Abstract
Acute kidney injury (AKI) is associated with high mortality rates and predisposes development of chronic kidney disease (CKD). Distant organ damage, particularly in the lung, may contribute to mortality in AKI patients. Animal models of AKI demonstrate an increase in pulmonary infiltration of lymphocytes and reveal an acute compromise of lung function, but the chronic effects of AKI on pulmonary inflammation are unknown. We hypothesized that in response to renal ischemia/reperfusion (I/R), there is a persistent systemic increase in Th17 cells with potential effects on pulmonary structure and function. Renal I/R injury was performed on rats, and CKD progression was hastened by unilateral nephrectomy and exposure to 4.0% sodium diet between 35 and 63 days post-I/R. Th17 cells in peripheral blood showed a progressive increase up to 63 days after recovery from I/R injury. Infiltration of leukocytes including Th17 cells was also elevated in bronchiolar lavage (BAL) fluid 7 days after I/R and remained elevated for up to 63 days. Lung histology demonstrated an increase in alveolar cellularity and a significant increase in picrosirius red staining. Suppression of lymphocytes with mycophenolate mofetil (MMF) or an IL-17 antagonist significantly reduced Th17 cell infiltration and fibrosis in lung. In addition, tracheal smooth muscle contraction to acetylcholine was significantly enhanced 63-days after I/R relative to sham-operated controls. These data suggest that AKI is associated with a persistent increase in circulating and lung Th17 cells which may promote pulmonary fibrosis and the potential alteration in airway contractility.
Keywords: kidney, lymphocyte, inflammation, ischemia reperfusion, trachea
INTRODUCTION
Acute kidney injury (AKI) is caused by multiple etiologies including hypoperfusion, sepsis, or nephrotoxicity and results in a rapid reduction in renal function (1). AKI rarely occurs in isolation and extra renal complications increase mortality (6, 11). Pulmonary complications are well described in the setting of AKI and may result from fluid overload, left ventricular cardiac dysfunction, increased lung capillary permeability, or acute lung injury (ALI) secondary to inflammation (11, 13, 14). AKI can lead to pulmonary edema and dubbed “uremic lung” and can increase AKI-associated mortality by up to ~80% (9).
Recent studies suggest that increased proinflammatory mediators produced by injured kidney promote the development of ALI. Klein et al. (19, 24) have demonstrated that AKI increases lung vascular permeability, inflammation, and dysfunction after renal ischemia/reperfusion (I/R) injury. Studies using IL-6-deficient mice or antibody blockade suggest a central role for IL-6 in the development of ALI in models of kidney injury (24). Several studies have outlined the rapid infiltration of inflammatory cells, including CD8+ and CD4+ T cells, and have implicated a role for these cells in lung injury during AKI (16, 17, 25, 27). For example, lung injury characterized by apoptosis and caspase-3 activation is reduced in T cell-deficient mice and restored by adoptive transfer of T cells (20).
AKI also increases the risk of developing chronic kidney disease (CKD) and end-stage renal disease (ESRD). To the best of our knowledge, lung-kidney cross-talk has been studied primarily during the early injury phase to AKI (i.e., 1–7 days after I/R injury) with little attention given to persistent lung alterations following recovery from AKI and/or subsequent development of CKD. The possibility that AKI can promote sustained alterations in distant organs is supported by studies demonstrating that skeletal muscle blood vessels elicit altered vascular responses for at least 5 wk after recovery from AKI (38). Because respiratory failure represents a significant risk factor for patients with CKD, it is possible that sustained effects of renal I/R injury may also promote sustained alterations in lung function.
We and others have suggested that sustained T-lymphocyte activity after recovery from AKI may contribute to the development of CKD and hypertension (7, 31, 42). With the use of an I/R model of AKI in rats, renal function based on serum creatinine returns to sham-operated control levels within ~1 wk, whereas renal tubular structure is also largely restored within 2–4 wk (1, 3, 4). Nevertheless, structural alterations persist in recovered kidneys (1, 3, 4), and subsequent exposure of rats to high-Na+ diet beginning at 5 wk after recovery from I/R promotes renal infiltration of immune cells and hastens the development of proteinuria, interstitial fibrosis, and hypertension (31, 42). Recent studies from our laboratory and others indicate that Th17 cells represent the primary lymphocyte population in kidney following I/R, where they contribute to both acute and progressive renal injury (8, 31). Th17 cells are CD3+/CD4+ cells that express the cytokine IL-17 and have been suggested to promote fibrosis in both kidney and lung (30, 40). We hypothesized that lymphocyte infiltration in lungs after I/R persists following recovery from AKI and may reflect an abundance of Th17 cells or other IL17-expressing cells (such as natural killer cells), as observed in kidneys (30). We further hypothesize that increased dietary Na+ following recovery from AKI may further exacerbate lung infiltration of Th17 cells, which may promote further inflammation or fibrosis. Therefore, we conducted the following studies to evaluate the potential role of lymphocytes on lung structure and inflammation in a model of the AKI-to-CKD transition.
METHODS
Animals.
Rats were maintained in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by Institutional Animal Care and Use Committees at Indiana University. Study I used male Sprague-Dawley rats (250–300g) that were purchased from Harlan (Indianapolis, IN). An additional strain of rats was used for study II and study III, which represent the control strain for athymic rats (Foxn1rnu-/rnu-) purchased from Harlan. These studies used the corresponding euthymic wild-type littermates (Foxn1rnu-/rnu+). Rats were used at a starting weight of ~200–250 g.
Study protocols.
