Abstract
The extracellular matrix (ECM) modulates brain maturation and plays a major role in regulating neuronal plasticity during critical periods of development. We examined 1) whether there is a critical postnatal period of ECM expression in brain stem cardiorespiratory control regions and 2) whether the attenuated hypoxic ventilatory response (HVR) following neonatal sustained (5 days) hypoxia [SH (11% O2, 24 h/day)] exposure is associated with altered ECM formation. The nucleus tractus solitarius (nTS), dorsal motor nucleus of the vagus, hypoglossal motor nucleus, cuneate nucleus, and area postrema were immunofluorescently processed for aggrecan and Wisteria floribunda agglutinin (WFA), a key proteoglycan of the ECM and the perineuronal net. From postnatal day (P) 5 (P5), aggrecan and WFA expression increased postnatally in all regions. We observed an abrupt increase in aggrecan expression in the nTS, a region that integrates and receives afferent inputs from the carotid body, between P10 and P15 followed by a distinct and transient plateau between P15 and P20. WFA expression in the nTS exhibited an analogous transient plateau, but it occurred earlier (between P10 and P15). SH between P11 and P15 attenuated the HVR (assessed at P16) and increased aggrecan (but not WFA) expression in the nTS, dorsal motor nucleus of the vagus, and area postrema. An intracisternal microinjection of chondroitinase ABC, an enzyme that digests chondroitin sulfate proteoglycans, rescued the HVR and the increased aggrecan expression. These data indicate that important stages of ECM formation take place in key brain stem respiratory neural control regions and appear to be associated with a heightened vulnerability to hypoxia.
Keywords: brain stem extracellular matrix, breathing, development, hypoxia
INTRODUCTION
Postnatal development of the central nervous system (CNS) is strongly influenced by an animal’s external environment and exhibits a profound capacity to exhibit plasticity (3). Experiences in early life induce morphological and functional changes within neural systems that persist into adulthood, revealing various forms of developmental plasticity (3, 7, 37). The CNS demonstrates a unique capacity for plasticity during relatively narrow developmental windows, called critical periods (3). During these windows, which remain largely unexplored, appropriate sensory experiences can be essential for appropriate organization of mature, properly functioning neuronal networks (23, 51). It follows that adverse experiences or the absence of correct experiences during these periods may lead to maladaptive neural development, giving rise to functional abnormalities.
In the respiratory neural control system of the rat, the end of second postnatal week of life appears to represent a critical period of development (30, 33). Wong-Riley and Liu identified an abrupt shift within brain stem centers of respiratory control toward a more inhibitory neurochemical profile at or about postnatal day (P) 12 (P12) (28, 29, 54). They also showed that both respiratory frequency (fR) and minute ventilation (V̇e) reach peak values at P13 (25). Interestingly, however, the ventilatory response to acute hypoxia (HVR) is absent at P13, which coincides with natural and, in some cases, transient changes in brain stem neurochemistry (24, 26–28, 54, 55). We previously demonstrated that exposure to sustained hypoxia [SH (11% O2, 24 h/day, 5 days] from P11 through P15, which encompasses the P12/P13 critical window, attenuated the acute HVR (30, 33); such a deleterious effect on respiratory control was not observed in rats exposed to SH at earlier or later time points (33). Thus the critical period is characterized not only by normal maturational changes, but also by enhanced vulnerability to environmental stressors, including hypoxia and inflammatory stimuli (47).
Mechanisms underlying the heightened vulnerability to hypoxia during the critical period remain unknown. A possible contributing factor is the extracellular matrix (ECM) surrounding neurons, which serves important neuromodulatory functions, including regulation of synaptogenesis, axon guidance, and neurotransmitter expression during brain development (1). The ECM of the CNS is rich in chondroitin sulfate proteoglycans (CSPGs) of the lectican family (aggrecan, neurocan, brevican, and versican) (31, 36). CSPGs consist of a core protein to which chondroitin sulfate glycosaminoglycan (GAG) chains are covalently bound by serine residues (23, 31, 38). CSPGs are involved in neuronal proliferation, contribute to neuronal polarization, and function as axon guidance molecules (4, 11, 19–21, 41). The ECM of the brain is also affected by the external environment, such that expression patterns of CSPGs are altered by states of sensory deprivation, enrichment, and injury (1, 17, 35, 50). Furthermore, formation of specialized ECM structures, called perineuronal nets (PNNs), helps define the ends of critical periods. PNNs consist of lectican CSPGs complexed to a cell membrane-bound hyaluronic acid backbone and stabilized by link proteins and tenascin-R (6, 9, 23, 53). Formation of PNNs around neuronal cell bodies and proximal dendrites limits the neuronal plasticity that characterizes critical periods of development, thus defining the ends of such periods (8, 44, 45). We propose that ECM formation within distinct brain stem cardiorespiratory control regions during critical periods early in postnatal development may be sensitive to exogenous challenges or insults. A disruption or modification of ECM formation at a time when it may be playing a particularly important role in CNS development, therefore, could have profound neurodevelopmental and functional consequences. We hypothesize that the heightened vulnerability of the respiratory control system to SH during such a critical window could be the result of aberrant ECM formation in key brain stem (cardio)respiratory control regions.
In this study, therefore, we investigated 1) the temporal development of CSPG expression within specific brain stem respiratory (and non-respiratory)-related regions to determine whether there are critical windows of ECM formation, 2) whether SH (previously shown to attenuate the HVR) increases brain stem ECM formation, and 3) whether the attenuated HVR and increased ECM formation can be rescued by treatment with chondroitinase ABC (ChABC), an enzyme that digests components of the CSPGs and the PNN.
MATERIALS AND METHODS
Experiments were performed on male Lewis rats (colony PO6, Charles River Laboratories). All procedures were carried out in accordance with the National Institutes of Health guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
Immunohistochemistry and semiquantitative assessment of brain stem ECM.
