Abstract
We investigated the effect of shed antigen mesothelin on the tumor uptake of amatuximab, a therapeutic anti-mesothelin mAb clinically tested in mesothelioma patients. The B3 mAb targeting a nonshed antigen was also analyzed for comparison. The mouse model implanted with A431/H9 tumor, which expresses both shed mesothelin and nonshed Lewis-Y antigen, provided an ideal system to compare the biodistribution and PET imaging profiles of the two mAbs. Our study demonstrated that the tumor and organ uptakes of 89Zr-B3 were dose-independent when 3 doses, 2, 15, and 60 μg B3, were compared at 24 h after injection. In contrast, tumor and organ uptakes of 89Zr-amatuximab were dose-dependent, whereby a high dose (60 μg) was needed to achieve tumor targeting comparable to the low dose (2 μg) of 89Zr-B3, suggesting that shed mesothelin may affect amatuximab tumor targeting as well as serum half-life. The autoradiography analysis showed that the distribution of 89Zr-B3 was nonuniform with the radioactivity primarily localized at the tumor periphery independent of the B3 dose. However, the autoradiography analysis for 89Zr-amatuximab showed dose-dependent distribution profiles of the radiolabel; at 10 μg dose, the radiolabel penetrated toward the tumor core with its activity comparable to that at the tumor periphery, whereas at 60 μg dose, the distribution profile became similar to those of 89Zr-B3. These results suggest that shed antigen in blood may act as a decoy requiring higher doses of mAb to improve serum half-life as well as tumor targeting. Systemic mAb concentration should be at a severalfold molar excess to the shed Ag in blood to overcome the hepatic processing of mAb-Ag complexes. On the other hand, mAb concentration should remain lower than the shed Ag concentration in the tumor ECS to maximize tumor penetration by passing binding site barriers.
1. Introduction
Monoclonal antibody- (mAb-) based solid tumor therapy is challenging due to various parameters that can impede the tumor delivery and penetration of mAb. Some parameters are related to tumor environment factors, including vascular and stroma density, interstitial pressure, and tumor binding site barriers [1–3]. In addition, the antigen- (Ag-) mediated tumor targeting of mAb may be hampered by the presence of high levels of shed Ag in blood which could act as a decoy preventing mAbs from binding to antigens expressed on tumor cells [4–7]. We previously reported that shed mesothelin (MSLN) in blood circulation negatively affected the tumor targeting of amatuximab labeled with 111In or 64Cu by increasing its liver and spleen uptakes while decreasing its blood retention and tumor uptake when the injection dose of amatuximab was not sufficient to saturate the shed MSLN in blood circulation [5, 6]. In contrast to the negative effect of shed MSLN in blood circulation, a mathematical simulation suggested that the shed MSLN in the extracellular space (ECS) could positively affect the tumor uptake by improving the penetration of the antibody toward tumor core [8].
In this study, we investigated the effects of shed Ag on the tumor targeting and penetration of mAb. To achieve this goal, we used a nude mouse model implanted with A431/H9 tumor that overexpresses both shed MSLN (5 million Ag molecules/cell) and nonshed Lewis-Y (4 million Ag molecules/cell). MSLN is a membrane glycoprotein of 40 kDa that is actively internalized into the cell's cytosol as well as shed from the tumor cell surface, generating soluble MSLN in the tumor's interstitial space and blood circulation with its concentrations proportional to the size of tumor [5, 7, 9]. Lewis-Y is a carbohydrate antigen that is not actively internalized nor shed from the tumor surface [9–13]. As model antibodies, we used two mAbs: anti-MSLN mAb amatuximab (mouse/human chimeric antibody with 82.6% amino acid sequence identity to a human IgG1κ and 10−9 M KD binding affinity), a therapeutic mAb currently investigated in mesothelioma patients, and control anti-Lewis-Y mAb B3 (murine IgG1κ with 10−8 M KD binding affinity). Comparative studies using this system enabled us to define the effects of shed Ag on the tumor uptake and penetration of mAb apart from the effects of other factors such as vascular density, high interstitial fluid pressure (IFP), and extracellular protein contents.
The two mAbs were labeled with 89Zr (decay half-life, 78.4 h), which decays with a low positron emission energy of 395.5 keV, allowing for PET imaging with higher resolution [14–16]. 89Zr can enhance PET radioimmune detection of labeled mAbs and extend autoradiography preparation time due to the longer half-life and higher imaging resolution compared with 111In and 64Cu. In addition to the biodistribution (BD), PET imaging, and autoradiography studies for the two 89Zr-labeled mAbs, here we also report a new autoradiography analysis method to define the tumor uptake profile of the two 89Zr mAbs irrespective of tumor size and shape.
2. Materials and Methods
Amatuximab was obtained from Morphotek, Inc. (Exton, PA), and B3 was provided by Dr. Ira Pastan (LMB, NCI, NIH). p-Isothiocyanatobenzyl-desferrioxamine (p-SCN-Df) was purchased from Macrocyclics, Inc. (Dallas, TX). Zirconium-89 (89Zr) was produced at the National Institute of Health (Bethesda, MD) cyclotron facility using a 16.5 MeV proton cyclotron (PET trace, General Electric, Fairfield, CT) by proton irradiation (beam energy; 14 MeV, current; 20 µA) (p, n) reaction (2~5 h) on 89Y-metal mesh (200 mg, 4N purity, American Elements). 89Zr was separated as 89Zr-oxalate from irradiated 89Y-metal mesh using 0.1 M oxalic acid solution [16].
2.1. Conjugation of p-SCN-Df to Amatuximab or B3 Antibody
The mAbs were radiolabeled with 89Zr using desferrioxamine (Df) with an isothiocyanate linker as a chelating agent following a method of Vosjan et al. [17]. Briefly, amatuximab or B3 was reacted with p-SCN-Df at a molar ratio of 1 : 3 in 0.1 M sodium bicarbonate, at pH 9.5 at 37°C. The Df-amatuximab or Df-B3 conjugate was purified with a size exclusion PD-10 column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and concentrated with a Microcon® filter with a 30 kDa cutoff (Millipore, Bedford, MA). The column or the filter was pretreated with 25 mg BSA containing 1 µmol DTPA to block nonspecific protein binding sites and remove potential metal contaminants and then washed with metal-free sodium acetate (0.25 M, pH 5.5). The mAb concentrations were measured according to the method of Bradford [18]. The level of p-SCN-Df conjugated per mAb was determined by the percent 89Zr distribution between the peaks corresponding to Df-mAb and free DF on the size exclusion HPLC when the product mixture was radiolabeled as described below.
