstrated by the asymmetry of the funnel plot (figure 3). Of note, the smallest study showed the largest positive effect.25 It may be that smaller projects with less robust methods that did not show a positive effect were not published.
Figure 3.
Funnel plot of SE of risk ratio (RR) versus risk ratio.
Conclusions
This systematic review has shown that pharmacists can identify and resolve discrepancies while completing medication reconciliation after hospital discharge; however, the clinical relevance of these discrepancies has rarely been reported. The evidence does not support a reduction in readmission rates and there is not consistent evidence that other measures of healthcare utilisation, such as emergency department attendance and GP appointments, are reduced. Future research in this area should compare the clinical relevance of discrepancies identified and measure if this process reduces workload and thus frees clinical time in primary care.
Acknowledgments
The authors would like to thank Dr Lynda Cochrane for her guidance on the statistics used in this paper and Allan Gillies, NHS Education for Scotland, for assistance in designing the search strategy.
Footnotes
Contributors: DM and MR performed title, abstract and full-text screening. All authors discussed disagreements to reach consensus. DM extracted data from all included studies. A second data extraction was completed independently for each included study. This was divided evenly between PB, AR, GM and JM. All authors reviewed and contributed to writing the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Search syntax for all databases and all versions of citation lists are available from DM.
References
- 1. World Health Organization. Action on patient safety – high 5 s, 2014. http://www.who.int/patientsafety/implementation/solutions/high5s/High5_InterimReport.pdf?ua=1 (accessed 11 Oct 2017).
- 2. Institute for Healthcare Improvement. Medication reconciliation to prevent adverse drug events. http://www.ihi.org/topics/adesmedicationreconciliation/Pages/default.aspx (accessed 11 Oct 2107).
- 3. The Joint Commission. Using medication reconciliation to prevent errors. 2006. http://www.jointcommission.org/assets/1/18/SEA_35.pdf (accessed 11 Oct 2017). [PubMed]
- 4. ihub. Scottish patient safety programme in primary care. http://www.scottishpatientsafetyprogramme.scot.nhs.uk/programmes/primary-care (accessed 11 Oct 2017).
- 5. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150:178–87. 10.7326/0003-4819-150-3-200902030-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence. Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. London. 2015. https://www.nice.org.uk/guidance/ng5 (accessed 11 Oct 2017). [PubMed]
- 7. Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open 2016;6:e010003 10.1136/bmjopen-2015-010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehnbom EC, Stewart MJ, Manias E, et al. Impact of medication reconciliation and review on clinical outcomes. Ann Pharmacother 2014;48:1298–312. 10.1177/1060028014543485 [DOI] [PubMed] [Google Scholar]
- 9. Almanasreh E, Moles R, Chen TF. The medication reconciliation process and classification of discrepancies: a systematic review. Br J Clin Pharmacol 2016;82:645–58. 10.1111/bcp.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michaelsen MH, McCague P, Bradley CP, et al. Medication reconciliation at discharge from hospital: a systematic review of the quantitative literature. Pharmacy 2015;3:53–71. 10.3390/pharmacy3020053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Royal Pharmaceutical Society and Royal College of General Practitioners. Joint policy statement on general practice based pharmacists. 2015. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/01/building-the-workforce-new-deal-gp.pdf (accessed 11 Oct 2107).
- 12. The Scottish government. Prescription for excellence: a vision and action plan for the right pharmaceutical care through integrated partnerships and innovation. 2013. http://www.gov.scot/resource/0043/00434053.pdf (accessed 11 Oct 2107).
- 13. The Royal Pharmaceutical Society and Royal College of General Practitioners Joint policy statement on general practice based pharmacists. https://www.rpharms.com/Portals/0/RPS_document_library/Open_access/Policy_statements/rcgps-rps-joint-statement-on-general-practice-based-pharmacists.pdf (accessed 11 Oct 2107).
- 14. Bayoumi I, Howard M, Holbrook AM, et al. Interventions to improve medication reconciliation in primary care. Ann Pharmacother 2009;43:1667–75. 10.1345/aph.1M059 [DOI] [PubMed] [Google Scholar]
- 15. Nazar H, Nazar Z, Portlock J, et al. A systematic review of the role of community pharmacies in improving the transition from secondary to primary care. Br J Clin Pharmacol 2015;80:936–48. 10.1111/bcp.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holland R, Lenaghan E, Harvey I, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ 2005;330:293 10.1136/bmj.38338.674583.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barker A, Barlis P, Berlowitz D, et al. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. Int J Cardiol 2012;159:139–43. 10.1016/j.ijcard.2011.02.034 [DOI] [PubMed] [Google Scholar]
- 19. Refworks. www.refworks.com(accessed 11 Oct 2017)
- 20. Critical Appraisal Skills Programme (CASP). http://www.casp-uk.net/#!casp-tools-checklists/c18f8 (accessed 11 Oct 2107).
- 21. Cochrane Community. RevMan 5. Review manager 5 (RevMan 5) is the software used for preparing and maintaining cochrane Reviews. http://community.cochrane.org/tools/review-production-tools/revman-5 (accessed 11 Oct 2017).