Rats at five postnatal ages [postnatal days (P) 5 (P5), P10, P15, P20, and P90 (adult)] were euthanized via a lethal intraperitoneal injection of urethane anesthesia and immediately transcardially perfused with cold phosphate-buffered saline (PBS, pH 7.4) and then with 4% paraformaldehyde. Brains were removed and postfixed for 4 h in paraformaldehyde and then submerged in 30% sucrose solution at 4°C until saturated (48–72 h). Brain stems were removed, and a cryostat (model CM 1850, Leica) was used to cut the tissue into 20-μm sections, which were mounted onto glass slides. Slide-mounted sections were washed in PBS three times for 10 min and blocked in 5% normal goat serum, 0.1% bovine serum albumin, and 0.1% Triton X-100 in PBS for 2 h at room temperature. Wisteria floribunda agglutinin (WFA) and aggrecan immunohistochemistry were then performed. WFA is a plant lectin that binds N-acetylgalactosamine (one of the sugar moieties of the disaccharide units that make up GAG chains) on CSPGs and, therefore, is a broad-spectrum marker for CSPGs (5). Sections were incubated in biotinylated WFA agglutinin (1:100 dilution; Sigma) or rabbit polyclonal anti-aggrecan antibody (1:150 dilution; Millipore). For aggrecan immunohistochemistry, sections were preincubated with ChABC (0.02 U/ml; Seikagaku) in chondroitinase buffer (0.1 M Tris·HCl and 0.03 M sodium acetate, pH 8) for 1 h at 37°C. Sections were incubated in primary antibodies overnight at 4°C and then in the secondary antibodies streptavidin-Alexa Fluor 594 conjugate (1:500 dilution; Life Technologies) for WFA and Alexa Fluor 594-conjugated AffiniPure goat anti-rabbit IgG (1:200 dilution; Jackson ImmunoResearch) for aggrecan for 2 h at room temperature. All slides were stained simultaneously and imaged in a single session using a fluorescence microscope (model DMLB, Leica) at ×20 magnification (1,024 × 1,024 resolution) and QCapture Pro software. Brain regions of interest (ROIs), including the nucleus tractus solitarius (nTS, commissural-medial regions), the adjacent dorsal motor nucleus of the vagus (DMNV), the hypoglossal (XII) motor nucleus, and two nonrespiratory-related regions, the area postrema (AP) and the cuneate nucleus (CN), were located according to the atlas of Paxinos and Watson (43), as previously described (30). Semiquantification of WFA and aggrecan expression was performed via fluorescence densitometry within a designated ROI. Images were loaded into ImageJ, and the background was corrected to the same level for all images. A circular ROI (100-pixel diameter for nTS, XII, and CN and 50-pixel diameter for DMNV and AP) was then placed in the intended brain region, and the average pixel intensity within the circle was measured. Densitometry was performed using ImageJ software. For quantification of the postnatal changes in ECM expression, three to five images were collected for each animal and averaged to obtain a single value per animal (n = 1). The number of animals (n) for each age group is as follows: P5 = 6, P10 = 9, P15 = 8, P20 = 7, and P90 = 5. For the SH/ChABC experiments, two to four sections were averaged for each animal to obtain the following values for n: normoxia + saline = 9, normoxia + ChABC = 9, SH + saline = 8, and SH + ChABC = 9.
SH exposure.
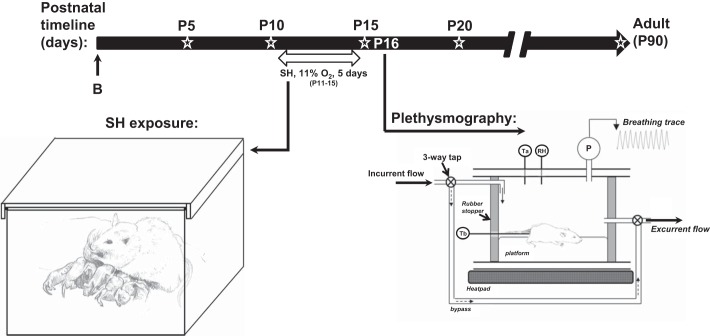
On P11, dams and their pups were assigned to one of two treatment groups, SH or normoxia. The SH group received 11% O2 for 5 consecutive days from P11 through P15 (Fig. 1). The normoxia group received 21% O2 throughout the study period. SH was achieved by placement of the mother and pups inside a Plexiglas chamber connected to adjustable rotameters for mixing air and N2. O2 levels were monitored (model TED 60T, Teledyne Analytical Instruments) daily and maintained constant at a flow rate of 3 l/min. On the 4th day of exposure (P14), pups were briefly removed from the chamber and prepared for an intracisternal microinjection of saline or ChABC into the fourth ventricle (see below). At the end (~5 PM) of the fifth day of exposure (P15), the rats were removed from the chamber and maintained in room air overnight before whole body plethysmography assessment for the acute HVR on the following day (P16). After measurement of the acute HVR, rat pups were euthanized and perfused, and brain stem sections were obtained for immunohistochemical analysis of brain stem ECM components (see above).
Fig. 1.
Timeline and experimental setup used to expose rats to sustained hypoxia [SH (11% O2, 24 h/day) between postnatal days (P) 11 (P11) and P15]. At the end of P15, rats were removed from the SH chamber, and the acute hypoxic ventilatory response (HVR) was assessed by whole body plethysmography on the following day (P16). Open star designates time points at which semiquantification of Wisteria floribunda agglutinin and aggrecan was carried out within brain stem regions (see Figs. 2 and 3). B, birth.
Intracisternal injection of ChABC.
On P14, individual pups were removed from the litter and anesthetized with isoflurane (3.5–4% isoflurane-balance O2). The fur over the upper back was shaved, and the skin was sterilized with 10% povidone-iodine antiseptic solution (Medline). The animal was placed on a heating pad, a small (~0.5-cm) skin incision was made at the base of the head, and the overlying fascia was retracted slightly to expose the posterior atlanto-occipital membrane. A 27-gauge needle was inserted through the membrane into the cisterna magna, and a Hamilton syringe was used to inject 3 μl of ChABC (0.02 U/μl) or 0.9% saline (control group) directly into the fourth ventricle (30). ChABC is a bacterial eliminase enzyme that digests the GAG chains of CSPGs into their basic disaccharide subunits, thus removing the GAG chain from the core protein (23, 56). Tracer experiments using microinjection of pontamine sky blue dye were used to confirm delivery and penetration of the injection site within the vicinity of the brain stem, particularly the nTS (see Fig. 9). After the injection, the incision was sutured, and the animal was returned to the litter after a period of recovery. The duration of the surgical procedure was <20 min; the rats recovered quickly on a heating pad and were returned to the dam to finish the remaining SH protocol. Normoxia-treated rats that underwent the same procedure (saline or ChABC microinjection and housing conditions) served as control groups. Whole body plethysmography was performed at P16.
