Abstract
Interleukin (IL)-27 is a pleiotropic cytokine that regulates multiple aspects of innate and adaptive immunity, but whose role in immune protection of the female reproductive tract is unknown. Although not constitutively expressed by human uterine epithelial cells and fibroblasts in culture, IL-27 secretion was upregulated after treatment with the viral ligand poly (I:C) in a type I interferon (IFN)-dependent manner, with higher levels measured in fibroblasts than epithelial cells. Estradiol increased poly (I:C)-induced IL-27 production by fibroblasts, but not epithelial cells. While both cell types expressed the IL-27 receptor, only fibroblasts responded to recombinant IL-27 with increased expression of the antiviral genes, APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G) and MxA, and the tryptophan-catabolizing enzyme, indoleamine 2,3-dioxygenase (IDO). Estradiol inhibited IL-27-mediated induction of IDO in fibroblasts through estrogen receptor alpha, but had no effect on APOBEC3G. IL-27 pretreatment also potentiated poly (I:C) upregulation of the antiviral genes, OAS2 and APOBEC3G, in fibroblasts. Thus, IL-27 is part of the antiviral response by uterine cells against potential pathogens. The effect of estradiol on IL-27 production and sensitivity by fibroblasts demonstrates a selective hormone action on individual cell types in the uterus and suggests that IL-27 may have differential effects during the menstrual cycle.
Keywords: : uterus, epithelial cells, stromal fibroblasts, estradiol, poly (I:C), IL-27
Introduction
The mucosal barrier of the female reproductive tract (FRT) is the first line of defense against sexually transmitted pathogens that cause morbidity and mortality in millions of women. In the uterine endometrium, the mucosa comprises a protective single layer of columnar epithelial cells supported by a dense layer of stromal fibroblasts with a dynamic population of immune cells interspersed throughout the tissue. Both epithelial cells and fibroblasts, which constitute the majority of nonhematopoietic cells in the endometrium, protect the FRT either by directly eliminating incoming pathogens or indirectly by secreting multiple cytokines and chemokines that in turn recruit and regulate immune cells. Defining the immune regulatory functions of epithelial cells and fibroblasts is essential for understanding their role in protecting the FRT.
IL-27 is a pleiotropic cytokine that regulates multiple aspects of innate and adaptive immunity (Yoshida and Hunter 2015). It is a member of the IL-12 family of cytokines and is primarily produced by a variety of immune cell types, including macrophages, monocytes, and dendritic cells (Yoshida and Hunter 2015). IL-27 comprises 2 subunits: Epstein–Barr virus-induced gene 3 and IL-27 p28 (Pflanz and others 2002); and signals through a heterodimeric receptor consisting of IL-27Rα (WSX-1/TCCR) and gp130 (Pflanz and others 2004). Receptor engagement by IL-27 leads to activation of downstream JAK/STAT (Hibbert and others 2003; Takeda and others 2003) and MAPK (Owaki and others 2006) signaling pathways. IL-27 was first described as a proinflammatory cytokine that promoted the differentiation of naïve T cells into Th1 cells (Pflanz and others 2002). Other inflammatory effects included decreasing Treg frequency (Stumhofer and others 2007; Huber and others 2008; Tait Wojno and others 2011) and activating proliferation of naïve T cells. However, other work demonstrated that IL-27 also had anti-inflammatory functions. It promoted production of the anti-inflammatory cytokine IL-10 by CD4+ T cells (Awasthi and others 2007; Freitas do Rosário and others 2012), suppressed differentiation of Th17 T cell subsets (Batten and others 2006; Stumhofer and others 2006), and inhibited pathology associated with an excessive immune response (Villarino and others 2003). However, no studies have addressed the role and regulation of IL-27 in the FRT.
IL-27 is also part of the immune response to incoming pathogens. Previous studies have shown that IL-27 is induced by stimulation of multiple Toll-like receptors (TLRs), including TLR3 (de Groot and others 2012) and TLR4 (Molle and others 2007). IL-27 inhibits HIV infection of macrophages and CD4+ T cells through the induction of antiviral genes such as APOBEC3G and bone marrow stromal antigen 2 (BST-2)/tetherin (Imamichi and others 2008; Greenwell-Wild and others 2009; Chen and others 2013; Dai and others 2013), which are also classical interferon-stimulated genes (ISGs). IL-27 also enhances the secretion of antimicrobial protein human beta-defensin 2 by keratinocytes (Kanda and Watanabe 2008). In addition, IL-27 is protective against malaria (Ayimba and others 2011), herpes simplex virus 1 (HSV-1) (Liao and others 2014), hepatitis C virus (HCV) (Frank and others 2010), and influenza A virus (Liu and others 2012, 2014). Whether IL-27 has a protective role in the FRT against potential pathogens is unknown.
Unique among mucosal sites, the immune system in the FRT is precisely regulated by the sex hormones, estradiol (E2) and progesterone (Wira and others 2015). Previous studies using epithelial cells and stromal fibroblasts determined that E2 regulates multiple aspects of immune function, including TLR, cytokine, and growth factor expression, suggesting that hormonal effects may vary across the menstrual cycle. However, our understanding of the role of E2 in IL-27 regulation is sparse. In previous studies, E2 increased IL-27 production by monocyte-derived dendritic cells (Kyurkchiev and others 2007). However, no studies have addressed IL-27 production by cells from the human FRT and its role in immune protection at this mucosal site. Therefore, the purpose of this study was to determine if uterine epithelial cells and fibroblasts produce and respond to IL-27, whether it is induced by viral dsRNA, and if the sex hormone E2 regulates its secretion and action.
Materials and Methods
Source of uterine tissue
Human uterine tissue was obtained from women undergoing hysterectomy surgery at Dartmouth-Hitchcock Medical Center (Lebanon, NH). All tissues used in this study were distal to the sites of pathology and were determined to be unaffected by disease upon inspection by a pathologist. All investigations involving human subjects were conducted according to the principles expressed in the Declaration of Helsinki and carried out with the approval from the Committee for the Protection of Human Subjects, Dartmouth-Hitchcock Medical Center, and with written informed consent obtained from the patients before surgery.
