Abstract
Background: During the conflicts in Iraq and Afghanistan, more than 52,000 U.S. military members were wounded in action. The battlefield mortality rate was lower than in past conflicts, however, those surviving often had complex soft tissue and bone injuries requiring multiple surgeries. This report describes the rates, types, and risks of infections complicating the care of combat casualties.
Patients and Methods: Infection and microbiology data obtained from the Trauma Infectious Disease Outcomes Study (TIDOS), a prospective observational study of infections complicating deployment-related injuries, were used to determine the proportion of infection, types, and associated organisms. Injury and surgical information were collected from the Department of Defense Trauma Registry. Multivariable Cox proportional hazards and logistic regression models were used to evaluate potential factors associated with infection.
Results: From 2009–2012, 1,807 combat casualties were evacuated to U.S. TIDOS-participating hospitals. Among the 1,807 patients, the proportion of overall infections from time of injury through initial U.S. hospitalization was 34% with half being skin, soft tissue, or bone infections. Infected wounds most commonly grew Enterococcus faecium, Pseudomonas aeruginosa, Acinetobacter spp. or Escherichia coli. In the multivariable model, amputation, blood transfusions, intensive care unit admission, injury severity scores, mechanical ventilation, and mechanism of injury were associated with risk of infection.
Conclusions: One-third of combat casualties from Iraq and Afghanistan develop infections during their initial hospitalization. Amputations, blood transfusions, and overall injury severity are associated with risk of infection, whereas more easily modifiable factors such as early operative intervention or antibiotic administration are not.
Keywords: : combat trauma, military health, trauma-related infections, wound infections
Infections are a known complication of battlefield injuries that can lead to substantial morbidity and mortality [1–3]. During World War I, there was a 5% incidence of gas gangrene with a 28% mortality rate. With the advent of antibiotic agents and decreasing time from injury to definitive surgical care, incidence and mortality decreased to 0.3%–1.5% and 15% in World War II, respectively [4,5]. Although management of combat casualties continued to advance during the Vietnam War, sepsis was still the third leading cause of death [6].
More than 52,000 U.S. military personnel were wounded in action in Iraq (Operation Iraqi Freedom [OIF]) and Afghanistan (Operation Enduring Freedom [OEF]) [7]. Deployment of forward surgical assets, utilization of tourniquets, rapid evacuation, and use of improved body armor resulted in a greater percentage of combat casualties surviving their initial injuries compared with past conflicts [8–10]. Reduced mortality rate, however, is coupled with major challenges in subsequent care because of massive blood loss, soft tissue and bone injuries, and extensive wound contamination requiring frequent surgeries.
Previous studies have examined the rate and types of infections complicating combat injuries during OIF/OEF. In 2003, 211 combat casualties cared for on the U.S. Naval Ship Comfort were evaluated for evidence of infection and the resulting rate was 27% with the majority being wound infections (84%) followed by blood stream infections (38%). Acinetobacter spp. were the most commonly grown isolate associated with infection followed by Escherichia coli and Pseudomonas spp. Infection risk factors included wound type, mechanism of injury, injury severity score, delay in time from injury to ship arrival, and having an external fixator device [11]. In a limited trauma registry analysis of 720 combat casualties with traumatic amputations (99% the result of improvised explosive devices [IEDs]), 17% developed wound infections and the proportion of infections increased with the number of amputations (15%–18% with single and double amputations to 25%–67% with triple and quadruple amputations). Moreover, 11% of the infections were associated with Acinetobacter spp. [12]. Another investigation documented a significant increase in rates of Acinetobacter infections after the start of OIF compared to historical trends [13].
Although these studies provide some information on the types of infections observed in patients injured during OIF/OEF, they were single site or limited trauma registry studies conducted over short periods of time without following patients through different levels of care. The Trauma Infectious Diseases Outcomes Study (TIDOS) was initiated in 2009 to prospectively collect standardized infection data from point of injury in Iraq or Afghanistan through Landstuhl Regional Medical Center (LRMC), a U.S.-run medical center in Germany where all combat casualties transit through for stabilization before returning to the United States, and during initial hospitalization at TIDOS-participating U.S. hospitals. This report describes the proportion of patients with infections, types of infections, associated organisms, and risk factors for infection in combat casualties followed in TIDOS from 2009–2012.
Patients and Methods
TIDOS study design
The Trauma Infectious Diseases Outcomes Study is an observational study of short- and long-term infectious disease complications of deployment-related traumatic injuries [14]. In addition, TIDOS serves as the infectious disease (ID) module of the Department of Defense Trauma Registry (DoDTR) [15]. Eligibility criteria include: age 18 years or older with injury during deployment to Iraq or Afghanistan requiring evacuation to LRMC for care. Trauma Infectious Diseases Outcomes Study-participating hospitals in the United States include: San Antonio Military Medical Center (San Antonio, TX); Walter Reed Army Medical Center (Washington, D.C.); and National Naval Medical Center (Bethesda, MD). The latter two merged in 2011, creating Walter Reed National Military Medical Center (Bethesda, MD). Standardized information collected from injury through hospitalization at U.S. facilities includes: laboratory values; vital signs; evidence of infection; microbiology; antibiotic administration; and operating room visits. Patient trauma history, injury severity score (ISS; e.g., anatomic classifications of injury severity [16]), and surgical history were obtained from the DoDTR. For this study, all patients with combat-related injuries admitted to LRMC between June 1, 2009, which was the start of TIDOS, and May 31, 2012 were included.
Infections were classified using a combination of clinical findings, laboratory and other test results, and applying a priori standardized definitions from the National Healthcare Safety Network [17], as described previously [14]. In addition, a physician's diagnosis plus directed antibiotic therapy (5 days or more for skin and soft tissue infections [SSTIs] and 21 days or more for osteomyelitis unless surgical cure performed with amputation) without an alternative diagnosis was also considered an infection. Microbiologic evaluation was performed at the discretion of the clinical team and was not dictated by TIDOS. Antibiotic susceptibility was determined by each institution's clinical microbiology laboratory. Organisms were classified as multi-drug–resistant (MDR) if they were resistant to 3 or more classes of antibiotic agents (aminoglycosides, β-lactams, carbapenems, and fluoroquinolones) or if they expressed extended-spectrum β-lactamases or carbapenemases. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus were also considered MDR.
