Abstract
Estrogen has been shown to affect vascular reactivity. Here, we assessed the estrogen receptor-α (ERα) dependency of estrogenic effects on vasorelaxation via a rapid nongenomic pathway in both male and ovary-intact female mice. We compared the effect of a primary estrogen, 17β-estradiol (E2) or 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT; selective ERα agonist). We found that E2 and PPT induced greater aortic relaxation in female mice than in male mice, indicating ERα mediation, which was further validated by using ERα antagonism. Treatment with 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP dihydrochloride; ERα antagonist) attenuated PPT-mediated vessel relaxation in both sexes. ERα-mediated vessel relaxation was further validated by the absence of significant PPT-mediated relaxation in aortas isolated from ERα knockout mice. Treatment with a specific ERK inhibitor, PD-98059, reduced E2-induced vessel relaxation in both sexes but to a lesser extent in female mice. Furthermore, PD-98059 prevented PPT-induced vessel relaxation in both sexes. Both E2 and PPT treatment activated ERK as early as 5–10 min, which was attenuated by PD-98059 in aortic tissue, cultured primary vascular smooth muscle cells (VSMCs), and endothelial cells (ECs). Aortic rings denuded of endothelium showed no differences in vessel relaxation after E2 or PPT treatment, implicating a role of ECs in the observed sex differences. Here, our results are unique to show estrogen-stimulated rapid ERα signaling mediated by ERK activation in aortic tissue, as well as VSMCs and ECs in vitro, in regulating vascular function by using side-by-side comparisons in male and ovary-intact female mice in response to E2 or PPT.
NEW & NOTEWORTHY Here, we assessed the estrogen receptor-α dependency of estrogenic effects in vasorelaxation of both male and ovary-intact female mice by performing side-by-side comparisons. Also, we describe the connection between estrogen-stimulated rapid estrogen receptor-α signaling and downstream ERK activation in regulating vascular function in male and ovary-intact female mice.
Keywords: estrogen, estrogen receptor-α, extracellular signal-regulated kinase, vascular function, endothelial cells
INTRODUCTION
The incidence of cardiovascular disease (CVD) is lower in women with premenopausal status compared with age-matched men (17, 49). Furthermore, after menopause, the risk of CVD for women are nearly equal to men 10 yr later in life (17). The decreased prevalence of CVD has been attributed to endogenous estrogen present in premenopausal women (17, 49). Although the impact of estrogen replacement therapy on treating cardiovascular diseases is controversial from key clinical trials (28, 40), it is clear that estrogen has significant effects on vascular physiology in health and disease. These findings highlight the need for additional research into the roles estrogen and its receptors play in the cardiovascular system and in what ways these roles are sex specific (30, 44).
The distinct contributions of estrogen receptors (ERs) in regulating vascular function, and the sex differences involved in each of their roles, have become the subject of increasing interest. The two classic ER subtypes, ERα and ERβ, have been identified as direct mediators of 17β-estradiol (E2) action in endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) (18–20, 22, 26, 48). Also, the more recently characterized G protein-coupled estrogen receptor (GPER) has been shown to have functional activity in ECs and VSMCs (14, 16, 25, 32, 42).
ERs can exert their influence by two different mechanisms: genomic signaling and rapid, acute nongenomic signaling (30). In addition to the classical genomic signaling that directly regulates gene transcription, studies have demonstrated the importance of rapid, nonnuclear signaling that is facilitated by estrogen binding to ERs (1, 31, 46, 47, 50). A role of rapid ER signaling has been identified in mediating vascular gene regulatory responses to E2 (1). Furthermore, rapid ER signaling pathways may be required for eliciting several protective effects of E2 on vascular cells and tissues (1). ERα has been shown from previous studies using knockout mice to be necessary for E2-induced protection in response to vascular injury by attenuating smooth muscle cell proliferation, medial layer thickening, and fibrosis (4, 18, 36).
The role of rapid ERα signaling pathways has recently become an important cornerstone for understanding the preventive effects of E2 in vascular pathogenesis (1, 5). For example, when rapid ER signaling is disrupted, the ability of E2 to inhibit VSMC proliferation is compromised (1). Rapid ERα signaling is also important for the effects of E2 on ECs, including reendothelialization and decreased vascular inflammation (27). The physiologically relevant roles of rapid ERα estrogen signaling in blood vessels include the regulation of vascular injury (1, 5), endothelial healing in atherosclerosis (2), and acute activation of endothelial nitric oxide synthase (eNOS) for vascular reactivity (7). Previous findings have demonstrated that activation of ERs by E2 rapidly initiates mitogen-activated protein kinase (MAPK) signaling in ECs (7, 13), and inhibition of the MAPK pathway prevents E2 activation of eNOS (7). ERα located at the cell membrane triggers rapid MAPK and phosphatidylinositol 3-kinase activation in E2-exposed arteries of ovariectomized female mice (13).
ERα and ERβ can act as mediators of the vasorelaxatory effects of estrogen (8, 13, 43, 51, 53). In addition, GPER has been shown to influence many rapid biological responses to estrogen (39), including vasodilation and improved vascular function (23–25, 32). However, there have been few studies that have investigated the molecular mechanisms of rapid ERα signaling that contribute to estrogen-induced vascular relaxation. We hypothesized that ERK as a mediator of ERα signaling plays a critical role in E2-regulated vascular tone. We also aimed to identify the impact of rapid ERα signaling in possible sex differences in vascular reactivity by comparing E2- and ERα-specific agonist 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT)-induced relaxation of precontracted aortic rings isolated from male and female C57BL/6J or ERα knockout (KO) mice.
From January 2016, National Institutes of Health (NIH) grant applications now require investigators studying vertebrate animals to explain how their study design will account for sex as a biological variable. Our side-by-side comparison of vessel tone and ERK activation in males and females throughout the study is novel. Greater understanding of rapid ERα signaling pathways for vascular protection and regulating proper vasoreactivity may lead to new drug targets for treating vascular injury and arteriosclerotic diseases associated with impaired vascular function in both men and women.
METHODS
Animals.
C57BL/6J and ERα KO mice were purchased from The Jackson Laboratory. Mice were kept at the Tulane University School of Medicine vivarium. Mice were maintained on a normal diet with access to food and water ad libitum. The animal care and experiments were approved by the Tulane University Institutional Animal Care and Use Committee and were in accordance with NIH guidelines.
Preparation of mouse aortas.
Twelve-week-old male and female C57BL/6J mice were used throughout the study except for one vessel tension experiment that used ERα KO animals. Mice were euthanized by deep anesthesia with a ketamine (100–200 mg/kg) and xylazine (5–16 mg/kg) combination injected intraperitoneally based on body weight. Under a dissecting microscope, thoracic aortas were excised and fat and connective tissue were removed and cut transversely into rings (3–4 mm in length) with extreme care to preserve the endothelium. For experiments assessing vessel tone without an intact endothelium, ECs were denuded from aortas before immersion into Krebs-Henseleit (KH) buffer.
Vessel tension measurements using an organ bath.
Aortic rings were mounted vertically by sliding them into two wire hooks. Aortic rings were immersed in 2 ml KH buffer. KH buffer contained the following (in mM): 118 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose, and 2.5 CaCl2. pH of the buffer was adjusted and maintained at 7.4 with 95% O2-5% CO2 at 37°C. Aortic rings were equilibrated for 90 min with a resting tension of 1 g. After baseline tension was determined, vessels were precontracted with phenylephrine (PE; 10−6 M) followed by administration of acetylcholine (ACh), an indicator of endothelium-dependent relaxation. To determine sex differences in estrogen-induced vessel relaxation and the role of ERα signaling, aortas were stimulated with increasing concentrations of E2 or the specific ERα agonist PPT (10−12−10−5 M) through cumulative addition after a supramaximal response to PE and transient relaxation by ACh. At each E2 and PPT concentration, changes in vessel tension were time dependently monitored (15 min each) and tension was recorded through the AD Instruments data-acquisition system (Colorado Springs, CO). For experiments examining ERα signaling and the potential downstream target ERK in vasorelaxation, aortic rings were pretreated with the ERα antagonist 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole (MPP) dihydrochloride (3 nM) or the ERK inhibitor PD-98059 (10 µM) before stimulation with E2 or PPT.