Isolation of uterine epithelial cells and uterine fibroblasts
Tissues were minced under sterile conditions into 1–2-mm fragments and subjected to enzymatic digestion using an enzyme mixture that contained final concentrations of 3.4 mg/mL pancreatin (Invitrogen Life Technologies, Carlsbad, CA), 0.1 mg/mL hyaluronidase (Worthington Biochemical, Lakewood, NJ), 1.6 mg/mL collagenase (Worthington Biochemical), and 2 mg/mL D-glucose, in 1× HBSS (Invitrogen). After enzymatic digestion for 1 h at 37°C, cells were dispersed through a 250-μm mesh screen, washed, and suspended in Hank's Balanced Salt Solution (Thermo Scientific, Logan, UT).
Epithelial cell sheets were separated from stromal fibroblasts by filtration through a 20-μm nylon mesh filter (Small Parts, Miami Lakes, FL). Epithelial sheets were retained on the 20-μm filter, while the stromal fraction containing fibroblasts passed through and were collected as part of the filtrate. Epithelial sheets were recovered by rinsing and backwashing the filter with DMEM/F12, centrifuged (500 g, 10 min), and analyzed for cell number and viability.
Uterine epithelial cell and uterine fibroblast cell culture
To establish an in vitro cell culture system of polarized human uterine epithelial cells with both apical and basolateral surfaces, uterine epithelial cells were incubated in Falcon cell culture inserts coated with Human Extracellular Matrix (Becton Dickinson, Franklin Lakes, NJ) in 24-well culture plates (Fisher Scientific, Pittsburgh, PA). Apical and basolateral compartments had 300 and 500 μL of complete medium, respectively. Complete medium consisted of DMEM/F12 supplemented with 20 mM HEPES (Invitrogen), 2 mM L-glutamine (Invitrogen), 50 mg/mL primocin (Invivogen), and 10% heat-inactivated defined fetal bovine serum (FBS) (Thermo Scientific). The medium was changed every 2 days.
To establish a purified population of uterine fibroblasts, the stromal filtrate was centrifuged (500 g, 10 min) and the pellet resuspended in complete media, placed in a 75-cm2 cell culture flask (Fisher) in complete medium until they reached confluence, with the medium changed every 2 days. After reaching confluence, the cells were trypsinized and 1 × 106 cells added to a fresh 75-cm2 flask. This was repeated at least once more before the cells were recovered and plated (1 × 106 cells/mL) in 24-well cell culture dishes (Fisher) in 500 μL of complete medium with charcoal–dextran-stripped FBS for at least 48 h before treatment.
Poly (I:C), IFNAR, interleukin-27, Raloxifene, and estradiol treatment
Cells were stimulated with poly (I:C) (Sigma) at 0.25–25 μg/mL for up to 24 h. Recombinant human IL-27 (Bio-Techne, Minneapolis, MN) was used at 1–100 ng/mL for up to 48 h. For polarized epithelial cells, poly (I:C) was added to the apical compartment only, while IL-27 was added to both the apical and basolateral compartments. Interferon receptor blockade experiments were conducted using a mouse monoclonal anti-human interferon receptor 2 (IFNAR2)-blocking antibody (Bio-Techne).
For all hormone experiments, 17β-estradiol (E2) (Calbiochem, Gibbstown, NJ) and Raloxifene (Rx) (Tocris Bioscience) were dissolved in 100% ethanol at an initial concentration of 1 × 10−3 M, evaporated to dryness, and resuspended in complete media containing charcoal–dextran-stripped FBS to a concentration of 1 × 10−5 M. Further dilutions were made to achieve a final working concentration of 5 × 10−8 M for E2 and 1 × 10−6 M for Rx. As a control, an equivalent amount of 100% ethanol without hormones was initially evaporated. For polarized uterine epithelial cells, E2 was added to both the apical and basolateral compartments. Rx was added at least 1 h before addition of E2. Both E2 and Rx were maintained in the cell culture media throughout the experiment.
In all experiments with poly (I:C), IL-27, E2, and Rx, complete medium containing 10% heat-inactivated defined FBS was replaced with 10% heat-inactivated charcoal/dextran-treated stripped FBS (Gemini, West Sacramento, CA) to remove any steroid hormones present in FBS that otherwise would confound the effects of the exogenous hormone.
TaqMan real-time RT-PCR
Total mRNA was isolated and purified using an RNeasy mini kit (Qiagen, Valencia, CA) with on-column DNase digestion using the RNase-free DNase set (Qiagen) according to the manufacturer's recommendations. Four hundred nanograms of total RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's recommendations. Relative mRNA expression levels of genes of interest were measured using the 5′ fluorogenic nuclease assay in real-time quantitative PCR using TaqMan chemistry on the ABI 7300 Prism real-time PCR instrument (Applied Biosystems, Carlsbad, CA). PCR was conducted using the following cycle parameters: 95°C, 12 min for 1 cycle (95°C, 20 s; 60°C, 1 min), for 40 cycles. Analysis was conducted using the sequence detection software supplied with ABI 7300. Relative expression levels were expressed as a fold increase in mRNA expression and calculated using the formula 2–ΔΔCt.
ELISA
Secretion of IL-27 in culture media was measured by Duoset ELISA (Bio-Techne) as per the manufacturer's instructions.
Statistical analysis
A 2-tailed paired t-test or a 1-way analysis of variance (ANOVA) was performed using GraphPad Prism, version 5.0, software (GraphPad Software, San Diego, CA). A P-value less than 0.05 was considered statistically significantly different. Comparison of 3 or more groups was performed by applying the Kruskal–Wallis test for nonmatched samples or Friedman test for matched samples, followed by Dunn's post-test for multiple comparison correction.
