Abstract
Environmental pollutants acting as endocrine-disrupting chemicals (EDCs) are recognized as potential contributors to metabolic disease pathogenesis. One such pollutant, arsenic, contaminates the drinking water of ~100 million people globally and has been associated with insulin resistance and diabetes in epidemiological studies. Despite these observations, the precise metabolic derangements induced by arsenic remain incompletely characterized. In the present study, the impact of arsenic on in vivo metabolic physiology was examined in 8-wk-old male C57BL/6J mice exposed to 50 mg/l inorganic arsenite in their drinking water for 8 wk. Glucose metabolism was assessed via in vivo metabolic testing, and feeding behavior was analyzed using indirect calorimetry in metabolic cages. Pancreatic islet composition was assessed via immunofluorescence microscopy. Arsenic-exposed mice exhibited impaired glucose tolerance compared with controls; however, no difference in peripheral insulin resistance was noted between groups. Instead, early insulin release during glucose challenge was attenuated relative to the rise in glycemia. Despite decreased insulin secretion, pancreatic β-cell mass was not altered, suggesting that arsenic primarily disrupts β-cell function. Finally, metabolic cage analyses revealed that arsenic exposure induced novel alterations in the diurnal rhythm of food intake and energy metabolism. Taken together, these data suggest that arsenic exposure impairs glucose tolerance through functional impairments in insulin secretion from β-cells rather than by augmenting peripheral insulin resistance. Further elucidation of the mechanisms underlying arsenic-induced behavioral and β-cell-specific metabolic disruptions will inform future intervention strategies to address this ubiquitous environmental contaminant and novel diabetes risk factor.
Keywords: arsenic, β-cell, diabetes, glucose intolerance, insulin
INTRODUCTION
Type 2 diabetes mellitus (T2DM) poses a growing burden to both individuals and healthcare systems worldwide (17a). Current estimates place the annual cost of diabetes at $245 billion in the United States alone (1). Because diabetes is the leading cause of adult blindness, kidney failure, and nontraumatic amputations as well as a potent driver of cardiovascular disease (3, 17a), understanding the factors that promote diabetes pathogenesis is crucially important. Sedentary lifestyles and unhealthy, destructive dietary consumption patterns are undoubted drivers of diabetes risk; however, these factors are not the only environmental threats to metabolic health (18). An area of increasing interest in recent years is the contribution of environmental pollutants acting as endocrine-disrupting chemicals (EDCs) to diabetes risk (16). The increasing body of evidence linking various pollutants to diabetes mandates investigation into the mechanisms of how EDCs alter normal energy physiology (14).
Arsenic is one such EDC that is listed by the World Health Organization (WHO) as a top 10 environmental contaminant of public health concern. Approximately 100,000,000 people worldwide are chronically exposed to unsafe levels of arsenic in their drinking water (30). Populations in areas of endemic arsenic contamination (e.g., Bangladesh, Taiwan) are exposed to drinking water arsenic levels that exceed the WHO-recommended upper limit (10 μg/l) by an order of magnitude or more (22, 39). A 2012 meta-analysis sponsored by the National Toxicology Program concluded that there exists limited-to-sufficient support for an association between arsenic and diabetes in populations with relatively high exposure levels (≥150 μg/l in drinking water) (20). Since the publication of this report, additional epidemiological studies have provided further evidence to support links between arsenic exposure and diabetes risk (21). Importantly, the conclusion of the National Toxicology Program report highlighted the need for greater understanding of the mechanisms linking arsenic exposure to metabolic dysfunction, a finding supported by a recent assessment of research gaps in the field (8).
In vitro and animal models have previously been used to understand the multitude of negative effects arising from arsenic exposure (10). In vitro studies using adipocyte cell lines have demonstrated that low-dose arsenite (As3+) attenuates insulin signaling (40), suggesting that arsenic may disrupt glucose homeostasis by interfering with insulin action in its target tissues. Additionally, in vitro studies employing β-cell model systems have suggested that arsenic may attenuate insulin secretion (12). Animal models of exposure have shown that rodents exposed to arsenic through drinking water exhibit impaired glucose homeostasis (25–27). However, the specific mechanisms by which arsenic exposure exerts these effects in vivo remain incompletely understood.
The present study was conducted to illuminate the relative contribution of the effects of arsenic on insulin action versus insulin secretion in whole body glucose homeostasis. Chronic, subtoxic arsenite ingestion in adult male mice promoted diabetogenic effects, including impaired glucose metabolism; however, systemic insulin action was unaffected. Instead, glucose-stimulated insulin release was attenuated, despite preservation of pancreatic islet endocrine cells, suggesting that arsenic contributes to diabetes pathogenesis primarily through functional impairments of insulin release as opposed to β-cell destruction.
MATERIALS AND METHODS
Animal care and arsenic exposure.
Seven- to eight-week-old male C57BL6/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in pairs under 12-h light-dark cycles at 22.2 ± 1.1°C. All animals received a chow diet (Teklad Global Diet 2018; Envigo, Madison, WI) ad libitum. The arsenic-exposed group was provided reverse-osmosis-purified bottled drinking water supplemented with 50 mg/l sodium arsenite (As3+) (Sigma Aldrich, St. Louis, MO). The control group received the same water without arsenite supplementation. Body weight, food consumption, and water intake were measured weekly throughout the studies. Animals were treated humanely in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Tissue harvest and preparation.
After 8 wk of exposure, animals were fasted for 5–6 h and then euthanized using isoflurane anesthesia followed by exsanguination via cardiac puncture. Tissues were dissected, weighed, and flash frozen in liquid nitrogen. Tissue samples were stored at −80°C until the time of processing.
Sample preparation and analysis of murine livers for arsenic content.
