Abstract
Paraquat (PQ) is a herbicide that is widely used in developing countries, and pulmonary fibrosisis one of the most typical features of PQ poisoning. The molecular mechanism underlying PQ toxicity is largely unknown, which makes it difficult to treat. In the present study, western blot analysis, reverse transcription-quantitative polymerase chain reaction and fluorescent immunostaining were used to analyze the effects of rapamycin on PQ-induced epithelial-mesenchymal transition (EMT) in A549 and MRC-5 cells. It was revealed that rapamycin significantly downregulated the mesenchymal cell marker, α-smooth muscle actin, and significantly upregulated the epithelial cell marker, E-cadherin, at mRNA and protein expression levels compared with the PQ group. Treatment with PQ significantly increased Wnt1, low-density lipoprotein receptor-related protein (LRP)5, LRP6 and β-catenin expression levels in A549 cells, while rapamycin significantly inhibited these effects of PQ. Activation of the Wnt signaling pathway using lithium chloride attenuated the inhibitory effects of rapamycin on PQ-induced EMT. In conclusion, rapamycin protects against PQ-induced pulmonary EMT via the Wnt/β-catenin signaling pathway.
Keywords: rapamycin, paraquat, epithelial mesenchymal transition, Wnt/β-catenin pathway
Introduction
Paraquat (PQ; 1,1′-dimethyl-4,4′-bipyridinium) is a herbicide that is widely used in developing countries worldwide, which may cause severe toxicity in animals and humans (1). PQ poisoning was reported to account for up to a third of all suicides around the world (2). PQ poisoning may cause patients to develop acute multi-organ failure, pulmonary fibrosis and finally mortality due to respiratory failure (3). Pulmonary fibrosis is a typical feature of PQ poisoning, the onset of which may be several days or weeks following ingestion of PQ (4). Although PQ-induced pulmonary fibrosis has a high mortality rate, the molecular mechanisms underlying its toxicity are largely unknown, which makes it particularly hard to treat.
Epithelial-mesenchymal transition (EMT) has been reported to be associated with pulmonary fibrosis following PQ exposure (3). It has also been reported that factors, including epithelial growth factor, transforming growth factor-β1 (TGF-β1), insulin-like growth factor and interleukin-17, may induce EMT (5). Myofibroblasts are key mediators in fibrosis. In murine models of hepatic and renal fibrosis, ~40% of α-smooth muscle actin (SMA)-positive myofibroblasts were derived from epithelial cells via EMT (6). However, to the best of our knowledge, the roles and mechanisms of rapamycin in the EMT process of A549 and MRC-5 cells remain unknown.
In the present study, the roles and mechanisms of rapamycin on PQ-induced pulmonary fibrosis were investigated. It was revealed that rapamycin alleviated PQ-induced EMT in A549 and MRC-5 cells, and PQ activated the Wnt signaling pathway. Rapamycin inhibited the effects of PQ, and the activation of the Wnt signaling pathway attenuated the inhibitory effects of rapamycin on PQ-induced EMT.
Materials and methods
Reagents
PQ, rapamycin and lithium chloride (LiCl) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies against E-cadherin (ab1416), β-actin (ab8227) and α-SMA (ab7817) were purchased from Abcam (Cambridge, UK). Antibodies against Wnt1, low-density lipoprotein receptor-related protein (LRP)5 and LRP6 were provided by Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). Antibodies against β-catenin were purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA).
Cell culture and treatment
A549 (human lung adenocarcinoma epithelial cells) and MRC-5 (human fetal lung fibroblast cells) cells were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere.
A previous study demonstrated that 300 µmol/l PQ treatment for 6 days induced the EMT of A549 cells; therefore, this concentration was selected for use within the present study (3). Prior to treatment, A549 cells and MRC-5 cells were incubated in serum-free DMEM for 12 h at 37°C. To evaluate the effects of Wnt signaling on EMT, 300 µmol/l PQ, 100 nM rapamycin and 10 µmol/l LiCl were added to the cells for 48 h. All the reagents were added again when the medium was changed every day.
