Abstract
Recent studies demonstrate that maternal hypertension during pregnancy sensitizes an angiotensin (ANG) II-induced increase in blood pressure (BP) in adult male offspring that was associated with upregulation of mRNA expression of several renin-angiotensin-aldosterone system (RAAS) components and NADPH oxidase in the lamina terminalis (LT) and paraventricular nucleus (PVN). The purpose of the present study was to test whether there are sex differences in the maternal hypertension-induced sensitization of ANG II hypertension, and whether sex hormones are involved in the sensitization process. Male offspring of hypertensive dams showed an enhanced hypertensive response to systemic ANG II when compared with male offspring of normotensive dams and to female offspring of either normotensive or hypertensive dams. Castration did not alter the hypertensive response to ANG II in male offspring. Intact female offspring had no upregulation of RAAS components and NADPH oxidase in the LT and PVN, whereas ovariectomy (OVX) upregulated mRNA expression of several RAAS components and NADPH oxidase in these nuclei and induced a greater increase in the pressor response to ANG II in female offspring of hypertensive dams compared with female offspring of normotensive dams. This enhanced increase in BP was partially attenuated by 17β-estradiol replacement in the OVX offspring of hypertensive dams. The results suggest that maternal hypertension induces a sex-specific sensitization of ANG II-induced hypertension and mRNA expression of brain RAAS and NADPH oxidase in offspring. Female offspring are protected from maternal hypertension-induced sensitization of ANG II hypertension, and female sex hormones are partially responsible for this protective effect.
Keywords: E2 replacement, gonadectomy, maternal hypertension, offspring, sensitization of angiotensin II hypertension
INTRODUCTION
Epidemiological and experimental studies have shown that an adverse uterine environment increases the risk of cardiovascular diseases, including hypertension in the offspring (2, 3). The insults experienced in the prenatal period include maternal undernutrition, hypertension/preeclampsia, placental insufficiency, high-salt intake, high-fat diet, hypoxia, glucocorticoid, and nicotine exposure. Sex of the offspring is an important consideration with regard to disease susceptibility. Although both sexes are susceptible to insults during pregnancy, studies that investigate sex differences in the timing of onset and severity of hypertension consistently indicate that female offspring are protected as compared with male offspring (7, 8, 11, 13, 14, 16–22, 24–26). These studies also suggest a role of the sex hormones in modulating the long-term changes in blood pressure (BP) in response to these prenatal insults. For example, ovariectomy (OVX) in the female offspring of maternal undernutrition, high-salt intake, high-fat diet, hypoxia, glucocorticoid, or nicotine exposure led to an increase in BP or an increased BP response to acute ANG II treatment, which was reversed by replacement of estrogen (7, 8, 11, 13, 14, 16, 18, 20–22, 24–26). Castration blocked the increase in BP in spontaneously hypertensive rat offspring (19). Moreover, both OVX and castration abolished the sex difference in BP in the offspring of placental insufficiency model (16, 17). These studies suggest that both estrogen and testosterone contribute to the sexual dimorphism of BP in the animal models of fetal programming.
Preeclampsia and other types of gestational hypertension are common conditions affecting 5 to 7% of all pregnancies (12). Our previous studies showed that maternal gestational hypertension generated by ANG II infusion produced sensitization of the ANG II-elicited hypertensive response in male offspring when they were tested as adults between 10 to 12 wk of age. At 10 wk of age, these males also had upregulation of mRNA expression for hypertensive components of the brain renin-angiotensin-aldosterone system (RAAS) and of pro-inflammatory markers in structures along the lamina terminalis (LT) and in the hypothalamic paraventricular nucleus (PVN). This sensitized male hypertensive response was blocked either by 5 wk (weaning to 8 wk of age) of treatment with an angiotensin converting-enzyme (ACE) inhibitor or by renal denervation at 8 wk of age. These same interventions also reduced the expression of mRNA message for RAAS components and for inflammatory markers in the same brain structures (30). The effect of maternal hypertension on programming of the sensitization of the hypertensive response to ANG II in adult female offspring has not been studied.
Sex steroid hormones play an important role in regulating cardiovascular responses, and ovarian and testicular function may be altered by an adverse uterine environment (4, 27). In the present series of studies, we tested whether sex and sex hormones play a role in hypertensive response sensitization in the offspring of mothers with ANG II hypertension. In addition, we examined the effects of maternal gestational hypertension and sex hormones on the expression of mRNA for components of the brain RAAS and NADPH oxidase in adult male and female offspring.
METHODS
Animals
All animals were maintained in a temperature (23 ± 2°C) and light (12-h light/dark cycle) controlled facility. Twenty female and twenty male rats (10 wk old, Sprague-Dawley, Harlan) were used for breeding. Half of the females were chronically treated with vehicle (saline) and considered as normotensive (NT) dams, whereas the other half were treated with ANG II (250 ng·kg−1·min−1 sc, model 2004, 4 wk, Alzet) throughout mating and pregnancy and considered as hypertensive (HT) dams as previously described (30). The offspring were weighed and counted at birth, and the litter sizes reduced at 3 days of age to eight pups; four male and four female offspring. All offspring were weaned at 3 wk of age and provided with rat chow (7013 NIH-31 modified rat diet, 0.25% NaCl) ad libitum. A total of 25 male and 42 female offspring of NT dams and same numbers of male and female offspring of HT dams were used in the present experiments. Each experimental group was composed of individual subjects that were randomly selected from different litters. Figure 1 shows representative timeline of the study design.
Fig. 1.
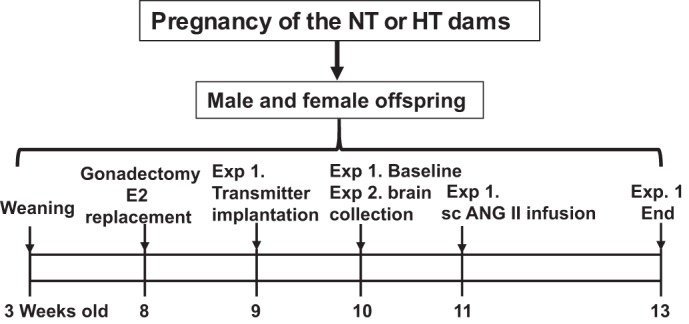
Representative time line of the study design. Male and female offspring of normotensive (NT) or hypertensive (HT) dams were weaned at 3 wk of age and gonadectomized at 8 wk of age. A portion of ovariectomized female offspring was supplemented with 17β-estradiol (E2). Experiment 1 (Exp. 1): intact or gonadectomized male and female offspring were instrumented with the telemetry transmitters followed by osmotic pump implantation to subcutaneously (sc) deliver angiotensin II (ANG II). Experiment 2 (Exp. 2): at 10 wk of age, intact male offspring and intact and ovariectomized female offspring from either NT or HT dams were euthanized to collect brain tissues for assessing mRNA expression.