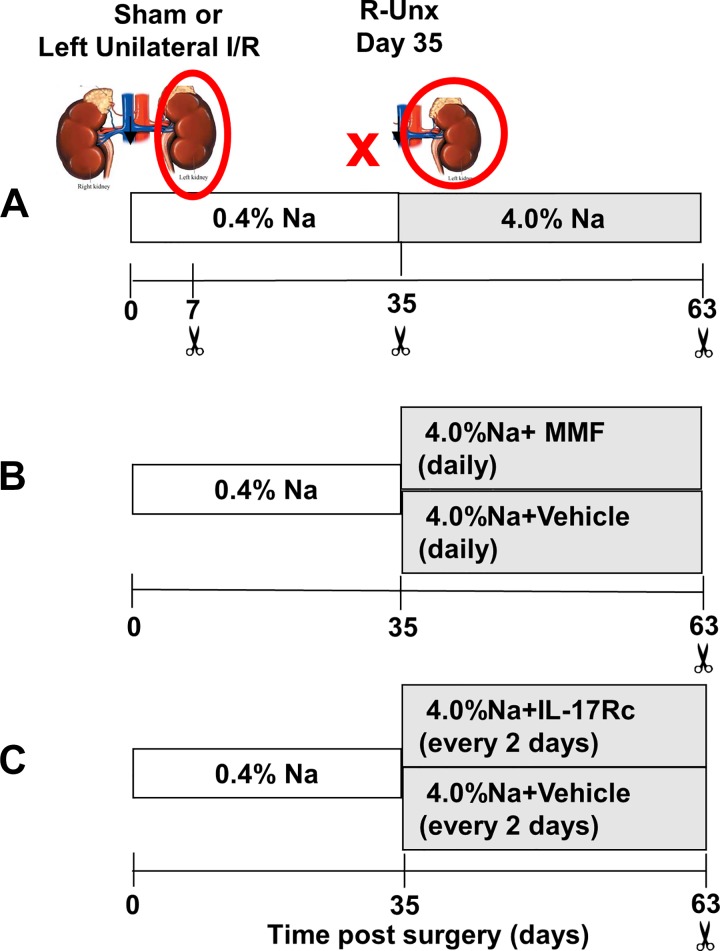
Study I was designed to evaluate the infiltration of immune cells into lungs in response to renal I/R as function of time. Rats were subjected to unilateral (left) I/R injury by clamping the renal pedicle for 40 min using a surgical approach described previously and then allowed to recover for 7, 35, or 63 days after surgery and were provided a standard diet (AIN 76A, Dyets, Bethlehem, PA) containing 0.4% NaCl (Fig. 1A). Some of rats were euthanized at day 7 or 35 days postsurgery, the remaining rats were subjected to unilateral nephrectomy (UNx) of the contralateral noninjured kidney at 33 days; rats were subsequently exposed to elevated dietary Na+(4% NaCl) for ~4 wk. Sham-control rats were not subjected to renal pedicle clamping but were subjected to UNx. The degree of renal injury and recovery by 35 days, the subsequent progression of renal fibrosis and proteinuria in response to high-salt diet, were originally described in a previous publication (4).
Fig. 1.
Experimental schema of studies to investigate the effect of renal ischemic injury on immune cell infiltration in lungs. The studies presented utilize a model of chronic kidney disease (CKD) after recovery from renal ischemia/reperfusion (I/R) injury. A: study I investigated the infiltration of immune cells in the lung after renal ischemic injury in rats as a function of time. Renal injury was induced by unilateral I/R injury to the left kidney by clamping the renal pedicle for 40 min (red circle). Rats were allowed to recover on 0.4% NaCl diet. On day 33, rats were subjected to unilateral nephrectomy (UNx) (indicated by red X) and subsequently placed on a high-salt diet (day 35; 4% NaCl). Sham-operated rats received similar treatment without clamping on day 0 but with UNx at day 33. Tissues were harvested at 7, 35, or 63 days postsurgery. B: study II investigated the effect of T cell inhibition on cell infiltration in the lung after recovery from renal I/R. Rats were treated during the high-salt phase from day 35 to day 63 with either mycophenolate mofetil (MMF, 30 mg·kg−1·day−1) or vehicle daily as described previously (30). C: study III was designed to investigate the effect of IL-17 antagonism on persistent lung infiltration after recovery from I/R injury by treating rats with IL-17RC or vehicle during the high-salt phase from day 35 to day 63 as described previously (30).
Study II was designed to evaluate the role of T-lymphocyte activity in pulmonary fibrosis after renal I/R. To inhibit T cell activity, rats were randomly treated from 35 to 63 days with either mycophenolate mofetil (MMF, 30 mg·kg−1·day−1; Accord Healthcare, Durham NC) or vehicle (sugar-free chocolate pudding at 1 g/kg) once daily concomitant with introduction of high-salt diet (Fig. 1B). Rats were observed to ensure that the daily doses were ingested completely.
Study III was designed to study the effect of IL-17 antagonism on development of pulmonary fibrosis following recovery from renal I/R. To inhibit the effects of IL-17, mouse recombinant IL-17Rc-soluble receptor (2270-ml-050: 150 ng/day ip; R&D Systems) was administered to rats every other day beginning at the time of exposure to high-salt diet (from day 3 to day 63) (Fig. 1C) (10). No sham-operated rats were included in study III. Data pertaining to the progression of renal fibrosis in the same rats used in study II and study III were included in a recent publication (30).
FACS analysis.
At the end of all studies, rats were deeply anesthetized with 50–100 mg/kg pentobarbital sodium (Fatal Plus, Vortech, Dearborn, MI). Bronchoalveolar lavage (BAL) was collected by cannulating the trachea and washing the lungs using 10 ml of sterile PBS. All antibodies were obtained from BD Biosciences (San Jose, CA) unless otherwise indicated. To evaluate T lymphocytes in BAL fluid, cells were stained with antibodies against rat CD4 (PE-Cy7, clone OX53), CD8a (Alexa 647, clone OX-8), CD25 (FITC, clone OX-39), and CD62L (PE, clone HRL1). To evaluate cytokines secreted by T cells, cells were stained for CD4, permeabilized using saponin, and stained with antibodies against rat IFN-γ (FITC, clone DB-1), IL-4 (PE, clone OX-81), IL-17 (FITC clone eBio 17B7), and TNF-α (PE, eBiosciences, clone TN3-19). B cells were stained with antibody against RTIB (FITC, clone OX-6), and macrophages were stained using anti-CD103 (PE; clone OX62). The specificity of the IL17A antibody (clone eBio 17B7) in a fluorescence-activated cell sorting (FACS) application was demonstrated in a study in which the antibody detected a prominent IL17A signal in CD4 cells from wild-type mice with autoimmune encephalomyelitis but did not detect a signal in CD4 cells from IL17−/− mice (22). Additional information regarding the target specificity of antibodies used in this study is available on the manufacturers’ websites. The antibody concentrations used during staining were based on the manufacturers’ recommendations.
