Abstract
Evidence of sex-related differences in gastrointestinal (GI) functions has been reported in the literature. In addition, various GI disorders have disproportionate prevalence between the sexes. An essential step in the initiation of smooth muscle contraction is the phosphorylation of the 20-kDa regulatory myosin light chain (MLC20) by the Ca2+/calmodulin-dependent myosin light chain kinase (MLCK). However, whether male stomach smooth muscle inherits different contractile signaling mechanisms for the regulation of MLC20 phosphorylation from that in females has not been established. The present study was designed to investigate sex-associated differences in the regulation of MLC20 phosphorylation and thus muscle contraction in gastric smooth muscle cells (GSMCs). Experiments were performed on GSMCs freshly isolated from male and female rats. Contraction of the GSMCs in response to acetylcholine (ACh), a muscarinic agonist, was measured via scanning micrometry in the presence or absence of the MLCK inhibitor, ML-7. Additionally, the protein levels of MLC20, MLCK and phosphorylated MLC20 were measured by ELISA. The protein levels of MLC20 and MLCK were indifferent between the sexes. ACh induced greater contraction (P<0.05) as well as greater MLC20 phosphorylation (P<0.05) in male GSMCs compared with female. Pretreatment of GSMCs with ML-7 significantly reduced the ACh-induced contraction (P<0.05) and MLC20 phosphorylation (P<0.05) in the male and female cells, and notably, abolished the contractile differences between the sexes. In conclusion, MLC20 phosphorylation and thus muscle contraction may be activated to a greater extent in male rat stomach compared with that in females.
Keywords: contraction, myosin light chain, gastric, sex, smooth muscle
Introduction
It has been established in previous research that gastrointestinal (GI) motility function is affected by gender (1). Healthy women have also been identified to have slower gastric emptying of solids compared with men (2). This may explain why gastroparesis - a chronic stomach motility disorder in which there is a delayed gastric emptying of food without mechanical obstruction - is more common in women than men (3,4). Furthermore, previous studies assessing colonic motility have demonstrated faster colon transit (shorter transit time) in men compared with women (5–7). In one study, women exhibited less pressure activity in the colon, particularly in the transverse/descending colon, than men (8). Sex-associated differences were also evident in the anal sphincter contraction and anorectal motility (9). For instance, Sun and Read (10) identified that healthy men had stronger anal sphincter pressures compared with women. In the gallbladder, women have been observed to have a slower emptying rate than men under normal conditions, and this may explain the increased probability of gallstone development in women compared with men (11). Sex-dependent differences in esophageal motility in terms of duration and velocity of esophageal contraction have also been also reported (12).
In addition, sex-associated differences have been observed in various functional GI disorders and colorectal disturbances. For instance, inflammatory and irritable bowel syndromes, chronic functional abdominal pain, pelvic floor dysfunction, constipation, bloating, fecal incontinence, globus and dysphagia are more prevalent in women compared with men (13,14).
Physiologically, phosphorylation of the 20-kDa regulatory myosin light chain (MLC20) is considered an essential step in GI smooth muscle contraction (15). This phosphorylation is initiated and regulated by activation of the Ca2+/calmodulin-dependent myosin light-chain kinase (MLCK), which transfers the phosphate group from adenosine triphosphate (ATP) to the Ser19 hydroxyl group of MLC20 (16). This phosphorylation activates the actin-activated myosin ATPase and actin-myosin interaction, thereby initiating smooth muscle contraction (15,16).
Studies on sex differences in GI motility disorders have generally focused on sex hormone/receptor-mediated effects on tract function (3,17). However, these sex differences may also be associated with alterations in the signaling mechanisms of smooth muscle contractile machinery. For example, our group previously demonstrated greater gastric smooth muscle contraction in male rats compared with female (1). In association with this higher contraction, there was greater activation of the small G protein RhoA and its downstream effector, Rho-associated protein kinase (ROCK), an important pathway in developing and maintaining smooth muscle tone (18), in the male stomach muscle cells compared with female (1). The effect of gender on the expression and activity of other protein kinases and phosphatases that regulate smooth muscle contraction is less clear.
The aim of the present study was to determine whether the increased contractions of gastric muscle cells in males compared with females are attributable in part to sex differences in the phosphorylation of MLC20.