- 22. Nazareth I, Burton A, Shulman S, et al. A pharmacy discharge plan for hospitalized elderly patients--a randomized controlled trial. Age Ageing 2001;30:33–40. 10.1093/ageing/30.1.33 [DOI] [PubMed] [Google Scholar]
- 23. Ho PM, Lambert-Kerzner A, Carey EP, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med 2014;174:2706–7. 10.1001/jamainternmed.2013.12944 [DOI] [PubMed] [Google Scholar]
- 24. Duggan C, Feldman R, Hough J, et al. Reducing adverse prescribing discrepancies following hospital discharge. Int J Pharm Pract 1998;6:77–82. 10.1111/j.2042-7174.1998.tb00920.x [DOI] [Google Scholar]
- 25. Hawes EM, Maxwell WD, White SF, et al. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. J Prim Care Community Health 2014;5:14–18. 10.1177/2150131913502489 [DOI] [PubMed] [Google Scholar]
- 26. Shcherbakova N, Tereso G. Clinical pharmacist home visits and 30-day readmissions in medicare advantage beneficiaries. J Eval Clin Pract 2016;22:363–8. 10.1111/jep.12495 [DOI] [PubMed] [Google Scholar]
- 27. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc 2013;53:78–84. 10.1331/JAPhA.2013.11250 [DOI] [PubMed] [Google Scholar]
- 28. Setter SM, Corbett CF, Neumiller JJ, et al. Effectiveness of a pharmacist-nurse intervention on resolving medication discrepancies for patients transitioning from hospital to home health care. Am J Health Syst Pharm 2009;66:2027–31. 10.2146/ajhp080582 [DOI] [PubMed] [Google Scholar]
- 29. Boockvar KS, Carlson LaCorte H, Giambanco V, et al. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother 2006;4:236–43. 10.1016/j.amjopharm.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 30. Tedesco GW, McConaha JL, Skomo ML, et al. A pharmacist’s impact on 30-day readmission rates when compared to the current standard of care within a patient-centered medical home: a pilot study. J Pharm Pract 2016;29:368–73. 10.1177/0897190014568671 [DOI] [PubMed] [Google Scholar]
- 31. Polinski JM, Moore JM, Kyrychenko P, et al. An insurer’s care transition program emphasizes medication reconciliation, reduces readmissions and costs. Health Aff 2016;35:1222–9. 10.1377/hlthaff.2015.0648 [DOI] [PubMed] [Google Scholar]
- 32. Gray S, Urwin M, Woolfrey S, et al. Copying hospital discharge summaries to practice pharmacists: does this help implement treatment plans? Qual Prim Care 2008;16:327–34. [PubMed] [Google Scholar]
- 33. ZeitounI R, Saha A, Gettys KE, et al. Improving transition of care for patients with high risk for readmission. J Gen Intern Med 2014;29:S486–S487. [Google Scholar]
- 34. Vuong V, O’Donnell D, Navare H, et al. BOOMR: better coordinated cross-sectoral medication reconciliation for residential care. Healthc Q 2017;20:34–9. 10.12927/hcq.2017.25075 [DOI] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 36. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med 2012;172:1057–69. 10.1001/archinternmed.2012.2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med 2013;158:397–403. 10.7326/0003-4819-158-5-201303051-00006 [DOI] [PubMed] [Google Scholar]
- 38. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev 2013;2:CD008986 10.1002/14651858.CD008986.pub2 [DOI] [PubMed] [Google Scholar]
- 39. Huiskes VJ, Burger DM, van den Ende CH, et al. Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Fam Pract 2017;18:5 10.1186/s12875-016-0577-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med 2012;157:1–10. 10.7326/0003-4819-157-1-201207030-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forster AJ, Clark HD, Menard A, et al. Effect of a nurse team coordinator on outcomes for hospitalized medicine patients. Am J Med 2005;118:1148–53. 10.1016/j.amjmed.2005.04.019 [DOI] [PubMed] [Google Scholar]
- 42. Bell BG, Reeves D, Marsden K, et al. Safety climate in English general practices: workload pressures may compromise safety. J Eval Clin Pract 2016;22:71–6. 10.1111/jep.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hodson K, Smith M, Blenkinsopp A, et al. Evaluation of the discharge medicines review service in wales: content analysis of discharge medicines reviews. http://www.cpwales.org.uk/Contract-support-and-IT/Advanced-Services/Discharge-Medicines-Review- (accessed11 Oct 2017).
- 44. Bailey JE, Surbhi S, Bell PC, et al. Using pharmacy technicians in a novel role as community health workers to improve transitions of care. J Am Pharm Assoc 2016;56:73–81. 10.1016/j.japh.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 45. Crotty M, Rowett D, Spurling L, et al. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother 2004;2:257–64. 10.1016/j.amjopharm.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 46. Bolas H, Brookes K, Scott M, et al. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharm World Sci 2004;26:114–20. 10.1023/B:PHAR.0000018601.11248.89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqs-2017-007087supp001.docx (14.2KB, docx)