2.2. Radiolabeling
Purified Df-amatuximab or Df-B3 (1.0 mg/ml, 6.9 µM) was labeled with 89Zr (344 MBq/0.15 M oxalic acid for Df-amatuximab and 148 MBq/0.15 M oxalic acid for Df-B3), which was neutralized with a solution containing sodium carbonate (0.135 M)/HEPES buffer (pH = 7, 0.25 M) and d-mannitol (5.5 mg/mL) at 25°C for 1 h. One mL of 0.25 M sodium acetate containing 5 mg/mL gentisic acid (pH 5.5) was then added to the reaction solution. The labeled product was purified with PD 10 columns eluted with metal-free elution buffer (0.25 M sodium acetate containing 5 mg/mL gentisic acid, pH 5.5). Each PD 10 column was pretreated with 25 mg BSA containing 1 µmol DTPA to block nonspecific protein binding sites and remove potential metal contaminants and then washed with metal-free elution buffer. The radiolabeling yield and the radiochemical purity were assessed by analytical size exclusion HPLC (Gilson, Middleton, WI) before and after the purification (please see the Materials and Methods section in the previous paper for detailed information [5]). The radiolabeling yield was determined based on the distribution of 89Zr between 89Zr-labeled amatuximab (retention time: 8.53 min) and unbound 89Zr (retention time: 9.45 min) on the HPLC profiles.
2.3. Immunoreactivity Determination
The immunoreactivity of 89Zr-amatuximab or B3 was determined by a modified cell-binding assay of Lindmo and Bunn [19], as previously reported [5]. Aliquots (5 ng/50 µL) of the conjugate samples were incubated side-by-side with an increasing number of A431/H9 cells (positive for both mesothelin and Lewis-Y; 2 × 104–1 × 106 cells) in 100 µL of PBS with 1% BSA at 4°C for 3 hours. Nonspecific binding to the cells was determined by performing the cell-binding assays under a condition of excess amount of antibodies (50 µg amatuximab and 50 µg B3).
2.4. Tumor Model
A431/H9 is a derivative of the A431 epidermoid carcinoma cell line that has been stably transfected with vectors for human mesothelin and grown in media supplemented with 750 mg/mL G-418 (Geneticin) for selection [20]. This cell line was grown at 37°C with 5% CO2 in media supplemented with 10% FBS, 2 mmol/L L-glutamine, 100 U penicillin, and 100 mg streptomycin (Invitrogen Corporation). The cell line was authenticated at the source and grown from frozen stocks prepared from an early passage of the original line [21].
2.5. Biodistribution Studies
For the BD studies with 89Zr-labeled amatuximab or B3 conjugate with Df molecules, groups (n = 4-5 mice/group) of mice were injected (i.v.) with 89Zr-labeled mAb conjugates (111 kBq for 89Zr-amatuximab; 74 kBq for 89Zr-B3) mixed with corresponding unlabeled intact antibodies (2, 10, or 60 µg amatuximab; 2, 15, or 60 µg B3) in 0.2 mL PBS containing 1% BSA. The tumor sizes at the time of the BD studies were as follows: 245.5 ± 6.5, 190.8 ± 5.0, and 226.4 ± 7.1 mm3 for 2, 10, and 60 µg amatuximab, respectively, and 205.9 ± 3.4, 321.2 ± 9.0, and 372.5 ± 8.2 mm3 for 2, 15, and 60 µg B3, respectively. The animals were euthanized at 24 hours by CO2 inhalation and exsanguination by cardiac puncture. We performed the BD studies as described previously (please see the Materials and Methods section in the previous paper for detailed information [5]). All animal experiments were performed under a protocol approved by the NIH Animal Care and Use Committee.
2.6. PET Imaging
PET imaging studies were performed as described in the previous study of 64Cu-NOTA-amatuximab (please see PET Imaging section in the previous paper [5]). The mice (n = 5) with A431/H9 tumor were injected (i.v.) with 89Zr-amatuximab (2.96 MBq/10 or 60 µg total amatuximab) or 89Zr-B3 (2.22 MBq/15 or 60 µg total B3) in 0.2 ml of normal saline through the tail vein and then 15 min static PET scans were performed at 3, 24, and 48 h p.i. The tumor sizes at the time of the PET imaging were as follows: 429 ± 141 mm3 (range: 253–599 mm3) for 10 µg amatuximab and 406 ± 23 mm3 (range: 385–440 mm3) for 60 µg amatuximab; 700 ± 220 mm3 (range: 436–1042 mm3) for 15 µg B3 and 441 ± 126 mm3 (range: 304–630 mm3) for 60 µg B3.