Materials and methods
Materials
A DC protein assay kit (500–0119) was obtained from Bio-Rad Laboratories, Inc., Hercules, CA, USA. An MLCK ELISA kit (CSB-EL015320RA) was purchased from Cusabio Technology LLC, Baltimore, MD, USA. A phospho-MLC20 (pSer19 in rat) cell-based ELISA kit (ABIN1380310) was purchased from antibodies-online, Inc., Atlanta, GA, USA. An MLC20 ELISA kit (rat MLC polypeptide 9; MBS7201038) was purchased from MyBioSource, San Diego, CA, USA. The MLCK inhibitor, ML-7 (ab120848) was purchased from Abcam, Cambridge, MA, USA. A 500-µm Nitex mesh was purchased from Amazon, Seattle, WA, USA. All remaining chemicals were obtained from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany. Dimethylsulfoxide was used to prepare a stock solution of ML-7.
Preparation of freshly dispersed gastric smooth muscle cells (GSMCs)
Young mature male and female Sprague-Dawley rats (~12 weeks of age, 250–300 g, n=37; 20 males and 17 females) were provided by the animal house of the Jordan University of Science and Technology, Irbid, Jordan. The animals were housed under standardized conditions (temperature 20–22°C, humidity 50–60% and 12-h light/dark cycle) and allowed free access to food and tap water throughout the experiments. Rats were euthanized by inhalation of CO2 (4.5 l/min flow rate) in a CO2 chamber (30 × 30 × 25 cm; 2 animals per exposure) for at least 5 min. To further confirm euthanasia an incision was made through the diaphragm with a scalpel blade. Following euthanasia the stomach was immediately excised. The current study protocols were approved and followed the guidelines of the Animal Care and Use Committee at Jordan University of Science and Technology.
GSMCs were isolated from the circular muscle layer of the rat stomach by sequential enzymatic digestion, filtration and centrifugation as described previously (19). Briefly, strips of circular muscle free of mucosa from all regions of the stomach were dissected and incubated at 31°C for 30 min in HEPES medium (pH was adjusted to 7.4) containing 120 mM NaCl, 4 mM KCl, 2.0 mM CaCl2, 2.6 mM KH2PO4, 0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose, 2.1% Eagle's essential amino acid mixture, 0.1% collagenase and 0.01% soybean trypsin inhibitor. The tissue was continuously gassed with 100% oxygen throughout the isolation procedure. Following two washes of the partially digested strips with 50 ml of enzyme-free medium, the muscle cells were allowed to disperse spontaneously for 30 min. The cells were harvested by filtration through a 500-µm Nitex mesh and centrifuged twice at 350 × g for 10 min to eliminate broken cells and organelles. A dye exclusion test was used following cell collection to determine the number of viable cells present in the collected cell suspension. Briefly, the cell suspension was mixed for less than 3 min at room temperature with trypan blue dye and then visually examined with an inverted Nikon TMS-F microscope (Nikon Corporation, Tokyo, Japan) to determine whether cells took up or excluded the dye; live cells possess intact cell membranes that exclude trypan blue whereas dead cells do not. The cells were counted in a hemocytometer and it was estimated that 95% of the cells excluded trypan blue. This cell isolation procedure consistently yielded spindle-shaped and viable GSMCs that exhibited notable contraction in response to contractile stimuli. All the experiments were performed within 2–3 h of cell dispersion.
Detection of MLCK and MLC20 protein levels by ELISA
A total of three repeated freeze-thaw cycles were used to break up the membranes of the isolated GSMCs. Briefly, in each cycle, cells were rapidly frozen on dry ice (−78.5°C) and left for 3 min, then thawed immediately at room temperature for 30 min. Following centrifugation of the lysates at 20,000 × g for 10 min at 4°C, the protein concentration of the supernatant was determined with the DC protein assay kit from Bio-Rad Laboratories, Inc. Samples of equal amounts of protein were quantitated for MLCK and MLC20 by ELISA according to the manufacturers' instructions.
MLC20 phosphorylation assay
The phosphorylation level of MLC20 (pSer-19) was measured by using the phospho-MLC20 ELISA kit. The assay was performed according to the manufacturer's protocol, using 10 µl of protein lysate. The total starting protein concentration for all samples was 1 mg/ml.
