Abstract
Impaired microvascular insulin signaling may develop before overt indices of microvascular endothelial dysfunction and represent an early pathological feature of adolescent obesity. Using a translational porcine model of juvenile obesity, we tested the hypotheses that in the early stages of obesity development, impaired insulin signaling manifests in skeletal muscle (triceps), brain (prefrontal cortex), and corresponding vasculatures, and that depressed insulin-induced vasodilation is reversible with acute inhibition of protein kinase Cβ (PKCβ). Juvenile Ossabaw miniature swine (3.5 mo of age) were divided into two groups: lean control (n = 6) and obese (n = 6). Obesity was induced by feeding the animals a high-fat/high-fructose corn syrup/high-cholesterol diet for 10 wk. Juvenile obesity was characterized by excess body mass, hyperglycemia, physical inactivity (accelerometer), and marked lipid accumulation in the skeletal muscle, with no evidence of overt atherosclerotic lesions in athero-prone regions, such as the abdominal aorta. Endothelium-dependent (bradykinin) and -independent (sodium nitroprusside) vasomotor responses in the brachial and carotid arteries (wire myography), as well as in the skeletal muscle resistance and 2A pial arterioles (pressure myography) were unaltered, but insulin-induced microvascular vasodilation was impaired in the obese group. Blunted insulin-stimulated vasodilation, which was reversed with acute PKCβ inhibition (LY333-531), occurred alongside decreased tissue perfusion, as well as reduced insulin-stimulated Akt signaling in the prefrontal cortex, but not the triceps. In the early stages of juvenile obesity development, the microvasculature and prefrontal cortex exhibit impaired insulin signaling. Such adaptations may underscore vascular and neurological derangements associated with juvenile obesity.
Keywords: brain, juvenile obesity, skeletal muscle, vascular insulin resistance
INTRODUCTION
Over four decades ago, an editorial in the Lancet emphasized the importance of preventing juvenile obesity (3). Since then, the problem has worsened steadily throughout the world (20), and current estimates indicate the prevalence of obesity has increased (severalfold) to ~20% in the United States among juvenile boys and girls (ages 6–13 yr old) (58). Consumption of a poor diet and increasing sedentary behavior contribute to juvenile obesity and associated health consequences, including, but not limited to, the cardiovascular system (i.e., endothelial dysfunction) (42, 94), skeletal muscle (i.e., metabolic dysfunction) (88, 98), and the brain (i.e., psychosocial and neurological dysfunction) (20, 22, 44, 91, 98). Furthermore, juvenile obesity may increase the risk of chronic disease, premature illness, and mortality in adulthood (28). Indeed, in a special report in the New England Journal of Medicine, Olshansky et al. (75) predicted that on the basis of increased rates of childhood obesity and associated long-term consequences, today’s children may represent the first generation to experience a decline in life expectancy. Juveniles in the early stages of obesity development frequently exhibit only moderate metabolic derangement and seldom display symptoms consistent with long-standing Type 2 diabetes, cardiovascular disease, or dementia (66, 89, 98). Thus, the pathophysiology of vascular, metabolic, and neurological impairments in this population remains difficult to elucidate. The concept of insulin resistance, as an early onset underlying cause of preclinical microvascular and metabolic impairments, reconciles the above-mentioned cluster of health derangements (29, 47). However, this prospect has not received much attention in the context of juvenile obesity.
Vascular insulin resistance results in reduced insulin-stimulated vasodilation, mediated in part by decreased insulin receptor substrate 1 (IRS-1)-induced activation of phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt)/nitric oxide (NO) signaling (10, 29, 47, 72). Evidence suggests that impaired vasodilator actions of insulin decrease glucose and insulin delivery to end organs and, as a result, impairs skeletal muscle glucose uptake (4, 51, 52), as well as insulin delivery and uptake in the brain (29, 39, 65). Among potential molecular targets responsible for inducing this adaptation, available data indicate that activation of PKCβ may directly reduce vascular NO bioavailability (31, 80) and impair vascular insulin signaling in an insulin-resistant state (73, 92). In addition to the microvasculature, both skeletal muscle and brain tissue respond to insulin directly, with decreased IRS-1/PI3K/Akt signaling implicated in reduced insulin-mediated skeletal muscle glucose uptake (72) and impaired prefrontal cortex metabolism (49, 65). Of note, a poor diet resulting in altered lipid profiles may also contribute to depressed insulin signaling in the vasculature, as well as in the end-organ tissue it supplies (15, 36, 59). Given that insulin resistance precedes the overt clinical manifestation of Type 2 diabetes and subsequent cardiovascular disease (47, 72), examining insulin signaling in the microvasculature and corresponding tissue it supplies may provide novel insight into the microvascular and metabolic impairments associated with early, preclinical stages of obesity development.
Therefore, this study examined the relationship between insulin resistance and early-stage obesity development using a translational porcine model of juvenile obesity. We tested the hypotheses that 1) obese juvenile pigs display impaired insulin-stimulated resistance artery vasodilation in the skeletal muscle and brain coupled with impaired insulin-stimulated Akt phosphorylation in the skeletal muscle and brain tissue itself, and 2) acute PKCβ inhibition restores insulin-induced vasodilation in obese pigs.
METHODS
Study design.
All experimental procedures described herein were approved by the Animal Care and Use Committee at the University of Missouri. Intact female and male Ossabaw miniature swine (n = 12; RRID:NSRRC:0008) were housed under temperature-controlled conditions, with a 12:12-h light-dark cycle. After being weaned, all pigs were group housed and consumed a standard commercially available chow diet (5L80, Laboratory Diet; 3.03 kcal/g; carbohydrate, 71%; protein, 18.5%; fat, 10.5%) until 3.5 mo of age. Thereafter, pigs were transitioned into individual pens and were randomly assigned into two groups: 1) juvenile lean control (n = 6; 4 female/2 male); and 2) short-term diet-induced juvenile obesity (n = 6; 3 female/3 male). No differences in outcome variables were observed between female and male swine. Although the lean control group continued to ingest standard chow, at 3.5 mo of age, their counterparts were fed a Western diet high in fat, high-fructose corn syrup, and cholesterol (5B4L, Laboratory Diet; 4.14 kcal/g; carbohydrate, 40.8% (17.8% of total calories from high-fructose corn syrup), protein, 16.2%; fat, 43%; 2%, cholesterol wt/wt; 1,000 g/day) for the remaining 2.5 mo of the study (from age 3.5 to 6 mo) to induce the early stages of juvenile obesity development. All pigs were fed once per day in the morning. At 6 mo of age, after a 20-h fast, pigs were anesthetized for in vivo hemodynamic evaluation and then euthanized for ex vivo vascular/metabolic experiments and tissue harvesting.
Spontaneous physical activity.
Two weeks before euthanasia, for 10 days, pigs were outfitted with an omnidirectional Actical B-series accelerometer (Koninklijke Phillips, Amsterdam, The Netherlands) placed between the midline and caudal to the forelimbs. Accelerometers were initialized to save data in one-minute averages. Data over the 10 days were averaged.
Hemodynamic data collection.
Pigs were sedated first using a Telazol (5 mg/kg)-xylazine (2.25 mg/kg) mixture, and anesthesia was maintained using propofol (6–15 mg·kg−1·min−1 iv). At this dose, propofol does not impair cerebral autoregulation in pigs (53, 67). Blood was sampled from the jugular vein in an EDTA-coated Vacutainer and centrifuged at 4°C at 5,000 rpm for 10 min, and the plasma was stored for future analysis. Heart rate (ECG), body temperature (rectal probe; maintained using heating pads), and mean arterial pressure (MAP; fluid filled 6-F arterial catheter) were monitored continuously. Middle cerebral blood flow velocity was examined using transcranial Doppler ultrasound (Multigon). Briefly, a 2-MHz probe was placed on the fronto-orbital region, and the angular orientation of the probe and signal depth were adjusted to obtain an optimal signal. An inherent limitation of transcranial Doppler ultrasound is that cerebral blood flow velocity must be used to estimate cerebral perfusion, as in vivo arterial diameters cannot be resolved. Thereafter, pigs were placed in the supine position, and brachial artery blood flow was measured using Doppler ultrasound (Phillips HDI 5000; Bothel, WA). Forelimb blood flow was calculated as the product of the measured blood flow velocity and cross-sectional area of the artery, as determined from the measured diameter. Vascular resistance was calculated as the quotient of MAP and arterial blood flow (blood flow velocity for the middle cerebral artery). Vascular data were averaged over ~60 cardiac cycles.
Ex vivo vascular reactivity of the conduit and downstream resistance arteries.
At euthanasia, the brachial artery and the medial portion of the long head of the triceps, as well as the carotid artery and a portion of the lateral aspect of the brain (including the middle cerebral artery and corresponding branches) were harvested immediately and placed in ice-cold physiological saline solution (PSS: 145 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4 with 10 g/l albumin added) with a pH of 7.4. The brachial and carotid arteries were cleaned of surrounding adipose and cut into ~3-mm-long arterial rings. The rings were mounted on wire brackets connected to an isometric force transducer and submerged in a bath containing 20 ml of oxygenated (95% O2-5% CO2) physiological Krebs solution (pH = 7.4) and maintained at 37°C and optimal tension, as previously described (62, 63, 95, 101). Viability of arterial rings was confirmed by constriction to 80 mM KCl. Arterial rings were preconstricted with prostaglandin PGF2-α (PGF; 30 μM) and dose-response curves for bradykinin (to assess endothelium-dependent vasorelaxation; whole log doses; 1e−9 – 1e−4 M), and sodium nitroprusside (to assess endothelium-independent vasorelaxation; SNP; whole log doses; 1e−9 – 1e−4 M) were examined. Vasorelaxation at each concentration was measured and expressed as percent maximum relaxation, where 100% is equivalent to the loss of all tension developed in response to PGF.
