Abstract
Maintaining a balance of ω-6 and ω-3 fatty acids is essential for cardiac health. Current ω-6 and ω-3 fatty acids in the American diet have shifted from the ideal ratio of 2:1 to almost 20:1; while there is a body of evidence that suggests the negative impact of such a shift in younger organisms, the underlying age-related metabolic signaling in response to the excess influx of ω-6 fatty acids is incompletely understood. In the present study, young (6 mo old) and aging (≥18 mo old) mice were fed for 2 mo with a ω-6-enriched diet. Excess intake of ω-6 enrichment decreased the total lean mass and increased nighttime carbohydrate utilization, with higher levels of cardiac cytokines indicating low-grade chronic inflammation. Dobutamine-induced stress tests displayed an increase in PR interval, a sign of an atrioventricular defect in ω-6-fed aging mice. Excess ω-6 fatty acid intake in aging mice showed decreased 12-lipoxygenase with a concomitant increase in 15-lipoxygenase levels, resulting in the generation of 15(S)-hydroxyeicosatetraenoic acid, whereas cyclooxygenase-1 and -2 generated prostaglandin E2, leukotriene B4, and thromboxane B2. Furthermore, excessive ω-6 fatty acids led to dysregulated nuclear erythroid 2-related factor 2/antioxidant-responsive element in aging mice. Moreover, ω-6 fatty acid-mediated changes were profound in aging mice with respect to the eicosanoid profile while minimal changes were observed in the size and shape of cardiomyocytes. These findings provide compelling evidence that surplus consumption of ω-6 fatty acids, coupled with insufficient intake of ω-3 fatty acids, is linked to abnormal changes in ECG. These manifestations contribute to functional deficiencies and expansion of the inflammatory mediator milieu during later stages of aging.
NEW & NOTEWORTHY Aging has a profound impact on the metabolism of fatty acids to maintain heart function. The excess influx of ω-6 fatty acids in aging perturbed electrocardiography with marked signs of inflammation and a dysregulated oxidative-redox balance. Thus, the quality and quantity of fatty acids determine the cardiac pathology and energy utilization in aging.
Keywords: aging, fatty acids, inflammation, lipid signaling, metabolism
INTRODUCTION
Aging is associated with changes in metabolic, cellular, and molecular signaling pathways, which inevitably increase the risks of morbidity and mortality. In an obesogenic setting, aging is prone to induce sustained low-grade inflammation and oxidative stress (9, 19, 37). The impact of the aging process is an independent and cumulative factor in cardiovascular disease (CVD) and is incompletely understood (11). Over the past 70 yr, due to the surplus consumption of processed food and an increasingly sedentary lifestyle, obesity has become more prevalent. It thus presents as a major risk factor in CVD, especially in elderly populations. To maintain homeostasis and reduce cardiovascular disease, the recommended ratio of ω-6 to ω-3 fatty acids is ~2:1. In obese individuals, this ratio is alarmingly skewed up to a ratio of 20:1 (2).
There has been a >1,000-fold increase in the estimated per capita consumption of soybean oil from 0.006% to 7.38% of energy in the United States food supply (2). This increased consumption of soybean oil (ω-6 enriched) has led to an increase in dietary linoleic acid (LA) that far exceeds changes seen in all other essential fatty acids (32). A global consortium of 19 studies from 16 countries indicated that marine food- and plant-derived ω-3 fatty acids are associated with a significant risk reduction of fatal CVDs (7). Additionally, excess fatty acids can be oxidized to generate either potent proinflammatory mediators [e.g., leukotriene B4 (LTB4) and thromboxane (TBX)] or proresolving lipid mediators (e.g., lipoxins and resolvins) that have a strong immunomodulatory capacity (30).
In the process of aging, cardiovascular tissues undergo metabolic changes that may have an immediate effect on heart. For example, aging is known to have an effect on heart rate variability. Heart rate is influenced not only by the loss of cells in the sinoatrial node (responsible for controlling heart rate) but also by structural changes in the heart, such as fibrosis and hypertrophy, which slow the propagation of electric impulses throughout the heart coordinated by macrophages (12, 23). Furthermore, aging has a profound impact on the progressive atrioventricular conduction affecting the sinoatrial node, atrioventricular node, and His bundle, indicating slow conduction (R). Age-related ECG alterations and their relation to pathophysiological changes in the heart are understudied in the context of excess ω-6 consumption, even though these effects are observed worldwide. Therefore, it is of paramount importance to determine the electrophysiological changes and the state of redox homeostasis in progressive aging.
METHODS
Animal compliance.
All protocols involving animals conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Revised 2015) and were approved by the Animal Care and Use Committee of the University of Alabama at Birmingham.
Mice and diet maintenance.
Male C57BL/6J mice at ages of 6 mo old (young) and 18 mo old (aging) were sourced from the National Institute of Aging colony. Mice were maintained with free access to water and diet under a constant temperature of 19.8–22.2°C. Young adult and aging mice were randomized into the following two groups: 1) standard laboratory chow (LC; maintenance laboratory chow, American Institute of Nutrition 93M diet, approximately wt/wt: 14% protein, 73% carbohydrate, and 4% fat) and 2) safflower oil (SO; approximately wt/wt: 15% protein, 66% carbohydrate, and 10% SO ω-6-enriched diet) for 2 mo (Fig. 1A, study design).
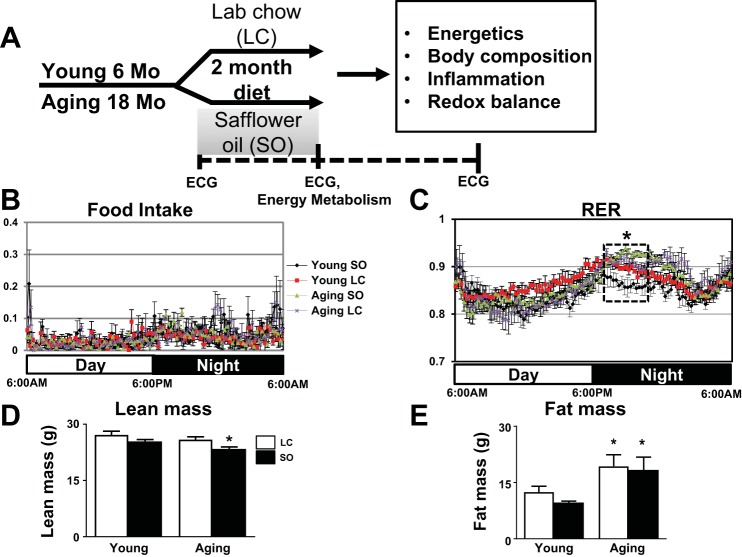
Fig. 1.