Mouse liver samples were weighed and predigested overnight with 1.5 ml of concentrated nitric acid (HNO3). Another 1.5 ml of HNO3 were added to each sample bringing the total acid volume to 3 ml. The samples were digested with microwave-assisted heating (MARS V; CEM, Matthews, NC), but without pressurizing them, then allowed to cool to room temperature, and diluted to 10 ml with double-deionized (>18 MΩ·cm) water. Digested liver samples, calibration standards, and several digested reference materials were diluted 1 + 24 with a reagent containing a gallium internal standard, 0.005% TX-100, and 2% HNO3 and analyzed for total As on a PE ELAN DRC II Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) (PerkinElmer, Shelton, CT). The ICP-MS instrument was operated in dynamic reaction cell (DRC) mode with a mixture of 10% (vol/vol) H2 gas in argon to eliminate a polyatomic interference (40Ar35Cl) at the same mass-to-charge ratio as 75As. The ICP-MS method limit of detection for As in liver was 0.12 μg/g, while method repeatability (%relative standard deviation) was 4.1%. Levels below the method limit of detection were assigned a value equal to the limit of detection divided by the square root of two (0.12 μg/g ÷ 1.41 = 0.08 μg/g). Validation for As in liver was established using NRC Certified Reference Material TORT 3-Lobster Hepatopancreas (National Research Council, Ontario, Canada) and New York State Caprine Liver Reference Materials, G99-3 and G99-14 (New York State Dept. of Health; Wadsworth Center, Albany, NY).
Serum collection, preparation, and analysis.
At the time of death, exsanguination via cardiac puncture was performed to collect blood. Whole blood was collected in microfuge tubes, allowed to clot at room temperature for 30 min, and then centrifuged at 1,500 g for 15 min to collect serum. Commercially available assays for blood urea nitrogen (BUN; ab83362; Abcam, Cambridge, UK) and creatinine (ab65340; Abcam) were performed as per the manufacturer’s instructions.
Intraperitoneal glucose tolerance tests.
After 8 wk of exposure, mice were fasted for 6 h, at which point baseline fasting blood glucose readings were obtained from all mice via tail vein sampling after application of local anesthetic (2% viscous lidocaine; Water-Jel, Carlstadt, NJ). Dextrose was injected intraperitoneally at a concentration of 2 g/kg body wt. Blood glucose levels were measured at 10, 20, 30, 40, 60, 90, and 120 min following injection using a Freestyle Lite glucometer (Abbott Laboratories, Abbott Park, IL). The mean absolute relative difference between blood glucose measurements for the Freestyle Lite glucometer is 4.9% (35). Blood samples were collected from the tail vein into heparinized microtainer tubes (Sarstedt, Numbrecht, Germany) at 0, 10, 30, and 60 min for determination of insulin concentrations. Tubes were placed immediately on ice and centrifuged at 1,500 g for 15 min at 4°C, and plasma was collected. Plasma insulin concentrations were measured using the Mouse Ultrasensitive Insulin ELISA Kit according to the manufacturer’s instructions (ALPCO, Salem, NH). The sensitivity of the ELISA was determined by the manufacturer to be 0.115 ng/ml for the 5-μl sample size, and the intra-assay coefficient of variation was determined to be between 4.53 and 9.30%. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using fasting blood glucose and fasting plasma insulin levels as previously described (19).
Intraperitoneal insulin tolerance tests.
After 8 wk of exposure, mice were fasted for 3 h, and fasting blood glucose levels were measured via tail vein sampling after application of local anesthetic. Mice were then injected intraperitoneally with Humalog insulin (0.5 U/kg body wt; Eli Lilly, Indianapolis, IN). Serial blood glucose readings were taken at 15, 30, 45, 60, 90, and 120 min after injection.
Pancreatic histology and immunohistochemistry.
At the time of terminal harvest, the pancreas was dissected, weighed, and fixed in 4% paraformaldehyde overnight and paraffin embedded. Tissue sections (5 μm in thickness) were immunostained with the following primary antibodies (all at 1:500 dilution): polyclonal guinea pig anti-porcine insulin (DAKO, Carpinteria, CA), mouse monoclonal anti-human glucagon (Sigma-Aldrich), polyclonal goat anti-somatostatin (Santa Cruz Biotechnology, Santa Cruz, CA), and DAPI (Invitrogen, Carlsbad, CA). The primary antibodies were detected using a combination of DyLight 488-, 549-, and 649-conjugated secondary antibodies (1:200, Jackson Immuno Research Laboratory, West Grove, PA). Antibodies used for these studies have been previously validated (28, 29).
Image capture and islet quantification.
As previously described (28, 29), microscopic images of pancreatic sections were taken with an Olympus IX8 DSU spinning disk confocal microscope (Melville, NY) with Stereo Investigator imaging software (SI; Micro Bright Field, Williston, VT). A modified method of “virtual slice capture” was used. Quantification of cellular composition (i.e., each area of β-, α-, and δ-cell populations, and islet area by automated contouring of each islet) was carried out using custom-written scripts for Fiji/ImageJ (https://rsbweb.nih.gov/ij/). MATLAB (MathWorks, Natick, MA) was used for mathematical analyses. Pancreatic endocrine cell and total islet masses were calculated by multiplying cellular or total islet area by pancreas mass.
Histopathological review of pancreatic sections.
Five micrometer-thick hematoxylin and eosin-stained sections from harvested pancreatic tissue were reviewed by a clinical pathologist on an Olympus BX41 microscope at ×40, ×100, ×200, and ×400 original magnifications (OMs). All islets and ducts were examined for histologic changes, particularly lymphocytic inflammatory infiltrates. Inflammatory infiltration was scored systematically in all visible islets for each histological section. The exocrine parenchyma was examined at ×40 OM entirely and was additionally reviewed to quantify mitotic figures in acinar cells in 10 high-power fields at ×400 OM.
Metabolic cage housing and energy expenditure measurements.
After 8 wk of exposure, indirect calorimetric measurements were carried out using the LabMaster System (TSE Systems, Chesterfield, MO) on individually housed mice and maintained under otherwise standard housing conditions (12-h light-dark cycle; 22.2 ± 1.1°C). Mice were provided ad libitum access to food and water with arsenic exposure continuing for the duration of analyses. After a 2-day acclimation period, O2 consumption, CO2 production, energy expenditure, locomotor activity (x–y-axis movement activity and z-axis rearing activity), as well as food and water consumption, were monitored over 30-min periods for 5–6 consecutive light-dark cycles over 3 successive days. The respiratory exchange ratio (RER) was calculated as the ratio of O2 consumption to CO2 production over 30-min periods. These values were then averaged for each mouse during each 12-h cycle.
Overall approach to understanding arsenic-induced metabolic dysfunction.