Skeletal muscle resistance arteries and 2A pial arteries were dissected using the aid of an Olympus microscope, transferred to a Plexiglas chamber filled with PSS and cannulated with two glass micropipettes (60–75 µm) filled with PSS. The chambers were then transferred to the stage of an inverted microscope (Nikon Diaphot 200) attached to a video camera (Javelin Electronics, Los Angeles, CA), video micrometer (Microcirculation Research Institute, Texas A&M University) and a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO), as previously described (18, 19, 68, 69, 79). Fluid-filled reservoirs were used to set intraluminal pressure at 60 cmH2O, and luminal diameter was monitored throughout the experiment. After cannulation, arteries were allowed 30 min to stabilize, at which point maximal arterial vasoconstriction in response to 80 mM KCl was determined. Thirty minutes later, arteries were preconstricted with U46119 (thromboxane A2 analogue; 1e−7 − 1e−4 M) and dose-response curves for bradykinin (half-log doses; 1e−9 – 1e−5 M), insulin (1e−9 – 1e−4 M) and SNP (whole-log doses; 1e−9 – 1e−4 M) were examined under untreated conditions. In a second artery, to test the hypothesis that acute PKCβ inhibition reverses vascular insulin resistance in obese juvenile pigs, insulin-induced dilation was examined after 20 min of pretreatment with PKCβ1 and PKCβ2 inhibitor (LY333-531; 1e−6 M; no SML0693-1MG; Sigma-Aldrich). LY333-531 was brought to solution with DMSO (0.5% final concentration) (37, 40, 83, 90). Previous studies (37, 40, 83, 90) and pilot data from our group indicate this DMSO concentration does not alter vasomotor reactivity to endothelium-dependent vasodilators in isolated arteries. In addition, data from the current study indicate that similar to untreated conditions, acute PKCβ inhibition (i.e., LY333-531 prepared in DMSO) had no effect on basal skeletal muscle or cerebral resistance artery diameters in lean or obese swine. After the final dose-response curve, vessels were washed twice with Ca2+-free PSS to determine maximal diameter. Vasomotor responses were expressed as percent possible dilation (i.e., the quotient of ∆ diameter-baseline diameter and ∆ maximal Ca2+-free diameter-baseline diameter, multiplied by 100%). Resistance arteries that did not obtain a stable preconstriction diameter were removed from the analyses (see figure captions for details).
Additional skeletal muscle resistance and 2A pial arterial segments (~2 mm long) were mounted on 17-µm stainless-steel wires in oxygenated Krebs PSS (95% O2-5% CO2) in a small vessel wire myograph for isometric tension recording (Danish Myo Technology, Aarhus, Denmark), as previously described (8, 71). Vessel length was measured after mounting with a calibrated lens in the dissection scope. After warming to 37°C and equilibration, normalization was performed, as previously described (86), and vessels were stretched to achieve an internal circumference corresponding to a transmural pressure of 90 mmHg. Vessel viability was subsequently assessed by exposure to 80 mM KCl Krebs PSS. After washing was completed, vasocontractile dose-response curves to phenylephrine (PE; 10−9 – 10−4 M), the thromboxane A2 analog U46119 (10−8–10−6 M), and endothelin-1 (ET-1; 10−10–10−8 M) were assessed in untreated conditions. Vasocontractile responses were expressed as developed tension from baseline normalized to vessel length (mN/mm).
End-organ insulin signaling studies.
After euthanasia, triceps, prefrontal cortex, and omental fat samples were harvested and underwent ex vivo insulin signaling experiments. Tissues were cut into ~2-mm pieces and placed in a 1.5-ml tube containing 1 ml DMEM (Gibco no. 11965-092; Thermo Scientific) + 0.1% FBS and allowed to incubate for 3 h at 37°C on a rocking platform. Next, tissues underwent one of the following three treatments for 30 min: 0 nM insulin, 10 nM insulin, and 100 nM insulin (14). After incubation, tissues were removed from the DMEM media and placed in a clean collection tube for storage in a −80°C freezer until analyzed.
Triton X-100 tissue lysates of the aforementioned insulin signaling tissues were prepared in 1:1 Laemmli buffer. Prepared protein samples (10 µg/lane muscle and brain, and 8 µg/lane omental adipose tissue) were separated via Criterion Tris-glycine eXtended-PAGE precast gels (Bio-Rad, Hercules, CA). Proteins were transferred onto polyvinylidene difluoride membranes and blocked overnight in 5% nonfat dry milk. Membranes were probed for total PKCβ2 (1:1,000, no. 32026; Abcam, Cambridge, MA), total Akt (1:500; no. 54691; Cell Signaling, Danvers, MA) and phosphorylated Akt (Ser473) (1:250; no. 4060; Cell Signaling). Omental adipose samples were also probed for β-tubulin (no. 2146, 1:1,000; Cell Signaling). Intensity of individual protein bands were quantified via densitometry using FluoroChem HD2 (AlphaView, version 3.4.0.0) and were expressed as the ratio of phosphorylated to total Akt, normalized to gel and relative to the control group. Lean and obese samples were run on the same immunoblots. Total protein stain for skeletal muscle and brain samples was determined on an unexposed region of the PVDF membrane to serve as a loading control (1% amido-black; Sigma-Aldrich). Total protein stain was not available for adipose tissue; therefore, β-tubulin protein content is presented instead.
Gene expression.
Skeletal muscle and brain resistance arteries were homogenized in TRIzol reagent (TissueLyser LT; Qiagen, Valencia, CA), and total RNA was isolated using the Qiagen’s RNeasy lipid tissue homogenization kit according to the manufacturerʼs instructions. RNA concentration and purity were assessed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). First-strand cDNA was synthesized from total RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed using the ABI StepOne Plus sequence detection system (Applied Biosystems). Primers were purchased from Integrated DNA Technologies (Coralville, IA) or Sigma Aldrich (St. Louis, MO). A 20-μl reaction mixture containing iTaq UniverSYBR Green SMX (Bio-Rad), 1,000 nmol of gene-specific primers, cDNA template (1,000 μg/μl), and RNase free water were loaded in a single well of a 96-well plate. The list of gene primer sequences are available upon request. Polymerase chain reactions were performed in duplicate under thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 45 s. A dissociation melt curve analysis was performed to verify the specificity of the PCR products, and 18S was used as a housekeeping control gene. mRNA expression values were calculated by the ∆∆ CT method (57) and analyzed as a fold difference compared with the lean group, which was set at 1.0.
Prefrontal cortex and skeletal muscle fatty acid analysis.
Frozen triceps muscle and prefrontal cortex were homogenized in 10 volumes of Tris·HCl buffer (pH 8.0) and used for lipid analysis. The resultant homogenate was transferred into Kimax tubes, and total lipids were extracted using chloroform/methanol (2:1), as previously described (27). Isolated samples were spiked with tridecanoic acid (13:0; internal standard) and were methylated (61), and then the fatty acid composition of each was analyzed by gas chromatography, as previously described (13). Briefly, the fatty acid methyl esters were separated on an UFM-RTX WAX analytical column (Thermo Electron, Milan, Italy) using a gas chromatograph (Trace GC Ultra, Thermo Electron) that was fitted with a fast-flame ionization detector, a split-splitless injector, and a Triplus AS autosampler. Fatty acids were identified by retention time as compared with known standards (Supelco 37 component FAME mix; Supelco, Bellefonte, PA), and the absolute amount of each individual fatty acid was calculated using tridecanoic acid.
Adipose tissue and atherosclerosis histological assessment.
Formalin-fixed omental adipose samples were processed through paraffin embedment, sectioned at 5 μm and stained with hematoxylin and eosin. Sections were evaluated via an Olympus BX34 photomicroscope (Olympus, Melville, NY), and images were taken via an Olympus SC30 Optical Microscope Accessory CMOS color camera using the ×10 objective. Adipocyte size was calculated from the average of 100 adipocytes per animal. Briefly, cross-sectional areas of the adipocytes perimeter tracings were obtained and performed using ImageJ software, and the distribution of adipocytes per discrete cell size was examined, as previously described (54, 55, 100). All procedures were performed by an investigator who was blinded to the groups.
The abdominal aorta was harvested and fixed in 10% formalin. The aorta samples were opened longitudinally, pinned to a paraffin tray, stained with Sudan IV, and photographed digitally for determination of atherosclerotic plaques, as previously described (95).
Blood analyses.
Plasma glucose, total cholesterol, LDL, HDL, and triglycerides were determined using commercially available assays (Beckman-Coulter, Brea, CA) on an automated clinical chemistry analyzer (Beckman-Coulter AU680). Plasma nonesterified fatty acids were also determined on the automated clinical chemistry platform using a commercially available assay (Randox Laboratories, Kearneysville, WV). Plasma insulin (Crystal Chem, Downers Grove, IL) and leptin (LifeSpan Biosciences, Seattle WA) concentrations were determined using a species-specific, commercially available ELISA kit.
Statistical analyses.
Power analyses were conducted to determine the appropriate number of pigs per group as recommended by Kim and Seo (46) using the Sealed Envelope Power Calculator (https://www.sealedenvelope.com/power/continuous-superiority/). For input, we used currently unpublished data obtained from 14-mo-old Ossabaw pigs fed a control or Western diet for 9 mo. In particular, we used skeletal muscle resistance artery insulin-stimulated vasodilation from five healthy lean and four aortic-banded diet-induced obese female pigs. Significance level was set to 5%, power to 80%, mean outcome in control group = 24.1, mean outcome in experimental group = 0, and standard deviation = 13.7. The power analysis revealed that n = 6 per group would be sufficient to detect between-group differences in insulin-stimulated resistance artery vasodilation; thus, our group n’s were based on this calculation. Body mass throughout the study was compared using a repeated-measures ANOVA (group × time). Between-group comparisons at the final time point were made using an unpaired, two-tailed t-test. Vasomotor responses to pharmacological, vasodilator, and vasoconstrictor agents, and ex vivo insulin signaling responses were compared using a repeated-measures ANOVA (group × dose). The relative change in the fatty acid concentration in the triceps and prefrontal cortex of obese animals was compared using a paired, two-tailed t-test. The significance level was set at P ≤ 0.05, and pairwise comparisons were performed using a post hoc Student-Newman-Keuls test. All data are presented as means ± SE.