In the present study, four treatment groups were established to study the effects of rapamycin on the PQ-induced EMT and the Wnt signaling pathway in A549 and MRC-5 cells: The negative control group, the PQ-treated group, the rapamycin-treated group and the PQ + rapamycin-treated group. Subsequently, five groups were established to confirm the roles of Wnt signaling pathway in the inhibitory effects of rapamycin on PQ-induced EMT in A549 and MRC-5 cells: The negative control group, the PQ-treated group, the LiCl-treated group, the PQ + rapamycin-treated group and the LiCl + PQ + rapamycin-treated group.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549 and MRC-5cells using a total RNA rapid extraction kit (BioTeke Corporation, Beijing, China) according to manufacturer's protocol. A total of 1 µg RNA was reverse transcribed into cDNA using M-MLV reverse transcriptase (BioTeke Corporation) in the presence of oligo (dT) and random 50 primers (Invitrogen; Thermo Fisher Scientific, Inc.). The reaction program was 42°C for 50 min, and then 72°C for 10 min. The instruments in this section were pre-treated with surface RNase Erase (Tiandz, Inc., Beijing, China) and the reagents were RNase-free. The cDNA (1 µl for each reaction) was used for qPCR to detect the gene expression levels using 2XPower Taq PCR Master mix (BioTeke Corporation) and SYBR Green (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) with GAPDH as the internal control. The PCR procedure was set as follows: 95°C for 10 min, followed by 38 cycles of 95°C for 12 secs 60°C for 18 sec and 72°C for 30 sec, and finally 4°C for 5 min. Calculations were performed using the 2−ΔΔCq method described previously (7). The primers used were are as follows: GAPDH, forward 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse 5′-GGCTGTTGTCATACTTCTCATGG-3′; E-cadherin, forward 5′-AAGGCACGCCTGTCGAAGCA-3′ and reverse 5′-ACGTTGTCCCGGGTGTCATCCT-3′; α-SMA, forward 5′-TACTACTGCTGAGCGTGAGA-3′ and reverse 5′-CATCAGGCAACTCGTAACTC-3′; Wnt1, forward 5′-CCGATGGTGGGGTATTGTGAA-3′ and reverse 5′-TCCCCGGATTTTGGCGTATC-3′; β-catenin, forward 5′-GCCAGTGGATTCCGTACTGT-3′ and reverse 5′-GAGCTTGCTTTCCTGATTGC-3′; LRP5, forward 5′-GGGAGACGCCAAGACAGACAAGATCG-3′ and reverse 5′-GGTGAAGACCAAGAAGGCCTCAGG-3′; and LRP6, forward 5′-ATTGTAGTTGGAGGCTTGGAGGATGC-3′ and reverse 5′-CCATCCATTCCAGCACGTTCTATC-3′.
Western blot analysis
Protein was extracted from A549 and MRC-5cells using a whole-cell lysis kit (CWBio, Beijing, China, http://www.cwbiotech.com) and the concentration of protein was measured using a bichinchoninic acid protein quantitative kit (Beyotime Institute of Biotechnology, Haimen, China). Following denaturation by boiling for 5 min, the protein samples (40 µg for each lane) were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Merck KGaA). Following blocking with 5% skimmed milk at room temperature for 1 h, the membranes were incubated with the primary antibodies anti-E-cadherin (1:1,000), anti-α-SMA (1:1,000), anti-Wnt1 (1:2,000), anti-LRP5 (1:2,000), anti-LRP6 (1:1,000), anti-β-catenin (1:1,000) and anti-β-actin (1:5,000)at 4°C overnight. Following rinsing with Tris-buffered saline with Tween-20, the membranes were incubated, with goat anti-rabbit immunoglobulin (Ig) G labeled with horseradish peroxidase (HRP;sc-2007; dilution 1:5,000) or goat anti-mouse IgG-HRP (sc-2005; dilution 1:5,000; both Santa Cruz Biotechnology, Inc.) at 37°C for 45 min. The membranes were visualized using an enhanced chemiluminescent reagent (Thermo Fisher Scientific, Inc.). The optical density values of bands were analyzed using Image Lab software v.3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Fluorescent immunostaining
A549 cells were grown on cover slips at 37°C in DMEM cell culture medium, and then fixed with 4% paraformaldehyde for 10 min at room temperature, and blocked with 1% bovine serum albumin (Thermo Fisher Scientific, Inc.) in PBS for 1 h at room temperature, and then stained with primary antibodies against E-cadherin (dilution 1:500) overnight at 4°C. The cells were subsequently incubated with goat anti-mouse secondary antibody (dilution 1:2,000, ab150115, Abcam) for 1 h at room temperature. The nuclei were stained with 4′,6-diamidino-2-phenylindole for 1 h at room temperature. The fluorescence images were observed under a fluorescence microscope at a magnification of ×400.
