Abstract
Aim
To compare the cardiovascular autonomic reflex tests (CARTs) with cardiac sympathetic innervation imaging with 123I-metaiodobenzylguanidine (MIBG) in patients with type 1 diabetes mellitus (T1DM).
Patients and Methods
Forty-nine patients (29 males, mean age 36 ± 10 years, mean T1DM duration 19 ± 6 years) without cardiovascular risk factors were prospectively enrolled. Participants were evaluated for autonomic dysfunction by assessing the mean circular resultant (MCR), Valsalva maneuver (Vals), postural index (PI), and orthostatic hypotension (OH). Within one month from the performance of these tests, patients underwent cardiac MIBG imaging and the ratio of the heart to upper mediastinum count density (H/M) at 4 hours postinjection was calculated (abnormal values, H/M < 1.80).
Results
Twenty-nine patients (59%) had abnormal CARTs, and 37 (76%) patients had an H/M_4 < 1.80 (p = 0.456). MCR, PI, Vals, and OH were abnormal in 29 (59%), 8 (16%), 5 (10%), and 11 (22%) patients, respectively. When using H/M_4 < 1.80 as the reference standard, a cutoff point of ≥2 abnormal CARTs had a sensitivity of 100% but a specificity of only 33% for determining CAN.
Conclusions
CARTs are not closely associated with 123I-MIBG measurements, which can detect autonomic dysfunction more efficiently than the former. In comparison to semiquantitative cardiac MIBG assessment, the recommended threshold of ≥2 abnormal CARTs to define cardiovascular autonomic dysfunction is highly sensitive but of limited specificity and is independently determined by the duration of T1DM.
1. Introduction
Diabetic neuropathy is the most common cause of neuropathy worldwide and the most common chronic complication of diabetes mellitus (DM) [1, 2]. Autonomic neuropathy is an underestimated complication of DM and may remain asymptomatic in early stages, especially in young patients with type 1 DM (T1DM) [3]. In the setting of DM, cardiovascular autonomic neuropathy (CAN) is defined as the impairment of the autonomic control of the cardiovascular system after the exclusion of other causes [2, 4]. CAN is one of the most serious consequences of DM due to its life-threatening complications, including silent myocardial infarction, ventricular arrhythmias, intraoperative cardiovascular instability, and sudden cardiac death, as well as because of its relation with other microvascular comorbidities [5, 6].
Over the years, much attention has been directed to the early diagnosis of CAN using standardized cardiovascular reflex tests (CARTs) [7]. These tests assess the parasympathetic system by evaluating beat-to-beat variations during deep breathing, moving from the supine to the standing position and during the Valsalva maneuver (Vals) [8]. On the other hand, orthostatic hypotension (OH) and the blood pressure response to Vals mostly evaluate the sympathetic system [8].
Nuclear imaging is also useful for the assessment of cardiac sympathetic innervation, by visualizing and measuring the uptake and storage of radiolabeled neurotransmitters into the presynaptic nerve terminals [9]. Several studies using 123I-metaiodobenzylguanidine (123I-MIBG) imaging have demonstrated the presence of abnormalities in sympathetic adrenergic innervation in diabetic patients [10–13].
It is noteworthy that most data on CAN are based on older studies, at a time when the treatment of diabetic patients might not have been optimal or the tests for identifying CAN might have been limited in number, performed in a nonstandardized manner, or influenced by comorbidities or medications [13, 14]. Moreover, the relation between CARTs and the more objective 123I-MIBG scintigraphy, in terms of direct visualization and quantification of cardiac sympathetic innervation, is not well determined [10, 15, 16].
The aim of the present study was to assess the CAN by both CARTs and 123I-MIBG scintigraphy in well-characterized T1DM patients, with disease duration > 5 years, without complications or cardiovascular risk factors, and who were only receiving insulin.
2. Patients and Methods
2.1. Patient Recruitment
Over an 18-month period, consecutive adult patients with T1DM who fulfilled the following criteria were enrolled in the study: (a) regular attendance of the outpatient diabetes clinic of our hospital to ensure systematic monitoring of glycemic control and diabetic complications, (b) diabetes duration > 5 years [17], (c) no symptoms associated with CAN (e.g., exercise intolerance, orthostatic intolerance, and syncope), (d) no clinically overt diabetic complications, other than CAN, or known cardiovascular risk factors and hence treated only with insulin, and (e) a normal adenosine myocardial perfusion scintigraphy. Ten patients had mild background retinopathy, and 6 patients had microalbuminuria. Thyroid stimulating hormone and thyroid hormones were measured in all patients because Hashimoto's thyroiditis is common in patients with T1DM. The levels of both thyroid stimulating hormone and thyroid hormones were within the normal range in all patients.
