Abstract
Sudden infant death syndrome (SIDS) is associated with serotonin (5-HT) neuron abnormalities. There is evidence of autonomic dysfunction during sleep in infants eventually succumbing to SIDS, as well as cardiovascular collapse before death. Neonatal rodents deficient in central 5-HT display hypotension and bradycardia. We hypothesized that central 5-HT reduces cardiac vagal tone and increases sympathetic vascular tone and, given the firing pattern of 5-HT neurons, that these effects are greater in quiet sleep (QS) than in active sleep (AS). We tested these hypotheses using 2-wk-old male and female rat pups lacking tryptophan hydroxylase-2 (TPH2−/−) and wild-type (WT) littermates. Arterial blood pressure (ABP) and heart rate (HR) were measured over 3 h during periods of QS and AS. We also gave atropine or atenolol (each 1 mg/kg iv), or phentolamine (5, 50, and 500 μg/kg iv) to separate groups to assess the effects 5-HT deficiency on autonomic tone to the heart or sympathetic vascular tone, respectively. Compared with WT, male and female TPH2−/− pups had reduced ABP in QS but not in AS. Atropine induced a greater HR increase in female TPH2−/− than in female WT pups, an effect absent in male TPH2−/− pups. Both genotypes experienced the same atenolol-induced drop in HR. In males only, phentolamine induced a smaller decrease in the ABP of TPH2−/− pups compared with WT. These data suggest that central 5-HT maintains ABP in QS, and HR in both states. In males, central 5-HT facilitates sympathetic vascular tone, and in females it reduces cardiac vagal drive.
Keywords: autonomic, blood pressure, cardiovascular, CNS, serotonin
INTRODUCTION
Sudden infant death syndrome (SIDS), the leading cause of death in the postneonatal period, occurs during sleep periods and has a higher incidence in males than in females (12). There is evidence of autonomic nervous system dysfunction in infants eventually succumbing to SIDS; some studies have demonstrated this dysfunction is more apparent in specific states of vigilance (21, 22); and records from SIDS cases indicate that death is preceded by overt cardiovascular collapse [i.e., a substantial loss of heart rate (HR) and presumably blood pressure] (18, 25). Immunohistochemical and audioradiographic abnormalities within brain stem serotonin (5-hydroxytryptamine, 5-HT) neurons are common in SIDS cases, including an increased proportion of immature 5-HT neurons, reduced tryptophan hydroxylase (TPH) expression, and 5-HT content and reduced 5-HT1A receptors (11, 13, 26). There is a vast amount of evidence from adult animals that brain stem 5-HT neurons influence the autonomic control of cardiovascular function (19). In addition, there is mounting evidence that serotonergic defects underpin the autonomic and/or cardiorespiratory dysfunction associated with SIDS. For example, a loss of serotonergic neurons or central 5-HT content in the early postnatal period leads to lower resting arterial blood pressure (ABP) and HR and leads to cardiovascular collapse during exposure to severe hypoxia (4, 5, 14, 27). This latter phenotype in particular is reminiscent of the pathophysiology of SIDS.
The activity of brain stem serotonergic neurons changes across states of vigilance. Their activity is highest in wakefulness and lowest in REM sleep [i.e., active sleep (AS) in infants], with intermediate levels of activity in NREM sleep [i.e., quiet sleep (QS) in infants] (15). With this in mind, it may be that serotonergic abnormalities underlie not only the autonomic and cardiovascular dysfunction apparent in SIDS cases but also the sleep state dependency of this dysfunction that has been previously reported (21, 22).
This study addresses two hypotheses: first, that a loss of central 5-HT leads to bradycardia and hypotension due to altered parasympathetic and sympathetic tone to the heart and vasculature, respectively; and second, given the firing pattern of serotonergic neurons, that the influences of 5-HT on ABP and HR are greater in QS than in AS. To test these hypotheses, we conducted experiments on tryptophan hydroxylase 2 knockout (TPH2−/−) rat pups that had a specific loss of central 5-HT and increased mortality in the second postnatal week [10% vs. 1% for wild-type (WT) controls] (10), an age that may be akin to human infancy (24). Our data lend partial support to these hypotheses and, surprisingly, indicate that the effects of 5-HT on the control of ABP and HR depend on sex. For example, central 5-HT facilitates sympathetic vascular tone in males but not in females, and reduces cardiac vagal drive in females but not in males. The implications of these findings for the role of serotonergic dysfunction in male and female SIDS cases are discussed.
METHODS
Ethical approval.
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Missouri at Columbia, MO, in accordance with national guidelines.
Animals.
This study utilizes TPH2−/− and WT rat pups at postnatal days 14–16. The generation and genotyping of the TPH2−/− rat line on Dark Agouti background has been previously described (10, 28). TPH2−/− animals have a specific loss of central 5-HT, with unchanged central catecholamines (10). Adult male and female TPH2+/− rats were used as breeding pairs to generate WT and TPH2−/− pups. TPH2+/− dams were fed ad libitum on standard rat chow and kept on a 121:12-h light-dark cycle. Pups were fed ad libitum until they were removed from the dams for surgery and testing. We used a total of 69 TPH2−/− pups (n = 34 males and 35 females) and 69 age-matched WT littermates (n = 38 males and 31 females). TPH2−/− pups weighed significantly less than controls (TPH2−/−, 17.0 ± 0.4 g; WT, 26.7 ± 0.7 g; P < 0.001), an observation that has been previously reported (10, 28).
Determining vigilance state.