Excess ω-6 fatty acids in aging increased carbohydrate use. A: study design indicating young (6 mo old) and aging (18 mo old) mice supplied ω-6 fatty acid-enriched safflower oil (SO) diet for 2 mo. Activity monitored using the comprehensive laboratory animal monitoring system (CLAMS) indicated no change in food intake (B) and an increase in the respiratory exchange ratio (RER; C) in the SO-aging group in the nighttime. D and E: body composition analysis of lean body mass (D) and fat mass (E) of young and aging mice fed with standard laboratory chow (LC) or the ω-6-enriched diet. Red, young-LC group; black, young-SO group; purple, aging-LC group; green, aging-SO group. Values are means ± SE; n = 8 mice/group. *P < 0.05 vs. the young-LC group.
Measurements of fat and lean mass using quantitative MRI.
Young and aging mice fed either excess ω-6 and maintenance diets were subjected to whole body composition measurements using quantitative MRI (QMRI instrument, Echo Medical System) as previously described (19).
Comprehensive Laboratory Animal Monitoring System.
The Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments) is a set of live-in cages for automated, noninvasive, and simultaneous monitoring of horizontal and vertical activity, feeding and drinking, and O2 consumption and CO2 production. Young and aging mice either excess ω-6 and maintenance diets were individually placed in CLAMS cages and monitored over a 7-day period. Food and water consumption were measured directly as accumulated data. The hourly file displays all measurements for each of the following parameters: volume of O2 consumed (V̇o2; in ml·kg−1·h−1), volume of CO2 produced (V̇co2; in ml·kg−1·h−1), respiratory exchange ratio (RER), heat (in Kcal/h), accumulated food (in g), accumulated drink (in g), and XY total activity (all horizontal beam breaks in counts). Light and feeding conditions were kept the same as in the home cages (3).
Baseline ECG recordings in young and aging mice with and without ω-6 supplementation.
Mice were anesthetized with 2% with a 100% oxygen mix and, after 5 min, placed on the platform. Three color-coded recording electrodes were subdermally inserted into both upper limbs and the left lower limb. The ambient room temperature was monitored using temperature controller (LA CROSSE) technology. Mice that had an average heart beat below 400 beats/min were excluded from the study. The recording electrodes were connected to an ix-228/S data acquisition unit (iWorx Systems). Signals were filtered between 3 and 500 Hz. The input range was within 5 mV. Signals were also digitized with 16-bit precision at a sampling rate of 1,000 samples/s. The ECG signal was continuously recorded for 5 min for baseline analysis. Only data from recordings of stable ECG signals were used in the analyses. Data were then analyzed with Labchart 8 software (AD Instruments).
ECG recording during dobutamine-induced stress.
The dobutamine stress test was performed to determine exercise-induced changes in the ECGs of young and aging mice with and without ω-6 enrichment. Mice were anesthetized under 2% isoflurane in a 100% O2 mix. Baseline ECGs were recorded for each mouse before the dobutamine injection. The dobutamine solution of 3 μg/kg body wt was prepared in normal saline and injected intraperitoneally. The dobutamine response was measured postinjection for 20 min, and ECGs were analyzed using LabChart 8 software (AD Instruments).
ECG analyses.
Each signal was analyzed using LabChart 8 (AD Instruments). The selected ECG cycles were averaged, and abnormal beats or noise-containing waveforms were excluded from the analyses. Parameters such as P, Q, R, S, and T amplitudes and RR, PR, JT, QT, and corrected QT (QTc) time intervals were tabulated. Waterfall plots (three-dimensional waveform analysis) were generated from the calculated parameters. The baseline rodent ECG was selected from the menu, and beats were averaged to four beats for calculations. The typical QRS width was kept at 10 ms and R waves at 10 ms. The pre P baseline was selected as 10 ms, maximum RT as 40 ms, and maximum PR as 50 ms. The ST height was selected as 10 ms from alignment. All of these parameters are typically found in a normal ECG of the rodent. Therefore, the deflections were calculated according to set parameters.
Lipid mediator analyses in the left ventricle, spleen, and plasma.
Samples remained on ice during the entirety of the extraction procedure except for centrifugation. For the left ventricle (LV) and spleen, ~20 mg of tissue were transferred to a microtube and the corresponding weights were recorded. Extraction of lipid mediators was initiated with the addition of 1× PBS (pH 7.4) containing isotope-labeled internal standards with 20 mg/0.5 ml of solvent normalization criteria observed. Tissue was disrupted using a probe sonicator set at 40% output power and 20% duty cycle in 20-s bursts. For plasma, 50 μl of plasma were placed in a microtube, 0.950 ml of the PBS solution were added, and samples were vortexed thoroughly to mix. After those steps, all samples were incubated on ice for 10 min. Meanwhile, 60 mg/3 ml Strata-X SPE columns (Phenomenex, Torrance, CA) were prewashed and conditioned according to the manufacturer’s directions and set aside. Samples were loaded on the SPE column, and vacuum suction was applied to draw samples completely through column. Columns containing samples were washed with 2 ml of 10% methanol, a vacuum was applied to completely wash through, and columns were collected postfilter and discarded. A column containing samples was then eluted with the addition of 1 ml of methanol vacuum, and the subsequent sample was collected postfilter in a microtube. Samples were dried in a speedvac at 45°C and then redissolved in 100 μl mobile phase A (60:40:0.2 water-acetonitrile-acetic acid), vortexed briefly to aid dissolution, and then centrifuged briefly. The redissolved sample was then placed into an autosampler vial for mass spectrometry analysis. A small volume of each sample was removed to create a pooled sample for quality control purposes. A series of calibration standards was prepared along with samples to quantify metabolites.
Liquid chromatography-mass spectrometry analyses.
Liquid chromatography-mass spectrometry (LC-MS) analysis was performed on an Agilent system consisting of a 1260 UPLC module coupled with a QQQ 6490 mass spectrometer (Agilent Technologies, Santa Clara, CA). The instrument was tuned and calibrated according to procedures recommended by the manufacturer. Eicosanoids were separated on a 150 × 2-mm Luna 3-μ C18 column (Phenomenex). Mobile phase A consisted of 60:40 (vol/vol) water-acetonitrile with 0.05% acetic acid; mobile phase B consisted of 50:50 (vol/vol) of isopropanol-acetonitrile. The flow rate was 0.25 ml/min, and the gradient was 10–60% B over 10 min, 60–100% over 2 min, and isocratic at 100% B for 3 min followed by a return to starting conditions (10% B) and equilibration of the system for 10 min. The mass spectrometer was operated in ESI- mode with a gas and sheath temperatures of 275 and 250°C, respectively, and gas and sheath flow rates of 15 and 11 l/min. The fragmentor, capillary, and nozzle voltages were 380, 3,500, and 2,000, respectively. Data were processed using MassHunter Quantitative analysis version B.07.00. Spleen and LV samples were normalized to the nearest isotope-labeled internal standard and quantitated using two replicated injections of five standards to create a linear calibration curve with accuracy > 80% for each standard. Plasma eicosanoids were normalized and quantitated to the nearest internal standard.