To ascertain the effects of arsenic on metabolic function, adult male C57BL/6J mice were exposed to arsenic for 8 wk. To accommodate the various complimentary measures of energy homeostasis assessed herein, four separate cohorts of mice were studied as outlined below. Weight gain as well as food and water intake were monitored throughout the exposure period (cohort 1). After 8 wk of exposure, glucose tolerance was assessed (cohorts 1 and 2). To determine whether alterations in glucose tolerance were due to insulin resistance, systemic insulin sensitivity was assessed by intraperitoneal insulin tolerance tests (IP-ITTs; cohort 3). Conversely, effects of arsenic on insulin secretion that could contribute to glucose intolerance were assessed by measuring insulin secretion during intraperitoneal glucose tolerance tests (IP-GTTs; cohort 1). Indirect calorimetry was used to assess effects on global energy handling, including shifts in circadian rhythms (cohort 4). To determine whether reduced insulin secretion was due to disruption of pancreatic islets, loss of insulin-secreting β-cells, or immune infiltration of the pancreas, histological examination of pancreata was conducted (cohort 1).
Statistical analyses.
Unless otherwise noted, data are presented as means ± SE. For glucose and insulin tolerance tests, area-under-the-curve of blood glucose over time was calculated using the trapezoidal rule. Statistical significance was tested using two-tailed, unpaired Student’s t-tests to compare control and arsenic-exposed groups, unless otherwise noted. F-testing was applied to account for differences in variance; for F < 0.05, t-tests were performed with Welch’s correction, and when F > 0.05, standard Student’s t-tests were performed. One-way ANOVA with repeated measures was used when comparing experimental groups over time. All statistical analyses were performed using GraphPad Prism, version 6.0. P < 0.05 was considered statistically significant for all experiments.
RESULTS
Chronic ingestion of arsenic does not alter weight gain or total food consumption.
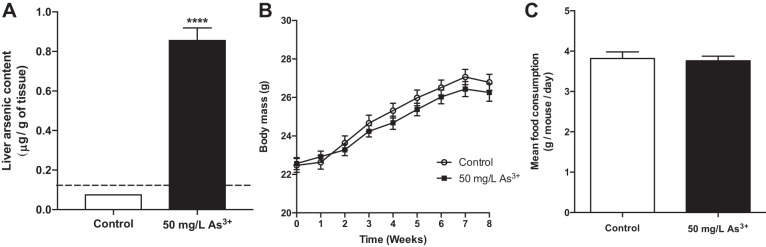
The in vivo effects of chronic arsenic exposure on energy metabolism were studied using a modified paradigm from previously published studies (25). To facilitate comparisons with existing literature, hepatic arsenic content was quantified by ICP-MS to provide an internal measure of exposure. While every sample from the control group fell below the limit of detection, mice in the arsenic-exposed group had liver arsenic levels that averaged 0.86 μg/g (Fig. 1A), which approximates levels observed in previous mouse studies (24) and is below levels observed in human livers from a highly exposed population (15). At this level of exposure, no significant differences in body weight were found between the control and arsenic-exposed groups throughout the study nor were there differences in final weight at the time of sacrifice (Fig. 1B). Additionally, there was no difference in total food consumption between the two groups (Fig. 1C).
Fig. 1.
Arsenic exposure does not affect weight gain or food consumption. A: quantification of total internal arsenic levels as measured in liver samples. Samples below the method detection limit (dashed line) assigned as described in materials and methods; n = 10 mice per group. B: total body weight measured weekly over the course of the study and (C) per mouse average total food consumption measured over the course of the study; n = 16 mice per group (cohort 1). Data are means ± SE; ****P < 0.0001.
Arsenic reduces water intake but does not cause significant dehydration.
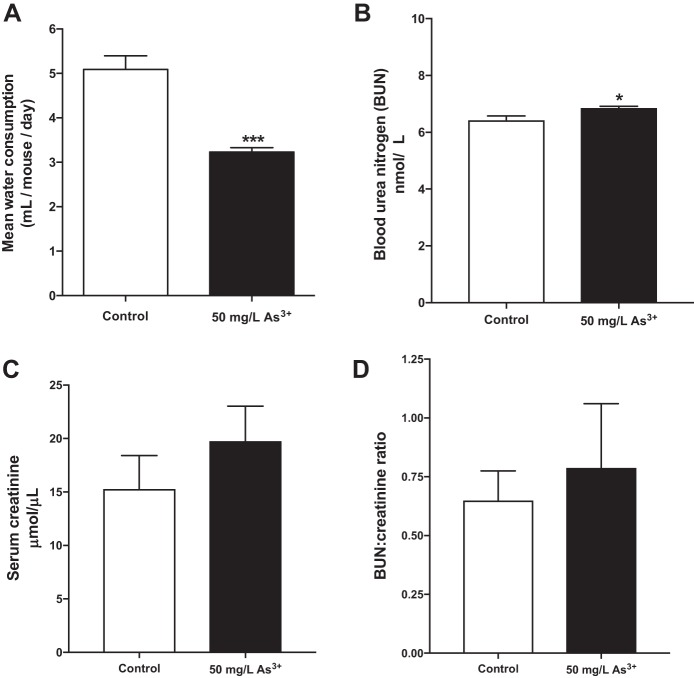
As noted in prior studies, water consumption was significantly lower for the arsenic-exposed animals in the present study (Fig. 2A), with animals in the arsenic-exposed group consuming 37% less water on average. This result is consistent with results from previous rodent models using similar exposures (27). No outward signs of dehydration in the mice were observed, and the water intake for arsenic-exposed mice remained within the normal range of daily water intake for rodents of this strain (4). To specifically address hydration status in the mice, BUN and serum creatinine levels were quantified. Arsenic exposure raised BUN levels by a modest 6.7% (Fig. 2B, P = 0.01); however, serum creatinine (Fig. 2C) and the BUN-to-creatinine ratio (Fig. 2D) were unaffected by arsenic exposure (P = 0.33 and P = 0.65, respectively). These data suggest that reduced water intake did not lead to significant dehydration in arsenic-exposed mice.
Fig. 2.