Measurement of contraction in dispersed smooth muscle cells
Contraction in freshly dispersed GSMCs was determined by scanning micrometry (20). Aliquots (0.4 ml) of cells containing approximately 104 cells/ml were prepared and distributed into either male or female groups. Cells were stimulated with acetylcholine (ACh; 0.1 µM) for 1 min in the presence or absence of the MLCK inhibitor, ML-7 (1 µM), and the reaction was terminated with 1% acrolein at a final concentration of 0.1%. Acrolein kills and fixes cells without affecting the cell length. The cells were viewed using a 10× or 20× objective of the inverted Nikon TMS-F microscope, and cell images were acquired using a Canon digital camera (DS126291; Canon, Inc., Tokyo, Japan) and ImageJ acquisition software v1.45 (National Institutes of Health, Bethesda, MD, USA). The resting cell length was determined in control experiments in which muscle cells were not treated with ACh. The mean length of at least 50 muscle cells was measured with ImageJ from each group. The contractile response to ACh was defined as the decrease in the mean length of at least 50 cells and expressed as the percent of change in length relative to mean resting length.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Each experiment was performed on single gastric muscle cells collected from at least 5–10 different rats of each sex. Statistical analyses were performed using Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between two groups were performed by unpaired Student's t-tests. Comparisons between more than two groups were performed by one-way analysis of variance followed by Fisher's post-hoc analysis. P<0.05 was considered to indicate statistical significance.
Results
MLC20 phosphorylation
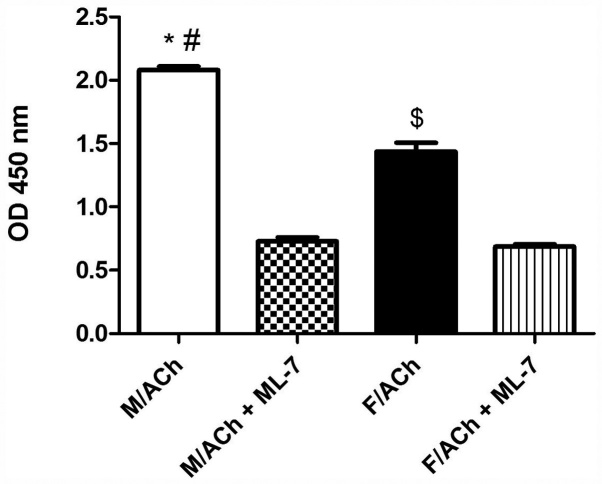
Treatment of freshly dispersed male or female gastric muscle cells with ACh, a Gαq/13-coupled receptor agonist (21), for 1 min significantly increased MLC20 phosphorylation above the basal level (P<0.05; data not shown). Notably, ACh-induced MLC20 phosphorylation was greater in male cells compared with female cells (P<0.05; Fig. 1). The basal MLC20 phosphorylation level was similar in the male and female groups (data not shown). To determine whether the sex-dependent phosphorylation of MLC20 was due to the effect of MLCK activity, GSMCs were treated the MLCK inhibitor, ML-7. ACh-induced MLC20 phosphorylation was significantly inhibited by ML-7 in both sexes (P<0.05), and most notably, sex differences were abolished (Fig. 1).
Figure 1.
ACh-induced MLC20 phosphorylation in the GSMCs of male and female rats. Phosphorylation of MLC20 was detected from absorbance at 450 nm. Treatment of GSMCs with ACh significantly increased MLC20 phosphorylation in each sex. The ACh-induced phosphorylation of MLC20 was significantly higher in male cells compared with female cells. ML-7 significantly inhibited ACh-induced MLC20 phosphorylation in the GSMCs of male and female rats. ML-7 abolished the sex differences in phosphorylation. *P<0.05 vs. F/ACh; #P<0.05 vs. M/ACh + ML-7; $P<0.05 vs. F/ACh + ML-7. MLC20, 20-kDa regulatory myosin light chain; ACh, acetylcholine; ML-7, myosin light chain kinase inhibitor; GSMC, gastric smooth muscle cell; F, female; M, male.