LV RNA isolation and real-time PCR.
Postnecropsy, frozen LV samples were processed for RNA extraction. Briefly, tissue was homogenized with a sonic dismembrator (Fisher Scientific) at an amplitude between 10 and 100 Hz in 500 µl TRIzol (Invitrogen). RNA was then extracted and isolated. cDNA synthesis was performed using 2 µg total RNA using SuperScript VILO cDNA synthesis kit (Invitrogen). Quantitative PCR (qPCR) for the genes TNF-α, IL-6, IL-1β, and arachidonate lipoxygenase (Alox)-12, Alox-15, and Alox-5 was done using TaqMan probes (Applied Biosystems) on master cycler ABI, 7900 HT. qPCR for nuclear factor erythroid 2-like (Nrf)2, Kelch-like ECH associated protein 1 (Keap-1), glutathione peroxidase 1 (GPX-1), glutathione-S-transferase-α4 (Gsta4), NAD(P)H quinone dehydrogenase 1 (Nqo-1), glutathione-disulfide reductase (GSR), glutamate-cysteine ligase catalytic subunit (Gclc), glutamate-cysteine ligase modifier subunit (Gclm), glucose-6-phosphate dehydrogenase (G6PD), catalase (Cat), superoxide dismutase (SOD)1, and SOD2 was done using SYBER green. Gene levels were normalized to hypoxanthine phosphoribosyltransferase (Hprt-1) as a housekeeping gene. Data are reported and analyzed as (ΔΔCT) values (where CT is threshold cycle).
5-Lipoxygenase DNA methylation assay.
The 5-lipoxygenase (5-LOX) DNA methylation in LV samples was done using the EZ-96-DNA Methylation Direct kit (D5022) from Zymo Research as per the manufacturer’s instructions. The PCR was done using the following primers: forward 5′-TGATGTGGCTGGCCTCTTATGTGA-3′ and reverse 5′-ACTGGGACTGAGTGCAGGAAATGT-3′ (14).
Protein extraction and immunoblot analysis.
The protein extraction and electrophoresis of LV tissues were performed as previously described (15). The blot was probed with primary antibody (5-LOX: 1:1,000; β-actin: 1:5,000) overnight at 4°C followed by secondary antibody (Bio-Rad). Proteins were detected using a Femto chemiluminescence detection system (Pierce Chemical, Rockford, IL). Densitometry was performed using ImageJ software (National Institutes of Health).
Statistical analysis.
Data are expressed as means ± SE. Statistical analyses were performed using GraphPad Prism 7. Two-way ANOVA was used for comparisons between young-LC, young-SO, aging-LC, and aging-SO groups. The basic parametric tests applied to metabolomics data and all metabolites included in study were taken above the range of detection limit. Lipid metabolites below the limit of detection were excluded from the analysis to eliminate false discovery metabolite measurements, and one-way ANOVA was used for statistical significance. All immunoblot densitometry data were normalized to total protein per lane. P < 0.05 was considered as statistically significant.
RESULTS
Surplus ω-6 fatty acid elevated carbohydrate utilization in aging.
To determine the impact of high ω-6 fatty acids intake on energy balance in young and aging C57BL/6J mice, we maintained mice for 2 mo on a LC diet and ω-6-enriched diet using the SO diet (Fig. 1A). To test whether aging superimposed on ω-6 enrichment impacts the metabolic and energetic responses in mice, the metabolic cage system (CLAMS) was used. There was no significant difference observed in the daily food intake in the four groups (Fig. 1B). Aging mice fed with excess of ω-6 displayed an increase in whole body RER. The ratio of 0.9 indicates an increase in carbohydrate utilization during the night (Fig. 1C). Both high ω-6-fed and standard LC diet-fed aging mice showed an age-related increase in fat mass compared with LC diet-fed young mice (P < 0.05). However, there was a significant decrease (~8%) in lean mass in aging mice fed the excess ω-6 diet compared with young mice (Fig. 1D). Thus, high-ω-6 diet-fed aging mice showed a 2% increase in fat mass compared with young mice (Fig. 1E), and the overall results indicated that aging increases carbohydrate utilization with a decrease in lean mass in ω-6 diet-fed mice.
Excess intake of ω-6 in aging mice results in signs of atrioventricular defect.
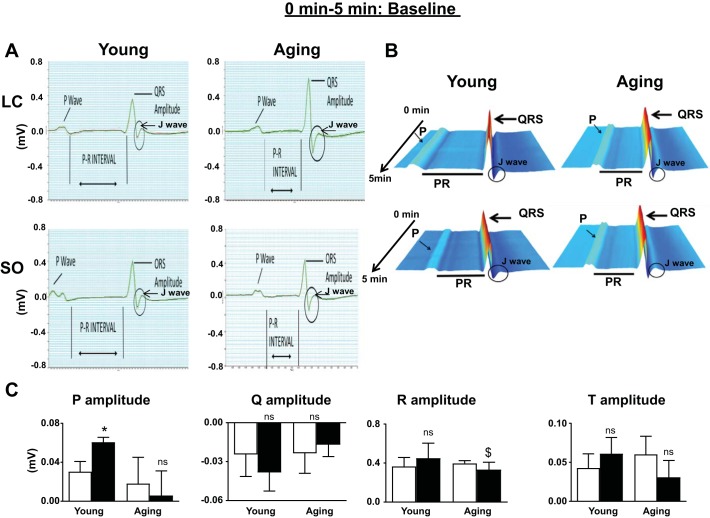
To understand how excess intake of ω-6 fatty acids influences the electrical conductance of the heart, ECGs were acquired before and after 2 mo of the dietary intervention protocol in young and aging mice. ECG recordings showed that the P wave duration and atrioventricular conduction interval (PQ interval) were increased in SO diet-fed aging mice compared with SO diet-fed young mice. There were no changes in ventricular conduction time (QRS complex width). However, QRS amplitude was observed to increase in aging mice, resulting in widening of the J wave. Also, all young mice had a PR interval of between 40 and 42 ms (normal range). Both young and aging mice who were supplemented with an excess ω-6 diet displayed a bifurcation of the P wave (Fig. 2, A and B). ω-6-diet fed mice displayed a further decrease in the PR interval to 35 ms. The P amplitude was increased in young mice fed the ω-6 diet (P < 0.05) but displayed a decreasing trend (P = not significant) in ω-6 diet-fed aging mice (Fig. 2C). Thus, the ECG analyses suggested that excess feeding of ω-6 fatty acid may increase the incidence of atrioventricular defects during aging.
