Abstract
In pathological populations, elevated sympathetic activity is associated with increased activity of individual sympathetic neurons. We used custom action potential detection software to analyze multiunit sympathetic activity in 18 normotensive pregnant women (third trimester; 33 ± 5 wk) and 19 nonpregnant women at rest and a subset (10 and 13, respectively) during a cold pressor challenge. Although the number of action potentials per burst and number of active amplitude-based “clusters” were not different between groups, the total number of sympathetic action potentials per minute was higher in pregnant women at rest. Individual clusters were active predominately once per burst, suggesting they represent single neurons. Action potentials occurred in closer succession in normotensive pregnant (interspike interval 36 ± 10 ms) versus nonpregnant women (50 ± 27 ms; P < 0.001) at rest. Pregnant women had a lower total peripheral resistance (11.7 ± 3.0 mmHg·l−1·min) than nonpregnant women (15.1 ± 2.7 mmHg·l−1·min; P < 0.001), indicating a blunted neurovascular transduction. The cold pressor reduced the number of action potentials per burst in both groups due to shortening of the R-R interval in conjunction with increased burst frequency; total neural firing per minute was unchanged. Thus elevated sympathetic activity during normotensive pregnancy is specific to increased incidence of multiunit bursts. This is likely due to decreased central gating of burst output as opposed to generalized increases in central drive. These data also reinforce the concept that pregnancy appears to be the only healthy state of chronic sympathetic hyperactivity of which we are aware.
Keywords: action potentials, microneurography, neural recruitments, pregnancy, sympathetic activity
INTRODUCTION
There is limited but consistent evidence that sympathetic nerve activity (SNA) is significantly elevated in normotensive pregnancies (9, 19, 25, 26), which manifests as early as the first trimester (9). However, the transduction of sympathetic activity to vascular outcomes, such as vascular resistance or stiffness, appears to be offset or blunted at rest and during periods of stress (9, 26). Thus in contrast to other clinical populations (e.g., chronic obstructive pulmonary disease, sleep apnea, obesity) (1, 4, 11, 15), elevated sympathetic activity does not appear to have negative cardiovascular consequences during pregnancy. This makes pregnancy one of the only “healthy” physiological states associated with persistent sympathetic excitation.
Analysis of integrated SNA demonstrates increases in burst frequency (bursts/minute) and burst incidence [bursts/100 heartbeats (hb)] (9, 19, 25, 26) during pregnancy. However, integrated sympathetic recordings are associated with an inherent loss of information regarding the activity, recruitment, and firing characteristics of individual or groups of sympathetic neurons (12, 23). Indeed, the firing probability of individual neurons can occur independent of the probability of integrated bursts of activity. Macefield and Wallin (13) demonstrated that in healthy individuals with “low” levels of sympathetic activity (~21 bursts/100 hb), individual neurons were active in ~100% of bursts. In contrast, single neurons were active only in ~47% of bursts in healthy individuals with “high” levels of sympathetic activity (~75 bursts/100 hb) (13). These data indirectly suggest a larger population of active neurons (recruitment) in individuals with higher sympathetic activity. To study the activity of single sympathetic neurons in normal and hypertensive pregnant women, Greenwood and colleagues (6–8) have applied the same single-unit neuronal recording technique. Their data suggest an increase in the firing frequency (per minute or per 100 cardiac cycles) of individual sympathetic neurons in healthy pregnant women. However, it appears that the increase in the firing frequency of individual neurons was dependent on the concomitant increase in the number of integrated bursts of activity. What remains unclear is whether the total number of active neurons changes with pregnancy, whether given neurons fire more often within a burst of activity, and how changes in single-unit firing may relate to the activity of other neurons. As traditional measures of integrate burst frequency and incidence do not account for these complexities, such distinctions may provide additional insight into the central mechanism of apparent sympathetic hyperactivity during pregnancy. These data may also help to define the relationship between apparent increases in integrated sympathetic activity during normotensive pregnancy and sympathetic hyperactivity occurring during pregnancy-related hypertensive disorders (10, 18). Recently, techniques to analyze the simultaneous activity of many individual sympathetic neurons within the integrated, multiunit signal have been developed (21, 23). Steinback et al. (23) used this approach previously to identify elevated firing frequencies and the recruitment of neurons during acute periods of increased, integrated, multiunit sympathetic activity. Therefore, the aim of this study was to assess the firing patterns of groups of sympathetic neurons during normotensive pregnancy, an established period of persistent sympathetic excitation. We hypothesized that action potential content within sympathetic bursts would be elevated (increased multiple firing, increased recruitment) in pregnant women relative to nonpregnant women.
METHODS
The present study was part of a larger investigation of neurovascular regulation during pregnancy. Here, we report descriptive metrics of integrated sympathetic activity used to provide similar information in our previous reports (25, 26). The novel aspects of this study focus on the activation and firing patterns of individual and groups of sympathetic neurons at rest and in a subset of women, in response to a standard sympathetic stressor, a cold pressor test (CPT).
Participants.