Preparation of primary aortic VSMCs and immunofluorescence.
For isolation of aortic VSMCs, thoracic aortas were removed after gentle perfusion with PBS (GIBCO, Grand Island, NY) plus heparin (1,000 U/ml) and transferred to plates containing fresh 20% FBS-DMEM-F-12 with heparin. Adventitial fat and connective tissue were removed using collagenase (type II, LS-004176, Worthington Biochemical, Lakewood, NJ) under a dissecting microscope. Isolated male and female mouse thoracic aortas were surgically opened along the longitudinal axis. The endothelial layer was removed by gently rubbing the inside of the vessel. Aortic tissue was transferred into fresh media containing DMEM-nutrient mixture F-12 without phenol red (DMEM-F-12; no. 21041-025, GIBCO), 10% charcoal-stripped FBS (no. 12676011, GIBCO), 1% (vol/vol) penicillin-streptomycin (no. 15070063, GIBCO), collagenase (374 U/mg, LS-004176, Worthington Biochemical), and elastase (6 U/mg, LS-002279, Worthington Biochemical) and incubated at 37°C (5% CO2) overnight. Cells were concentrated by centrifugation at 1,500 rpm for 2 min in a table top centrifuge. VSMCs were collected after centrifugation and transferred to a cell culture plate with fresh media [DMEM-F-12 (no phenol red) with 10% charcoal-stripped FBS, 2 mM l-glutamine, 25 mM HEPES, 100 μg/ml heparin, and 100 μg/ml ECGS]; 2 × 106 cells were used for experiments. Early passage (passages 3–7) VSMCs were used for the experiments. In certain experiments, VSMCs were preincubated with 10 µM of the ERK inhibitor for 30 min before treatment with E2 or PPT. E2- or PPT-stimulated (1 µM) VSMCs were harvested at various time points (0, 5, 10, 30, 60, and 120 min). For immunocytochemistry, VSMCs were plated on Laboratory-Tek chamber slides (Nunc, Grand Island, NY), washed twice with PBS, and then fixed with phosphate-buffered 4% paraformaldehyde for 15 min. Nonspecific binding was blocked by incubation in 5% goat serum in PBS (pH 7.4) containing 0.1% Tween 20 and 0.3% Triton X-100. Slides were then incubated with α-smooth muscle actin FITC-conjugated antibody (F-3777, Sigma-Aldrich, St. Louis, MO, 1:500,) (15). Images were captured with Olympus TH4-100 External Power Supply (Olympus, Tokyo, Japan) and Olympus IX73 Fluorescence Microscope (Olympus).
Preparation of primary aortic ECs and immunofluorescence.
For collection of aortic ECs, thoracic aortas were removed after gentle perfusion with PBS (GIBCO) plus heparin (1,000 U/ml) and transferred to plates containing fresh 20% FBS-DMEM-F-12 with heparin. Adventitial fat and connective tissue were removed using collagenase under a dissecting microscope. To collect ECs from isolated male and female thoracic aortas, a 24-gauge cannula was inserted into the proximal portion of the aorta (21). After ligation at the site with a silk thread, the inside of the lumen was washed with serum-free DMEM-F-12 without phenol red. Aortas with collagenase type II solution (2 mg/ml, dissolved in serum-free DMEM-F-12) were incubated for 45 min at 37°C. ECs were removed from the aorta by flushing with 5 ml DMEM-F-12 containing 20% FBS and cultured with growth media [20% FBS, 1% (vol/vol) penicillin-streptomycin, 2 mM l-glutamine, 25 mM HEPES, 100 μg/ml heparin, 100 μg/ml ECGS, and DMEM-F-12 without phenol red] on 0.1% gelatin-coated dishes. ECs were collected by centrifugation at 1,500 rpm for 2 min using a table top centrifuge; 2 × 106 cells were used for experiments. Early passage (passages 2–5) ECs were used for experiments and maintained in DMEM-F-12 (no phenol red) with 10% charcoal-stripped FBS, 2 mM l-glutamine, 25 mM HEPES, 100 μg/ml heparin, and 100 μg/ml ECGS. In certain experiments, ECs were preincubated for 30 min with 10 µM of the ERK inhibitor before treatment with E2 or PPT. E2- or PPT-stimulated (1 µM) ECs were harvested at various time points (0, 5, 10, 15, 30, 60, and 120 min). For ECs, active uptake of Dil-labeled acetylated low-density lipoprotein (LDL) was performed with the Dil-Ac-LDL uptake assay (022K, Cell Applications, San Diego, CA). Images were captured with Olympus TH4-100 External Power Supply (Olympus) and Olympus IX73 Fluorescence Microscope (Olympus).
Western blot analysis.
E2- or PPT-stimulated thoracic aortas were collected at various time points (0, 5, 10, 30, 60, and 120 min) and homogenized in lysis buffer. In certain experiments, aortas were treated for 30 min with 10 µM of the ERK inhibitor before treatment with 1 µM E2 or PPT. Proteins from aortic tissue, VSMCs, or ECs were extracted by sonication in RIPA lysis buffer (Santa Cruz Biotechnology, Dallas, TX) by the Branson Sonifier 250 (Emerson Industrial Automation, Danbury, CT). Protein concentrations in tissues and cells were determined by the Protein Assay Dye Reagent Concentrate (no. 500-0006, Bio-Rad, Hercules, CA). Equal amounts of protein were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes (GE Healthcare, Pittsburgh, PA). Membranes were washed with Tris-buffered saline containing Tween 20 (TBST) and blocked in TBST-5% dry milk or TBST-3% BSA at room temperature for 1 h with shaking. After the blocking buffer, each membrane was washed three times for 10 min with TBST and then incubated with diluted primary antibody overnight at 4°C with shaking. Blots were incubated with antibodies against ERα (ab37438, Abcam, Cambridge, MA, 1:3,000) (45), phospho-ERK1/2 (no. 9101, Cell Signaling, Boston, MA, 1:4,000) (6), ERK1/2 (no. 9102, Cell Signaling, 1:4,000) (6), β-actin (A5441, Sigma-Aldrich, 1:5,000) (33), GAPDH (PA1-987, ThermoFisher Scientific, Waltham, MA, 1:5,000) (3), and β-tubulin (RB-9249-P0, ThermoFisher Scientific, 1:5,000) (9). After overnight incubation, each membrane was washed three times for 10 min with TBST and incubated with species-specific horseradish peroxidase-conjugated secondary antibody at room temperature for 2 h with shaking. Membranes were then washed three times for 10 min with TBST, and blots were treated with an enhanced chemiluminescence detection reagent. Pierce ECL 2 Western Blotting Substrate (no. 32132, ThermoFisher Scientific) and SuperSignal West Pico Chemiluminescent Substrate (no. 34080, ThermoFisher Scientific) were used as chemiluminescence detection systems. Films were scanned on a HP Scanjet G4050 scanner (HP, Palo Alto, CA). Bands were analyzed using ImageJ software (NIH) for densitometry. ERα expression was normalized to GAPDH. The level of phosphorylated ERK1/2 was normalized to that of total ERK1/2.
Drugs.
(17β)-Estra-1,3,5(10)-triene-3,17-diol (E2), ERα agonist PPT, ERα antagonist MPP dihydrochloride, and ERK 1/2 inhibitor PD-98059 were purchased from Tocris Bioscience (Minneapolis, MN). R-(−)-phenylephrine hydrochloride (PE) and ACh were purchased from Sigma-Aldrich. E2, PPT, MPP, and PD-98059 were dissolved in DMSO (ThermoFisher Scientific) vehicle. The final concentration of DMSO vehicle was 0.05% in the organ bath chamber with no effect by itself on the aortic rings.
Data and statistical analysis.
Concentration-response curves for muscle relaxation were constructed by cumulative addition of the agonist in the organ bath. Data were analyzed by GraphPad Prism statistical software (version 5.0, GraphPad Software, La Jolla, CA). Statistical significance between means was determined using two-way ANOVA and Bonferroni posttests. P < 0.05 was considered as significant. Western blot experiments were also analyzed by GraphPad Prism statistical software. Statistical significance was determined by one-way ANOVA and Bonferroni’s comparison test for unpaired data. P < 0.05 was considered as significant. All values are presented as means ± SE; n is the number of animals or experiments.