2.7. Autoradiography and Its Analysis
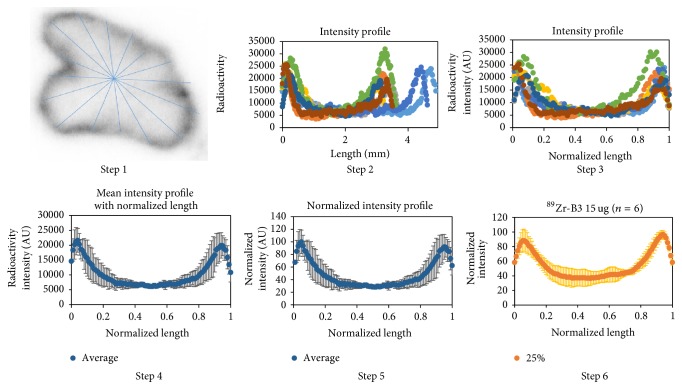
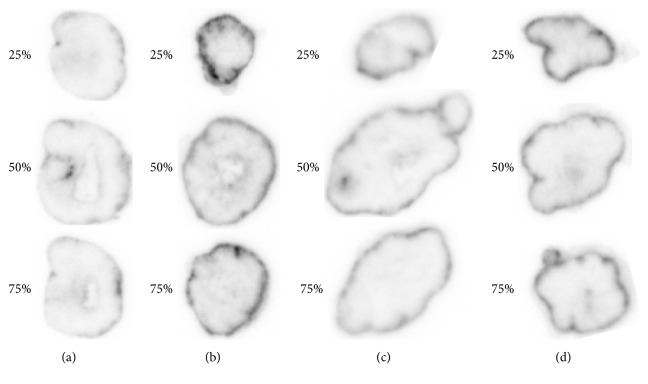
For Ex vivo autoradiography, the mice were selected according to tumor volume by PET and euthanized immediately after 48 h PET imaging session and the tumors were excised. The tumors with the following sizes were used for autoradiography studies: 285 ± 46 mm3 (range: 252–318 mm3; n = 2) and 388 ± 5 mm3 (range: 385–392 mm3; n = 2) for 10 and 60 µg amatuximab, respectively, and 574 ± 121 mm3 (range: 437–666 mm3; n = 3) and 364 ± 60 mm3 (range: 304–424 mm3; n = 3) for 15 and 60 µg B3, respectively. The tumors were embedded and frozen in Tissue-Tek® CRYO-OCT compound (Sakura® Finetek USA Inc., Torrance, CA, USA) at −20°C for 3 h. Serial 20 µm thick short axis sections were cut in 400 µm intervals covering the entire tumor. Two or three consecutive tumor slices were selected at 3 tumor regions (25%, 50%, and 75% long axis regions from the tumor surface) as representative sections throughout the tumor and exposed on the phosphor screen for 16 h. Signals were obtained by the use of the Typhoon FLA 7000 (GE Healthcare Life Sciences, Pittsburgh, PA, USA) with 25 µm pixel resolution and analyzed with Image Quant TL8.1 software. Values were grouped together from the 3 tumor regions to represent a tumor. Each tumor was treated as an independent sample. To analyze the microdistribution of the radioactivity in the tumor sections, we introduced a normalized length analysis method as described below. The first line was drawn along the longest axis, and the second line was drawn along a short axis perpendicularly at the center of the first longest line (see Figure 1). The center was selected as the point where the two lines meet. Additional lines were drawn evenly and continuously between the two original lines passing through the same center point (total of 8 lines). Radioactivity profile of each line was analyzed with ImageJ (NIH, Bethesda, MD) and exported into Excel files to redefine values with Matlab's interpolation function interp1. The maximum length of each line in x-axis was normalized to 1 to correct for the differences in the length of each line for reconstruction of the radioactivity versus tumor penetration distance profiles of each tumor section. The maximum signal intensity within each tumor section in y-axis was also normalized to 100 to correct for the differences in the signal intensity between each tumor section. Mean radioactivity versus distance profiles with standard deviation were then reconstructed for tumor sections obtained at 25%, 50%, and 75% regions.
Figure 1.
Procedure of a new normalized length analysis method for autoradiography. Step 1: draw lines on autoradiography image imported into ImageJ. Step 2: export each line plot data file and plot the original intensity versus line length profile. Step 3: normalize each length and replot with the interpolation function, interp1, in Matlab. Step 4: plot mean and standard deviation intensity profile with normalized length. Step 5: plot normalized intensity profile with normalized length. Step 6: repeat steps 1–5 for each tumor section and get mean normalized intensity profile to represent tumor sections cut at 25%, 50%, and 75% regions of the long tumor axis.
2.8. Statistical Analysis
Statistical analysis was performed using ANOVA for comparing multiple groups, and Student's t-test was performed for unpaired data between two groups. All tests were two-sided, and a probability value (p) of less than 0.05 was considered significant.
3. Results
3.1. Characterization of 89Zr mAbs
The level of Df conjugation was 1.6 ± 0.3 (n = 3) for B3 and 0.9 ± 0.2 (n = 3) for amatuximab. The 89Zr-labeled mAbs were purified on PD-10 columns eluted with acetate buffer (pH 5.5) containing gentisic acid at 5 mg/ml. The purified products were >95% radiochemically pure based on the size exclusion HPLC profiles. The specific activities of the purified product were 296 kBq/µg for 89Zr-amatuximab and 148 kBq/µg for 89Zr-B3. The immunoreactivities of 89Zr-amatuximab and 89Zr-B3 were 84.0 ± 2.2% (n = 2) and 70.0 ± 1.0% (n = 2), respectively.
3.2. Biodistribution Studies
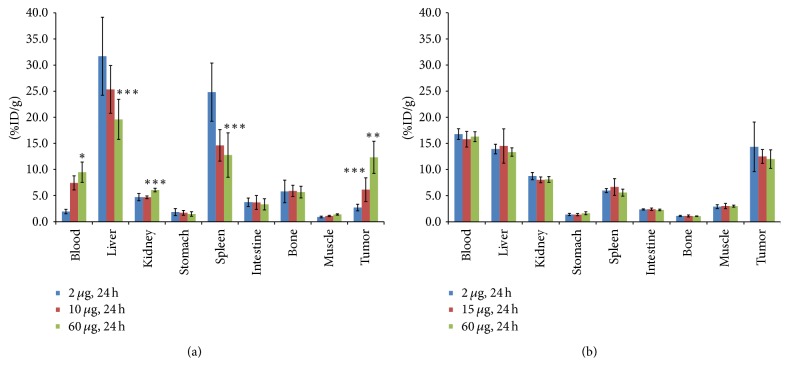
The results of comparative BD studies at 24 h indicated that the uptake of 89Zr-amatuximab in tumor, liver, spleen, and blood directly correlated with dose levels whereas the uptake of anti-Lewis-Y antibody 89Zr-B3 in these organs was dose-independent. In fact, 89Zr-amatuximab tumor uptake and blood retention increased as the injection dose increased (Figure 2(a) and Table 1). However, the liver and spleen uptake decreased as the injection dose increased. The tumor-to-organ ratios increased and conversely the tumor-to-blood ratio decreased as the dose increased, as previously reported for 64Cu-NOTA-amatuximab [5]. In contrast, a dose effect on tumor uptake, blood retention, and liver uptake, as well as the tumor-to-organ and the tumor-to-blood ratios for 89Zr-B3, was not appreciable (Figure 2(b) and Table 1).
Figure 2.