MLC20 expression
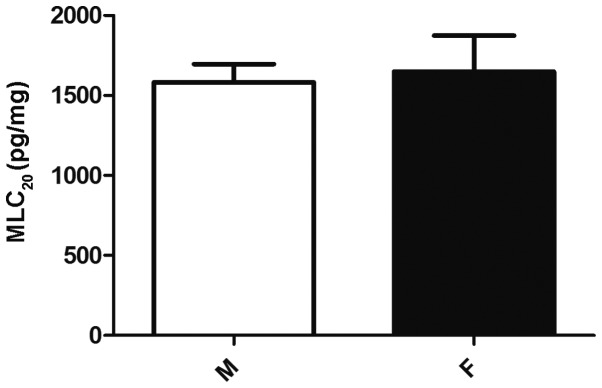
To determine whether the MLC20 protein expression profile correlated with the phosphorylation differences in MLC20, the protein level of MLC20 protein level was compared between male and female gastric muscle cells by ELISA. Despite the higher level of agonist-stimulated MLC20 phosphorylation in male muscle cells compared with female cells, the expression of MLC20 protein was similar in both sexes (P>0.05; Fig. 2). This indicates the possibility that gender differences in MLC20 phosphorylation may be due to an effect of other upstream kinases, particularly of the key enzyme MLCK, on MLC20 phosphorylation.
Figure 2.
Protein expression of MLC20 in the GSMCs of male and female rats. The protein expression of MLC20 did not differ between male and female GSMCs. MLC20 protein levels are expressed as pg/mg of total protein. Values shown are representative of four independent experiments performed in triplicate. Data represent the means ± standard error of the mean. MLC20, 20-kDa regulatory myosin light chain; GSMC, gastric smooth muscle cell; F, female; M, male.
MLCK expression
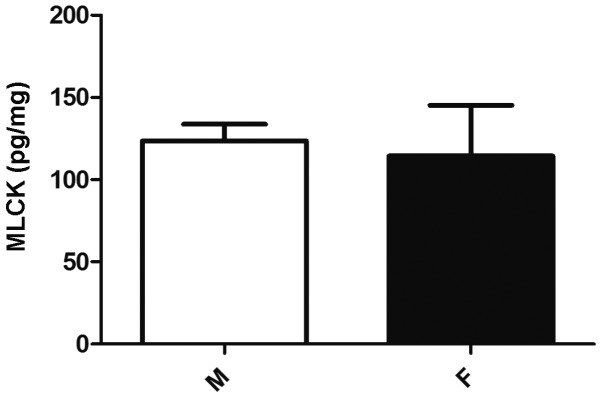
Despite the greater effect of MLCK inhibition on MLC20 phosphorylation in male compared with female cells, the protein expression of MLCK did not differ significantly between the groups of cells (P>0.05; Fig. 3).
Figure 3.
Expression of MLCK protein in the GSMCs of male and female rats. The protein expression of MLCK did not differ between male and female GSMCs. MLCK protein levels are expressed as pg/mg of total protein. Values shown are representative of four independent experiments performed in triplicate. Data represent the means ± standard error of the mean. MLCK, myosin light chain kinase; GSMC, gastric smooth muscle cell; F, female; M, male.
Smooth muscle contraction
The change in gastric muscle cell length in response to ACh treatment was measured by scanning micrometry. Resting (not treated with ACh) muscle cell lengths did not differ (P>0.05) between the sexes (data not shown). ACh caused muscle cell contraction in both the male and female groups. Notably, contraction in response to ACh was significantly greater in male cells compared with female cells (P<0.05; Fig. 4). To assess the effect of MLCK on the sex-dependent muscle cell contraction, GSMCs were treated with ML-7. Preincubation of cells with ML-7 significantly reduced ACh-induced contraction in the male and female cells (P<0.05), and this inhibition was to a greater extent in male compared with female cells (Fig. 4). Most notably, ML-7 abolished the sex differences in cell contraction (Fig. 4).
Figure 4.