Statistical analysis
The data in the present study were presented as the mean ± standard deviation of three or five individual experiments. The results were analyzed by one-way analysis of variance with post hoc comparisons with Tukey's honest significant difference test using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Rapamycin alleviates PQ-induced EMT in A549 and MRC-5 cells
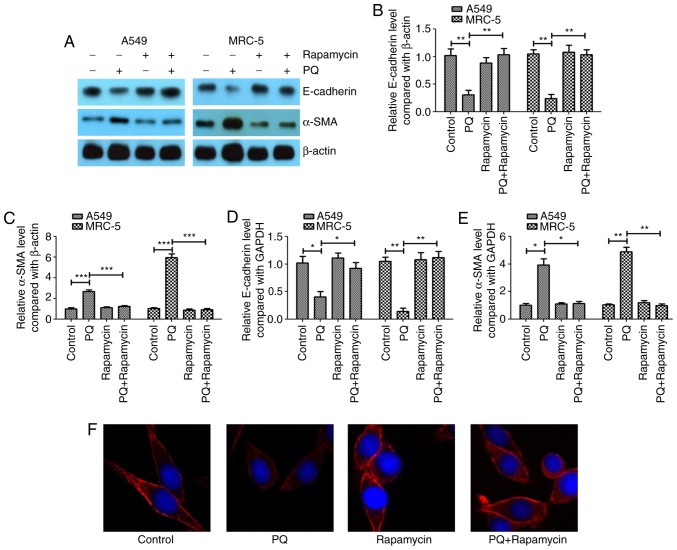
In the present study, A549 and MRC-5 cells were exposed to 300 µmol/l PQ for 6 days as previously described (3). A western blotting assay revealed that PQ induced EMT in A549 and MRC-5 cells (Fig. 1A), as the epithelial cell marker E-cadherin was significantly downregulated in the PQ group compared with the level in the control (Fig. 1B). Furthermore, the mesenchymal cell marker α-SMA was significantly upregulated in the PQ group compared with the level in the control (Fig. 1C). It was observed that rapamycin may inhibit the EMT process that was induced by PQ as E-cadherin was significantly increased and α-SMA was significantly decreased in the PQ + rapamycin group compared with the level in the PQ group.
Figure 1.
Rapamycin alleviates PQ-induced EMT in A549 and MRC-5 cells. (A) Western blot analysis of the EMT markers E-cadherin and α-SMA. The western blotting results of (B) E-cadherin and (C) α-SMA were quantified. Reverse transcription-quantitative polymerase chain reaction was performed to measure the mRNA expression levels of (D) E-cadherin and (E) α-SMA. (F) Immunofluorescent staining was performed to detect E-cadherin in A549 cells (magnification, ×400). *P<0.05, **P<0.01 and ***P<0.001 as indicated. PQ, paraquat; EMT, epithelial-mesenchymal transition; SMA, smooth muscle actin.
The mRNA expression levels of E-cadherin and α-SMA were investigated using RT-qPCR, and it was observed that PQ significantly decreased the mRNA expression level of E-cadherin (Fig. 1D) and significantly increased the mRNA level of α-SMA (Fig. 1E) compared with the levels observed in the control group. Rapamycin significantly inhibited the effect of PQ on α-SMA and E-cadherin in A549 and MRC-5 cells. Using immunofluorescent staining, it was further demonstrated that rapamycin notably upregulated the expression of E-cadherin compared with the level in the PQ group in A549 cells (Fig. 1F).