Effects of total injection dose of mAb on the BD of 89Zr-mAb in nude mice (n = 4-5 per group) with A431/H9 tumor: (a) the BD data from 89Zr-amatuximab (111 kBq) with different injection doses of amatuximab (2 µg, 10 µg, and 60 µg) are compared at 24 h p.i. in nude mice with tumor sizes 245.5 ± 6.5, 190.8 ± 5.0, and 226.4 ± 7.1 mm3 for 2, 10, and 60 µg amatuximab. The results demonstrate that tumor uptake and blood retention significantly increased whereas liver uptake decreased as the antibody dose was increased; (b) the BD data from 89Zr-B3 (74 kBq) with different injection doses of B3 (2 µg, 15 µg, and 60 µg) are compared at 24 h p.i. in nude mice with tumor sizes 205.9 ± 3.4, 321.2 ± 9.0, and 372.5 ± 8.2 mm3 for 2, 15, and 60 µg. The results demonstrate that tumor uptake, blood retention, and liver uptake were not affected by the antibody dose. The data are mean ± SD. Analysis of statistical significance in each organ uptake data compared to the data from 2 µg injection: ∗p < 0.001, 0.001 < ∗∗p < 0.01, and 0.01 < ∗∗∗p < 0.05; column: mean; bar: SD.
Table 1.
Effect of mAb dose on tumor-to-blood and tumor-to-organ uptake ratios of 89Zr-amatuximab (111 kBq/2, 10 or 60 µg) or 89Zr-B3 (74 kBq/2, 15 or 60 µg) in nude mice with A431/H9 tumor. All values are reported as mean ± standard deviation (n = 5).
| mAb | Time | Injection dose | Tumor/blood | Tumor/liver | Tumor/kidney | Tumor/spleen | Tumor/muscle |
|---|---|---|---|---|---|---|---|
| Amatuximab | 24 h | 2 µg | 2.66 ± 0.43 | 0.11 ± 0.06 | 0.73 ± 0.32 | 0.23 ± 0.10 | 3.67 ± 0.86 |
| Amatuximab | 24 h | 10 µg | 2.30 ± 1.35 | 0.24 ± 0.07 | 1.32 ± 0.49 | 0.38 ± 0.25 | 5.54 ± 2.00 |
| Amatuximab | 24 h | 60 µg | 1.37 ± 0.54 | 0.66 ± 0.22 | 2.05 ± 0.54 | 1.01 ± 0.28 | 8.97 ± 2.44 |
| B3 | 24 h | 2 µg | 0.98 ± 0.34 | 1.00 ± 0.27 | 1.59 ± 0.39 | 2.71 ± 0.88 | 4.58 ± 1.23 |
| B3 | 24 h | 15 µg | 0.80 ± 0.17 | 0.89 ± 0.19 | 1.57 ± 0.22 | 1.95 ± 0.44 | 4.23 ± 0.81 |
| B3 | 24 h | 60 µg | 0.74 ± 0.10 | 0.90 ± 0.13 | 1.48 ± 0.18 | 2.15 ± 0.26 | 4.01 ± 0.57 |
3.3. PET Imaging Studies
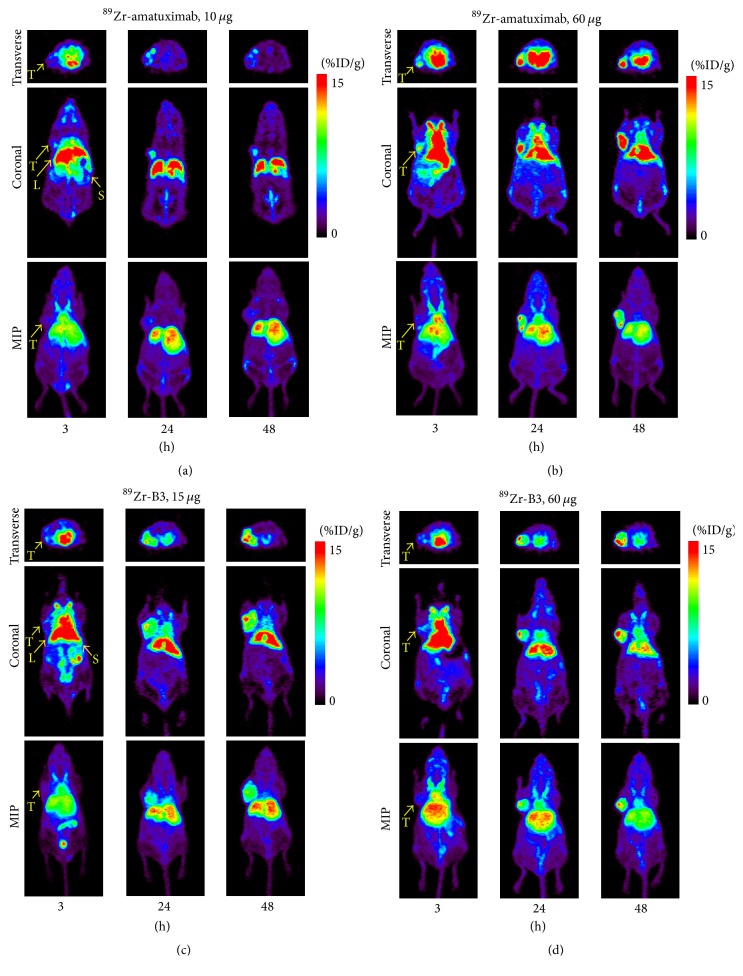
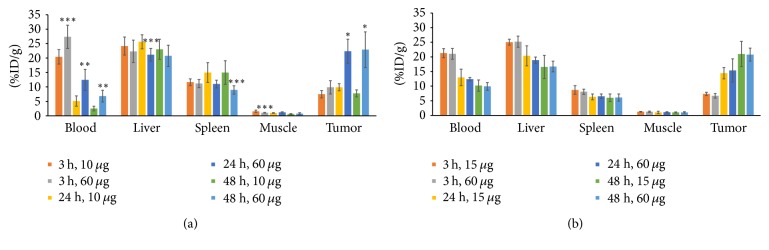
The findings from the BD studies were supported by the PET imaging results. 89Zr-amatuximab tumor uptake was visualized as early as 3 h postinjection (p.i.) at both dose levels, while the majority of radioactivity was still localized in heart (blood pool) and liver (Figure 3(a)). During the 3–48 h period, 89Zr-amatuximab was cleared rapidly from blood at the 10 µg dose while tumor uptake remained unchanged (Figures 3(a) and 4(a)). In contrast, at the 60 µg dose 89Zr-amatuximab cleared more gradually from blood and produced a drastic increase in tumor uptake (%ID/g) at 24 h and 48 h (Figure 4). The tumor uptake (%ID/g) was 7.46 ± 1.26, 9.88 ± 1.25, and 7.74 ± 1.22 at 3, 24, and 48 h, respectively, for 10 µg and 9.85 ± 21.3, 22.37 ± 4.18, and 22.90 ± 6.18 at 3, 24, and 48 h, respectively, for 60 µg. Blood retention (%ID/g) was 20.43 ± 2.49, 5.09 ± 1.78, and 2.52 ± 0.78 at 3, 24, and 48 h, respectively, for 10 µg and 27.37 ± 4.03, 12.44 ± 3.66, and 6.80 ± 2.04 at 3, 24, and 48, respectively, for 60 µg. Liver uptake (%ID/g) was 24.17 ± 3.11, 25.64 ± 2.43, and 23.03 ± 3.51 at 3, 24, and 48 h, respectively, for 10 µg and 22.33 ± 3.89, 21.14 ± 2.21, and 20.78 ± 3.67 at 3, 24, and 48 h, respectively, for 60 µg. Thus, these data indicate that a higher injection dose of amatuximab is advantageous for the tumor visualization by PET. The tumor-to-organ and the tumor-to-blood ratios from PET imaging had similar values observed in 64Cu-NOTA-amatuximab study [5]. The tumor-to-liver ratio showed a value >1 at 24 and 48 h p.i. for 60 µg but a value <1 for 10 µg (Table 2), suggesting that it is feasible to visualize tumors in the upper abdomen with the higher dose.