Experiment 1.
In the functional experiments, at 11 wk of age, male and female offspring of both NT dams and HT dams were used to determine if there were sex differences in maternal hypertension-induced sensitization of the hypertensive response to ANG II and to evaluate the effects of sex hormones on sensitization. The rats were divided into 14 groups: 1,2) intact/sham NT-dam male offspring; 3,4) intact/sham HT-dam male offspring; 5) castrated NT-dam male offspring; 6) castrated HT-dam male offspring; 7,8) intact/sham NT-dam female offspring; 9,10) intact/sham HT-dam female offspring; 11) OVX NT-dam female offspring; 12) OVX HT-dam female offspring; 13) OVX NT-dam female offspring with 17β-estradiol (E2) replacement; and 14) OVX HT-dam female offspring with E2 replacement. All of the groups were infused subcutaneously with a slow pressor dose of ANG II (120 ng·kg−1·min−1) for 2 wk. Out of a total 94 offspring studied, there were 10 animals that did not show any changes in BP after infusion of ANG II. We could not identify whether it was because the animals had no response to ANG II or whether it was due to osmotic pump failure. Because even offspring from NT dams consistently showed a BP response to the ANG II infusion, we excluded animals showing no increase in BP from analysis. The BP data reported were obtained from a total of 84 animals (6 offspring/group).
Experiment 2.
At 10 wk of age without ANG II treatment, intact male and female offspring from NT or HT dams were euthanized and had their brains collected for analyses of mRNA expression of the RAAS components and NADPH oxidase (n = 5 per group). Similarly, 10-wk-old intact female offspring from either NT or HT dams and female offspring with OVX performed at 8 wk of age were also used to determine the changes in RAAS components and NADPH oxidase in the brain (n = 5 per group). The structures lying along the LT [i.e., the subfornical organ (SFO), median preoptic nucleus (MnPO), and organum vasculosum of the lamina terminalis (OVLT)] and the PVN were used for these analyses.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Iowa Animal Care and Use Committee.
Gonadectomy and E2 Replacement
Because rats are considered prepubertal stage before 8 wk of age, gonadectomy was performed on 8-wk-old offspring as previously described (16, 25, 32). Briefly, in the females, a single 2- to 3-cm dorsal midline incision was made in the skin and underlying muscles. The ovaries were isolated, tied off with sterile suture, and removed, and the incisions were closed. In the males, a single incision was made in the skin covering the scrotum. The testicles were exteriorized, tied off, and removed, and the skin was sutured. In sham surgeries, the ovaries or testicles were visualized but not removed. E2 valerate minipellets (5 mg, 60 days release; Innovative Research of American) were used for continuous release of the hormone at a dose to maintain E2 concentration in the physiological range (16, 25). Pellets were implanted immediately after OVX.
Telemetry Probe and Osmotic Pump Implantation
Ten days after gonadectomy, rat BP transmitters (TA11PA-C40, DSI, St. Paul, MN) were used to directly measure arterial pressure in individual animals. The rats were anesthetized with a ketamine-xylazine mixture (90% ketamine and 10% xylazine), and the femoral artery was accessed with a ventral incision. The right femoral artery was isolated, and the catheter of a telemetry probe was inserted into the vessel. Through the same ventral incision, a pocket along the right flank was formed. The body of the transmitter was slipped into the pocket and secured with tissue adhesive. The ventral incision was then closed with suture.
After baseline BP and heart rate (HR) recordings were obtained, osmotic pumps (model 2002, ALZET) containing ANG II (120 ng·kg−1·min−1, Sigma) were implanted subcutaneously in the back of both male and female offspring.
Real-time RT-PCR Analysis
Total RNA was isolated from the LT by using the TRIzol method (Invitrogen) and treated with DNase I (Invitrogen). RNA integrity was checked by gel electrophoresis. Total RNA was reverse transcribed using random hexamers following the manufacturer’s instructions (Applied Biosystems). Real-time PCR was conducted using 200–300 ng of cDNA and 500 nM of each primer in a 20-μl reaction with iQ SYBR Green Supermix (Bio-Rad). Amplification cycles were conducted at 95°C for 3 min, followed by 40 cycles of 95°C for 15 s, and annealing/extension at 60°C for 30 s. Reactions were performed in duplicate and analyzed using a C1000 thermocycler system (Bio-Rad). mRNA levels for RAAS components [renin, angiotensinogen (AGT), ACE1, ANG II type 1receptor (AT1-R), mineralocorticoid receptor (MR)], NADPH oxidase (NOX2), and GAPDH were analyzed with SYBR Green real-time RT-PCR. The values were corrected by GAPDH, and the final concentration of mRNA was calculated using the formula x = 2−ΔΔCt, where x is fold difference relative to control. Primers were purchased from Integrated DNA Technologies (Coralville, IA). The sequences of the primers are shown in Table 1.
Table 1.
Primer sequences for real-time RT-PCR
| Gene | Forward Primer | Reverse Primer | Product Size, bp |
|---|---|---|---|
| GAPDH | TGACTCTACCCACGGCAAGTTCAA | ACGACATACTCAGCACCAGCATCA | 141 |
| Renin | CTGCCACCTTGTTGTGTGAG | ACCTGGCTACAGTTCACAACG | 154 |
| AGT | TCCCTCGCTCTCTGGACTTA | AAGTGAACGTAGGTGTTGAAA | 209 |
| ACE1 | GTGTTGTGGAACGAATACGC | CCTTCTTTATGATCCGCTTGA | 187 |
| AT1R | CTCAAGCCTGTCTACGAAAATGAG | GTGAATGGTCCTTTGGTCGT | 188 |
| MR | GCCCGGCAAATCTCAACAACTCAA | TTAGGGAAAGGAACGTCGTGAGCA | 235 |
| NOX2 (gp91phox) | CAAGATGGAGGTGGGACAGT | GCTTATCACAGCCACAAGCA | 170 |
AGT, angiotensinogen; ACE1, angiotensin-converting enzyme 1; AT1R, angiotensin II type 1 receptor; MR, mineralocorticoid receptor; NOX2, NADPH oxidase 2.