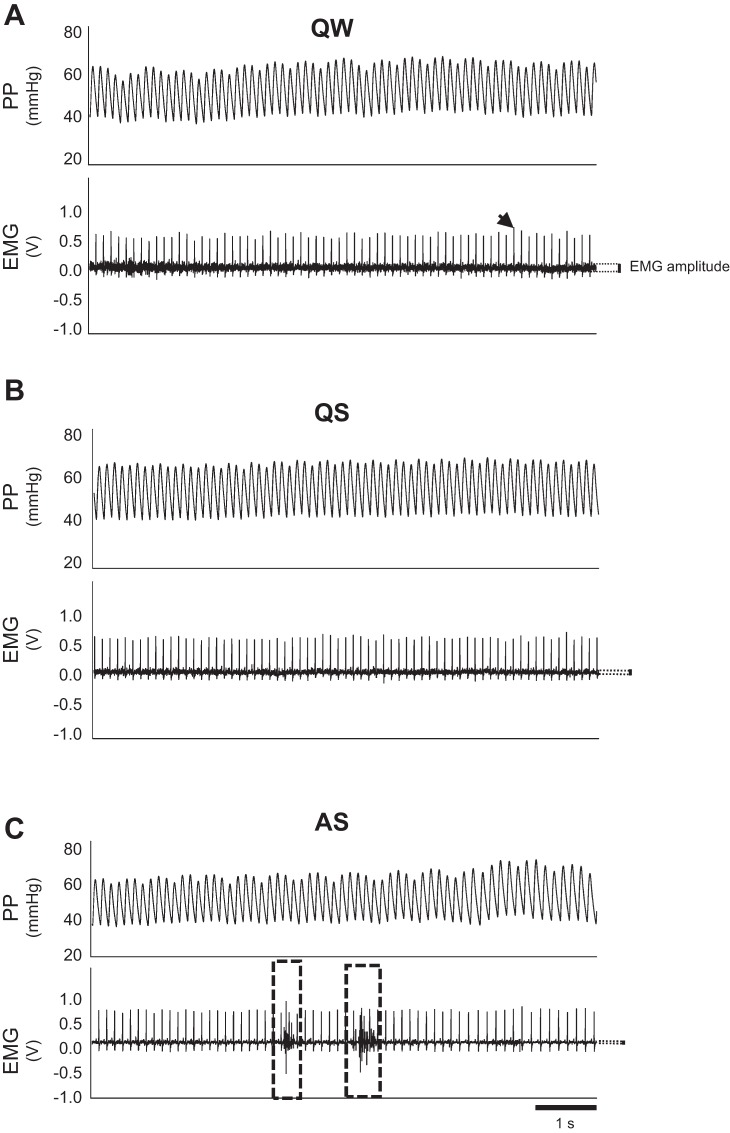
Wakefulness, QS, and AS were determined using a combination of nuchal electromyography (EMG) along with established behavioral criteria that we and others have previously described (6, 28). Specifically, quiet wakefulness (QW) was associated with relatively high EMG amplitude (Fig. 1A). When awake, pups typically displayed voluntary exploratory behavior within the chamber. This unfortunately introduced significant artifacts in the ABP and HR records, preventing us from being able to include cardiovascular data from this state. QS was associated with reduced EMG amplitude (Fig. 1B) and complete immobility without the presence of myoclonic twitching, with the head resting on the forelimbs or floor of the chamber. EMG amplitude decreased further in AS (Fig. 1C), when involuntary, myoclonic twitching of the facial muscles, limbs, and trunk musculature was observed; this muscle activity generated bursts of activity on the nuchal EMG record (Fig. 1C).
Fig. 1.
Arterial pulse pressure (PP) and nuchal electromyographic (EMG) records obtained from short periods of quiet wakefulness (QW, A), quiet sleep (QS, B), and active sleep (AS, C). Amplitude of nuchal EMG record (dark black section of trace) is indicated by dotted lines with the black vertical bar. When compared with QW, EMG amplitude is lower in QS and is reduced further in AS. During AS, burst of activity associated with myoclonic twitching appears in the EMG record (dashed boxed regions). Nuchal EMG is superimposed on higher amplitude R-waves of the electrocardiographic record (indicated by arrowhead).
In preliminary experiments, we noted that the implantation of catheters and EMG electrodes inhibited the appearance of AS, at least compared with previously published data from our laboratory (26). Thus, to facilitate the appearance of AS, we implanted both EMG electrodes and catheters in only a subset of animals (n = 6 TPH2−/−, n = 6 WT), to confirm the behavior normally associated with QS and AS (28). For all subsequent experiments, we utilized behavioral observation alone (see animal numbers below for groups 1–4). For EMG electrode implantation, pups were removed from the dams and anesthetized using isoflurane (induction with 3%, maintained on ~2%). Noxious paw pinch was used to assess anesthesia depth. Two insulated stainless steel wire electrodes, with suture pads at the end, were used for each animal (part no. E363/76; PlasticsOne, Roanoke, VA); pad dimension were thickness 0.66 mm, width 1 mm, length 3.18 mm. Wire dimensions were diameter 0.25 mm, length 37.5 mm. Electrode pads were sutured in place, one under the nuchal muscle and the other in flank muscles (ground). Lidocaine (~100–200 μl of 20 mg/ml solution) was applied to each of the electrode sites.
Femoral artery and venous catheterization.
Femoral arterial and venous catheters were implanted into pups immediately before testing, as described previously (14). Briefly, catheters consisting of PE10 tubing were heated, stretched, and filled with sterile heparinized 0.9% saline (100 μl/ml) before surgery. While the pups were under ~2% isoflurane, a skin incision was made in the left groin for visualization and dissection of the left femoral artery under an ×20 dissecting microscope. With the use of 5-0 surgical suture, the artery was tied just distal to the epigastric branch, and an incision was made in the artery for insertion of the PE10 catheter. The tip of the catheter was advanced near the inguinal ligament (~0.6–0.8 cm). Femoral venous catheters were also implanted in pups from groups 2–4 for drug delivery (see Experimental groups and protocols). After surgery, lidocaine was applied to the surgical site.
Experimental setup.
Data were recorded from unrestrained animals while they cycled naturally between wakefulness, QS, and AS within a water-perfusable glass chamber (volume: 100 ml) attached to a programmable water bath/pump. Chamber temperature, monitored with a thermocouple and digital thermometer (Omega Engineering, Norwalk, CT), was maintained at 31°C, within the thermoneutral range for pups this age (16, 28). We have previously demonstrated that under these conditions TPH2−/− and WT pups have the same metabolic rate (28). Air (21% O2, balance N2) from a gas cylinder or wall air was passed through a flowmeter before entering the chamber via a 20-gauge needle pushed through one of the rubber stoppers that seal the chamber. Chamber pressure was kept near atmospheric by pulling the gas from the opposite end of the chamber with a pump, also through a 20-gauge needle. Air flow through the chamber was held constant at 300 ml/min. The femoral arterial catheter was exteriorized via a separate 20-gauge needle and attached to a blood pressure transducer, which was calibrated each experimental day with a sphygmomanometer. Analog signals from both arterial pressure and respiratory transducers were fed into a Powerlab data acquisition system (ADInstruments, Colorado Springs, CO) and analyzed in LabChart 7.3.7 (ADInstruments).
Experimental groups and protocols.
Experiments were performed during the daytime (8 AM to 5 PM) on four groups of animals. For all groups, animals were allowed to recover from isoflurane anesthesia for 30 min. In our hands, 30 min are sufficient for the full recovery of HR, ABP, breathing, and metabolic rate (14, 28). Pups started displaying QS at this time, and usually the first AS episode occurred within an hour of recovery. Group 1 animals (TPH2−/−, n = 11 male, 12 female; WT, n = 12 male, 11 female), left unperturbed in the chamber for a total of 3 h, were used solely to investigate how central 5-HT deficiency affected BP and HR in QS and AS, under resting conditions. Animals behaved freely in the chamber for 2–3 h, to enable acquisition of data across at least one episode of QS and AS.