Previously collected data (25, 26) were assessed for suitability for analysis, and data from 18 healthy, normotensive pregnant women and 19 healthy, nonpregnant female controls were included. All women were between the ages of 18 and 40 yr, with a body mass index (BMI) <30 kg/m2 and no history of cardiovascular, neurological, or respiratory diseases and had participated in research studies after providing written, informed consent. Pregnant women were tested in the third trimester (33 ± 4 wk), and all had singleton pregnancies with no history of gestational diabetes, gestational hypertension, or pre-eclampsia. Nine of 18 pregnant women were primigravid. Nonpregnant women (including those taking hormonal contraceptives; n = 5) were tested during the early follicular phase of the menstrual cycle. This study was approved by the Health Research Ethics Board at the University of Alberta.
Participants were tested in the morning after a 12-h fast and having abstained from caffeine, nicotine, alcohol, and strenuous exercise for 12 h. Height and weight were recorded upon arrival, and participants then received a light, standardized meal, including a multigrain bagel with sugar-free jam and a juice box. They were then instructed to void their bladder before instrumentation.
Instrumentation.
Testing occurred with participants seated in a semirecumbent position in a dentist-style chair in a laboratory maintained at 20°C. Heart rate was measured using a standard lead II electrocardiogram. Blood pressure was measured continuously using photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). The pressure waveform was calibrated using the device-specific calibration procedure (“return to flow”) and confirmed using manual sphygmomanometry. The calibrated waveform was analyzed to determine mean, systolic, and diastolic blood pressures. Cardiac output was assessed using the Modelflow algorithm (Finometer), and total peripheral resistance (TPR) was calculated as mean pressure/cardiac output.
Microneurography.
As described previously (25), microneurography was used to measure multiunit sympathetic neural activity (3, 23). Microneurography electrodes (UNP35F2T; FHC, Bowdoin, ME) were 200 μm epoxylite-coated tungsten needles with an impedance of 2 MΩ. Briefly, the recording electrode was inserted percutaneously within the fibular nerve. A bare tungsten reference electrode was inserted 1–3 cm from the recording site. The recording electrode was then manipulated to record from muscle sympathetic neurons, which were identified by a pulse-synchronous signal that increased in response to voluntary apnea but not to auditory arousal. The raw sympathetic signal was amplified [preamplifier (1,000×) and variable-gain isolated amplifier (10,000×; model 662C-3; Engineering Electronics Shop, College of Engineering, The University of Iowa, Iowa City, IA)], band-pass filtered (700–2,000 Hz), and recorded at 10,000 Hz for subsequent multiunit analysis. The raw signal was also rectified and integrated (decay constant 0.1 s) to produce an integrated neurogram with a characteristic bursting pattern.
After all instrumentation was complete, participants rested for a minimum of 10 min. Based on variations in search time for a suitable SNA signal, the start of data collection varied from 30 to 60 min following the provided meal. After the 10-min rest period, participants underwent a CPT that involved the placement of their hand (up to the wrist) into an ice-water bath for 3 min.
Data analysis.
All data were stored for offline data analysis (PowerLab/16SP with LabChart v8.0; ADInstruments, Colorado Springs, CO). Integrated bursts of sympathetic activity were initially detected by a semiautomated peak-detection algorithm (Chart v8.0; ADInstruments). Bursts were subsequently confirmed by a trained observer (C. W. Usselman/C. D. Steinback) using the raw signal and the timing, with respect to the cardiac cycle as guides. Integrated activity was quantified as burst frequency (bursts/minute), burst incidence (bursts/100 hb), and burst amplitude. Burst amplitudes were normalized by identification of the largest burst within the baseline period and the assignment of it with a value of 100; all other burst amplitudes were expressed in a relative fashion. Burst amplitude distributions were constructed by binning normalized bursts into 10 arbitrary unit (au) bins. Total normalized activity was determined as the product of normalized burst amplitude (percent) and frequency (bursts/minute).
Resting action potential firings were characterized after a period of 10 min of rest. To standardize the amount of data from each individual incorporated in analyses, a total of 100 consecutive sympathetic bursts from each participant were exported and subsequently run through custom multiunit sympathetic action potential detection software (APD v2.1) (21). Briefly, the raw neurogram was de-noised, and extracellular sympathetic action potentials were identified based on wavelet matching and counted. Identified action potentials were extracted within 3.2-ms windows and sorted based on peak-to-peak amplitude. Action potentials were subsequently binned (“clustered”), based on Scott’s rule (22), and overlaid to generate a mean waveform (Figs. 1 and 2). Only action potential waveforms, which could be reliably separated and classified (separation >1.4 ms), were included in the cluster analysis. Overlapping waveforms (0.7–1.4 ms separation) were counted but not clustered [see Salmanpour and coworkers (20, 21, 23) for further methodological detail]. Based on previous work, amplitude-based clusters of action potentials were assumed to represent a single neuron or a small number of neurons with similar action potential amplitudes (23). Similar to the normalization procedure for integrated burst amplitudes, action potential amplitudes were normalized with respect to the largest sympathetic action potential within the recording. Multiunit sympathetic neuron activity was then quantified as the following: 1) the average number of action potentials per burst, 2) the average number of active clusters per burst, 3) the occurrence of single and multiple firings of individual clusters within a burst, 4) the interspike interval between any two action potential firings within a burst, 5) action potential firing frequency (number/minute), and 6) action potential incidence (number/100 hb).