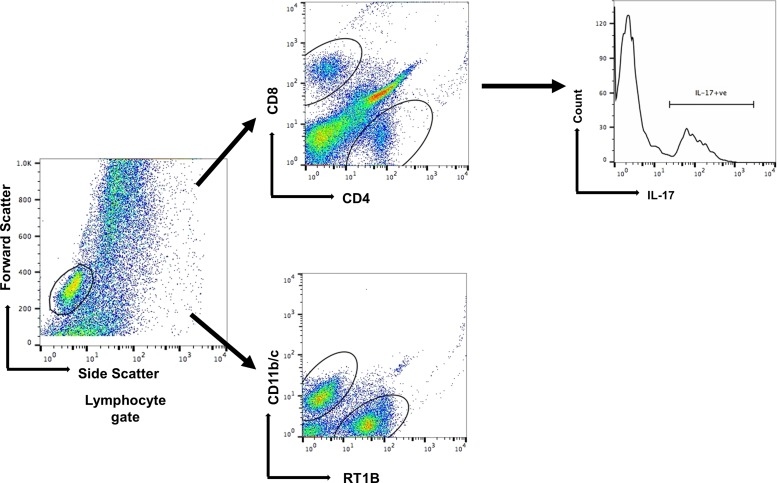
Cells were scanned using flow cytometry (FACSCalibur, BD Biosciences), and scans were analyzed using Flowjo software (Tree Star, Ashland, OR). Lymphocyte gating strategy was described previously (30). A representative series of FACS scans from BAL fluid is shown in Fig. 2. The data are expressed a percentage of the grandparent (lymphocyte gating) as calculated by the Flowjo software.
Fig. 2.
Representative fluorescence-activated cell sorting (FACS) analysis of cells obtained from bronchoalveolar lavage (BAL) fluid of rats after ischemia/reperfusion (I/R). Gating strategies for the phenotypic analysis of infiltrating CD4+ T cells in the BAL fluid. Lymphocyte gating is defined based on the forward scatter versus side scatter, which is further gated on CD4+, CD8, RT1B cells, and CD11b/c (DCs). The CD4+ T cells were analyzed further based on the IL-17 production.
Lung histology.
At the time of tissue harvest, one lobe of the lung was inflated and fixed with 10% formalin and embedded in paraffin, and 5-µm sections were stained with either hematoxylin and eosin (H&E) or picrosirus red to assess fibrosis. For quantitative analysis, five random images of the airways and the adjacent parenchyma were obtained using Leica DMLB (Scientific Instruments, Columbus, OH) microscope with a ×20 objective. The percent area of picrosirus red stain was calculated relative to total parenchyma area using ImageJ (NIH) similar to methods described previously (30). To quantify H&E sections, computer-aided image analysis (Image J, NIH) was used to apply an arbitrary grid (8×10) to define regions within the photomicrograph, and the number of cells and alveoli were manually counted in a subset of 6–8 regions, which were selected a priori based on their location within the photomicrograph. However, in an effort to focus on alveolar structure, regions containing larger airways were excluded. The size of each region counted was ~1.2% of the entire area of the image. Data are presented as the average number cells per region.
Airway contractility.
Tracheas from post-AKI or sham-operated rats were excised and cut into 4- to 5-mm rings. Tracheal rings were then suspended in 25-ml organ baths filled with physiological saline solution (composition in mM: 110 NaCl, 3.4 KCl, 2.4 CaCl2, 0.8 MgSO4, 25.8 NaHCO3, 1.2 KH2PO4, and 5.6 glucose) at 37°C and bubbled with gas containing 95% O2-5% CO2. Force was measured during isometric contractions by attaching the tissues to Grass force-displacement transducers. Before the beginning of each experimental protocol, the resting tension on the tracheal ring was increased to a preload of 1 g. After a 15-min equilibration period, concentration-response curves to acetylcholine (ACh) were performed by the cumulative addition of increasing concentrations of ACh. Active tension for each concentration was calculated as the increase in contractile force in response to ACh above the preload force.
Statistical analysis.
All data are expressed as means ± SE. Differences in means were established by Student's t-test or one-way ANOVA with Tukey’s multiple comparison test as indicated in the figure legends. Analysis was done with the aid of SigmaPlot software (Version 11.0, Systat, San Jose, CA).
RESULTS
Persistent systemic lymphocyte activity and lung infiltration following AKI.
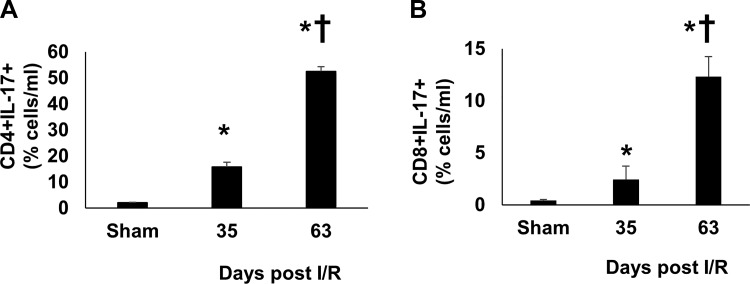
We initially sought to determine if the rat AKI-to-CKD model (Fig. 1A) was associated with a systemic increase in Th17 cells. Peripheral blood from post-AKI rats was analyzed by flow cytometry, which demonstrated an increase in both Th17 cells (CD4+/IL-17+) cells and CD8+/IL-17+ cells at 35 days post-I/R (Fig. 3, A and B). Interestingly, upon exposure to a high-salt diet for an additional 28 days, a maneuver previously shown to result in hypertension and CKD progression, the number of circulating IL-17+ cells was significantly enhanced relative to the 35-day post-I/R time point (Fig. 3, A and B).