RESULTS
Physical characteristics and cardiometabolic risk factors.
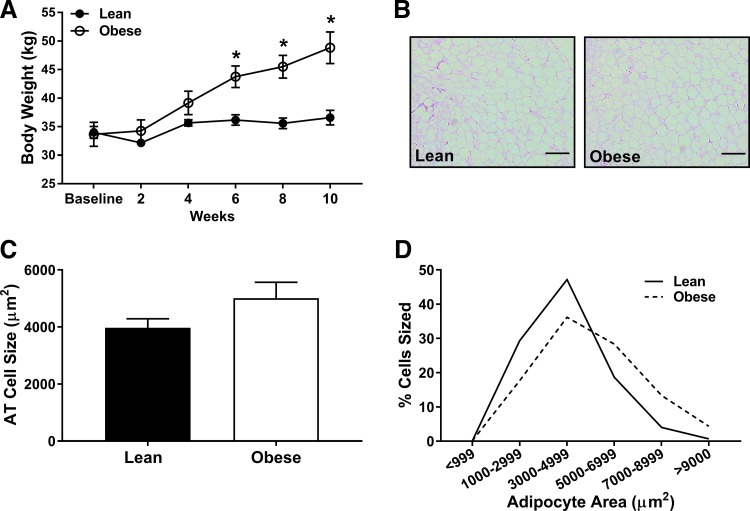
Body mass was similar between groups from baseline through week 4 and greater in juvenile obese vs. lean pigs from week 6 to 10 of the intervention (P < 0.01; Fig. 1A). Although average adipocyte size was not significantly different between groups (P = 0.13; Fig. 1, B and C), obese pigs appeared to have a greater percentage of larger adipocytes (Fig. 1D). No evidence of crown-like structures were noted in either group upon examination of histological images. Brain mass was not different between groups (wet weight: lean = 88 ± 2 vs. obese = 86 ± 2 g; P = 0.29).
Fig. 1.
Body mass in lean (solid) and obese (open) juvenile swine over 10 wk of normal chow vs. Western diet feeding, from 4 to 6.5 mo of age. Histological images (B: scale bar = 200 µM) and analysis (C) of omental adipocyte size and adipocyte area distribution (D). *Significantly different from lean (P ≤ 0.05).
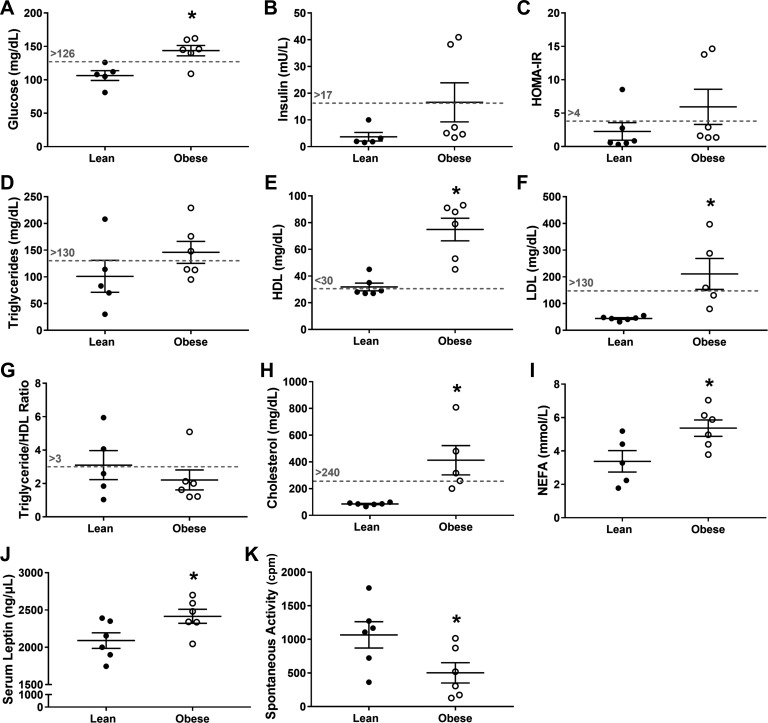
Although plasma glucose was greater (P = 0.01; Fig. 2A), plasma insulin and homeostatic model assessment of insulin resistance scores were not significantly different (P ≥ 0.13; Fig. 2, B and C) in the early stages of obesity development. Also, plasma cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and nonesterified fatty acids (NEFA) were greater (P ≤ 0.03; Fig. 2, E–H) in the obese group, but triglycerides and the HDL:triglyceride ratio were not significantly different (P ≥ 0.23; Fig. 2, D and G). Corresponding with increased body mass, plasma leptin was higher, and daily cage activity was lower in the juvenile obese group (P ≤ 0.05; Fig. 2, J–K). There was no evidence of atherosclerosis in either group, indicated by negative Sudan IV staining in the abdominal aorta (data not shown).
Fig. 2.
Cardio-metabolic risk factors (A–H; scored according to Refs. 66 and 74), nonesterified fatty acids (NEFA; I) serum leptin (J), spontaneous activity (cpm; K) in lean (solid) and obese (open) juvenile swine at the end point of the study. Dashed lines represent clinical cut-offs for cardiovascular disease risk in juvenile obesity (66, 74). *Significantly different from lean (P ≤ 0.05). Values that were below or above the detectable range were excluded from analyses.
Hemodynamics and ex vivo vascular reactivity along the arterial tree.
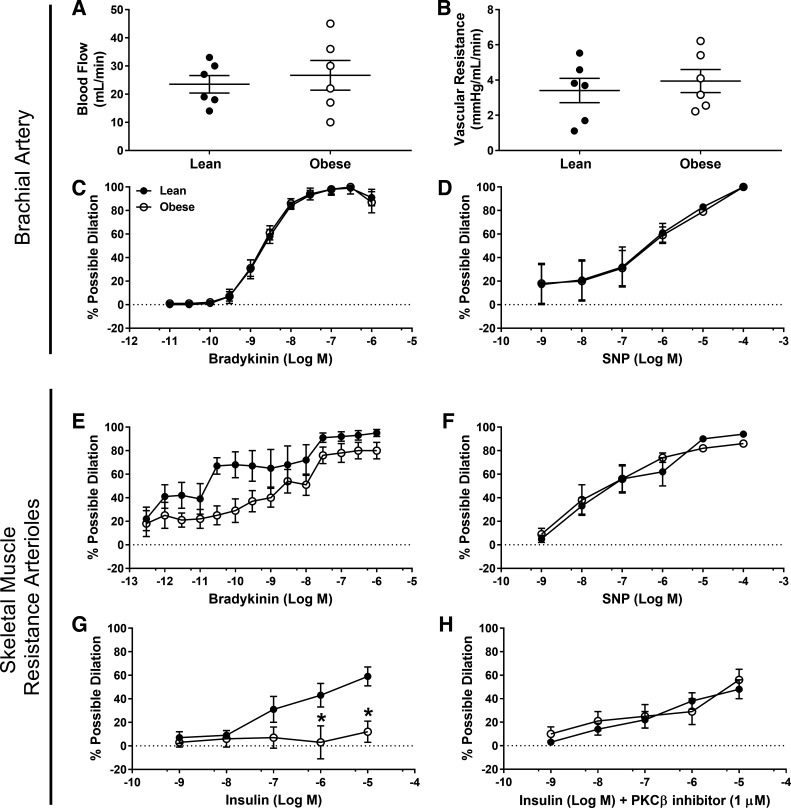
Systolic (lean = 77 ± 11 vs. obese = 99 ± 5 mmHg; P = 0.11), diastolic (lean = 62 ± 10 vs. obese = 72 ± 5 mmHg; P = 0.38) and mean arterial pressure (lean = 70 ± 11 vs. obese = 88 ± 5 mmHg, P = 0.17) were not significantly different; however, pulse pressure (lean = 15 ± 2 vs. obese = 23 ± 3 mmHg; P = 0.03) was greater in the early stages of Western diet-induced obesity development. Resting brachial artery blood flow and vascular resistance were similar between groups (P ≥ 0.58; Fig. 3, A and B). Brachial artery vasorelaxation to bradykinin and SNP were similar among groups (P ≥ 0.96; Fig. 3, C and D). Though there was a trend toward a main effect of juvenile obesity, skeletal muscle resistance artery vasodilation to bradykinin was not significantly different (P = 0.07; Fig. 3E), and vasodilation to SNP was similar among groups (P = 0.81; Fig. 3F). Sample size calculation reveals that 16 pigs per group would have been necessary to detect group differences in bradykinin-induced vasodilation. Overall, endothelium-dependent and -independent responses along the vascular tree appeared relatively preserved in the initial stages of obesity development. However, insulin-induced vasodilation in skeletal muscle resistance arteries was markedly decreased in juvenile obese pigs (P < 0.05; Fig. 3G). Depressed insulin-induced vasodilation in skeletal muscle arterioles was reversed with acute PKCβ inhibition (Fig. 3H). However, neither gene expression (lean = 1.0 ± 0.3 vs. obese = 1.0 ± 0.2; P = 0.96) nor protein content of PKCβ (lean = 1.0 ± 0.1 vs. obese = 1.3 ± 0.2; P = 0.25) in skeletal muscle resistance arteries was different between groups. Arteriole vasocontractile responses to the α1 adrenergic receptor agonist PE, thromboxane A2 analog U46119, and ET-1 were similar between groups (P ≥ 0.61, data not shown).
Fig. 3.
Brachial blood flow (A), vascular resistance (B), brachial artery vasomotor responses to bradykinin (C) and sodium nitroprusside (SNP; D), skeletal muscle resistance artery vasomotor responses to bradykinin (E), SNP (F), insulin (G), and insulin + 1 µM PKCβ inhibitor (H). *Significantly different from lean (P ≤ 0.05). Inability to obtain a stable preconstriction diameter resulted in an n = 5 per group for G and for the control group in H.