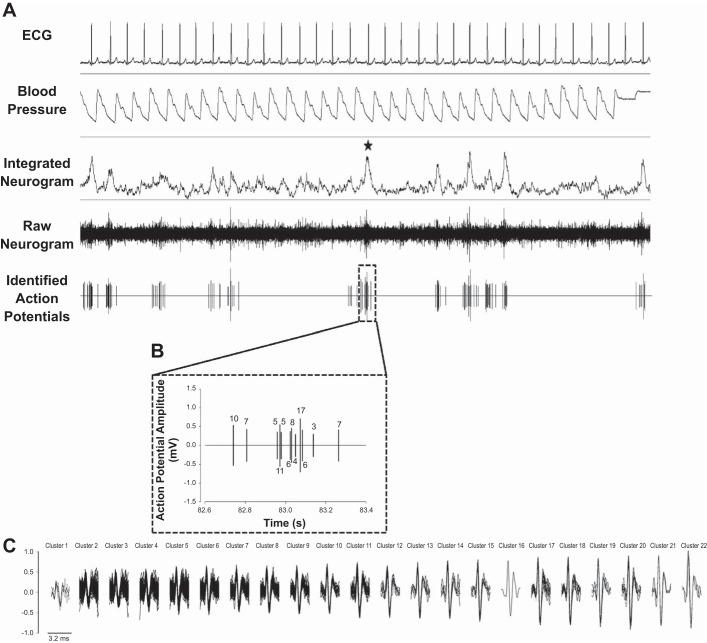
Fig. 1.
Schematic of raw data from 1 nonpregnant woman. A: 30 s of continuous data, including ECG (lead II), blood pressure, integrated sympathetic nerve activity (SNA), raw SNA, and the de-noised neurogram, indicating identified sympathetic action potentials. The star identifies a burst of interest. B: the sympathetic action potentials identified within the burst of interest. Numbers refer to the cluster to which each action potential belongs. C: all action potential clusters identified across the entire analysis for this individual (i.e., 100 bursts). Clusters have been organized based on peak-to-peak amplitude, and action potentials belonging to each cluster have been overlaid to represent the mean waveform. In this individual, 9 unique action potential clusters were active within the identified burst of interest, with 3 of the clusters firing twice with the burst and 6 firing only once.
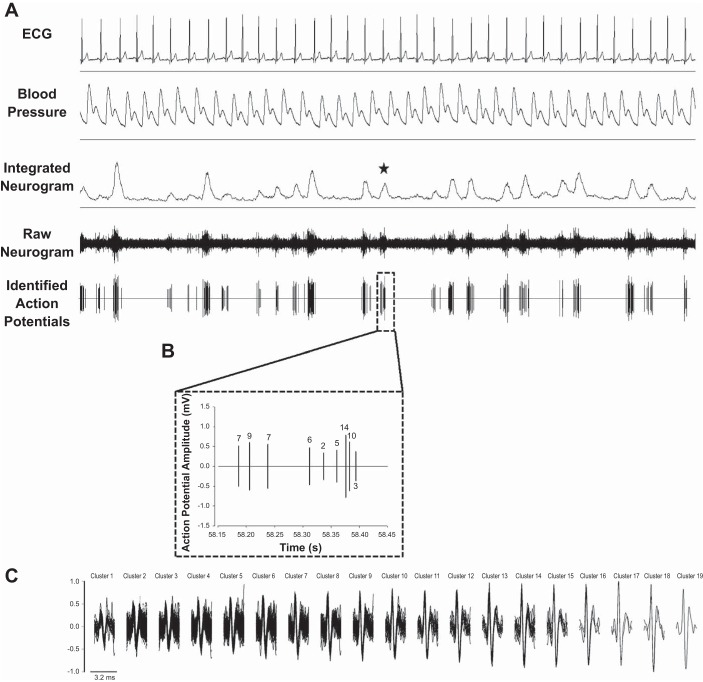
Fig. 2.
Schematic of raw data from 1 pregnant woman. A: 30 s of continuous data, including ECG (lead II), blood pressure, integrated sympathetic nerve activity (SNA), raw SNA, and the de-noised neurogram, indicating identified sympathetic action potentials. The star identifies a burst of interest. B: the sympathetic action potentials identified within the burst of interest. Numbers refer to the cluster to which each action potential belongs. C: all action potential clusters identified across the entire analysis for this individual (i.e., 100 bursts). Clusters have been organized based on peak-to-peak amplitude, and action potentials belonging to each cluster have been overlaid to represent the mean waveform. In this individual, 8 unique action potential clusters were active within the identified burst of interest, with 1 of the clusters firing twice with the burst and 7 firing only once.
To assess changes in action potential firing during sympathetic activation, data corresponding to the cold pressor challenge were analyzed in a subset of women (nonpregnant, n = 13; pregnant, n = 10). Hemodynamic data were averaged over the entire 3 min of CPT for comparison with sympathetic analyses.
Statistical analyses.