Statistical analysis
Differences between subjects transferred to TIDOS-participating and non-participating U.S. sites were compared using χ2 testing for categorical indicators and two sample t-test (or non-parametric testing) for continuous characteristics to examine demographic, traumatic injury, early clinical- and hospitalization-related characteristics. Among patients transferred to participating U.S. sites, patients who experienced any infection were compared with those without infection. Demographics, injury cause and type, early clinical- and hospitalization-related characteristics were considered as potential risk factors and examined through the univariable and multivariable regression models. Covariates with p ≤ 0.2 from the univariable model were considered in the initial full multivariable models. Time-to-event modeling using Cox proportional hazards models were used to examine the relation of potential risk factors from the time of injury to first infection. Covariates that might be confounding were examined for interactions (e.g., amputation, shock index, first 24-hour blood transfusion, and ISS).
Logistic regression analyses were performed by defining the infection as a binary outcome variable (1 or more infection versus no infection) and examining only risk factors present prior to transferring to U.S. hospitals. Stepwise, backward, and forward model selections were conducted to choose the final multivariable model. All variables in the final model meet the criteria of being significant at p < 0.05.
Results
Study population
During the study period, 3,304 patients were admitted to LRMC with combat-related injuries. Of those patients, 55% transferred to TIDOS-participating U.S. hospitals, 41% went to other hospitals in the United States, 4% returned to duty, and 1% died at LRMC. Overall, the combat casualties were mostly young, male, enlisted members injured in Afghanistan with two-thirds being in the Army (Table 1). More than half of those admitted to LRMC were injured from IEDs and approximately 14% suffered at least one traumatic amputation and 69% at least one bone fracture. The ISS indicated that 17% and 10% of patients sustained severe and life-threatening injuries, respectively. In the first 24 hours post-injury, 14% received at least 10 units of red blood cells.
Table 1.
Characteristics of Wounded Military Personnel Admitted to Landstuhl Regional Medical Center between June 2009 and May 2012 by Dispositiona
| Total (n = 3,304) | Return to duty (n = 121) | Died at LRMC (n = 34) | Transferred to TIDOS-participating sites (n = 1,807) |
Transferred to non-participating sites (n = 1,342) |
pb | |
|---|---|---|---|---|---|---|
| Operational theater, No. (%) | 0.009 | |||||
| Operation Iraqi Freedom (OIF) | 200 (6.1) | 6 (5.0) | 2 (5.9) | 93 (5.1) | 99 (7.4) | |
| Operation Enduring Freedom (OEF) | 3,027 (91.6) | 113 (93.4) | 32 (94.1) | 1,677 (92.9) | 1,205 (89.8) | |
| Non-OIF/OEF deploymentc | 76 (2.3) | 2 (1.7) | 0 | 36 (2.0) | 38 (2.8) | |
| Missing | 1 | 0 | 0 | 1 | 0 | |
| Male | 3,253 (98.5) | 115 (95.0) | 34 (100) | 1,784 (98.7) | 1,320 (98.4) | 0.392 |
| Median age at time of injury (IQR) | 24.4 (21.9–28.3) | 26.3 (22.7–32.0) | 23.5 (21.9–28.7) | 24.2 (21.8–28.1) | 24.6 (22.0–28.1) | 0.377 |
| Branch of service, No. (%) | <0.0001 | |||||
| Army | 2,167 (66.7) | 98 (84.5) | 22 (66.7) | 1,100 (61.8) | 947 (71.7) | |
| Marine | 897 (27.6) | 11 (9.5) | 11 (33.3) | 566 (31.8) | 309 (23.4) | |
| Air Force | 74 (2.3) | 3 (2.6) | 0 | 45 (2.5) | 26 (2.0) | |
| Navy | 92 (2.8) | 1 (0.9) | 0 | 57 (3.2) | 34 (2.6) | |
| Other | 18 (0.6) | 3 (2.6) | 0 | 11 (0.6) | 4 (0.3) | |
| Missing | 53 | 5 | 1 | 27 | 20 | |
| Military Rank, No. (%) | 0.007 | |||||
| Enlisted | 2,994 (93.1) | 97 (82.9) | 33 (100) | 1,615 (92.5) | 1,249 (94.7) | |
| Warrant/Officer | 201 (6.3) | 13 (11.1) | 0 | 121 (6.9) | 67 (5.1) | |
| Civilian | 20 (0.6) | 7 (6.0) | 0 | 10 (0.6) | 3 (0.2) | |
| Missing | 89 | 4 | 1 | 61 | 23 | |
| Mechanism of injury, No. (%) | <0.0001 | |||||
| Blast alone | 2,307 (69.8) | 82 (67.8) | 21 (61.8) | 1,379 (76.3) | 825 (61.5) | |
| IED | 1,803 (54.6) | 62 (51.2) | 17 (50.0) | 1,116 (61.8) | 608 (45.3) | |
| Non-IED blast | 504 (15.3) | 20 (16.5) | 4 (11.8) | 263 (14.6) | 217 (16.2) | |
| Non-blastd | 997 (30.2) | 39 (32.2) | 13 (38.2) | 428 (23.7) | 517 (38.5) | |
| Traumatic Amputations, No. (%)e | <0.0001 | |||||
| Any distal amputation | 189 (5.7) | 1 (0.8) | 1 (2.9) | 167 (9.3) | 20 (1.5) | |
| Any proximal amputation | 267 (8.1) | 0 | 9 (26.5) | 243 (13.5) | 15 (1.1) | |
| No amputation or digit only amputation | 2,845 (86.2) | 119 (99.2) | 24 (70.6) | 1,395 (77.3) | 1,307 (97.4) | |
| Missing | 3 | 1 | 0 | 2 | 0 | |
| Fractures, No. (%)f | <0.0001 | |||||
| Closed | 1,032 (31.2) | 49 (40.5) | 10 (29.4) | 534 (29.6) | 439 (32.7) | |
| Open only/both open and closed | 1,241 (37.6) | 16 (13.2) | 19 (55.9) | 787 (43.6) | 419 (31.2) | |
| Abbreviated Injury Scale Upper Extremity Maximum severity, No. (%)g | <0.0001 | |||||
| None | 1,508 (45.6) | 70 (57.9) | 20 (58.8) | 739 (40.9) | 679 (50.6) | |
| 1 | 923 (27.9) | 37 (30.6) | 6 (17.6) | 518 (28.7) | 362 (27.0) | |
| 2 | 585 (17.7) | 10 (8.3) | 3 (8.8) | 352 (19.5) | 220 (16.4) | |
| 3 | 225 (6.8) | 4 (3.3) | 2 (5.9) | 144 (8.0) | 75 (5.6) | |
| 4 | 59 (1.8) | 0 | 3 (8.8) | 51 (2.8) | 5 (0.4) | |