RESULTS
E2 stimulation more effectively relaxes aortic rings from female mice.
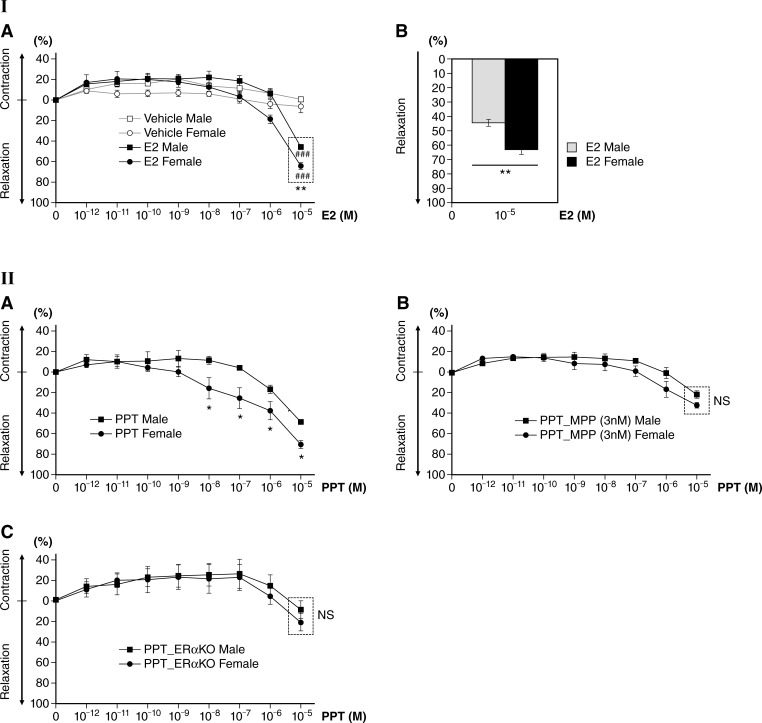
Estrogen has been shown to affect vascular tone. First, we examined the vessel tension of isolated aortic rings from male and ovary-intact female mice in response to E2 exposure in the organ bath system, as described above in detail in methods. We first determined baseline tension by equilibration for 90 min with a resting tension of 1 g and then precontracted the vessels with 10−6 M PE. To study the effects of E2 on vessel tension, we stimulated aortas with increasing concentrations of E2 (10−12−10−5 M) through cumulative addition after a supramaximal response to PE (PE-induced precontraction) and transient relaxation by ACh. At each E2 concentration, changes in vessel tension were measured time dependently (15 min each). E2 was titrated into the organ bath, and relaxation of the rings was measured as a function of E2 concentration. Under these conditions, E2 administration caused rapid vessel relaxation at 10−5 M in both sexes compared with vehicle controls (DMSO only) of the same sex (P < 0.001, E2 vs. vehicle; Fig. 1I,A). However, E2-stimulated female aortic rings (62.95 ± 3.27%) showed significantly greater relaxation than those of male aortic rings (44.44 ± 2.12%; P < 0.01, male vs. female aortic rings; Fig. 1I,A and B).
Fig. 1.
Dose-dependent effect of 17β-estradiol (E2) on male and female mouse aortic relaxation. I,A: percent relaxation after E2 (10−12−10−5 M) treatment. E2 significantly increased vessel relaxation at 10−5 M in both male and female aortic rings compared with vehicle controls. E2-induced relaxation was greater in female aortic rings (62.95 ± 3.27%) than those of male aortic rings (44.44 ± 2.12%) at 10−5 M, showing sex differences. Vehicle controls: n = 3 for both male and female mice; E2 treatment: n = 5 for both male and female mice. ###P < 0.001, E2 treatment vs. vehicle controls of the same sex; **P < 0.01, E2-treated female vs. male mice at 10−5 M. I,B: bar graph of I,A at 10−5 M E2. Percent differences in relaxation are shown between female and male aortic rings treated with E2 at 10−5 M. **P < 0.01, E2-treated female vs. male mice (n = 5 per sex). II,A: dose-dependent effect of the estrogen receptor-α (ERα) agonist 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT) on male and female mouse aortic relaxation; percent relaxation after PPT (ERα agonist, 10−12−10−5 M) treatment is shown. PPT-induced relaxation was greater in female than male aortic rings (n = 4 per sex, *P < 0.05, PPT-treated female vs. male mice at 10−8−10−5 M). II,B: dose-dependent effect of the ERα antagonist 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole (MPP) on male and female mouse aortic relaxation; preincubation of aortas with the ERα antagonist MPP (3 nM) for 30 min greatly attenuated the effect of PPT-induced vessel relaxation, and there was no significant difference in vessel tension between male and female mice (n = 3 per sex in each treatment group). II,C: aortic rings from ERα knockout (KO) mice did not show vessel relaxation after PPT treatment, and there was no significant difference in vessel tension between male and female mice (n = 3 per sex). Data are expressed as means ± SE.
ERα-specific agonist PPT stimulation more effectively relaxes aortic rings from female mice.
Estrogens influence gene expression, cell signaling, and function in target cells and tissue through activation of ERs (11). There is considerable evidence implicating a role of ERα in vascular function (19, 48). To focus on estrogenic effects mediated through ERα, we compared isometric force responses of PE-induced, precontracted aortic rings from both male and female mice to the ERα-specific agonist PPT (10−12−10−5 M). Similar to the E2-exposed aortas shown in Fig. 1I,A and B, female aortic rings showed greater relaxation than those of male aortic rings in response to PPT (P < 0.05; Fig. 1II,A). To further validate the sex differences in PPT-induced vessel relaxation, we next examined the effect of a highly specific ERα antagonist, MPP. When aortic rings were pretreated with 3 nM MPP (Ki for ERα), aortic relaxation was only obvious at 10−5 M PPT and the sex difference was abolished (Fig. 1II,B). These results affirm sex differences in estrogen-stimulated ERα signaling in vasorelaxation.
PPT-induced vessel relaxation in mouse aortic rings is negligible in male and female ERα KO mice.
To further validate ERα signaling on the regulation of vasomotor function, we used aortic rings from ERα KO mice. When we administered PPT at concentrations of 10−12−10−6 M to PE-precontracted aortic rings from ERα KO mice, vessel relaxation was absent in both male and female aortic rings. Although there was minimal relaxation of vessels at 10−5 M PPT, there were no significant differences between male and female mice (Fig. 1II,C). These data, along with selective pharmacological inhibition of the ERα pathway by MPP, clearly demonstrate ERα-mediated stimulation of vessel relaxation where PPT-induced relaxation of mouse aortas exerts greater relaxation in female vessels compared with male vessels.
ERK inhibitor PD-98059 decreases E2- and PPT-induced vessel relaxation.
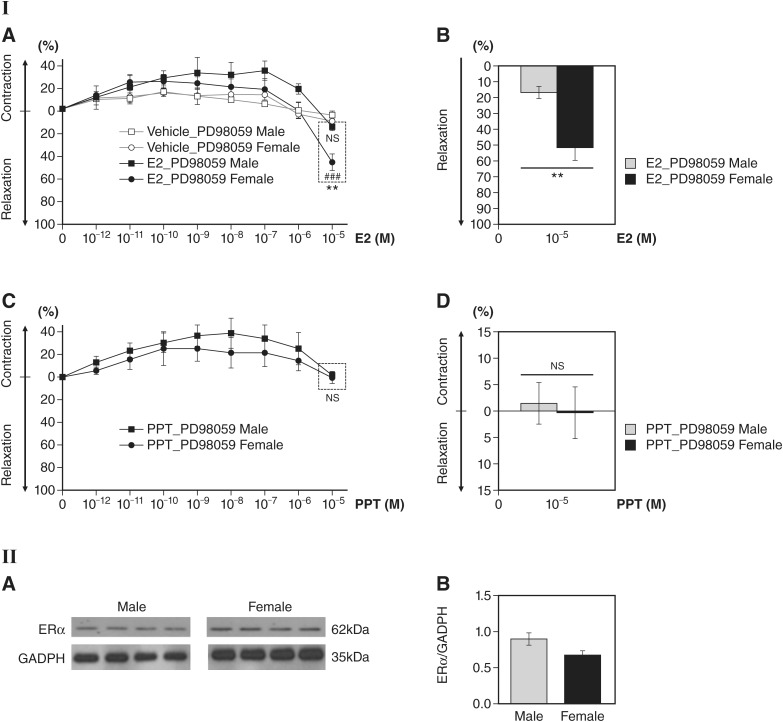
Next, we investigated the signaling mechanisms by which estrogen promotes vascular relaxation through rapid ERα signaling. In vitro, ERα-ERK dependent signaling has been implicated in eNOS activation in pulmonary artery ECs (7). To assess the role of ERK in rapid downstream signaling of ERα, we pretreated aortic vessels with 10 μM PD-98059, a known inhibitor of the ERα target ERK. We then stimulated these vessels with 10−12−10−5 M E2. At E2 concentrations ranging from 10−12 to 10−7 M, male and female E2-stimulated vessels did not show relaxation when preincubated with PD-98059 (Fig. 2I,A). At 10−5 M E2, PD-98059-preincubated female aortas (48.11 ± 7.47%) still displayed greater relaxation than male aortas (16.03 ± 3.43%, P < 0.01; Fig. 2I,A and B). Next, we assessed the effect of PD-98059 on PPT-treated aortas. Aortic vessels were pretreated with PD-98059 and stimulated with PPT (10−12−10−5 M). PD-98059 abolished PPT-induced vessel relaxation in both sexes (Fig. 2I,C and D), suggesting ERα ERK-dependent signaling in the regulation of vasomotor function.