Group 2 pups (TPH2−/−, n = 8 male, 7 female; WT, n = 11 male, 6 female) were used to assess the effects of central 5-HT deficiency on sympathetic tone to the vasculature. Pups behaved naturally in the chamber until an episode of AS was observed (i.e., to ensure pups were cycling naturally between vigilance states). Again, in most animals this occurred within 1 h. If AS was not observed by 1.5 h, the experiment commenced regardless. Autonomic tone to the heart was first blocked by simultaneously administering atropine methyl nitrite and atenolol (both at 1 mg/kg iv; Sigma-Aldrich, St. Louis, MO). Atropine and atenolol were allowed to act for 15 min to reach the steady-state HR response. We then sequentially injected three increasing doses of phentolamine (Sigma-Aldrich), an α-adrenoreceptor blocker (5, 50, and 500 μg/kg iv), to reduce vascular tone and thus induce a fall in ABP. These doses were determined empirically; at concentrations higher than 500 μg/kg, the drop in blood pressure induced by phentolamine lasted longer than 15 min, making it difficult to give multiple doses to the animal. Animals received doses of phentolamine in QS, except for 2 of 17 WT and 1 of 15 TPH2−/− pups that had reduced sleep following autonomic blockade and therefore received 5 and 500 μg/kg doses while in wakefulness. We gave 15 min between each dose to allow for recovery of blood pressure. The maximum fall in blood pressure occurred within ~30 s of each injection. Given the transient nature of the drop in ABP, as well as the fact that all animals woke up upon injection, we were unable to report the ABP response to phentolamine in either QS or AS. In a subset of pups, we injected additional doses of atropine and atenolol (1 mg/kg) at the end of the experiment; the lack of a change in HR confirmed that autonomic blockade at the level of the heart was maintained throughout the experiment.
Group 3 (TPH2−/−, n = 6 male, 6 female; WT, n = 5 male, 7 female) and group 4 (TPH2−/−, n = 10 male, 10 female; WT, n = 8 male, 6 female) were used to assess the effects of central 5-HT deficiency on sympathetic and vagal drive to the heart, respectively. As with group 2 pups, baseline cardiovascular variables were recorded for 1.5 h to allow for the appearance of AS. Subsequently, we administered either atropine methyl nitrate or atenolol, as a 10-μl intravenous bolus dissolved in saline (final dose for each: 1 mg/kg) during QS. To ensure that drugs acted effectively during the experiment, the same dose of atropine or atenolol was given ~30 min following the initial injection. HR and ABP were measured over the course of following 1 h, across multiple episodes of QS and AS (but see below regarding effect of atropine on AS occurrence). Saline alone was injected into a cohort of animals (TPH2−/−, n = 6; WT, n = 5) to control for the effects of the injection alone on ABP and HR.
Some WT and TPH2−/− pups did not display any AS episodes after atropine treatment. Despite the reduction in animals' numbers, we still report the change in HR in AS (TPH2−/−, n = 4 males, 3 females; WT, n = 5 males, 6 females). At the end of each experiment, intrinsic HR (HRINT; i.e., HR following complete autonomic blockade) was determined by the administration of atenolol to pups treated initially with atropine and atropine to pups treated initially with atenolol.
Because we did not observe any significant differences in resting cardiovascular variables between the four groups, the predrug periods from animals in groups 2–4 (1–1.5 h) were combined with group 1 data (3-h recordings) for analyses (total animals for baseline variables in QS and AS (Fig. 2: TPH2−/−, n = 34 male, 35 female; WT, n = 38 male, 31 female).
Fig. 2.
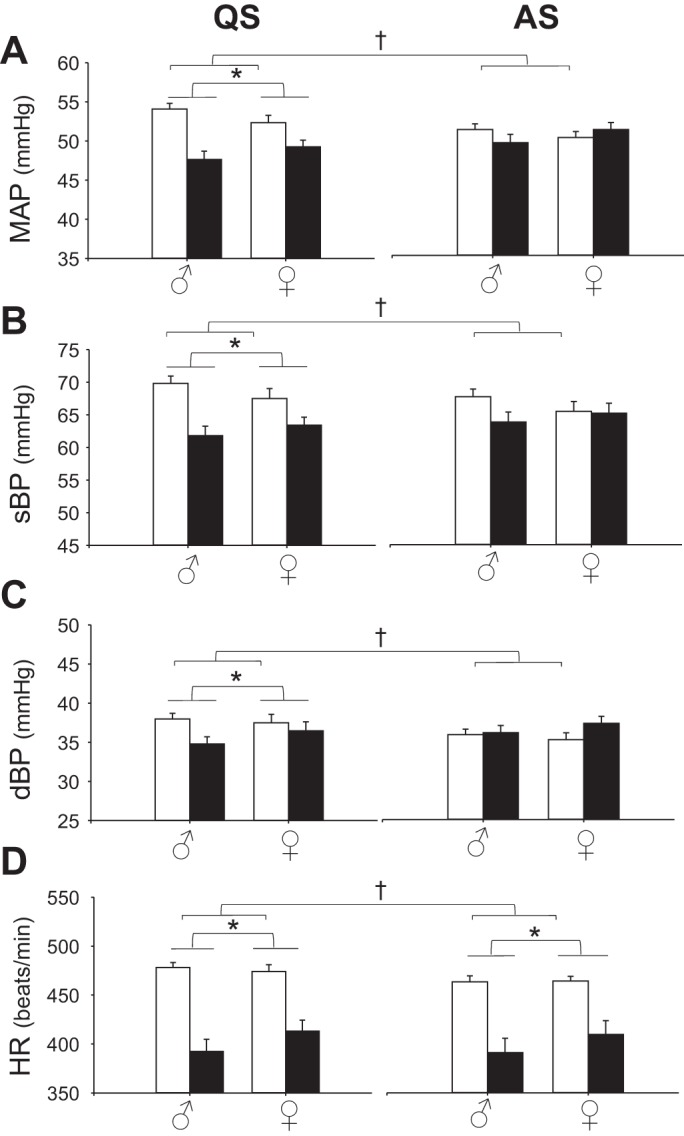
Central serotonin (5-hydroxytryptamine, 5-HT) deficiency leads to reduced blood pressure in quiet sleep (QS), and reduced heart rate (HR) in both sleep states. Shown are mean arterial pressure (MAP; A), systolic blood pressure (sBP; B), diastolic blood pressure (dBP; C), and HR (D) for 5-HT-deficient (TPH2−/−) rat pups (solid bars; n = 34 male, 35 female) and wild-type (WT) littermates (open bars; n = 38 male, 31 female) in QS; left) and AS; right). *Significant effect of genotype [2-factor ANOVA (2FA); P < 0.01 for all variables]; †significant interaction between genotype and sleep state, testing data from animals displaying both QS and AS [3-factor, repeated-measures ANOVA (3FRMA; P < 0.001 for all variables]. For this and subsequent figures, data are mean values ± SE.