Fig. 2.
ECGs from young and aging mice fed with standard laboratory chow (LC) or excess ω-6 diet. A: ECG analyses displaying the change in QRS amplitude. B: waterfall plots displaying the changes in ECG traces from 0 to 5 min. C: bar graph of P, Q, R, and T amplitude from ECG traces of young and aging mice fed with standard LC or ω-6-enriched diet. ECGs were recorded after stabilizing mice for 5 min at room temperature. Values are means ± SE; n = 8 mice/group. *P < 0.05 vs. the young-LC group; $P < 0.05 vs. the aging-LC group; ns, not significant as analyzed by two-way ANOVA.
Surplus intake of ω-6 fatty acid in aging mice resulted in variability in electrical conductance with dobutamine stress.
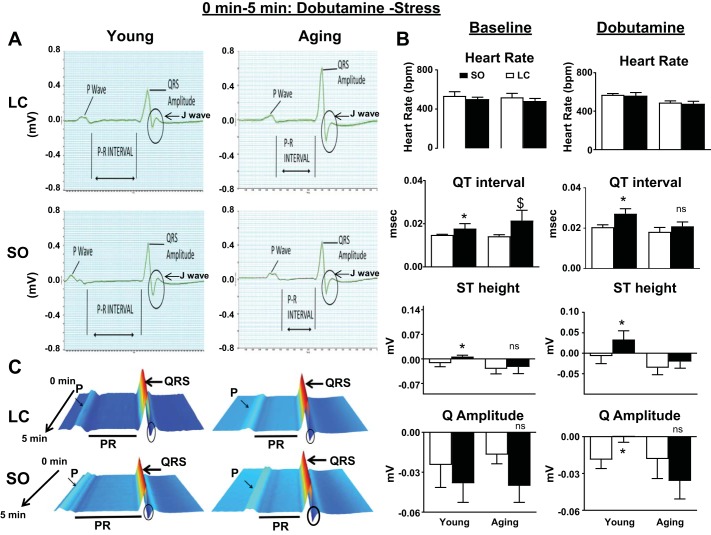
To mimic an exercise-induced stress test in human hearts, we used the pharmacological agent dobutamine and simultaneously recorded ECGs for young and aging mice. There was an immediate increase in heart rate after dobutamine administration. The heart rate reached 600 ± 4 beats/min in all animals during the first 5 min after dobutamine injection (Fig. 3B). Both young and aging mice supplemented with excess ω-6 showed a change in ST height, specifically in young mice (P < 0.5). ECG analyses of dobutamine-injected mice displayed a decrease in PR and QT intervals in both ω-6 diet-fed young and aging mice, indicating prolonged conduction times. This, in turn, indicates an atrioventricular conduction defect compared with the respective standard diet-fed mouse groups, thus suggesting that conduction between atria and ventricles of the heart is impaired. The dobutamine-induced stress test displayed a decreasing trend in the Q amplitude compared with the baseline in ω-6 diet-fed young mice but an increase in aging mice.
Fig. 3.
ECGs from young and aging mice fed with standard laboratory chow (LC) or surplus ω-6 intake subjected to dobutamine stress. A: ECG measurement displaying the change in the increase in the PR interval. B: a bar graph showing heart rate, QT interval, ST height, and Q amplitude of baseline and dobutamine-stressed mice. C: waterfall plots displaying changes in ECG traces from 0 to 5 min after dobutamine injection. ECGs were recorded after acclimatizing mice on the acquisition platform for 5 min at room temperature. Values are means ± SE; n = 8 mice/group. *P < 0.05 vs. the young-LC group; $P < 0.05 vs. the aging-LC group; ns, not significant as analyzed by two-way ANOVA.
Excess ω-6 increased proinflammatory LV gene expression in aging mice.
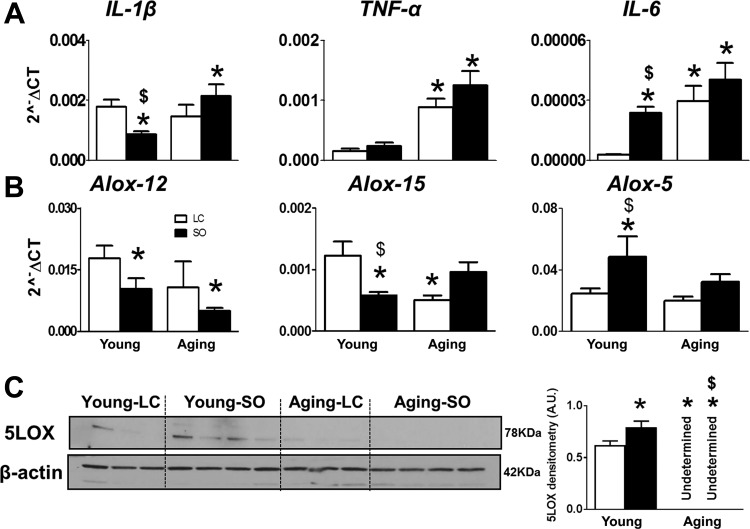
To determine age-related low-grade chronic inflammation, TNF-α, IL-1β, and IL-6 expression was measured in the LV. ω-6 diet-fed young mice had reduced levels of IL-1β, indicating an anti-inflammatory property as the essential nature of ω-6 fatty acids in physiology. In contrast, IL-6 levels were increased, indicating differential effects of the ω-6 diet in young and aging mice. Aging mice fed with standard diet or ω-6 enrichment displayed signs of LV low-grade chronic inflammation with upregulated TNF-α (3.2-fold in the aging-LC group and 4.7-fold in the aging-SO group, P < 0.01) and IL-6 (2.1-fold in the aging-LC group and 4.3-fold in the aging-SO group, P < 0.01) compared with the respective young-LC groups. However, IL-1β was upregulated in aging-SO mice (3.1-fold, P < 0.01) compared with aging-LC mice (Fig. 4A). Thus, despite the essential role of ω-6 fatty acids, excess intake in aging mice leads to low-grade chronic inflammation.