Data Analysis
Mean arterial pressure (MAP) and HR are presented as mean daily values. Differences for MAP and HR were calculated for each animal based on the mean of a 5-day baseline subtracted from the mean of the final 5 days of ANG II treatment. Two-way ANOVA for the experimental groups was then conducted on daily MAP, HR, or the means of calculated differences (The factors were sex and ANG II treatment). After a significant ANOVA was established, post hoc analyses were performed with Tukey multiple comparison tests between pairs of mean changes. One-way ANOVAs and post hoc Tukey analyses were used to test for the differences in the mean of the 5-days basal MAP and HR between groups and in mRNA expression of the RAAS components and NADPH oxidase in the LT and PVN. All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Effects of Hypertension During Pregnancy on the Neonates
We have previously shown that BP was significantly increased with no changes in HR in dams during pregnancy that received ANG II infusions when compared with females treated with saline (30). In the present study, 10 hypertensive dams produced a total of 113 pups including 54 males and 59 females, whereas 10 normotensive dams produced a total of 119 pups including 57 males and 62 females. There were no significant differences in litter sizes (11.3 ± 0.6 pups vs. 11.9 ± 0.5 pups, P > 0.05) or birth weights of the pups (5.99 ± 0.45 g/pup vs. 6.11 ± 0.42 g/pup, P > 0.05) from HT dams and NT dams, respectively.
Sex Differences in Maternal Hypertension-Induced Sensitization of ANG II Hypertension in Adult Offspring
At 10 wk of age, there were no significant differences in basal MAP and HR between male offspring from NT dams (108.5 ± 1.1 mmHg and 345.7 ± 8.8 beats/min) and HT dams (109.3 ± 1.2 mmHg and 345.2 ± 7.9 beats/min) or between female offspring from NT dams (99.8 ± 2.1 mmHg and 398.1 ± 5.2 beats/min) and HT dams (101.5 ± 1.8 mmHg and 394.2 ± 3.4 beats/min). However, female offspring exhibited significantly lower MAP and higher HR compared with male offspring (P < 0.05).
When tested beginning at 11 wk of age, male offspring of HT dams showed an enhanced hypertensive response to systemic ANG II (Δ37.8 ± 2.6 mmHg, P < 0.05) compared with male offspring of NT dams (Δ22.8 ± 1.3 mmHg). In contrast, ANG II treatment produced only a slight but comparable increase in MAP in female offspring of both HT (Δ9.5 ± 2.1 mmHg) and NT dams (Δ12.2 ± 2.1 mmHg, Fig. 2, A and B). The increase in female BP was significantly less than that seen in male offspring of both NT and HT dams (P < 0.05). Conversely, ANG II induced greater decreases in HR in female offspring (Δ37.0 ± 6.6 and Δ34.8 ± 3.6 beats/min, P < 0.05) than those in male offspring (Δ18.4 ± 5.5 and Δ21.1 ± 3.4 beats/min) regardless of whether the offspring were from either NT dams or HT dams (Fig. 2, C and D).
Fig. 2.
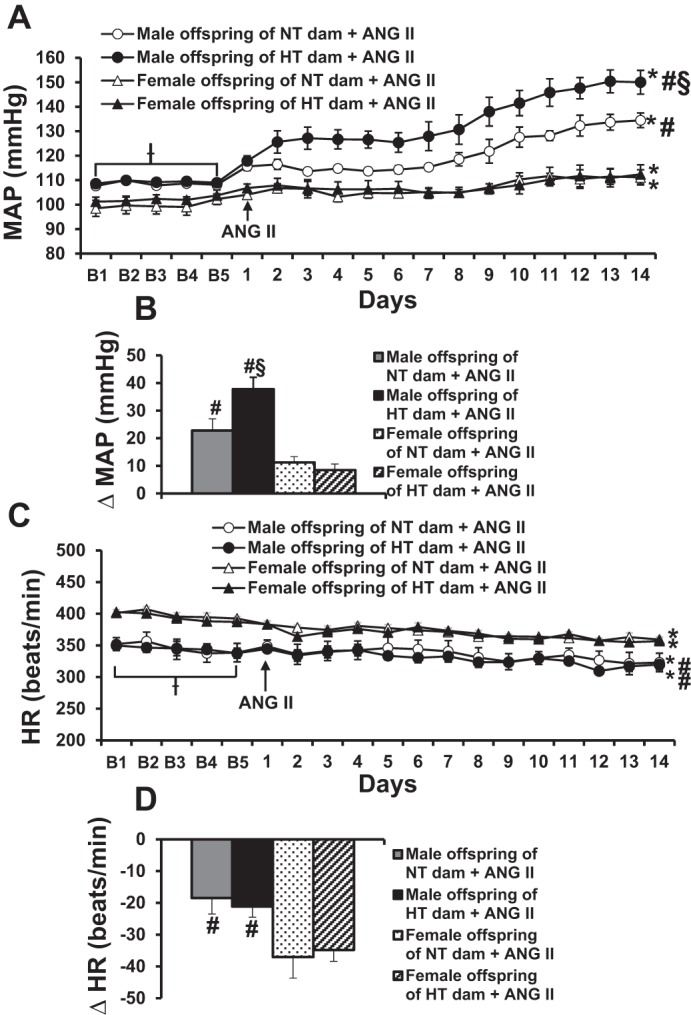
Sex differences in maternal hypertension-induced sensitization of angiotensin II (ANG II) hypertension. Daily mean arterial pressures (MAP) (A) and heart rate (HR) (C) before and during systemic infusion of ANG II in intact offspring of normotensive (NT) and hypertensive (HT) dams. B and D: average changes in MAP and HR induced by ANG II treatment in all groups. Baseline recording days are denoted by B letters, followed by 14 days of systemic ANG II infusion (n = 6/group; ƗP < 0.05 vs. basal MAP or HR of female offspring, *P < 0.05 vs. baseline, #P < 0.05 vs. female offspring, §P < 0.05 vs. male offspring of NT dams).