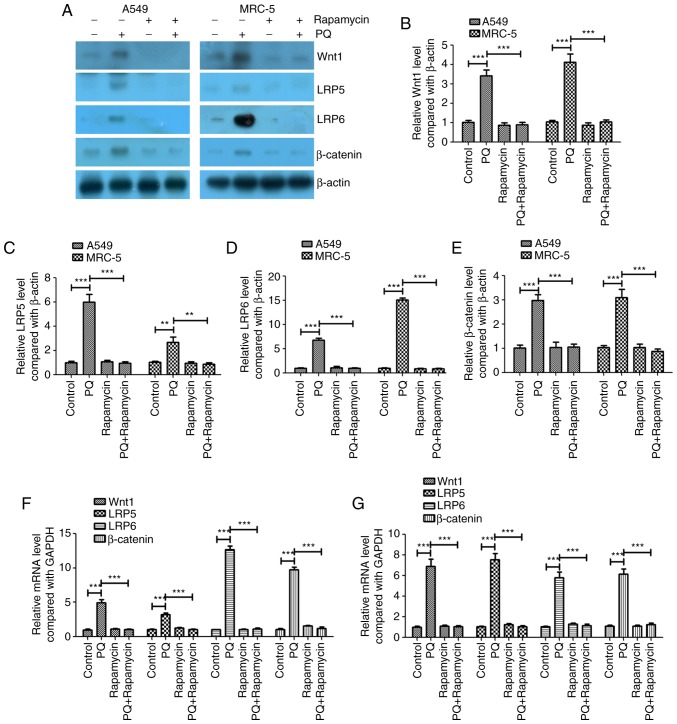
PQ activates the Wnt signaling pathway in A549 and MRC-5 cells, and rapamycin inhibits these effects of PQ
The present study investigated whether the Wnt signaling pathway served an important role in the PQ-induced EMT process. The protein and mRNA expression levels of Wnt1, LRP5 and LRP6 were investigated and it was revealed that PQ significantly increased the protein and mRNA expression levels of Wnt1, LRP5 and LRP6 compared with the levels in the control group (Fig. 2). Rapamycin significantly decreased the protein and mRNA expression levels of Wnt1, LRP5 and LRP6 compared with the levels in the PQ group in A549 and MRC-5 cells (Fig. 2). PQ also significantly upregulated the key regulator of the Wnt signaling pathway, β-catenin, at the mRNA and protein levels, and rapamycin significantly decreased β-catenin expression compared with the level observed in the PQ group in A549 and MRC-5 cells (Fig. 2). These results indicate that PQ activated the Wnt signaling pathway in A549 and MRC-5 cells and rapamycin inhibited the effects of PQ.
Figure 2.
PQ activates the Wnt signaling pathway in A549 and MRC-5 cells and rapamycin inhibits these effects. (A) Western blot analysis of the Wnt signaling pathway genes Wnt1, LRP5, LRP6 and β-catenin. The western blotting results of (B) Wnt1, (C) LRP5, (D) LRP6 and (E) β-catenin were quantified. Reverse transcription-quantitative polymerase chain reaction was performed to measure the mRNA expression levels of Wnt1, LRP5, LRP6 and β-catenin in (F) A549 and (G) MRC-5 cells. **P<0.01 and ***P<0.001 as indicated. PQ, paraquat; LRP, low-density lipoprotein receptor-related protein.
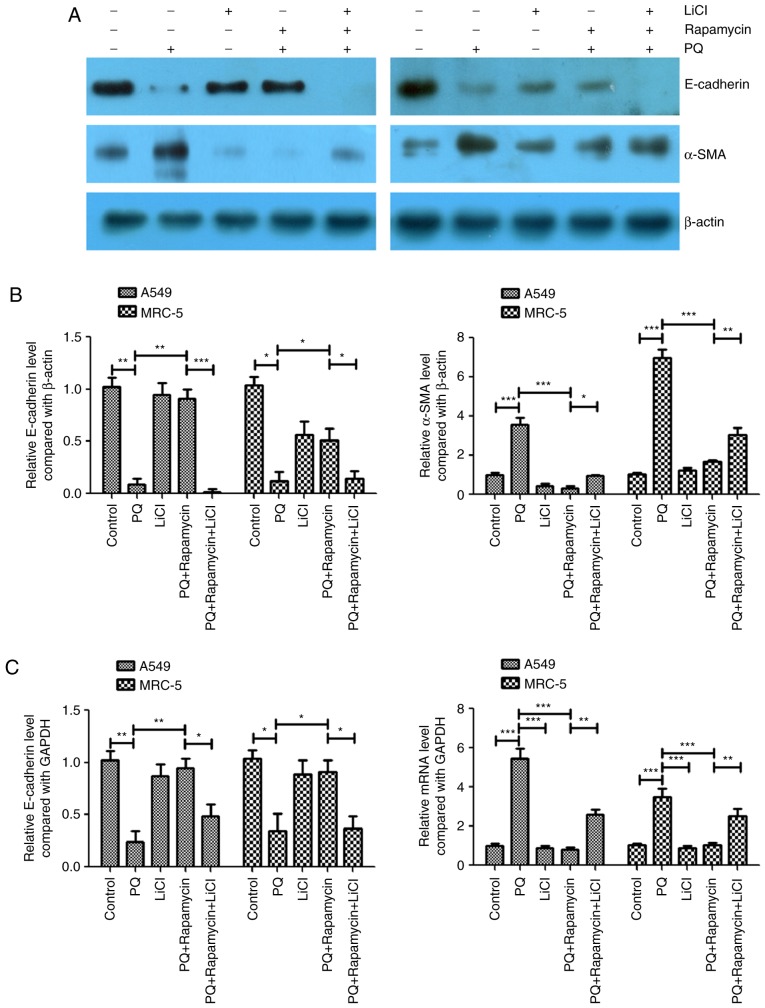
Activation of the Wnt signaling pathway attenuates the inhibitory effects of rapamycin on PQ-induced EMT
LiCl is an activator of the Wnt/catenin signaling pathway (6–9) and this activator was used to further study the association between the Wnt signaling pathway and PQ-induced EMT in the present study. In the PQ-treated A549 and MRC-5 cells, rapamycin inhibited PQ-induced EMT by significantly upregulating E-cadherin and significantly downregulating α-SMA protein levels compared with the levels in the PQ only group (Fig. 3A-C). However, LiCl significantly inhibited the effects of rapamycin as it caused a significant decrease in the protein expression level of E-cadherin and a significant increase in the protein expression of α-SMA compared with the levels observed in the PQ+ rapamycin group (Fig. 3A and B). The mRNA expression levels of E-cadherin and α-SMA were measured using RT-qPCR and similar results were observed. Rapamycin significantly increased the mRNA expression level of E-cadherin and significantly decreased the level of α-SMA compared with the levels in the PQ only group, while LiCl significantly inhibited the effects of rapamycin compared with the PQ + rapamycin group (Fig. 3B and C).