Fig. 3.
Increased expression of Th17 lymphocytes in circulating blood of rats after recovery from ischemia/reperfusion (I/R). Lymphocytes were isolated from peripheral blood collected from renal I/R-injured rats 35 and 63 days after surgery and from sham-operated rats. Total CD4+IL-17+ (A) and CD8+IL-17+ (B) double positive cells are shown. Data are means ± SE. *P < 0.05 vs. sham group; †P < 0.05 35 days vs. 63 days, by one ANOVA and Tukey’s post-hoc test (n = 3–5 animals/group).
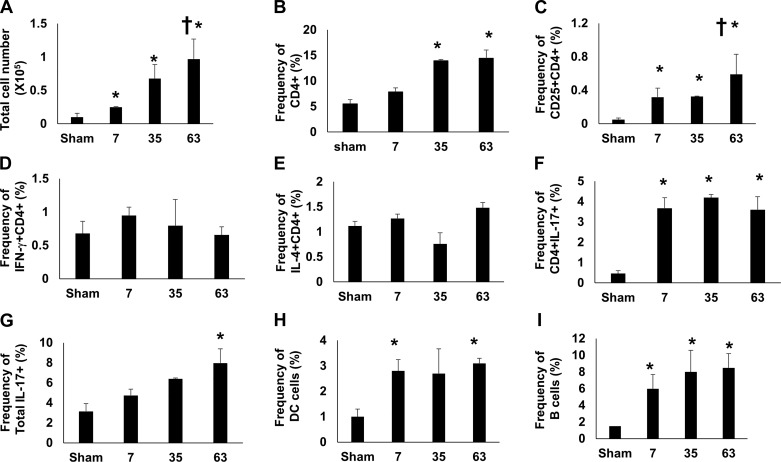
Within the lung, there was a modest but significant increase in BAL fluid cell infiltration relative to sham-control rats at 7 days post-I/R. With prolonged recovery, BAL fluid infiltration remained significantly elevated versus sham at 35 and 63 days post-I/R. The progressive increase in infiltrating cells was significantly higher at 63 days post-I/R versus 7 days post-I/R, whereas the intermediate level of infiltration at 35 days post-I/R was not statistically significant versus day 7 (Fig. 4A).
Fig. 4.
Phenotypic analysis of infiltrating cells in bronchoalveolar lavage (BAL) fluid after recovery from renal ischemia/reperfusion (I/R). BAL fluid was isolated from rats after sham surgery or 7, 35, and 63 days afterg recovery from renal I/R. A: total number of cells recovered in the BAL fluid of renal-injured rats. B: frequency of CD4+ cells, expressed as a percentage of cells in BAL fluid. The frequency of double-positive CD4+ cells are shown for CD25+CD4+ (C) IFN-γ+ CD4+ (Th1) (D), IL4+CD4+ (Th2) (E), and IL17+CD4+ (Th17) (F). G: percentage of all IL17+-expressing cells. H: percentage of B cells in BAL. I: percentage of dendritic cells (DCs) in BAL. Data are means ± SE. *P < 0.05 vs. sham group; †P < 0.05 in 63 -days vs. 7 days post I/R in A and 63 day vs. 7 and 35 days in C, by one-way ANOVA and Tukey’s post hoc test (n = 3–6 animals/group).
The percent frequency of CD4+ cells within the BAL fluid was significantly elevated at both 35 and 63 days relative to sham, whereas a trend of CD8+ cells relative to sham was not statistically significant (not shown). Within the CD4+ population, the frequency of activated T cells (CD4+CD25+) was significantly elevated at 7 and 35 days and further enhanced following high-salt diet by day 63 (Fig. 4C). Analysis of T-helper subpopulations indicated that Th1 (IFN-γ+) and Th2 cells (IL-4+) were unchanged by renal I/R (Fig. 4, D and E). However, the percentage of Th17 cells (as CD4+/IL17+) in BAL fluid was significantly increased at all time points post-I/R relative to sham (Fig. 4F), whereas the percentage of all cells expressing IL-17 was significant at 63 days (Fig. 4G). In addition, both B cells and dendritic cells (DCs) were significantly enhanced at all time points after recovery from I/R (Fig. 4, H and I).
Alterations in the lung structure in response to renal I/R.
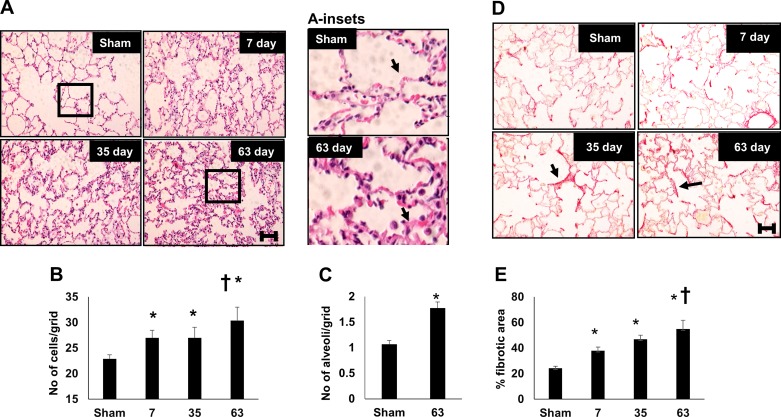
Figure 5 illustrates alterations in lung structure following recovery from I/R. Increased cell density within the alveolar wall was consistently evident at both 7 and 35 days post-I/R, and cell density was further increased at day 63 after exposure to a high-salt diet (Fig. 5, A and B). This increase in cell density can be appreciated by the apparent increase in alveolar wall thickness (black arrows in Fig. 5A, inset). In addition, there was a decrease in alveolar size post-I/R relative to sham (Fig. 5C). Picrosirius red staining, illustrative of collagen deposition, was evident in lung of post-I/R rats and localized primarily near the alveolar wall (Fig. 5D, small arrow). The degree of picrosirius red staining progressively increased with the duration of recovery from renal I/R injury (Fig. 5E).