| 5 | 4 (0.1) | 0 | 0 | 3 (0.2) | 1 (0.1) | |
| Abbreviated Injury Scale Lower Extremity Maximum Severity, No. (%)g | <0.0001 | |||||
| None | 970 (29.4) | 43 (35.5) | 15 (44.1) | 456 (25.2) | 456 (34.0) | |
| 1 | 747 (22.6) | 43 (35.5) | 4 (11.8) | 327 (18.1) | 373 (27.8) | |
| 2 | 595 (18.0) | 23 (19.0) | 2 (5.9) | 271 (15.0) | 299 (22.3) | |
| 3 | 447 (13.5) | 11 (9.1) | 1 (2.9) | 279 (15.4) | 156 (11.6) | |
| 4 | 263 (8.0) | 1 (0.8) | 1 (2.9) | 219 (12.1) | 42 (3.1) | |
| 5 | 282 (8.5) | 0 | 11 (32.4) | 255 (14.1) | 16 (1.2) | |
| Injury Severity Score, No. (%)h | <0.0001 | |||||
| 0–9 (mild) | 1,718 (52.1) | 89 (74.2) | 0 | 635 (35.2) | 994 (74.1) | |
| 10–15 (moderate) | 690 (20.9) | 22 (18.3) | 2 (5.9) | 426 (23.6) | 240 (17.9) | |
| 16–24 (severe) | 565 (17.1) | 6 (5.0) | 5 (14.7) | 477 (26.4) | 77 (5.7) | |
| ≥ 25 (life-threatening) | 329 (10.0) | 3 (2.5) | 27 (79.4) | 268 (14.8) | 31 (2.3) | |
| Missing | 2 | 1 | 0 | 1 | 0 | |
| Shock index,i median (IQR) | 0.7 (0.5–0.8) | 0.6 (0.5–0.7) | 0.8 (0.7–1.0) | 0.7 (0.6–0.8) | 0.6 (0.5–0.7) | <0.0001 |
| Blood product transfusions within first 24 hours, No. (%)j | ||||||
| Zero or missing units | 2,229 (67.5) | 116 (95.9) | 8 (23.5) | 923 (51.1) | 1,182 (88.1) | <0.0001 |
| 1–9 units | 613 (18.6) | 4 (3.3) | 8 (23.5) | 473 (26.2) | 128 (9.5) | |
| 10–20 units | 252 (7.6) | 1 (0.8) | 7 (20.6) | 228 (12.6) | 16 (1.2) | |
| > 20 units | 210 (6.4) | 0 | 11 (32.4) | 183 (10.1) | 16 (1.2) | |
| LRMC admission unit, No. (%) | <0.0001 | |||||
| Intensive care unit | 1,117 (33.9) | 8 (6.8) | 33 (97.1) | 954 (52.9) | 122 (9.1) | |
| Ward | 2,176 (66.1) | 110 (93.2) | 1 (2.9) | 849 (47.1) | 1,216 (90.9) | |
| Missing | 11 | 3 | 0 | 4 | 4 | |
| Mechanical ventilation at LRMC, No. (%)k | 741 (22.4) | 2 (1.7) | 34 (100) | 651 (36.0) | 54 (4.0) | <0.0001 |
Data are missing for some variables. Percentages are calculated excluding the missing data from the denominator.
p value compares data from the TIDOS-participating sites to the non-participating sites. Missing values are not included in p value calculation.
Non-OIF/OEF deployment includes Operation New Dawn, which began in Iraq on September 1, 2010 and continued until the end of military operations in Iraq on December 15, 2011.
Non-blast mechanisms include burns, gunshot wounds, motor vehicle crashes, and falls.
Amputation data collected from LRMC. Distal amputations include below the knee or below the elbow. Proximal amputations include through the knee, above the knee, or above the elbow. Digit only amputations are excluded from the distal and proximal categories.
Digit-only fractures are not included. Fracture data obtained from LRMC.
The Abbreviated Injury Scale is an anatomically based severity scoring system that classifies traumatic injuries by body region using an ascending scale of injury severity (0 = none; 5 = critical injury) [16].
The Injury Severity Score is an overall measure calculated for each patient based on the top three maximum Abbreviated Injury Scale anatomic region values [44].
Shock index is defined as the heart rate divided by systolic blood pressure. It was documented at the initial support hospital following injury.
Blood transfusion data are missing for 2,116 patients. Missing blood transfusion data are not randomly distributed. Patients with missing blood data are characterized by lower injury severity scores and shock indices. In addition, the majority of patients with missing blood data did not sustain a traumatic amputation and were not admitted to the LRMC intensive care unit.
Mechanical ventilation data missing three patients who transferred to a TIDOS-participating site and one at a non-participating site
IED = improvised explosive device; IQR = interquartile range; LRMC = Landstuhl Regional Medical Center; TIDOS = Trauma Infectious Disease Outcomes Study.
Patients transferred to a TIDOS-participating hospital had a higher proportion of injuries because of IED blasts in Afghanistan, any amputation, an ISS greater than 10, and a greater number of blood transfusions in the first 24 hours in those who went on to non-TIDOS–participating hospitals (Table 1).
Proportion of infections, types, and associated organisms
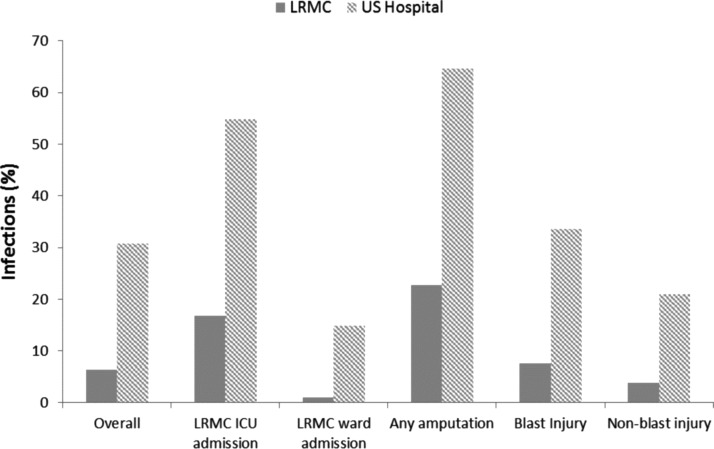
The median number of days between injury and arrival at LRMC was 2.0 (interquartile range [IQR]: 2.0–3.0) and the duration of hospitalization at LRMC was 2.0 (IQR: 1.0–3.0) days. The overall infection rate at LRMC for all patients with combat-related injuries was 6.4% (Fig. 1). Among the 1,807 patients who transferred to TIDOS-participating U.S. sites, 9.9% at LRMC were diagnosed with any infection. The median duration of hospitalization at TIDOS-participating U.S. sites was 18.0 (IQR: 9.0–36.0) days and the overall infection rate was 30.7%. At both LRMC and the TIDOS-participating U.S. sites, infections were more common in patients with amputations as well as those admitted to the intensive care unit (ICU). Including infections at both LRMC and the U.S., 34% of patients who went to a TIDOS-participating U.S. site had at least one infection. Of those patients with infections, more than half had more than one infection (24% had two infections, 14% had three infections, and 19% had four or more infections). The infection incidence density rate (first infection/100-person days, 95% confidence interval) was 1.9 (1.7–2.0).