All patients had good glycemic control using insulin alone according to widely accepted standards of care [18]. Patients were well characterized and recruited for a broader study on diabetic autonomic dysfunction, which had been approved by the Ethics Committee of the Aristotle University of Thessaloniki. All patients provided informed consent.
2.2. Cardiovascular Autonomic Reflex Testing
Patients were submitted to a battery of CARTs early in the morning, as endorsed by international guidelines [7, 8, 19]. Subjects were instructed to abstain from caffeine or alcoholic beverages and smoking for a minimum of 8 h prior to testing and also from strenuous exercise for at least 24 h prior to testing. Patients with arrhythmias, fever, hypoglycemia, and emotional distress on the day before testing were excluded from testing. The following CARTs were performed:
The heart rate variation during deep breathing at 6 cycles per minute for 5 minutes, over 120 consecutive beats, was assessed with the mean circular resultant (MCR), which was measured by a vector analysis technique, as described elsewhere [18]. Patients, while lying down, were ordered to perform a deep and slow inspiration up to the maximum total lung capacity for 5 seconds, which was followed by a forced expiration down to the residual volume for 5 seconds. The time to alternate the respiratory cycle was signalled directly to the patient by the attending physician.
The postural index (PI) was calculated by measuring the heart rate response to the change of position from recumbent to standing over 180 seconds after getting up. This was calculated as the “30–15 ratio,” defined as the longest RR interval of beats 20–40 divided by the shortest RR interval of beats 5–25, starting from the first beat during the process of standing up.
For the assessment of heart rate variability during Vals, patients with the nose closed were asked to exhale into a mouthpiece connected to a manometer and to perform a continuous expiratory effort, which was equivalent to an intraoral pressure of 40 mmHg, for 15 seconds. The expiratory overstrain was then released, and patients were asked to breathe regularly until the end of the test. The ratio of the longest RR interval following the pressure release to the shortest RR interval during the maneuver was determined. The test was performed 3 times, and the mean value was used in the analyses.
For the OH test, blood pressure was measured with the patients in recumbent position, every minute for three minutes, and then while standing, every minute for 5 minutes. A drop of ≥20 mmHg in systolic blood pressure or ≥10 mmHg in diastolic blood pressure was considered abnormal.
Age-specific reference values were applied [20]. The first two CARTs address parasympathetic function, and Vals evaluates both parasympathetic and sympathetic function, whereas OH assesses sympathetic integrity.
2.3. 123I-MIBG Acquisition and Analysis
Within 1 month from performing the CARTs, patients were submitted to cardiac MIBG imaging. For this purpose, patients were treated with 130 mg potassium iodide orally for 3 days, starting on the day before tracer injection. There was no need to withhold medications interfering with MIBG imaging, since all patients were receiving only insulin [9]. 185 MBq of 123I-MIBG was administered slowly via a secured intravenous line in a peripheral vein.
Imaging was performed with a single-headed, large field of view ADAC Genesys SPECT gamma camera (ADAC Labs., Milpitas, CA, USA), equipped with a 3/8″ sodium iodide scintillation crystal and low-energy high-resolution collimator and interfaced with a Pegasys processing workstation. Anterior cardiac images were acquired at 15 minutes and 4 hours postinjection on a 256 × 256 matrix and an acquisition time of 600 sec, using a 15% energy window centered at the 159 keV photopeak of 123I.
The heart-to-mediastinum (H/M) count density ratio was calculated in a standardized manner, using an elliptical region of interest (ROI) placed over the heart and a rectangular ROI drawn over the upper mediastinum. Both the acquisition and measurement methodology are in line with official recommendations and have been previously validated in our institution [21–23]. For the purposes of this study, the H/M at 4 hours postinjection (H/M_4) was used in analysis, and the cutoff point of abnormal values was set at H/M_4 < 1.80, based on the published data [22, 24].
2.4. 51Cr-EDTA Glomerular Filtration Measurements
Within one month from the performance of CARTs, glomerular filtration rate (GFR) was measured in the morning, using the slope intercept and two sample technique, as described elsewhere [25]. In brief, blood samples were collected at 120 and 240 min after an intravenous injection of 3-4 MBq 51Cr-EDTA. A scintillation well counter (Cobra II, Packard, Meriden, CT, USA) was set to count plasma 51Cr, and the quadratic Brochner-Mortensen correction was applied in calculations [26]. GFR was measured with this technique because of the inaccuracy of various equations for the evaluation of GFR [27] and because CAN appears to predict the development of diabetic nephropathy [28].