Fig. 4.
Aging and excess ω-6 drives the proinflammatory milieu with increased 15-lipoxygenase (LOX) expression in the left ventricle (LV). A and B: mRNA expression of IL-1β, TNF-α, and IL-6 (A) and arachidonate lipoxygenase (Alox)-12, Alox-15, and Alox-5 (B) in the LV. C: immunoblot analysis and densitometry of 5-LOX in the LV. Values are means ± SE; n = 4 mice/group. *P < 0.05 vs. the young-laboratory chow (LC) group; $P < 0.05, LC vs. safflower oil (SO).
Excess ω-6 decreased LOX expression in LVs of aging mice.
LOXs are lipid-metabolizing enzymes used in the metabolism of ω-6 fatty acid to form eicosanoids and bioactive products required for cellular and molecular actions. ω-6 diet-fed aging mice exhibited a significant decrease in Alox-12 (2.7-fold) compared with LC diet-fed young mice and an increase in Alox-15 (1.7-fold) compared with SO diet-fed young mice. Interestingly, young mice fed on excess of ω-6 fatty acid displayed an increase in Alox-5 expression compared with young mice on a standard LC diet. However, no significant change was observed in mRNA levels of Alox-5 in either LC-fed or ω-6-fed aging mice (Fig. 4B). In contrast, protein levels of 5-LOX were undetectable in aging mice fed the LC diet and ω-6-supplied mice (Fig. 4C). These results indicate that ω-6 feeding widely impacted the levels of LOX enzymes in aging mice, which alters the synthesis of bioactive lipid mediators.
Surplus ω-6 intake in aging increased proinflammatory lipid mediators.
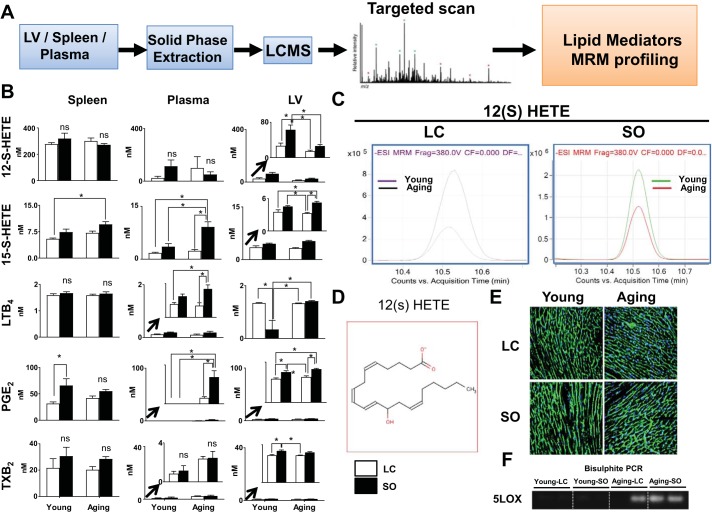
Analyses of LV gene expression for cytokines and LOXs in aging suggested low-grade chronic inflammation in the ω-6-fed group compared with LC diet-fed young mice. Therefore, to confirm the LOX activity in these groups, we comprehensively determined ω-6-derived lipid mediators in the spleen, LV, and plasma. As expected, targeted LC-MS/MS-based lipidomic analysis showed elevated levels of arachidonic acid-derived lipid metabolites in aging mice fed with ω-6 fatty acids (Fig. 5A). Compared with plasma and the LV, the spleen displayed higher levels of lipid mediators, indicating the enrichment of lipid mediators in immune cells. Excess ω-6 fatty acid-fed aging mice displayed high levels of the lipid mediators 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE], LTB4, PGE2, and TXB2 in the spleen, plasma, and LV compared with LC-fed mice (Fig. 5B). Levels of the arachidonic acid metabolite 12(S)-HETE were significantly (P < 0.05) in the LV of mice fed excess ω-6 fatty acid compared with the respective normal chow-fed young and aging mice. However, 12(S)-HETE levels were decreased (P < 0.05) in the LV of the young-LC group compared with the young-SO group. Aging-SO mice showed significantly increased levels of 15(S)-HETE, LTB4, and PGE2 in the LV; moreover, TXB2 was significantly (P < 0.05) increased in young mice after dietary intervention (Fig. 5, B and C). The chemical structure of 12(S)-HETE is shown in Fig. 5D. Furthermore, to determine the effect of aging or ω-6 supplementation on LV structure, we assessed the myocyte cross-sectional area. Wheat germ agglutinin staining showed that myocyte area was not different between the young and aging groups with or without ω-6 supplementation (Fig. 5E). However, supplementation of ω-6 in aging mice resulted in relatively higher thickening of the myocyte outer wall compared with LC diet-fed young mice. Likewise, to determine the effect of aging or ω-6 supplementation on epigenetic modification of LOXs, we performed bisulphite PCR analysis, which revealed the presence of CpGs, indicating methylation of 5-LOX DNA during aging (Fig. 5F). Thus, surplus intake of ω-6 fatty acids in aging mice showed profound diversity in the lipid mediator milieu in aging mice without major alterations in the LV myocardium structure.
Fig. 5.
ω-6 supplementation increases proinflammatory lipid mediators with no structural changes in the left ventricle (LV). A: schematic representation of targeted liquid chromatography-mass spectrometry analyses (LC/MS) analysis. B: quantitative analysis of 12(S)-HETE, 15(S)-HETE, leukotriene B4 (LTB4), PGE2, and thromboxane B2 (TXB2) in young and aging mice fed with standard laboratory chow (LC) or excess ω-6 diet. Red, young-LC group; black, young-safflower oil (SO) group; purple, aging-LC group; green, aging-SO group. C: multiple reaction monitoring (MRM) peaks of 12(S)-HETE in young and aging mice with and without ω-6 supplementation. D: structure of 12(S)-HETE. E: representative wheat germ agglutinin (WGA) membrane and Hoechst nuclear-stained images showing LV cardiomyocytes from young and aging mice fed with LC or ω-6-enriched diets. F: 5-lipoxygenase (5-LOX) methylation in aging by bisulphite PCR. Values are means ± SE; n = 5 mice/group. *P < 0.05 as analyzed by one-way ANOVA.
Excess ω-6 intake impaired nuclear erythroid 2-related factor 2/antioxidant-responsive element signaling in aging.