Figure 3.
Representative PET images of 89Zr-mAb in nude mice with A431/H9 tumor: (a) 89Zr-amatuximab (2.96 MBq) coinjected with 10 µg and 60 µg amatuximab; (b) 89Zr-B3 (2.22 MBq) coinjected with 15 µg and 60 µg B3. Fifteen-minute static PET scans were performed at 3, 24, and 48 h p.i. PET images demonstrate that (a) the tumor uptake significantly increased when the injection dose of 89Zr-amatuximab was 60 µg compared with 10 µg dose and (b) there was no significant dose effect on the tumor uptake of 89Zr-B3 and the tumor uptake gradually increased over time.
Figure 4.
Effect of mAb dose on accumulation of 89Zr-mAb in nude mice (n = 5 per group) with A431/H9 tumor by PET analysis: (a) effect of amatuximab dose (10 µg and 60 µg) on accumulation of 89Zr-amatuximab (2.96 MBq) in nude mice with tumor (429 ± 141 mm3; range: 253–599 mm3) for 10 µg amatuximab and (406 ± 23 mm3; range: 385–440 mm3) for 60 µg amatuximab. (b) Effect of B3 antibody dose (15 µg and 60 µg) on accumulation of 89Zr-B3 (2.22 MBq) in nude mice with tumor (700 ± 220 mm3; range: 436–1042 mm3) for 15 µg B3 and (441 ± 126 mm3; range: 304–630 mm3) for 60 µg B3. The uptake value (%ID/g) was calculated by ROI analysis of PET images. Analysis of statistical significance in each organ uptake data (a) compared to the data from 10 µg for amatuximab or (b) 15 µg for B3. ∗p < 0.001, 0.001 < ∗∗p < 0.01, and 0.01 < ∗∗∗p < 0.05.
Table 2.
Effect of mAb dose on tumor-to-blood and tumor-to-organ uptake ratios of 89Zr-amatuximab (2.96 MBq/10 or 60 µg) or 89Zr-B3 (2.22 MBq/15 or 60 µg) in nude mice with A431/H9 tumor. The ratios were calculated by maximum uptake values from ROI analysis of PET images. Blood-H represents the blood activity in the heart. All values are reported as mean ± standard deviation (n = 5).
| mAb | Time | Injection dose | Tumor/liver | Tumor/spleen | Tumor/muscle | Tumor/blood-H |
|---|---|---|---|---|---|---|
| Amatuximab | 3 h | 10 µg | 0.31 ± 0.06 | 0.65 ± 0.14 | 5.03 ± 1.53 | 0.38 ± 0.10 |
| Amatuximab | 24 h | 10 µg | 0.40 ± 0.03 | 0.69 ± 0.21 | 10.20 ± 1.05 | 2.10 ± 0.64 |
| Amatuximab | 48 h | 10 µg | 0.35 ± 0.02 | 0.54 ± 0.21 | 13.31 ± 1.68 | 3.21 ± 0.77 |
| Amatuximab | 3 h | 60 µg | 0.36 ± 0.03 | 0.84 ± 0.18 | 9.57 ± 3.52 | 0.36 ± 0.03 |
| Amatuximab | 24 h | 60 µg | 1.04 ± 0.07 | 1.98 ± 0.63 | 18.90 ± 1.57 | 1.68 ± 0.17 |
| Amatuximab | 48 h | 60 µg | 1.02 ± 0.20 | 2.68 ± 0.86 | 31.16 ± 5.58 | 3.46 ± 0.69 |
| B3 | 3 h | 15 µg | 0.30 ± 0.03 | 0.89 ± 0.14 | 6.11 ± 0.45 | 0.35 ± 0.03 |
| B3 | 24 h | 15 µg | 0.72 ± 0.10 | 2.15 ± 0.29 | 14.26 ± 3.60 | 1.09 ± 0.15 |
| B3 | 48 h | 15 µg | 1.30 ± 0.01 | 3.67 ± 1.34 | 20.00 ± 4.08 | 2.15 ± 0.74 |
| B3 | 3 h | 60 µg | 0.28 ± 0.03 | 0.86 ± 0.19 | 5.51 ± 1.84 | 0.32 ± 0.02 |
| B3 | 24 h | 60 µg | 0.82 ± 0.21 | 2.40 ± 0.68 | 14.53 ± 5.49 | 1.24 ± 0.34 |
| B3 | 48 h | 60 µg | 1.21 ± 0.05 | 3.12 ± 0.35 | 19.03 ± 1.59 | 1.98 ± 0.15 |
The PET images of 89Zr-B3 showed tumor uptake as early as 3 h p.i. at both 15 and 60 µg doses. Compared to 89Zr-amatuximab, the PET images from 89Zr-B3 did not show any significant dose effects on its uptake and the clearance pharmacokinetics from tumor, blood, and liver (Figure 3(b)). The tumor uptake of the 89Zr-B3 increased steadily over a 48 h period while clearing gradually from the blood and the liver as follows (Figure 4): tumor uptake (%ID/g) of 7.36 ± 0.54, 14.43 ± 1.94, and 20.99 ± 4.33 at 3, 24, and 48 h, respectively, for 15 µg and 6.71 ± 0.78, 15.36 ± 3.98, and 20.78 ± 2.21 at 3, 24, and 48 h, respectively, for 60 µg; blood retention (%ID/g) of 21.28 ± 1.54, 12.99 ± 2.83, and 10.21 ± 1.92 at 3, 24, and 48 h, respectively, for 15 µg and 21.05 ± 1.85, 12.40 ± 0.56, and 9.92 ± 1.33 at 3, 24, and 48 h, respectively, for 60 µg; liver uptake (%ID/g) of 25.04 ± 1.03, 20.37 ± 3.4, and 16.55 ± 3.98 at 3, 24, and 48 h, respectively, for 15 µg and 25.21 ± 1.90%ID/g, 18.90 ± 1.06, and 16.72 ± 1.83 at 3, 24, and 48 h, respectively, for 60 µg. The tumor-to-organ and the tumor-to-blood ratios from PET imaging did not change as the dose increased (Table 2).