Data and statistical analysis.
For all experimental groups, we recorded raw pulse pressure, from which systolic (sBP), diastolic (dBP), and HR were derived. We recorded and analyzed cardiovascular variables after the 30-min settling period, from QS and AS episodes lasting at least 10 s in duration. Statistical analyses on variables of interest were performed using the averages for each animal across each sleep state. Significant effects of genotype, sleep state, and sex on sleep variables (number and duration of AS and QS episodes), resting MAP, sBP, dBP, and HR (and interactions) were resolved using three-factor, repeated-measures analyses of variance (3FRMA; IBM SPSS software, Armonk, NY). Given that state was the within-animal factor, 3FRMA was performed only on pups displaying both QS and AS (TPH2−/−, n = 17 male, 19 female; WT, n = 28 male, 23 female). Two-factor ANOVA (2FA; Sigmaplot 12.5) was used to assess the effects of genotype and sex on ABP and HR within QS and AS, using the data collected from all animals, irrespective of whether they experienced an AS episode or not (TPH2−/−, n = 34 male, 35 female; WT, n = 38 male, 31 female). Effects of genotype and sex on the change in HR following atropine and atenolol treatment were resolved with 2FA (Sigmaplot 12.5). Effects of genotype, sex and the dose of phentolamine on the drop in MAP were resolved with a 3FRMA (IBM SPSS software) and 2FRMA (Sigmaplot 12.5) within each sex. When significant main effects were resolved, pairwise multiple comparisons were performed using Tukey’s post hoc analyses. Regression analyses were also performed to examine the potential relationship between cardiovascular variables of TPH2−/− pups and their reduced body mass.
RESULTS
Sleep architecture during baseline conditions.
We analyzed the number of AS and QS episodes, the duration of these episodes, as well as the percent total time group 1 WT and TPH2−/− pups spent in AS and QS (Table 1). Most animals displayed at least one episode of AS and QS (1/8 male WT, 1/9 female WT; 2/7 male TPH2−/−, and 1/10 female TPH2−/− did not experience an AS episode). Both male and female TPH2−/− animals had significantly fewer episodes of QS and AS over the 3-h period (3FRMA, genotype: P = 0.01) and tended to have longer episodes of AS compared with WT littermates (P = 0.05). However, as they had fewer episodes of QS and AS, TPH2−/− pups spent ~10–15% more time in wakefulness compared with WT (3FRMA, genotype × state: P < 0.001).
Table 1.
Sleep architecture of group 1 WT and TPH2−/− pups
| QS |
AS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Sex | W (%Time) | No. of episodes | Episode duration, s | %Time | No. of episodes | Episode duration, s | %Time |
| WT | ♂ n = 12 | 58.0 ± 3.8 | 90.3 ± 13.0 | 39.9 ± 4.1 | 34.8 ± 3.2 | 7.5 ± 2.3 | 66.7 ± 7.6 | 7.2 ± 2.4 |
| ♀ n = 11 | 53.5 ± 3.6 | 64.8 ± 9.5 | 46.1 ± 4.0 | 36 ± 1.9 | 9.4 ± 2.2 | 82.3 ± 8.7 | 10.5 ± 3.1 | |
| TPH2−/− | ♂ n = 11 | 67.9 ± 5.8† | 38.6 ± 5.5* | 52.4 ± 9.2 | 26.9 ± 4.9† | 3.6 ± 1.6* | 92.6 ± 26 | 5.3 ± 2.2† |
| ♀ n = 12 | 67.5 ± 3.1† | 42.7 ± 5.0* | 55.3 ± 9.8 | 26.3 ± 2.7† | 5.1 ± 1.1* | 103.1 ± 8.6 | 6.3 ± 1.2† | |
Values are means ± SE; n, number of pups. Shown are variables for periods of wakefulness (W), quiet sleep (QS), and active sleep (AS). WT, wild type; TPH2−/−, serotonin (5-hydroxytryptamine, 5-HT) deficient.
Significant effect of genotype, indicating that TPH2−/− pups have fewer episodes of QS and AS.
Significant genotype × state interaction, indicating that TPH2−/− spend more time in W, and less time in QS and AS, vs. WT. Significance at P < 0.05.
Central 5-HT maintains arterial blood pressure during quiet sleep.
We hypothesized that central 5-HT prevents ABP and HR from falling during QS, when serotonergic neurons are firing and releasing synaptic 5-HT (15). There was no significant influence of genotype on sBP, dBP, or MAP during AS (Fig. 2, A–C, and Table 2). On the other hand, the sBP, dBP, and MAP of TPH2−/− pups were all significantly reduced during QS compared with WT (P < 0.03 for all; Fig. 2, A–C, and Table 2). The effect of genotype on blood pressure emerged in QS because in WT pups pressure was elevated in QS compared with AS, whereas in TPH2−/− pups pressure was unaffected by state (genotype × state interaction: P < 0.001 for all; Fig. 2A and Table 2). Our statistical analyses indicated that both male and female TPH2−/− pups had reduced MAP compared with WT controls (genotype × sex interaction: P = 0.06). However, on average, the difference in MAP between male TPH2−/− and WT pups during QS (~7 mmHg), was greater than the difference between female TPH2−/− and WT pups (~2 mmHg) (Fig. 2A and Table 2).
Table 2.
Baseline MAP and HR for group 1 WT and TPH2−/− pups
| QS |
AS |
||||
|---|---|---|---|---|---|
| Genotype | Sex | MAP, mmHg | HR, beats/min | MAP, mmHg | HR, beats/min |
| WT | ♂ n = 12 | 54.1 ± 0.7† | 478 ± 5 | 51.3 ± 0.8 | 465 ± 6 |
| ♀ n = 11 | 52.4 ± 0.9† | 474 ± 7 | 50.3 ± 0.8 | 466 ± 5 | |
| TPH2−/− | ♂ n = 11 | 47.6 ± 1.1 | 393 ± 13* | 50.0 ± 1.3 | 406 ± 16* |
| ♀ n = 12 | 49.3 ± 0.9 | 413 ± 11* | 51.3 ± 0.9 | 411 ± 15* | |
Values are means ± SE; n, number of pups. Shown are variables during periods of quiet sleep (QS) and active sleep (AS). MAP, mean arterial pressure; HR, heart rate; WT, wild-type.
Significant effect of genotype, indicating lower HR in TPH2−/− pups in both states.
Significant genotype × state interaction, indicating that the MAP of WT pups is elevated in QS vs. AS, an effect absent in TPH2−/− pups. Significance at P < 0.05.