Fig. 5.
Renal ischemia-reperfusion (I/R) injury results in persistent alterations in lung structure. A: representative hematoxylin and eosin (H&E)-stained images of lung parenchyma obtained from rats after sham surgery or after recovery from renal I/R. Inset: a higher magnification is also shown. Quantification of cell density expressed as number per arbitrary grid (B) or the number of alveoli per arbitrary grid (C) is shown. D: representative images of picrosirus red-stained lung cross section. E: percent picrosirius red-stained relative to total cellular area. Magnification bar (100 µm) is shown in bottom panels. Data are means ± SE. For B and E, *P < 0.05 I/R groups vs. sham; †P < 0.05 vs. 7 day post-I/R, by one-way ANOVA and Tukey’s post hoc test. For C, *P < 0.05 by Student’s t-test. (n = 3–4 rats per group).
Blockade of lung inflammation attenuates changes in lung morphology after AKI.
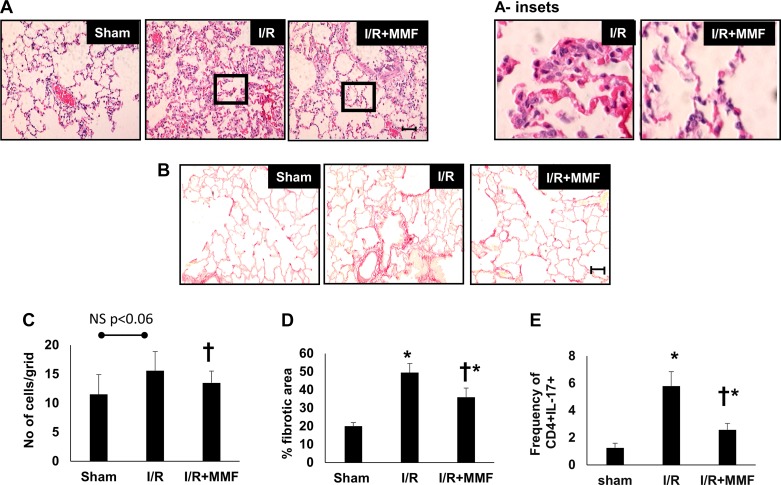
Study II was conducted to investigate the effect of lymphocyte inhibition during the AKI-to-CKD transition in rats (see Fig. 1B). MMF significantly attenuated the frequency of CD4+/IL-17+ cells in BAL fluid (Fig. 6E) but did not influence the frequency of total CD4 cells (not shown). In addition, both B cells and DCs were significantly reduced by MMF treatment in postischemic rats (Table 1). MMF treatment reduced cell density in the lung parenchyma measured in H&E-stained sections, relative to vehicle-treated post-AKI rats (Fig. 6, A and C). Additionally, picrosirius red staining of collagen in the lungs was also significantly reduced by MMF treatment relative to vehicle controls (Fig. 6, B and D).
Fig. 6.
Effect of lymphocyte inhibition on Th17 cell infiltration and lung fibrosis after recovery from renal ischemia-reperfusion (I/R). Postischemic rats were treated with vehicle (I/R) or mycophenolate mofetil (MMF) (I/R + MMF) from day 35 to day 63, and lung infiltration and structure were examined. Representative hematoxylin and eosin (H&E)-stained (A) and picrosirus red-stained lung sections (B) are shown from sham, I/R-vehicle, I/R MMF-treated rats. Inset: a higher magnification. Magnification bar is 100 µm. C: quantification of cell density from H&E-stained sections. D: quantification of fibrosis based on picrosirius red staining. E: frequency of Th17 cells (CD4+/IL-17+) in the bronchoalveolar lavage (BAL) fluid. Data are means ± SE. *P < 0.05 I/R vehicle vs. sham group; †P < 0.05 I/R MMF vs. I/R vehicle using ANOVA and Tukey’s post hoc test (n = 5–8 animals/group).
Table1.
Frequency of bronchoalveolar lavage fluid-derived B cells and dendritic cells after recovery from ischemia-reperfusion and effects of mycophenolate mofetil or IL17RC
| Frequency of DC | Frequency of B cells | |
|---|---|---|
| Study II | ||
| Sham | 0.75 ± 0.01 | 0.9 ± 0.1 |
| I/R+vehicle | 4.5 ± 2.6* | 10.3 ± 1.6* |
| I/R+MMF | 1.9 ± 1.5† | 3.5 ± 0.9† |
| Study III | ||
| IR-vehicle | 3.9 ± 1.4 | 9.4 ± 1.8 |
| IR+ IL-17Rc | 1.3 ± 0.6‡ | 2.6 ± 1.4‡ |
Data are expressed as percent frequency and are means ± SE. Study II, mycophenolate mofetil; study III, IL17RC.
P < 0.05 sham vs. I/R vehicle;
P < 0.05 I/R MMF vs. I/R vehicle, by one-way ANOVA and Tukey’s post-hoc test.
P < 0.05 IL17RC vs. vehicle, by Student’s t-test.