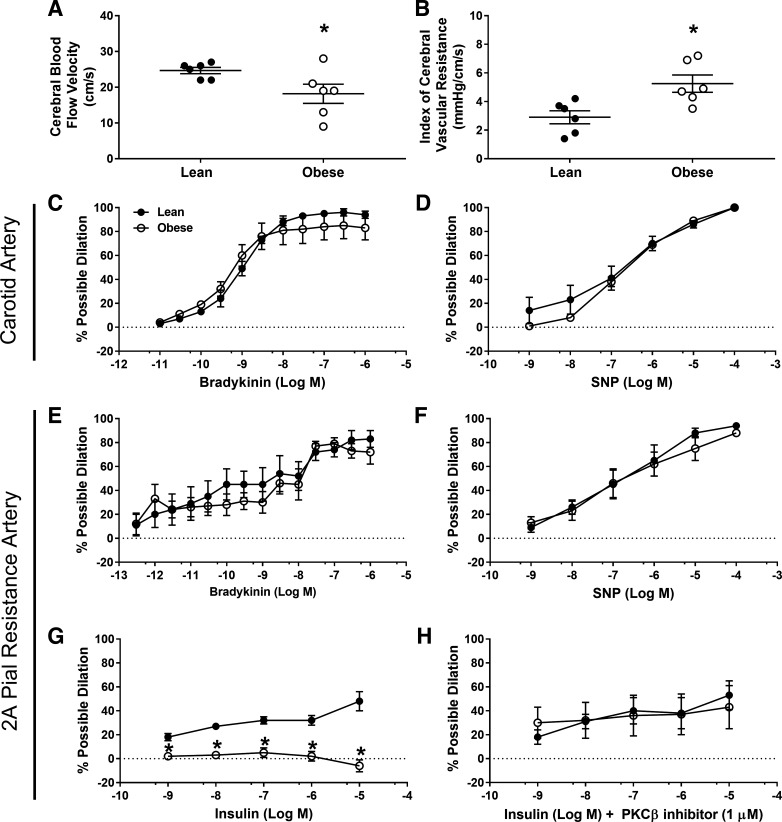
Resting middle cerebral blood flow velocity and the index of vascular resistance were decreased and increased, respectively, in the juvenile obese pigs fed a Western diet (P ≤ 0.05; Fig. 4, A and B). Carotid artery vasorelaxation and 2A pial arterial vasodilation to bradykinin and SNP were similar among groups (P ≥ 0.68; Fig. 4, C–F), indicating preserved endothelium-dependent and -independent responses along the vascular tree. Consistent with observations in the skeletal muscle arterioles, insulin-induced vasodilation in 2A pial arteries was prominently decreased in early stages of obesity development (P < 0.05; Fig. 4G), and blunted vasodilatory responses were reversed with acute PKCβ inhibition (Fig. 4H). Similar to skeletal muscle resistance arteries, neither gene expression (lean = 1.0 ± 0.2 vs. obese = 1.2 ± 0.2; P = 0.51) nor protein content of PKCβ (lean = 1.0 ± 0.1 vs. obese = 1.0 ± 0.1; P = 0.83) in 2A pial arteries was different between groups. Pial artery contractile responses to PE, U46119, and ET-1 were similar between groups (P ≥ 0.38; data not shown). Both 2A pial and skeletal muscle resistance arteriole characteristics (i.e., passive diameters, wall thickness, wall:lumen ratio, KCl-induced vasoconstriction, and average % preconstriction) were similar between groups (P ≥ 0.24) and are presented in Table 1.
Fig. 4.
Middle cerebral artery blood flow velocity (A), indices of vascular resistance (B), carotid artery vasomotor responses to bradykinin (C), and sodium nitroprusside (SNP; D), 2A pial artery vasomotor responses to bradykinin (E), SNP (F), insulin (G), insulin + 1 µM PKCβ inhibitor (H). *Significantly different from lean (P ≤ 0.05).
Table 1.
Microvessel characteristics
| Lean | Obese | P Value | |
|---|---|---|---|
| Passive diameter in Ca2+ free, µM | |||
| TRI-RA | 170 ± 14 | 174 ± 18 | 0.87 |
| 2A Pial | 221 ± 14 | 260 ± 29 | 0.24 |
| Wall thickness, µM | |||
| TRI-RA | 61 ± 3 | 58 ± 5 | 0.63 |
| 2A Pial | 42 ± 3 | 39 ± 3 | 0.51 |
| Wall-to-lumen ratio | |||
| TRI-RA | 0.39 ± 0.04 | 0.34 ± 0.02 | 0.38 |
| 2A Pial | 0.21 ± 0.01 | 0.18 ± 0.02 | 0.38 |
| KCl-induced % vasoconstriction | |||
| TRI-RA | 71 ± 5 | 72 ± 2 | 0.84 |
| 2A Pial | 55 ± 5 | 46 ± 7 | 0.34 |
| Average % preconstriction | |||
| TRI-RA | 35 ± 2 | 34 ± 3 | 0.67 |
| 2A Pial | 38 ± 3 | 41 ± 2 | 0.46 |
Values are means ± SE. TRI-RA, triceps resistance arteriole.
Ex vivo insulin signaling in skeletal muscle, prefrontal cortex, and omental fat tissues.
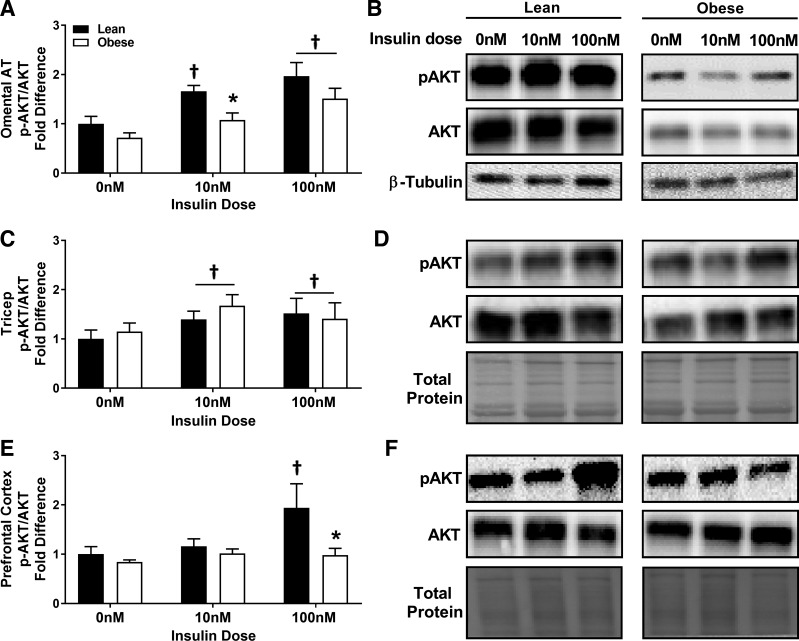
Insulin stimulation increased the ratio of phosphorylated to total Akt in omental adipose tissue from both groups, and insulin-stimulated phosphorylation of Akt in omental adipose was depressed with obesity at 10 nM of insulin (P < 0.01; Fig. 5, A and B). Insulin stimulation increased the ratio of phosphorylated to total Akt in whole skeletal muscle tissue in both groups (P = 0.01; Fig. 5, C and D). Insulin stimulation increased the ratio of phosphorylated to total Akt in whole prefrontal cortex tissue in lean, but not juvenile obese pigs at 100 nM of insulin (P < 0.05; Fig. 5, E and F).
Fig. 5.
Basal and insulin-induced phosphorylated Akt-to-total Akt ratio in omental adipose tissue (A; representative immunoblots presented in B), skeletal muscle (C, representative immunoblots presented in D), and prefrontal cortex (E; representative immunoblots presented in F). *Significantly less than lean (P < 0.05). †Significantly greater than 0 nM insulin (P ≤ 0.05).
Fatty acid profile in triceps and the prefrontal cortex.
Western diet-induced juvenile obesity altered the triceps fatty acid profile indicated by significant increases in the following fatty acids: 14:0, 15:0, 16:0, 18:0, 22:0, 17:1, and 18:3n6 and (P ≤ 0.04; Table 2). Furthermore, obesity-induced increases in the following fatty acids approached significance: 12:0, 24:0, 16:1, and 18:1 (P ≤ 0.10; Table 2). These changes corresponded with significant increases in total lipid concentration (P = 0.03; Table 2), increased saturated fat concentration (P = 0.02; Table 2), and a trend toward increased monounsaturated fat concentration (P = 0.09; Table 2). The ratio of n3:n6 fatty acids in the triceps was lower in juvenile obese pigs (P = 0.05, Table 2).
Table 2.