FIG. 1.
Proportion of infections by facility (i.e., Landstuhl Regional Medical Center [LRMC] or a participating hospital in the United States) at the time of diagnosis. ICU = intensive care unit.
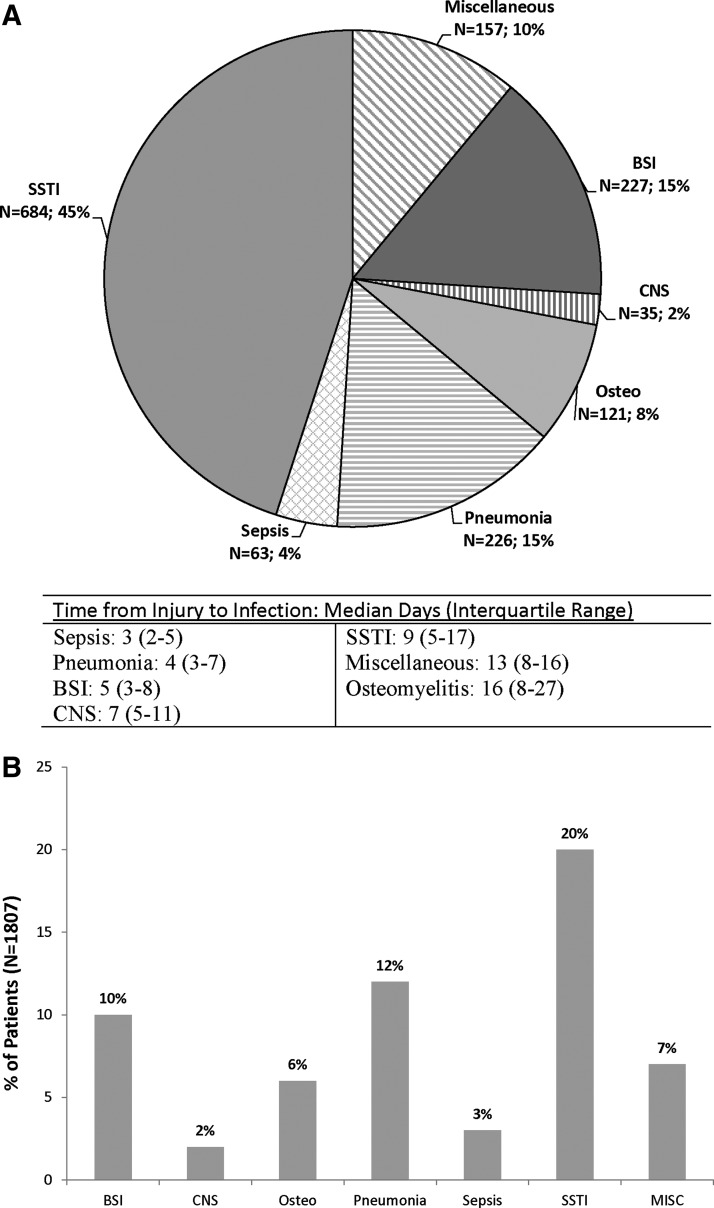
Among the patients transferred to TIDOS-participating U.S. sites, there were a total of 1,514 infections in theater, LRMC, and the United States, of which 45% were SSTIs, 15% pneumonia, 15% blood stream infections, and 8% osteomyelitis (Fig. 2A). Central nervous system infections and sepsis not linked to another infection type were uncommon. On a patient level, of the 1,807 patients, 20% developed a SSTI during their initial hospitalization, 12% developed pneumonia, 10% a blood stream infection, and 6% osteomyelitis (Fig. 2B). Time to first infection varied by infection type (Fig. 2A). Table 2 presents the microbiologic profile associated with SSTI, osteomyelitis, and blood stream infections and the percentage of organisms that were MDR. Although the majority of the Acinetobacter spp. and Escherichia coli isolates were MDR, the opposite was true of Pseudomonas aeruginosa and Enterococcus faecium.
FIG. 2.
Distribution of types of infections from the inpatient period at participating sites in the United States. (A) Data are on a per infection basis. Miscellaneous infections are urinary tract infections (5.7%), intra-abdominal infections (2.3%), Clostridium difficile (1.0%), sinusitis (0.6%), bronchitis (0.3%), eye infections (0.1%), and otitis (0.1%). Percentages are calculated based upon the total number of infections during the inpatient period (n = 1,514). (B) Data are on a per subject basis. Percentages are calculated based upon the total number of patients admitted to participating sites in the United Sates (n = 1,807). BSI = blood stream infection; CNS = central nervous system; MISC = miscellaneous; Osteo = osteomyelitis; SSTI = skin and soft-tissue infection.
Table 2.
Microbiologic Profile of Common Trauma-Related Infectionsa
| Organism | Percentage of total organisms per infection syndrome | Percentage classified as multidrug-resistant |
|---|---|---|
| Blood stream infection organisms (n = 313) | ||
| Gram-positive bacteria | 47.6 | 6.7 |
| Coagulase-negative Staphylococcus | 33.2 | 0 |
| Enterococcus faecium | 6.1 | 26.3 |
| Gram-negative bacteria | 41.5 | 42.3 |
| Acinetobacter calcoaceticus baumannii complex | 9.0 | 92.9 |
| Escherichia coli | 7.0 | 90.9 |
| Pseudomonas aeruginosa | 6.7 | 4.8 |
| Anaerobes | 3.2 | 0 |
| Osteomyelitis organisms (n = 263) | ||
| Gram-positive bacteria | 23.2 | 21.3 |
| Enterococcus faecium | 8.0 | 23.8 |
| Coagulase-negative Staphylococcus | 5.7 | 0 |
| Gram-negative bacteria | 46.8 | 47.2 |
| Acinetobacter calcoaceticus baumannii complex | 11.0 | 86.2 |
| Pseudomonas aeruginosa | 10.3 | 18.5 |
| Escherichia coli | 8.8 | 78.3 |
| Enterobacter cloacae | 6.1 | 12.5 |
| Anaerobes | 12.5 | 0 |
| Skin and soft-tissue infection organisms (n = 1,341) | ||
| Gram-positive bacteria | 26.9 | 13.9 |
| Enterococcus faecium | 9.9 | 17.3 |
| Gram-negative bacteria | 46.5 | 41.8 |
| Pseudomonas aeruginosa | 9.6 | 10.2 |
| Escherichia coli | 9.6 | 73.4 |
| Acinetobacter calcoaceticus baumannii complex | 8.7 | 94.8 |
| Enterobacter cloacae | 6.3 | 1.2 |
| Anaerobes | 7.6 | 0 |
Includes organisms contributing ≥5% to the microbiologic profile; organisms were detected in cultures but may not be the etiologic agent for the infection.