Mice exposed to arsenic exhibit decreased water intake but do not display marked differences in serum hydration measures. A: per mouse average water consumption measured over the course of the study; n = 16 mice per group. B: blood urea nitrogen (BUN) levels. C: serum creatinine levels. D: BUN-to-creatinine ratio; n = 13 mice per group (cohort 1). Data are means ± SE; *P < 0.05, ***P < 0.001.
Arsenic exposure impairs glucose tolerance but does not impact whole body insulin sensitivity.
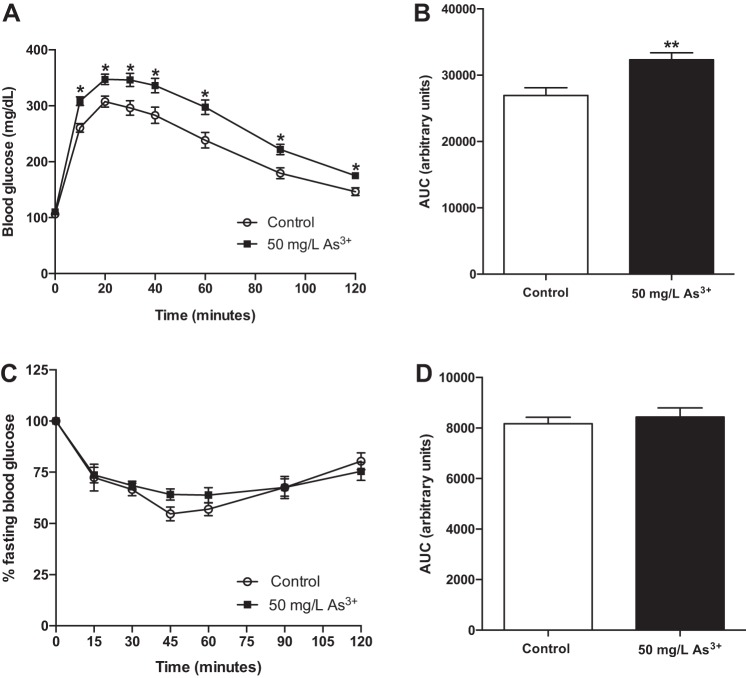
Based on dynamic measures of glucose homeostasis using IP-GTTs, arsenic-exposed mice exhibited clear glucose intolerance following 8 wk of exposure (Fig. 3A), with a 20% increase in glucose area under the curve in arsenic-exposed animals compared with controls (Fig. 3B). Despite observed glucose intolerance, arsenic did not significantly alter global insulin sensitivity as assessed by IP-ITTs (Fig. 3, C and D), suggesting that systemic insulin resistance was not the primary mechanism of arsenic-induced glucose intolerance in this model.
Fig. 3.
Arsenic exposure impairs glucose tolerance but does not alter insulin sensitivity. A: intraperitoneal glucose tolerance tests (IP-GTTs) performed after 8 wk of exposure (n = 24–26 mice per group; cohorts 1 and 2). B: area-under-the-curve (AUC) from the IP-GTT. C: intraperitoneal insulin tolerance tests (IP-ITTs) performed after 8 wk of exposure using separate cohorts of mice (n = 9–12 mice per group; cohort 3). D: AUC from the IP-ITT. Data are means ± SE; *P < 0.05, **P < 0.01.
Arsenic dysregulates steady-state glucose homeostasis.
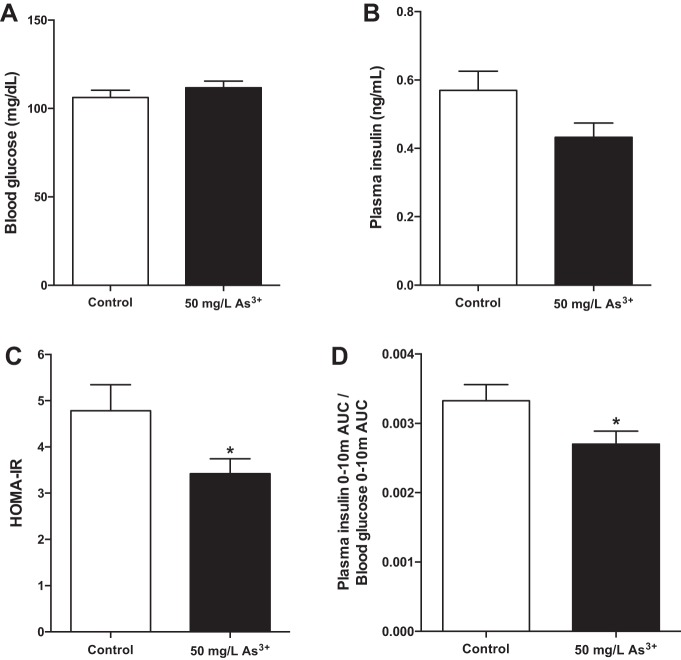
To determine the effect of arsenic exposure on steady-state glucose homeostasis, measures of fasting glucose homeostasis were calculated. After a 6-h fast, arsenic-exposed mice showed no significant difference in fasting glucose compared with control mice (Fig. 4A) with a near-significant reduction in fasting plasma insulin levels (Fig. 4B, P = 0.06), leading to a statistically significant 28% decrease in HOMA-IR (Fig. 4C, P < 0.05). Although a putative index of insulin resistance, evidence suggests that the HOMA-IR fails in states of β-cell decompensation (7). With evidence that arsenic exposure does not alter system insulin sensitivity (Fig. 3, C and D), the reduction in HOMA-IR suggests that the primary defect leading to arsenic-induced glucose intolerance is likely related to impaired insulin secretion from pancreatic β-cells.
Fig. 4.
Arsenic exposure induces metabolic dysfunction. A: fasting glucose after a 6-h fast. B: fasting insulin collected from peripheral blood after a 6-h fast. C: homeostatic model assessment of insulin resistance (HOMA-IR) values calculated using fasting blood glucose and fasting plasma insulin measures. D: change in insulin (AUC) relative to the change in blood glucose (AUC) from 0 to 10 min during an IP-GTT; n = 14–16 mice per group (cohort 1). Data are means ± SE; *P < 0.05.
Arsenic exposure impairs insulin release in response to a glucose challenge.