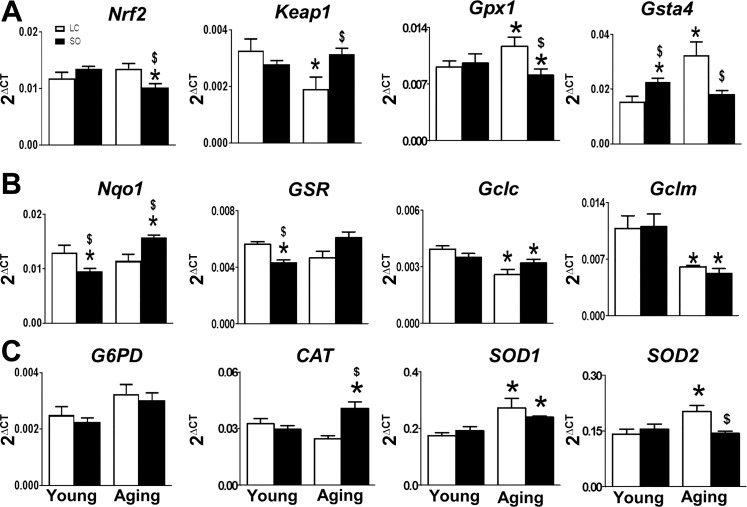
The balance of oxidative and redox stress signaling is essential to maintain the antioxidant-regulating element in aging. In young mice, excess ω-6 acts as an antioxidant and reduces the radical stress indicated by decreased levels of Nqo-1 and Gsr-1. To determine the oxidative and redox balance, we determine the expression of Nrf2/antioxidant-responsive element (ARE)-related genes, e.g., Nrf2, Keap-1, and Nrf2-targeted antioxidant genes, to study the impact of ω-6 surplus intake during aging. There were no obvious changes observed in Nrf2 and Keap-1 mRNA levels between groups, whereas a significant decrease in Nqo-1 (2.2-fold) and GSR (1.8-fold) and an increase Gsta4 (1.7-fold, P < 0.05) gene expression were observed in ω-6 diet-fed young mice (Fig. 6). Nrf2/ARE expression selectively decreased in ω-6 diet-fed aging mice compared with mice fed the LC diet. ω-6 diet-fed aging mice displayed a decrease (0.8-fold) in Nrf2 and an increase in Keap-1 (3.1-fold) gene levels (P < 0.05). This is in contrast to ω-6 diet-fed young mice, where Gclc, Gclm, GPX-1, Cat, G6PD, and SOD1 remained unaltered except for SOD2, which increased (1.8-fold). Aging mice fed the ω-6 diet displayed a significant (P < 0.05) increase in Nqo-1, GSR, G6PD, SOD1, and Cat and decrease in Gclc, Gclm, GPX-1, and Gsta4. These findings suggest that Nrf2/ARE signaling is perturbed in the LV by ω-6-enriched diet during aging, thereby leading to a redox imbalance.
Fig. 6.
Supplementation of excess ω-6-in aging disrupts nuclear erythroid 2-related factor 2 (Nrf2)-mediated antioxidant signaling. A−C: mRNA expression of Nrf2, Kelch-like ECH associated protein 1 (Keap-1), glutathione peroxidase 1 (GPX-1), and glutathione-S-transferase-α4 (Gsta4) (A), NAD(P)H quinone dehydrogenase 1 (Nqo-1), glutathione-disulfide reductase (GSR), glutamate-cysteine ligase catalytic subunit (Gclc), glutamate-cysteine ligase modifier subunit (Gclm) (B); and glucose-6-phosphate dehydrogenase (G6PD), catalase (Cat), superoxide dismutase (SOD)1, and SOD2 (C) in young and aging mice fed with standard laboratory chow (LC) or excess ω-6 diet. Values are means ± SE; n = 4 mice/group. *P < 0.05 vs. the young-LC group; $P < 0.05 LC vs. safflower oil (SO).
DISCUSSION
The present study shows that the surplus intake of ω-6 fatty acids in aging leads to a differential low-grade chronic inflammation and an impaired oxidative-redox balance, which, in turn, leads to early pathological signs in ECG under stress (Fig. 7). In contrast, the excess intake of ω-6 diet in young mice supported maintenance of homeostasis under stress. The short-term surplus intake of ω-6-enriched diet in aging mice showed 1) increased carbohydrate utilization during active time; 2) decreased atrioventricular conduction interval (PR interval); 3) increased levels of proinflammatory lipid mediators 15(S)-HETE, LTB4, PGE2, and TXB2 and cytokines IL-1β, TNF-α, and IL-6; and 4) impaired Nrf2/ARE antioxidant signaling.) Our results suggest that excess intake of ω-6 contributes to functional deficiencies and expansion of the inflammatory mediator milieu during later stages of aging (Fig. 7).
Fig. 7.
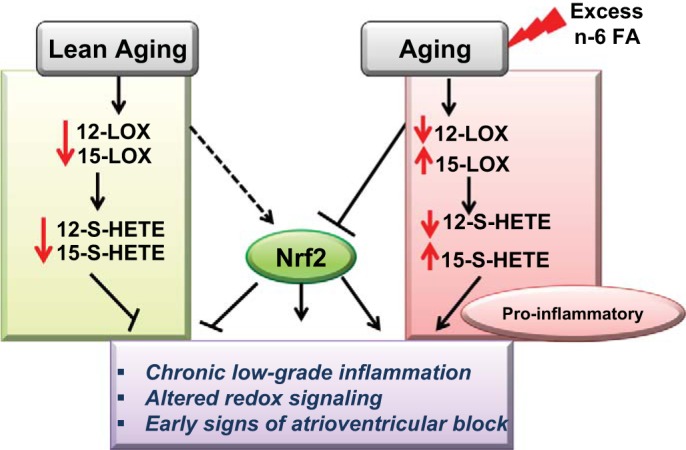
Schematic summary displaying the effect of surplus intake of ω-6 leading to chronic inflammation, ECG defects, and dysregulated oxidative-redox balance in aging with marked perturbation in nuclear erythroid 2-related factor 2/antioxidant-responsive element (Nrf2/ARE) signaling. FA, fatty acid.