These results suggest a significant difference in biodistribution characteristics between 89Zr-amatuximab and 89Zr-B3, which may be due to the presence of shed mesothelin in blood and tumor tissue that may affect the serum half-life as well as Ag-specific tumor uptake of radiolabeled amatuximab as previously reported [5].
3.4. Autoradiography Studies
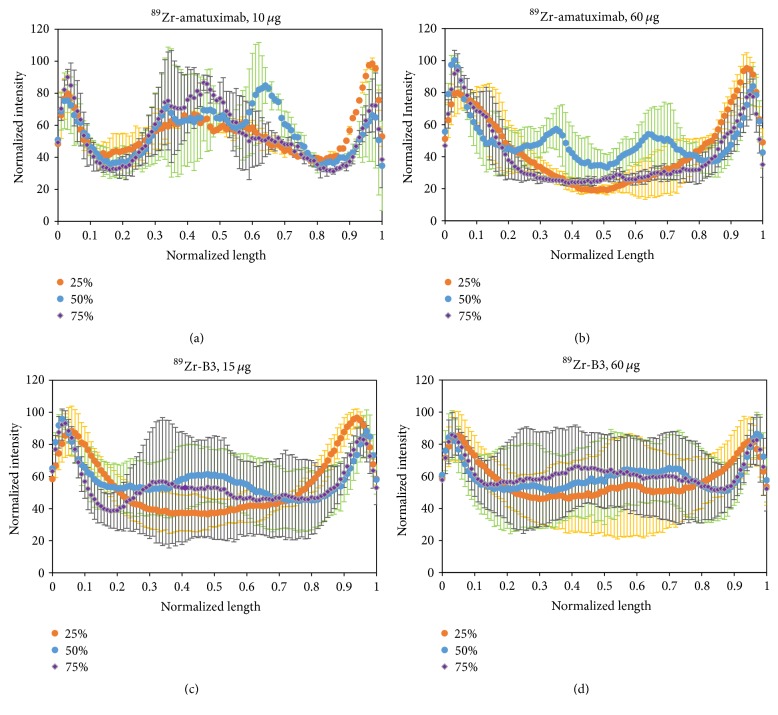
To investigate if the shed mesothelin in the ECS of tumor could affect the penetration of 89Zr-amatuximab, we performed the autoradiography of tumor segments at 48 h p.i. immediately after the completion of the PET imaging studies. The data of autoradiography images analysis (see Figure 1 for image analysis method) demonstrated that 89Zr-amatuximab had similar radioactivity intensity at the tumor core and the periphery when the injection dose was 10 µg (Figures 5(a) and 6(a)). However, increasing the dose to 60 µg increased the uptake to the periphery but not to the center of the tumor (Figures 5(b) and 6(b)). In contrast, the radioactivity data and the normalized radioactivity versus distance profiles for 89Zr-B3 showed that the uptake peaked at the tumor periphery while it decreased rapidly toward the tumor core at either dose levels (Figures 5(c), 5(d), 6(c), and 6(d)).
Figure 5.
Representative autoradiography images of 89Zr-labeled antibody. 89Zr-amatuximab (2.96 MBq) coinjected with (a) 10 µg and (b) 60 µg amatuximab; 89Zr-B3 (2.22 MBq) coinjected with (c) 15 µg and (d) 60 µg B3 mAb.
Figure 6.
Autoradiography analysis of 89Zr-mAbs depicted as normalized intensity versus normalized length profiles: 89Zr-amatuximab (2.96 MBq) was coinjected with (a) 10 µg and (b) 60 µg amatuximab. 89Zr-B3 (2.22 MBq) was coinjected with (c) 15 µg and (d) 60 µg B3. The results demonstrate that the normalized radioactivity of 89Zr-amatuximab at the tumor core and the periphery was similar with the low dose of amatuximab but the relative 89Zr intensity at the tumor periphery was higher as the injection dose was increased to 60 µg. In contrast, the radioactivity profile of 89Zr-B3 was not affected by the B3 dose between 15 and 60 µg B3 and the radioactivity preferentially accumulated in the tumor periphery.
4. Discussion
The studies reported here highlight an important property of anti-mesothelin mAb amatuximab as compared to anti-Lewis-Y mAb B3 for the Ag-mediated targeting of A431/H9 tumor overexpressing both a shed Ag, mesothelin, and a nonshed Ag, Lewis-Y, in a mouse model. The comparative BD, PET imaging, and autoradiography studies between these two mAbs allowed us to define the effect of shed Ag on the tumor targeting and penetration, apart from the effects of other factors related to the tumor microenvironment. While the biodistribution and tumor uptake of 89Zr-B3 were not significantly affected by the injected dose, the tumor uptake of 89Zr-amatuximab increased whereas the uptake in liver and spleen decreased as the injected dose increased. This finding is consistent with the previously reported dose-dependent effect on the biodistribution and tumor uptake of 64Cu-NOTA-amatuximab [5]. The BD and tumor uptake of 89Zr-B3 were not appreciably affected by the injection dose levels because its target, Lewis-Y Ag, is not shed by tumor cells. The ROI analysis of PET images corresponded to the BD results and is consistent with the hypothesis of a decoy effect caused by soluble shed Ag in blood and tumor.