Demographic, cardiovascular, and integrated sympathetic burst data (i.e., burst frequency, burst incidence, normalized mean burst amplitude, and normalized median burst amplitude) were compared between groups and conditions using two-way mixed-model ANOVAs. Significant differences were assessed using Holm-Sidak post hoc tests. As the signal-to-noise ratio of the raw sympathetic neurogram influences the ability to detect action potentials, multiunit action potential outcomes were compared between groups using analysis of covariance controlling for signal to noise. The mean interspike interval (duration between any two action potentials within a burst) was compared between groups using an analysis of covariance controlling for the signal-to-noise ratio of the raw neurogram, as well as the number of detected action potentials within a burst. Relationships between variables were assessed using Pearson correlations. Data are presented at means ± SD. Significance was set at P ≤ 0.05 for all analyses.
RESULTS
Demographic and cardiovascular characteristics of participants are expressed in Table 1. Pregnant women were slightly, but significantly, older (31 ± 3 yr) than nonpregnant controls (27 ± 5 yr; P < 0.01). However, age was not correlated to any cardiovascular or neural outcomes when considering either group individually or as a combined cohort. Weight, at the time of the study, was significantly higher in pregnant women, as expected. For comparative purposes, recalled weight was used to calculate prepregnant BMI and was not different between pregnant and nonpregnant women (Table 1). Blood pressure was not different between groups, but TPR was lower in pregnant women compared with controls. Pregnant women also demonstrated a characteristically elevated heart rate and cardiac output compared with nonpregnant women (Table 1).
Table 1.
Participant demographic and cardiovascular characteristics
| Nonpregnant (Early Follicular) |
Pregnant (3rd Trimester) |
|||
|---|---|---|---|---|
| Baseline (n = 19) | Cold pressor (n = 13) | Baseline (n = 18) | Cold pressor (n = 10) | |
| Age, yr | 27 ± 5 | 31 ± 3* | ||
| Gestational age, wk | 33 ± 5 | |||
| Height | 166 ± 5 | 166 ± 6 | ||
| Weight, kg | 66 ± 14 | 75 ± 10*† | ||
| Non/prepregnant BMI, kg/m2 | 23.7 ± 4.9 | 23.9 ± 3.1‡ | ||
| Heart rate, beats/min | 66 ± 8 | 77 ± 11§ | 85 ± 9* | 91 ± 13*§ |
| Arterial pressure, mmHg | ||||
| Mean | 84 ± 7 | 96 ± 8§ | 87 ± 7 | 98 ± 17§ |
| Systolic | 111 ± 8 | 121 ± 18§ | 112 ± 10 | 126 ± 23§ |
| Diastolic | 67 ± 8 | 76 ± 6§ | 71 ± 8 | 79 ± 14§ |
| Cardiac output, l/min | 5.7 ± 1.2 | 6.8 ± 1.2§ | 7.7 ± 1.6* | 8.1 ± 1.8§ |
| Total peripheral resistance, mmHg·l−1·min | 15.1 ± 2.7 | 14.4 ± 2.5 | 11.7 ± 3.0* | 12.5 ± 3.0 |
| SNA burst frequency, bursts/min | 23 ± 6 | 28 ± 13 | 38 ± 11* | 49 ± 13*§ |
| SNA burst incidence, bursts/100 heartbeats | 35 ± 8 | 37 ± 15 | 45 ± 12* | 57 ± 11*§ |
| Total SNA, au/min | 1,053 ± 263 | 1,442 ± 943 | 2,062 ± 757* | 2,226 ± 664*§ |
All data are presented as means ± SD; n, number of women. au, arbitrary units.
Significantly different between groups during same condition; P < 0.05.
Difference in weight due to the influence of pregnancy.
Prepregnancy BMI (using recalled prepregnant weight) was used as an assessment of typical nonpregnant weight status in pregnant women.
Significantly different from baseline within the same group; P < 0.05.
Action potential firing characteristics at rest.
The duration of data required to obtain 100 bursts of sympathetic activity differed between groups (267 ± 79 vs. 163 ± 46 s for nonpregnant and pregnant groups, respectively; P < 0.001). The signal-to-noise ratio for raw sympathetic activity was not different between groups (4.6 ± 0.6, range: 4.0–6.2 for nonpregnant women; 4.3 ± 0.4, range: 3.6–5.1 for pregnant women). Integrated sympathetic burst frequency, burst incidence, and total sympathetic activity were significantly higher at rest in the pregnant group (Table 1). Distribution analysis also demonstrated larger sympathetic bursts in pregnant women, with the median burst amplitude at 43 ± 8 au in nonpregnant and 50 ± 8 au in pregnant women (P < 0.01).
At rest, there was no difference in the average number of action potentials per burst (14 ± 8 vs. 12 ± 6 for nonpregnant and pregnant women, respectively). However, in conjunction with an elevated, integrated burst frequency, there was a significantly higher action potential frequency in pregnant women (447 ± 274 action potential/min) versus. nonpregnant women (303 ± 207 action potential/min; P < 0.05). The number of unique, active clusters per burst (6 ± 2 vs. 5 ± 2) and the distribution of action potential amplitudes (mean 44 ± 14 vs. 44 ± 11 au) were not different between nonpregnant and pregnant women, respectively. The overall average firing frequency of unique action potential clusters across bursts was higher with pregnant women (22 ± 9 action potentials/min) compared with nonpregnant women (14 ± 8 action potentials/min; P < 0.05). However, when cluster firing was normalized per 100 bursts, the frequency of unique clusters was no longer different; 59 ± 25 vs. 55 vs. 18 action potentials/100 bursts in nonpregnant and pregnant women, respectively. The firing occurrence of individual clusters within a burst of activity was not different between groups, with individual clusters typically firing only once when active (60 ± 11% of occurrences in nonpregnant women and 62 ± 9% of occurrences in pregnant women; see Fig. 3A).