Central 5-HT-deficient pups had reduced HR compared with WT, but, unlike MAP, the magnitude of this effect did not depend on sleep state. In both states, the HR of male and female TPH2−/− pups was ~50–100 beats/min lower than that of WT littermates (P < 0.001 in both states; Fig. 2D). Similarly to ABP, the HR of WT pups was higher in QS compared with AS, whereas the HR of TPH2−/− was not influenced by state (genotype × state interaction: P < 0.001; Fig. 2D).
Central 5-HT facilitates sympathetic vascular tone in infant males.
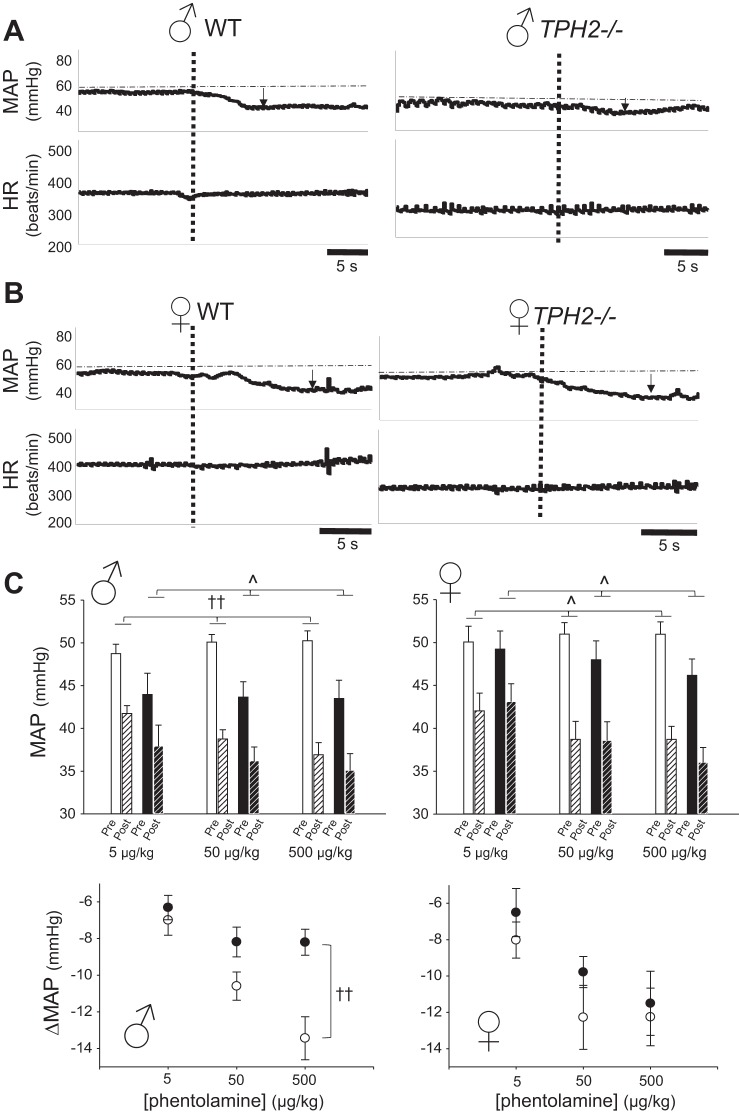
To test the hypothesis that central 5-HT facilitates sympathetic vascular tone in QS, we treated male and female TPH2−/− and WT littermates with increasing doses of phentolamine (5, 50, and 500 μg/kg iv in saline) to block vascular α1-adrenoreceptors, reduce vascular tone, and decrease ABP. Surprisingly, the ABP response to phentolamine depended not only on genotype but also on sex. Representative traces from male and female WT and TPH2−/− pups are shown in Fig. 3, A and B, demonstrating the fall in pressure induced by 500 μg/kg phentolamine. In males, this dose of phentolamine induced a larger decrease in the MAP of WT pups (~13 mmHg) compared with TPH2−/− littermates (~8 mmHg, P < 0.001; Fig. 3C). In contrast, female pups experienced the same drop in MAP irrespective of genotype (~12 mmHg; Fig. 3, B and C). Across all three doses, male TPH2−/− pups had reduced sensitivity to phentolamine compared with male WT (2FRMA, genotype × dose: P = 0.004; Fig. 3C), whereas the sensitivity of female pups did not depend on genotype (2FRMA, genotype × dose: P = 0.70; 3FRMA, genotype × dose V sex interaction: P = 0.04; Fig. 3C). The injection of saline alone had no significant effect on MAP (TPH2−/− in QS: 3 ± 2 mmHg; WT in QS: −1 ± 1 mmHg).
Fig. 3.
Loss of central 5-hydroxytryptamine (5-HT) reduces sympathetic tone to the vasculature in males. Typical responses of mean arterial pressure (MAP) and heart rate (HR) to phentolamine for male wild-type (WT) and TPH2−/− pups (A) and female WT and TPH2−/− pups (B). Note that phentolamine injection (at vertical dashed line) induced a smaller decrease in MAP in the male TPH2−/− pup vs. its WT counterpart, an effect not seen in females. C, top: average pretreatment values for the MAP of WT [open, nonhatched bars, n = 11 males (left) and 6 females (right)] and TPH2−/− pups [solid, nonhatched bars; n = 8 males (left) and 7 females (right)] as well as values, postphentolamine treatment, at 5, 50, and 500 μg/kg, indicated by the open, hatched (WT) and closed, hatched (TPH2−/−) bars. ^Significant effect of dose (3FRMA, P < 0.001); ††significant interaction between genotype, sex, and dose on the fall in MAP (3FRMA, P = 0.04). Bottom: same data as in top, expressed as change in MAP from pre- to post-phentolamine treatment, in WT (open circles) and TPH2−/− pups (closed circles).