Fatty acid profile in the triceps
| Fatty Acid, µmol/g wet wt | Lean | Obese | P Value |
|---|---|---|---|
| 8:0 | 0.07 ± 0.03 | 0.24 ± 0.11 | 0.17 |
| 10:0 | 0.09 ± 0.07 | 0.07 ± 0.02 | 0.71 |
| 11:0 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.42 |
| 12:0 | 0.05 ± 0.01 | 0.18 ± 0.07 | 0.07 |
| 14:0 | 0.39 ± 0.06 | 1.0 ± 0.19* | 0.01 |
| 15:0 | 0.06 ± 0.01 | 0.10 ± 0.01* | 0.01 |
| 16:0 | 7.79 ± 1.17 | 14.58 ± 2.26* | 0.02 |
| 17:0 | 0.20 ± 0.02 | 0.50 ± 0.21 | 0.19 |
| 18:0 | 4.70 ± 0.82 | 7.51 ± 0.87* | 0.04 |
| 20:0 | 0.06 ± 0.01 | 0.13 ± 0.07 | 0.36 |
| 22:0 | 0.02 ± 0.01 | 0.07 ± 0.02* | 0.03 |
| 23:0 | 0.16 ± 0.02 | 0.19 ± 0.05 | 0.68 |
| 24:0 | 0.02 ± 0.01 | 0.06 ± 0.02 | 0.06 |
| 14:1 | 0.16 ± 0.03 | 0.18 ± 0.04 | 0.81 |
| 15:1 | 0.09 ± 0.05 | 0.11 ± 0.06 | 0.80 |
| 16:1 | 0.84 ± 0.14 | 1.44 ± 0.26 | 0.07 |
| 17:1 | 0.16 ± 0.02 | 0.29 ± 0.03* | 0.01 |
| 18:1 | 9.61 ± 1.87 | 15.69 ± 2.73 | 0.10 |
| 20:1 | 0.18 ± 0.03 | 0.18 ± 0.06 | 0.92 |
| 22:1 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.94 |
| 18:3n3 | 0.22 ± 0.03 | 0.30 ± 0.04 | 0.15 |
| 20:3n3 | 1.69 ± 0.40 | 2.04 ± 0.49 | 0.60 |
| 20:5n3 | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.77 |
| 22:6n3 | 0.03 ± 0.02 | 0.00 ± 0.00 | 0.19 |
| 18:2n6 | 5.98 ± 1.02 | 9.16 ± 1.78 | 0.15 |
| 18:3n6 | 0.06 ± 0.01 | 0.15 ± 0.04* | 0.03 |
| 20:2n6 | 0.19 ± 0.03 | 0.20 ± 0.04 | 0.96 |
| 20:3n6 | 0.20 ± 0.04 | 0.32 ± 0.08 | 0.25 |
| 20:4n6 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.57 |
| 22:2n6 | 0.05 ± 0.01 | 0.10 ± 0.05 | 0.34 |
| Total lipids | 33.2 ± 5.0 | 54.5 ± 7.0* | 0.03 |
| Total saturates | 13.6 ± 2.0 | 24.6 ± 3.6* | 0.02 |
| Total monoenes | 11.0 ± 2.0 | 17.9 ± 3.1 | 0.09 |
| Total polyenes | 8.5 ± 1.5 | 12.4 ± 2.4 | 0.20 |
| n3 polyenes | 2.0 ± 0.4 | 2.4 ± 0.5 | 0.57 |
| n6 polyenes | 6.5 ± 1.1 | 10.0 ± 1.9 | 0.14 |
| n3:n6 ratio | 0.3 ± 0.0 | 0.2 ± 0.0* | 0.05 |
Values are means ± SE. *Significantly different from lean (P ≤ 0.05).
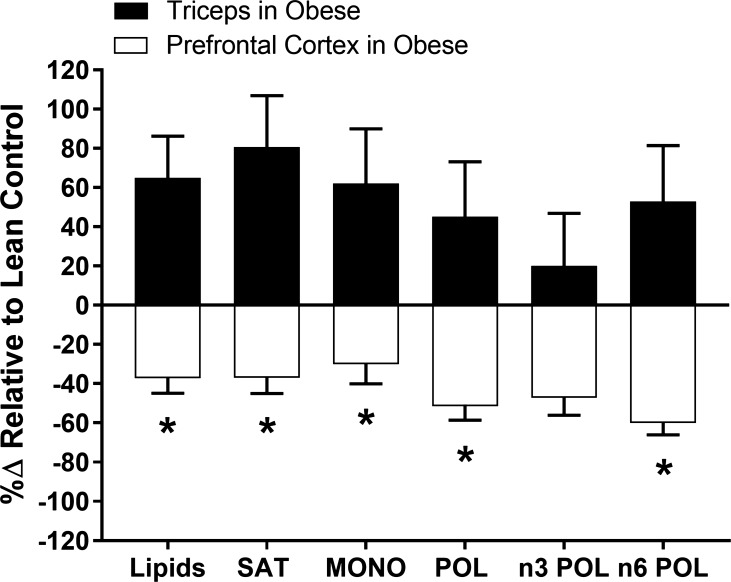
Western diet-induced juvenile obesity did not significantly alter prefrontal cortex fatty acid profile. However, obesity-induced decreases in the following fatty acids approached significance: 23:0, 24:0, 17:1, 18:3n3, and 20:3n3 (P ≤ 0.10; Table 3). The effect of obesity on lipid accumulation appeared to be tissue-dependent; whereas obesity increased lipid concentration in the triceps, it tended to decrease lipid concentration in the prefrontal cortex (see Fig. 6; P < 0.05).
Table 3.
Fatty acid profile in the prefrontal cortex
| Fatty Acid, µmol/g wet wt | Lean | Obese | P Value |
|---|---|---|---|
| 8:0 | 0.57 ± 0.33 | 0.44 ± 0.20 | 0.74 |
| 10:0 | 0.30 ± 0.22 | 0.09 ± 0.05 | 0.38 |
| 11:0 | 0.18 ± 0.07 | 0.08 ± 0.04 | 0.23 |
| 12:0 | 0.05 ± 0.01 | 0.08 ± 0.03 | 0.32 |
| 14:0 | 0.93 ± 0.22 | 0.72 ± 0.09 | 0.39 |
| 15:0 | 0.22 ± 0.05 | 0.16 ± 0.03 | 0.28 |
| 16:0 | 31.50 ± 6.20 | 21.91 ± 3.39 | 0.20 |
| 17:0 | 0.71 ± 0.15 | 0.51 ± 0.10 | 0.29 |
| 18:0 | 35.21 ± 6.93 | 22.07 ± 3.11 | 0.11 |
| 20:0 | 0.41 ± 0.08 | 0.30 ± 0.06 | 0.34 |
| 22:0 | 0.41 ± 0.14 | 0.31 ± 0.11 | 0.57 |
| 23:0 | 4.18 ± 0.86 | 2.49 ± 0.34 | 0.10 |
| 24:0 | 7.96 ± 2.01 | 3.57 ± 0.74 | 0.07 |
| 14:1 | 0.20 ± 0.08 | 0.15 ± 0.03 | 0.53 |
| 15:1 | 0.27 ± 0.11 | 0.22 ± 0.10 | 0.77 |
| 16:1 | 2.68 ± 0.57 | 1.90 ± 0.24 | 0.24 |
| 17:1 | 1.16 ± 0.24 | 0.65 ± 0.10 | 0.08 |
| 18:1 | 28.21 ± 5.87 | 19.861 ± 3.11 | 0.24 |
| 20:1 | 0.76 ± 0.18 | 0.58 ± 0.12 | 0.42 |
| 22:1 | 0.10 ± 0.06 | 0.27 ± 0.12 | 0.22 |
| 18:3n3 | 0.34 ± 0.11 | 0.14 ± 0.02 | 0.10 |
| 20:3n3 | 11.15 ± 2.66 | 5.82 ± 0.91 | 0.09 |
| 20:5n3 | 0.03 ± 0.02 | 0.04 ± 0.04 | 0.83 |
| 22:6n3 | 0.76 ± 0.47 | 0.57 ± 0.46 | 0.76 |
| 18:2n6 | 2.03 ± 0.64 | 1.15 ± 0.14 | 0.21 |
| 18:3n6 | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.33 |
| 20:2n6 | 0.84 ± 0.26 | 0.36 ± 0.08 | 0.11 |
| 20:3n6 | 1.31 ± 0.62 | 0.48 ± 0.16 | 0.22 |
| 20:4n6 | 1.25 ± 1.17 | 0.04 ± 0.01 | 0.32 |
| 22:2n6 | 0.34 ± 0.09 | 0.30 ± 0.08 | 0.76 |
| Total lipids | 134.1 ± 27.9 | 85.2 ± 11.3 | 0.14 |
| Total saturates | 82.6 ± 16.2 | 52.7 ± 7.2 | 0.12 |
| Total monoenes | 33.4 ± 6.9 | 23.6 ± 3.6 | 0.24 |
| Total polyenes | 18.1 ± 5.4 | 8.9 ± 1.4 | 0.13 |
| n3 polyenes | 12.3 ± 3.0 | 6.6 ± 1.2 | 0.11 |
| n6 polyenes | 5.8 ± 2.7 | 2.4 ± 0.4 | 0.23 |
| n3:n6 ratio | 2.9 ± 0.4 | 2.9 ± 0.4 | 0.92 |
Values are means ± SE.
Fig. 6.
%∆ relative to lean control in total lipid, saturates (SAT), monoenes (MON), polyenes (POL), n3 polyenes, and n6 polyenes in the triceps and prefrontal cortex of obese animals. *Significantly different from triceps (P < 0.05).
DISCUSSION
The major finding of the current study indicates that in the early stage of Western diet-induced juvenile obesity development, characterized by excess body mass, a shift toward a greater number of larger adipocytes and hyperglycemia, impaired insulin-induced vasodilation in skeletal muscle resistance arteries and 2A pial arteries occurs independent of overt endothelium-dependent and -independent vascular dysfunction (i.e., preserved vasomotor responses to bradykinin and sodium nitroprusside in conduit and resistance arteries). Acute PKCβ inhibition restored insulin-induced dilation, highlighting the potential therapeutic efficacy of targeted PKCβ inhibitors (i.e., ruboxistaurin) in the treatment of vascular insulin resistance in in Western diet-induced juvenile obesity. Functional microvascular insulin resistance coincided with decreased cerebral blood flow velocity and altered insulin-stimulated Akt signaling in the prefrontal cortex, but not skeletal muscle. These data implicate the brain and corresponding vasculature as early targets for detecting insulin resistance in the initial stages of juvenile obesity development.