Fig. 3.
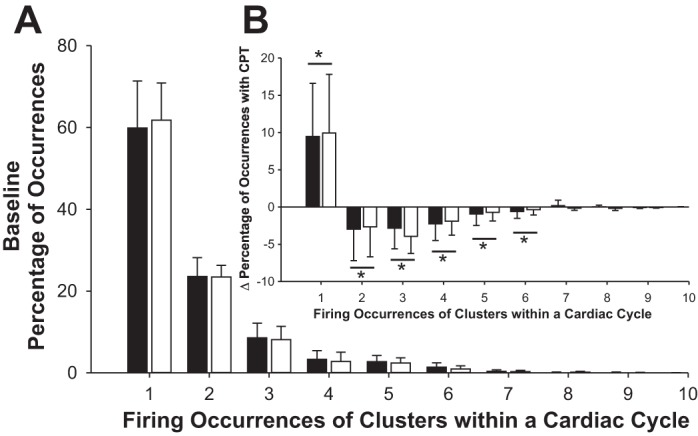
A: histograms showing proportion of occurrences of a given action potential cluster associated with single or multiple firings in pregnant (white bars) and nonpregnant (black bars) women at rest. No differences in firing occurrence were found between groups. When a cluster was present within a burst of activity, the majority of these occurrences (~60%) was associated with only a single action potential. The occurrence of multiple firings from a given cluster was relatively low, with clusters contributing 2 action potentials ~23% of the time, 3 action potentials ~8% of the time, and 4+ action potentials ~9% of the time. B: during the cold pressor test (CPT), there was a reduction in the occurrence of multiple firings and an increase in a singular event. This was consistent between pregnant (n = 10) and nonpregnant women (n = 13). *Significant change in the percentage of occurrences during CPT, P < 0.01.
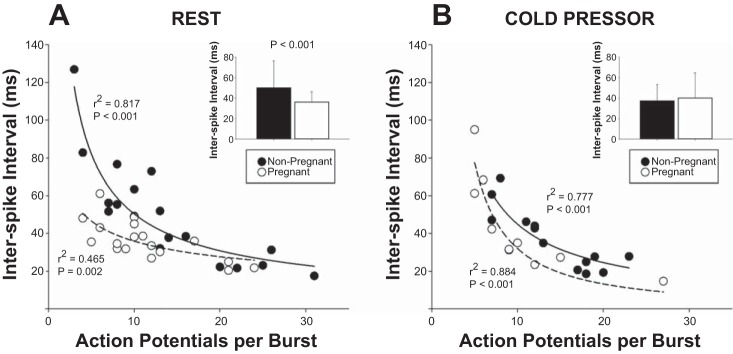
Within a burst of activity, pregnant women had a significantly shorter interspike interval (36 ± 10 ms) compared with nonpregnant women (50 ± 27 ms; P < 0.001), even after controlling for the relationship between signal to noise and the number of action potentials within bursts (Fig. 4A). The variability in the interspike interval across bursts within individuals was also decreased, as indicated by narrower interquartile ranges in pregnant (39 ± 15 ms) versus nonpregnant (54 ± 38 ms) women (P < 0.05). Across both groups, the interspike interval was inversely correlated with burst amplitude (r2 = 0.152; P < 0.05), but this relationship was more pronounced in pregnant (r2 = 0.454; P < 0.05) vs. nonpregnant (r2 = 0.150; P = 0.102) women.
Fig. 4.
A: at rest, the number of action potentials per burst was not different between pregnant (C) and nonpregnant (@) women. However, even after controlling for the influence of the number of action potentials per burst, the interspike interval between action potentials was significantly lower in pregnant women (inset). B: during the cold pressor test, the interspike interval was reduced in nonpregnant women, and there were no longer any differences between groups. Scatter plots represent the mean values for each woman, including the regression between action potentials per burst and the interspike interval. Bar graph data are presented as means ± SD for each group.
Action potential firing characteristics during the cold pressor (subanalysis).
The CPT increased heart rate, blood pressure, and cardiac output in both groups. However, TPR remained unchanged during the challenge in both pregnant and nonpregnant women (Table 1). Similar to our previously reported data (26), increases in integrated sympathetic burst frequency were more pronounced in pregnant women during the CPT (Table 1). During the CPT, median burst amplitude was no longer different between nonpregnant (43 ± 3) and pregnant (46 ± 11) women.