Sex Differences in mRNA Expression of RAAS Components and NADPH Oxidase in the Brain of Adult Offspring Without ANG II Treatment
The mRNA expression data in female offspring were collected as part of a larger study in which two groups of male offspring from NT and HT dams were cohorts. The results of the male expression were published previously (30). In brain tissues collected at 10 wk of age, the male offspring of HT dams showed significant upregulation of mRNA expression of the RAAS components (renin, AGT, ACE1, AT1R, and MR) in the LT but not in the PVN, whereas mRNA expression of NADPH oxidase NOX2 was upregulated in both the LT and PVN (published in Ref. 30). In contrast to the results from males, female offspring of HT dams did not exhibit upregulation of these genes (P > 0.05).
Effects of ANG II Treatment on BP and HR in Sham and Castrated Male Offspring
Over 14 days of ANG II treatment, sham castrated male offspring of HT dams progressively developed a greater increase in MAP (Δ35.1 ± 2.7 mmHg, P < 0.05) than that seen in sham-operated male offspring of NT dams (Δ20.7 ± 2.6 mmHg). Castration did not alter the hypertensive responses to ANG II in either the male offspring of HT dams (Δ35.3 ± 2.8 mmHg) or the male offspring of NT dams (Δ23.9 ± 2.3 mmHg, Fig. 3, A and B).
Fig. 3.
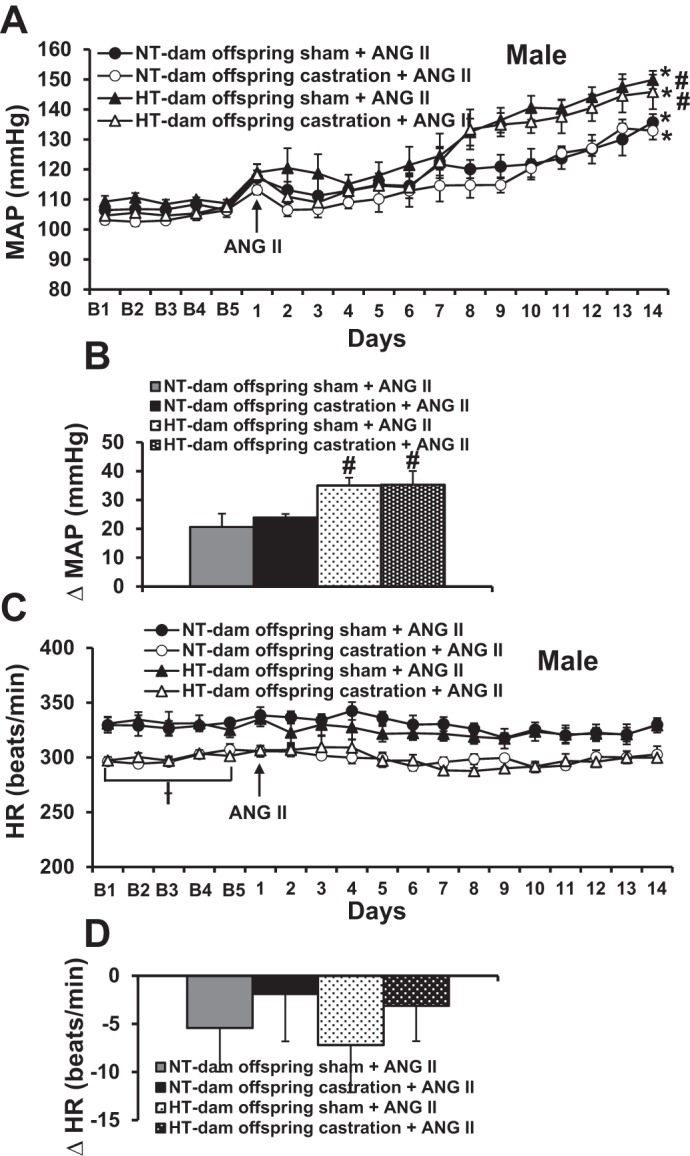
Castration failed to prevent maternal hypertension-induced sensitization of hypertension initiated by angiotensin II (ANG II) in male offspring of hypertensive (HT) dams (A), but reduced heart rate (HR) (C) in both groups of male offspring. B and D show the changes in mean arterial pressures (MAP) and heart rate (HR) after infusion of ANG II in all groups [B = baseline days; n = 6/group; ƗP < 0.05 vs. basal HR of both male and female sham offspring, *P < 0.05 vs. baseline, #P < 0.05 vs. male offspring of normotensive (NT) dams].
Although castration significantly reduced basal HR in both the male offspring of NT dams (sham 329.5 ± 6.9 vs. castrated 299.3 ± 2.8, P < 0.05) and the male offspring of HT dams (sham 330.9 ± 6.3 vs. castrated 299.9 ± 3.5, P < 0.05), chronic ANG II infusion produced slight and similar decreases in HR in all groups (P > 0.05, Fig. 3, C and D).
Effects of ANG II Treatment on BP and HR in Sham and OVX Female Offspring
There were no significant differences in basal MAP and HR between sham OVX female offspring of NT dams (101.1 ± 1.3 mmHg and 386.4 ± 7.3 beats/min) and HT dams (99.6 ± 1.5 mmHg and 383.3 ± 7.9 beats/min). OVX resulted in a significant increase in basal MAP but reduced basal HR in the offspring of both NT dams (106.3 ± 1.1 mmHg and 344.2 ± 2.7 beats/min, P < 0.05) and HT dams (106.4 ± 1.2 mmHg and 344.1 ± 5.1 beats/min, P < 0.05).
After 14 days of treatment with a slow pressor dose of ANG II, MAP was slightly and significantly increased in sham offspring of both NT dams (∆13.5 ± 2.2 mmHg) and HT dams (∆15.8 ± 3.3 mmHg). OVX elicited an augmented hypertensive response to ANG II infusion in the offspring of HT dams (∆34.8 ± 2.8 mmHg, P < 0.05) when compared with that seen in the offspring of NT dams (∆23.9 ± 1.1 mmHg, Fig. 4, A and B). In contrast, an ANG II-induced decrease in HR was only evident in the sham OVX female offspring (NT-dam ∆34.2 ± 5.6 beats/min and HT-dam ∆26.5 ± 4.7 beats/min) but not in the OVX female offspring (Fig. 4, C and D).