Fig. 2.
Effect of the ERK1/2 inhibitor PD-98059 on 17β-estradiol (E2)-induced or 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT)-induced vessel relaxation in male and female mouse aortas. I,A: preadministration with PD-98059 (10 μM) for 30 min in E2 (10−12−10−5 M)-treated aortic rings reduced vessel relaxation in both male and female aortic rings at 10−5 M E2, but female aortic rings showed significantly greater relaxation than male aortic rings (n = 3 per sex in each treatment group, ###P < 0.001, E2 treatment vs. vehicle controls in female aortic rings; **P < 0.01, E2-treated female vs. male aortic rings). No significant difference in vessel relaxation was observed between E2- and vehicle-treated male aortic rings pretreated with PD-98059 (n = 3 per sex). I,B: bar graph of I,A at 10−5 M E2. n = 3 per sex, **P < 0.01, E2-treated female vs. male mice. Percent differences in relaxation are shown between male and female aortas preincubated with PD-98059 at 10−5 M E2. I,C: pretreatment with PD-98059 (10 µM) in PPT-treated aortic rings showed no significant vessel relaxation at 10−5 M PPT in both male and female mice (n = 5 per sex). I,D: bar graph of I,C at 10−5 M PPT. No significant difference in vessel tension was found between female and male mice following PPT treatment in PD-98059-pretreated groups (n = 5 per sex). II,A: estrogen receptor-α (ERα) expression levels in male and female mouse aortas; representative Western blots are shown for ERα levels normalized to the GAPDH loading control. There was no significant difference in aortic ERα levels of male compared with female mice (n = 4 per sex). II,B: ERα expression levels in male and female mouse aortas (quantitation of II,A). No significant difference was found in ERα levels of protein between male and female aortas (n = 4 per sex). Data are presented as means ± SE.
E2 and PPT induced rapid ERK phosphorylation in male and female aortas.
Next, we assessed the protein levels of ERα in male and female mouse aortas. Western blot analysis showed no significant statistical difference of ERα expression in aortas from male and ovary-intact female mice (Fig. 2II,A and B). While there is no significant sex difference in the ERα expression level, there may be potential sex differences in estrogen’s modulation of ERα functional activity and the ERα-mediated vasorelaxatory response in male and female vascular tissue.
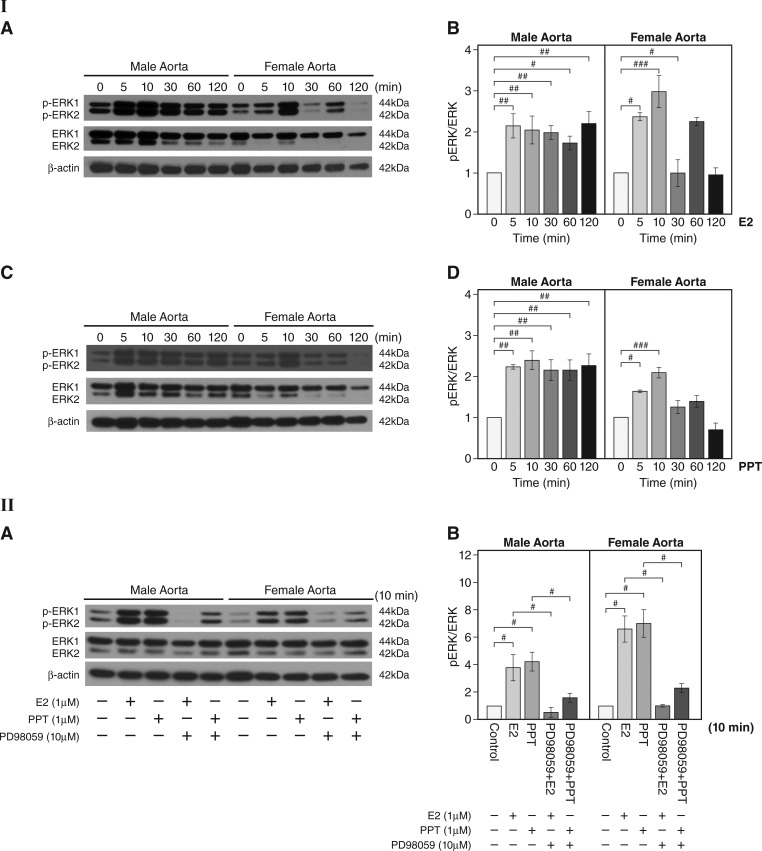
The capability of E2 to rapidly activate ERK in cannulated carotid and femoral arteries from female ovariectomized mice and contribute to beneficial vessel relaxation has been previously shown (13). We tested whether E2 treatment activates ERK signaling in aortas from male and ovary-intact female mice. Western blot analysis showed that E2 rapidly induced phosphorylation of ERK at 5 and 10 min in male and female aortas (n = 3 experiments, P < 0.05, P < 0.01, and P < 0.001, E2 vs. vehicle; Fig. 3I,A and B). To determine the effects of rapid ERα signaling on ERK activity, we assessed the effects of PPT on the level of phosphorylation of ERK1/2. PPT stimulation led to a significant increase in phosphorylation of ERK1/2 at 5 and 10 min in male and female vessels and reached a peak at 10 min in both sexes (n = 3 experiments, P < 0.05, P < 0.01, and P < 0.001, PPT vs. vehicle; Fig. 3I,C and D). These data demonstrate estrogen-mediated increases in ERK activity are mediated through rapid ERα signaling in both male and female aortas.
Fig. 3.
Increased phosphorylation of ERK after 17β-estradiol (E2) or 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT) treatment in male and female aortas. I,A: representative Western blots for levels of phosphorylated (p)ERK1/2 and total ERK1/2 at different time points after E2 (1 µM) stimulation in aortas. β-Actin is shown as the loading control. I,B: quantitation of I,A. pERK was normalized to total ERK1/2 (n = 3 experiments, #P < 0.05, ##P < 0.01, and ###P < 0.001, E2 vs. vehicle). I,C: representative Western blots for the levels of pERK1/2 and total ERK1/2 at different time points after PPT (1 µM) stimulation in aortas. I,D: quantitation of I,C. pERK1/2 was normalized to total ERK1/2 (n = 3 experiments, #P < 0.05, ##P < 0.01, and ###P < 0.001, PPT treatment vs. vehicle controls). II,A: effect of the ERK inhibitor PD-98059 on pERK1/2 after E2 or PPT treatment in male and female mouse aortas. Representative Western blots are shown for pERK1/2 and total ERK1/2 after E2 (1 µM) or PPT (1 µM) stimulation for 10 min with or without PD-98059 preincubation at 10 µM for 30 min. II,B: effect of the ERK inhibitor PD-98059 on pERK after E2 or PPT treatment in male and female mouse aortas (quantitation of II,A). The level of pERK1/2 was normalized to that of total ERK1/2 (n = 3 experiments, #P < 0.05). Data are expressed as means ± SE.