Relative to the rise in blood glucose induced by intraperitoneal glucose, insulin release over the first 10 min of the IP-GTT was reduced in the arsenic-exposed group by 19% (Fig. 4D). This suggests a specific impairment in first-phase insulin release under conditions of arsenic exposure, as plasma insulin measured at later time points over the course of the IP-GTT was unchanged (data not shown). Collectively, these data suggest that defective insulin secretion, rather than peripheral insulin resistance, drives arsenic-induced glucose tolerance.
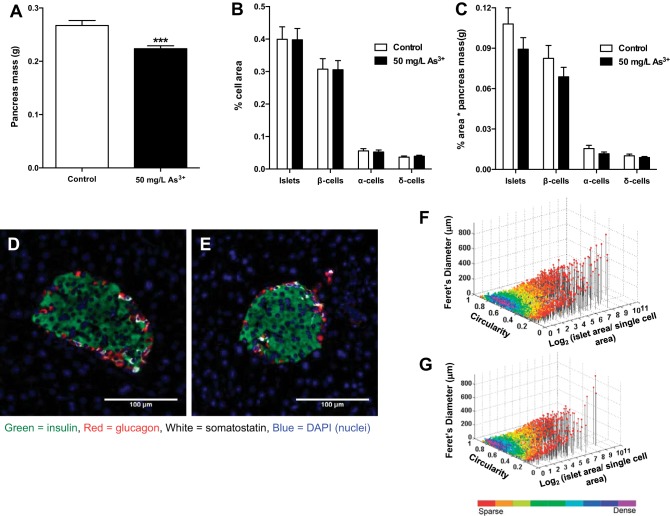
Arsenic does not alter relative pancreatic islet endocrine cell area.
Interestingly, the total pancreas weight of arsenic-exposed mice was significantly lower than that of control mice (Fig. 5A). To determine possible direct adverse effects of arsenic on the endocrine pancreas, the pancreatic endocrine cell/islet area was quantitatively analyzed in the whole pancreas. No differences were observed in β-cell, α-cell, δ-cell, or total islet area or mass between the groups (Fig. 5, B–E). Furthermore, there were no differences in islet cell composition, islet size distribution, or cellular composition (Fig. 5, F and G), suggesting that the effect of arsenic exposure on glucose intolerance is not the result of a loss of β-cells/islets. To discern whether or not inflammation or immune cell infiltration may be driving the observed insulin secretion phenotype, histological sections were scored by a pathologist for evidence of inflammation, both in the endocrine islets and in the exocrine acinar cells. No infiltration of immune cells was appreciated in or around any of the islets reviewed for any section in either exposure group. Similarly, a systematic review of inflammatory infiltration in the exocrine pancreas revealed no significant differences in inflammation between groups, suggesting that immune infiltration was not a major driver of the observed insulin secretory defect. Furthermore, there was no histological evidence of acinar death that would explain the loss of pancreatic mass.
Fig. 5.
Arsenic exposure does not affect islet morphology or endocrine cell composition. A: pancreas mass measured immediately after each mouse was euthanized. B: histological sections of pancreas from each mouse were immunostained, and endocrine cell area [β-cell, α-cell, δ-cell, and islet (additive)] was calculated relative to total area analyzed. C: islet mass as measured by %cell area multiplied by pancreas mass for each animal. D: representative image of a single islet from control mouse. E: representative image of a single islet from arsenic-exposed mouse. F: scatter plot of individual islet morphology from all control mouse slides. G: scatter plot of individual islet morphology from all arsenic-exposed mouse slides; n = 16 slides per group (cohort 1). Each slide is representative of 1 individual mouse. Data are means ± SE; ***P < 0.001.
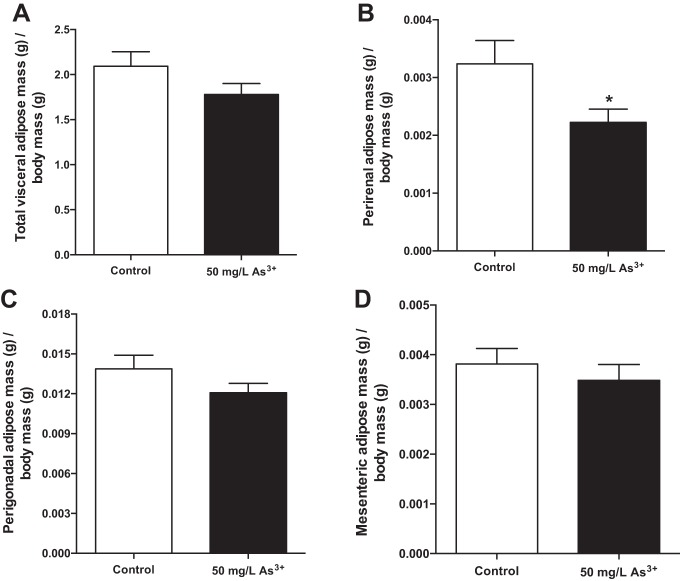
Arsenic exposure alters visceral adiposity in a depot-specific manner.
Both adipose tissue mass and its distribution across different anatomical depots (e.g., visceral vs. subcutaneous) have profound effects on whole body energy metabolism, glucose handling, and insulin sensitivity (18). After normalization for body weight, a trend toward decreased total visceral adiposity in arsenic-exposed mice was observed compared with controls (Fig. 6A, P = 0.13). In particular, relative perirenal adipose tissue mass decreased 31% in arsenic-exposed mice (Fig. 6B, P < 0.05). The perigonadal (Fig. 6C) and mesenteric (Fig. 6D) adipose tissue masses did not differ significantly between the groups (P = 0.16 and P = 0.47, respectively).
Fig. 6.
Arsenic exposure decreases visceral adiposity. Visceral adipose depots were collected upon euthanasia, and adipose depot weight was normalized to body weight for each individual mouse for total visceral adiposity (A) and its components, perigonadal (B), perirenal (C), and mesenteric (D) depots; n = 16 mice per group (cohort 1). Data are means ± SE; *P < 0.05.