These findings could be explained by a threshold effect whereby 89Zr-amatuximab is mostly bound to shed MSLN in blood at the lower dose (10 µg) and sequestered into the reticular endothelial system of liver and spleen, thereby lowering both blood retention and tumor uptake. Based on this knowledge, we hypothesized that a dose of 60 µg could be sufficiently high to saturate shed MSLN as well as create an excess of 89Zr-amatuximab available for a more effective tumor targeting. This effect could be explained by correlating the concentration of shed MSLN in the blood and in the ECS of A431/H9 tumor, as estimated in previous studies [8, 22, 23], similarly to that of 64Cu-NOTA-amatuximab. 89Zr-amatuximab injected at 10 µg dose results in an estimated blood concentration of 43.3 nM (assuming blood volume of 1.6 mL for a 20 g mouse) immediately after injection and 1.75 nM at 48 h (based on 2.52%ID/g blood in Figure 3). Because the average concentration of shed MSLN in the blood is ~6 nM in a tumor of ~300 mm3, 89Zr-amatuximab (1.75 nM) would be mostly bound to shed MSLN in blood during a 48 h period and this complex sequestered into liver and spleen. At 48 h, unbound 89Zr-amatuximab would have crossed the tumor vasculature and diffused into tumor ECS (5.36 nM based on 7.74%ID/g tumor at 48 h and 53.6 nM in the ECS, assuming that ECS constitutes 10% of total tumor volume). Because the concentration of shed MSLN is ~300 nM in the ECS of a tumor of 300 mm3, 89Zr-amatuximab (53.6 nM) in the ECS would mostly exist as an antibody-Ag complex distributed throughout the entire tumor, presumably bypassing the binding sites on the surface of tumor cells nearest to the vasculature. On the other hand, 89Zr-amatuximab injected at 60 µg dose results in an estimated blood concentration of 260 nM immediately after injection and 28.3 nM at 48 h (6.8%ID/g blood), that is, at a molar excess compared to the average concentration of shed MSLN in the blood (~6 nM). Consequently, 89Zr-amatuximab would remain mostly unbound, overcoming the sequestration into the hepatic reticuloendothelial system during a 48 h period. Therefore, a larger portion of the injected dose (95.2 nM based on 22.90%ID/g tumor at 48 h and 952 nM in the ECS) would have crossed the tumor vasculature and diffused into the tumor ECS. This concentration (952 nM) is larger than the shed MSLN concentration in the tumor ECS. The excess of 89Zr-amatuximab not bound to shed MSLN would bind to MSLN on tumor cells in the periphery rather than in the tumor core, as observed for 89Zr-B3 as well as Alexa-labeled B3 [24]. According to Boucher et al. the interstitial fluid pressure is often elevated in solid tumors but declines in the tumor periphery in the outer 0.2–1.1 mm [25]. Therefore, the accumulation in the tumor periphery might be favored by the lower interstitial fluid pressure in this region allowing for more antibody extravasation than in tumor core.
It is noteworthy that our PET study demonstrated that the tumor localized outside of the abdomen could be visualized even with the lower dose (10 µg) at 24 h and thereafter because the tumor-to-blood and tumor-to-muscle ratios of 89Zr-amatuximab were higher than 2. However, it would be necessary to inject the higher dose (60 µg) to visualize the tumor in the abdominal area. The PET study also suggests that 89Zr-amatuximab could be useful in a clinical setting because 89Zr with a long half-life (78.4 h) would be more suitable for the detection of tumor and the tumor-to-background ratio increased over time with the injection of the higher dose (60 µg).
In this study, we demonstrated the effect of shed mesothelin on the tumor targeting and tumor microdistribution of anti-mesothelin mAb amatuximab in A431/H9 tumor using anti-Lewis-Y B3 as a negative control. However, it is possible that other shed antigens with a different binding epitope or a different affinity for the targeting mAb might show a different degree of shed antigen effects. Thus, careful investigation of other shed antigen systems would be needed to determine whether our findings could be generalized.
5. Conclusion
The use of A431/H9 tumor which overexpresses both the shed Ag mesothelin and the nonshed Ag Lewis-Y as a tumor model and the use of anti-mesothelin mAb amatuximab and anti-Lewis-Y mAb B3 as model mAbs for the BD, PET, and autoradiography studies allowed us to make a direct assessment on the effect of the shed Ag on the tumor and organ uptakes and tumor penetration, apart from the effects of other factors related to the tumor microenvironment. In addition, the use of 89Zr with a 3.4-day half-life to label the mAbs for PET imaging and autoradiography studies provided the advantages of high image sensitivity and resolution which enabled us to quantify the tumor and organ uptakes as well as tumor penetration of the mAbs.
The findings of this study imply that the systemic mAb concentration should be at a severalfold molar excess to the shed Ag concentration in the blood to increase the concentration of free mAb available for the tumor uptake and reduce the fraction of the mAb bound to the shed Ag in the blood and the subsequent hepatic processing of the mAb-Ag complexes. However, the mAb concentration in the tumor ECS should remain lower than the shed Ag concentration in the tumor ECS to maximize the tumor penetration of the mAb-Ag complexes by bypassing the binding site barrier. It will be the future challenge for both antibody engineers and clinical investigators to find this balance.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH Clinical Center and funded in part with federal funds from the National Cancer Institute, NIH (under Contract no. HHSN261200800001E) (Dr. Insook Kim). The authors would like to acknowledge Dr. Ira Pastan for his support.
Contributor Information
Jae-Ho Lee, Email: jaeho.jhlee@gmail.com.
Chang H. Paik, Email: cpaik@mail.nih.gov.
Disclosure
The authors disclose that the preliminary work of this research was presented as an abstract at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging, 2017.
Conflicts of Interest
The authors have declared that no conflicts of interest exist.