Next, we validated the role of ERK activation as a mediator of rapid vascular ERα signaling. We chose 10 min as our assessment time point because the E2- or PPT-induced rapid increase in phospho-ERK1/2 peaks at 10 min (Fig. 3I,A–D). Western blot analysis showed that pretreatment with the ERK inhibitor PD-98059 abolished the rapid phosphorylation of ERK1/2 in E2- or PPT-treated aortas in both sexes (n = 3 experiments, P < 0.05; Fig. 3II,A and B). These findings demonstrate that ERK activation mediates rapid vascular ERα signaling in both sexes.
E2 and PPT induced rapid ERK phosphorylation in male and female VSMCs.
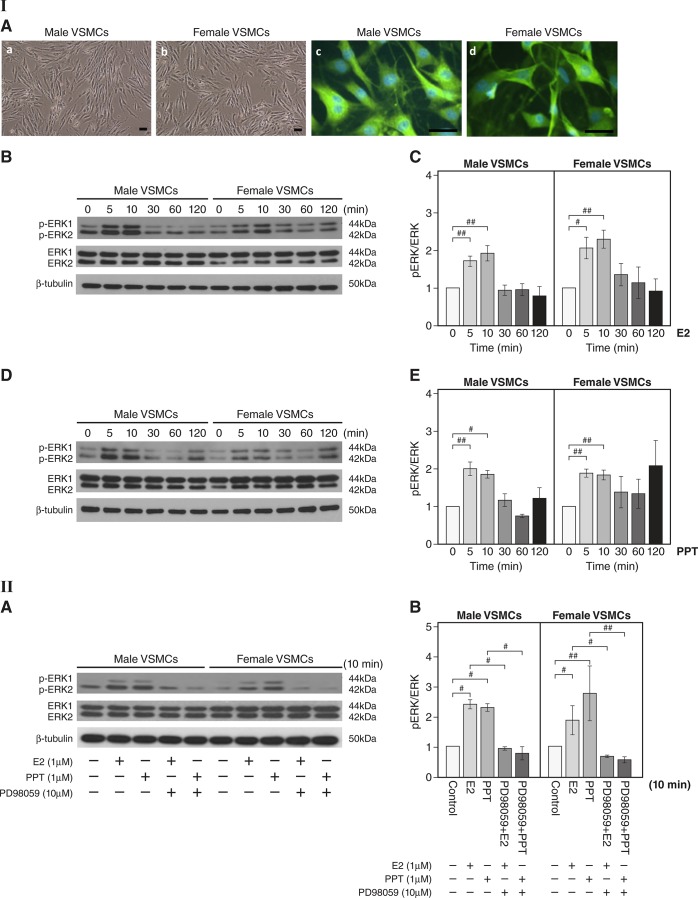
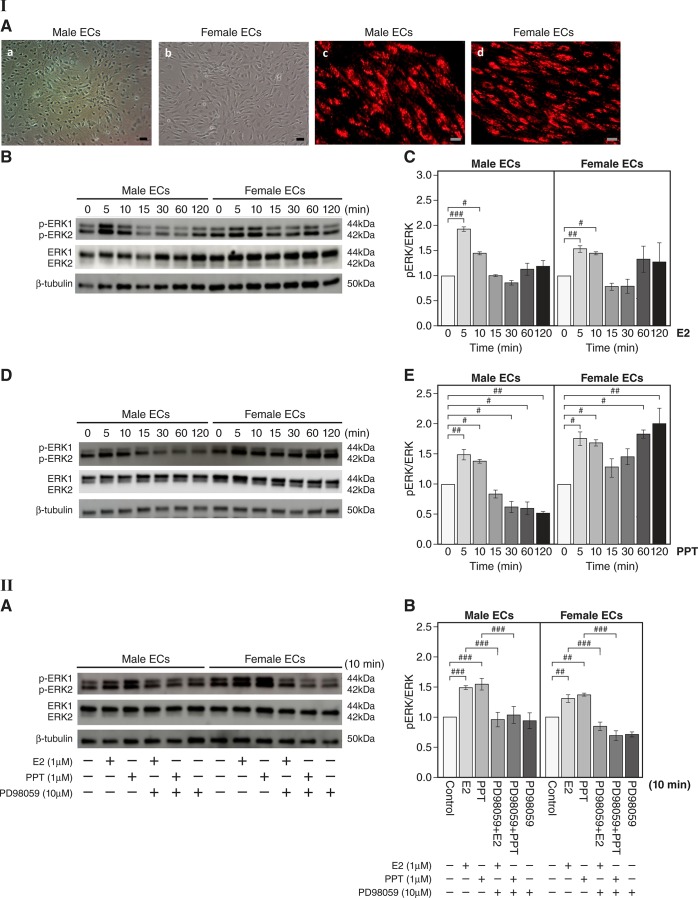
Next, we determined whether the mechanism of action for estrogen-induced ERα-mediated activation of ERK found in aortas is also evident in male and female VSMCs. In vitro, cultured primary VSMCs isolated from aortas showed positive immunoreactivity for α-smooth muscle actin (Fig. 4I,A green). First, we determined whether E2 treatment activates ERK signaling in male and female VSMCs. Western blot analysis showed that E2 rapidly induced phosphorylation of ERK1/2 in male and female VSMCs at 5 and 10 min (n = 3 experiments, P < 0.05 and P < 0.01, E2 vs. vehicle; Fig. 4I,B and C). To determine the effect of rapid ERα signaling on ERK activation in VSMCs, we determined the level of phosphorylation of ERK1/2 after PPT stimulation of VSMCs. PPT stimulation led to a rapid increase in the phosphorylation of ERK1/2 at 5 and 10 min and reached a peak at 10 min in both sexes (n = 3 experiments, P < 0.05 and P < 0.01, PPT vs. vehicle; Fig. 4I,D and E). Together, these data demonstrate estrogen-mediated increases in ERK activity are mediated through rapid ERα signaling in both male and female VSMCs.
Fig. 4.
Activation of ERK following estradiol (E2) or 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT) treatment in primary male and female vascular smooth muscle cells (VSMCs). I,A: representative photomicrographs of aortic VSMCs (a and b). Scale bar = 200 μm. Fluorescent images of immunoreactive VSMCs against α-smooth muscle actin antibody (c and d, green) are shown. Cells were visualized by nuclear DAPI staining (blue). Scale bar = 50 μm. I,B: representative Western blots for the levels of phosphorylated (p)ERK1/2 and total ERK1/2 after E2 (1 μM) stimulation of male and female VSMCs. β-Tubulin is shown as a loading control. I,C: quantitation of I,B. The level of pERK1/2 was normalized to that of total ERK1/2 (n = 3 experiments, #P < 0.05 and ##P < 0.01, E2 treatment vs. vehicle controls). I,D: representative Western blots for the levels of pERK1/2 and total ERK1/2 after PPT (1 µM) stimulation of VSMCs. I,E: quantitation of I,D. pERK1/2 expression was normalized to that of total ERK1/2. Data were collected from 3 independent experiments where primary VSMCs were generated for each experiment (n = 3 experiments, #P < 0.05 and ##P < 0.01, PPT treatment vs. vehicle controls). II,A: effect of the ERK inhibitor PD-98059 on expression of pERK following E2 or PPT stimulation in primary male and female mouse VSMCs. Representative Western blots are shown for levels of pERK1/2 and total ERK1/2 after E2 (1 µM) or PPT (1 µM) stimulation for 10 min with or without PD-98059 (10 µM) preincubation for 30 min. II,B: effect of the ERK inhibitor PD-98059 on the expression of pERK after E2 or PPT stimulation in primary male and female mouse VSMCs (quantitation of II,A). The level of pERK1/2 was normalized to that of total ERK1/2 (n = 3 experiments, #P < 0.05 and ##P < 0.01). Data are expressed as means ± SE.
Next, we determined whether E2- and PPT-induced phosphorylation of ERK is attenuated in male and female VSMCs after pretreatment with the ERK inhibitor PD-98059. We preincubated VSMCs with PD-98059 for 30 min before E2 or PPT treatment. Western blot analysis showed that E2 or PPT stimulation increased phosphorylation of ERK1/2 at 10 min (n = 3 experiments, P < 0.05 and P < 0.01, E2 or PPT vs. vehicle; Fig. 4II,A), but PD-98059 treatment prevented phosphorylation of ERK1/2 in VSMCs from both sexes (P < 0.05 and P < 0.01; Fig. 4II,A and B), suggesting marked suppression of activation of ERK. These findings clearly demonstrate the role of ERK as a mediator of E2- and PPT-stimulated signaling in VSMCs.