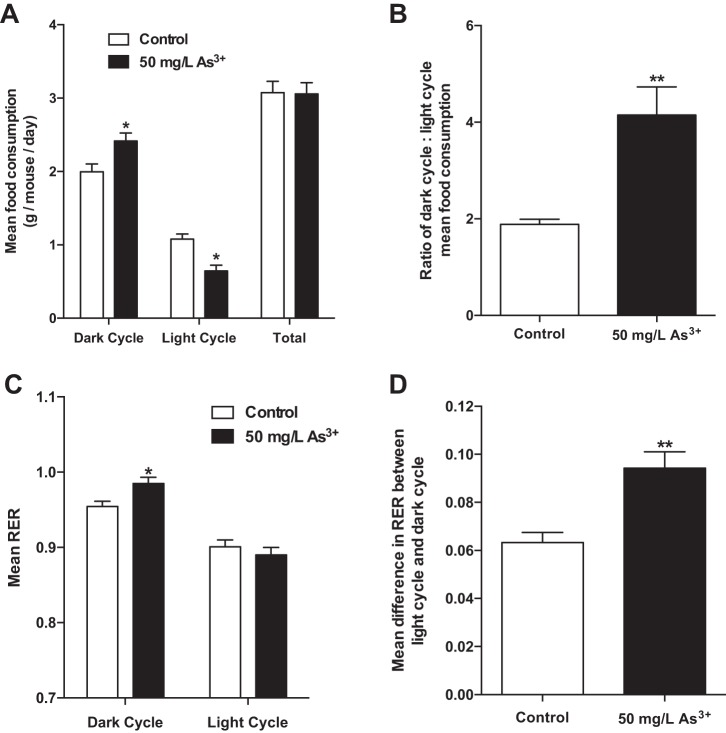
Arsenic exposure alters behavioral and metabolic circadian rhythms.
Arsenic exposure markedly increased the fraction of food consumed during the normal feeding dark cycle without altering total food intake (Fig. 7, A and B). Indirect calorimetry measurements showed differences in energy utilization between the two groups. Specifically, arsenic exposure significantly increased the RER during the dark cycle (Fig. 7C). Notably, no differences in locomotor activity (x–y-axis movement) were observed between the two groups (data not shown). Furthermore, arsenic exposure increased the difference in RER between the light and dark periods (Fig. 7D). These results demonstrate that arsenic disrupts the circadian rhythmicity of key metabolic parameters in exposed mice.
Fig. 7.
Arsenic exposure alters metabolic circadian rhythm. A: mean food consumption was calculated for 2–3 alternating 12-h light-dark cycles. B: mean ratio of dark cycle-to-light cycle food consumption. C: mean respiratory exchange ratio (RER) measured within 12-h light-dark cycles. D: mean change in 12-h RER between successive light and dark cycles; n = 6 mice per group (cohort 4). Data are means ± SE; *P < 0.05, **P < 0.01.
DISCUSSION
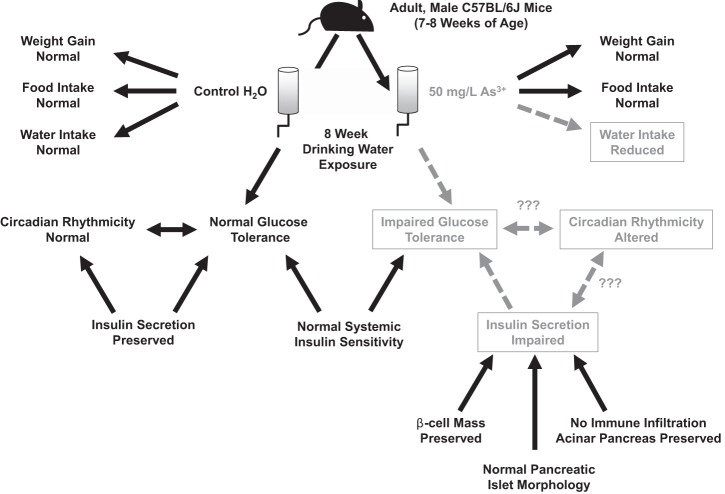
Arsenic exposure remains an important threat to human health and an underappreciated contributor to the current epidemic of diabetes. This mouse model of chronic arsenic exposure provided additional insights into the links between arsenic exposure and metabolic dysfunction (Fig. 8). Despite normal patterns of total food intake and weight gain, arsenic exposure impaired glucose tolerance following 8 wk of exposure without effects on systemic insulin sensitivity. Metabolic cage analyses further support the hypothesis that arsenic does not promote hyperglycemia via the induction of insulin resistance, as arsenic-exposed mice exhibited an increase in RER rather than the typical decrease observed in insulin-resistant states (36). Rather, insulin secretion was decreased during glucose challenge, suggesting that the primary cause of arsenic-induced hyperglycemia is due to an insulin secretion defect. Importantly, these disruptions do not appear to arise from a diminution of β-cell mass or alteration in islet morphology but likely arise from defects in β-cell function. Furthermore, these studies revealed novel alterations in circadian rhythms of energy metabolism induced by drinking water arsenic exposure, thereby expanding upon previously published work.
Fig. 8.
Schematic of experimental design and summary of interrelated results. Abnormalities induced by arsenic exposure shown in gray with dashed lines, whereas key findings that were unaltered with arsenic exposure are shown in black with solid lines.
In the present studies, the metabolic impact of arsenic was studied using exposure to sodium arsenite (As3+) via drinking water. The exposure paradigm used herein was modeled on previously published work that demonstrated that mice exposed to 50 mg/l arsenite had internal levels comparable to those in highly exposed human populations (15, 26, 32) and utilized the most relevant species of arsenic for human exposure (33). Importantly, rodents are generally more resistant to the toxic effects of arsenic (26); therefore, higher concentrations are required to study its biological effects in murine models. Importantly, however, hepatic arsenic concentrations in the present model were lower than those reported in a highly exposed human population (15). Consistent with prior studies, mice exposed to arsenic-supplemented water in the present study consumed less water than controls (27), effectively lowering the exposure relative to drinking water measurements. While mice did not display marked signs of dehydration, whether decreased water intake per se impacts the observed metabolic phenotype remains to be determined.