Results
Poly (I:C) induces IL-27 in uterine epithelial cells and fibroblasts
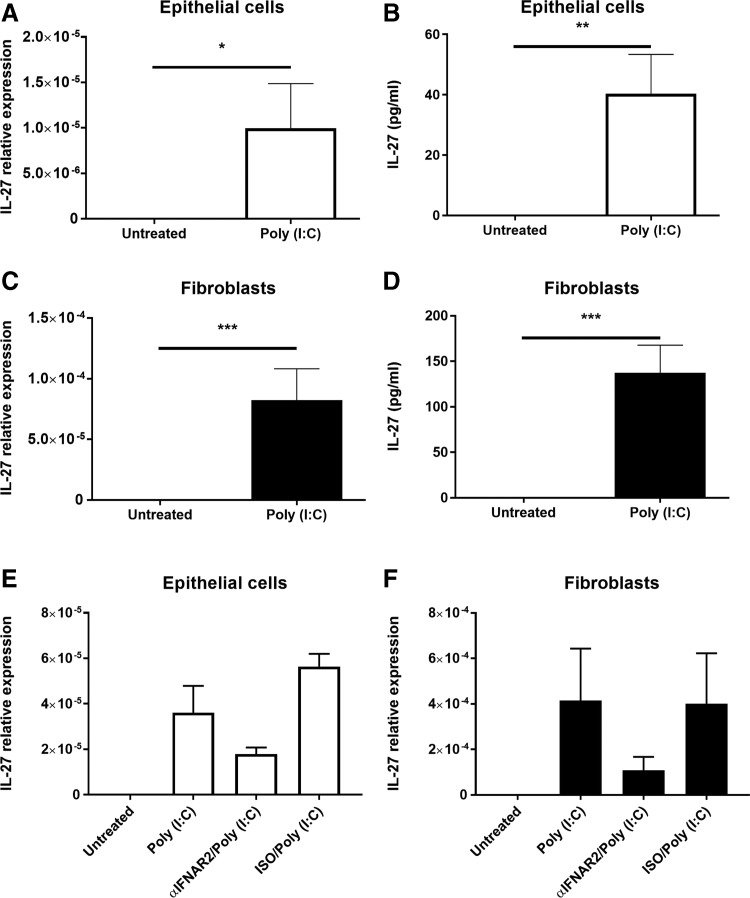
To investigate the role of IL-27 in the uterine mucosa, we isolated and grew to confluence purified populations of epithelial cells and stromal fibroblasts from uterine hysterectomy tissues recovered from both pre- and postmenopausal women. We found that neither epithelial cells nor fibroblasts constitutively secreted IL-27. To determine if IL-27 expression was inducible, epithelial cells and stromal fibroblasts were incubated with the viral ligand/TLR3 agonist poly (I:C) for 24 and 48 h. As seen in Figure 1A and C, IL-27 mRNA was induced at 24 h relative to untreated controls for both cell types. Also shown in Figure 1B and D is the stimulatory effect of poly (I:C) on IL-27 secretion measured at 48 h for both cell types. IL-27 protein was not detectable in 24-h supernatants and only became measurable after 48 h of poly (I:C) exposure. As seen in Figure 2, when fibroblasts from the endocervix and ectocervix were incubated with poly (I:C), IL-27 secretion measured at 48 h was markedly increased, indicating that fibroblasts from the upper and lower FRT are responsive to viral pathogens.
FIG. 1.
Poly (I:C) induces IL-27 mRNA expression and secretion in uterine epithelial cells (A, B) and stromal fibroblasts (C, D). Confluent monolayers of epithelial cells and fibroblasts were treated with 25 μg/mL of poly (I:C). mRNA (A, C) and secretions (B, D) were recovered after 24 and 48 h, respectively, and levels of IL-27 measured by RT-PCR and ELISA (n = 4–5). For type I interferon (IFN) receptor complex (IFNAR) blockade (E, F), both epithelial cells and fibroblasts were pretreated with αIFNAR2 or IgG control (ISO) for 1 h before poly (I:C) treatment for the following 24 h. αIFNAR2 was maintained in cell culture media throughout the experiment (n = 3). mRNA expression is normalized to levels of β-actin. *P < 0.05; **P < 0.01; ***P < 0.001.
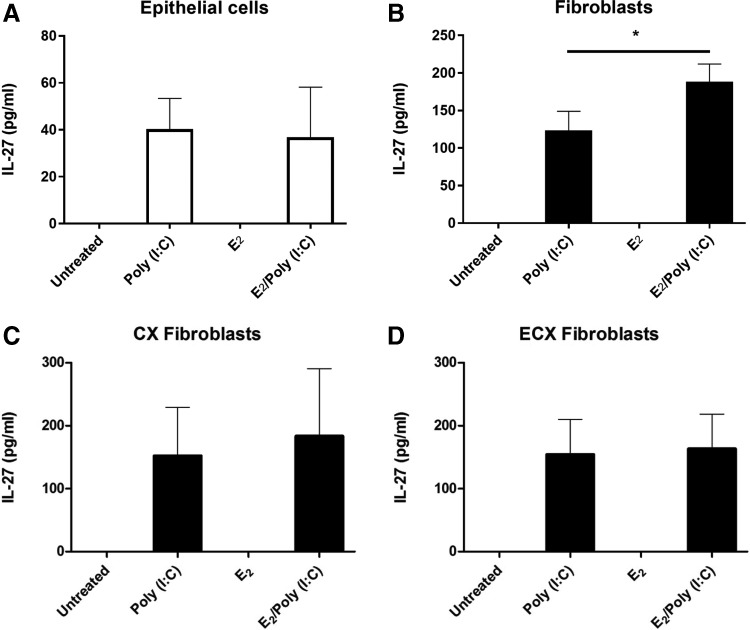
FIG. 2.
Estradiol upregulates poly (I:C)-induced secretion of IL-27 by uterine stromal fibroblasts. Confluent monolayers of uterine epithelial cells (A), uterine fibroblasts (B), endocervical fibroblasts (C), and ectocervical fibroblasts (D) were pretreated with estradiol (E2) at 5 × 10−8 M for 48 h before washout and subsequent treatment with E2 and poly (I:C) (25 μg/mL) for 48 h. IL-27 levels were measured in cell secretions by ELISA (n = 4–5). *P < 0.05.