Fig. 9.
Rostral-caudal images of dorsal brain stem 45 min following a 3-μl microinjection of pontamine sky blue dye into the 4th ventricle of a 14-day-old rat. Note localized penetration of dye, especially into the nTS and DMNV. Scale bar = 200 μm.
Whole body plethysmography for assessment of the acute HVR.
On P16, individual pups were removed from the litter and placed inside a custom-made Perspex plethysmograph chamber (Fig. 1). Airflow through the chamber was held constant (450 ml/min) using a mass flow controller (Aalborg). Before each experiment, the chamber was assessed for adequate seal by observation of the stability of the square pressure change following injection of a calibration volume (50 μl) using a glass microsyringe (Hamilton, Harvard Apparatus). V̇e was measured at baseline and at minutes 1, 2, 3, 4, and 5 of acute hypoxia (10% O2), as described previously (33, 34). Baseline V̇e was first measured by momentarily (for 20 s) sealing the chamber using stopcocks located up- and downstream of the plethysmograph; the incurrent gas was immediately changed to a premixed 10% O2-balance N2 gas mixture for rapid purging of the plethysmograph with hypoxia. The pressure oscillations associated with breathing were recorded and later analyzed for breath-by-breath analysis of the respiratory waveform and measurement of V̇e, tidal volume (Vt), and fR. Vt was calculated from the pressure change associated with microinjection of a calibration volume (50 μl) using the glass microsyringe calibration injection. A fine temperature thermocouple (Cole-Parmer, Vernon Hills, IL) was secured to the base of the tail with tape and used to measure rectal temperature throughout the experiment. An identical thermocouple was placed inside the chamber to monitor the ambient temperature, which was held constant (~28°C) using a water bath (Isotemp 3013S, Fisher Scientific, Pittsburgh, PA) that circulated water to a heating pad located underneath the plethysmograph. Relative humidity was also measured for calculation of Vt, which was temperature-corrected, as described previously (15, 40). The magnitude of the HVR was calculated as the difference between the level of normoxic ventilation (before acute hypoxia) and values during each time point during hypoxia (i.e., Δbaseline). After the measurements were completed, the rats were euthanized via urethane overdose, and brain stems were removed for immunohistochemical assessment of ECM, as described above.
Statistical analysis.
Statistical analysis was conducted with SigmaPlot12 software. Densitometry data between age groups and SH-exposed animals (at P16) were compared using a one-way ANOVA. Multiple brain stem images were utilized as independent samples for each animal. The magnitude of the HVR at various time points of hypoxia (as well as fR and Vt) was compared between treatment groups using a two-way, repeated-measures ANOVA. Differences were considered significant at P < 0.05. Values are means ± SE.
RESULTS
Postnatal expression of brain stem ECM.
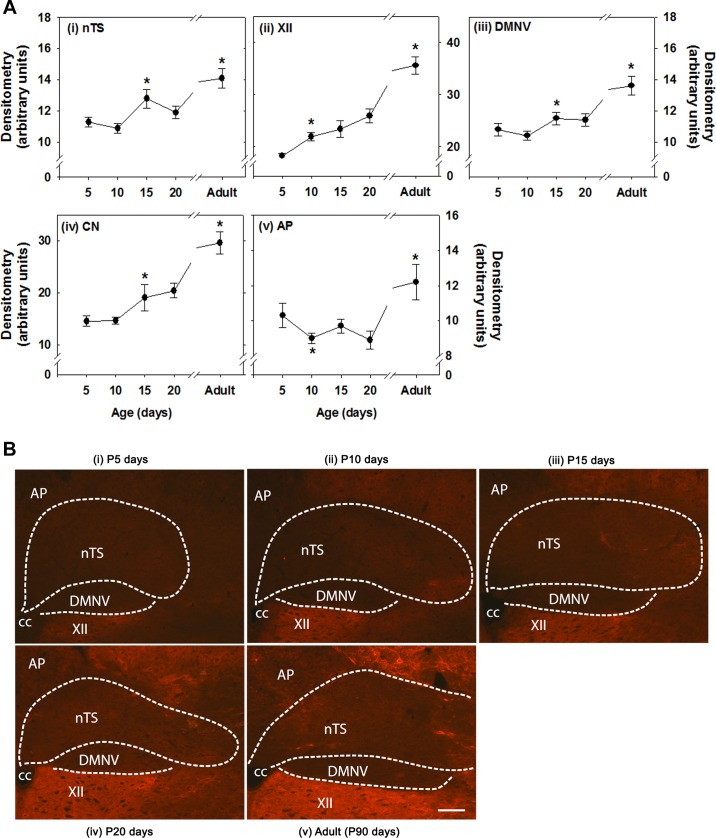
Immunofluorescent staining specific for aggrecan expression within various brain regions across several postnatal ages is shown in Fig. 2. Overall, in the youngest (P5) age group, aggrecan expression was relatively low in all brain regions but increased with development, reaching peak levels at adulthood (P90). In the nTS and DMNV, however, a significant increase in aggrecan expression between P10 and P15 was followed by a transient plateau between P15 and P20 and another increase in the adult (Fig. 2A). A similar temporal profile of expression occurred in the CN and XII, although in the latter the initial rise in aggrecan increased sooner (between P5 and P10). The AP also exhibited a decrease in expression between P5 and P10 and then an extended plateau (vs. the other regions) to P20 (Fig. 2A). High-resolution representative images from each age group are shown in Fig. 2B.
Fig. 2.
Aggrecan immunoreactivity in various brain stem regions at various postnatal ages. A: densitometry. Brain regions include nucleus tractus solitarius (nTS), hypoglossal motor nucleus (XII), dorsal motor nucleus of the vagus (DMNV), cuneate nucleus (CN), and area postrema (AP). CC, central canal. Values are means ± SE; n = 6 at P5, 9 at P10, 8 at P15, 7 at P20 , and 5 at P90. *Statistically different from preceding (younger) age group (P < 0.05, by One-way ANOVA). B: representative images of brain stem regions at P5, P10, P15, P20, and P90 (adult). Scale bar = 100 μm.