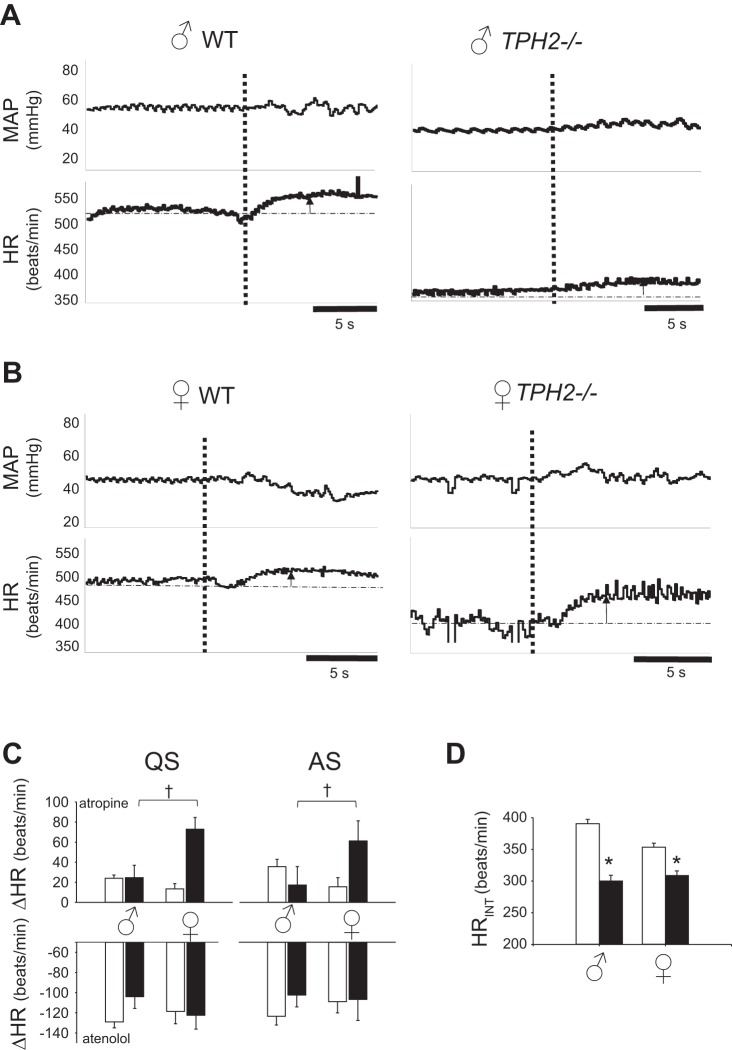
Central 5-HT reduces cardiac vagal drive in infant females.
To test the hypothesis that central 5-HT maintains HR in QS, we treated both TPH2−/− and WT pups with atropine and atenolol. Like the ABP response to phentolamine, the HR response to atropine depended on genotype and sex. The rise in HR after intravenous administration of atropine was greater in female TPH2−/− pups than in female WT, an effect not observed in male TPH2−/− animals. In both QS and AS, the HR of female TPH2−/− pups increased ~50 beats/min more than that of WT littermates (genotype × sex P = 0.016 and 0.028 in QS and AS, respectively; Fig. 4C), an effect unrelated to their smaller body size (R2 = 0.20, P = 0.22). These data suggest that central 5-HT reduces vagal drive to the heart in females but not in males. There was no effect of either genotype (P = 0.38) or sex (P = 0.73) on the HR response to atenolol [nor was there any significant interaction (P = 0.24)]. The injection of saline alone had no significant effect on HR (TPH2−/− in QS, −8 ± 13 beats/min; WT in QS, −1 ± 11 beats/min). Intrinsic HR (HRINT) was revealed in each group following complete autonomic blockade with atropine and atenolol. HRINT of male and female TPH2−/− pups was lower than that of their WT counterparts (genotype: P < 0.001; Fig. 4D). Regression analyses showed a relatively weak but significant correlation between the body mass of TPH2−/− pups and their HRINT; smaller pups had lower HRINT than larger pups (R2 = 0.27; P = 0.04).
Fig. 4.
Loss of central 5-hydroxytryptamine (5-HT) increases cardiac vagal tone in females. Typical responses of mean arterial pressure (MAP) and heart rate (HR) to atropine for male wild-type (WT) and TPH2−/− pups (A) and female WT and TPH2−/− pups (B). Note that atropine injection (at vertical dashed line) induced a larger HR increase in the female TPH2−/− pup vs. its WT counterpart, an effect not seen in the male pups. C: change in HR in response to atropine (top) of WT (open bars; n = 5 male, 7 female) and TPH2−/− pups (closed bars; n = 6 male, 6 female) and atenolol (bottom) (WT: n = 8 male, 6 female; TPH2−/−: n = 10 male, 10 female) in quiet sleep (QS) and active sleep (AS). †Significant genotype × sex interaction (2FA, P = 0.016 QS; P = 0.028, AS). D: intrinsic HR (HRINT) of male and female WT (open bars) and TPH2−/− pups (closed bars). *Significant effect of genotype (2FA, P < 0.001).
DISCUSSION
In this study, we investigated whether the bradycardia and hypotension evident in pups deficient in central 5-HT manifests in a sleep state-dependent fashion and whether sympathetic or parasympathetic dysfunction might underlie these phenotypes. We addressed two specific hypotheses: first, that central 5-HT reduces vagal drive to the heart and facilitates sympathetic vascular tone; and second, that central 5-HT has a greater influence in QS, when serotonergic neurons fire, than in AS, when the neurons are silent. Our experiments lend partial support to these hypotheses. First, both male and female TPH2−/− pups had reduced MAP in QS, but not in AS; HR, on the other hand, was lower in TPH2−/− pups in both sleep states. Second, we showed evidence that cardiac vagal drive was higher, and sympathetic vascular tone lower, in TPH2−/− pups compared with controls. Another important, unexpected finding was that the degree to which central 5-HT influenced autonomic drive to the heart and vasculature depended on the animal's sex. Central 5-HT facilitates sympathetic vascular tone only in males and inhibits cardiac vagal drive only in females.
Central 5-HT maintains arterial blood pressure in QS but not AS.
We (14) previously showed that ABP was reduced in 2-wk-old rat pups following a partial loss of 5-HT neurons. As 5-HT neurons synthesize and release numerous neuromodulators, the present study is the first to demonstrate that 5-HT is necessary for the maintenance of ABP in infancy. Previous studies examining the role of 5-HT in ABP regulation in adult animals have produced varying results. Alenina et al. (1) showed that adult male and female TPH2−/− mice have low MAP and HR. Interestingly, the phenotypes of adult TPH2−/− mice are most obvious in the afternoon and early evening and, generally, occur coincidentally with decreased activity. Although here we did not ask about a circadian influence, we show that, in male and female pups alike, a loss of central 5-HT leads to reduced MAP solely in QS. The difference in MAP between the genotypes during QS exists essentially because the MAP of WT pups is elevated in QS compared with AS, whereas no such increase occurs in TPH2−/− pups. This observation strongly suggests that the increase in MAP experienced by WT pups in QS is due to increased release of synaptic 5-HT by serotonergic neurons coincident with their increased firing in QS, an event that does not occur in 5-HT-deficient TPH2−/− animals (15).
Central 5-HT facilitates sympathetic drive to the vasculature in males.