Since previous studies have demonstrated a role for type I IFNs in the upregulation of IL-27 (Pirhonen and others 2007), we then investigated whether type I IFN signaling is necessary for the upregulation of IL-27 following exposure to poly (I:C). As seen in Figure 1E and F, blockade of the IFN receptor (IFNAR) complex using an antibody against IFNAR2 inhibits upregulation of IL-27 expression by both epithelial cells and fibroblasts following treatment with poly (I:C) for 24 h.
Estradiol upregulates IL-27 secretion by uterine fibroblasts
Previously, we found that E2 is a potent regulator of uterine epithelial cell and fibroblast cytokine secretion (Fahey and others 2008; Coleman and others 2009). To determine if E2 regulates the expression and secretion of IL-27, we pretreated epithelial cells and stromal fibroblasts with E2 for 48 h, followed by stimulation with poly (I:C) for an additional 24–48 h. E2 alone did not induce IL-27 in either cell type (Fig. 2). However, E2 significantly, but modestly, increased IL-27 secretion by stromal fibroblasts beyond that seen with poly (I:C) alone from ∼100 to 150 pg/mL (Fig. 2B). In contrast, E2 had no effect on poly (I:C)-induced epithelial cell secretion of IL-27 (Fig. 2A). To determine if this response was specific for uterine fibroblasts, we used matched tissue samples of cervical (CX) and ectocervical (ECX) fibroblasts. As seen in Figure 2C and D, in 4 experiments, E2 had no effect on IL-27 secretion by CX or ECX fibroblasts either alone or in the presence of poly (I:C), indicating that the effect of E2 in the presence of poly (I:C) is unique to uterine stromal fibroblasts.
IL-27 induces ISG expression in uterine fibroblasts
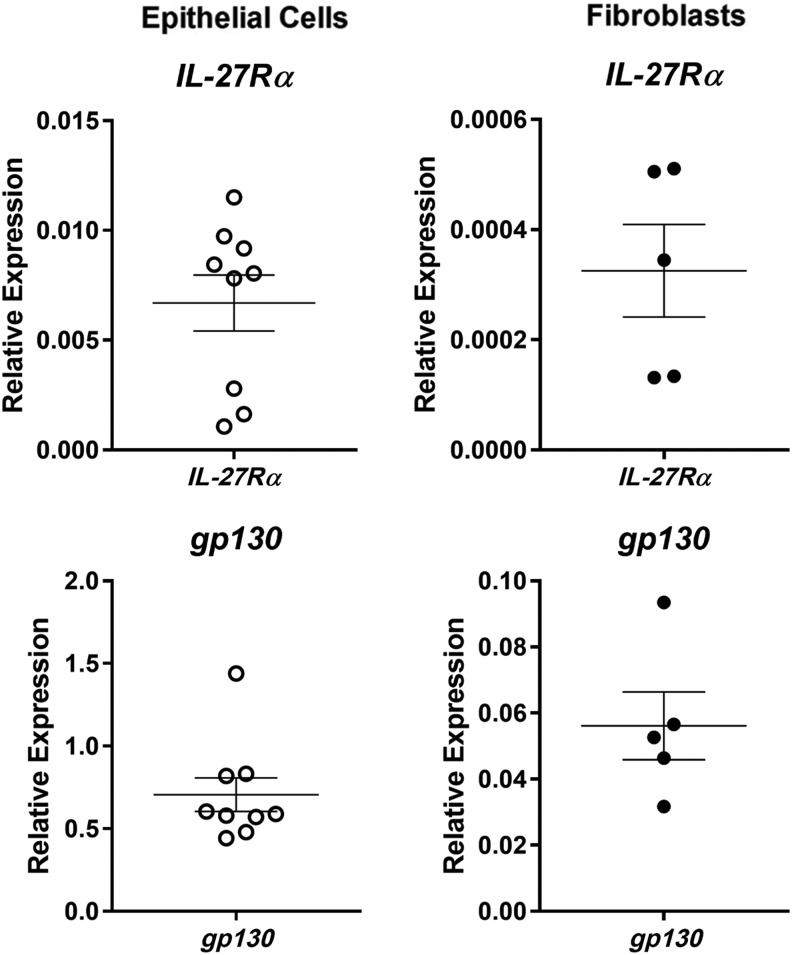
As a pleiotropic cytokine, IL-27 has a range of varying effects (Yoshida and Hunter 2015). To determine if uterine epithelial cells or fibroblasts are potentially responsive to IL-27, we analyzed the mRNA recovered from both cell types for the presence of IL-27Rα and gp130, the subunits of the IL-27 heterodimeric receptor complex. As seen in Figure 3, both IL-27Rα and gp130 are expressed in epithelial cells and fibroblasts, suggesting that they are potential targets for IL-27 in the FRT.
FIG. 3.
IL-27 receptor expression in uterine epithelial cells and stromal fibroblasts. mRNA was recovered from untreated confluent monolayers of epithelial cells and fibroblasts, and levels of IL-27Rα and gp130 were determined by RT-PCR. Expression levels of IL-27Rα and gp130 are both normalized to levels of β-actin in each cell type.