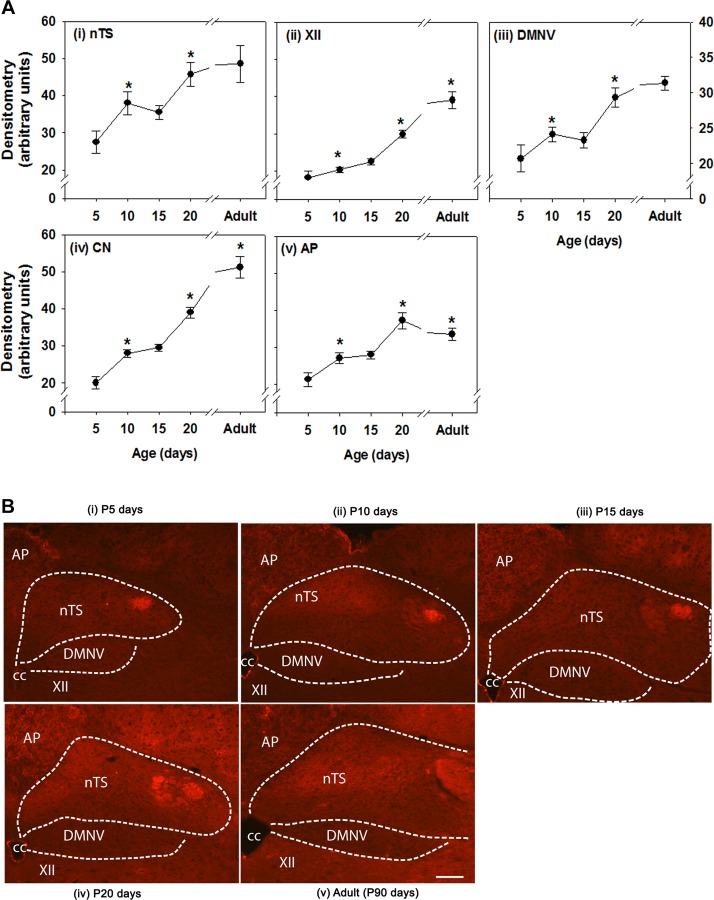
Immunofluorescent staining for CSPGs with WFA also reveals distinct temporal changes in expression within various brain stem respiratory control regions (Fig. 3). Similar to aggrecan, WFA immunoreactivity also increased postnatally in all brain regions, although subtle differences in temporal profile were observed in certain brain regions. Specifically, there was an increase in WFA between P5 and P10 in the nTS and DMNV followed by a transient plateau to P15, which paralleled the pattern of aggrecan expression, although these events occurred sooner (Fig. 3A). WFA expression in the AP was also similar to that in the nTS and DMNV and reached a peak at P20 in the AP, nTS, and DMNV. There were large increases in WFA expression in the XII and CN between P15 and adulthood.
Fig. 3.
Wisteria floribunda agglutinin (WFA) immunoreactivity in various brain stem regions at various postnatal ages. A: densitometry. Brain regions include nTS XII, DMNV, CN, and AP. Values are means ± SE; n = 6 at P5, 9 at P10, 8 at P15, 7 at P20 , and 5 at P90. *Statistically different from preceding (younger) age group (P < 0.05, by One-way ANOVA). B: representative images of brain stem regions at P5, P10, P15, P20, and P90 (adult). Scale bar = 100 μm.
Effects of SH on the acute HVR and brain stem ECM expression.
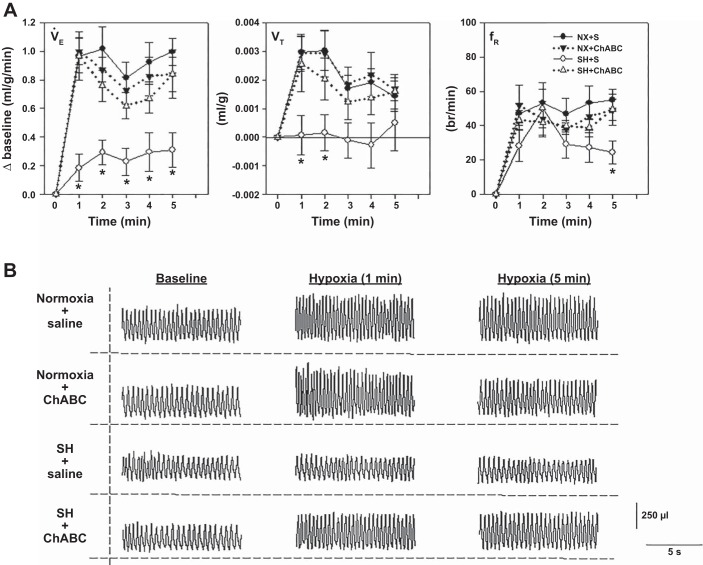
The HVR was assessed at P16 after SH between P11 and P15. Rats reared in normoxia exhibited a robust HVR (Fig. 4A). As shown previously (30, 33), SH induced a significant reduction in the HVR, which was mediated primarily via an attenuated Vt response; fR was also reduced, but only during the later phase (5th min) of the HVR. In contrast, the HVR was rescued in rats that had received an intracisternal microinjection of ChABC on the fourth day of SH (Fig. 4, A and B); the protective effects of ChABC on the HVR were mediated by restoration of Vt and fR, neither of which was statistically different from normoxia-reared rats that also received intracisternal saline or ChABC. Representative breathing traces from animals for each treatment group are shown in Fig. 4B.
Fig. 4.
HVR at P16 in rats following SH exposure between P11 and P15 with or without microinjection of chondroitinase ABC (ChABC) into the 4th ventricle. A: changes in ventilation (V̇e, i.e., HVR), tidal volume (Vt), and respiratory frequency (fR) of rats exposed to SH between P11 and P15 after microinjection of saline [SH + S (○)] or ChABC [SH + ChABC (Δ)]. Control groups include rats exposed to normoxia between P11 and P15 following microinjection of saline [NX + S (●)] or ChABC [NX + ChABC (▼)].Values are means ± SE; n = 12 NX + S, 11 NX + ChABC, 12 SH + S, and 11 SH + ChABC. *Significantly different from NX + S for a given time point (P < 0.05, by 2-way, repeated-measures ANOVA). B: representative breathing traces from animals at baseline and minutes 1 and 5 of acute hypoxia for each treatment group.