Fig. 4.
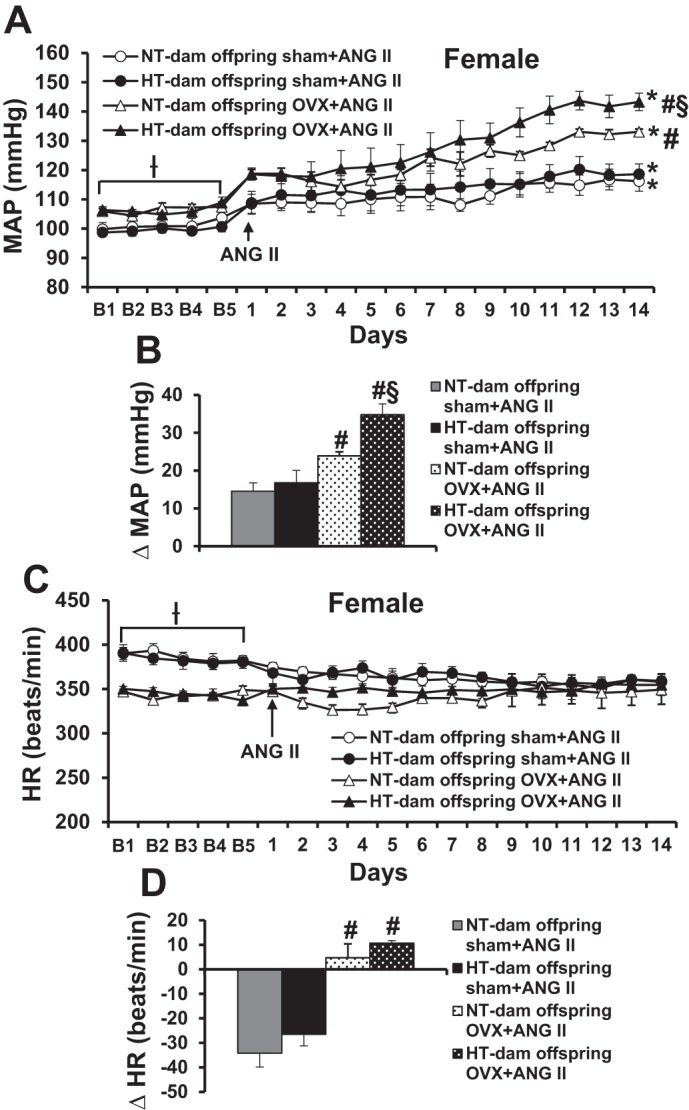
Ovariectomy (OVX) induced a greater hypertensive response to angiotensin II (ANG II) in female offspring of hypertensive (HT) dams compared with female offspring of normotensive (NT) dams. Daily mean arterial pressure (MAP) (A) and heart rate (HR) (C) before and during systemic infusion of ANG II in sham and OVX female offspring. B and D: average changes in MAP and HR across days induced by ANG II infusion in all groups. Baseline recording days are denoted by B letters, followed by 14 days of systemic ANG II infusion (n = 6/group; ƗP < 0.05 vs. basal MAP or HR of sham female offspring, *P < 0.05 vs. baseline, #P < 0.05 vs. sham female offspring, §P < 0.05 vs. OVX female offspring of NT dams).
Effects of OVX on mRNA Expression of RAAS Components and NADPH Oxidase in the Brain of Adult Offspring Without ANG II Treatment
In brain tissues collected at 10 wk of age, OVX female offspring from HT dams exhibited increased mRNA expression of RAAS components and NADPH oxidase (renin, ACE1, and MR in the LT, and ACE1, AT1R, and NOX2 in the PVN) when compared with sham OVX female offspring of either NT or HT dams (P < 0.05, Fig. 5, A and B). In contrast, OVX only upregulated mRNA expression of ACE1 in the LT of female offspring from NT dams (P < 0.05, Fig. 5A).
Fig. 5.
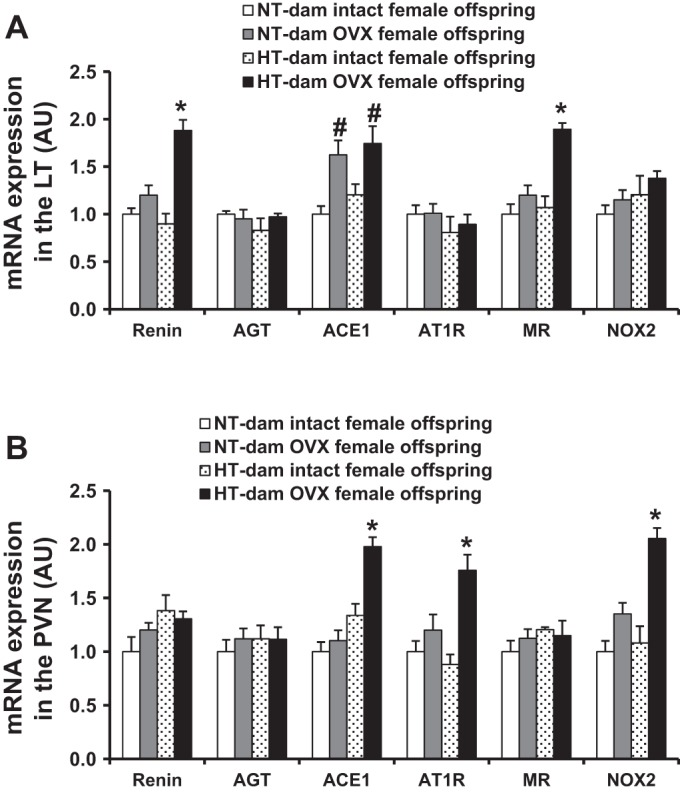
Comparison of the mRNA expression of renin-angiotensin-aldosterone system components and NADPH oxidase in the lamina terminalis (LT) (A) and paraventricular nucleus (PVN) (B) in intact or ovariectomized (OVX) female offspring from both normotensive (NT) and hypertensive (HT) dams (n = 5/group; *P < 0.05 vs. intact and OVX female offspring of NT dams and intact female offspring of HT dams, #P < 0.05 vs. intact female offspring of either NT dams or HT dams).