Figure 3.
Activation of the Wnt signaling pathway attenuates the inhibitory effects of rapamycin on PQ-induced EMT. (A) Western blot analysis of the EMT markers E-cadherin and α-SMA. (B) The western blot results of E-cadherin and α-SMA were quantified. (C) Reverse transcription-quantitative polymerase chain reaction was performed to measure the mRNA expression levels of E-cadherin and α-SMA. *P<0.05, **P<0.01 and ***P<0.001 as indicated. PQ, paraquat; EMT, epithelial-mesenchymal transition; SMA, smooth muscle actin; LiCl, lithium chloride.
Discussion
Pulmonary fibrosis is a chronic lung disease, which causes fibrosis of the lung parenchyma and loss of lung function (10). Aging, smoking and infection have been reported as risk factors for pulmonary fibrosis (11). The pathological characteristics of pulmonary fibrosis are repetitive microscopic alveolar epithelial cell injury, dysregulated repair, fibroblast proliferation and accumulation of extracellular matrix, which ultimately result in respiratory failure (12). EMT is an important mechanism of lung fibrogenesis through the generation of mesenchymal-type myofibroblasts from lung epithelial cells (13). EMT is also considered to be the key process that leads to end-stage lung fibrosis, which occurs in a number of different types of inflammatory interstitial lung diseases and chronic obstructive pulmonary disease (5).
PQ may induce EMT-like cellular responses resulting in fibrogenesis and the prevention of apoptosis in human pulmonary epithelial cells (14). Previous studies reported that PQ promoted the EMT process by activating the TGF-β/Smad signaling pathway (15,16). Lysyl oxidase may promote EMT during PQ-induced pulmonary fibrosis (17). In PQ poisoning-induced early pulmonary fibrosis, hypoxia-inducible factor-1α enhanced Snail and β-catenin and promoted EMT (18). The results of the present study demonstrated that PQ significantly upregulated the expression levels of Wnt1, LRP5, LRP6 and β-catenin. Rapamycin was revealed to inhibit these effects of PQ on the Wnt signaling pathway genes. When the Wnt signaling pathway was activated using LiCl, EMT was promoted. These findings suggest that rapamycin protects against PQ-induced pulmonary EMT by suppressing the Wnt/β-catenin signaling pathway.