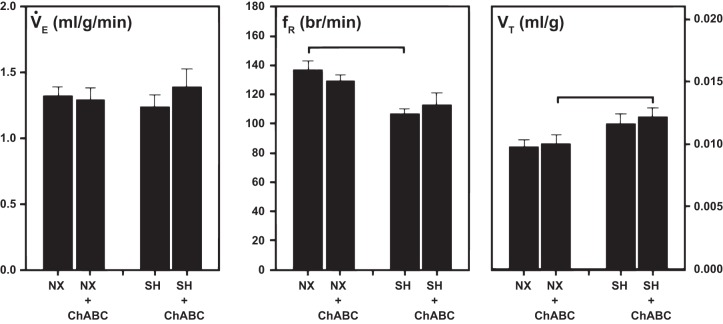
Although there was no significant effect of SH or ChABC injection on baseline (normoxia) breathing (V̇e) (Fig. 5), there were some changes in breathing pattern. In the saline-treated groups, SH exposure significantly decreased fR compared with normoxia-exposed rats, but the corresponding lack of an effect on V̇e was offset by a nonsignificant increase in Vt (Fig. 5). Similarly, in ChABC-treated rats, baseline Vt was significantly enhanced by SH compared with normoxia, although the lack of an effect on V̇e in this case was due to a nonsignificant decrease in fR.
Fig. 5.
Baseline (normoxia) V̇e, fR, and Vt of rats raised in normoxia or exposed to SH between P11 and P15 after prior (at P14) intracisternal microinjection of saline or ChABC. Statistical significance (P < 0.05, by Two-way ANOVA) between groups as indicated.
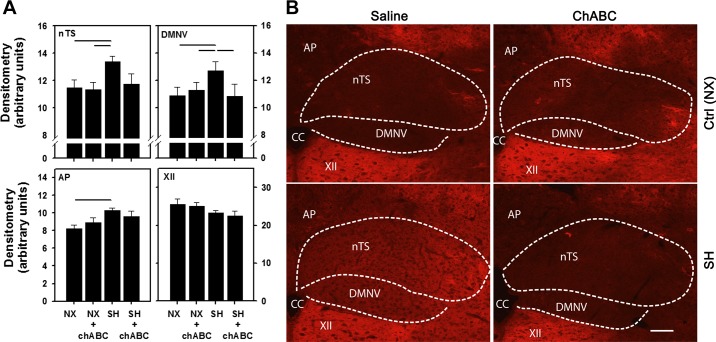
The attenuated HVR following SH between P11 and P15 was associated with increased aggrecan expression compared with normoxia-reared rats, although significant changes were seen only within the nTS, DMNV, and AP (Fig. 6A). Aggrecan expression within the XII [and CN (data not shown)] was not affected by SH. Interestingly, ChABC reversed the increase in aggrecan expression in the nTS and DMNV in the SH-exposed rats. In normoxia- or SH-exposed rats, ChABC injection had no effect on aggrecan expression in the AP or XII. Higher-magnification images for all four treatment groups (normoxia + saline, normoxia + ChABC, SH + saline, and SH + ChABC) are shown in Fig. 6B.
Fig. 6.
Changes in aggrecan immunoreactivity in various brain stem regions following SH and ChABC microinjection. A: increase in aggrecan expression in nTS, DMNV, and AP, but no effect in XII, following SH. Increased expression in nTS and DMNV was reduced by microinjection of ChABC into the 4th ventricle. Values are means ± SE; n = 9 NX + S, 9 NX + ChABC, 8 SH + S, and 9 SH + ChABC. Statistical significance (P < 0.05, by Two-way ANOVA) between groups as indicated. B: representative images of brain stem regions. Scale bar = 100 μm.
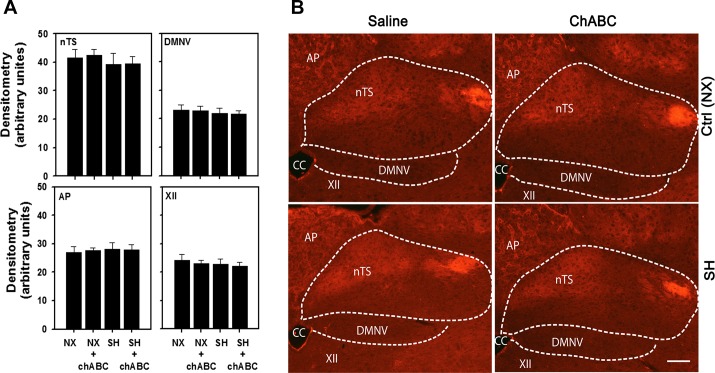
In contrast to the changes in brain stem aggrecan, SH did not affect WFA expression in any brain stem regions (Fig. 7, A and B). Similarly, ChABC microinjection also did not affect WFA expression in SH- or normoxia-exposed rats compared with saline-treated animals.
Fig. 7.
WFA immunoreactivity in various brain stem regions following SH exposure and microinjection of ChABC into the 4th ventricle. A: no effect of SH or ChABC on WFA immunoreactivity in nTS, DMNV, AP, or XII. Values are means ± SE; n = 9 NX + S, 9 NX + ChABC, 8 SH + S, and 9 SH + ChABC. B: representative images of brain stem regions. Ctrl (NX), normoxia treated rats. Scale bar = 100 μm.
Relative expression of brain stem ECM across various brain stem regions.