2.5. Statistical Analysis
All data were analyzed with the statistical package SPSS (version 17.0; SPSS, Chicago, IL, USA). Data are presented as percentages for categorical variables and as mean and standard deviation for continuous variables. Differences between groups were assessed with paired or independent sample t-test, as appropriate, for continuous variables, and with chi-square test or Fisher's exact test, as appropriate, for categorical variables. One-way ANOVA was used when categorical or ordinal variables were compared between three or more groups, and Levene's test was used for evaluating the equality of variances. Stepwise backward binary logistic regression analysis was used to determine independent predictors, and a p value < 0.20 in univariate analysis was required for a variable to enter the multivariate model. A value of p < 0.05 was used to define statistical significance.
3. Results
Forty-nine patients with T1DM were enrolled in the study (29 males, mean age 36 ± 10 years (range, 19–62 years), mean DM duration 19 ± 6 years (range, 7–31 years)). Patients' characteristics are shown in Table 1. HbA1c was also measured within 1 month from CARTs. Blood samples from 37 patients were analyzed in our institution, whereas the rest were tested elsewhere because of temporary logistic constraints. As deviations in the latter HbA1c measurements were strongly suspected subsequently, only results from our laboratory were taken into account in this study, which are presented in a supplementary analysis. Similarly, 51Cr-EDTA GFR measurements within 1 month from CARTs were available for 46 patients, and thus renal function is analyzed separately, together with the 37 patients in whom HbA1c was measured in our institution.
Table 1.
Patients' characteristics.
| Males (%) | 59.2 |
|---|---|
| Mean age (years) | 36 ± 10 |
| Mean duration of diabetes mellitus (years) | 19 ± 6 |
| Mean systolic blood pressure (mmHg) | 115 ± 10 |
| Mean diastolic blood pressure (mmHg) | 78 ± 8 |
| Mean body mass index (kg/m2) | 25.2 ± 3.1 |
| Retinopathy (%) | 20.4 |
| Microalbuminuria (%) | 12.2 |
| Mean HbA1c (%) | 6.9 ± 1.5 |
| Mean hematocrit (%) | 42.1 ± 1.1 |
| Mean aspartate transaminase (IU/L) | 27.3 ± 1.9 |
| Mean alanine transaminase (IU/L) | 28.8 ± 1.3 |
| Mean triglycerides (mg/dL) | 117.8 ± 20.2 |
| Mean total cholesterol (mg/dL) | 159.4 ± 14.5 |
| Mean high-density lipoprotein cholesterol (mg/dL) | 48.1 ± 13.3 |
Twenty-nine patients (59%) had abnormal CARTs, and 37 patients (76%) had an H/M_4 < 1.80 (p = 0.456). MCR, PI, Vals, and OH were abnormal in 29 (59%), 8 (16%), 5 (10%), and 11 (22%) patients, respectively. The MIBG assessment of CAN as a function of the number of abnormal CARTs is presented in Table 2. Among the 36 patients with 0 or 1 abnormal CARTs, 24 patients (67%) also had sympathetic impairment in cardiac MIBG scintigraphy.
Table 2.
Cardiac sympathetic innervation imaging with 123I-metaiodobenzylguanidine results as a function of the number of abnormal cardiovascular autonomic reflex tests (CARTs).
| Number of abnormal CARTs | p | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Patients with H/M_4 < 1.80 |
14/20 (70%) |
10/16 (63%) |
5/5 (100%) |
6/6 (100%) |
2/2 (100%) |
0.198 |
| H/M_4 | 1.68 ± 0.17 | 1.68 ± 0.26 | 1.51 ± 0.21 | 1.50 ± 0.08 | 1.64 ± 0.20 | 0.170 |
H/M_4: ratio of the heart to upper mediastinum count density at 4 hours postinjection.
All 16 patients with only 1 abnormal CART had abnormal MCR. Abnormality in any one of the remaining CARTs was invariably accompanied by at least a second abnormal test. There were also 11 patients with abnormal OH test (H/M_4 = 1.50 ± 0.14), which were accompanied by 1 additional abnormal CART in 4 patients (H/M_4 = 1.45 ± 0.20) and 2 or more additional abnormal CARTs in 7 patients (H/M_4 = 1.53 ± 0.10). There was no significant difference between this latter group of 7 patients and the rest of the patients (1.66 ± 0.21, p = 0.115). When a more stringent cutoff point was used to assess advanced CAN, 5/7 (71%) patients with OH had an H/M_4 < 1.60 versus 19/42 patients (45%, p = 0.247).