Yang et al (19) revealed that silencing the mechanistic target of rapamycin (mTOR) using small interfering RNA effectively inhibited the expression level of mTOR in the lung tissues of PQ-poisoned rats and further decreased the fibrosis of lung tissues caused by PQ. Rapamycin is an inhibitor of mTOR and may activate autophagy (20,21). The present study revealed that rapamycin significantly inhibited PQ-induced EMT and the Wnt signaling pathway. However, whether mTOR is associated with the regulation of PQ-induced EMT requires further study to confirm.
Using a murine model, Chung et al (22) demonstrated that rapamycin treatment reduced inflammatory cytokine expression, extracellular matrix production and senescence in type II pneumocytes, and that rapamycin protected against radiation-induced pulmonary fibrosis. Phosphoinositide 3-kinase (PI3K) was reported as a promising therapeutic target for idiopathic pulmonary fibrosis (23). Rapamycin may increase connective tissue growth factor expression in lung fibroblasts by regulating the PI3K signaling pathway (24). However, the association between the Wnt and PI3K/mitogen-activated protein kinase signaling pathways requires further investigation.
Snail and Twist are important transcription factors of EMT associated with pulmonary fibrosis (25,26). In further studies, which transcription factor is involved in the process of PQ-induced EMT should be studied.
In conclusion, the results of the present study suggested that rapamycin alleviated PQ-induced EMT in A549 and MRC-5 cells. It was also observed that PQ activated the Wnt signaling pathway in A549 and MRC-5 cells and that rapamycin inhibited these effects of PQ. Activation of the Wnt signaling pathway attenuated the inhibitory effects of rapamycin on PQ-induced EMT. Further investigation into the effects of PQ and rapamycin on EMT are required, particularly on the expression of additional EMT-associated proteins, including zonula occludens-1, cytokeratin, vimentin and N-cadherin. The role of autophagy in PQ-induced pulmonary fibrosis should also be investigated.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81560015 and 81360015), Yunnan Applied Basic Research Projects (grant no. 2013FB049) and Yunnan Applied Basic Research Projects-Joint Special Project (established by Yunnan Provincial Science and Technology Department and Kunming Medical University) [grant nos. 2014FB046 and 2017FE468 (−005)].
Competing interests
The authors declare that they have no competing interests.
References
- 1.Weng CH, Chen HH, Hu CC, Huang WH, Hsu CW, Fu JF, Lin WR, Wang IK, Yen TH. Predictors of acute kidney injury after paraquat intoxication. Oncotarget. 2017;8:51345–51354. doi: 10.18632/oncotarget.17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med J. 2010;51:496–500. [PubMed] [Google Scholar]
- 3.Li T, Yang X, Xin S, Cao Y, Wang N. Paraquat poisoning induced pulmonary epithelial mesenchymal transition through Notch1 pathway. Sci Rep. 2017;7:924. doi: 10.1038/s41598-017-01069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/S0300-483X(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 5.Willis BC, Borok Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Hao Y, Zhang H, Ying W, Li D, Ge Y, Ying B, Cheng B, Lian Q, Jin S. Posttreatment with Protectin DX ameliorates bleomycin-induced pulmonary fibrosis and lung dysfunction in mice. Sci Rep. 2017;7:46754. doi: 10.1038/srep46754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 8.Xia MY, Zhao XY, Huang QL, Sun HY, Sun C, Yuan J, He C, Sun Y, Huang X, Kong W, Kong WJ. Activation of Wnt/β-catenin signaling by lithium chloride attenuates d-galactose-induced neurodegeneration in the auditory cortex of a rat model of aging. FEBS Open Bio. 2017;7:759–776. doi: 10.1002/2211-5463.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Tang S, Dai C, Li D, Zhang S, Deng S, Zhou Y, Xiao X. Quinocetone induces mitochondrial apoptosis in HepG2 cells through ROS-dependent promotion of VDAC1 oligomerization and suppression of Wnt1/β-catenin signaling pathway. Food Chem Toxicol. 2017;105:161–176. doi: 10.1016/j.fct.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Lucarini L, Durante M, Lanzi C, Pini A, Boccalini G, Calosi L, Moroni F, Masini E, Mannaioni G. HYDAMTIQ, a selective PARP-1 inhibitor, improves bleomycin-induced lung fibrosis by dampening the TGF-β/SMAD signalling pathway. J Cell Mol Med. 2017;21:324–335. doi: 10.1111/jcmm.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, Egan JJ, Lambrecht BN, Lories R, Parfrey H, et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur Respir J. 2013;41:1207–1218. doi: 10.1183/09031936.00073012. [DOI] [PubMed] [Google Scholar]