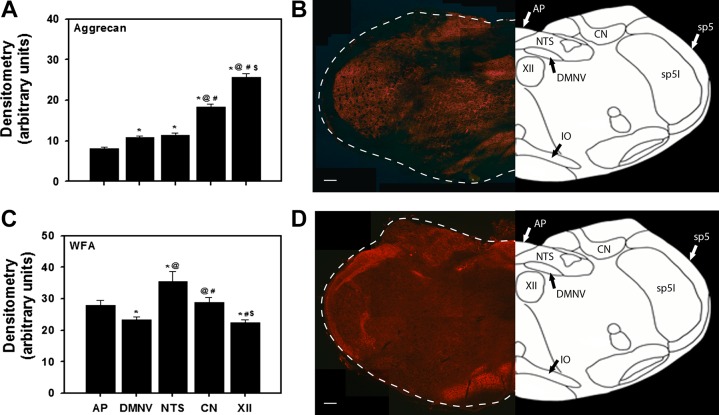
After assessing the effects of SH (and ChABC injection) on the expression of aggrecan and WFA in the brain stem of P15 rats, we also assessed the relative expression of aggrecan and WFA across the different regions (in normoxia-reared rats from Figs. 2 and 3; n = 8 animals). Aggrecan expression was highest in the XII and lowest in the AP, nTS, and DMNV (Fig. 8, A and B). In contrast, WFA expression was lowest in the XII and DMNV and highest in the nTS (Fig. 8, C and D). Low-magnification representative images demonstrate the distinct regional staining within the brain stem for aggrecan (Fig. 8B) and WFA (Fig. 8D).
Fig. 8.
A and C: aggrecan and WFA immunoreactivity showing relative expression across various brain regions in P15 normoxic rats. Values are means ± SE; n = 8. *P < 0.05 vs. AP; @P < 0.05 vs. DMNV; #P < 0.05 vs. nTS; $P < 0.05 vs. CN (by 1-way ANOVA). B and D: representative images from single rats. AP, area postrema; DMNV, dorsal motor nucleus of the vagus; nTS, nucleus tractus solitarius; CN, cuneate nucleus; XII, hypoglossal motor nucleus; IO, inferior olive; sp5, spinal trigeminal tract; sp5I, spinal 5 interpolar. Scale bar = 200 μm.
DISCUSSION
The ECM is an important modulator of brain development, in that it plays a crucial role in proliferation and differentiation of neural progenitor cells, axon guidance, and synaptic stabilization (4, 11, 19–21, 41). During critical periods, neural development can be particularly sensitive to sensory experiences (23, 51). In rats, the end of the second postnatal week has been identified as a critical period of respiratory neural development, characterized by changes in brain stem neurochemistry (25, 26, 28, 54, 55) and increased vulnerability to hypoxic (30, 33) and inflammatory-related (47) stressors. In this study we observed complex changes in ECM formation, characterized by a transient quiescent period (i.e., a plateau) of CSPG deposition during the neonatal period, although the specific expression patterns varied depending on the type of CSPG, anatomic location, and timing of deposition. Perhaps the most notable pattern of constitutive expression was the plateau in aggrecan levels between P15 and P20 that occurred within the nTS; the profile for WFA expression was similar to that for aggrecan expression, but with an earlier onset (between P10 and P15). Such changes in the nTS (and, perhaps, comparable changes in the DMNV) could be significant to the heightened vulnerability of the HVR to SH during the same period. Furthermore, the unique changes in CSPG expression coincide with changes in expression of various neurotransmitters and their receptors within specific respiratory neural control regions (28, 29, 54, 55). The results of the current study indicate a likely role for CSPGs in the normal maturational processes of the (cardio)respiratory neural circuitry.
An important feature of some critical periods is that neuronal development is dependent on sensory experience. The CNS during these periods exhibits a high degree of plasticity in response to environmental stimuli, such that appropriate experiences drive the organization of mature, properly functioning neural networks, while adverse experiences lead to potentially debilitating faults in the developing neural circuitry (23, 51). In this study we have shown that an adverse consequence of SH during a critical period of respiratory neural development is an aberrant increase in the expression of CSPG (specifically, aggrecan) in key (cardio)respiratory control regions, in association with a disturbance in the neonate’s ability to mount an adequate HVR. This is consistent with our prior study, which further showed that younger and older rats exposed to the same SH challenge outside of the P11 and P15 period, were relatively more resistant (33). In the current study it is unclear how SH affects the ECM and also whether its expression would be similarly affected in younger or older animals that appear relatively resistant to SH (33). SH could affect the ECM directly, although this seems unlikely, since aggrecan expression in other brain stem regions (e.g., XII and CN) was not affected by SH. We speculate that, at least with regard to aggrecan expression between P11 and P15, SH may have an indirect effect on ECM formation (specifically, aggrecan) and, likely, involves intermediary mechanisms that may not be as prominent in younger or older age groups. The result is a premature upregulation of the ECM that could occur only during a specific stage of development, resulting in a disturbance in key neurodevelopmental/chemical events in specific CNS regions.
In many cortical brain areas, aggrecan expression is affected by the postnatal environment in which an animal is reared. McRae et al. demonstrated that sensory deprivation by whisker trimming from P0 through P30 is associated with decreased aggrecan mRNA and aggrecan protein expression in the mouse barrel cortex (35). Similarly, sensory deprivation by rearing an animal in darkness is associated with decreased aggrecan-containing PNNs within the lateral geniculate nucleus and visual cortex in cats (17, 50). In our study we observed an increase in aggrecan expression within the nTS, DMNV, and AP after SH in the early postnatal period. Interestingly, we did not observe SH-associated changes in PNN expression in WFA-stained sections. This is consistent with the findings of McRae et al. that sensory deprivation was associated with aggrecan-specific changes, but not with significant alterations in expression of WFA-labeled CSPGs (35). These findings indicate that expression of aggrecan, but not other CSPGs, may be uniquely influenced by environmental stimuli during early postnatal life. Alternatively, because WFA binds to a variety of CSPGs, changes in overall CSPG expression may not be detectable if one or more specific types of CSPGs are upregulated and others are downregulated. Furthermore, the effects of SH and other environmental conditions on the ECM may also be mediated by structural alterations in organization of CSPGs or by changes in sulfation patterns, such that solely quantitative measures of CSPG expression may be inadequate. Further studies are necessary to delineate the specific effects of sensory input on expression of individual ECM components. Interestingly, we also did not see an effect of ChABC on WFA or aggrecan expression in normoxia-reared rats, nor did we see an effect on baseline ventilation [although ChABC did modify normoxic breathing pattern of SH rats (Fig. 5)]. The lack of an effect of ChABC on ECM expression of normoxic rats is difficult to explain, but the finding that its effects are unique to SH-exposed rats suggests that ChABC only prevents formation of new CSPGs. Alternatively, the lack of an effect of ChABC on CSPG expression could be related to the timing of injection relative to tissue collection. Specifically, an injection of ChABC at P14 could have allowed sufficient time for a significant rebound in CSPG expression at the time of tissue collection at P16. It is unclear if ChABC alone may have had a more immediate effect on CSPG expression or the acute HVR in normoxia-reared rats, and further investigation is warranted. These studies may further help in understanding the significance of constitutive ECM expression during postnatal development and the effects it might have in a “normally” developing animal.