Effects of E2 Replacement on ANG II-Induced BP and HR in OVX Female Offspring
E2 replacement restored basal MAP and HR and ANG II-induced decrease in HR only in NT-dam OVX offspring (baseline, 99.5 ± 2.0 mmHg and 371.2 ± 3.2 beats/min; ANG II treatment, ∆21.5 ± 3.4 beats/min, P < 0.05, Fig. 6) but not in HT-dam OVX offspring. Furthermore, this E2 replacement blocked the OVX-augmented increase in BP during ANG II infusion in the offspring of NT dams (∆7.1 ± 1.9 mmHg, P < 0.05), whereas the E2 replacement only partially restored protective effect against ANG II-induced hypertension in offspring of HT dams (∆34.8 ± 2.8 vs. ∆23.1 ± 3.3 mmHg, P < 0.05, Fig. 6, A and B).
Fig. 6.
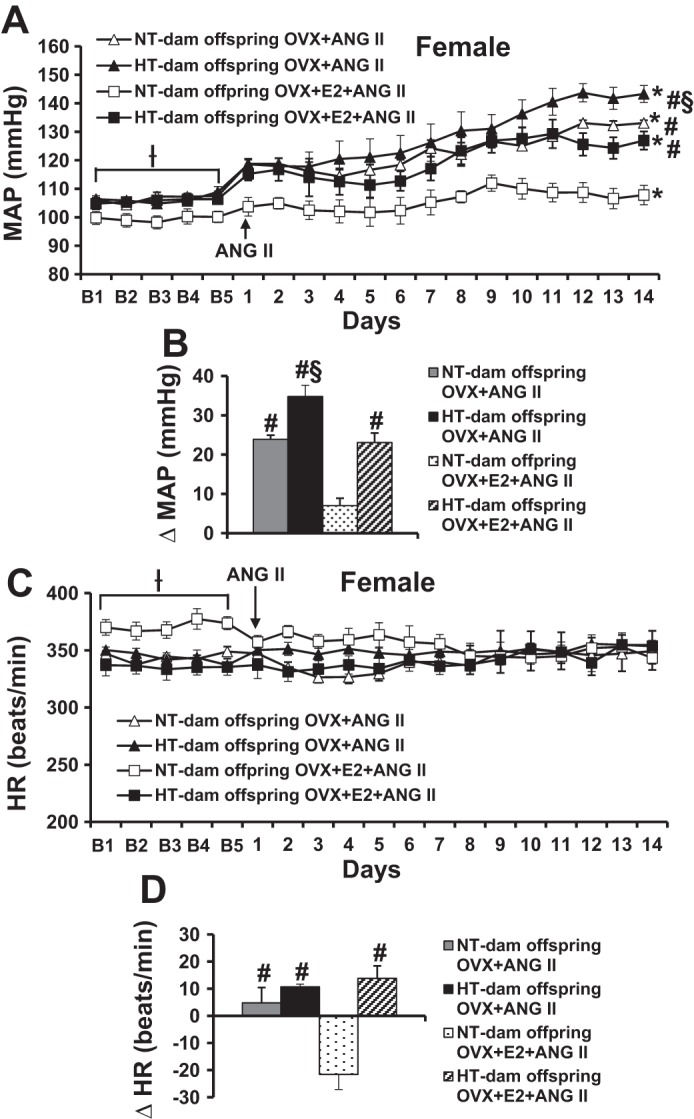
17β-Estradiol (E2) supplement partially attenuated ovariectomy (OVX)-augmented pressor effects in female offspring of hypertensive (HT) dams, whereas this E2 supplement completely restored female protective effect in the offspring of normotensive dams. Daily mean arterial pressures (MAP) (A) and heart rates (HR) (C) before and during systemic infusions of angiotensin II (ANG II). B and D: average changes in mean arterial pressure (MAP) and heart rate (HR) induced by ANG II infusion in all groups (B = baseline days; n = 6/group; Ɨ P < 0.05 vs. basal MAP or HR of OVX+E2 offspring of NT dams, *P < 0.05 vs. baseline, #P < 0.05 vs. OVX+E2+ANG II offspring of NT dams, §P < 0.05 vs. OVX+E2+ANG II offspring of HT dams or OVX+ANG II offspring of NT dams).
DISCUSSION
The major findings of the present study are the following: 1) female offspring of dams with gestational hypertension did not display sensitization of the hypertensive response as found in male offspring; 2) castration did not alter the hypertensive response to ANG II in male offspring from either NT dams or HT dams; 3) in contrast, OVX in female offspring resulted in a sensitized hypertensive response; 4) OVX upregulated several brain RAAS components and NADPH oxidase in both the LT and PVN; and 5) E2 replacement beginning at the time of OVX partially reduced the sensitized response in the female offspring.
In previous studies in adult animals, we used an Induction-Delay-Expression experimental design to study sensitization of the hypertensive response. In these studies, during Induction rats were exposed over a relatively short period of time to various types of challenges that did not produce by themselves any sustained effect on BP. Then after a period of Delay, a sensitized hypertensive response to a slow pressor dose of ANG II was observed in these rats when challenged during a period of Expression (29, 31, 33, 34). Furthermore, using a gestational hypertension model to address whether sensitization of the hypertensive response could be produced in the next generation, we found that maternal hypertension during pregnancy sensitized the ANG II-induced hypertensive response through mechanisms that required intact renal nerves and RAAS components in adult male offspring (30). In the present study, we demonstrated that unlike male offspring, the female offspring of HT dams did not respond to ANG II with a greater hypertensive response than the female offspring of NT dams, suggesting that the female offspring are protected from maternal hypertension-induced sensitization of ANG II hypertension as compared with their male littermates. This finding is consistent with many other prenatal insult models, showing that males and females are not equally affected; usually, but not always, males experience more profound effects (2, 3).