The data presented support the conclusion that the mechanisms of arsenic-induced glucose intolerance differ from those underlying typical T2DM. In the present study, steady-state measures of insulin resistance in the fasting state (i.e., HOMA-IR) indicated that arsenic does not promote insulin resistance. The observed reduction in HOMA-IR is in agreement with epidemiological evidence showing that greater arsenic exposure in human populations negatively correlates with HOMA-IR (9). Rather than a typical T2DM model in which glycemic control deteriorates due to an initial loss of insulin sensitivity, arsenic likely induces glucose intolerance via a deficiency in insulin release (18). Indeed, impairments in insulin secretion from β-cells were confirmed during IP-GTTs during which early, first-phase insulin release was reduced relative to the rise in blood glucose. Additionally, evidence that arsenic exposure tends to reduce rather than increase adiposity, an effect in agreement with prior studies (27), argues against a model of obesity-driven metabolic deterioration. Rather arsenic appears to promote a metabolically dysfunctional state similar to monogenic forms of diabetes that are characterized by primary impairments in β-cells or early type 1 diabetes (T1DM) in which insulin secretory capacity is reduced. However, unlike T1DM, arsenic exposure does not appear to promote dysglycemia via immune destruction of pancreatic β-cells.
In the present study, impaired insulin release and subsequent glucose intolerance were not due to overt changes in the relative area or composition of islet endocrine cells (β-cells, α-cells, or δ-cells). These data suggest that arsenic induces glucose intolerance through a disruption in β-cell function that alters normal stimulus-secretion coupling rather than a depletion of β-cell mass. In support of this hypothesis, a prior study using a cellular model of exposure demonstrated that arsenic impaired glucose-stimulated insulin secretion in a dose- and time-dependent manner (12). Similarly, a study using isolated murine pancreatic islets found that exposure to subtoxic concentrations of trivalent arsenic species inhibited glucose-stimulated insulin secretion (11). Importantly, while β-cell mass was not altered by arsenic exposure in our model, total pancreatic weight was reduced by ~16%. Since endocrine cell mass and total body weight were unchanged, this suggests that arsenic exposure may specifically reduce exocrine pancreatic mass; however, no histological evidence of acinar cell death was observed. Interestingly, in models of T1DM in which diabetes arises from selective destruction of β-cells, pancreatic mass has similarly been shown to be reduced, possibly secondary to the loss of the trophic effects of insulin on the acinar pancreas (6). While this does not exclude the possibility that arsenic is directly toxic to the exocrine pancreas, the current data are consistent with an induction of β-cell physiological dysfunction as the primary defect in arsenic-induced metabolic dysfunction.
Classically, arsenic has been characterized as a metabolic toxin based on its ability to inhibit intermediary metabolism at different points depending on the species under investigation (10); this includes classical evidence demonstrating inhibition of pyruvate dehydrogenase (PDH) (37). In the current model, indirect calorimetry data demonstrated an increase in RER during the dark (feeding) phase and an accentuation of metabolic flexibility across fed-fasted transitions. These data suggest the preferential utilization of carbohydrates for fuel during the fed state, an outcome likely to impact global metabolic parameters given prior studies in rodent models of diabetes (5). This provides indirect evidence that arsenic exposure in the present model does not globally impair PDH, which is predicted to reduce the efficiency of carbohydrate metabolism (23); however, tissue-specific impairments in PDH remain possible. Indeed, the current data are in agreement with studies showing that β-cell-specific ablation of PDH results in glucose intolerance with concomitant hypoinsulinemia and reduced glucose-stimulated insulin secretion (31). This raises the possibility that arsenic may have tissue-specific effects on PDH function that are not observed globally, potentially due to selective enrichment of arsenic in β-cells. Further work is required to understand the precise mechanisms by which arsenic can affect macronutrient utilization in both fed and fasted states.
While the present studies provide critical validation of earlier models of arsenic-induced glucose intolerance and shed new light on the metabolic defects that give rise to this disruption in energy homeostasis, there remain several limitations. Importantly, the dose dependence of arsenic exposure was not assessed, and the impact of other arsenic species was not determined. Given that human populations are exposed to a multitude of arsenic species, future studies should utilize environmentally relevant mixtures of arsenicals (8). The current studies were also limited to male mice. Additional studies in female mice will be critical to better understand the impact of arsenic on diabetes risk in human populations given evidence that estrogen may modulate arsenic toxicity (17). Despite these limitations, the findings presented herein provide critical additional support to the hypothesis that chronic arsenic ingestion augments diabetes risk.
Perspectives and Significance
Millions of people worldwide currently consume arsenic-contaminated drinking water that exceeds WHO safety limits, and arsenic contamination of groundwater remains prevalent in the United States (38). Moreover, given recent evidence of additional arsenic ingestion from grains such as rice (34) and previously unappreciated exposures in populations such as in East Chicago, Indiana (13), the metabolic risk imposed by arsenic is likely underestimated. To better appreciate this risk, future studies should explore arsenic’s effects on hepatic and lipid metabolism, potential synergy with other physiological stressors (e.g., caloric excess), and the molecular pathways by which arsenic disrupts β-cell stimulus-secretion coupling. Such insights will be critical for designing strategies to mitigate the deleterious effects of this ubiquitous pollutant and novel diabetes risk factor.
GRANTS
This work was supported by the National Institutes of Health Grants R21-ES-021354 and P30-ES-027792 (to R. M. Sargis), T32-HL-007009 (to S. M. Regnier), and P60-DK-020595 (to the University of Chicago Diabetes Research and Training Center). Support was also provided by a Junior Faculty Development Award from the American Diabetes Association (1-17-JDF-033 to R. M. Sargis). C. M. Carmean is supported as an Overseas Researcher by a Postdoctoral Fellowship from the Japan Society for the Promotion of Science. Institutional support from the University of Chicago (to R. M. Sargis) is gratefully acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.G.K., C.M.C., P.J.P., and R.M.S. conceived and designed research; A.G.K., D.R., H.Y., S.M.R., A.P., W.K., and A.A.R. performed experiments; A.G.K., C.M.C., A.P., M.H., D.N.J., A.A.R., P.J.P., S.S., and R.M.S. analyzed data; A.G.K., M.H., D.N.J., P.J.P., S.S., and R.M.S. interpreted results of experiments; A.G.K. and R.M.S. prepared figures; A.G.K. and R.M.S. drafted manuscript; A.G.K., C.M.C., M.H., D.N.J., P.J.P., and R.M.S. edited and revised manuscript; A.G.K., C.M.C., M.H., S.S., and R.M.S. approved final version of manuscript.