Activation of ERK after E2 or PPT treatment in male and female vascular ECs.
We evaluated whether the mechanism of action for estrogen-induced ERα-mediated activation of ERK found in aortas and VSMCs is also evident in ECs. In vitro, active uptake of acetylated LDL is a well-known characteristic of ECs, and our Dil-Ac-LDL uptake assay showed that LDL was taken into ECs (Fig. 5I,A, red). We tested whether E2 treatment activates ERK signaling in ECs. Western blot analysis showed that E2 rapidly induced phosphorylation of ERK1/2 at 5 and 10 min in male and female ECs (n = 3 experiments, P < 0.05, P < 0.01, and P < 0.001, E2 vs. vehicle; Fig. 5I,B and C). Next, we determined the effect of rapid ERα signaling on ERK activation in ECs by detecting the phosphorylation level of ERK1/2 after PPT stimulation of ECs. Incubation of ECs with PPT resulted in a rapid increase in ERK1/2 phosphorylation at 5 and 10 min in both sexes (n = 3 experiments, P < 0.05 and P < 0.01, PPT vs. vehicle; Fig. 5I,D and E). Together, these data demonstrate that estrogen-stimulated increases in ERK activity are mediated through rapid ERα signaling in both male and female ECs. Next, we determined whether E2- and PPT-stimulated activation of ERK is attenuated in male and female ECs after pretreatment with PD-98059. We preincubated primary ECs with PD-98059 for 30 min followed by the addition of E2 or PPT. Western blot analysis showed that E2 or PPT treatment increased ERK1/2 phosphorylation at 10 min (n = 3 experiments, P < 0.01 and P < 0.001, E2 or PPT vs. vehicle) but PD-98059 treatment prevented phosphorylation of ERK1/2 in ECs (P < 0.001; Fig. 5II,A and B). These findings delineate the role of ERK in E2- or PPT-stimulated ECs and rapid ERα signaling in the endothelium.
Fig. 5.
Activation of ERK following estradiol (E2) or 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT) treatment in primary male and female vascular endothelial cells (ECs). I,A: representative photomicrographs of aortic vascular ECs (a and b). Scale bar = 200 μm. The Dil-Ac-LDL uptake assay shows LDL uptake by ECs (c and d, red). Scale bar = 50 μm. I,B: representative Western blots for the levels of phosphorylated (p)ERK1/2 and total ERK1/2 after E2 (1 µM) stimulation. I,C: quantitation of I,B. pERK1/2 expression was normalized to that of total ERK1/2 (n = 3 experiments, #P < 0.05, ##P < 0.01, and ###P < 0.001, E2 treatment vs. vehicle). I,D: representative Western blots for the levels of pERK1/2 and total ERK1/2 after PPT (1 µM) stimulation of ECs. I,E: quantitation of I,D. pERK1/2 expression was normalized to that of total ERK1/2. Data were collected from 3 independent experiments where primary ECs were generated for each experiment (n = 3 experiments, #P < 0.05 and ##P < 0.01, PPT treatment vs. vehicle controls). II,A: effect of the ERK inhibitor PD-98059 on pERK after E2 or PPT stimulation in primary male and female mouse ECs. Representative Western blots are shown for the levels of pERK1/2 and total ERK1/2 after E2 (1 µM) or PPT (1 µM) stimulation for 10 min with or without PD-98059 (10 µM) preincubation for 30 min. II,B: effect of the ERK inhibitor PD-98059 on pERK after E2 or PPT stimulation in primary male and female mouse ECs (quantitation of II,A). pERK1/2 expression was normalized to that of total ERK1/2 (n = 3 experiments, ##P < 0.01 and ###P < 0.001). Data are expressed as means ± SE.
Vascular response of endothelium-denuded aortas after E2 or PPT stimulation in male and female mice.
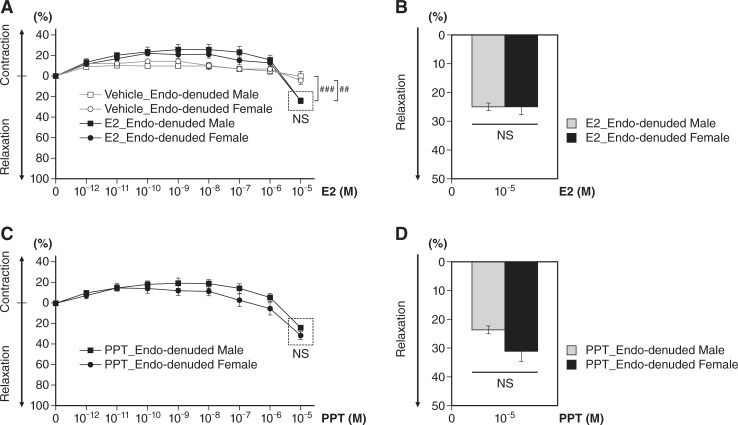
We next assessed the extent of E2- or PPT-mediated vessel relaxation on endothelium-denuded aortas. ECs were removed from isolated aortic rings, and all subsequent treatments and timelines were the same as those of aforementioned EC-intact aortic rings. We found vessel relaxation at 10−5 M E2 compared with vehicle controls in both male and female mice (n = 3 per sex in each treatment group, P < 0.01, E2 vs. vehicle in female mice; P < 0.001, E2 vs. vehicle in male mice; Fig. 6A). PPT evoked a relaxation response in EC-denuded aortas (Fig. 6, C and D), similar to E2 (Fig. 6, A and B). However, there was no sex difference in E2 (Fig. 6, A and B)- or PPT-induced (Fig. 6, C and D) vessel relaxation in EC-denuded aortas, implicating the role of ECs and ERα mediation in the sex differences in vessel relaxation.
Fig. 6.
Dose-dependent effect on aortas without endothelial cells after 17β-estradiol (E2) or 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl)tris-phenol (PPT) treatment in male and female mice. A: increased vessel relaxation at 10−5 M E2 compared with vehicle controls in both male and female mice was found (n = 3 per sex in each treatment group, ##P < 0.01, E2 treatment vs. vehicle controls in female mice; ###P < 0.001, E2 treatment vs. vehicle controls in male mice). However, there was no sex difference in E2-induced vessel relaxation in endothelium-denuded aortas (n = 3 per sex). B: bar graph of A at 10−5 M E2. C: the estrogen receptor-α agonist PPT induced vessel relaxation in aortas without endothelial cells from both wild-type male and female mice. However, no significant differences were found between male and female mice in PPT-induced vessel relaxation (n = 3 per sex). D: bar graph of C at 10−5 M PPT.
DISCUSSION
Estrogen’s short-term vasodilatory responses within minutes are too fast to be dependent on changes in gene expression (31, 50). This is likely the result of estrogen’s activation of specific protein kinases via rapid, nonnuclear ER signaling pathways. Here, we tested these assumptions and investigated ERα-mediated vasomotor function and the downstream signaling target involved in rapid vascular ERα signaling. In this study, we focused on the biological significance of rapid nongenomic ERα-ERK signaling in vasomotor tone using both male and ovary-intact female mice. Use of castrated male or ovariectomized female mice is beyond the scope of this study.
Evaluating the vasorelaxatory responses of precontracted vascular rings toward increasing E2 concentrations is an accepted model system for examining rapid ER effects on blood vessels. For vascular function experiments, we assessed the role of ERα activation in the relaxation of PE-contracted aortic vascular rings from male and ovary-intact female mice after treatment with E2 and the highly selective ERα agonist PPT (41). Regarding the ligand concentrations, several reports have used E2 and PPT ligand concentrations up to the 10 micromolar (10−5 M) range (23–25, 29, 34, 37, 38). A limitation of our study is that the maximal effects of E2 were observed at micromolar concentrations, several orders higher than physiological plasma E2 levels. It is important to mention that the acute vascular effects from E2 ex vivo are not fully representative of vascular responses from chronic exposure to E2. It is conceivable that lengthened exposure to physiological concentrations of E2 in vivo would result in an accumulation of E2 levels comparable to those in our vascular experiments. E2 is lipophilic with limited aqueous solubility (10). E2 can bind to the plasma membrane or readily diffuse across the plasma membrane and bind to membrane-associated ERs in the vasculature (35). Therefore, further assessments of E2 pharmacodynamics and ERs are necessary for future studies.