Infection risk factors
The Cox model was used to examine unadjusted and adjusted association of time to infection with potential risk factors among patients with combat injuries who were transferred to TIDOS-participating U.S. sites (Table 3). In the multivariable model, amputation (both distal and proximal), blood transfusions in the first 24 hours post-injury, LRMC ICU admission, severe or life-threatening ISS, and mechanical ventilation were associated with increased risk of infection (Table 3). In addition, non-IED blasts were associated with lower infection risk compared with those with non-blast injuries. The risk factor analysis was repeated using logistic regression to evaluate for risk factors associated with any infection and the results were similar (data not shown).
Table 3.
Analysis of Risk Factors for the Development of Infections among Military Personnel with Combat-Related Injuries
| Risk factor | Total (n = 1,807); No. (% of total) | Patients with ≥1 infection (n = 612); No. (% of row) | Patients with no infection (n = 1,195); No. (% of row) | Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Operational theater | |||||
| Operation Iraqi Freedom | 93 (5.1) | 31 (33.3) | 62 (66.7) | 1.0 | — |
| Operation Enduring Freedom | 1,677 (92.9) | 573 (34.2) | 1,104 (65.8) | 1.10 (0.77–1.59) | — |
| Non-OIF/OEF deployment | 36 (2.0) | 8 (22.2) | 28 (77.8) | 0.72 (0.33–1.58) | — |
| Gender | |||||
| Female | 23 (1.3) | 4 (17.4) | 19 (82.6) | 1.0 | — |
| Male | 1,784 (98.7) | 608 (34.1) | 1,176 (65.9) | 2.15 (0.81–5.76) | — |
| Branch of service | |||||
| Army | 1,100 (60.9) | 348 (31.6) | 752 (68.4) | 1.0 | — |
| Marine | 566 (31.3) | 224 (39.6) | 342 (60.4) | 1.31 (1.10–1.54) | — |
| Air Force | 45 (2.5) | 12 (26.7) | 33 (73.3) | 0.72 (0.41–1.29) | — |
| Navy | 57 (3.2) | 17 (29.8) | 40 (70.2) | 1.01 (0.62–1.65) | — |
| Other | 39 (2.2) | 11 (28.2) | 28 (71.8) | 0.96 (0.53–1.75) | — |
| Military rank | |||||
| Enlisted | 1,637 (93.0) | 548 (33.5) | 1,089 (66.5) | 1.0 | — |
| Warrant / Officer | 123 (7.0) | 45 (36.6) | 78 (63.4) | 0.99 (0.73–1.34) | — |
| Civilian / Missing | 47 | 19 | 28 | NA | — |
| Mechanism of injury | |||||
| Non-blast | 428 (23.7) | 102 (23.8) | 326 (76.2) | 1.0 | 1.0 |
| IED | 1,116 (61.8) | 461 (41.3) | 655 (58.7) | 1.80 (1.45–2.23) | 1.07 (0.84–1.35) |
| Non-IED blast | 263 (14.6) | 49 (18.6) | 214 (81.4) | 0.73 (0.52–1.03) | 0.69 (0.49–0.97) |
| Traumatic Amputationsa | |||||
| No amputation or digits only amputation | 1,395 (77.3) | 332 (23.8) | 1,063 (76.2) | 1.0 | 1.0 |
| Any distal amputation excluding digits | 169 (9.4) | 90 (53.3) | 79 (46.7) | 2.09 (1.66–2.64) | 1.46 (1.11–1.92) |
| Any proximal amputation excluding digits | 241 (13.4) | 190 (78.8) | 51 (21.2) | 4.67 (3.90–5.59) | 1.57 (1.23–2.01) |
| Any fracture, excluding digitsb | |||||
| None | 441 (24.4) | 91 (20.6) | 350 (79.4) | 1.0 | — |
| Closed only | 435 (24.1) | 121 (27.8) | 314 (72.2) | 1.36 (1.04–1.79) | — |
| Open +/- closed | 931 (51.5) | 400 (43.0) | 531 (57.0) | 2.01 (1.60–2.53) | — |
| Abbreviated Injury Scale Upper Extremity Maximum Severityc | |||||
| 0 (none) | 739 (40.9) | 189 (25.6) | 550 (74.4) | 1.0 | — |
| 1 | 518 (28.7) | 164 (31.7) | 354 (68.3) | 1.29 (1.05–1.59) | — |
| 2 | 352 (19.5) | 167 (47.4) | 185 (52.6) | 1.97 (1.60–2.42) | — |
| 3 | 144 (8.0) | 60 (41.7) | 84 (58.3) | 1.68 (1.26–2.25) | — |
| 4 | 51 (2.8) | 30 (58.8) | 21 (41.2) | 2.53 (1.72–3.72) | — |
| 5 | 3 (0.2) | 2 (66.7) | 1 (33.3) | 5.45 (1.35–21.99) | — |
| Abbreviated Injury Scale Lower Extremity Maximum Severityc | |||||
| 0 (none) | 456 (25.2) | 99 (21.7) | 357 (78.3) | 1.0 | — |
| 1 | 327 (18.1) | 58 (17.7) | 269 (82.3) | 0.79 (0.57–1.09) | — |
| 2 | 271 (15.0) | 55 (20.3) | 216 (79.7) | 0.77 (0.56–1.08) | — |
| 3 | 279 (15.4) | 108 (38.7) | 171 (61.3) | 1.39 (1.06–1.83) | — |
| 4 | 219 (12.1) | 99 (45.2) | 120 (54.8) | 1.78 (1.35–2.36) | — |
| 5 | 255 (14.1) | 193 (75.7) | 62 (24.3) | 4.26 (3.34–5.43) | — |
| Injury Severity Scored | |||||
| 0–9 (mild) | 635 (35.2) | 68 (10.7) | 567 (89.3) | 1.0 | 1.0 |
| 10–15 (moderate) | 426 (23.6) | 119 (27.9) | 307 (72.1) | 2.37 (1.76–3.20) | 1.21 (0.88–1.67) |
| 16–24 (severe) | 477 (26.4) | 241 (50.5) | 236 (49.5) | 5.21 (3.98–6.83) | 1.46 (1.06–2.00) |
| ≥ 25 (life-threatening) | 268 (14.8) | 184 (68.7) | 84 (31.3) | 8.37 (6.34–11.06) | 1.79 (1.29–2.49) |
| Shock indexe | |||||
| <0.6 (lowest quartile) | 457 (25.3) | 79 (17.3) | 378 (82.7) | 1.0 | — |
| 0.6–0.8 (IQR) | 820 (45.4) | 242 (29.5) | 578 (70.5) | 1.57 (1.22–2.03) | — |
| >0.8 (highest quartile) | 528 (29.3) | 291 (55.1) | 237 (44.9) | 3.22 (2.51–4.13) | — |
| Blood product transfusions within first 24 hoursf | |||||
| Zero or missing units | 923 (51.1) | 107 (11.6) | 816 (88.4) | 1.0 | 1.0 |
| 1–9 units | 473 (26.2) | 190 (40.2) | 283 (59.8) | 3.21 (2.53–4.07) | 1.80 (1.40–2.33) |
| 10–20 units | 228 (12.6) | 153 (67.1) | 75 (32.9) | 6.36 (4.96–8.15) | 2.00 1.48–2.70) |
| > 20 units | 183 (10.1) | 162 (88.5) | 21 (11.5) | 13.48 (10.53–17.24) | 3.19 (2.30–4.42) |
| LRMC admission unit | |||||
| Ward | 849 (47.1) | 88 (10.4) | 761 (89.6) | 1.0 | 1.0 |
| Intensive care unit | 954 (52.9) | 522 (54.7) | 432 (45.3) | 6.47 (5.16–8.11) | 1.92 (1.42–2.60) |
| Mechanical ventilation at LRMC | 651 (36.0) | 421 (64.7) | 230 (35.3) | 5.40 (4.55–6.41) | 2.12 (1.70–2.65) |
| Any operating room visit in theater | 1,535 (84.9) | 580 (37.8) | 955 (62.2) | 2.96 (2.07–4.23) | — |