Surprisingly, the CPT tended to reduce the number of total action potentials per burst (main effect of condition; P = 0.053). This reduction in action potential firing was related to a shift in the firing occurrence of individual clusters toward single events (Fig. 3B). With both groups considered together, the shift in firing occurrence was weakly (but nonsignificantly; r2 = 0.13; P = 0.066) related to the shortening of the R-R interval during the CPT. When pregnant women were considered separately, both the reduction in the number of action potentials per burst (r2 = 0.531; P < 0.05) and the shift in firing occurrence toward single events (r2 = 0.452; P < 0.05) were significantly related to the shortening of the R-R interval during CPT. These relationships were not observed in nonpregnant women. Given that the reduction in the number of action potentials per burst occurred in conjunction with an increase in burst frequency, there was no net change in the number of action potentials occurring per minute in either nonpregnant (402 ± 242 spikes/min at rest vs. 420 ± 291 spikes/min during CPT) or pregnant (519 ± 311 spikes/min at rest vs. 520 ± 356 spikes/min during CPT) women.
During the cold pressor, the interspike interval was also no longer different between groups, although this was due to a specific reduction in the interspike interval in nonpregnant women (44 ± 20–37 ± 16 ms; P = 0.050; controlling for the signal-to-noise ratio and the number of action potentials within bursts; Fig. 4B).
DISCUSSION
Pregnancy is a unique, healthy state of persistent sympathetic excitation. In other examples of persistent sympathoexcitation (e.g., chronic obstructive pulmonary disease, obstructive sleep apnea, and obesity), not only is there an increase in the number of integrated bursts of activity, but a given neuron fires more often within a given burst. This contributes to an overall elevated number of firings per minute or per 100 hb (1, 4, 11).
To determine if the augmented integrated sympathetic activity observed in pregnant women is also associated with similar changes in neuronal activity, we compared the firing characteristics of populations of sympathetic neurons in normotensive pregnant women in the third trimester with nonpregnant controls. Despite higher baseline burst frequency and incidence in pregnant women, we observed that the number of action potentials and action potential clusters within individual bursts remained the same between groups. There were also no differences in the relative size of normalized recorded action potentials or in the occurrence of multiple firings of individual action potential clusters.
To assess whether neural firing and recruitment during stress were altered during pregnancy, we had a subset of women perform a cold pressor challenge. The CPT is a nonspecific, nociceptive stressor designed to cause sympathetic excitation. Although we observed greater increases in integrated burst frequency and incidence in the pregnant women, contrary to our expectation, action potentials per burst were reduced, not elevated, during the CPT in both groups. Additional analyses indicated that the reduction in neuronal firing per burst was related to R-R interval shortening and that this effect was most pronounced in pregnant women. Although this result was unexpected, it is consistent with the work of Tsukahara and Mano, who demonstrated that the firing frequency of single sympathetic neurons is positively related to the R-R interval (24a). Macefield and Elam (12a) also demonstrated this phenomenon in α-motoneurons, which had lower firing frequencies in relation to burst duration. This suggests that this principle is common across types of neurons.
Together, our data indicate that the activation of individual neuron firing within bursts of integrated sympathetic activity is not affected by pregnancy. Thus elevated sympathetic activity during normotensive pregnancy is specific to increased incidence of multiunit bursts. This is likely due to decreased central baroreflex gating of sympathetic burst output as opposed to generalized increases in central drive (17). This is in keeping with our recent data, demonstrating baroreflex resetting during pregnancy, favoring elevated burst incidence for a given arterial pressure (25) compared with age-matched, nonpregnant controls.
In this study, we have attempted to quantify aspects of neural signaling related to the burst amplitude component of the muscle SNA signal. Quantification of the amplitude component of the integrated muscle SNA signal, although intrinsically related to the number of active neurons, is complicated by the fact that absolute integrated burst amplitude is also affected by the size of neurons within the recording field, their proximity to the recording electrode, and the temporal relationship between action potentials (23). However, after the application of a normalization method (24), we demonstrate that pregnant women exhibit increased burst amplitudes (a right-skewed distribution) compared with controls. In the absence of an increased number or size of action potentials within a burst, the increase in average burst amplitude appears to be related specifically to neuronal firing occurring in closer temporal succession, observed as the inverse correlation between amplitude and interspike interval. As conduction velocity is an intrinsic property of neurons, which is dependent on neuron size (2, 23), it is unlikely that pregnancy affects the conduction velocity of neurons, such that action potentials arrive at the peripheral recording site in a closer temporal pattern. As such, the mechanism causing a reduced interspike interval in pregnancy remains unclear. However, closer neural firing raises the interesting question as to what the functional implications may be. In the neuromotor system, high-frequency firings of the same neuron result in a greater force of contraction (5). In the neurovascular system, higher impulse frequency is also associated with greater vasoconstriction (10, 18). This would suggest that an increased temporal association between action potential firing in healthy, pregnant individuals would tend to promote greater sympathetic vasoconstriction, directly contrary to the decrease in neurovascular transduction observed with pregnancy. These data further support the notion that sympathetic vasoconstriction is directly countered or offset during pregnancy at the level of the vasculature and that the magnitude of this offset is greater than previously quantified.
Considerations.