Authors' Contributions
Conceptualization was the responsibility of Jae-Ho Lee and Chang H. Paik. Data curation was the responsibility of Heejung Kim, Zhengsheng Yao, Insook Kim, Jae-Ho Lee, and Chang H. Paik. Formal analysis was the responsibility of Heejung Kim and Jae-Ho Lee. Funding acquisition was the responsibility of Chang H. Paik. Investigation was the responsibility of Heejung Kim, Zhengsheng Yao, Insook Kim, and Jae-Ho Lee. Methodology was the responsibility of Heejung Kim, Zhengsheng Yao, Jae-Ho Lee, and Chang H. Paik. Project administration was the responsibility of Chang H. Paik. Resource was the responsibility of Lawrence P. Szajek and Luigi Grasso. Supervision was the responsibility of Chang H. Paik. Validation was the responsibility of Jae-Ho Lee and Chang H. Paik. Visualization was the responsibility of Heejung Kim and Jae-Ho Lee. Writing the original draft was the responsibility of Jae-Ho Lee and Chang H. Paik. Writing, reviewing, and editing the manuscript were the responsibility of Heejung Kim, Zhengsheng Yao, Insook Kim, Lawrence P. Szajek, Jae-Ho Lee, Luigi Grasso, and Chang H. Paik.
References
- 1.Scott A. M., Wolchok J. D., Old L. J. Antibody therapy of cancer. Nature Reviews Cancer. 2012;12(4):278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 2.Junttila M. R., de Sauvage F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 3.Jones P. A., Issa J. J., Baylin S. Targeting the cancer epigenome for therapy. Nature Reviews Genetics. 2016;17(10):630–641. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- 4.Houghton J. L., Zeglis B. M., Abdel-Atti D., Sawada R., Scholz W. W., Lewis J. S. Pretargeted immuno-PET of pancreatic cancer: Overcoming circulating antigen and internalized antibody to reduce radiation doses. Journal of Nuclear Medicine. 2016;57(3):453–459. doi: 10.2967/jnumed.115.163824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.-H., Kim H., Yao Z., et al. Tumor and organ uptake of 64Cu-labeled MORAb-009 (amatuximab), an anti-mesothelin antibody, by PET imaging and biodistribution studies. Nuclear Medicine and Biology. 2015;42(11):880–886. doi: 10.1016/j.nucmedbio.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin I. S., Lee S.-M., Kim H. S., et al. Effect of chelator conjugation level and injection dose on tumor and organ uptake of 111In-labeled MORAb-009, an anti-mesothelin antibody. Nuclear Medicine and Biology. 2011;38(8):1119–1127. doi: 10.1016/j.nucmedbio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pimm M. V. Circulating antigen: Bad or good for immunoscintigraphy? Nuclear Medicine and Biology. 1995;22(2):137–145. doi: 10.1016/0969-8051(94)00098-5. [DOI] [PubMed] [Google Scholar]
- 8.Pak Y., Pastan I., Kreitman R. J., Lee B. Effect of antigen shedding on targeted delivery of immunotoxins in solid tumors from a mathematical model. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0110716.e110716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westwood J. A., Murray W. K., Trivett M., et al. The lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. Journal of Immunotherapy. 2009;32(3):292–301. doi: 10.1097/CJI.0b013e31819b7c8e. [DOI] [PubMed] [Google Scholar]
- 10.Camera L., Kinuya S., Paik C. H., Carrasquillo J. A. Preclinical Evaluation of 111 In-labeled B3 Monoclonal Antibody: Biodistribution and Imaging Studies in Nude Mice Bearing Human Epidermoid Carcinoma Xenografts. Cancer Research. 1993;53(12):2834–2839. [PubMed] [Google Scholar]
- 11.Pastan I., Lovelace E. T., Gallo M. G., Rutherford A. V., Magnani J. L., Willingham M. C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Research. 1991;51(14):3781–3787. [PubMed] [Google Scholar]
- 12.Pai-Scherf L. H., Carrasquillo J. A., Paik C., et al. Imaging and phase I study of 111In- and 90Y-labeled anti-LewisY monoclonal antibody B3. Clinical Cancer Research. 2000;6(5):1720–1730. [PubMed] [Google Scholar]
- 13.Yao Z., Zhang M., Axworthy D. B., et al. Radioimmunotherapy of A431 xenografted mice with pretargeted B3 antibody-streptavidin and90Y-labeled 1, 4, 7, 10-tetraazacyclododecane-N, N′, N′, N‴-tetraacetic acid (DOTA)-biotin. Cancer Research. 2002;62(20):5755–5760. [PubMed] [Google Scholar]
- 14.Holland J. P., Caldas-Lopes E., Divilov V., et al. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/ neu expression in mice using 89Zr-DFO-trastuzumab. PLoS ONE. 2010;5(1) doi: 10.1371/journal.pone.0008859.e8859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verel I., Visser G. W., Boellaard R., Stigter-van Walsum M., Snow G. B., van Dongen G. A. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. Journal of Nuclear Medicine. 2003;44(8):1271–1281. [PubMed] [Google Scholar]
- 16.Holland J. P., Sheh Y., Lewis J. S. Standardized methods for the production of high specific-activity zirconium-89. Nuclear Medicine and Biology. 2009;36(7):729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vosjan M. J. W. D., Perk L. R., Visser G. W. M., et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nature Protocols. 2010;5(4):739–743. doi: 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- 18.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Lindmo T., Bunn P. A. [65] Determination of the True Immunoreactive Fraction of Monoclonal Antibodies after Radiolabeling. Methods in Enzymology. 1986;121(C):678–691. doi: 10.1016/0076-6879(86)21067-8. [DOI] [PubMed] [Google Scholar]
- 20.Ho M., Hassan R., Zhang J., et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clinical Cancer Research. 2005;11(10):3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 21.Weldon J. E., Xiang L., Zhang J., et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Molecular Cancer Therapeutics. 2013;12(1):48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Hansen J. K., Xiang L., et al. A flow cytometry method to quantitate internalized immunotoxins shows that taxol synergistically increases cellular immunotoxins uptake. Cancer Research. 2010;70(3):1082–1089. doi: 10.1158/0008-5472.CAN-09-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Xiang L., Hassan R., Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proceedings of the National Acadamy of Sciences of the United States of America. 2007;104(43):17099–17104. doi: 10.1073/pnas.0708101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Shin I. S., Hancock H., et al. Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. Journal of Controlled Release. 2012;162(1):218–224. doi: 10.1016/j.jconrel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucher Y., Baxter L. T., Jain R. K. Interstitial Pressure Gradients in Tissue-isolated and Subcutaneous Tumors: Implications for Therapy. Cancer Research. 1990;50(15):4478–4484. [PubMed] [Google Scholar]