Statistically our data suggest that a loss of central 5-HT affects the MAP of males and females to the same degree in QS (sex × genotype: P = 0.06). However, it may be that 5-HT makes a stronger contribution to the regulation of MAP in males compared with females. We showed, for example, that the vascular component of sympathetic drive is facilitated in males by the action of central 5-HT, evidenced by the larger drop in ABP following phentolamine treatment in WT compared with littermate TPH2−/− pups. In fact, phentolamine essentially eliminated the difference in ABP between the two genotypes that existed before treatment. This effect was absent in females. This finding is consistent with pharmacological data obtained from adult cats (20); vascular resistance significantly increased in these animals following the application of a 5-HT2A receptor agonist to the rostral ventrolateral medulla (RVLM), without any chronotropic or ionotropic effects on the heart (20). Reduced vascular resistance in male TPH2−/− pups may therefore be due to a lack of 5-HT2A receptor signaling in the RVLM. But why is sympathetic vascular tone reduced only in male TPH2−/− pups? At this point we can only speculate. We may assume that the expression of α1-adrenoreceptors in vascular smooth muscle is not influenced by a loss of central 5-HT (although this needs testing). Differences in the levels of sex hormones, even at these young ages, could be a contributing factor. The reduced vascular tone of TPH2−/− males may be related to the vasodilating properties of testosterone (3) which, even at these young ages, is found at higher concentrations in males than in females (8). Still, an interaction between central 5-HT and estrogens in male pups cannot be fully discounted; indeed, there may be slightly reduced plasma estradiol in males compared with females at these ages, and the sympathoinhibitory role of estrogens has been well described (9). Exactly how sex-specific factors influence the function of central 5-HT in the control of blood pressure awaits further investigation.
Central 5-HT inhibits cardiac vagal drive in females.
We showed that, in both QS and AS, pups deficient in central 5-HT have reduced HR compared with WT. We then assessed the extent to which each arm of the autonomic nervous system contributed to this cardiac phenotype. We demonstrated that in both states the atropine-induced rise in HR was greater in female TPH2−/− than in WT pups (an effect not observed in male TPH2−/− pups), suggesting that in infant females a loss of central 5-HT ultimately leads to enhanced vagal drive to the heart, reducing HR in both states. There are multiple 5-HT receptors expressed on cardiac vagal neurons, including inhibitory 5-HT1A receptors, and it has been shown that 5-HT1A signaling can suppress excitatory glutamatergic drive to these neurons following hypoxic and hypercapnic conditions (7). Recently, we showed that TPH2−/− pups experience prolonged apnea during AS and hypoventilate in both states (28). Increased glutamatergic drive to cardiac vagal neurons, subsequent to chronic hypoxia and hypercapnia, may therefore contribute to the increased vagal drive and bradycardia displayed by female 5-HT-deficient animals. Of course, this does not explain why there is increased vagal drive solely in females. There are relatively recent data suggesting that estrogens are capable of depolarizing cardiac vagal neurons in the nucleus ambiguus via G protein-bound estrogen receptors (2). As mentioned, serum estradiol concentrations may be higher in 2-wk-old female rats compared with age-matched males (8). Although speculative, it may be that a loss of inhibitory 5-HT1A signaling tips the balance toward estrogen-mediated excitation, increasing vagal nerve activity.
Unlike sympathetic drive to the vasculature, there was little influence of central 5-HT on sympathetic drive to the heart, given that the drop in HR following atenolol treatment was the same in TPH2−/− and WT pups. Following complete autonomic blockade, HR remained lower in both male and female TPH2−/− pups compared with WT, indicating reduced HRINT in TPH2−/− pups. That there is a small yet statistically significant correlation between HRINT and the body size of TPH2−/− pups implies that there are yet-to-be identified developmental factors that also contribute to the lower resting HR of TPH2−/− pups.
Perspectives and Significance
SIDS remains a leading cause of infant death and occurs more frequently in males than in females. Perhaps as high as ~70% of SIDS cases have at least one defect within the serotonergic system of the brain stem, including reduced 5-HT and expression of TPH2 (12). There is considerable evidence of autonomic and cardiovascular dysfunction in infants eventually succumbing to SIDS (18, 21–23, 25). These infants ultimately succumb during periods of sleep; in some cases, death follows overt cardiovascular collapse (18, 25). A better understanding of how 5-HT contributes to cardiovascular control during sleep is therefore important in our efforts to reduce SIDS incidence, including the potential for 5-HT to function differently in males than in females or between different states of vigilance.
Herein, we show that, in males, central 5-HT increases vascular tone to prevent a fall in ABP in quiet sleep (Fig. 5A). Furthermore, in females, our data suggest that central 5-HT dampens vagal drive in order to increase heart rate (Fig. 5B). Given that TPH2−/− rat pups are smaller than wild-type controls, it may be that altered development within the CNS or in peripheral tissues contributes to these phenotypes (17). However, we note that, at the same age, rat pups in which 5-HT neurons are acutely lesioned with 5,7-dihydroxytryptamine (a selective 5-HT neurotoxin) also have reduced ABP and HR. Thus, it seems likely that 5-HT operates physiologically to facilitate the maintenance of ABP and HR in infancy during sleep. Serotonergic dysfunction may therefore put male infants at risk during quiet sleep, especially in the presence of other factors that could lower vasomotor tone and/or ABP (e.g., severe hypoxia). Females may be at risk during both quiet and active sleep due to inappropriately elevated vagal drive and bradycardia.
Fig. 5.
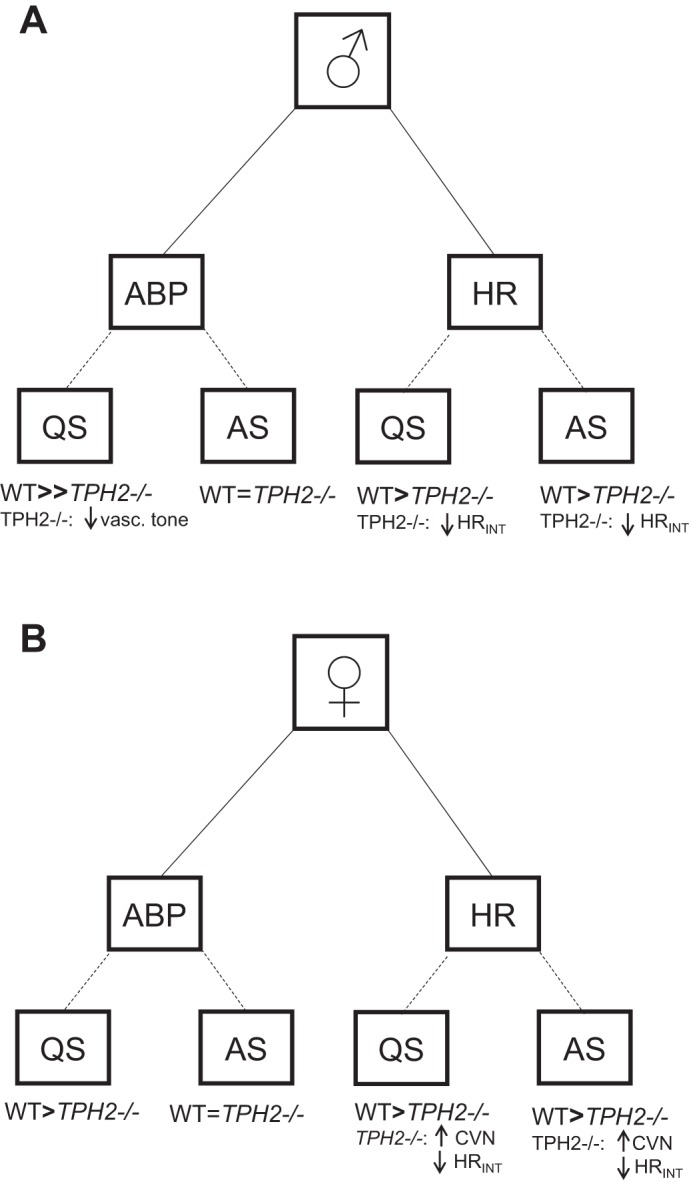
Summary of main findings. In both sexes, TPH2−/− pups have reduced arterial blood pressure (ABP) in quiet sleep (QS) but not in active sleep (AS). In males, but not females, central 5-hydroxytryptamine (5-HT) helps maintain ABP by increasing sympathetic vascular tone. Both male and female TPH2−/− pups have reduced heart rate (HR) compared with wild-type (WT) littermates, in part because of reduced intrinsic HR (HRINT). In both QS and AS, increased cardiac vagal nerve activity (CVN) contributes to the reduced HR of TPH2−/− females.
