Abstract
Changes in the environment cause both short-term and long-term changes in an animal's behavior. Here we show that specific sensory experiences cause changes in chemosensory receptor gene expression that may alter sensory perception in the nematode Caenorhabditis elegans. Three predicted chemosensory receptor genes expressed in the ASI chemosensory neurons, srd-1, str-2, and str-3, are repressed by exposure to the dauer pheromone, a signal of crowding. Repression occurs at pheromone concentrations below those that induce formation of the alternative dauer larva stage, suggesting that exposure to pheromones can alter the chemosensory behaviors of non-dauer animals. In addition, ASI expression of srd-1, but not str-2 and str-3, is induced by sensory activity of the ASI neurons. Expression of two receptor genes is regulated by developmental entry into the dauer larva stage. srd-1 expression in ASI neurons is repressed in dauer larvae. str-2 expression in dauer animals is induced in the ASI neurons, but repressed in the AWC neurons. The ASI and AWC neurons remodel in the dauer stage, and these results suggest that their sensory specificity changes as well. We suggest that experience-dependent changes in chemosensory receptor gene expression may modify olfactory behaviors.
Animals modify their olfactory behaviors in response to short-term changes in their environment, and as part of hard-wired developmental programs. For example, Drosophila temporarily changes its preference for odors that have been paired with a repellent shock (1, 2). Drosophila also demonstrates olfactory preferences that differ from the larval to the adult stage (3, 4). Alterations in higher processing centers underlie some forms of olfactory plasticity, but the contributions of specific olfactory neurons and their receptors to short- and long-term behavioral modifications are unknown. The olfactory systems in flies, mice, and worms contain many different cell types that can be distinguished by the receptors they express (5–8). Unlike other sensory systems, the olfactory system uses receptors that are specialized for particular odor stimuli. Therefore, changes in receptor expression have the potential to alter the stimuli detected by the olfactory system and the perception of those stimuli.
The nematode Caenorhabditis elegans demonstrates behavioral plasticity in response to its environment and as part of a developmental program. C. elegans is exquisitely sensitive to chemical, thermal, and mechanical stimuli and alters its responses to those stimuli based on its experience (9). C. elegans also displays predictable behavioral changes through its lifetime. One distinct life period is the dauer larval phase, an alternative form of the normal third larval stage that is induced by high temperature, limited food, and high population density (10–12). The decision to make a dauer larva is initiated during the first larval stage, when animals assess population density by detecting dauer pheromone, an uncharacterized chemical cue constitutively secreted by all animals in the population (13, 14). The dauer stage is characterized by morphological changes, developmental arrest, extended lifespan, and resistance to harsh environmental conditions (15). Dauer larvae also display markedly different behavior patterns that allow them to disperse and seek out better conditions. When conditions improve, and particularly when pheromone levels drop, animals recover from the dauer stage and continue development to the adult stage.
Dauer pheromone and other chemosensory cues are detected by the bilaterally symmetric amphid sensory organs, which each contain twelve ciliated chemosensory and thermosensory neurons (8, 16). Each bilateral pair of chemosensory neurons has a characteristic function that is reproducible between animals. The ASI neurons have two distinct functions: they mediate chemotaxis to some water-soluble attractants, and also prevent entry into the dauer stage (8, 17). The ASI neurons prevent dauer entry by secreting the transforming growth factor (TGF)-β homolog DAF-7, which signals through the heteromeric TGF-β receptor complex of DAF-1 and DAF-4 (18–21). DAF-1 and DAF-4 are expressed by other neurons, and are thought to regulate gene expression and production of a second nonautonomous signal that acts on many tissues to result in anatomical and behavioral changes (15, 22–24). Cell ablation and genetic studies suggest that ASI has a basal level of DAF-7-secreting activity in the absence of sensory inputs (17). Dauer pheromone is thought to inhibit this basal activity, allowing dauer formation, whereas attractive food cues might antagonize pheromone action by stimulating ASI activity and DAF-7 release. Other sensory neurons, including ADF, ASG, and ASJ, also regulate dauer entry through mechanisms that are not as well understood, but may include secretion of insulin-like peptides (17, 21, 25).