The intake of a diet containing balanced ω-3 and ω-6 fatty acids is important for maintaining the integrity of the cell membrane and molecular signaling (6). The human and rodent lipidome is established through internal (LOX regulation) and external (diet and environment) factors. Although both ω-3 and ω-6 are polyunsaturated fatty acids, they have a unique conformation and breakdown to specific eicosanoids or bioactive lipid species depending on the physiological and pathological setting (4). The ability of the body to use or metabolize dietary fat is a critical determinant for homeostatic energy balance and regulation (8, 35). Previous reports have indicated that long-term exposure of ω-6 fatty acids in aging impaired neutrophil clearance after myocardial infarction, with signatures of nonresolving inflammation as the major hallmark of chronic inflammation (19, 25). Since impaired bioenergetics lead to defective metabolic mechanism and altered physiological response, the way in which aging impacts heart energy utilization is unknown. Here, we demonstrated the systemic age-related calorimetric food intake and RER to dissect energy utilization in young versus aging. ω-6 fatty acid-fed aging mice showed an increased utilization of carbohydrates without direct impact in total food intake. The presented outcome is also supported by previous studies that showed an impairment in energy intake and dysregulation of the β3-adrenergic receptor, leptin, food intake, and adiposity in aging in humans (5, 16). Thus, a decrease in fat oxidation with no change in net food intake may be one of the factors associated with impaired energy metabolism leading to multifactorial chronic disease in aging. LV function is influenced primarily by aging, with a decrease in elasticity and the ability to respond to changes in pressure of the arterial system. Some studies have reported that aging mice showed increase in wall thickness with no change in function (17, 18, 28). Anversa et al. (1) reported that age-related cardiac hypertrophy is a compensatory mechanism in aging rats. As the aging population is prone to defective electrophysiological properties or LV function, it is essential to delineate the mechanism of the early predisposition to developing heart diseases (22). However, there is no evidence for any functional defects or increases in the myocyte cross-sectional area in young versus aging mice with ω-6 enrichment compared with standard diet-fed mice. Aging mice are prone to display a change in electrical conductance, e.g., atrial fibrillation and atrioventricular block (20), and ECG analysis showed that aging mice supplemented with ω-6 displayed an increased PR interval (>0.20 ms), displayed signs of first degree atrioventricular defects, and simultaneously sustained the QRS complex and prevented cardiac hypertrophy. The pharmacological exercise stress test (dobutamine) in aging mice with ω-6 supplementation increased in R amplitude, which is also a sign of ischemic myocardium in humans, and shortened QT interval, a parameter known to cause sudden death (29). Recent studies have shown that the resident macrophages, which are present in abundance in the distal atrioventricular node, contribute to cardiac conduction. The macrophage ablation induces progressive atrioventricular defects, indicating that the inflammatory response can alter electrical conductance (12). The present results support the evidence of high-fat diet-induced alterations of atrial electrical activities in mice (38). In addition, recent studies have suggested both immune cells as well as a high-fat diet lead to the change in electrical conductance (12). The present study suggests that the aging might contribute to the impact of high-fat-diet intake on electrical conductivity and thereby ECG dysfunction.
Aging is characterized by progressive defects in the metabolic signaling of the immune system, resulting in a greater susceptibility to pathology as a consequence of inflammation (34, 36). This phenomenon of chronic, low-grade inflammation is termed “inflammaging.” The present results suggest that surplus intake of ω-6 superimposed on aging lead to chronic inflammation, but not in young mice. To support the chronic inflammation outcome in aging mice, our previous results suggest that chronic enrichment of ω-6 reduced 5-LOX levels, thereby magnifying the postmyocardial infarction inflammatory response in aging mice (19). Human data indicate that comprehensive measurement of lipids is crucial, instead of mere measurements of total triglycerides and total cholesterol obtained from classical blood biochemistry. This was endorsed by Valsesia et al. (33) using specific lipid signature of low-caloric diet in obese, nondiabetic patients. Furthermore, specific lipid mediator changes during an 8-wk low-caloric diet helped in predicting insulin-resistant patients after 6 mo of weight maintenance. This suggests that lipid mediators are important indicators in predicting the disease state and metabolism. ω-6-generated lipid mediators are biologically active, and these “eicosanoids” or bioactive lipid mediators are involved in various pathological processes of inflammatory conditions such as atherosclerosis, obesity, and myocardial infarction (6, 19). 12(S)-HETE and LTB4 play an important role in pathological processes, leading to increasing in prominent inflammatory cytokines such as TNF-α, IL-6, and IL-1β that were increased with age in ω-6-fed mice. The LV in aging mice showed a robust decrease in 5-LOX lipid-metabolizing enzyme, which further regulates the inflammatory response, in mice with and without ω-6-supplementation, indicating a change in the lipid mediator response. Dysregulation of 5-LOX is associated with an increase in methylation on the promoter of the CpG island (methylation) of 5-LOX DNA during aging. Decreased expression of 5-LOX is regulated by epigenetic modifications of DNA methylation in the 5-LOX promoter (27). Epigenetic mechanisms play a significant role in rodent and human aging (24, 31). Thus, the present study provides evidence that the chronic-low grade inflammation accelerated in aging with enrichment of ω-6 diet may be related to epigenetic changes in 5-LOX or other lipid-metabolizing enzymes.
Impaired redox signaling is one of the major phenomena of the antioxidant regulating system and one of the prime contributors of age-related cardiac pathologies. Oxidative and antioxidative balance is essential for maintaining cellular redox homeostasis (26). It has been reported that the antioxidant system progressively declines with aging. Nrf2/ARE signaling regulates the antioxidant defense machinery at the transcriptional level, and excess ω-6 has been shown to diminish Nrf2/ARE binding activity in the heart (10). Miller et al. (21) demonstrated that Nrf2 knockout mice exhibit decreased expression of antioxidant genes. In contrast to previous reports, we have not seen any decrease in Nrf2 levels in aging mice at 18 mo of age. The possible reason may be that in previous studies mice were senescent (24 mo old) and both male and female mice were used. Nrf2 contributes to the anti-inflammatory process, and aging mice with high ω-6 intake displayed a decrease in Nrf2 levels. Therefore, decreased levels of Nrf2, GPX-1, Gclc, Gclm, and Gsta4 in ω-6-supplemented aging mice magnified the inflammaging mechanism.
In conclusion, our study illustrated changes in metabolic defects due to the surplus intake of ω-6 in both young and aging mice. ω-6 fatty acids appear to be essential in dose ranges of 4–5% to maintain homeostatic heart health at a young age. However, excessive intake leads to proinflammation in aging mice with prominent increases in proinflammatory lipid mediators, which, in turn, leads to the development of chronic low-grade inflammation. Thus, to maintain cardiovascular health, the expression and flux capacity of fatty acid-metabolizing LOX enzymes and the oxidative/redox balance are essential to delay ECG pathological defects in aging.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants AT-006704 and HL-132989 (G. V. Halade), analytical support from the Michigan Regional Comprehensive Metabolomics Resource Core (NIH Grant DK-097153), American Heart Association Postdoctoral Fellowship POST31000008 (V. Kain), and NIH Grant HL-118067 (N. S. Rajasekaran).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.V.H. conceived and designed research; V.K., K.A.I., M.K., H.B., G.S., N.S.R., M.E.Y., and G.V.H. performed experiments; V.K., K.A.I., and G.V.H. interpreted results of experiments; V.K. and G.V.H. prepared figures; V.K. and G.V.H. drafted manuscript; G.V.H. edited and revised manuscript; G.V.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the help of Alondria McNair for echocardiogram analyses.