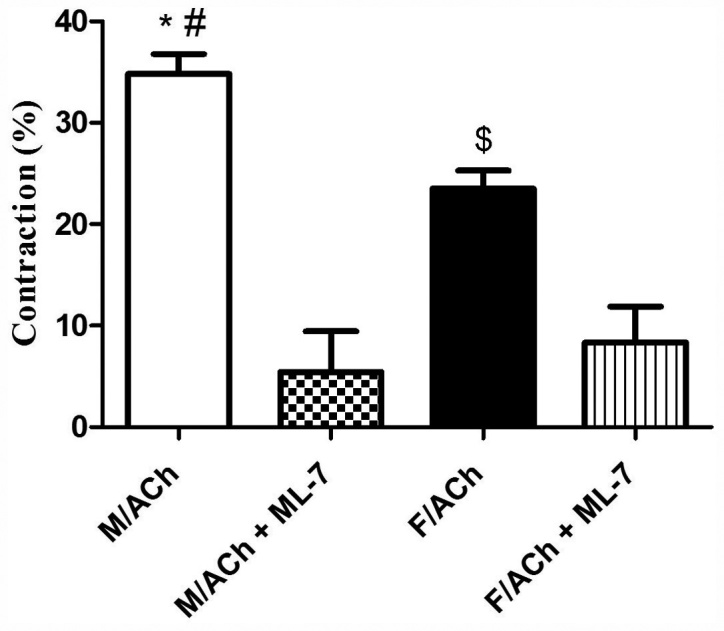
Effect of the MLCK inhibitor, ML-7, on ACh-induced contraction in the GSMCs of male and female rats. GSMCs of male or female rats were stimulated with ACh in the presence or absence of ML-7 and were viewed under an inverted phase microscope. Images of treated and non-treated single cells were acquired and the extent of cell contraction was measured. Contraction in response to ACh was significantly greater in male cells compared with female cells. MLCK inhibitor significantly inhibited ACh-induced contraction in the GSMCs of male and female rats. ML-7-induced inhibition of gastric muscle contraction and abolished the sex differences in the ACh-induced contractions. *P<0.05 vs. F/ACh; #P<0.05 vs. M/ACh + ML-7; $P<0.05 vs. F/ACh + ML-7. ACh, acetylcholine; GSMC, gastric smooth muscle cell; F, female; M, male.
Discussion
In the present research, elevated MLC20 phosphorylation and contraction in response to ACh were identified in GSMCs from male rats compared with those from females. However, the protein levels of MLC20 were did not differ between males and females. Inhibition of MLCK reduced MLC20 phosphorylation and ACh-induced contraction in both sexes and abolished sex-dependent differences. These findings suggest that phosphorylation of MLC20 by MLCK is regulated differently in GSMCs from males versus females.
Sex differences in smooth muscle function have been reported in various tissue organs and in different species. For example, greater myogenic tone has been identified in the arteries of male rats over that in females (22,23). Furthermore, women have increased asthma prevalence with changes in pulmonary smooth muscle contractile behavior compared with men (24). Most notably, our group recently reported greater muscle contraction in male stomach muscle cells compared with the female counterparts (1).
Indeed, gender differences in smooth muscle reactivity may be related to differential expression and/or activity of sex hormone receptors (25), effects of sex hormones on the gene expression of the specific receptors of contractile agonists (26), or sex-related differences in the signaling mechanisms of smooth muscle contraction downstream from receptor activation (1). It is established that phosphorylation of Ser19 on the regulatory light chain of myosin II by Ca2+/calmodulin-dependent MLCK is essential for the initiation of smooth muscle contraction (27). MLC20 phosphorylation may also be increased through inhibition of MLC phosphatase by Rho kinase and other important kinases, which sustains smooth muscle contraction without a change in intracellular Ca2+ level (28).
Research on gender-related differences in the signaling mechanisms of smooth muscle contraction in the GI tract is limited. In the vasculature, studies have demonstrated a smaller basal intracellular Ca2+ level in female rats compared with males, suggesting gender differences in Ca2+-handling mechanisms (29,30). In addition, stronger vasodilator response to the Rho kinase inhibitor, Y27632, has been observed in the cerebral circulation of male rats compared with females, although the measured protein levels of RhoA and Rho kinase did not differ (31). Other studies have demonstrated that gender differences in vascular reactivity reflect differences in the expression and activity of PKC isoforms in vascular smooth muscle (32–34). Whether these sex variations exist in the GI tract muscle is currently unknown.
Parallel to previous findings outside the GI tract, our group recently reported an increased contraction attributable to greater RhoA/ROCK activation in the stomach smooth muscle cells of male rats compared with females (1). This provided a basis for investigating sex differences in other contraction signaling pathways in the GI tract smooth muscle. To the best of our knowledge, the present study is the first to indicate differential activation of the MLCK/MLC20 pathway and its effect on contraction of single GSMCs between the sexes in rats.
As sex-related differences in gastric contraction may be due to differences in various types of stomach cells, studying the muscle contraction and the regulation of the MLCK/MLC20 pathway in a multicellular preparation may be difficult and non-specific. For this reason, the present study was performed on single smooth muscle cells freshly isolated from the stomach of rat to avoid the contribution of other non-muscle cell types.