| Splenectomy performed prior to first infection | 3 (0.2) | 3 (100) | 0 | 3.59 (1.15–11.16) | — |
| Any antibiotic use in the first 48 hours | 1,466 (81.1) | 559 (38.1) | 907 (61.9) | 2.38 (1.79–3.15) | — |
| Colonization with MDROs prior to first infection | 344 (19.0) | 149 (43.3) | 195 (56.7) | 1.33 (1.10–1.60) | — |
Traumatic amputations recorded at Landstuhl Regional Medical Center. Distal amputations include below the knee or below the elbow. Proximal amputations include through the knee, above the knee, or above the elbow.
Fracture data collected from all levels of care.
The Abbreviated Injury Scale is an anatomically based severity scoring system that classifies traumatic injuries by body region using an ascending scale of injury severity (0 = none; 5 = critical injury) [16].
The Injury Severity Score is an overall measure calculated for each patient based on the top three maximum Abbreviated Injury Scale anatomic region values [44].
Shock index is defined as the heart rate divided by systolic blood pressure. It was documented at the initial support hospital following injury.
Blood transfusion data are missing for 2,116 patients. Missing blood transfusion data are not randomly distributed. Patients with missing blood data are characterized by lower injury severity scores and shock indices. In addition, the majority of patients with missing blood data did not sustain a traumatic amputation and were not admitted to the LRMC intensive care unit.
CI = confidence interval; IED = improvised explosive device; IQR = interquartile range; LRMC = Landstuhl Regional Medical Center; MDRO = multidrug-resistant organism.
Discussion
The Trauma Infectious Diseases Outcomes Study is the first study to evaluate comprehensively combat trauma infection rates, types, and risks by collecting standardized data from across multiple levels of care from the combat theater to the United States. One-third of combat casualties treated at TIDOS-participating U.S. sites develop infections during their initial hospitalization with more than half being SSTI or bone infections. Among those with amputations, two-thirds develop infections. The most common organisms cultured from infected wounds include Enterococcus faecium, Pseudomonas aeruginosa, MDR Acinetobacter spp., and ESBL-producing Escherichia coli. Risk factors for developing infection include having a traumatic amputation, receiving blood transfusions within 24 hours of injury (particularly if more than 10 units), ICU admission, early mechanical ventilation, and having a moderate to severe ISS. Less risk of infection was associated with the non-IED injury mechanism relative to other blast trauma.
It is difficult to compare the rate of infection during OIF/OEF with that of other conflicts because past conflicts have not had similar registries that were able to follow patients from the battlefield through initial hospitalization in the United States. For example, a study evaluating 17,726 casualties from the Vietnam War found an overall infection rate of 3.9% [2]. This study, however, only evaluated patients during their hospitalization in Vietnam (mean duration: 9 days) with more than one-third evacuated from Vietnam in less than 5 days and almost three-quarters in less than 15 days. In our analysis, SSTIs and osteomyelitis accounted for more than half of the infections and these did not occur until a median of 9–16 days post-injury. Therefore, the infection rate reported in the Vietnam study is not comparable to the one in our study. The 34% infection rate observed in the current analysis, however, is higher than the 8%–9% infection rate associated with non-combat trauma [18–20] and the 4% reported overall U.S. hospital-acquired infection rate [21].
An earlier analysis of TIDOS data that included only three months of data collection (June 2009 to August 2009) found an infection rate of 27% among those transferred from LRMC to U.S. TIDOS-participating sites [14], which is similar to the rate observed in the current three-year analysis. This is in contrast to an analysis that used the DoDTR to investigate infection rates in deployment-injured patients from March 2003 to April 2009 and found an overall infection rate of 5.5% [22]. The latter evaluation pre-dated the TIDOS ID module of the DoDTR in which there were limited infection-related outcomes data captured. Given the risk factors associated with infection, the combat-related injuries are likely to have higher rates of infection.
Organisms cultured from infected sites in patients injured in OIF/OEF are similar to ones observed in patients injured in Vietnam. A study of 30 U.S. Marines injured in Vietnam found that on admission wounds initially grew gram-positive skin flora along with Enterobacter, Escherichia coli, Klebsiella pneumoniae, and members of what was called the Mimaea-Herellea-Bacterium-Alcaligenes group (may have included Acinetobacter spp.) [23]. By the fifth hospital day, wound bacteriology changed with increased isolation of Pseudomonas aeruginosa and Proteus. All of the gram-negative bacteria were resistant to penicillin, the antibiotic agent given to the combat casualties. The wounds in that study were not necessarily infected, but rather sampled to understand the bacteriology of war wounds over time [23]. Likewise, a study of 100 Vietnam casualties found that the most common bacterial species isolated from war wounds requiring amputation included Pseudomonas aeruginosa, Staphylococcus aureus, Proteus, and Klebsiella [24].