Upregulation of aggrecan in response to noxious stimuli may serve neuroprotective functions in adult animals, although this appears unlikely in the current study, since enzymatic degradation of the ECM in the nTS (and DMNV) following microinjection of ChABC rescued the HVR and, thus, appears to have a protective effect against SH. Nevertheless, evidence suggests that neurons ensheathed in aggrecan-rich PNNs may be more resistant to the effects of oxidative stress and microglial activation related to aging and neurodegenerative diseases (39, 48). In developing animals, however, aggrecan upregulation may adversely affect neuronal adaptations to noxious stimuli. In many areas of the brain, the appearance of CSPGs associated with PNNs marks the close of critical periods (18, 22, 44, 46, 57), which could be consistent with the plateaus in aggrecan and WFA expression in some brain stem regions in the current study. PNN formation helps stabilize established neuronal networks and limits plastic changes to future experiences (23). The possible mechanisms by which CSPGs may limit plasticity are formation of a physical barrier around neurons that prevents formation of new synaptic contacts by advancing axons, serving as a substrate for the binding of growth-inhibitory molecules, and restriction of cell surface receptor mobility (10, 13, 14, 16, 23, 35, 42, 49, 51). While the exact mechanisms remain unknown, multiple experiments have shown that disruption of CSPGs with ChABC can reopen critical periods and restore neuronal plasticity by allowing for new neurite outgrowth and synaptogenesis (2, 12, 32, 44, 45, 52). In our model of SH-induced respiratory dysfunction, aberrant and/or premature upregulation of aggrecan at an inappropriate stage of PNN formation may disrupt formation of neural connections that are necessary for normal maturation. Enzymatic disruption of CSPGs with ChABC may destabilize these improper connections and allow for establishment of new neural connections, although further studies are necessary to determine the specific effect of these changes in CSPGs on neural development. For example, we showed previously that SH increased microglia expression specifically within the nTS and DMNV, whereas other brain stem regions showed no changes in response to SH (30). Such changes that were selective for the nTS and DMNV are, in part, consistent with the results of the current study, in which the effects of SH on aggrecan expression were seen predominantly (but not exclusively) in the nTS and DMNV (and also the AP), whereas aggrecan expression in the XII and CN were not affected by SH. These observations raise the question of whether there is an interaction between ECM formation and microglia activity and warrant further investigation. Finally, from experiments involving microinjections of pontamine sky blue dye into the fourth ventricle of an anesthetized 14-day-old rat (Fig. 9), we were able to demonstrate fairly concentrated penetration of the dye into the dorsal brain stem region, particularly the nTS, DMNV, and AP, relative to other brain regions such as the XII. However, despite very little evidence of dye spread to other brain regions, we should not exclude the possibility that other (cardio)respiratory control regions were not affected by ChABC treatment.
Perspectives and Significance
Early life experiences have a profound impact on the developing nervous system. Critical periods represent particularly important developmental windows, characterized by maturation of neural networks, the capacity for plasticity, and vulnerability to physiological stress. In this study we revealed a role for the ECM in hypoxia-induced respiratory dysfunction in rats exposed to SH during a critical period of respiratory neural development, although we were able to rescue such a deleterious effect of SH following enzymatic degradation of the ECM. We speculate that, during normal development, the plateau in ECM formation likely serves a deliberate and important maturational purpose necessary for appropriate development of the neural architecture within key cardiorespiratory control regions. An aberrant increase in ECM formation at a critical stage of development may have severe unintended consequences, leading to failure of the respiratory control system to respond adequately to hypoxic challenges. However, the mechanisms whereby SH and other types of adverse challenges affect ECM formation beyond the brain stem offer important avenues for future studies.
GRANTS
This work was funded by the Department of Pediatrics, Case Western Reserve University (CWRU), Rainbow Babies & Children’s Hospital. D. Camperchioli was the recipient of a Summer Undergraduate Research Award from the Department of Pediatrics, CWRU.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S., D.W.C., C.A.M., and P.M.M. performed experiments; C.S., D.W.C., C.A.M., and P.M.M. analyzed data; C.S., W.J.A., and P.M.M. interpreted results of experiments; C.S., C.A.M., and P.M.M. prepared figures; C.S. and P.M.M. drafted manuscript; C.S., D.W.C., C.A.M., W.J.A., R.J.M., and P.M.M. edited and revised manuscript; C.S., D.W.C., C.A.M., W.J.A., R.J.M., and P.M.M. approved final version of manuscript; P.M.M. conceived and designed research.
ACKNOWLEDGMENTS
We thank Steve Torontali for construction of the plethysmography chambers and Mia Freer for animal illustration.
Present address of W. J. Alilain: Spinal Cord and Brain Injury Research Center, Department of Neuroscience, University of Kentucky, Lexington, KY.