We found that E2-induced greater vessel relaxation of aortic rings obtained from ovary-intact female mice than male mice, despite the presence of similar levels of ERα, as shown by Western blot analysis. We observed a similar sex-related difference in aortic relaxation induced with the selective ERα agonist PPT, suggesting that ERα-mediated signaling plays a major role in the vasorelaxatory difference observed in male and female aortas. Treatment of aortic rings with the ERα antagonist MPP or KO of the gene encoding ERα eliminates differences in PPT-induced relaxation in aortic rings from both male and female mice, suggesting that the sex differences in estrogen-stimulated aortic relaxation are linked to the activation of ERα, which appears to induce greater aortic relaxation in female mice than in male mice. Other preclinical investigations have demonstrated sex differences in the acute effect of E2 on vascular function. For instance, a study on hypertensive Dahl rats reported that E2 had no effect on renal artery contraction or relaxation in males but antagonized increases in vascular tone in hypertensive females through suppression of endothelium-derived contracting factor mechanisms and enhancement of nitric oxide-mediated relaxation (52). However, these studies did not assess cell-signaling mechanisms that lead to observed rapid actions of estrogen in the vasculature.
We examined vascular ERα-ERK signaling mechanisms by pretreating aortic rings with the ERK inhibitor PD-98059. PD-98059 treatment attenuated aortic relaxation induced by E2 in both male and female mice, suggesting that ERK signaling plays a role in E2-mediated aortic relaxation. PD-98059 attenuation of vessel relaxation was greater in E2-treated aortic rings from male mice than from those of female mice. In contrast to E2-treated aortas, the ERK inhibitor abrogated PPT-induced vessel relaxation in both male and female aortic rings and there was no sex difference. Although ERK inhibition completely abolished PPT-induced aortic relaxation, it is important to mention that E2-mediated relaxation was greatly reduced but not completely obviated in aortic rings pretreated with PD-98059. This observation suggests that estrogen signaling may influence vasomotor tone through mechanisms separate from ERK activation through ERα. For instance, other laboratories have reported that selective ERβ activation promotes vasodilation through endothelium-dependent hyperpolarization (43).
We also examined the expression levels of phosphorylated ERK in aortas in response to E2 and PPT. Western blot analysis showed increased phosphorylation of ERK in aortic tissue after E2 treatment. A similar effect of ERK activation was shown in PPT-stimulated aortas, suggesting ERα mediation. However, pretreatment with the ERK inhibitor PD-98059 prevented ERK activation after E2 and PPT treatment in the aortas of both male and ovary-intact female mice. These data are consistent with our vessel tension experiments and clearly implicate the role of ERα-ERK in mediating vasodilation. Downstream targets of phosphorylated ERK in aortas remain to be elucidated. It is important to mention that, even though sex differences in relaxation were observed in E2- and PPT-treated intact aortas, the extent of ERα-mediated ERK activation appeared to be similar between male and female aortic tissue in our experiments. Therefore, other endothelium-dependent mechanisms, such as eNOS activation through the Akt pathway (12), may be responsible for greater vascular reactivity in response to estrogen signaling in female mice.
Most studies investigating estrogen-induced vasodilation have historically focused on ECs and have not taken into consideration the possible effects of ER activation in VSMCs. Our data indicate that VSMCs are important targets for estrogen-induced vasodilation. Both E2 and PPT treatment resulted in significantly increased phosphorylation of ERK in male and female VSMCs at 5 and 10 min posttreatment. ERK activation was significantly blunted when male and female VSMCs were pretreated with the ERK inhibitor PD-98059. These data demonstrate that ERα stimulates phosphorylation of ERK in both male and female VSMCs. Further studies are needed to determine the downstream targets of phosphorylated ERK to fully understand the signaling cascades responsible for both rapid and genomic ERα-mediated vasodilation in VSMCs.
We found that aortas denuded of ECs showed much less vessel relaxation after E2 or PPT treatment, implicating a role of ECs for the increased sensitivity to rapid estrogen-stimulated ERα in female mice in response to changes in vasomotor tone. Our study revealed that, in vitro, rapid ERK activation after E2 or PPT treatment was evident in cultured ECs from aortas of both sexes and that the ERK inhibitor prevented increased phosphorylation of ERK in both sexes. Rapid ERα signaling is likely to be critical for numerous cellular responses to estrogen. Further investigation of rapid ERα activation in ECs and VSMCs will continue to enhance our understanding of the role of rapid ERα signaling in vascular biology and also its potential role in vascular pathophysiology, in part through pharmacological inhibition, through gene silencing, or by using animal models that disrupt nonnuclear ERα signaling.
Aortas are classified as conduit arteries and are not as heavily involved in resistance as the arteries of the peripheral vasculature. However, the distribution and concentration of different ER subtypes have been shown to vary between different vascular beds (38). Here, we chose to use aortic rings for the analysis of vasomotor tone, as they still demonstrate contractile and dilatory responses and display higher expression levels of ERα compared with other vessels (38). However, future studies should also focus on estrogen signaling in other conduit arteries such as carotids or in resistance vessels of both males and females. Since ER subtypes are not evenly distributed between different vascular beds, estrogen signaling may regulate vasomotor tone in the peripheral vasculature differently than aortic rings.
Our in vitro findings of E2 and PPT-mediated phosphorylation and activation of ERK in ECs and VSMCs, along with vessel tension studies involving ERK inhibition, reveal that ERK signaling plays an important role in ERα-mediated vasomotor function. However, our data suggest that E2-mediated relaxation proceeds through at least two separate pathways, only one of which involves ERα-mediated ERK phosphorylation. The exact molecular mechanisms of ERα-mediated vascular protection in both sexes need to be further investigated to ascertain the specific points in which the male and female pathways diverge.
Impaired vascular tone is a characteristic of many vascular diseases, such as atherosclerosis. We focused on identifying sex differences in rapid ERα-mediated ERK activation during vascular function, but in the future we need to broaden our scope to examine sex differences in rapid ER signaling in vascular remodeling diseases. Finally, driven by our increased understanding of rapid ER signaling in vascular function, there is growing interest in the sex differences in rapid vascular-selective ER mechanisms and signaling, which will likely be exploitable for novel treatment of vascular diseases, including atherosclerosis, hypertension, vascular lesion formation, stroke, and aortic aneurysms.
GRANTS
This work was supported by the Tulane University Building Interdisciplinary Research Careers in Women's Health (National Institute of Child Health and Human Development Grant 2-K12-HD-043451-11) and faculty startup funds from the Tulane University School of Medicine Department of Pharmacology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.D. and M.H.H. conceived and designed research; S.C.K., A.C.B., M.H.M., and R.M.C. performed experiments; S.C.K., L.C., and M.H.H. analyzed data; S.C.K., L.C., P.D., J.-P.L., and M.H.H. interpreted results of experiments; S.C.K. prepared figures; S.C.K., A.C.B., J.-P.L., and M.H.H. drafted manuscript; S.C.K., A.C.B., P.D., K.-J.Y., J.-P.L., and M.H.H. edited and revised manuscript; S.C.K., A.C.B., M.H.M., R.M.C., L.C., P.D., K.-J.Y., J.-P.L., and M.H.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Margaret Hartney and Dr. Habiburrahman Ansari for providing technical assistance and Carol Chen for assistance with statistical analysis.