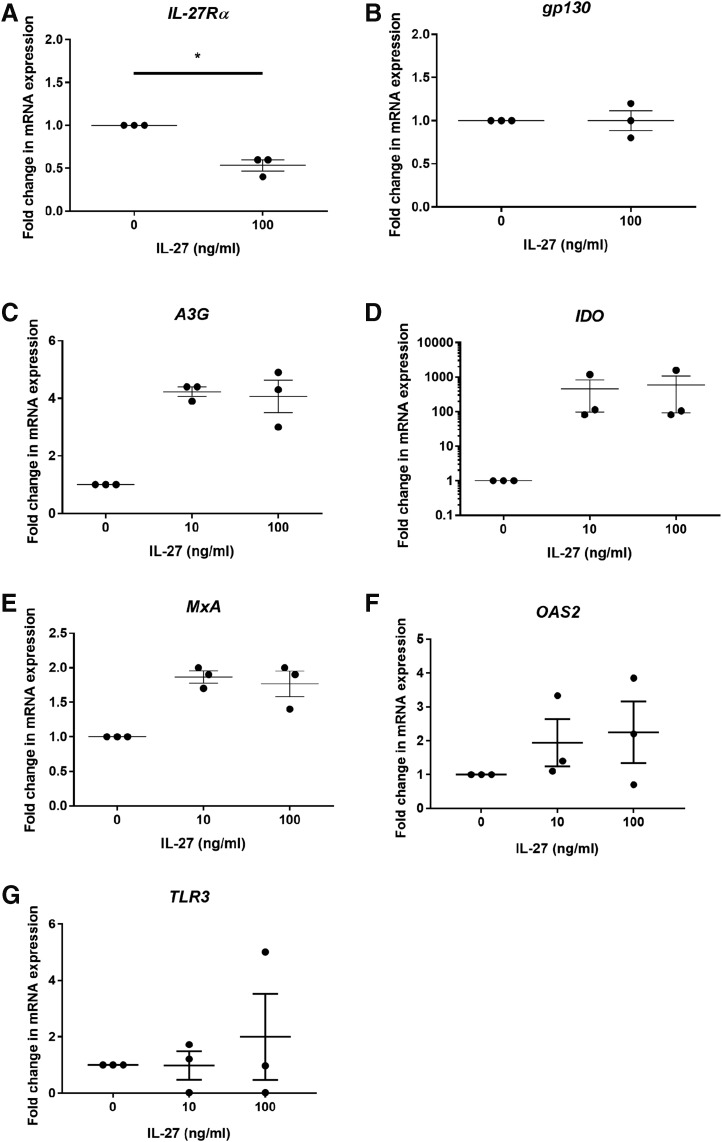
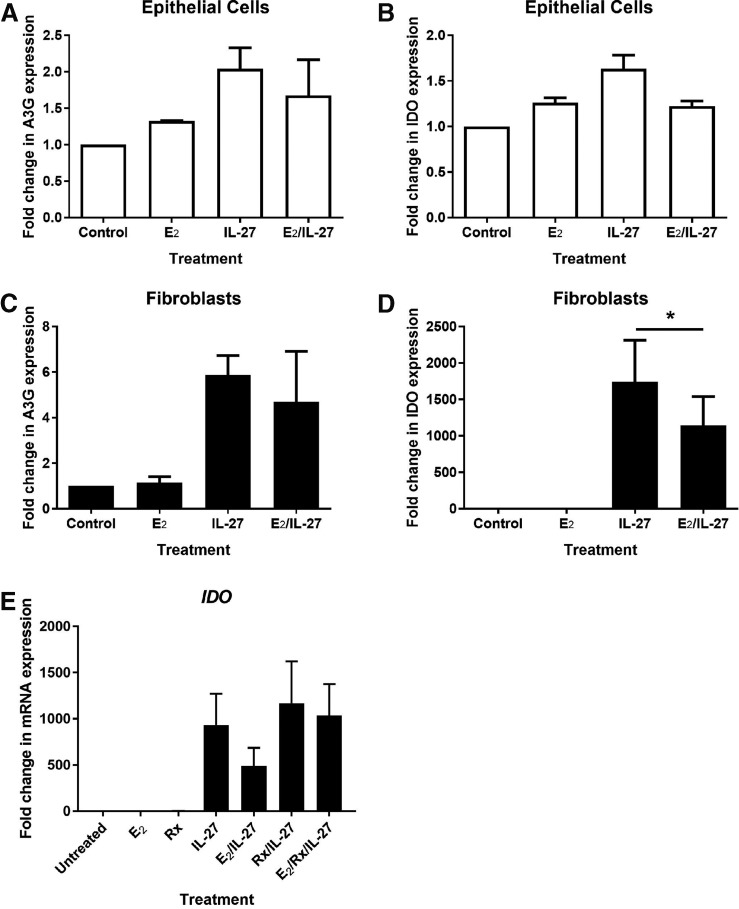
Uterine epithelial cells and fibroblasts were then incubated with 10 and 100 ng/mL of recombinant IL-27 for 24 h before mRNA recovery and analysis by RT-PCR. As seen in Figure 4A and B, IL-27 significantly reduced mRNA expression of the IL-27Rα subunit in fibroblasts by ∼50%, but had no effect on gp130 expression. IL-27 upregulated the fibroblast expression of the tryptophan-catabolizing enzyme, indoleamine 2,3-dioxygenase (IDO), (Fig. 4D) by 600-fold over untreated cells and APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G) (Fig. 4C), which plays an important role in innate antiviral immunity, by ∼4-fold over untreated cells. IL-27 weakly upregulated the expression of MxA (Fig. 4E) and had no effect on the expression of either OAS2 (Fig. 4F) or TLR3 (Fig. 4G). In contrast to the fibroblasts, there was no effect of IL-27 on uterine epithelial cell expression of IL-27Rα, gp130, IDO, APOBEC3G, MxA, OAS2, or TLR3 (data not shown).
FIG. 4.
IL-27 reduces IL-27Rα and induces IDO and A3G expression in uterine stromal fibroblasts. Confluent monolayers of fibroblasts were treated with recombinant human IL-27 for 24 h before mRNA recovery and analysis for IL-27Rα (A), gp130 (B), IDO (C), APOBEC3G (A3G) (D), MxA (E), OAS2 (F), and TLR3 (G) by RT-PCR. *P < 0.05. APOBEC3G, apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G; IDO, indoleamine 2,3-dioxygenase.