Consistent with previous observations in lean and obese miniature swine (96) and humans (99), in the current study, juvenile pigs in the initial stages of diet-induced obesity development displayed a reduced index of cerebral blood flow. Cross-sectional adult human data from Ainslie et al. (2) reveal an annual age-related decline in cerebral blood flow velocity of ~0.76 cm/s; thus, in the context of the present study, obesity development may predispose juveniles to accelerated cerebrovascular aging. In contrast to the cerebral circulation, resting brachial artery blood flow appeared unaffected by juvenile obesity. Karpoff et al. (42) reported no difference in lower limb blood flow scaled to thigh muscle mass in moderately obese juvenile boys (~12 yr old, BMI = 23.9 ± 0.8), whereas Ribeiro et al. (85) observed decreases in forearm blood flow in severely obese juvenile boys and girls (~10 yr old, BMI = 29 ± 0.3). Thus, the vascular bed, degree of adiposity, and load-bearing status of the interrogated limb may contribute to divergent findings concerning the effects of juvenile obesity on basal skeletal muscle perfusion. We examined blood flow in a weight-bearing limb in pigs during the early stages of diet-induced obesity development and did not scale brachial blood flow to forelimb mass, all of which may limit the likelihood of observing a flow deficit. Nevertheless, when contrasted with the cerebral blood flow velocity data, it appears that decrements in estimated cerebral blood flow likely occurred to a greater extent than any potential changes in forelimb blood flow in the early stages of diet-induced obesity development.
In the early stages of diet-induced obesity development, on the basis of ex vivo/in vitro vascular experiments, neither the carotid nor brachial arteries, nor corresponding downstream vasculature, displayed overt endothelium-dependent or -independent vasodilatory dysfunction (i.e., preserved vasomotor responses to bradykinin and sodium nitroprusside in conduit and resistance arteries). Yet, consistent with previous observations that indicate vascular insulin resistance represents an early feature of endothelial dysfunction (9, 23, 43), both downstream vascular beds exhibited evidence of vascular insulin resistance, indicated by impaired insulin-induced vasodilation. Functionally, this defect may reduce insulin delivery and disrupt transendothelial insulin transport in the brain (29, 39, 65), as well as impair glucose disposal in skeletal muscle (4, 51, 52), independent of changes in bulk flow (17). Notably, acute PKCβ inhibition rescued insulin-mediated vasodilation in both the skeletal muscle and cerebrovascular beds. Reports indicate hyperglycemia (6) and elevated leptin (82), both of which were present in the juvenile obese group in the current study, impair endothelium-dependent vasorelaxation through a PKCβ-dependent mechanism. Furthermore, treatment (for 6 wk) with PKCβ inhibition improves brachial artery flow-mediated dilation in patients with Type 2 diabetes (64). The current data suggest the detrimental effects of insulin resistance and PKCβ on vasomotor control at an early developmental stage of obesity manifest without evidence of overt endothelial dysfunction or increases in vascular PKCβ content. Taken together with previous findings, this highlights the potential utility of PKCβ inhibitors, such as ruboxistaurin, for treating vascular dysfunction in the early and advanced stages of disease pathology, even when vascular PKCβ content may not be increased. Establishing the effectiveness of PKCβ inhibition on vascular insulin resistance in the clinical setting is of particular importance, as previous studies indicate controlling blood glucose alone in the setting of Type 1 or 2 diabetes does not prevent impairments in insulin-induced vasodilation (18, 19, 77, 78).
At the end-organ level, the prefrontal cortex and omental adipose tissue, but not the skeletal muscle, exhibited evidence of impaired insulin signaling (i.e., attenuated insulin-induced Akt phosphorylation (Ser473) highlighting the potential vulnerability of the brain and visceral fat depots to short-term perturbations in metabolic status. Both systemic (1, 16, 49, 60, 70) and local prefrontal cortex insulin resistance appears central to impaired prefrontal cortex function, notably inhibitory control (49, 50, 56), the outcome of which could negatively affect scholarly success during developmentally sensitive periods (44, 84) and promote unfavorable behavioral modifications, such as decreased physical activity (5, 41) and enhanced food cravings (49, 50). Of note, cognitive dysfunction, disinhibited food intake, and physical (in)activity appear functionally linked or interrelated (32, 41, 44, 48, 49, 60, 84), such that systemic and prefrontal cortex insulin resistance may represent an underlying process in the development of each abnormality, and pathological changes in one outcome may directly or indirectly affect the other outcomes. The underlying cause of insulin resistance in the present study remains unresolved. Evidence indicates insulin resistance in the brain may relate to depressed insulin delivery (65), as well as altered IRS-1 tyrosine vs. serine phosphorylation status (11, 34). Specifically, metabolic derangement and activation of the JNK-TNF-α pathway have been implicated in impaired transendothelial transport of insulin into the brain (65), as well as decreased IRS-1 tyrosine phosphorylation in favor of serine phosphorylation, which inhibits subsequent downstream signaling of the PI3K/Akt pathway (11, 34). In the context of the current study, despite evidence of microvascular insulin resistance, because prefrontal cortex insulin signaling experiments were performed ex vivo and in vitro, transendothelial insulin transport was likely not the cause of the observed deficit in insulin-stimulated Akt signaling.
In stark contrast to the brain, the juvenile obese group displayed robust skeletal muscle fatty acid accumulation, mainly due to elevated saturated, and to a lesser extent monounsaturated, fatty acid concentrations. Increased fatty acid intake or synthesis (30), but not enhanced triglyceride hydrolysis, likely mediated the aforementioned effect on skeletal muscle, as juvenile obese pigs ingested a Western diet and simultaneously exhibited increased sedentary behavior. Current literature indicates that skeletal muscle fatty acid accumulation, notably 16:0 (palmitate) (30), as well as a depressed n3:n6 ratio, contributes to skeletal muscle insulin resistance (48, 87). In the current study, although increased fatty acid concentrations (including 16:0) and reduced n3:n6 ratio (being driven by a nonsignificant increase in n6) did not coincide with blunted insulin responses ex vivo, they did correspond with fasting hyperglycemia, the most prevalent metabolic risk factor among the Western diet-induced juvenile obese group and a primary clinical indicator of insulin resistance. Potentially, the fatty acid profile (30, 48, 87) and microvascular insulin resistance (4, 51, 52) represent two distinct mechanisms that contribute to fasting and postprandial (76) indices of skeletal muscle insulin resistance. Combined with the prefrontal cortex ex vivo insulin signaling data, these data suggest that the molecular basis of insulin resistance involves multiple pathways and may exhibit heterogeneity across organs (87). One potential mechanism that might explain the divergent responses to insulin between the prefrontal cortex vs. skeletal muscle may relate to inherent differences in glucose transport between these tissues. The brain relies almost extensively on GLUT 1 and GLUT 3 receptors, which combined facilitate glucose uptake independent of insulin and even during periods of low circulating glucose, whereas, skeletal muscle relies predominantly on GLUT 4 receptors, which are often sequestered inside the cell, and their translocation is regulated by insulin (7, 33). Given that the Western diet-fed obese pigs were hyperglycemic, conceivably, hyperglycemia-induced increases in cellular glucose content were greater in the neurons vs. skeletal myocytes, and by extension, the effect of hyperglycemia to attenuate insulin-stimulated Akt signaling (12, 19a, 25, 45) was more pronounced in the prefrontal cortex than in skeletal muscle. The endothelium and adipocytes also express GLUT 1 and GLUT 3 receptors (7, 24, 33), respectively, which may have contributed to the vulnerability of resistance arteries and adipose tissue to Western diet-induced obesity-related impairments in insulin responsiveness.
In the visceral adipose tissue, blunted insulin signaling, a primary culprit of adipose tissue dysfunction (97), occurred independently of overt adipocyte hypertrophy. Marked adipocyte enlargement develops at later stages of obesity in this pig model (81, 93, 97); thus, adipose tissue insulin resistance may reflect an initial pathological adaptation in the early stages of juvenile obesity development.
Limitations.
In the current study, although pharmacological inhibition of PKCβ improved insulin-induced vasodilation in preobese skeletal muscle and cerebral resistance arteries, neither vascular gene expression of PKCβ nor protein levels of PKCβ appeared different between groups. These results are consistent with the lack of effects of PKCβ on resting vasomotor tone, highlighting the possibility that while basal PKCβ activity may remain unaltered in preobesity, insulin-stimulated PKC activity may be altered (21, 38). A similar effect has been observed in isolated coronary arteries from obese adult Ossabaw swine; namely, PKCβ inhibition did not alter the endothelium-dependent response to bradykinin, but did prevent endothelial impairment induced by incubation with perivascular adipose tissue (82). The current data provide the rationale to conduct future experiments investigating the role of insulin stimulation on PKCβ activity in resistance arteries, and the tissues they supply, in the early stages of obesity development. To extend on the current data, it would be advantageous to include physiological concentrations of insulin in such experiments to enhance the translational relevance and to preserve insulin-stimulated tissues for immunohistochemical analyses to allow for examination of localization of PKCβ activity or Akt phosphorylation across the various cell types within whole tissues.
A major translational strength of the current study was the dual examination of in vivo and ex vivo (in vitro) vascular function, in two different vascular beds, using a large animal model of juvenile obesity development. However, the sample size, particularly for independent analyses of sex-related differences in the development of vasomotor dysfunction, was limited. Given that age and sex interactions may influence vascular function in children and adolescents (26), validation of the present findings in a clinical population, including boys and girls, is necessary to confirm their translational relevance, particularly at the mechanistic level.
Perspectives and Significance
Data from the current study reveal vascular insulin resistance occurs early in the development of Western diet-induced obesity, and independently of overt endothelium-dependent and -independent microvascular dysfunction in the skeletal muscle and cerebral microvasculature. Blunted insulin-stimulated vasodilation, which was reversed with PKCβ inhibition, occurred concurrently with reductions in estimated tissue perfusion, as well as depressed insulin-stimulated Akt signaling in the prefrontal cortex, but not the skeletal muscle. Such adaptations at the level of the brain and corresponding vasculature may underscore vascular and neurological derangements associated with juvenile obesity (20, 35).