Interestingly, the ASI sensory neurons themselves are remodeled in the dauer stage. In dauer larvae, the ciliated endings of the ASI neurons withdraw from the pore through which chemical stimuli are sensed, and the ASG, AFD, and AWC neurons also change their cilia morphologies (12). Dauer animals sense improved environmental conditions that allow them to resume normal development mostly by means of the ASJ sensory neurons (17). Their sensitivity to dauer pheromone changes over a prolonged period in the dauer stage, making recovery more likely after extended times (14).
C. elegans detects its chemical environment by using a large number of chemosensory receptor genes. The largest chemoreceptor gene family, the ODR-10 superfamily, consists of 831 genes and pseudogenes that encode predicted G protein-coupled, 7-transmembrane domain receptors (26, 27). This gene family is named for its founding member ODR-10, the receptor for the odorant diacetyl, which was identified in a genetic screen for olfaction-defective mutants (28). Several additional gene families are also considered candidate chemoreceptors based on their expression in chemosensory neurons and their sequences, leading to a total of over 1,000 candidate chemoreceptor genes (29, 30). After excluding those genes that are not expressed in sensory neurons or that appear to be pseudogenes, C. elegans may have 500 different chemosensory receptors. Individual chemosensory receptor genes are typically expressed in a small set of chemosensory neurons. For example, the odr-10 gene is expressed specifically in the AWA olfactory neurons, the str-1 gene is expressed specifically in the AWB olfactory neurons, and the str-2 gene is expressed strongly in one AWC olfactory neuron and weakly in both ASI neurons (28, 31, 32). To accommodate the vast repertoire of receptors, each C. elegans chemosensory neuron expresses many different receptor genes. The ASI neurons express the receptors sra-6, srd-1, str-2, str-3, and at least three other members of the odr-10 gene family.
The identification of the chemosensory receptors provides an opportunity to investigate their contribution to plasticity of olfactory behavior under different environmental conditions and at different developmental stages. Artificially altering expression of the odr-10 receptor by introducing it into AWB instead of AWA changes the behavioral response to diacetyl from attraction to repulsion (31). Thus, changes in receptor expression can have significant behavioral consequences. Here we describe regulated expression of three predicted chemosensory receptors in the ASI neurons, and find that they change their expression both in response to an environmental cue, dauer pheromone, and as a consequence of a developmental change, entry into the dauer stage. srd-1 is expressed in the ASI neurons of well fed animals, but is undetectable in pheromone-treated or dauer animals. str-2 is expressed mainly in AWC neurons in well fed adults, but only in ASI neurons in dauer animals. Expression of str-3 in the ASI neurons is high in both adult and dauer animals, but is lost in response to pheromone. Changes in the spatial pattern of chemosensory receptor gene expression may contribute to behavioral differences between sparse, crowded, and dauer animals.
Materials and Methods
Strains and Genetics.
Wild-type nematodes were C. elegans variety Bristol, strain N2 grown on plates under standard uncrowded and well fed conditions at 20°C unless otherwise noted (33). Promoter–green fluorescent protein (GFP) fusion genes were used to track chemosensory receptor expression. Integrated srd-1∷GFP I, str-2∷GFP X, and str-3∷GFP X fusion genes were examined for expression in the ASI neurons (29, 32, 34). An integrated daf-7∷GFP strain was generously provided by P. Ren and D. L. Riddle (20). Mutants included the following: che-3(e1124), daf-22(m130), egl-19(n582), egl-19(ad695), osm-6(p811), tax-2(p691), tax-4(p678), unc-2(e55), and unc-36(e251).
Analysis of Gene Expression.