Estradiol inhibits IL-27-induced expression in uterine fibroblasts
Recognizing that IL-27 signals through the PI3K, STAT, and NF-kB pathways, which can be influenced by E2 (Björnström and Sjöberg 2005), we pretreated matched sets of uterine epithelial cells and fibroblasts with E2 for 48 h before stimulation with IL-27 for a subsequent 24 h. As seen in Figure 5, E2 inhibited the IL-27-induced upregulation of IDO in uterine fibroblasts, but had no effect on epithelial cells. Interestingly, E2 had no effect on APOBEC3G expression in either cell type, nor did it affect the mRNA expression of IL-27Rα or gp130 (data not shown).
FIG. 5.
Estradiol inhibits IL-27-induced IDO expression through ERα in uterine stromal fibroblasts. Confluent monolayers of matched epithelial cells (A, B) and fibroblasts (C, D) were pretreated with estradiol (E2) at 5 × 10−8 M for 48 h before washout and subsequent treatment with E2 or IL-27 (100 ng/mL) for 24 h, after which mRNA expression levels for APOBEC3G (A3G) and IDO were measured by RT-PCR (n = 4–5). For ER blockade, Rx was added to confluent monolayers of fibroblasts (E) at 5 × 10−6 M for 1 h before addition of E2 at 5 × 10−8 M for a further 48 h, followed by washout and treatment with E2 or IL-27 (100 ng/mL) for a further 24 h. Rx was maintained in the media at 5 × 10−6 M for the entire duration of the experiment (n = 3). *P < 0.05. ER, estrogen receptor; Rx, Raloxifene.
To determine the pathway of E2 regulatory control, we treated uterine fibroblasts with Rx, an E2 antagonist that is a competitor of E2 for estrogen receptor (ER) α. As seen in Figure 5E, Rx alone had no effect on IDO expression in uterine fibroblasts. Neither did Rx affect the upregulation of IDO following treatment with IL-27. However, Rx blocked E2-induced downregulation of IDO following treatment with IL-27, thus demonstrating that E2 modulates IL-27 signaling through ERα.
IL-27 potentiates the antiviral response of uterine fibroblasts to poly (I:C)
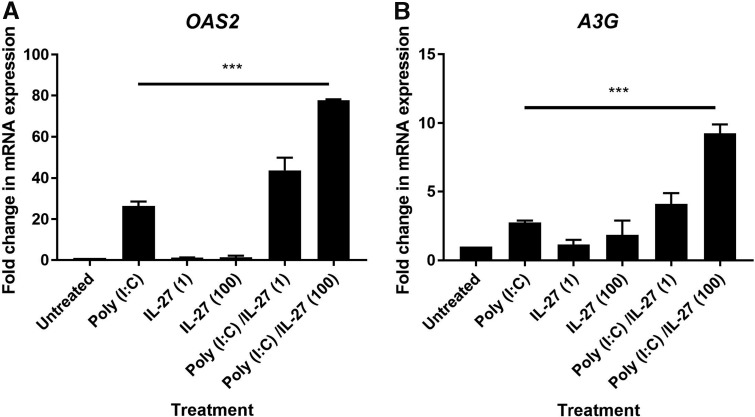
Previous studies have demonstrated that exposure to IL-27 alters the response to TLR ligands in other cell types such lung fibroblasts (Su and others 2016). To determine if IL-27 regulates the antiviral response to poly (I:C), we pretreated uterine fibroblasts with IL-27 for 48 h before washout and stimulation with poly (I:C) for a subsequent 24 h. As seen in Figure 6, pretreatment of uterine fibroblasts with IL-27 led to upregulation of OAS2 and APOBEC3G upon exposure to poly (I:C). In the presence of 100 ng/mL of IL-27, OAS2 expression was ∼70-fold greater than the untreated control, while poly (I:C) alone only increased OAS2 expression by ∼30-fold. Similarly, APOBEC3G expression was upregulated ∼10-fold by poly (I:C) following pretreatment with 100 ng/mL IL-27. In contrast, the expression of IDO was unaffected by poly (I:C) despite pretreatment with IL-27 (data not shown). The effect of IL-27 was dose dependent as fibroblasts pretreated with 10 ng/mL IL-27 only weakly upregulated OAS2 and APOBEC3G. Overall, these findings indicate that IL-27 potentiates the upregulation of OAS2 and APOBEC3G in uterine stromal fibroblasts in response to poly (I:C).
FIG. 6.
IL-27 potentiates the upregulation of OAS2 and APOBEC3G (A3G) in uterine stromal fibroblasts. Confluent monolayers of fibroblasts were pretreated with IL-27 (1 and 100 ng/mL) for 48 h before washout and subsequent treatment with poly (I:C) (25 μg/mL) for 24 h, after which mRNA expression levels for OAS2 (A) and A3G (B) were measured by RT-PCR (n = 4). ***P < 0.001.
Discussion
The present study demonstrates that IL-27 is expressed by epithelial cells and fibroblasts in the human FRT in response to the TLR3 agonist poly (I:C) in a type I IFNAR-dependent manner. Recombinant IL-27 induced IDO, APOBEC3G, and MxA gene expression in uterine fibroblasts, but not epithelial cells. While E2 had no direct effect on IL-27 secretion or IL-27 receptor subunit expression, it potentiated the increase in IL-27 secretion induced by poly (I:C) from uterine fibroblasts, but not epithelial cells. However, E2 also inhibited the increase in IDO expression through ERα after IL-27 treatment of fibroblasts. IL-27 also potentiated the response of fibroblasts to poly (I:C). Together, these results demonstrate that uterine fibroblasts are both producers of and responsive to IL-27 in the human FRT. Since both cell types express the IL-27 receptors, but are not equally responsive to IL-27, it suggests that regulatory steps beyond receptor binding regulate cellular responsiveness to IL-27.