It has been shown that the presence of testosterone in the male may promote the development of hypertension, whereas estrogens in females tend to protect against the hypertensive effects of an adverse fetal environment (2, 3). Estrogen and testosterone, either alone or together, are responsible for the sex difference in the development of hypertension in offspring of different prenatal insult models. Castration has been shown to abolish the development of hypertension in offspring from spontaneously hypertensive rats and in offspring in a model of placental insufficiency (17, 19), whereas castration failed to prevent prenatally programmed hypertension induced by modest maternal protein restriction or cortisol exposure (15, 23). In contrast, OVX in the female offspring that experienced placental insufficiency, maternal undernutrition, high-salt intake, high-fat diet, hypoxia, glucocorticoid, or nicotine exposure resulted in increases in BP or increased BP responses to acute ANG II treatment (7, 8, 11, 13, 14, 16, 18, 22, 24–26). In the present study, we confirmed that OVX induced an enhanced ANG II-induced pressor response in the female offspring of mothers with gestational hypertension, whereas castration had no effect on this form of gestational-induced sensitization. This suggests that female sex hormones, but not male sex hormones, contribute to the sexual dimorphism of hypertensive response sensitization in this model of hypertension during pregnancy.
To further determine the protective role of estrogen against maternal hypertension-induced sensitization of ANG II pressor response, E2 replacement was applied in the OVX offspring. In contrast to the findings in the offspring of NT dams showing that E2 replacement completely restored increased BP to the level in sham female offspring during ANG II infusion, E2 replacement in OVX female offspring of HT dams only partially buffered the sensitized BP increase during ANG II treatment. These results suggest that E2 replacement reversed only the OVX-induced increase in the BP response, and that maternal hypertension during pregnancy per se might also be a candidate involved in the sensitization process. Indeed, it has been demonstrated that in the prenatal hypoxia model, E2 replacement had no effects on OVX-mediated BP response to acute ANG II in the offspring (24), and that E2 replacement only partially attenuated OVX-induced increase in BP in protein-restricted offspring (22). The authors suggested that these prenatal insults might have severely impacted the developing cardiovascular system and/or the target of ovarian hormones, resulting in a less responsive offspring to E2 (22, 24). If either is the case in our maternal hypertension model, chronic ANG II infusion should have induced a greater increase in BP in sham OVX female offspring of HT dams. However, we found a similar increase in BP during ANG II in both sham-operated female offspring from HT dams and NT dams. The reasons for this result in the present study are not entirely clear. Future studies to dissect the mechanisms involved in initiating programming in utero as well as events secondary to the altered hypertensive response are warranted.
It is well established that male animals exhibit higher basal BP and lower basal HR compared with female animals, and that both testosterone and estrogen play key roles in maintaining these differences (5, 9, 28). Consistent with adult animals including humans (5, 6, 29), in the present study, we also found that OVX increased baseline BP and decreased baseline HR in female offspring, whereas castration only reduced baseline HR in male offspring of NT dams and HT dams. Furthermore, ANG II treatment induced a greater reduction in HR in female offspring than that in male offspring, which may be indicative of female offspring being able to buffer the hypertensive response to ANG II better than the male offspring. This is in contrast to the findings in prenatal nicotine-treated offspring exhibiting impaired baroreflex sensitivity in both males and females (26). However, in females in a perinatal growth restriction model, a baseline increase in baroreceptor reflex sensitivity and high-frequency spectral power was evident, which was indicative of a vagal compensatory mechanism that was lost following OVX and partially restored by E2 replacement (8). Similarly, in the present study, E2 replacement only restored baseline BP and HR and the HR response to ANG II infusion in offspring of NT dams, but not in offspring of HT dams. This might account for the partial reduction of ANG II-augmented BP increase in the OVX offspring of HT dams with E2 replacement. Further study is warranted to directly investigate the changes in baroreflex sensitivity in both male and female offspring of HT dams.
It has been demonstrated that both male and female sex hormones contribute to the development and progression of hypertension through interactions with other regulatory pathways, including the RAAS and oxidative stress (2, 27, 32). Our previous studies showed that there is a sex difference in the sensitizing effects of ANG II in adult rats, and that the interactions between central estrogen and the RAAS are involved in this sensitization process (33). Maternal hypertension during pregnancy also sensitizes ANG II-elicited hypertension in male offspring of HT dams, which is associated with upregulation of the central RAAS (renin, AGT, ACE1, AT1R, and MR), NADPH oxidase NOX2, and proinflammatory cytokine components. Blockade of the RAAS and renal denervation reversed the sensitized hypertensive response to ANG II in these male offspring (30). Notably, these manipulations also are capable of normalizing the BP of ovariectomized or aging female offspring from several kinds of fetal insult (10, 16). In the present study, the mRNA expression data from the offspring of NT and HT dams was obtained when the females were 10 wk of age, which was before the time of beginning the slow pressor ANG II infusion in the animals. There was no upregulation in the expression of any of the components of the RAAS or NADPH oxidase in the two groups of female offspring in either the LT or PVN. The expression data in the two groups of females were generated in conjunction with a larger study that included groups of littermate males as cohorts. Moreover, OVX upregulated several RAAS components and NADPH oxidase in both the LT and PVN of the offspring from HT dams in comparison with only upregulation of ACE1 expression in the LT of the OVX offspring from NT dams. Comparison of the expression data of the present study with those of males studied under identical conditions (30) suggests that activation of the RAAS and increase in reactive oxygen species in males and OVX females from HT dams may mediate the sensitized hypertensive response, and that in intact females, sensitization does not occur because E2 protects against the increased RAAS and NADPH oxidase activity. Therefore, it is possible that differences in expression of these genes in the brain produced by each sex or OVX may account for the sex differences or the protective effects of the female sex or female sex hormones against sensitization of ANG II-induced hypertension in the offspring of HT dams.
Perspectives and Significance
Sex-specific effects are widely observed in perinatal developmental programming studies. The present study demonstrated that female offspring of HT dams are protected from maternal hypertension-induced sensitization of ANG II hypertension, and that female sex hormones account for the sex-specific sensitization of the hypertensive response to ANG II, which may be associated with estrogen downregulation of expression of the brain RAAS prohypertensive components. Previous studies have demonstrated that estrogen upregulates brain ACE2/ANG-(1–7)/Mas-R expression to offset the sensitizing effects induced by nonpressor dose of ANG II in adult female rats (33). Whether estrogen-mediated increase in activity of the antihypertensive components of the RAAS plays a protective role against sensitization of ANG II-induced hypertension in female offspring of HT dams needs to be studied further. Nevertheless, our findings provide insight into the cardiovascular protective mechanisms of female sex or female sex hormones in antagonizing increased risk of the development of hypertension induced by maternal hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-14388, HL-84207 and HL-98207.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.X. and A.K.J. conceived and designed research; B.X., T.G.B., and F.G. performed experiments; B.X. and A.K.J. analyzed data; B.X. and A.K.J. interpreted results of experiments; B.X. prepared figures; B.X. drafted manuscript; B.X. and A.K.J. edited and revised manuscript; B.X., T.G.B., F.G., and A.K.J. approved final version of manuscript.