REFERENCES
- 1.Bernelot Moens SJ, Schnitzler GR, Nickerson M, Guo H, Ueda K, Lu Q, Aronovitz MJ, Nickerson H, Baur WE, Hansen U, Iyer LK, Karas RH. Rapid estrogen receptor signaling is essential for the protective effects of estrogen against vascular injury. Circulation 126: 1993–2004, 2012. doi: 10.1161/CIRCULATIONAHA.112.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billon-Galés A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci USA 108: 13311–13316, 2011. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisson JA, Mills B, Paul Helt JC, Zwaka TP, Cohen ED. Wnt5a and Wnt11 inhibit the canonical Wnt pathway and promote cardiac progenitor development via the caspase-dependent degradation of AKT. Dev Biol 398: 80–96, 2015. doi: 10.1016/j.ydbio.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation 103: 423–428, 2001. doi: 10.1161/01.CIR.103.3.423. [DOI] [PubMed] [Google Scholar]
- 5.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120: 2319–2330, 2010. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrasekar B, Mummidi S, Valente AJ, Patel DN, Bailey SR, Freeman GL, Hatano M, Tokuhisa T, Jensen LE. The pro-atherogenic cytokine interleukin-18 induces CXCL16 expression in rat aortic smooth muscle cells via MyD88, interleukin-1 receptor-associated kinase, tumor necrosis factor receptor-associated factor 6, c-Src, phosphatidylinositol 3-kinase, Akt, c-Jun N-terminal kinase, and activator protein-1 signaling. J Biol Chem 280: 26263–26277, 2005. doi: 10.1074/jbc.M502586200. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103: 401–406, 1999. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbacho AM, Eiserich JP, Zuniga LA, Valacchi G, Villablanca AC. Compromised aortic vasoreactivity in male estrogen receptor-alpha-deficient mice during acute lipopolysaccharide-induced inflammation. Endocrinology 148: 1403–1411, 2007. doi: 10.1210/en.2006-0399. [DOI] [PubMed] [Google Scholar]
- 9.Cottle DL, Ursino GM, Ip SC, Jones LK, Ditommaso T, Hacking DF, Mangan NE, Mellett NA, Henley KJ, Sviridov D, Nold-Petry CA, Nold MF, Meikle PJ, Kile BT, Smyth IM. Fetal inhibition of inflammation improves disease phenotypes in harlequin ichthyosis. Hum Mol Genet 24: 436–449, 2015. doi: 10.1093/hmg/ddu459. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham AR, Klopman G, Rosenkranz HS. A dichotomy in the lipophilicity of natural estrogens, xenoestrogens, and phytoestrogens. Environ Health Perspect 105, Suppl 3: 665–668, 1997. doi: 10.1289/ehp.97105s3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J 10: 615–624, 1996. [PubMed] [Google Scholar]
- 12.Florian M, Lu Y, Angle M, Magder S. Estrogen induced changes in Akt-dependent activation of endothelial nitric oxide synthase and vasodilation. Steroids 69: 637–645, 2004. doi: 10.1016/j.steroids.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem 280: 19704–19710, 2005. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 14.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, and Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 49: 1358–1363, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 303: F377–F385, 2012. doi: 10.1152/ajprenal.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 85: 447–452, 1976. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 18.Karas RH, Hodgin JB, Kwoun M, Krege JH, Aronovitz M, Mackey W, Gustafsson JA, Korach KS, Smithies O, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor beta-deficient female mice. Proc Natl Acad Sci USA 96: 15133–15136, 1999. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation 89: 1943–1950, 1994. doi: 10.1161/01.CIR.89.5.1943. [DOI] [PubMed] [Google Scholar]
- 20.Kim-Schulze S, McGowan KA, Hubchak SC, Cid MC, Martin MB, Kleinman HK, Greene GL, Schnaper HW. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation 94: 1402–1407, 1996. doi: 10.1161/01.CIR.94.6.1402. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb 12: 138–142, 2005. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 22.Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res 83: 224–229, 1998. doi: 10.1161/01.RES.83.2.224. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol 57: 598–603, 2011. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey SH, da Silva AS, Silva MS, Chappell MC. Reduced vasorelaxation to estradiol and G-1 in aged female and adult male rats is associated with GPR30 downregulation. Am J Physiol Endocrinol Metab 305: E113–E118, 2013. doi: 10.1152/ajpendo.00649.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 81: 99–102, 2014. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation 89: 1501–1510, 1994. doi: 10.1161/01.CIR.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 27.Lu Q, Schnitzler GR, Ueda K, Iyer LK, Diomede OI, Andrade T, Karas RH. ER alpha rapid signaling is required for estrogen induced proliferation and migration of vascular endothelial cells. PLoS One 11: e0152807, 2016. doi: 10.1371/journal.pone.0152807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson JE, Martin KA. Clinical practice. Postmenopausal hormone-replacement therapy. N Engl J Med 345: 34–40, 2001. doi: 10.1056/NEJM200107053450106. [DOI] [PubMed] [Google Scholar]
- 29.Mazzuca MQ, Mata KM, Li W, Rangan SS, Khalil RA. Estrogen receptor subtypes mediate distinct microvascular dilation and reduction in [Ca2+]i in mesenteric microvessels of female rat. J Pharmacol Exp Ther 352: 291–304, 2015. doi: 10.1124/jpet.114.219865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 31.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 32.Murata T, Dietrich HH, Xiang C, Dacey RG Jr. G protein-coupled estrogen receptor agonist improves cerebral microvascular function after hypoxia/reoxygenation injury in male and female rats. Stroke 44: 779–785, 2013. doi: 10.1161/STROKEAHA.112.678177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura S, Hayashi K, Iwasaki K, Fujioka T, Egusa H, Yatani H, Sobue K. Nuclear import mechanism for myocardin family members and their correlation with vascular smooth muscle cell phenotype. J Biol Chem 285: 37314–37323, 2010. doi: 10.1074/jbc.M110.180786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nechmad A, Merin G, Schwalb H, Shimon DV, Borman JB, Milgalter E, Mosseri M. Estrogen induces nitric oxide-mediated vasodilation of human mammary arteries in vitro. Nitric Oxide 2: 460–466, 1998. doi: 10.1006/niox.1998.0202. [DOI] [PubMed] [Google Scholar]
- 35.Oren I, Fleishman SJ, Kessel A, Ben-Tal N. Free diffusion of steroid hormones across biomembranes: a simplex search with implicit solvent model calculations. Biophys J 87: 768–779, 2004. doi: 10.1529/biophysj.103.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res 90: 1087–1092, 2002. doi: 10.1161/01.RES.0000021114.92282.FA. [DOI] [PubMed] [Google Scholar]
- 37.Raffetto JD, Qiao X, Beauregard KG, Khalil RA. Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins. J Vasc Surg 51: 972–981, 2010. doi: 10.1016/j.jvs.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reslan OM, Yin Z, do Nascimento GR, Khalil RA. Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat. J Cardiovasc Pharmacol 62: 26–40, 2013. doi: 10.1097/FJC.0b013e31828bc88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 40.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 41.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology 140: 800–804, 1999. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- 42.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun 240: 737–741, 1997. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 43.Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension 49: 1364–1370, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science 304: 1269–1273, 2004. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 45.Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PA, Thum T, Groner B, Chowdhury K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat Genet 42: 1101–1108, 2010. doi: 10.1038/ng.709. [DOI] [PubMed] [Google Scholar]
- 46.Ueda K, Karas RH. Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids 78: 589–596, 2013. doi: 10.1016/j.steroids.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Ueda K, Lu Q, Baur W, Aronovitz MJ, Karas RH. Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol 33: 1837–1843, 2013. doi: 10.1161/ATVBAHA.112.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venkov CD, Rankin AB, Vaughan DE. Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation 94: 727–733, 1996. doi: 10.1161/01.CIR.94.4.727. [DOI] [PubMed] [Google Scholar]
- 49.Villablanca AC, Jayachandran M, Banka C. Atherosclerosis and sex hormones: current concepts. Clin Sci (Lond) 119: 493–513, 2010. doi: 10.1042/CS20100248. [DOI] [PubMed] [Google Scholar]
- 50.Wessler S, Otto C, Wilck N, Stangl V, Fritzemeier KH. Identification of estrogen receptor ligands leading to activation of non-genomic signaling pathways while exhibiting only weak transcriptional activity. J Steroid Biochem Mol Biol 98: 25–35, 2006. doi: 10.1016/j.jsbmb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Widder J, Pelzer T, von Poser-Klein C, Hu K, Jazbutyte V, Fritzemeier KH, Hegele-Hartung C, Neyses L, Bauersachs J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension 42: 991–996, 2003. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Kosaka H. Sex-specific acute effect of estrogen on endothelium-derived contracting factor in the renal artery of hypertensive Dahl rats. J Hypertens 20: 237–246, 2002. doi: 10.1097/00004872-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]