Consistent with our previous study (1), treatment of muscle cells with ACh induced muscle contraction and significantly enhanced MLC20 phosphorylation level in male and female cells. Despite the differences in MLC20 phosphorylation level, the protein expression of MLC20 was indifferent between the sexes. Thus, differences in the expression of MLC20 did not account for the difference in MLC20 phosphorylation between the sexes. These results indicate the possibility that the sex-dependent elevation of MLC20 phosphorylation in males may be due to differences in the activation of other upstream regulators of myosin, such as MLCK.
To assess the role of MLCK in sex-dependent differential MLC20 phosphorylation and contraction, MLC20 phosphorylation and muscle contraction was measured in the presence or absence of the MLCK inhibitor, ML-7. ACh-induced muscle cell contraction was greater in male gastric cells compared with female cells. The MLCK inhibitor ML-7 inhibited both the phosphorylation of MLC20 and muscle contraction. Most notably, inhibition of MLCK by ML-7 abolished the observed sex differences and normalized contractions in response to ACh between male and female cells. This suggests that enhanced MLCK activity in the stomach of males may mediate the sex difference. Indeed, the binding affinity of ML-7 for smooth muscle MLCK is ~100 times higher than its affinity for other enzymes, and it is more specific than its parent form, ML-9, in inhibiting MLCK (35,36). However, its ability to inhibit other enzymes including cyclic adenosine monophosphate-dependent protein kinases, protein kinase C (PKC) and calcium phosphodiesterase can not be excluded (35). A recent and advanced method for testing the function of a specific enzyme is to treat cultured GSMCs with small interfering RNA (siRNA) to block expression of the target gene. However, due to limited laboratory facilities, these siRNA experiments could not be performed in the present study. Thus, future studies using siRNA and more specific inhibitors of MLCK should be performed.
Various receptor agonists generate both initial/transient (<1 min) Ca2+-dependent and sustained (>5 min) Ca2+-independent contraction in GI smooth muscle cells (37–39). As the present study treated single gastric muscle cells with agonist for 1 min, the phosphorylation assay and contraction results mostly represent sex differences in the initial phase of contraction. During this phase, MLC20 phosphorylation is mainly regulated by MLCK (38). Future measurement of MLCK activity by specific kinase assay techniques may aid to verify the present results.
When regarding the inhibitory effects of female steroid hormones, namely estrogen and progesterone, on muscle contraction (40), the present results are consistent with previous studies on human myometrial muscle, which demonstrated significant reduction in MLC20 phosphorylation upon KCl treatment in tissues from pregnant women compared with nonpregnant women (41). This KCl-mediated MLC20 phosphorylation depends on Ca2+ influx through voltage-sensitive Ca2+ channels (42). Both estrogen and progesterone have been demonstrated to inhibit Ca2+ influx in smooth muscle (43,44). In addition, the current findings are in parallel with other results on enhanced myosin phosphatase target subunit 1 expression and thus MLC phosphatase activity in pregnancy, which resulted in decreased basal levels of MLC20 phosphorylation (45).