REFERENCES
- 1.Abbott DH, Padmanabhan V, Dumesic DA. Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod Biol Endocrinol 4: 17, 2006. doi: 10.1186/1477-7827-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasinger JH, Alexander BT. Gender differences in developmental programming of cardiovascular diseases. Clin Sci (Lond) 130: 337–348, 2016. doi: 10.1042/CS20150611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasinger JH, Davis GK, Newsome AD, Alexander BT. Developmental programming of hypertension: physiological mechanisms. Hypertension 68: 826–831, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8: 127–141, 2007. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Mas MM, Afify EA, Mohy El-Din MM, Omar AG, Sharabi FM. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J Cardiovasc Pharmacol 38: 754–763, 2001. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension 31: 90–96, 1998. doi: 10.1161/01.HYP.31.1.90. [DOI] [PubMed] [Google Scholar]
- 7.Gray C, Gardiner SM, Elmes M, Gardner DS. Excess maternal salt or fructose intake programmes sex-specific, stress- and fructose-sensitive hypertension in the offspring. Br J Nutr 115: 594–604, 2016. doi: 10.1017/S0007114515004936. [DOI] [PubMed] [Google Scholar]
- 8.Haskell SE, Peotta V, Reinking BE, Zhang C, Zhu V, Kenkel EJ, Roghair RD. Oral oestrogen reverses ovariectomy-induced morning surge hypertension in growth-restricted mice. Clin Sci (Lond) 130: 613–623, 2016. doi: 10.1042/CS20150693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández I, Delgado JL, Díaz J, Quesada T, Teruel MJ, Llanos MC, Carbonell LF. 17β-Estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 279: R1599–R1605, 2000. doi: 10.1152/ajpregu.2000.279.5.R1599. [DOI] [PubMed] [Google Scholar]
- 10.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension 61: 828–834, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. doi: 10.1161/01.HYP.0000047511.97879.FC. [DOI] [PubMed] [Google Scholar]
- 12.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 53: 944–951, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 13.Maduwegedera D, Kett MM, Flower RL, Lambert GW, Bertram JF, Wintour EM, Denton KM. Sex differences in postnatal growth and renal development in offspring of rabbit mothers with chronic secondary hypertension. Am J Physiol Regul Integr Comp Physiol 292: R706–R714, 2007. doi: 10.1152/ajpregu.00458.2006. [DOI] [PubMed] [Google Scholar]
- 14.McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension 46: 1374–1380, 2005. doi: 10.1161/01.HYP.0000188702.96256.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moritz KM, Dodic M, Jefferies AJ, Wintour EM, DeMatteo R, Singh RR, Evans RG. Haemodynamic characteristics of hypertension induced by prenatal cortisol exposure in sheep. Clin Exp Pharmacol Physiol 36: 981–987, 2009. doi: 10.1111/j.1440-1681.2009.05180.x. [DOI] [PubMed] [Google Scholar]
- 16.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 50: 679–685, 2007. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R763, 2007. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 18.Ojeda NB, Royals TP, Alexander BT. Sex differences in the enhanced responsiveness to acute angiotensin II in growth-restricted rats: role of fasudil, a Rho kinase inhibitor. Am J Physiol Renal Physiol 304: F900–F907, 2013. doi: 10.1152/ajprenal.00687.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480–483, 2000. doi: 10.1161/01.HYP.35.1.480. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Rodríguez P, de Pablo AL, Condezo-Hoyos L, Martín-Cabrejas MA, Aguilera Y, Ruiz-Hurtado G, Gutierrez-Arzapalo PY, Ramiro-Cortijo D, Fernández-Alfonso MS, González MC, Arribas SM. Fetal undernutrition is associated with perinatal sex-dependent alterations in oxidative status. J Nutr Biochem 26: 1650–1659, 2015. doi: 10.1016/j.jnutbio.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Roghair RD, Segar JL, Volk KA, Chapleau MW, Dallas LM, Sorenson AR, Scholz TD, Lamb FS. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. Am J Physiol Regul Integr Comp Physiol 296: R651–R662, 2009. doi: 10.1152/ajpregu.90756.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring—influence of oestradiol. Br J Nutr 107: 665–673, 2012. doi: 10.1017/S0007114511003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods LL, Morgan TK, Resko JA. Castration fails to prevent prenatally programmed hypertension in male rats. Am J Physiol Regul Integr Comp Physiol 298: R1111–R1116, 2010. doi: 10.1152/ajpregu.00803.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao D, Huang X, Xue Q, Zhang L. Antenatal hypoxia induces programming of reduced arterial blood pressure response in female rat offspring: role of ovarian function. PLoS One 9: e98743, 2014. doi: 10.1371/journal.pone.0098743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao D, Huang X, Yang S, Zhang L. Estrogen normalizes perinatal nicotine-induced hypertensive responses in adult female rat offspring. Hypertension 61: 1246–1254, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension 51: 1239–1247, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol Regul Integr Comp Physiol 305: R459–R463, 2013. doi: 10.1152/ajpregu.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 29.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin–angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II–elicited hypertension. Hypertension 67: 163–170, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue B, Yin H, Guo F, Beltz TG, Thunhorst RL, Johnson AK. Maternal gestational hypertension-induced sensitization of angiotensin II hypertension is reversed by renal denervation or angiotensin-converting enzyme inhibition in rat offspring. Hypertension 69: 669–677, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue B, Yu Y, Zhang Z, Guo F, Beltz TG, Thunhorst RL, Felder RB, Johnson AK. Leptin mediates high-fat diet sensitization of angiotensin II–elicited hypertension by upregulating the brain renin–angiotensin system and inflammation. Hypertension 67: 970–976, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am J Physiol Heart Circ Physiol 307: H191–H198, 2014. doi: 10.1152/ajpheart.01012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)–initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension 59: 459–466, 2012. doi: 10.1161/HYPERTENSIONAHA.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II–induced hypertension. Hypertension 60: 1023–1030, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]