Little is known about the role of IL-27 in the FRT. Previous studies suggested that IL-27 is essential for successful reproduction. For example, neutralization of IL-27 in the murine placenta led to increased rates of abortion (Mas and others 2008). Pregnancy induces an immune response and IL-27 may modulate this to increase the likelihood of successful reproduction. However, the role of IL-27 in the nonpregnant endometrium remains equally unclear. Since neither uterine epithelial cells nor fibroblasts constitutively produce IL-27, this suggests that outside of immune protection in the nonpregnant endometrium, IL-27 has a restricted role. By demonstrating that IL-27 is specifically secreted in response to the viral mimic poly (I:C), our study expands the potential role of IL-27 in the FRT to include the immune response to viral pathogens. The presence of IL-27 in FRT secretions may thus be a useful marker for viral exposure in the FRT, unlike other inflammatory cytokines such as IL-8 and macrophage chemotactic protein 1 (MCP-1), which are constitutively produced by both FRT epithelial cells and fibroblasts (Koumas and others 2001; Schaefer and others 2005). Poly (I:C) is a synthetic viral mimic, and it is important to recognize that poly (I:C) treatment is only a substitute for live viral exposure and may not capture the full complexity of a viral infection. Therefore, future studies will need to move beyond the use of synthetic TLR ligands and use live pathogens.
The IL-27 secreted by epithelial cells and fibroblasts likely acts upon immune cells within the local mucosal environment to augment the antiviral state and protect against incoming pathogens. In response to IL-27, macrophages, monocytes, and CD4+ T cells inhibit HIV (Imamichi and others 2008; Greenwell-Wild and others 2009; Chen and others 2013; Dai and others 2013). IL-27 can also inhibit HCV (Frank and others 2010) and HSV-1 (Liao and others 2014) infection in vitro. Thus, IL-27 could be involved in pathogen protection in the FRT due to its ability to upregulate antiviral genes such as APOBEC3G in uterine fibroblasts. Upregulation of antiviral genes such as APOBEC3G likely extends the antiviral state and creates a hostile environment within FRT tissues long after the initial pathogen exposure. Our finding of induction of antiviral genes in the FRT is consistent with previous studies demonstrating that IL-27 can upregulate classical ISGs by signaling through the same pathway as the interferons. However, the pattern of gene expression varies with cell type, with macrophages upregulating more genes than CD4+ T cells (Imamichi and others 2008). Similarly, in our system, while fibroblasts upregulated APOBEC3G, IDO, and MxA in response to IL-27, there was no change in expression of these genes within epithelial cells. This contrasts with intestinal epithelial cells, which strongly upregulate IDO in response to IL-27 (Diegelmann and others 2012). This does not imply that epithelial cells are insensitive to IL-27, but rather that IL-27 regulates a different panel of genes in epithelial cells compared with fibroblasts and that the responsiveness of epithelial cells from different mucosal sites to IL-27 is unique.
Previous studies have shown that IL-27 enhances TLR4-mediated immune responses to lipopolysaccharide to induce a signaling cascade in monocytes and lung fibroblasts (Guzzo and others 2012; Su and others 2016). Our studies extend these findings by demonstrating that uterine fibroblasts respond to IL-27 to potentiate the antiviral response to poly (I:C). This further demonstrates that IL-27 enhances the immune response among distinct fibroblast populations. Taken together, these findings suggest that a major function of IL-27 is increasing sensitivity to potential pathogens that access the endometrium and breach the epithelial barrier. It further supports our hypothesis that IL-27 provides a protective measure against exposure to viral pathogens allowing for a rapid innate immune response, leading to intracellular protection. Whether this occurs at other mucosal sites, particularly among fibroblasts in the subepithelial stromal tissues, is unclear, but it could allow IL-27 to extend the duration of specific aspects of the antiviral state.
E2 is a potent regulator of immune function in the FRT (Wira and others 2015). The current study extends these findings by demonstrating that secretion of and responsiveness to IL-27 are regulated by E2 in uterine fibroblasts. In contrast to previous studies, in which constitutive and induced antimicrobial secretion increased with E2 (Hibbert and others 2003), we saw no effect of sex hormones alone on IL-27 secretion by epithelial cells and fibroblasts. Kyurkchiev and others (2007) reported that E2 upregulates IL-27 secretion by monocyte-derived DCs. Together with our results, this demonstrates that the effects of E2 on IL-27 are unique and distinct and vary with different cell types. Our findings also suggest that the production of IL-27 in response to pathogens, and the sensitivity of fibroblasts to IL-27, may vary in women across the menstrual cycle, particularly during periods when the concentration of E2 is high, such as mid-cycle and mid-secretory phases. Whether E2 affects IL-27 production and sensitivity of other cell types is unknown, but it is an important question since IL-27 restricts viral infection.
In conclusion, IL-27 is induced as part of an antiviral response in the endometrium and subsequently enhances responses to further stimulation by viral ligands. The effect of E2 suggests that IL-27 may play a role in immune protection during the menstrual cycle, potentially regulating immune responses to incoming pathogens. Whether other estrogenic compounds such as the ethinyl estradiol, in use in contraceptives, or selective ER modulators affect IL-27 production and sensitivity is unknown, but it is an important question given the numbers of women using these chemical contraceptives and hormone therapy worldwide.
Acknowledgments
This study was supported by NIH AI102838, AI071761, and AI117739 (Charles R. Wira).
Author Disclosure Statement
No competing financial interests exist.