We recognize that the amplitude of recorded action potentials will depend on the size of the neuron and proximity to the recording electrode. However, there was a strong relationship between the number of detected action potentials within a burst and the number of active clusters. These data suggest that the number of detected action potentials is related to the pool of active neurons as opposed to multiple firing of individual neurons (23). Our distribution analysis of action potential occurrences indicated that a given cluster fires predominantly once in the majority of bursts (~60% of occurrences) and that multiple firings occur much less frequently. This is similar to single-unit recordings (13, 14) and suggests that a given cluster likely represents a single neuron. Therefore, we believe that our multiunit data provide a robust analysis of the firing characteristics of the active pool of sympathetic neurons. To conduct our analyses, we standardized the amount of data extracted to 100 bursts of activity for each woman. Although this resulted in a shorter duration of data in the pregnant group, we believe this had little bearing on indices of integrated activity. In fact, if a longer duration (>5 min) were assessed, then resting burst frequency data were nearly identical to that calculated from the 100 bursts that we extracted (23 ± 6 vs. 24 ± 6 bursts/min, respectively, for nonpregnant women; 38 ± 11 vs. 38 ± 9 bursts/min, respectively, for pregnant women).
Perspectives and Significance
Previous analyses of sympathetic regulation during pregnancy have focused on integrated sympathetic activity and the relationship between integrated burst frequency and incidence and vascular outcomes. As the analysis of integrated burst frequency and incidence is essentially the binary quantification of the presence or absence of bursts over time, there is a large amount of data loss. In theory, the number of active neurons within a burst may be increased or reduced, independent of changes in the number of bursts of activity occurring per minute or per 100 hb. As the “strength” of the vasoconstrictor signal is intrinsically related to the firing characteristics of individual and groups of neurons, the explanation of this physiology has important implications for understanding how vascular resistance is controlled in healthy and patient populations. In patients with obstructive sleep apnea, chronic obstructive pulmonary disease, and chronic hypertension, there is an increase in integrated burst frequency, as well as an increase in multiple firings of a given neuron within a burst of activity (1, 11). Similarly, chronic heart failure is associated with elevated, integrated sympathetic activity and an overall recruitment of a larger pool of active neurons, resulting in more action potentials per burst of activity (16). In contrast, normotensive, healthy pregnant women exhibit elevated, integrated sympathetic activity but without apparent increases in burst firing frequency or neuronal recruitment. This is noteworthy, as this suggests that increased neuronal firing and/or recruitment (in addition to elevated, integrated burst frequency or incidence) may be important factors in cardiovascular pathology.
In the current study, pregnant women in the third trimester had decreased TPR, and maintenance of lower-to-normal blood pressure, despite elevated sympathetic vasoconstrictor nerve activity. This indicates an offsetting of neurovascular control in normotensive pregnant women at rest. We (26) and others (9) have demonstrated a decrease in transduction (e.g., prevailing blood pressure or vascular resistance in relation to prevailing sympathetic activity) in pregnant women in the resting state and during periods of sympathetic activation. At rest, we did not observe any differences in the total population of active neurons or the number of action potentials per burst with given bursts of activity. Thus the mechanism(s) resulting in a decrease in neurovascular transduction are likely to occur downstream of neural signaling (e.g., neurotransmitter release and/or receptor sensitivity). These data reinforce the concept that pregnancy appears to be the only healthy state of chronic sympathetic hyperactivity of which we are aware.
GRANTS
Funding for this research was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN 06637), Women and Children’s Health Research Institute (WCHRI; WCHRIIG RES0018745 and WCHRI-GAP RES0028401), Heart and Stroke Foundation of Canada (HSFC; G-16-00014033 and RES0033140), and University of Alberta (UOFAB-BF-VPA RES0028400).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.W.U., M.K.S., C.G.J., R.C., R.K., S.T.D., M.H.D., and C.D.S. conceived and designed the study; C.W.U. and M.H.D. performed experiments; S.M.L.S., C.W.U., E.M., and C.D.S. analyzed data; S.M.L.S., C.W.U., M.H.D., and C.D.S. interpreted results of experiments; S.M.L.S. and C.D.S. prepared figures; S.M.L.S. and C.D.S. drafted manuscript; S.M.L.S., C.W.U., E.M., M.K.S., C.G.J., R.S.C., R.K., S.T.D., M.H.D., and C.D.S. edited and revised manuscript; S.M.L.S., C.W.U., E.M., M.K.S., C.G.J., R.S.C., R.K., S.T.D., M.H.D., and C.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Research was conducted in the laboratory of M. H. Davenport and C. D. Steinback.