In Table 3, the H/M_4 ratio is compared between patients divided according to the number of abnormal CARTs. Based on cardiac 123I-MIBG, a threshold of ≥2 abnormal CARTs could determine significant differences in the assessment of CAN with cardiac MIBG imaging. When using H/M_4 < 1.80 as a reference standard, a cutoff point of ≥2 abnormal CARTs had a sensitivity of 100% but a specificity of 33% in determining CAN.
Table 3.
Comparison of cardiac sympathetic innervation imaging with 123I-metaiodobenzylguanidine (123I-MIBG) findings between groups of patients formed according to the number of abnormal cardiovascular autonomic tests (CARTs) versus those with fewer tests than the defined threshold of abnormality.
| Number of abnormal CARTs | 123I-MIBG H/M_4 | p | |
|---|---|---|---|
| Abnormal group | Normal group | ||
| ≥1 | 1.61 ± 0.22 | 1.68 ± 0.17 | 0.234 |
| (n = 29) | (n = 20) | ||
| ≥2 | 1.52 ± 0.14 | 1.68 ± 0.21 | 0.014 |
| (n = 13) | (n = 36) | ||
| ≥3 | 1.53 ± 0.09 | 1.66 ± 0.21 | 0.104 |
| (n = 8) | (n = 41) | ||
| ≥4 | 1.62 ± 0.11 | 1.64 ± 0.21 | 0.916 |
| (n = 2) | (n = 47) | ||
There was no difference between males and females in mean age (35 ± 8 versus 37 ± 11 years, resp.; p = 0.356), mean DM duration (18 ± 6 versus 19 ± 7 years, resp.; p = 0.520), number of abnormal CARTs (p = 0.335), H/M_4 (1.67 ± 0.23 versus 1.60 ± 0.17, resp.; p = 0.296), and 51Cr-EDTA GFR (102 ± 18 versus 93 ± 15, resp.; p = 0.086), but males had lower HbA1c than females (6.5% ± 1.4 versus 7.7% ± 2.0, resp.; p = 0.038).
Study participants with ≥2 abnormal CARTs were older (42 ± 11 versus 33 ± 8 years, p = 0.003) and had longer DM duration (25 ± 5 versus 16 ± 5 years, p < 0.001) than patients with <2 abnormal CARTs (Figure 1). In binary logistic regression analysis, only DM duration was a significant predictor of ≥2 abnormal CARTs in the entire study population (model's chi-square 21.010, p < 0.001).
Figure 1.
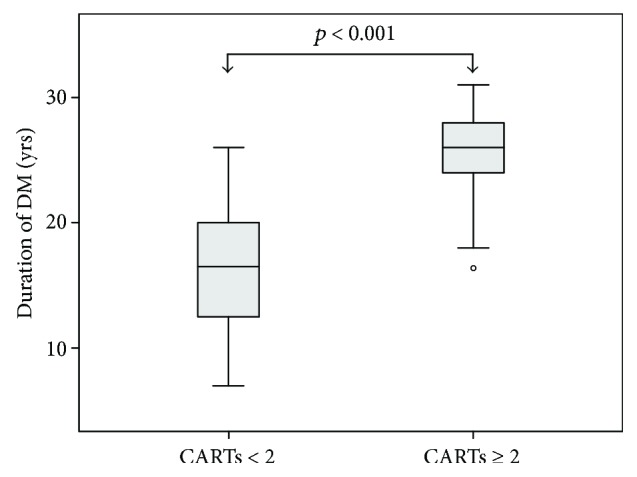
Box plots of the duration of type 1 diabetes mellitus in patients divided according to a cutoff point of ≥2 abnormal cardiovascular autonomic reflex tests (CARTs).
In the 37 patients with available HbA1c measurements, there were significant differences between those with ≥2 abnormal CARTs and those with 0-1 abnormal CARTs in HbA1c (7.7% ± 2.3 versus 6.5% ± 1.2, resp.; p = 0.044), 51Cr-EDTA GFR (86 ± 13 versus 101 ± 16 ml/min/1.73m2, resp.; p = 0.006), age (42 ± 11 versus 35 ± 8 years, resp.; p = 0.021), DM duration (25 ± 17 versus 17 ± 5 years, resp.; p < 0.001), and H/M_4 (1.52 ± 0.15 versus 1.70 ± 0.22, resp.; p = 0.012). In binary logistic regression analysis, both age and HbA1c were independent predictors of ≥2 abnormal CARTs (model's chi-square 30.541, p < 0.001).