In conclusion, the present study demonstrated that phosphorylation of MLC20 and thus smooth muscle contraction in response to ACh was greater in stomach cells from males compared with those from females. This sex-dependent phosphorylation was most probably mediated by increased activation of MLCK in males compared with females, as the sex differences were eliminated by the MLCK inhibitor ML-7. The exact mechanisms by which the MLCK/MLC20 pathway is differentially regulated between the sexes should be investigated in future studies. Sex differences are present across the GI system, in which the MLCK/MLC20 pathway serves a crucial role in GI motility function (46). Further understanding of the role of the MLCK/MLC20 pathway in modulating the normal physiological as well as the pathophysiological functions of the GI tract may enable more effective and sex-appropriate treatments for various GI motility disturbances.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MLC20
20-kDa regulatory myosin light chain
- GI
gastrointestinal
- MLCK
myosin light chain kinase
- GSMC
gastric smooth muscle cell
- ACh
acetylcholine
- ML-7
myosin light chain kinase inhibitor
- ROCK
Rho-associated protein kinase
- PKC
protein kinase C
- siRNA
small interfering RNA
Funding
The present work was supported by Jordan University of Science and Technology, Irbid, Jordan (grant no. 20150371).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Conception and design of the study, acquisition of data, and drafting of the manuscript were performed by OAA. Analysis and interpretation of data and revising the manuscript critically for intellectual content were performed by OAA, ANA, MAA and AGM. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study protocols were approved and followed the guidelines of the Animal Care and Use Committee at Jordan University of Science and Technology, Irbid, Jordan.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Al-Shboul O. The role of the RhoA/ROCK pathway in gender-dependent differences in gastric smooth muscle contraction. J Physiol Sci. 2016;66:85–92. doi: 10.1007/s12576-015-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennink R, Peeters M, Van den Maegdenbergh V, Geypens B, Rutgeerts P, De Roo M, Mortelmans L. Comparison of total and compartmental gastric emptying and antral motility between healthy men and women. Eur J Nucl Med. 1998;25:1293–1299. doi: 10.1007/s002590050298. [DOI] [PubMed] [Google Scholar]
- 3.Rao JN. Estrogens and gastroparesis: A clinical relevance. Dig Dis Sci. 2013;58:1449–1451. doi: 10.1007/s10620-013-2683-0. [DOI] [PubMed] [Google Scholar]
- 4.Oh JH, Pasricha PJ. Recent advances in the pathophysiology and treatment of gastroparesis. J Neurogastroenterol Motil. 2013;19:18–24. doi: 10.5056/jnm.2013.19.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teff KL, Alavi A, Chen J, Pourdehnad M, Townsend RR. Muscarinic blockade inhibits gastric emptying of mixed-nutrient meal: Effects of weight and gender. Am J Physiol. 1999;276:R707–R714. doi: 10.1152/ajpregu.1999.276.3.R707. [DOI] [PubMed] [Google Scholar]
- 6.Meier R, Beglinger C, Dederding JP, Meyer-Wyss B, Fumagalli M, Rowedder A, Turberg Y, Brignoli R. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterol Motil. 1995;7:235–238. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 7.Lampe JW, Fredstrom SB, Slavin JL, Potter JD. Sex differences in colonic function: A randomised trial. Gut. 1993;34:531–536. doi: 10.1136/gut.34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G629–G639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 9.Zakari M, Nee J, Hirsch W, Kuo B, Lembo A, Staller K. Gender differences in chronic constipation on anorectal motility. Neurogastroenterol Motil. 2017;29:29. doi: 10.1111/nmo.12980. [DOI] [PubMed] [Google Scholar]
- 10.Sun WM, Read NW. Anorectal function in normal human subjects: Effect of gender. Int J Colorectal Dis. 1989;4:188–196. doi: 10.1007/BF01649702. [DOI] [PubMed] [Google Scholar]
- 11.Chukwuka UA, Kalu AK, Erondu OF. Variabilities of gallbladder contraction indices and a simple regression model for gallbladder and gastric emptying ratio. Pan Afr Med J. 2011;9:11. doi: 10.4314/pamj.v9i1.71186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantas RO, Ferriolli E, Souza MA. Gender effects on esophageal motility. Brazilian journal of medical and biological research. Rev Bras Pesqui Med Biol. 1998;31:539–544. doi: 10.1590/s0100-879x1998000400011. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 14.Chial HJ, Camilleri M. Gender differences in irritable bowel syndrome. The journal of gender specific medicine. J Gend Specif Med. 2002;5:37–45. [PubMed] [Google Scholar]
- 15.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 16.Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991;266:1708–1715. [PubMed] [Google Scholar]
- 17.Yang X, Liu R, Dong Y. Regulative effects of ovarian steroids on rat gastric motility and sensitivity. Sheng li xue bao: Acta physiologica Sinica. 2006;58:275–280. [PubMed] [Google Scholar]