References
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 8(12):1380–1389 [DOI] [PubMed] [Google Scholar]
- Ayimba E, Hegewald J, Segbena AY, Gantin RG, Lechner CJ, Agosssou A, Banla M, Soboslay PT. 2011. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol 166(2):218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 7(9):929–936 [DOI] [PubMed] [Google Scholar]
- Björnström L, Sjöberg M. 2005. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19(4):833–842 [DOI] [PubMed] [Google Scholar]
- Chen Q, Swaminathan S, Yang D, Dai L, Sui H, Yang J, Hornung RL, Wang Y, Huang da W, Hu X, Lempicki RA, Imamichi T. 2013. Interleukin-27 is a potent inhibitor of cis HIV-1 replication in monocyte-derived dendritic cells via a type I interferon-independent pathway. PLoS One 8(3):e59194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KD, Wright JA, Ghosh M, Wira CR, Fahey JV. 2009. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertil Steril 92(3):1107–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Lidie KB, Chen Q, Adelsberger JW, Zheng X, Huang D, Yang J, Lempicki RA, Rehman T, Dewar RL, Wang Y, Hornung RL, Canizales KA, Lockett SJ, Lane HC, Imamichi T. 2013. IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation. J Exp Med 210(3):517–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R, van Beelen AJ, Bakdash G, Taanman-Kueter EW, de Jong EC, Kapsenberg ML. 2012. Viral dsRNA-activated human dendritic cells produce IL-27, which selectively promotes cytotoxicity in naive CD8+ T cells. J Leukoc Biol 92(3):605–610 [DOI] [PubMed] [Google Scholar]
- Diegelmann J, Olszak T, Goke B, Blumberg RS, Brand S. 2012. A novel role for interleukin-27 (IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory proteins. J Biol Chem 287(1):286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. 2008. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol 1(4):317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AC, Zhang X, Katsounas A, Bharucha JP, Kottilil S, Imamichi T. 2010. Interleukin-27, an anti-HIV-1 cytokine, inhibits replication of Hepatitis C virus. J Interferon Cytokine Res 30(6):427–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas do Rosário AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, Muller W, O'Garra A, Langhorne J. 2012. IL-27 promotes IL-10 production by effector Th1 CD4(+) T cells; a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 188(3):1178–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell-Wild T, Vazquez N, Jin W, Rangel Z, Munson PJ, Wahl SM. 2009. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood 114(9):1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo C, Ayer A, Basta S, Banfield BW, Gee K. 2012. IL-27 Enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J Immunol 188(2):864–873 [DOI] [PubMed] [Google Scholar]
- Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. 2003. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res 23(9):513–522 [DOI] [PubMed] [Google Scholar]
- Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. 2008. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol 20(2):223–234 [DOI] [PubMed] [Google Scholar]
- Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, Adelsberger JW, Lempicki RA, Baseler MW, Lane HC. 2008. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS 22(1):39–45 [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 2008. IL-12, IL-23, and IL-27 enhance human beta-defensin-2 production in human keratinocytes. Eur J Immunol 38(5):1287–1296 [DOI] [PubMed] [Google Scholar]
- Koumas L, King AE, Critchley HOD, Kelly RW, Phipps RP. 2001. Fibroblast heterogeneity. Am J Pathol 159(3):925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyurkchiev D, Ivanova-Todorova E, Hayrabedyan S, Altankova I, Kyurkchiev S. 2007. Female sex steroid hormones modify some regulatory properties of monocyte-derived dendritic cells. Am J Reprod Immunol 58(5):425–433 [DOI] [PubMed] [Google Scholar]
- Liao J, Jijon HB, Kim IR, Goel G, Doan A, Sokol H, Bauer H, Herrmann BG, Lassen KG, Xavier RJ. 2014. An image-based genetic assay identifies genes in T1D susceptibility loci controlling cellular antiviral immunity in mouse. PLoS One 9(9):e108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FDM, Kenngott EE, Schröter MF, Kühl A, Jennrich S, Watzlawick R, Hoffmann U, Wolff T, Norley S, Scheffold A, Stumhofer JS, Saris CJM, Schwab JM, Hunter CA, Debes GF, Hamann A. 2014. Timed action of IL-27 protects from immunopathology while preserving defense in influenza. PLoS Pathog 10(5):e1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cao Z, Chen J, Li R, Cao Y, Zhu C, Wu K, Wu J, Liu F, Zhu Y. 2012. Influenza A virus induces interleukin-27 through cyclooxygenase-2 and protein kinase A signaling. J Biol Chem 287(15):11899–11910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas AE, Petitbarat M, Dubanchet S, Fay S, Ledee N, Chaouat G. 2008. Immune regulation at the interface during early steps of murine implantation: involvement of two new cytokines of the IL-12 family (IL-23 and IL-27) and of TWEAK. Am J Reprod Immunol 59(4):323–338 [DOI] [PubMed] [Google Scholar]
- Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. 2007. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol 178(12):7607–7615 [DOI] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Fukai F, Mizuguchi J, Yoshimoto T. 2006. IL-27 induces Th1 differentiation via p38 MAPK/T-bet- and intercellular adhesion molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol 177(11):7579–7587 [DOI] [PubMed] [Google Scholar]
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. 2004. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol 172(4):2225–2231 [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16(6):779–790 [DOI] [PubMed] [Google Scholar]
- Pirhonen J, Siren J, Julkunen I, Matikainen S. 2007. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol 82(5):1185–1192 [DOI] [PubMed] [Google Scholar]
- Schaefer TM, Fahey JV, Wright JA, Wira CR. 2005. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly (I:C). J Immunol 174(2):992–1002 [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJM, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7(9):937–945 [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8(12):1363–1371 [DOI] [PubMed] [Google Scholar]
- Su Y, Yao H, Wang H, Xu F, Li D, Zhang X, Yin Y, Cao J. 2016. IL-27 enhances innate immunity of human pulmonary fibroblasts and epithelial cells through upregulation of TLR4 expression. Am J Physiol Lung Cell Mol Physiol 310(2):L133–L141 [DOI] [PubMed] [Google Scholar]
- Tait Wojno ED, Hosken N, Stumhofer JS, O'Hara AC, Mauldin E, Fang Q, Turka LA, Levin SD, Hunter CA. 2011. A role for IL-27 in limiting T regulatory cell populations. J Immunol 187(1):266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. 2003. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol 170(10):4886–4890 [DOI] [PubMed] [Google Scholar]
- Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19(5):645–655 [DOI] [PubMed] [Google Scholar]
- Wira CR, Rodriguez-Garcia M, Patel MV. 2015. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 15(4):217–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Hunter CA. 2015. The immunobiology of interleukin-27. Annu Rev Immunol 33:417–443 [DOI] [PubMed] [Google Scholar]