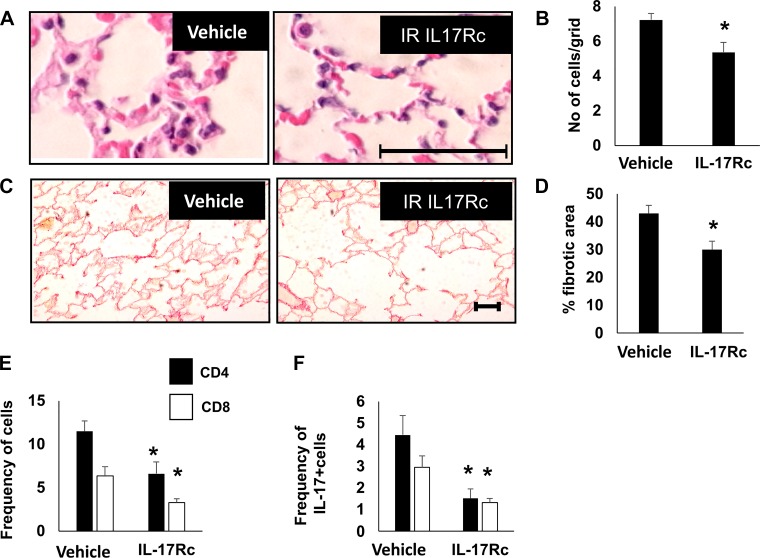
To assess whether infiltration of IL17+ cells influence the alterations in lung structure post-AKI, IL-17RC was administered to rats during the time of high-salt treatment, (Study III, Fig. 1C). Administration of IL-17RC in post-I/R rats significantly attenuated the degree of alveolar cellularity (Fig. 7, A and B) and picrosirius red staining relative to vehicle-treated post-I/R rats (Fig. 7, C and D). Treatment with IL-17RC significantly attenuated the frequency of CD4+ and CD8+ cells (Fig. 7E) as well as the frequency CD4+/IL17+ or CD8+/IL17 cells in BAL fluid (Fig. 7F). IL-17Rc treatment also reduced the frequency of B cells and DCs in the BAL fluid of postischemic rats (Table 1). Taken together, these data suggest that increased lymphocyte IL-17 activity following recovery from AKI promotes chronic alterations in lung structure.
Fig. 7.
Effect of systemic IL-17 blockade on infiltrating pulmonary immune cells and lung structure after recovery from renal ischemia-reperfusion (I/R). Representative hematoxylin and eosin (H&E)-stained (A) and picrosirius red-stained sections (C) through rat lung are shown from I/R+vehicle and I/R+IL17Rc-treated rats; Bar = 100 µm. Quantification of cell density based in H&E-stained sections (B) and fibrosis based on picrosirius red staining (D) is shown. E: frequency of CD4 and CD8 cells in bronchoalveolar lavage (BAL) fluid; F: frequency of CD4+IL-17+ and CD8+IL-17+. Data are means ± SE. *P < 0.05 I/R vehicle vs. IL17RC by Student’s t-test. n = 5–7 per group.
Altered airway contractility after AKI.
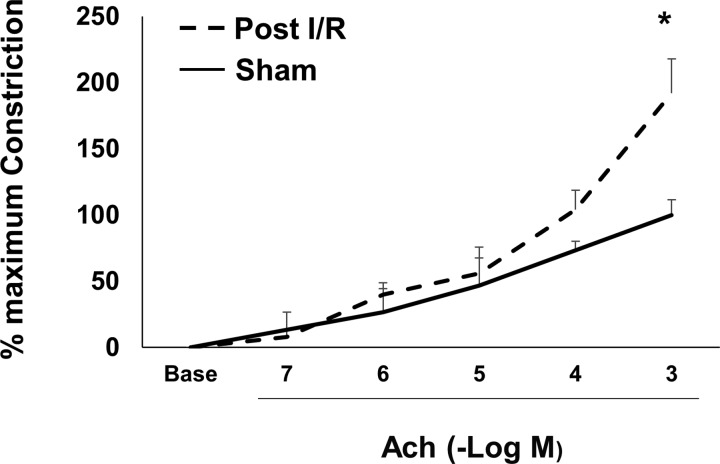
Inflammation is known to result in increased airway smooth muscle reactivity. To determine if recovery from AKI alters airway reactivity, constriction responses from trachea of rats at 63 days after recovery from I/R or sham surgery were determined. Figure 8 demonstrates that trachea from post-I/R rats manifests an enhanced constriction response to increasing concentrations of acetylcholine relative to sham-operated control rats.
Fig. 8.
Recovery from renal ischemia-reperfusion (I/R) alters airway reactivity. Rats were subjected to renal I/R and allowed to recover for 63 days outlined in Fig. 1. Rat tracheal rings were analyzed for constriction responses to increasing concentrations of acetylcholine (ACh). The maximal constriction response in trachea from sham-operated control rats was defined as 100%. Data are expressed as percent response at each dose. *P < 0.05 I/R vs. sham-operated group; (n = 4 rats/group).
DISCUSSION
AKI is a systemic disease often associated with dysfunction of multiple organs including brain, heart, lung, liver, and small intestine. In animal models of renal I/R, alterations in lung function and inflammation are well established; however, the current report suggests both systemic and lung inflammation are persistent despite the initial recovery from I/R and steadily increase during transition to CKD.
After renal I/R, inflammatory cytokines and chemokines such as IL-1β, IL-6, KC (IL-8), TNF-α, TNF-β, INF-γ, IL-17A, C5a, and MCP-1 are increased in serum as well as in distant organs such as the heart, which may potentially alter organ function (23). Increased expression of proinflammatory cytokines such as KC (IL-8) and granulocyte colony-stimulating factor has been measured in the brain in post-I/R rats (28). Changes in the brain microvasculature leading to increased susceptibility to stroke have been reported in AKI (29). Hepatic and small intestine dysfunction have also been observed in patients suffering from AKI (32, 34).
It is well known that AKI predisposes the progression of CKD and ESRD (1, 2). AKI also increases long-term mortality rates associated with cardiovascular and pulmonary diseases as well as deaths due to other unidentified causes (33). Increased pulmonary vascular permeability, inflammation, and apoptosis are thought to be important mechanisms connecting AKI to acute lung dysfunction (5, 41). Based on the data presented in the current report using a rat model of AKI, chronic impairment in lung function may represent an unidentified contributor toward increased mortality in post-I/R patients.
Distant organ effects may be attributable to sustained chronic inflammation despite the apparent resolution of renal function after AKI. For example, our laboratory demonstrated that unilateral renal I/R persistently increased CD4+ T cells and altered the function of the contralateral kidney up to 5 wk after injury (4). This activity may have promoted impaired renal hemodynamic responses, impaired sodium handling responses, and predisposition to hypertension (37). We also demonstrated that peripheral blood vessels manifest sustained oxidative stress for at least 5 wk after injury, which mediates increased constriction responses to ANG II (38).