GRANTS
The article was supported by the American Heart Association postdoctoral fellowship Grant 16POST27760052 (to T. D. Olver), National Institutes of Health K01 HL-125503 (to J. Padilla) and R01 HL-137769 (to J. Padilla), U42-ODO011140 (to NSRRC), Department of Veterans Affairs BLR&D CDA-2 IK2BX002030 (to S. B. Bender), and salary support from VA-Merit Grant I01BX003271-01 (to R. S. Rector). This work was also supported, in part, by the use of resources and facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.D.O., Z.I.G., T.J.J., M.H.L., R.S.R., S.B.B., E.M.W., C.A.E., and J.P. conceived and designed research; T.D.O., Z.I.G., T.J.J., R.E.M., P.J.L., T.R.S., A.M.C., S.B.B., E.M.W., and J.P. performed experiments; T.D.O., Z.I.G., T.J.J., R.E.M., P.J.L., S.B.B., and J.P. analyzed data; T.D.O., Z.I.G., T.J.J., R.E.M., P.J.L., M.H.L., S.B.B., and J.P. interpreted results of experiments; T.D.O., Z.I.G., T.J.J., and J.P. prepared figures; T.D.O. drafted manuscript; T.D.O., Z.I.G., T.J.J., R.E.M., P.J.L., M.H.L., R.S.R., S.B.B., E.M.W., C.A.E., and J.P. edited and revised manuscript; T.D.O., Z.I.G., T.J.J., R.E.M., P.J.L., T.R.S., A.M.C., M.H.L., R.S.R., S.B.B., E.M.W., C.A.E., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Pam Thorne and Jan Ivey for technical assistance with experimental testing, as well as Nathan C. Winn, Jimmy D. Browning, James R. Ball, T’keaya L. Gaines, Makenzie L. Woodford, and Michelle L. Gastecki for assistance with tissue processing.
REFERENCES
- 1.Abbatecola AM, Paolisso G, Lamponi M, Bandinelli S, Lauretani F, Launer L, Ferrucci L. Insulin resistance and executive dysfunction in older persons. J Am Geriatr Soc 52: 1713–1718, 2004. doi: 10.1111/j.1532-5415.2004.52466.x. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJA, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010, 2008. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous Infant and adult obesity. Lancet 1: 17–18, 1974. [PubMed] [Google Scholar]
- 4.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96: 786–792, 1995. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basso JC, Morrell JI. The medial prefrontal cortex and nucleus accumbens mediate the motivation for voluntary wheel running in the rat. Behav Neurosci 129: 457–472, 2015. doi: 10.1037/bne0000070. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase Cβ prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res 90: 107–111, 2002. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 7.Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care 13: 198–208, 1990. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- 8.Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Heart Circ Physiol 309: H574–H582, 2015. doi: 10.1152/ajpheart.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ, Sowers JR. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 65: 1082–1088, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes 62: 313–319, 2013. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel J, Decker H, Silverman M a, Kazi H, Melo HM, Mcclean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Invest 122: 1339–1353, 2012. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6: a009191, 2014. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley NS, Heigenhauser GJF, Roy BD, Staples EM, Inglis JG, LeBlanc PJ, Peters SJ. The acute effects of differential dietary fatty acids on human skeletal muscle pyruvate dehydrogenase activity. J Appl Physiol (1985) 104: 1–9, 2008. doi: 10.1152/japplphysiol.00636.2007. [DOI] [PubMed] [Google Scholar]
- 14.Bretón-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, Berk BD, Masaki N, Weisbrod RM, Inagaki E, Gokce N, Fuster JJ, Walsh K, Hamburg NM. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol 36: 561–569, 2016. doi: 10.1161/ATVBAHA.115.306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 278: 10297–10303, 2003. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 16.Cheke LG, Bonnici HM, Clayton NS, Simons JS. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia 96: 137–149, 2017. doi: 10.1016/j.neuropsychologia.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50: 2682–2690, 2001. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 18.Crissey JM, Jenkins NT, Lansford KA, Thorne PK, Bayless DS, Vieira-Potter VJ, Rector RS, Thyfault JP, Laughlin MH, Padilla J. Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. Am J Physiol Regul Integr Comp Physiol 306: R596–R606, 2014. doi: 10.1152/ajpregu.00493.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crissey JM, Padilla J, Jenkins NT, Martin JS, Rector RS, Thyfault JP, Harold Laughlin M. Metformin does not enhance insulin-stimulated vasodilation in skeletal muscle resistance arteries of the OLETF rat. Microcirculation 20: 764–775, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.De Nigris V, Pujadas G, La Sala L, Testa R, Genovese S, Ceriello A. Short-term high glucose exposure impairs insulin signaling in endothelial cells. Cardiovasc Diabetol 14: 114, 2015. doi: 10.1186/s12933-015-0278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet 360: 473–482, 2002. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 21.Egan JJ, Saltis J, Wek SA, Simpson IA, Londos C. Insulin, oxytocin, and vasopressin stimulate protein kinase C activity in adipocyte plasma membranes. Proc Natl Acad Sci USA 87: 1052–1056, 1990. doi: 10.1073/pnas.87.3.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson SJ, Robinson TN, Haydel KF, Killen JD. Are overweight children unhappy?: Body mass index, depressive symptoms, and overweight concerns in elementary school children. Arch Pediatr Adolesc Med 154: 931–935, 2000. doi: 10.1001/archpedi.154.9.931. [DOI] [PubMed] [Google Scholar]
- 23.Eringa EC, Stehouwer CDA, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 293: E1134–E1139, 2007. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 24.Farrell CL, Yang J, Pardridge WM. GLUT-1 glucose transporter is present within apical and basolateral membranes of brain epithelial interfaces and in microvascular endothelia with and without tight junctions. J Histochem Cytochem 40: 193–199, 1992. doi: 10.1177/40.2.1552163. [DOI] [PubMed] [Google Scholar]
- 25.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106: 466–472, 2002. doi: 10.1161/01.CIR.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 26.Fenwick N, Griffin G, Gauthier C. The welfare of animals used in science: how the “Three Rs” ethic guides improvements. Can Vet J 50: 523–530, 2009. [PMC free article] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 28.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 362: 485–493, 2010. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Z, Wu J, Nesil T, Li MD, Aylor KW, Liu Z. Long-term high-fat diet induces hippocampal microvascular insulin resistance and cognitive dysfunction. Am J Physiol Endocrinol Metab 312: E89–E97, 2017. doi: 10.1152/ajpendo.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funai K, Song H, Yin L, Lodhi IJ, Wei X, Yoshino J, Coleman T, Semenkovich CF. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J Clin Invest 123: 1229–1240, 2013. doi: 10.1172/JCI65726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319–1331, 2010. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol 3: 403–428, 2013. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochem J 295: 329–341, 1993. doi: 10.1042/bj2950329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445, 2011. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 35.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet 375: 1737–1748, 2010. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol 222: 187–194, 2010. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]
- 37.Ibayashi S, Ngai AC, Meno JR, Winn HR. Effects of topical adenosine analogs and forskolin on rat pial arterioles in vivo. J Cereb Blood Flow Metab 11: 72–76, 1991. doi: 10.1038/jcbfm.1991.8. [DOI] [PubMed] [Google Scholar]
- 38.Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes 49: 1353–1358, 2000. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]
- 39.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49: 1525–1533, 2000. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 40.Kaneda T, Sasaki N, Urakawa N, Shimizu K. Endothelium-dependent and -independent vasodilator effects of dimethyl sulfoxide in rat aorta. Pharmacology 97: 171–176, 2016. doi: 10.1159/000443894. [DOI] [PubMed] [Google Scholar]
- 41.Kang EB, Cho JY. Effects of treadmill exercise on brain insulin signaling and β-amyloid in intracerebroventricular streptozotocin induced-memory impairment in rats. J Exerc Nutrition Biochem 18: 89–96, 2014. doi: 10.5717/jenb.2014.18.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karpoff L, Vinet A, Schuster I, Oudot C, Goret L, Dauzat M, Obert P, Perez-Martin A. Abnormal vascular reactivity at rest and exercise in obese boys. Eur J Clin Invest 39: 94–102, 2009. doi: 10.1111/j.1365-2362.2008.02068.x. [DOI] [PubMed] [Google Scholar]
- 43.Katakam PVG, Tulbert CD, Snipes JA, Erdös B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol 288: H854–H860, 2005. doi: 10.1152/ajpheart.00715.2004. [DOI] [PubMed] [Google Scholar]
- 44.Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, Evans EM, Castelli DM, Hillman CH. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity (Silver Spring) 20: 2406–2411, 2012. doi: 10.1038/oby.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim F, Tysseling KA, Rice J, Gallis B, Haji L, Giachelli CM, Raines EW, Corson MA, Schwartz MW. Activation of IKKβ by glucose is necessary and sufficient to impair insulin signaling and nitric oxide production in endothelial cells. J Mol Cell Cardiol 39: 327–334, 2005. doi: 10.1016/j.yjmcc.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Seo BS. How to calculate sample size and why. Clin Orthop Surg 5: 235–242, 2013. doi: 10.4055/cios.2013.5.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award lecture. Diabetes 65: 1462–1471, 2016. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitessa SM, Abeywardena MY. Lipid-induced insulin resistance in skeletal muscle: the chase for the culprit goes from total intramuscular fat to lipid intermediates, and finally to species of lipid intermediates. Nutrients 8: 466, 2016. doi: 10.3390/nu8080466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring H-U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 96: 1169–1209, 2016. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 50.Kullmann S, Heni M, Veit R, Scheffler K, Machann J, Häring HU, Fritsche A, Preissl H. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care 38: 1044–1050, 2015. doi: 10.2337/dc14-2319. [DOI] [PubMed] [Google Scholar]