Although the types of organisms are similar to those that have been observed in the past, the antibiotic resistance patterns are different and are becoming more resistant over time [25,26]. Approximately 95% of the Acinetobacter spp. isolated from wounds was MDR and 75% of the Escherichia coli isolates produced ESBLs. Several previous studies have investigated the potential sources of these MDR bacteria. Studies of healthy service members in the United States have not demonstrated significant colonization with MDR Acinetobacter prior to deployment [27,28], however, one study showed an 11% prevalence of MDR Escherichia coli colonization in healthy U.S. service members who were in Afghanistan [29]. Also, MDR organisms have not been prevalent in cultures from soil in deployed locations [30] and war wounds have not been found to be colonized with MDR organisms at the time of injury [31]. There is evidence of nosocomial spread of Acinetobacter spp. [13], but less so for Escherichia coli [32] in deployed subjects and another potential source includes acquired resistance of endogenous flora because of antibiotic pressure. Over a three-year period (2009–2012), 14% of 2079 military trauma patients admitted to participating hospitals in the United States were colonized on hospital admission with MDR gram-negative bacilli with Escherichia coli being the most frequent MDR (or ESBL-producing) organism identified [33].
Although the Acinetobacter and Escherichia coli isolates associated with infection were usually MDR, the Pseudomonas and Enterococcus isolated from infected sites remained largely antibiotic susceptible. This may be secondary to the antibiotics that were given for post-injury prophylaxis [34], however, further investigation should be conducted on the potential association between post-injury antibiotic exposure and multidrug resistance.
Similar to other studies [2,19,35], factors associated with infection risk in this analysis were related mostly to injury pattern or severity. Blood transfusions, especially more than 10 units in the 24 hours post-injury, had the highest infection risk. Transfusion is a marker of severity of injury, however, in addition this may also be caused by the immunomodulatory effects of blood transfusions [36]. Several studies have also shown that transfusion of red blood cells collected more than 14 days prior is associated with increased rates of infection [37,38]. Since 2008, there has been a concerted effort by the Armed Services Blood Program to reduce the age of red blood cells available for transfusion in theater [39]. From 2007 to 2011, the average age of red blood cells received in theater decreased from 13 to 7 days and the average age upon transfusion to massively transfused (more than 10 units per 24 hours) patients decreased from 33 to 23 days. Because TIDOS does not collect information on the age of red blood cells transfused, the impact of this on infection could not be examined in this study.
Unfortunately most of the factors associated with infections in this analysis are not easily modifiable. For example, neither going to the operating room in the theater of combat (indicating early operative intervention) nor receiving antibiotic agents within 48 hours post-injury were associated with infection risk. The injuries in Afghanistan have been characterized by an increase in amputation rate along with abdominal–pelvic injuries sustained by service members injured by explosive devices while conducting dismounted (foot) patrols [10]. Our data suggest that in order to decrease the rate of infections, injury prevention is needed (perhaps through more mounted missions or further improvement in body armor). Another possible modification is decreasing the number of red blood cell transfusions by using post-injury tranexamic acid, an anti-fibrinolytic agent that has been shown to decrease mortality in bleeding trauma patients [40,41], or using younger blood products or blood product alternatives. It is not clear if any of these interventions would affect infection rates, however, one retrospective analysis that compared military trauma patients who did and did not receive tranexamic acid reported no significant association between tranexamic acid and infection risk [42]. Another study examining tranexamic acid and infection risk in civilian trauma patients had similar findings [43].
Several limitations of this analysis should be noted. Patients who were evacuated from LRMC to TIDOS-participating sites in the United States were injured more severely than those who went to other hospitals in the United States, therefore, the infection rates observed in this analysis may not be generalizable to all combat casualties. Still, more than half of the injured patients at LRMC went to one of the U.S. TIDOS-participating sites; therefore, these findings are still relevant to the majority of combat casualties. The information in this report may not be generalizable to most civilian trauma given the common mechanism of injury (blasts) and the setting requiring multiple evacuations for definitive care. Another limitation is that infected sites (often wounds) may be polymicrobial or grow contaminants or colonizing organisms. We do not have any way of determining which organisms were true pathogens. Any organism growing from infected wounds was considered associated with the infection. For blood stream infections, stricter criteria were used [14]. Strengths of this analysis include the fact that we used a priori criteria to define infections, collected standardized data across multiple levels of care from injury through U.S. hospitalization, and followed all combat injured who came through LRMC during the given time period.
Although the infection rate is high (34% overall; 68% in amputees), this only considers infections that occurred during initial hospitalization. Many of these patients require re-hospitalization and further surgical management of their injuries. The rate of incident infections after the initial hospitalization also needs to be determined to understand the full impact of infections complicating the care of combat casualties. Given the large number of patients with combat trauma injuries, high infection rate, and prevalence of MDR organisms, the cost of combat trauma infections to the healthcare system as well as the patient is high. Further research should focus on strategies for improving prevention and treatment of these infections.
Contributor Information
Collaborators: the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group
Acknowledgments
We are indebted to the Infectious Disease Clinical Research Program TIDOS study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
This work (IDCRP-024) was supported by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health [Inter-Agency Agreement Y1-AI-5072], and the Department of the Navy under the Wounded, Ill, and Injured Program [HU001-10-1-0014].