Patients with H/M_4 < 1.80 (n = 37) were older than those with a normal MIBG study (n = 12) (38 ± 9 versus 30 ± 9 years, resp.; p = 0.012, Figure 2), but there were no differences between the two groups in gender (56.7 versus 66.7% males, resp.; p = 0.544), DM duration (20 ± 6 versus 16 ± 6 years, p = 0.057) and the number of abnormal CARTs (14/20, 10/16, 5/5, 6/6, and 2/2 abnormal H/M_4 ratios in patients with 0, 1, 2, 3, and 4 abnormal CARTs, resp., p = 0.198). In binary logistic regression analysis, only age was found to represent an independent predictor of H/M_4 < 1.80 (model's chi-square 6.959, p = 0.008).
Figure 2.
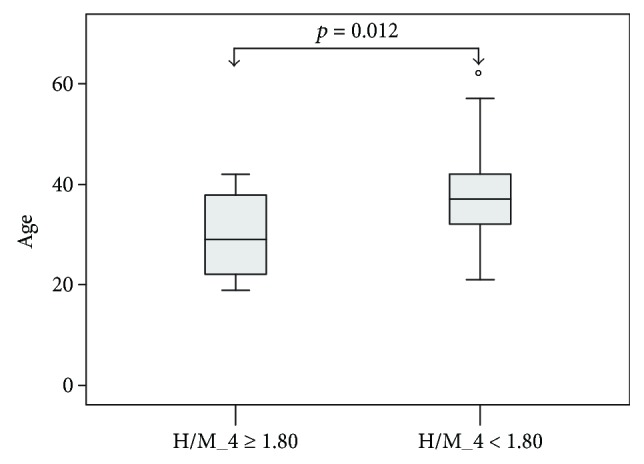
Box plots of age in patients divided according to a cutoff point of ratio of the heart to upper mediastinum count density at 4 hours postinjection (H/M_4) < 1.80 in cardiac 123I-metaiodobenzylguanidine scintigraphy.
In the 37 patients with available HbA1c measurements, patients with H/M_4 < 1.80 did not differ significantly from those with a normal ratio in age (p = 0.085), DM duration (p = 0.196), HbA1c (p = 0.435), 51Cr-EDTA GFR (p = 0.253), and the number of abnormal CARTs (p = 0.186). No significant predictors of H/M_4 < 1.80 were found when variables with a p < 0.20 were entered in binary logistic regression analysis.
4. Discussion
The present study compared clinical autonomic function testing and MIGB in T1DM patients with no overt complications or other comorbidities. Our findings suggest that clinical tests and MIBG measurements are not closely associated. A threshold of ≥2 abnormal CARTs determines significant changes in sympathetic integrity, as assessed with MIBG, and has high sensitivity but limited specificity to identify CAN. Among clinical variables, DM duration was identified as a significant determinant of ≥2 abnormal CARTs, whereas age was found to represent an independent predictor of abnormal MIBG findings.
The cardiovascular autonomic system plays a pivotal role in the regulation of heart rate, myocardial contractility, and blood pressure. Our group showed that the severity of CAN is associated with the severity of the left ventricular diastolic dysfunction in both patients with T1DM and type 2 DM [29, 30]. Indeed, CAN is a severe complication of DM with potentially deleterious effects on the outcome of these patients [3, 5, 6, 31]. Thus, early recognition of CAN may be crucial in the management of the disease because achieving normoglycemia may delay or prevent the onset of CAN in T1DM [32, 33]. Even though autonomic symptoms present more commonly in T1DM than in T2DM, these symptoms correlate weakly with deficits in early CAN [34]. The so-called Ewing battery of tests has been introduced many years ago to assess cardiovascular autonomic function and remains popular because these tests are noninvasive, simple, safe, reliable, reproducible, and standardized [7]. However, even though these tests are clinically relevant, they principally assess the parasympathetic system.
Myocardial 123I-MIBG allows direct visualization and quantification of sympathetic presynaptic neuronal integrity [9]. However, it should be mentioned that uptake measurements are semiquantitative and not an index of absolute neuronal retention. Besides, the delivery of tracers is influenced by myocardial perfusion, and measurements may be influenced by body weight, a number of diseases, and certain medications [2].
The prevalence of CAN detected in our cohort with either CARTs or MIBG is within the range reported in the literature [35]. Our results also demonstrate that MCR abnormalities precede other tests' impairment, whilst MIBG abnormalities occur in the majority of diabetic patients with no abnormal CARTs. Moreover, DM duration and HbA1c were significant predictors of the presence of ≥2 abnormal CARTs. Earlier work has shown inadequate glycemic control to be a leading cause for the development and progression of CAN and duration of the disease to be a significant risk factor [15, 36]. Previous data have also suggested that 123I-MIBG scintigraphy may be more sensitive than heart rate variability for detection of CAN in diabetic patients [36, 37]. Furthermore, it has been reported that heart rate variability in deep breathing heralds subclinical CAN and is the most sensitive test to diagnose autonomic dysfunction [2, 35]. Particularly in patients with T1DM, significant correlations were observed between CAN and age, duration of DM, HbA1c, retinopathy, microalbuminuria, hypoglycaemia, dyslipidemia, and diastolic blood pressure [14, 38].