- 12.Borensztajn K, Crestani B, Kolb M. Idiopathic pulmonary fibrosis: From epithelial injury to biomarkers-insights from the bench side. Respiration. 2013;86:441–452. doi: 10.1159/000357598. [DOI] [PubMed] [Google Scholar]
- 13.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix; Proc Natl Acad Sci USA; 2006; pp. 13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada A, Aki T, Unuma K, Funakoshi T, Uemura K. Paraquat induces epithelial-mesenchymal transition-like cellular response resulting in fibrogenesis and the prevention of apoptosis in human pulmonary epithelial cells. PLoS One. 2015;10:e0120192. doi: 10.1371/journal.pone.0120192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han YY, Shen P, Chang WX. Involvement of epithelial-to-mesenchymal transition and associated transforming growth factor-β/Smad signaling in paraquat-induced pulmonary fibrosis. Mol Med Rep. 2015;12:7979–7984. doi: 10.3892/mmr.2015.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie L, Zhou D, Xiong J, You J, Zeng Y, Peng L. Paraquat induce pulmonary epithelial-mesenchymal transition through transforming growth factor-β1-dependent mechanism. Exp Toxicol Pathol. 2016;68:69–76. doi: 10.1016/j.etp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Zhu Y, Tan J, Meng X, Xie H, Wang R. Lysyl oxidase promotes epithelial-to-mesenchymal transition during paraquat-induced pulmonary fibrosis. Mol Biosyst. 2016;12:499–507. doi: 10.1039/C5MB00698H. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Tan J, Xie H, Wang J, Meng X, Wang R. HIF-1α regulates EMT via the Snail and β-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol Med. 2016;20:688–697. doi: 10.1111/jcmm.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W, Zhao X, Liang R, Chen D. Effects of small RNA interference targeting mammalian target of rapamycin on paraquat-induced pulmonary fibrosis in rats. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:830–835. doi: 10.3760/cma.j.issn.2095-4352.2017.09.013. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 20.Chen LM, Song TJ, Xiao JH, Huang ZH, Li Y, Lin TY. Tripchlorolide induces autophagy in lung cancer cells by inhibiting the PI3K/AKT/mTOR pathway and improves cisplatin sensitivity in A549/DDP cells. Oncotarget. 2017;8:63911–63922. doi: 10.18632/oncotarget.19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Zhang P, Sun Y, Li X, Chen L, Xiao Y, Xing Y. AMPK activation-dependent autophagy compromises oleanolic acid-induced cytotoxicity in human bladder cancer cells. Oncotarget. 2017;8:67942–67954. doi: 10.18632/oncotarget.18980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung EJ, Sowers A, Thetford A, McKay-Corkum G, Chung SI, Mitchell JB, Citrin DE. Mammalian target of rapamycin inhibition with rapamycin mitigates radiation-induced pulmonary fibrosis in a murine model. Int J Radiat Oncol Biol Phys. 2016;96:857–866. doi: 10.1016/j.ijrobp.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, Franklin L, Nanthakumar CB, Man Y, Genovese F, et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax. 2016;71:701–711. doi: 10.1136/thoraxjnl-2015-207429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Dai H, Geng J, Wan X, Huang X, Li F, Jiang D, Wang C. Rapamycin increases CCN2 expression of lung fibroblasts via phosphoinositide 3-kinase. Lab Invest. 2015;95:846–859. doi: 10.1038/labinvest.2015.68. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Tai W, Qu X, Wu W, Li Z, Deng S, Vongphouttha C, Dong Z. Rapamycin protects against paraquat-induced pulmonary fibrosis: Activation of Nrf2 signaling pathway. Biochem Biophys Res Commun. 2017;490:535–540. doi: 10.1016/j.bbrc.2017.06.074. [DOI] [PubMed] [Google Scholar]
- 26.Pozharskaya V, Torres-Gonzalez E, Rojas M, Gal A, Amin M, Dollard S, Roman J, Stecenko AA, Mora AL. Twist: A regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS One. 2009;4:e7559. doi: 10.1371/journal.pone.0007559. [DOI] [PMC free article] [PubMed] [Google Scholar]