We propose that many of these persistent alterations in extra renal function may be due to an increase in Th17 activity. It is interesting that IL17-expressing cells appear to increase progressively in blood and BAL fluid. This is in contrast to results in which the renal expression of Th17 cells resolves within 7 days after renal I/R and is maintained at lower levels for up to 5 wk of recovery (31). However, renal Th17 cells are prominently enhanced in post-I/R kidneys after exposure to elevated dietary salt (31). This pattern of expression is consistent with the development of renal fibrosis and hypertension in response to a high-salt diet and the ability of MMF to prevent this response when provided simultaneously with a high-salt diet (36). Similarly, we recently showed that IL-17 blockade also prevented renal fibrosis and inflammation when provided over the same time period (30).
Th17 cells secrete the cytokine IL-17, which can contribute to multiple activities with pathological influence. For example, IL-17 stimulates neutrophil chemotaxis and has been shown to increase tissue fibrosis (15, 18). The current study supports the view that the rat model of AKI-to-CKD can be considered a systemic inflammatory disorder in which Th17 cells are enhanced both in circulating blood as well as distant organs such as lung. Furthermore, AKI was associated with detection of lung fibrosis and this was significantly, but not completely, attenuated by both lymphocyte inhibition and by IL-17 antagonism. This is consistent with prior studies indicating that Th17 cells are associated with pulmonary fibrosis (12, 35, 39, 43).
In the current study, two approaches, MMF and IL17Rc, mitigated fibrosis and Th17 cell infiltration. While the use of MMF reduces activity of all lymphocytes, IL17Rc is a competitive inhibitor of IL-17R, suggesting that IL17-expressing cells, including Th17 cells, influence lung structure post-IR injury. However, it is not known whether alterations in lung structure are due to the direct action of IL17 on lung parenchyma or secondary to recruitment of other cells types. Both approaches also attenuated the infiltration of B cells as well as dendritic cells. Therefore, it is also possible that IL-17 promotes infiltration of other cell types, which may mediate pathological effects. However, the current study cannot distinguish between these possibilities.
Finally, the model of AKI-to-CKD with a high-salt diet is associated with elevated blood pressure, which is partially mediated by immune cell activity (36). Therefore, it is also possible that the damaging effects of AKI on lung structure may occur secondary to changes in pulmonary blood pressure, which could be alleviated by suppression of immune cells. This possibility is one that deserves future consideration.
Whether or not pulmonary fibrosis leads to impaired lung function in the model of AKI-to-CKD is not yet clear. In an effort to determine if AKI might alter lung function, we demonstrated increased sensitivity of airway smooth muscle constriction after recovery from renal I/R. Whether this alteration is due to persistent inflammation and/or the activity of IL-17 is not yet clear; however it is important to note that IL-17 has also been shown to enhance airway smooth muscle contraction (26). Thus it can be envisioned that increased Th17 activity present following recovery from AKI may mediate alterations in lung function, a possibility that should be further investigated.
Perspectives and Significance
This study provides evidence of a sustained systemic inflammatory response characterized in part by an elevation in Th17 cells both in the circulating blood as well as the lung. Data using a rat model of AKI-to-CKD suggest there is a persistent nature of lung inflammation, which has not yet been previously appreciated. Whether these alterations translate to human disease are not yet known. It is of interest that dialysis patients have a higher incidence of asthma (21) and pulmonary hypertension (44), two conditions previously shown to be associated with increased Th17 activity. Therefore, there is potential that AKI effects on lung may be more long lasting than has previously been appreciated and should be examined more closely.
GRANTS
This work was supported by National Institutes of Health (NIH) DK063114 (to D. Basile) and by Ralph W. and Grace M. Showalter Research Trust Fund (to P. Mehrotra). Additional support was provided by Fortune Fry Funding from the Indiana University Research Foundation (to D. Basile). J. A. Collett was supported by NIH T32 5T32HL079995-10 and National Institutes of Health/National Center for Advancing Translational Science (NIH/NCATS) TL1 Postdoctoral Fellowship TL1TR001107 (to A. Shekar, PI). S. J. Gunst was supported by NIH HL-29289.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M., S.J.G., and D.P.B. conceived and designed research; P.M. and J.A.C. performed experiments; P.M. and S.J.G. analyzed data; P.M. and D.P.B. interpreted results of experiments; P.M. and J.A.C. prepared figures; P.M. and D.P.B. drafted manuscript; P.M., J.A.C., and D.P.B. edited and revised manuscript; P.M., J.A.C., S.J.G., and D.P.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Stone Chen and Lucia Grill of the Indianapolis Project STEM assistance in data analysis.