Interestingly, since cardiac MIBG scintigraphy addresses the sympathetic autonomic system and CARTs principally assess parasympathetic function, our findings contradict the general notion that parasympathetic (vagal) impairment precedes sympathetic dysfunction during the natural course of CAN in diabetic patients [39]. As denervation occurs in an ascending length-dependent manner, the vagus nerve is usually affected first in CAN, resulting in a relative predominance of the sympathetic tone [35]. At an early stage, this leads to baroreceptor impairment and changes in heart rate variability, but at later stages of the disease, cardiac involvement may become evident. In this respect, however, it has been suggested that MIBG assesses specifically the sympathetic innervation of the heart whereas heart rate variability indices are better markers of systemic autonomic function [35, 39].
The Toronto Consensus Panel required ≥2 abnormal CARTs to define CAN, one abnormal CART for possible CAN, and considered OH with ≥2 abnormal CARTs as indicative of advanced CAN [8]. In the present study, the criterion of ≥2 abnormal CARTs could only discern significant abnormality in cardiac MIBG findings (Table 2). Literally, all patients with ≥2 abnormal CARTs had scintigraphic evidence of sympathetic dysfunction. However, the presence of ≥2 abnormal CARTs had limited specificity, because the majority of T1DM patients with none or only one abnormal CARTs also had sympathetic impairment. Similarly, one abnormal CART was associated with a moderate (63%) likelihood for MIBG abnormality. Regarding the case of postural hypotension (which assesses sympathetic function) plus ≥ 2 abnormal CARTs, in our patients, an abnormal OH invariably was accompanied by one or more additional abnormal CARTs. In these patients, the H/M_4 tended to be low, but no presence of advanced CAN could be confirmed, even after applying a H/M_4 threshold of <1.60.
Age was found to represent an independent predictor of H/M_4 < 1.80. Earlier studies have reported a decrease of cardiac MIBG uptake as a physiological consequence of advancing age [40]. However, more recent reports have challenged this notion and support stability of H/M measurements in patients without coronary heart disease, because changes of numerator parallel those of denominator during ageing [41]. Whatever may be the underlying cause (a consequence of ageing alone or an effect of DM), our data show that advanced age is a marker of CAN in T1DM patients, as demonstrated by a lower cardiac MIBG uptake.
Limitations of the present study include the relatively small sample size, which is particularly important for multivariate analyses. Although a rule does not exist, statistical confidence is substantiated with larger numbers of cases. 123I-MIBG measurements were based on an H/M ratio at 4 hours alone, excluding other scintigraphic indices. However, this was preferred for reasons of simplicity in presenting the results, whereas recent data report very similar values between early and late H/M ratios. Notably, although cardiac 123I-MIBG testing offers improved sensitivity in detecting autonomic dysfunction and initiating timely therapeutic interventions, it is not known whether this type of assessment is accompanied with improved outcome compared to clinical autonomic testing alone.
Strengths of the present study include the enrollment of well-characterized and young patients with very good metabolic control, early CAN, long duration of DM, without cardiovascular disease, hypertension, or severe microvascular complications.