REFERENCES
- 1.Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 475: 196–200, 2011. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci 26: 10856–10867, 2006. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavis RW, MacFarlane PM. Developmental plasticity in the neural control of breathing. Exp Neurol 287: 176–191, 2017. doi: 10.1016/j.expneurol.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt RR, Schachner M. Chondroitin sulfates affect the formation of the segmental motor nerves in zebrafish embryos. Dev Biol 221: 206–219, 2000. doi: 10.1006/dbio.2000.9673. [DOI] [PubMed] [Google Scholar]
- 5.Brückner G, Brauer K, Härtig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8: 183–200, 1993. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 6.Brückner G, Grosche J, Schmidt S, Härtig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol 428: 616–629, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol (1985) 94: 375–389, 2003. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- 8.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133: 2331–2347, 2010. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 9.Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol 501: 83–94, 2007. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- 10.Celio MR, Blümcke I. Perineuronal nets—a specialized form of extracellular matrix in the adult nervous system. Brain Res Brain Res Rev 19: 128–145, 1994. doi: 10.1016/0165-0173(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 11.Chung KY, Taylor JS, Shum DK, Chan SO. Axon routing at the optic chiasm after enzymatic removal of chondroitin sulfate in mouse embryos. Development 127: 2673–2683, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Corvetti L, Rossi F. Degradation of chondroitin sulfate proteoglycans induces sprouting of intact Purkinje axons in the cerebellum of the adult rat. J Neurosci 25: 7150–7158, 2005. doi: 10.1523/JNEUROSCI.0683-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci 29: 40–55, 2005. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Deepa SS, Umehara Y, Higashiyama S, Itoh N, Sugahara K. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J Biol Chem 277: 43707–43716, 2002. doi: 10.1074/jbc.M207105200. [DOI] [PubMed] [Google Scholar]
- 15.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- 16.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12: 897–904, 2009. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 17.Guimarães A, Zaremba S, Hockfield S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J Neurosci 10: 3014–3024, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol 55: 505–514, 1990. doi: 10.1101/SQB.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- 19.Ichijo H, Kawabata I. Roles of the telencephalic cells and their chondroitin sulfate proteoglycans in delimiting an anterior border of the retinal pathway. J Neurosci 21: 9304–9314, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, Aono S, Fujita H, Fujiwara Y, Kaji T, Oohira A. Identification and functions of chondroitin sulfate in the milieu of neural stem cells. J Biol Chem 281: 5982–5991, 2006. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- 21.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44: 961–975, 2004. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Köppe G, Brückner G, Brauer K, Härtig W, Bigl V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res 288: 33–41, 1997. doi: 10.1007/s004410050790. [DOI] [PubMed] [Google Scholar]
- 23.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71: 1073–1089, 2011. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Fehring C, Lowry TF, Wong-Riley MT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J Appl Physiol (1985) 106: 1212–1222, 2009. doi: 10.1152/japplphysiol.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol 577: 957–970, 2006. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience 165: 61–78, 2010. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Wong-Riley MT. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J Comp Neurol 518: 1082–1097, 2010. doi: 10.1002/cne.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol (1985) 98: 1442–1457, 2005. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Wong-Riley MT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Bötzinger complex. J Appl Physiol (1985) 92: 923–934, 2002. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- 30.MacFarlane PM, Mayer C, Litvin D. Microglia modulate brainstem serotonergic expression following neonatal sustained hypoxia exposure: implications for sudden infant death syndrome. J Physiol. 594: 3079–3094, 2016. doi: 10.1113/JP271845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda N, Ishii M, Nishimura K, Kamimura K. Functions of chondroitin sulfate and heparan sulfate in the developing brain. Neurochem Res 36: 1228–1240, 2011. doi: 10.1007/s11064-010-0324-y. [DOI] [PubMed] [Google Scholar]
- 32.Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci 26: 4406–4414, 2006. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer CA, Di Fiore JM, Martin RJ, Macfarlane PM. Vulnerability of neonatal respiratory neural control to sustained hypoxia during a uniquely sensitive window of development. J Appl Physiol (1985) 116: 514–521, 2014. doi: 10.1152/japplphysiol.00976.2013. [DOI] [PubMed] [Google Scholar]
- 34.Mayer CA, Wilson CG, MacFarlane PM. Changes in carotid body and nTS neuronal excitability following neonatal sustained and chronic intermittent hypoxia exposure. Respir Physiol Neurobiol 205: 28–36, 2015. doi: 10.1016/j.resp.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci 27: 5405–5413, 2007. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milev P, Maurel P, Chiba A, Mevissen M, Popp S, Yamaguchi Y, Margolis RK, Margolis RU. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem Biophys Res Commun 247: 207–212, 1998. doi: 10.1006/bbrc.1998.8759. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94: 358–374, 2003. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 38.Morawski M, Brückner G, Arendt T, Matthews RT. Aggrecan: beyond cartilage and into the brain. Int J Biochem Cell Biol 44: 690–693, 2012. doi: 10.1016/j.biocel.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Morawski M, Brückner MK, Riederer P, Brückner G, Arendt T. Perineuronal nets potentially protect against oxidative stress. Exp Neurol 188: 309–315, 2004. doi: 10.1016/j.expneurol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol 76: 937–944, 1998. doi: 10.1139/y99-001. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura K, Ishii M, Kuraoka M, Kamimura K, Maeda N. Opposing functions of chondroitin sulfate and heparan sulfate during early neuronal polarization. Neuroscience 169: 1535–1547, 2010. doi: 10.1016/j.neuroscience.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Oohira A, Matsui F, Katoh-Semba R. Inhibitory effects of brain chondroitin sulfate proteoglycans on neurite outgrowth from PC12D cells. J Neurosci 11: 822–827, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248–1251, 2002. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 45.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci USA 103: 8517–8522, 2006. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat 204: 33–48, 2004. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rourke KS, Mayer CA, MacFarlane PM. A critical postnatal period of heightened vulnerability to lipopolysaccharide. Respir Physiol Neurobiol 232: 26–34, 2016. doi: 10.1016/j.resp.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Schüppel K, Brauer K, Härtig W, Grosche J, Earley B, Leonard BE, Brückner G. Perineuronal nets of extracellular matrix around hippocampal interneurons resist destruction by activated microglia in trimethyltin-treated rats. Brain Res 958: 448–453, 2002. doi: 10.1016/S0006-8993(02)03569-2. [DOI] [PubMed] [Google Scholar]
- 49.Snow DM, Brown EM, Letourneau PC. Growth cone behavior in the presence of soluble chondroitin sulfate proteoglycan (CSPG), compared to behavior on CSPG bound to laminin or fibronectin. Int J Dev Neurosci 14: 331–349, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Sur M, Frost DO, Hockfield S. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J Neurosci 8: 874–882, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res 349: 147–160, 2012. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31: 9332–9344, 2011. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margolis RU, Levinson SR, Shrager P, Montag D, Schachner M. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci 19: 4245–4262, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol 164: 28–37, 2008. doi: 10.1016/j.resp.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol 149: 83–98, 2005. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem 243: 1523–1535, 1968. [PubMed] [Google Scholar]
- 57.Zaremba S, Guimaraes A, Kalb RG, Hockfield S. Characterization of an activity-dependent, neuronal surface proteoglycan identified with monoclonal antibody Cat-301. Neuron 2: 1207–1219, 1989. doi: 10.1016/0896-6273(89)90305-X. [DOI] [PubMed] [Google Scholar]