REFERENCES
- 1.Anversa P, Hiler B, Ricci R, Guideri G, Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. J Am Coll Cardiol 8: 1441–1448, 1986. doi: 10.1016/S0735-1097(86)80321-7. [DOI] [PubMed] [Google Scholar]
- 2.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6-fatty acids in the United States during the 20th century. Am J Clin Nutr 93: 950–962, 2011. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes 37: 843–852, 2013. doi: 10.1038/ijo.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients 2: 355–374, 2010. doi: 10.3390/nu2030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das SK, Moriguti JC, McCrory MA, Saltzman E, Mosunic C, Greenberg AS, Roberts SB. An underfeeding study in healthy men and women provides further evidence of impaired regulation of energy expenditure in old age. J Nutr 131: 1833–1838, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J 1: 420–439, 2006. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- 7.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, Fretts AM, Guallar E, Matsumoto C, Prem K, Tanaka T, Wu JH, Zhou X, Helmer C, Ingelsson E, Yuan JM, Barberger-Gateau P, Campos H, Chaves PH, Djoussé L, Giles GG, Gómez-Aracena J, Hodge AM, Hu FB, Jansson JH, Johansson I, Khaw KT, Koh WP, Lemaitre RN, Lind L, Luben RN, Rimm EB, Risérus U, Samieri C, Franks PW, Siscovick DS, Stampfer M, Steffen LM, Steffen BT, Tsai MY, van Dam RM, Voutilainen S, Willett WC, Woodward M, Mozaffarian D; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) . ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 176: 1155–1166, 2016. doi: 10.1001/jamainternmed.2016.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flatt JP. Dietary fat, carbohydrate balance, and weight maintenance: effects of exercise. Am J Clin Nutr 45, Suppl: 296–306, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69, Suppl 1: S4–S9, 2014. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 10.Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KJ, Odelberg SJ, Firpo MA, Paine R 3rd, Hoidal JR, Abel ED, Rajasekaran NS. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One 7: e45697, 2012. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 12.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M. Macrophages facilitate electrical conduction in the heart. Cell 169: 510–522.e20, 2017. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imbesi M, Dzitoyeva S, Ng LW, Manev H. 5-Lipoxygenase and epigenetic DNA methylation in aging cultures of cerebellar granule cells. Neuroscience 164: 1531–1537, 2009. doi: 10.1016/j.neuroscience.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 84: 24–35, 2015. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar MV, Moore RL, Scarpace PJ. Beta3-adrenergic regulation of leptin, food intake, and adiposity is impaired with age. Pflugers Arch 438: 681–688, 1999. [PubMed] [Google Scholar]
- 17.Lin J, Lopez EF, Jin Y, Van Remmen H, Bauch T, Han HC, Lindsey ML. Age-related cardiac muscle sarcopenia: Combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol 43: 296–306, 2008. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res 66: 410–419, 2005. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol 308: H269–H280, 2015. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merentie M, Lipponen JA, Hedman M, Hedman A, Hartikainen J, Huusko J, Lottonen-Raikaslehto L, Parviainen V, Laidinen S, Karjalainen PA, Ylä-Herttuala S. Mouse ECG findings in aging, with conduction system affecting drugs and in cardiac pathologies: Development and validation of ECG analysis algorithm in mice. Physiol Rep 3: 3, 2015. doi: 10.14814/phy2.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller CJ, Gounder SS, Kannan S, Goutam K, Muthusamy VR, Firpo MA, Symons JD, Paine R 3rd, Hoidal JR, Rajasekaran NS. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim Biophys Acta 1822: 1038–1050, 2012. doi: 10.1016/j.bbadis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Executive Summary: Heart Disease and Stroke Statistics–2016 update: a report from the American Heart Association. Circulation 133: 447–454, 2016. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 23.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 110: 1097–1108, 2012. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal S, Tyler JK. Epigenetics and aging. Sci Adv 2: e1600584, 2016. doi: 10.1126/sciadv.1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6-polyunsaturated Fatty acids. J Nutr Metab 2012: 539426, 2012. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poljsak B. Suput D, Milisav I. Achieving the balance between ros and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013: 956792, 2013. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci 32: 332–341, 2007. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Rosa EF, Silva AC, Ihara SS, Mora OA, Aboulafia J, Nouailhetas VL. Habitual exercise program protects murine intestinal, skeletal, and cardiac muscles against aging. J Appl Physiol 99: 1569–1575, 2005. doi: 10.1152/japplphysiol.00417.2005. [DOI] [PubMed] [Google Scholar]
- 29.Schimpf R, Veltmann C, Papavassiliu T, Rudic B, Göksu T, Kuschyk J, Wolpert C, Antzelevitch C, Ebner A, Borggrefe M, Brandt C. Drug-induced QT-interval shortening following antiepileptic treatment with oral rufinamide. Heart Rhythm 9: 776–781, 2012. doi: 10.1016/j.hrthm.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 25: 101–137, 2007. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 31.Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One 2: e895, 2007. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 8: 128, 2016. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valsesia A, Saris WH, Astrup A, Hager J, Masoodi M. Distinct lipid profiles predict improved glycemic control in obese, nondiabetic patients after a low-caloric diet intervention: the Diet, Obesity and Genes randomized trial. Am J Clin Nutr 104: 566–575, 2016. doi: 10.3945/ajcn.116.137646. [DOI] [PubMed] [Google Scholar]
- 34.Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev 128: 83–91, 2007. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Vnotchenko SL, Aleksandrova GF, Mktrumova NA. [Indicators of the T- and B-systems of immunity in patients with diffuse toxic goiter]. Probl Endokrinol (Mosk) 29: 23–27, 1983. [PubMed] [Google Scholar]
- 36.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int 22: 1041–1050, 2009. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Xia S, Kalionis B, Wan W, Sun T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed Res Int 2014: 615312, 2014. doi: 10.1155/2014/615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F, Hartnett S, Sample A, Schnack S, Li Y. High fat diet induced alterations of atrial electrical activities in mice. Am J Cardiovasc Dis 6: 1–9, 2016. [PMC free article] [PubMed] [Google Scholar]