REFERENCES
- 1.Ashley C, Burton D, Sverrisdottir YB, Sander M, McKenzie DK, Macefield VG. Firing probability and mean firing rates of human muscle vasoconstrictor neurones are elevated during chronic asphyxia. J Physiol 588: 701–712, 2010. doi: 10.1113/jphysiol.2009.185348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clamann HP, Henneman E. Electrical measurement of axon diameter and its use in relating motoneuron size to critical firing level. J Neurophysiol 39: 844–851, 1976. [DOI] [PubMed] [Google Scholar]
- 3.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- 4.Elam M, McKenzie D, Macefield V. Mechanisms of sympathoexcitation: single-unit analysis of muscle vasoconstrictor neurons in awake OSAS subjects. J Appl Physiol (1985) 93: 297–303, 2002. doi: 10.1152/japplphysiol.00899.2001. [DOI] [PubMed] [Google Scholar]
- 5.Garland SJ, Griffin L. Motor unit double discharges: statistical anomaly or functional entity? Can J Appl Physiol 24: 113–130, 1999. doi: 10.1139/h99-010. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation 104: 2200–2204, 2001. doi: 10.1161/hc4301.098253. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood JP, Scott EM, Walker JJ, Stoker JB, Mary DA. The magnitude of sympathetic hyperactivity in pregnancy-induced hypertension and preeclampsia. Am J Hypertens 16: 194–199, 2003. doi: 10.1016/S0895-7061(02)03256-9. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood JP, Stoker JB, Walker JJ, Mary DA. Sympathetic nerve discharge in normal pregnancy and pregnancy-induced hypertension. J Hypertens 16: 617–624, 1998. doi: 10.1097/00004872-199816050-00009. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis SS, Shibata S, Bivens TB, Okada Y, Casey BM, Levine BD, Fu Q. Sympathetic activation during early pregnancy in humans. J Physiol 590: 3535–3543, 2012. doi: 10.1113/jphysiol.2012.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jendzjowsky NG, Delorey DS. Short-term exercise training enhances functional sympatholysis through a nitric oxide-dependent mechanism. J Physiol 591: 1535–1549, 2013. doi: 10.1113/jphysiol.2012.238998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 50: 862–868, 2007. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 12.Macefield VG. Firing patterns of muscle vasoconstrictor neurons in respiratory disease. Front Physiol 3: 153, 2012. doi: 10.3389/fphys.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Macefield VG, Elam M. Comparison of the firing patterns of human postganglionic sympathetic neurones and spinal alpha motoneurones during brief bursts. Exp Physiol 89: 82–88, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Macefield VG, Wallin BG. Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol 516: 293–301, 1999. doi: 10.1111/j.1469-7793.1999.293aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol 481: 799–809, 1994. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 16.Maslov PZ, Breskovic T, Brewer DN, Shoemaker JK, Dujic Z. Recruitment pattern of sympathetic muscle neurons during premature ventricular contractions in heart failure patients and controls. Am J Physiol Regul Integr Comp Physiol 303: R1157–R1164, 2012. doi: 10.1152/ajpregu.00323.2012. [DOI] [PubMed] [Google Scholar]
- 17.McAllen RM, Malpas SC. Sympathetic burst activity: characteristics and significance. Clin Exp Pharmacol Physiol 24: 791–799, 1997. doi: 10.1111/j.1440-1681.1997.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson H, Ljung B, Sjöblom N, Wallin BG. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiol Scand 123: 303–309, 1985. doi: 10.1111/j.1748-1716.1985.tb07592.x. [DOI] [PubMed] [Google Scholar]
- 19.Okada Y, Best SA, Jarvis SS, Shibata S, Parker RS, Casey BM, Levine BD, Fu Q. Asian women have attenuated sympathetic activation but enhanced renal-adrenal responses during pregnancy compared to Caucasian women. J Physiol 593: 1159–1168, 2015. doi: 10.1113/jphysiol.2014.282277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmanpour A, Brown LJ, Shoemaker JK. Detection of single action potential in multi-unit postganglionic sympathetic nerve recordings in humans: a matched wavelet approach. Proc IEEE Int Conf Acoust Speech Signal Process 2010, p. 554–557. doi: 10.1109/ICASSP.2010.5495604. [DOI] [Google Scholar]
- 21.Salmanpour A, Brown LJ, Shoemaker JK. Spike detection in human muscle sympathetic nerve activity using a matched wavelet approach. J Neurosci Methods 193: 343–355, 2010. doi: 10.1016/j.jneumeth.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Scott DW. On optimal and data-based histograms. Biometrika 66: 605–610, 1979. doi: 10.1093/biomet/66.3.605. [DOI] [Google Scholar]
- 23.Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. J Physiol 588: 4825–4836, 2010. doi: 10.1113/jphysiol.2010.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sverrisdóttir YB, Rundqvist B, Elam M. Relative burst amplitude in human muscle sympathetic nerve activity: a sensitive indicator of altered sympathetic traffic. Clin Auton Res 8: 95–100, 1998. doi: 10.1007/BF02267819. [DOI] [PubMed] [Google Scholar]
- 24a.Tsukahara R, Mano T. The recruitment pattern of single vasoconstrictor neurons in human. J Auton Nerv Syst 66: 26–34, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Usselman CW, Skow RJ, Matenchuk BA, Chari RS, Julian CG, Stickland MK, Davenport MH, Steinback CD. Sympathetic baroreflex gain in normotensive pregnant women. J Appl Physiol (1985) 119: 468–474, 2015. doi: 10.1152/japplphysiol.00131.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usselman CW, Wakefield PK, Skow RJ, Stickland MK, Chari RS, Julian CG, Steinback CD, Davenport MH. Regulation of sympathetic nerve activity during the cold pressor test in normotensive pregnant and nonpregnant women. Hypertension 66: 858–864, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05964. [DOI] [PubMed] [Google Scholar]