5. Conclusions
In T1DM patients with no complications or cardiovascular risk factors and treated with insulin alone, CAN is common and age is a strong predictor of its presence in cardiac 123I-MIBG imaging. Cardiovascular autonomic reflex tests are not closely associated with 123I-MIBG measurements, which can detect autonomic dysfunction more efficiently than the former. In comparison to the semiquantitative cardiac MIBG assessment, the recommended threshold of ≥2 abnormal tests is highly sensitive for identifying cardiovascular autonomic dysfunction but has limited specificity and is independently determined by the duration of T1DM. Therefore, MIBG might have to be used in combination with the CARTs for the diagnosis of cardiac autonomic neuropathy in patients with T1DM.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Said G. Diabetic neuropathy. Handbook of Clinical Neurology. 2013;115:579–589. doi: 10.1016/B978-0-444-52902-2.00033-3. [DOI] [PubMed] [Google Scholar]
- 2.Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. Journal of Cardiovascular Translational Research. 2012;5(4):463–478. doi: 10.1007/s12265-012-9367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verrotti A., Prezioso G., Scattoni R., Chiarelli F. Autonomic neuropathy in diabetes mellitus. Frontiers in Endocrinology. 2014;5:p. 205. doi: 10.3389/fendo.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesfaye S., Boulton A. J. M., Dyck P. J., et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinik A. I., Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 6.Gerritsen J., Dekker J. M., Ten Voorde B. J., et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn study. Diabetes Care. 2001;24(10):1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 7.Ewing D. J., Martyn C. N., Young R. J., Clarke B. F. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 8.Spallone V., Ziegler D., Freeman R., et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes/Metabolism Research and Reviews. 2011;27(7):639–653. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 9.Travin M. I. Cardiac autonomic imaging with SPECT tracers. Journal of Nuclear Cardiology. 2013;20(1):128–143. doi: 10.1007/s12350-012-9655-1. [DOI] [PubMed] [Google Scholar]
- 10.Kreiner G., Wolzt M., Fasching P., et al. Myocardial m-[123I]iodobenzylguanidine scintigraphy for the assessment of adrenergic cardiac innervation in patients with IDDM. Comparison with cardiovascular reflex tests and relationship to left ventricular function. Diabetes. 1995;44(5):543–549. doi: 10.2337/diab.44.5.543. [DOI] [PubMed] [Google Scholar]
- 11.Turpeinen A. K., Vanninen E., Kuikka J. T., Uusitupa M. I. Demonstration of regional sympathetic denervation of the heart in diabetes. Comparison between patients with NIDDM and IDDM. Diabetes Care. 1996;19(10):1083–1090. doi: 10.2337/diacare.19.10.1083. [DOI] [PubMed] [Google Scholar]
- 12.Muhr-Becker D. D., Weiss M., Tatsch K., Wolfram G., Standl E., Schnell O. Scintigraphically assessed cardiac sympathetic dysinnervation in poorly controlled type 1 diabetes mellitus: one-year follow-up with improved metabolic control. Experimental and Clinical Endocrinology & Diabetes. 1999;107(5):306–312. doi: 10.1055/s-0029-1212117. [DOI] [PubMed] [Google Scholar]
- 13.Nagamachi S., Fujita S., Nishii R., et al. Prognostic value of cardiac I-123 metaiodobenzylguanidine imaging in patients with non-insulin-dependent diabetes mellitus. Journal of Nuclear Cardiology. 2006;13(1):34–42. doi: 10.1016/j.nuclcard.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Kempler P., Tesfaye S., Chaturvedi N., et al. Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM complications study. Diabetic Medicine. 2002;19(11):900–909. doi: 10.1046/j.1464-5491.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 15.Schnell O., Muhr D., Dresel S., Weiss M., Haslbeck M., Standl E. Partial restoration of scintigraphically assessed cardiac sympathetic denervation in newly diagnosed patients with insulin dependent (type 1) diabetes mellitus at one-year follow-up. Diabetic Medicine. 1997;14(1):57–62. doi: 10.1002/(SICI)1096-9136(199701)14:1<57::AID-DIA297>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Giordano A., Calcagni M. L., Verrillo A., et al. Assessment of sympathetic innervation of the heart in diabetes mellitus using 123I-MIBG. Diabetes, Nutrition & Metabolism. 2000;13:350–355. [PubMed] [Google Scholar]
- 17.Buse J. B., Ginsberg H. N., Bakris G. L., et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115(1):114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2016;39(Supplement 1):S1–S112. [Google Scholar]
- 19.Spallone V., Bellavere F., Scionti L., et al. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(1):69–78. doi: 10.1016/j.numecd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Gerritsen J., TenVoorde B. J., Dekker J. M., et al. Measures of cardiovascular autonomic nervous function: agreement, reproducibility, and reference values in middle age and elderly subjects. Diabetologia. 2003;46(3):330–338. doi: 10.1007/s00125-003-1032-9. [DOI] [PubMed] [Google Scholar]
- 21.Flotats A., Carrió I., Agostini D., et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(9):1802–1812. doi: 10.1007/s00259-010-1491-4. [DOI] [PubMed] [Google Scholar]