REFERENCES
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 36: 1033–1046, 2013. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of Medical Care in Diabetes–2016. American Diabetes Association. Diabetes Care 39, Suppl. 1: S52–S59, 2016.26696682 [Google Scholar]
- 4.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32: 435–443, 2002. doi: 10.1023/A:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab 297: E1197–E1204, 2009. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreto SG, Carati CJ, Toouli J, Saccone GT. The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol 299: G10–G22, 2010. doi: 10.1152/ajpgi.00077.2010. [DOI] [PubMed] [Google Scholar]
- 7.Bergman RN, Zaccaro DJ, Watanabe RM, Haffner SM, Saad MF, Norris JM, Wagenknecht LE, Hokanson JE, Rotter JI, Rich SS. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes 52: 2168–2174, 2003. doi: 10.2337/diabetes.52.8.2168. [DOI] [PubMed] [Google Scholar]
- 8.Carlin DJ, Naujokas MF, Bradham KD, Cowden J, Heacock M, Henry HF, Lee JS, Thomas DJ, Thompson C, Tokar EJ, Waalkes MP, Birnbaum LS, Suk WA. Arsenic and Environmental Health: State of the Science and Future Research Opportunities. Environ Health Perspect 124: 890–899, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Razo LM, García-Vargas GG, Valenzuela OL, Castellanos EH, Sánchez-Peña LC, Currier JM, Drobná Z, Loomis D, Stýblo M. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health 10: 73, 2011. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz-Villaseñor A, Burns AL, Hiriart M, Cebrián ME, Ostrosky-Wegman P. Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus. Toxicol Appl Pharmacol 225: 123–133, 2007. doi: 10.1016/j.taap.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Douillet C, Currier J, Saunders J, Bodnar WM, Matoušek T, Stýblo M. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol 267: 11–15, 2013. doi: 10.1016/j.taap.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, Sun G, Andersen ME, Pi J. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect 118: 864–870, 2010. doi: 10.1289/ehp.0901608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodnough A. Their soil toxic, 1,100 Indiana residents scramble to find new homes. The New York Times. August 31, 2016.
- 14.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36: E1–E150, 2015. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guha Mazumder DN. Arsenic and liver disease. J Indian Med Assoc 99: 311–320, 2001. [PubMed] [Google Scholar]
- 16.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Saal FV. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68: 3–33, 2016. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CF, Yang CY, Chan DC, Wang CC, Huang KH, Wu CC, Tsai KS, Yang RS, Liu SH. Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect 123: 1138–1144, 2015. doi: 10.1289/ehp.1408663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.International Diabetes Federation IDF Diabetes Atlas. International Diabetes Federation (7th ed.). Brussels, Belguim: International Diabetes Federation, 2015. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventh-edition.html. [Google Scholar]
- 18.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120: 1658–1670, 2012. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121: 295–302, 2013. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan WC, Seow WJ, Kile ML, Hoffman EB, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Lu Q, Christiani DC. Association of low to moderate levels of arsenic exposure with risk of type 2 diabetes in Bangladesh. Am J Epidemiol 178: 1563–1570, 2013. doi: 10.1093/aje/kwt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel KP, O’Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab 106: 385–394, 2012. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 119: 1104–1109, 2011. doi: 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul DS, Harmon AW, Devesa V, Thomas DJ, Stýblo M. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect 115: 734–742, 2007. doi: 10.1289/ehp.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dedina J, Matousek T, Stýblo M. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol 222: 305–314, 2007. doi: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 119: 1104–1109, 2011. doi: 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poudel A, Fowler JL, Zielinski MC, Kilimnik G, Hara M. Stereological analyses of the whole human pancreas. Sci Rep 6: 34049, 2016. doi: 10.1038/srep34049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poudel A, Savari O, Striegel DA, Periwal V, Taxy J, Millis JM, Witkowski P, Atkinson MA, Hara M. Beta-cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine 49: 693–702, 2015. doi: 10.1007/s12020-015-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smedley PL, Kinniburgh DG. United Nations Synthesis Report on Arsenic in Drinking Water. Geneva, Switzerland: World Health Organization, 2001. [Google Scholar]
- 31.Srinivasan M, Choi CS, Ghoshal P, Pliss L, Pandya JD, Hill D, Cline G, Patel MS. ß-Cell-specific pyruvate dehydrogenase deficiency impairs glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 299: E910–E917, 2010. doi: 10.1152/ajpendo.00339.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.States JC, Singh AV, Knudsen TB, Rouchka EC, Ngalame NO, Arteel GE, Piao Y, Ko MS. Prenatal arsenic exposure alters gene expression in the adult liver to a proinflammatory state contributing to accelerated atherosclerosis. PLoS One 7: e38713, 2012. doi: 10.1371/journal.pone.0038713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74: 289–299, 2000. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 34.Sun GX, Williams PN, Carey AM, Zhu YG, Deacon C, Raab A, Feldmann J, Islam RM, Meharg AA. Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ Sci Technol 42: 7542–7546, 2008. doi: 10.1021/es801238p. [DOI] [PubMed] [Google Scholar]
- 35.Tack C, Pohlmeier H, Behnke T, Schmid V, Grenningloh M, Forst T, Pfützner A. Accuracy evaluation of five blood glucose monitoring systems obtained from the pharmacy: a European multicenter study with 453 subjects. Diabetes Technol Ther 14: 330–337, 2012. doi: 10.1089/dia.2011.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2011. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol 197: 67–83, 2004. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Welch AH, Westjohn DB, Helsel DR, Wanty RB. Arsenic in ground water of the United States–occurrence and geochemistry. Ground Water 38: 589–604, 2000. doi: 10.1111/j.1745-6584.2000.tb00251.x. [DOI] [Google Scholar]
- 39.Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, Lee TC. Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environ Health Perspect 109: 1011–1017, 2001. doi: 10.1289/ehp.011091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue P, Hou Y, Zhang Q, Woods CG, Yarborough K, Liu H, Sun G, Andersen ME, Pi J. Prolonged inorganic arsenite exposure suppresses insulin-stimulated AKT S473 phosphorylation and glucose uptake in 3T3-L1 adipocytes: involvement of the adaptive antioxidant response. Biochem Biophys Res Commun 407: 360–365, 2011. doi: 10.1016/j.bbrc.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]