REFERENCES
- 1.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C; ADQI XIII Work Group . Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 27: 687–697, 2016. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 4.Basile DP, Leonard EC, Tonade D, Friedrich JL, Goenka S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. Am J Physiol Renal Physiol 302: F625–F635, 2012. doi: 10.1152/ajprenal.00562.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu RK, Wheeler DS. Kidney-lung cross-talk and acute kidney injury. Pediatr Nephrol 28: 2239–2248, 2013. doi: 10.1007/s00467-012-2386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen D, Bihari D. Acute renal failure as a part of multiple organ failure: the slippery slope of critical illness. Kidney Int Suppl 66, Suppl: S25–S33, 1998. [PubMed] [Google Scholar]
- 7.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001. doi: 10.1172/JCI200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan AJ, Alikhan MA, Odobasic D, Gan PY, Khouri MB, Steinmetz OM, Mansell AS, Kitching AR, Holdsworth SR, Summers SA. Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am J Pathol 184: 1411–1418, 2014. doi: 10.1016/j.ajpath.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155: 1505–1511, 1995. doi: 10.1001/archinte.1995.00430140075007. [DOI] [PubMed] [Google Scholar]
- 10.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62: 1068–1073, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi K, Ishizu T, Fujita T, Noiri E. Lung injury following acute kidney injury: kidney-lung crosstalk. Clin Exp Nephrol 15: 464–470, 2011. doi: 10.1007/s10157-011-0459-4. [DOI] [PubMed] [Google Scholar]
- 12.Dong Z, Lu X, Yang Y, Zhang T, Li Y, Chai Y, Lei W, Li C, Ai L, Tai W. IL-27 alleviates the bleomycin-induced pulmonary fibrosis by regulating the Th17 cell differentiation. BMC Pulm Med 15: 13, 2015. doi: 10.1186/s12890-015-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faubel S. Acute kidney injury and multiple organ dysfunction syndrome. Minerva Urol Nefrol 61: 171–188, 2009. [PubMed] [Google Scholar]
- 14.Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis 15: 284–296, 2008. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Gifford AM, Chalmers JD. The role of neutrophils in cystic fibrosis. Curr Opin Hematol 21: 16–22, 2014. doi: 10.1097/MOH.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 16.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 19: 547–558, 2008. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurkan OU, He C, Zielinski R, Rabb H, King LS, Dodd-o JM, D’Alessio FR, Aggarwal N, Pearse D, Becker PM. Interleukin-6 mediates pulmonary vascular permeability in a two-hit model of ventilator-associated lung injury. Exp Lung Res 37: 575–584, 2011. doi: 10.3109/01902148.2011.620680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan SA, Eksteen B, Reid D, Paine HV, Alansary A, Johannson K, Gwozd C, Goring KA, Vo T, Proud D, Kelly MM. Role of IL-17A and neutrophils in fibrosis in experimental hypersensitivity pneumonitis. J Allergy Clin Immunol 131: 1663–1673, 2013. doi: 10.1016/j.jaci.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, Rabb H. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol 293: F30–F40, 2007. doi: 10.1152/ajprenal.00023.2007. [DOI] [PubMed] [Google Scholar]
- 20.Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol 297: F125–F137, 2009. doi: 10.1152/ajprenal.90666.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H-L, Ho S-Y, Li C-H, Chu F-Y, Ciou L-P, Lee H-C, Chen W-L, Tzeng N-S. Bronchial asthma is associated with increased risk of chronic kidney disease. BMC Pulm Med 14: 80, 2014. doi: 10.1186/1471-2466-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, Guralnik A, Bollig N, Jeltsch K, Heinemann C, Wittmann E, Buch T, Prazeres da Costa O, Brüstle A, Brenner D, Mak TW, Mittrücker H-W, Tackenberg B, Kamradt T, Lohoff M. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest 123: 247–260, 2013. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003. doi: 10.1097/01.ASN.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 24.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 74: 901–909, 2008. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 25.Ko GJ, Rabb H, Hassoun HT. Kidney-lung crosstalk in the critically ill patient. Blood Purif 28: 75–83, 2009. doi: 10.1159/000218087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554, 2012. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lie ML, White LE, Santora RJ, Park JM, Rabb H, Hassoun HT. Lung T lymphocyte trafficking and activation during ischemic acute kidney injury. J Immunol 189: 2843–2851, 2012. doi: 10.4049/jimmunol.1103254. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19: 1360–1370, 2008. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol 11: 707–719, 2015. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 30.Mehrotra P, Collett JA, McKinney SD, Stevens J, Ivancic CM, Basile DP. IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: compensatory role of natural killer cells in athymic rats. Am J Physiol Renal Physiol 312: F385–F397, 2017. doi: 10.1152/ajprenal.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehrotra P, Patel JB, Ivancic CM, Collett JA, Basile DP. Th-17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by AT-1R antagonism. Kidney Int 88: 776–784, 2015. doi: 10.1038/ki.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore JK, Love E, Craig DG, Hayes PC, Simpson KJ. Acute kidney injury in acute liver failure: a review. Expert Rev Gastroenterol Hepatol 7: 701–712, 2013. doi: 10.1586/17474124.2013.837264. [DOI] [PubMed] [Google Scholar]
- 33.Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, Hunn BH. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 28: 377–387, 2017. doi: 10.1681/ASN.2016010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SW, Chen SW, Kim M, Brown KM, Kolls JK, D’Agati VD, Lee HT. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest 91: 63–84, 2011. doi: 10.1038/labinvest.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paun A, Bergeron M-E, Haston CK. The Th1/Th17 balance dictates the fibrosis response in murine radiation-induced lung disease. Sci Rep 7: 11586, 2017. doi: 10.1038/s41598-017-11656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008. doi: 10.1152/ajpregu.00821.2007. [DOI] [PubMed] [Google Scholar]
- 37.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–R1363, 2009. doi: 10.1152/ajpregu.91022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips SA, Pechman KR, Leonard EC, Friedrich JL, Bian J-T, Beal AG, Basile DP. Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries. Am J Physiol Regul Integr Comp Physiol 298: R1682–R1691, 2010. doi: 10.1152/ajpregu.00448.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segawa S, Goto D, Iizuka A, Kaneko S, Yokosawa M, Kondo Y, Matsumoto I, Sumida T. The regulatory role of interferon-γ producing gamma delta T cells via the suppression of T helper 17 cell activity in bleomycin-induced pulmonary fibrosis. Clin Exp Immunol 185: 348–360, 2016. doi: 10.1111/cei.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, O’Brien RL, Fontenot AP. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol 182: 657–665, 2009. doi: 10.4049/jimmunol.182.1.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singbartl K. Renal-pulmonary crosstalk. Contrib Nephrol 174: 65–70, 2011. doi: 10.1159/000329237. [DOI] [PubMed] [Google Scholar]
- 42.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007. doi: 10.1152/ajprenal.00279.2006. [DOI] [PubMed] [Google Scholar]
- 43.Thakur C, Wolfarth M, Sun J, Zhang Y, Lu Y, Battelli L, Porter DW, Chen F. Oncoprotein mdig contributes to silica-induced pulmonary fibrosis by altering balance between Th17 and Treg T cells. Oncotarget 6: 3722–3736, 2015. doi: 10.18632/oncotarget.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, Reisner SA. Pulmonary hypertension in patients with end-stage renal disease. Chest 123: 1577–1582, 2003. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]