- 51.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 85: 1844–1852, 1990. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 53.Lagerkranser M, Stånge K, Sollevi A. Effects of propofol on cerebral blood flow, metabolism, and cerebral autoregulation in the anesthetized pig. J Neurosurg Anesthesiol 9: 188–193, 1997. doi: 10.1097/00008506-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 587: 3729–3739, 2009. doi: 10.1113/jphysiol.2009.172601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol (1985) 102: 1341–1347, 2007. doi: 10.1152/japplphysiol.01018.2006. [DOI] [PubMed] [Google Scholar]
- 56.Lee SH, Zabolotny JM, Huang H, Lee H, Kim YB. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol Metab 5: 589–601, 2016. doi: 10.1016/j.molmet.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 58.Long MW, Ward ZJ, Resch SC, Cradock AL, Wang YC, Giles CM, Gortmaker SL. State-level estimates of childhood obesity prevalence in the United States corrected for report bias. Int J Obes 40: 1523–1528, 2016. doi: 10.1038/ijo.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynn MA, Rupnow HL, Kleinhenz DJ, Kanner WA, Dudley SC, Hart CM. Fatty acids differentially modulate insulin-stimulated endothelial nitric oxide production by an Akt-independent pathway. J Investig Med 52: 129–136, 2004. doi: 10.1136/jim-52-02-22. [DOI] [PubMed] [Google Scholar]
- 60.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring) 19: 1382–1387, 2011. doi: 10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahadevappa VG, Holub BJ. Quantitative loss of individual eicosapentaenoyl-relative to arachidonoyl-containing phospholipids in thrombin-stimulated human platelets. J Lipid Res 28: 1275–1280, 1987. [PubMed] [Google Scholar]
- 62.Masseau I, Davis MJ, Bowles DK. Carotid inflammation is unaltered by exercise in hypercholesterolemic swine. Med Sci Sports Exerc 44: 2277–2289, 2012. doi: 10.1249/MSS.0b013e318266af0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol (1985) 82: 1438–1444, 1997. doi: 10.1152/jappl.1997.82.5.1438. [DOI] [PubMed] [Google Scholar]
- 64.Mehta NN, Sheetz M, Price K, Comiskey L, Amrutia S, Iqbal N, Mohler ER, Reilly MP. Selective PKCβ inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc Drugs Ther 23: 17–24, 2009. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meijer RI, Gray SM, Aylor KW, Barrett EJ. Pathways for insulin access to the brain: the role of the microvascular endothelial cell. Am J Physiol Heart Circ Physiol 311: H1132–H1138, 2016. doi: 10.1152/ajpheart.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michalsky MP, Inge TH, Simmons M, Jenkins TM, Buncher R, Helmrath M, Brandt ML, Harmon CM, Courcoulas A, Chen M, Horlick M, Daniels SR, Urbina EM; Teen-LABS Consortium . Cariovascular risk factors in severly obese adolescents: the teen longitudinal assessment of bariatric surgery (Teen-LABS) study. JAMA Pediatr 169: 438–444, 2015. doi: 10.1001/jamapediatrics.2014.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mikkelsen MLG, Ambrus R, Miles JE, Poulsen HH, Moltke FB, Eriksen T. Effect of propofol and remifentanil on cerebral perfusion and oxygenation in pigs: a systematic review. Acta Vet Scand 58: 42, 2016. doi: 10.1186/s13028-016-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol (1985) 109: 1203–1210, 2010. doi: 10.1152/japplphysiol.00064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mikus CR, Roseguini BT, Uptergrove GM, Morris EM, Rector RS, Libla JL, Oberlin DJ, Borengasser SJ, Taylor AM, Ibdah JA, Laughlin MH, Thyfault JP. Voluntary wheel running selectively augments insulin-stimulated vasodilation in arterioles from white skeletal muscle of insulin-resistant rats. Microcirculation 19: 729–738, 2012. doi: 10.1111/j.1549-8719.2012.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller AL, Lee HJ, Lumeng JC. Obesity-associated biomarkers and executive function in children. Pediatr Res 77: 143–147, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension 66: 988–997, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 73.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JRC, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase Cβ inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 55: 691–698, 2006. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 74.Cholesterol levels: what you need to know [Online]. NIH Medline 6–7, 2012. https://medlineplus.gov/magazine/issues/summer12/articles/summer12pg6-7.html.
- 75.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352: 1138–1145, 2005. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 76.Olver TD, Hazell TJ, Hamilton CD, Shoemaker JK, Lemon PWR. Impaired superficial femoral artery vasodilation and leg blood flow in young obese women following an oral glucose tolerance test. Appl Physiol Nutr Metab 37: 176–183, 2012. doi: 10.1139/h11-148. [DOI] [PubMed] [Google Scholar]
- 77.Olver TD, Laughlin HM. Endurance, interval sprint, and resistance exercise training: impact on microvascular dysfunction in type 2 diabetes. Am J Physiol Heart Circ Physiol 310: H337–H350, 2016. doi: 10.1152/ajpheart.00440.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olver TD, McDonald MW, Grisé KN, Dey A, Allen MD, Medeiros PJ, Lacefield JC, Jackson DN, Rice CL, Melling CWJ, Noble EG, Shoemaker JK. Exercise training enhances insulin-stimulated nerve arterial vasodilation in rats with insulin-treated experimental diabetes. Am J Physiol Regul Integr Comp Physiol 306: R941–R950, 2014. doi: 10.1152/ajpregu.00508.2013. [DOI] [PubMed] [Google Scholar]
- 79.Olver TD, McDonald MW, Klakotskaia D, Richardson RA, Jasperse JL, Melling CWJ, Schachtman TR, Yang HT, Emter CA, Laughlin MH. A chronic physical activity treatment in obese rats normalizes the contributions of ET-1 and NO to insulin-mediated posterior cerebral artery vasodilation. J Appl Physiol (1985) 122: 1040–1050, 2017. doi: 10.1152/japplphysiol.00811.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orzechowski H, Unther AG, Menzel S, Zimmermann A, Funke-kaiser H, Real R, Subkowski T, Zollmann FS, Paul M. Transcriptional mechanism of protein kinase C-induced isoform-specific expression of the gene for endothelin-1-converting enzyme-1 in human endothelial cells. Mol Pharmacol 60: 1332–1342, 2001. doi: 10.1124/mol.60.6.1332. [DOI] [PubMed] [Google Scholar]
- 81.Padilla J, Jenkins NT, Lee S, Zhang H, Cui J, Zuidema MY, Zhang C, Hill MA, Perfield JW II, Ibdah JA, Booth FW, Davis JW, Laughlin MH, Rector RS. Vascular transcriptional alterations produced by juvenile obesity in Ossabaw swine. Physiol Genomics 45: 434–446, 2013. doi: 10.1152/physiolgenomics.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-β pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717, 2010. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitts LH, Young AR, McCulloch J, MacKenzie E. Vasomotor effects of dimethyl sulfoxide on cat cerebral arteries in vitro and in vivo. Stroke 17: 483–487, 1986. doi: 10.1161/01.STR.17.3.483. [DOI] [PubMed] [Google Scholar]
- 84.Reyes S, Peirano P, Peigneux P, Lozoff B, Algarin C. Inhibitory control in otherwise healthy overweight 10-year-old children. Int J Obes 39: 1230–1235, 2015. doi: 10.1038/ijo.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribeiro MM, Silva AG, Santos NS, Guazzelle I, Matos LNJ, Trombetta IC, Halpern A, Negrão CE, Villares SMF. Diet and exercise training restore blood pressure and vasodilatory responses during physiological maneuvers in obese children. Circulation 111: 1915–1923, 2005. doi: 10.1161/01.CIR.0000161959.04675.5A. [DOI] [PubMed] [Google Scholar]
- 86.Richard V, Kaeffer N, Tron C, Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation 89: 1254–1261, 1994. doi: 10.1161/01.CIR.89.3.1254. [DOI] [PubMed] [Google Scholar]
- 87.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis 14: 121, 2015. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51: 1022–1027, 2002. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 89.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 346: 802–810, 2002. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 90.Sobey CG, Quan L. Impaired cerebral vasodilator responses to NO and PDE V inhibition after subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol 277: H1718–H1724, 1999. [DOI] [PubMed] [Google Scholar]
- 91.Strauss RS. Childhood obesity and self-esteem. Pediatrics 105: e15, 2000. doi: 10.1542/peds.105.1.e15. [DOI] [PubMed] [Google Scholar]
- 92.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase Cβ contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation 127: 86–95, 2013. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Toedebusch RG, Roberts MD, Wells KD, Company JM, Kanosky KM, Padilla J, Jenkins NT, Perfield JW II, Ibdah JA, Booth FW, Rector RS. Unique transcriptomic signature of omental adipose tissue in Ossabaw swine: a model of childhood obesity. Physiol Genomics 46: 362–375, 2014. doi: 10.1152/physiolgenomics.00172.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 358: 1400–1404, 2001. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 95.Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. Int J Exp Pathol 86: 335–345, 2005. doi: 10.1111/j.0959-9673.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Val-Laillet D, Layec S, Guérin S, Meurice P, Malbert C-H. Changes in brain activity after a diet-induced obesity. Obesity (Silver Spring) 19: 749–756, 2011. doi: 10.1038/oby.2010.292. [DOI] [PubMed] [Google Scholar]
- 97.Vieira-Potter VJ, Lee S, Bayless DS, Scroggins RJ, Welly RJ, Fleming NJ, Smith TN, Meers GM, Hill MA, Rector RS, Padilla J. Disconnect between adipose tissue inflammation and cardiometabolic dysfunction in Ossabaw pigs. Obesity (Silver Spring) 23: 2421–2429, 2015. doi: 10.1002/oby.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374, 2004. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 99.Willeumier KC, Taylor DV, Amen DG. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity (Silver Spring) 19: 1095–1097, 2011. doi: 10.1038/oby.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, Gaines TL, Woodford ML, Karasseva NG, Kanaley JA, Sacks HS, Padilla J. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol 312: R74–R84, 2017. doi: 10.1152/ajpregu.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. J Appl Physiol (1985) 94: 2017–2026, 2003. doi: 10.1152/japplphysiol.01025.2002. [DOI] [PubMed] [Google Scholar]