The views expressed are those of the authors and do not reflect the official views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, Walter Reed National Military Medical Center, Landstuhl Regional Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
A portion of this data was presented at the 2014 Military Health System Research Symposium, August 18–21, 2014, Fort Lauderdale, FL.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma 2008;64:S221–S231 [DOI] [PubMed] [Google Scholar]
- 2.Hardaway RM., 3rd. Viet Nam wound analysis. J Trauma 1978;18:635–643 [DOI] [PubMed] [Google Scholar]
- 3.Simchen E, Sacks T. Infection in war wounds: Experience during the 1973 October War in Israel. Ann Surg 1975;182:754–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North JP. Clostridial wound infections and gas gangrene; Arterial damage as a modifying factor. Surgery 1947;21:364–372 [PubMed] [Google Scholar]
- 5.Neel HB, Cole JP. Gas gangrene in amphibious warfare in the Pacific area. Am J Surg 1944;66:290–299 [Google Scholar]
- 6.Arnold K, Cutting RT. Causes of death in United States military personnel hospitalized in Vietnam. Mil Med 1978;143:161–164 [PubMed] [Google Scholar]
- 7.United States Department of Defense. OIF/OEF Casualty Status. www.defense.gov/casualty.pdf (Last accessed October13, 2017)
- 8.Gawande A. Casualties of war—Military care for the wounded from Iraq and Afghanistan. N Engl J Med 2004;351:2471–2475 [DOI] [PubMed] [Google Scholar]
- 9.Kelly JF, Ritenour AE, McLaughlin DF, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma 2008;64:S21–S26 [DOI] [PubMed] [Google Scholar]
- 10.Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield (2001–2011): Implications for the future of combat casualty care. J Trauma Acute Care Surg 2012;73:S431–S437 [DOI] [PubMed] [Google Scholar]
- 11.Petersen K, Riddle MS, Danko JR, et al. Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007;245:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey BW, Martin A, Chestovich PJ, et al. Patients with multiple traumatic amputations: An analysis of Operation Enduring Freedom Joint Theatre Trauma Registry data. Injury 2017;48;75–79 [DOI] [PubMed] [Google Scholar]
- 13.Scott P, Deye G, Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis 2007;44:1577–1584 [DOI] [PubMed] [Google Scholar]
- 14.Tribble DR, Conger NG, Fraser S, et al. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 2011;71:S33–S42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eastridge BJ, Jenkins D, Flaherty S, et al. Trauma system development in a theater of war: Experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2006;61:1366–1372 [DOI] [PubMed] [Google Scholar]
- 16.Champion HR, Holcomb JB, Lawnick MM, et al. Improved characterization of combat injury. J Trauma 2010;68:1139–1150 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. CDC/NHSN surveillance definitions for specific types of infections. www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf; (Last accessed June12, 2017)
- 18.Yun HC, Blackbourne LH, Jones JA, et al. Infectious complications of noncombat trauma patients provided care at a military trauma center. Mil Med 2010;175:317–323 [DOI] [PubMed] [Google Scholar]
- 19.Pories SE, Gamelli RL, Mead PB, et al. The epidemiologic features of nosocomial infections in patients with trauma. Arch Surg 1991;126:97–99 [DOI] [PubMed] [Google Scholar]
- 20.Lazarus HM, Fox J, Lloyd JF, et al. A six-year descriptive study of hospital-associated infection in trauma patients: Demographics, injury features, and infection patterns. Surg Infect 2007;8:463–473 [DOI] [PubMed] [Google Scholar]
- 21.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray CK, Wilkins K, Molter NC, et al. Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. J Trauma 2011;71:S62–73 [DOI] [PubMed] [Google Scholar]
- 23.Tong MJ. Septic complications of war wounds. JAMA 1972;219:1044–1047 [PubMed] [Google Scholar]
- 24.Heggers JP, Barnes ST, Robson MC, et al. Microbial flora of orthopaedic war wounds. Mil Med 1969;134:602–603 [PubMed] [Google Scholar]
- 25.Zapor MJ, Erwin D, Erowele G, et al. Emergence of multidrug resistance in bacteria and impact on antibiotic expenditure at a major army medical center caring for soldiers wounded in Iraq and Afghanistan. Infect Control Hosp Epidemiol 2008;29:661–663 [DOI] [PubMed] [Google Scholar]
- 26.Lesho E, Yoon EJ, McGann P, et al. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis 2013;208:1142–1151 [DOI] [PubMed] [Google Scholar]
- 27.Griffith ME, Ellis MW, Murray CK. Acinetobacter nares colonization of healthy US soldiers. Infect Control Hosp Epidemiol 2006;27:787–788 [DOI] [PubMed] [Google Scholar]
- 28.Griffith ME, Lazarus DR, Mann PB, et al. Acinetobacter skin carriage among US army soldiers deployed in Iraq. Infect Control Hosp Epidemiol 2007;28:720–722 [DOI] [PubMed] [Google Scholar]
- 29.Vento TJ, Cole DW, Mende K, et al. Multidrug-resistant gram-negative bacteria colonization of healthy US military personnel in the US and Afghanistan. BMC Infect Dis 2013;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keen EF, 3rd, Mende K, Yun HC, et al. Evaluation of potential environmental contamination sources for the presence of multidrug-resistant bacteria linked to wound infections in combat casualties. Infect Control Hosp Epidemiol 2012;33:905–911 [DOI] [PubMed] [Google Scholar]
- 31.Murray CK, Roop SA, Hospenthal DR, et al. Bacteriology of war wounds at the time of injury. Mil Med 2006;171:826–829 [DOI] [PubMed] [Google Scholar]
- 32.Mende K, Beckius ML, Zera WC, et al. Phenotypic and genotypic changes over time and across facilities of serial colonizing and infecting Escherichia coli isolates recovered from injured service members. J Clin Microbiol 2014;52:3869–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert LJ, Li P, Murray C, et al. Multidrug-resistant gram-negative bacilli colonization risk factors among trauma patients. Diagn Microbiol Infect Dis 2016;84:358–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd BA, Weintrob AC, Hinkle MK, et al. Adherence to published antimicrobial prophylaxis guidelines for wounded service members in the ongoing conflicts in southwest Asia. Mil Med 2014;179:324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papia G, McLellan BA, El-Helou P, et al. Infection in hospitalized trauma patients: incidence, risk factors, and complications. J Trauma 1999;47:923–927 [DOI] [PubMed] [Google Scholar]
- 36.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood 2001;97:1180–1195 [DOI] [PubMed] [Google Scholar]
- 37.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008;358:1229–1239 [DOI] [PubMed] [Google Scholar]
- 38.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg 2002;137:711–716 [DOI] [PubMed] [Google Scholar]
- 39.Rentas F, Lincoln D, Harding A, et al. The Armed Services Blood Program: Blood support to combat casualty care 2001 to 2011. J Trauma Acute Care Surg 2012;73:S472–S478 [DOI] [PubMed] [Google Scholar]
- 40.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010;376:23–32 [DOI] [PubMed] [Google Scholar]
- 41.Morrison JJ, Dubose JJ, Rasmussen TE, et al. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg 2012;147:113–119 [DOI] [PubMed] [Google Scholar]
- 42.Lewis CJ, Li P, Stewart L, et al. Tranexamic acid in life-threatening military injury and the associated risk of infective complications. Br J Surg 2016;103:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid uses in severely injured civilian patients and the effects on outcomes. A prospective cohort study. Ann Surg 2015;261:390–394 [DOI] [PubMed] [Google Scholar]
- 44.Linn S. The injury severity score—Importance and uses. Ann Epidemiol 1995;5:440–446 [DOI] [PubMed] [Google Scholar]