- 18.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/S0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 19.Murthy KS, Makhlouf GM. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am J Physiol. 1995;268:C171–C180. doi: 10.1152/ajpcell.1995.268.1.C171. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Mahavadi S, Sriwai W, Grider JR, Murthy KS. Cross-regulation of VPAC(2) receptor desensitization by M(3) receptors via PKC-mediated phosphorylation of RKIP and inhibition of GRK2. Am J Physiol Gastrointest Liver Physiol. 2007;292:G867–G874. doi: 10.1152/ajpgi.00326.2006. [DOI] [PubMed] [Google Scholar]
- 21.Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent M2-mediated inhibition via Galphai3 and m3-mediated stimulation via Gbetagammaq. J Biol Chem. 1997;272:21317–21324. doi: 10.1074/jbc.272.34.21317. [DOI] [PubMed] [Google Scholar]
- 22.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res. 1996;79:1024–1030. doi: 10.1161/01.RES.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 23.Huang A, Sun D, Koller A, Kaley G. Gender difference in myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NO. Am J Physiol. 1997;272:H1804–H1809. doi: 10.1152/ajpheart.1997.272.4.H1804. [DOI] [PubMed] [Google Scholar]
- 24.Fuseini H, Newcomb DC. Mechanisms Driving Gender Differences in Asthma. Curr Allergy Asthma Rep. 2017;17:19. doi: 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, McNeill JG, Poole-Wilson PA. 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.CIR.92.1.24. [DOI] [PubMed] [Google Scholar]
- 26.Nickenig G, Strehlow K, Wassmann S, Bäumer AT, Albory K, Sauer H, Böhm M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102:1828–1833. doi: 10.1161/01.CIR.102.15.1828. [DOI] [PubMed] [Google Scholar]
- 27.Dabrowska R, Hartshorne DJ. A Ca2+-and modulator-dependent myosin light chain kinase from non-muscle cells. Biochem Biophys Res Commun. 1978;85:1352–1359. doi: 10.1016/0006-291X(78)91152-X. [DOI] [PubMed] [Google Scholar]
- 28.Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25:613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- 29.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca(2+)](i) in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol. 2000;278:C834–C844. doi: 10.1152/ajpcell.2000.278.4.C834. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Khalil RA. Sex-related decrease in [Ca2+]i signaling and Ca2+-dependent contraction in inferior vena cava of female rat. Am J Physiol Regul Integr Comp Physiol. 2010;298:R15–R24. doi: 10.1152/ajpregu.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke. 2004;35:2200–2205. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- 32.Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol. 1998;25:974–985. doi: 10.1111/j.1440-1681.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Xiao X, Zhang J, Zhu Y, Hu Y, Zang J, Lu K, Yang T, Ge H, Peng X, et al. Age and sex differences in vascular responsiveness in healthy and trauma patients: Contribution of estrogen receptor-mediated Rho kinase and PKC pathways. Am J Physiol Heart Circ Physiol. 2014;306:H1105–H1115. doi: 10.1152/ajpheart.00645.2013. [DOI] [PubMed] [Google Scholar]
- 34.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280:C34–C45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 35.Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- 36.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: An update. Biochem J. 2003;371:199–204. doi: 10.1042/bj20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G849–G853. doi: 10.1152/ajpgi.00530.2004. [DOI] [PubMed] [Google Scholar]
- 38.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 39.Hu W, Mahavadi S, Li F, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1beta. Am J Physiol Cell Physiol. 2007;293:C1991–C2000. doi: 10.1152/ajpcell.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta S, Hey VM, Pleuvry BJ. Effects of pregnancy and associated hormones in mouse intestine, in vivo and in vitro. Pflugers Arch. 1974;346:87–95. doi: 10.1007/BF00587009. [DOI] [PubMed] [Google Scholar]
- 41.Word RA, Stull JT, Casey ML, Kamm KE. Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. J Clin Invest. 1993;92:29–37. doi: 10.1172/JCI116564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Himpens B, Matthijs G, Somlyo AP. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol. 1989;413:489–503. doi: 10.1113/jphysiol.1989.sp017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fomin VP, Cox BE, Word RA. Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Physiol. 1999;276:C379–C385. doi: 10.1152/ajpcell.1999.276.2.C379. [DOI] [PubMed] [Google Scholar]
- 44.Salom JB, Burguete MC, Pérez-Asensio FJ, Torregrosa G, Alborch E. Relaxant effects of 17-beta-estradiol in cerebral arteries through Ca(2+) entry inhibition. J Cereb Blood Flow Metab. 2001;21:422–429. doi: 10.1097/00004647-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Lontay B, Bodoor K, Weitzel DH, Loiselle D, Fortner C, Lengyel S, Zheng D, Devente J, Hickner R, Haystead TA. Smoothelin-like 1 protein regulates myosin phosphatase-targeting subunit 1 expression during sexual development and pregnancy. J Biol Chem. 2010;285:29357–29366. doi: 10.1074/jbc.M110.143966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.