- 22.Verberne H. J., Habraken J. B., van Eck-Smit B. L., Agostini D., Jacobson A. F. Variations in 123I-metaiodobenzylguanidine (MIBG) late heart mediastinal ratios in chronic heart failure: a need for standardisation and validation. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35(3):547–553. doi: 10.1007/s00259-007-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papanastasiou E., Moralidis E., Siountas A. The effect of scatter correction on planar and tomographic semiquantitative123I cardiac imaging. A phantom study. Hellenic Journal of Nuclear Medicine. 2017;20(2):154–159. [PubMed] [Google Scholar]
- 24.Nakajima K. Normal values for nuclear cardiology: Japanese databases for myocardial perfusion, fatty acid and sympathetic imaging and left ventricular function. Annals of Nuclear Medicine. 2010;24(3):125–135. doi: 10.1007/s12149-009-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arsos G., Moralidis E., Tsechelidis I., Sakagiannis G., Sidiropoulou V., Psarouli E. Measurement of glomerular filtration rate with chromium-51 ethylene diamino tetraacetic acid in the presence of gallium-67 citrate. Nuclear Medicine Communications. 2011;32(3):221–226. doi: 10.1097/MNM.0b013e328342b969. [DOI] [PubMed] [Google Scholar]
- 26.Fleming J. S., Zivanovic M. A., Blake G. M., Burniston M., Cosgriff P. S. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nuclear Medicine Communications. 2004;25(8):759–769. doi: 10.1097/01.mnm.0000136715.71820.4a. [DOI] [PubMed] [Google Scholar]
- 27.Iliadis F., Didangelos T., Ntemka A., et al. Glomerular filtration rate estimation in patients with type 2 diabetes: creatinine- or cystatin C-based equations? Diabetologia. 2011;54(12):2987–2994. doi: 10.1007/s00125-011-2307-1. [DOI] [PubMed] [Google Scholar]
- 28.Orlov S., Cherney D. Z. I., Pop-Busui R., et al. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clinical Journal of the American Society of Nephrology. 2015;10(7):1136–1144. doi: 10.2215/CJN.11441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Didangelos T. P., Arsos G. A., Karamitsos D. T., Athyros V. G., Karatzas N. D. Left ventricular systolic and diastolic function in normotensive type-1 diabetic patients with or without autonomic neuropathy. A radionuclide ventriculography study. Diabetes Care. 2003;26(7):1955–1960. doi: 10.2337/diacare.26.7.1955. [DOI] [PubMed] [Google Scholar]
- 30.Didangelos T. P., Arsos G., Karamitsos T., et al. Left ventricular systolic and diastolic function in normotensive type 2 diabetic patients with or without autonomic neuropathy: a radionuclide ventriculography study. Angiology. 2014;65(10):877–882. doi: 10.1177/0003319713510966. [DOI] [PubMed] [Google Scholar]
- 31.Soedamah-Muthu S. S., Chaturvedi N., Witte D. R., et al. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB prospective complications study (PCS) Diabetes Care. 2008;31(7):1360–1366. doi: 10.2337/dc08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the diabetes control and complications trial (DCCT) Diabetologia. 1998;41(4):416–423. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pop-Busui R., Low P. A., Waberski B. H., et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC) Circulation. 2009;119(22):2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low P. A., Benrud-Larson L. M., Sletten D. M., et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27(12):2942–2947. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 35.Fisher V. L., Tahrani A. A. Cardiac autonomic neuropathy in patients with diabetes mellitus: current perspectives. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2017;Volume 10:419–434. doi: 10.2147/DMSO.S129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler D., Weise F., Langen K. J., et al. Effect of glycaemic control on myocardial sympathetic innervation assessed by [123I] metaiodobenzylguanidine scintigraphy: a 4-year prospective study in IDDM patients. Diabetologia. 1998;41(4):443–451. doi: 10.1007/s001250050928. [DOI] [PubMed] [Google Scholar]
- 37.Schnell O., Hammer K., Muhr-Becker D., et al. Cardiac sympathetic dysinnervation in type 2 diabetes mellitus with and without ECG-based cardiac autonomic neuropathy. Journal of Diabetes and its Complications. 2002;16(3):220–227. doi: 10.1016/S1056-8727(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 38.Jaiswal M., Urbina E. M., Wadwa R. P., et al. Reduced heart rate variability among youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2012;36(1):157–162. doi: 10.2337/dc12-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jermendy G. Clinical consequences of cardiovascular autonomic neuropathy in diabetic patients. Acta Diabetologica. 2003;40:s370–s374. doi: 10.1007/s00592-003-0122-y. [DOI] [PubMed] [Google Scholar]
- 40.Estorch M., Carrio I., Berna L., Lopez-Pousa J., Torres G. Myocardial iodine-labeled metaiodobenzylguanidine 123 uptake relates to age. Journal of Nuclear Cardiology. 1995;2(2):126–132. doi: 10.1016/s1071-3581(06)80022-x. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson A. F., Chen J., Verdes L., Folks R. D., Manatunga D. N., Garcia E. V. Impact of age on myocardial uptake of 123I-mIBG in older adult subjects without coronary heart disease. Journal of Nuclear Cardiology. 2013;20(3):406–414. doi: 10.1007/s12350-013-9701-7. [DOI] [PubMed] [Google Scholar]