GRANTS
Funding for this work was provided by an American Heart Association Scientist Development Grant (14SDG18560022; principle investigator: K. J. Cummings) and an F31 Predoctoral Fellowship (1F31HL136067-01, J. Magnusson), University of Missouri College of Veterinary Medicine Faculty Research Grants (K. J. Cummings).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.M. and K.J.C. conceived and designed research; J.L.M. and K.J.C. performed experiments; J.L.M. and K.J.C. analyzed data; J.L.M. and K.J.C. interpreted results of experiments; J.L.M. and K.J.C. prepared figures; J.L.M. and K.J.C. drafted manuscript; J.L.M. and K.J.C. edited and revised manuscript; J.L.M. and K.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jane Chen for technical assistance and animal husbandry and Dr. Matthew Hodges (Medical College of Wisconsin) for providing the animal model and for helpful discussions throughout this study.
REFERENCES
- 1.Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boyé P, Vilianovitch L, Sohr R, Tenner K, Hörtnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106: 10332–10337, 2009. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brailoiu GC, Arterburn JB, Oprea TI, Chitravanshi VC, Brailoiu E. Bradycardic effects mediated by activation of G protein-coupled estrogen receptor in rat nucleus ambiguus. Exp Physiol 98: 679–691, 2013. doi: 10.1113/expphysiol.2012.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costarella CE, Stallone JN, Rutecki GW, Whittier FC. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther 277: 34–39, 1996. [PubMed] [Google Scholar]
- 4.Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruptions in rat pups with fewer brain stem 5-HT neurons. Am J Physiol Regul Integr Comp Physiol 296: R1783–R1796, 2009. doi: 10.1152/ajpregu.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonin-deficient Pet-1−/− mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol 298: R1333–R1342, 2010. doi: 10.1152/ajpregu.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darnall RA, Schneider RW, Tobia CM, Zemel BM. Arousal from sleep in response to intermittent hypoxia in rat pups is modulated by medullary raphe GABAergic mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R551–R560, 2012. doi: 10.1152/ajpregu.00506.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dergacheva O, Kamendi HW, Wang X, Mendelowitz D. 5HT1A receptors inhibit glutamate inputs to cardiac vagal neurons post-hypoxia/hypercapnia. Respir Physiol Neurobiol 179: 254–258, 2011. doi: 10.1016/j.resp.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology 97: 898–907, 1975. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 9.Hay M. Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin Sci (Lond) 130: 9–18, 2015. doi: 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan K, Echert AE, Massat B, Puissant MM, Palygin O, Geurts AM, Hodges MR. Chronic central serotonin depletion attenuates ventilation and body temperature in young but not adult Tph2 knockout rats. J Appl Physiol (1985) 120: 1070–1081, 2016. doi: 10.1152/japplphysiol.01015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol 4: 517–550, 2009. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med 361: 795–805, 2009. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kung LH, Scrogin KE. Serotonin nerve terminals in the dorsomedial medulla facilitate sympathetic and ventilatory responses to hemorrhage and peripheral chemoreflex activation. Am J Physiol Regul Integr Comp Physiol 301: R1367–R1379, 2011. doi: 10.1152/ajpregu.00576.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnusson J, Cummings KJ. Plasticity in breathing and arterial blood pressure following acute intermittent hypercapnic hypoxia in infant rat pups with a partial loss of 5-HT neurons. Am J Physiol Regul Integr Comp Physiol 309: R1273–R1284, 2015. [DOI] [PubMed] [Google Scholar]
- 15.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res 101: 569–575, 1976. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 16.Mortola JP, Naso L. Thermogenesis in newborn rats after prenatal or postnatal hypoxia. J Appl Physiol (1985) 85: 84–90, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Narboux-Nême N, Angenard G, Mosienko V, Klempin F, Pitychoutis PM, Deneris E, Bader M, Giros B, Alenina N, Gaspar P. Postnatal growth defects in mice with constitutive depletion of central serotonin. ACS Chem Neurosci 4: 171–181, 2013. doi: 10.1021/cn300165x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45: 350–354, 1999. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull 56: 425–439, 2001. doi: 10.1016/S0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- 20.Ramage AG, Daly MB. The central action of the 5-HT2 receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) on cardiac inotropy and vascular resistance in the anaesthetized cat. Br J Pharmacol 125: 1172–1179, 1998. doi: 10.1038/sj.bjp.0702183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep 11: 413–424, 1988. doi: 10.1093/sleep/11.5.413. [DOI] [PubMed] [Google Scholar]
- 22.Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Heart rate variation in normal infants and victims of the sudden infant death syndrome. Early Hum Dev 19: 167–181, 1989. doi: 10.1016/0378-3782(89)90077-7. [DOI] [PubMed] [Google Scholar]
- 23.Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP, Harper RM. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res 31: 606–612, 1992. doi: 10.1203/00006450-199206000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106-107: 1–16, 2013. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol 36: 113–122, 2003. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- 26.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557–618, 1981. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 27.Yang HT, Cummings KJ. Brain stem serotonin protects blood pressure in neonatal rats exposed to episodic anoxia. J Appl Physiol (1985) 115: 1733–1741, 2013. doi: 10.1152/japplphysiol.00970.2013. [DOI] [PubMed] [Google Scholar]
- 28.Young JO, Geurts AM, Hodges MR, Cummings KJ. Active sleep unmasks apnea and delayed arousal in infant rat pups lacking central serotonin. J Appl Physiol 123: 825–834, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]