Animals were examined as adults by using the 10× or 40× objective of an Axioplan II compound microscope (Zeiss). For quantitative analysis, animals were scored as GFP-positive if any GFP labeling was visible, regardless of apparent brightness. Statistical analysis was conducted by using PRIMER OF BIOSTATISTICS software (McGraw–Hill). Epifluorescence images were acquired by using an Axioplan II.
Pheromone Regulation of Gene Expression.
Dauer pheromone was prepared according to Golden and Riddle (14). To test the effects of pheromone on gene expression, 2 ml of nematode growth medium (NGM) agar, containing either 0 μl, 50 μl, or 100 μl of pheromone, was poured into 3-cm plates. Plates were allowed to dry overnight, then seeded with UV-killed concentrated OP50 bacteria. Adult srd-1∷GFP, str-2∷GFP, str-3∷GFP, or daf-7∷GFP animals were allowed to lay eggs onto the plates at 25° for 3–5 h, yielding ≈80 eggs per plate. The adults were then removed and the plates incubated at 25°C. Larval animals were removed at each stage for examination of GFP expression. Larvae that did not express a GFP fusion gene were transferred to nonpheromone plates and scored for GFP expression 24 and 48 h later. Adult animals grown in the presence of pheromone for their entire lifespan were also examined for GFP expression.
Experiments mixing daf-22 mutants and wild-type animals were performed as follows. For daf-22-alone plates, four daf-22; str-2∷GFP L4 animals were placed on a seeded 6-cm NGM agar plate and allowed to lay eggs, and 4 days later their progeny were examined as adults for str-2 expression in the ASI neurons. For daf-22 mutants mixed with wild-type animals, two daf-22; str-2∷GFP L4 and two dpy-20 L4 animals were placed on a seeded 6-cm NGM agar plate and allowed to lay eggs, and four days later the daf-22; str-2∷GFP adult progeny were examined for ASI str-2∷GFP expression. For both experiments several hundred animals developed on a single plate (moderate density). Under these conditions, expression of srd-1 or str-3 in wild-type animals was high. Under conditions in which several thousand animals were grown on one 6-cm NGM agar plate, expression of srd-1, str-3, and str-2 was repressed in ASI neurons in wild-type animals (high density/crowding).
Results
srd-1 Expression and str-2 Expression Are Altered in the Dauer Stage.
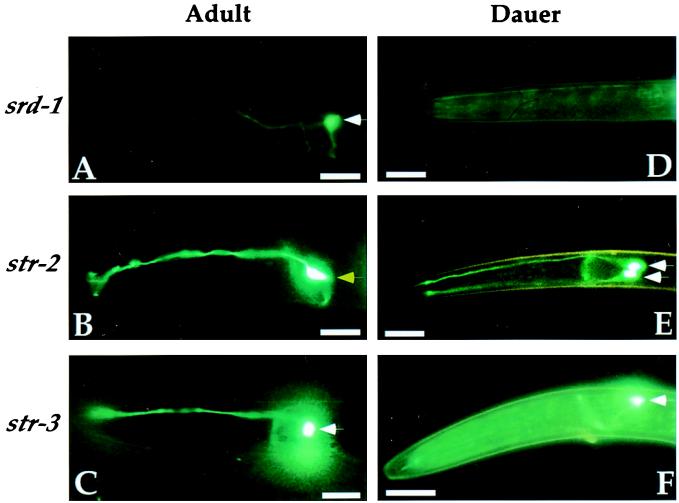
The expression of receptors in the ASI sensory neurons was examined indirectly by monitoring the pattern and intensity of GFP expression from promoter-GFP fusion genes (e.g., srd-1 expression = srd-1∷GFP expression). Three candidate receptors from the odr-10 superfamily, srd-1, str-2, and str-3, were examined during dauer and non-dauer development (29, 32, 34). Two of these genes, srd-1 and str-2, were differently expressed in dauer and non-dauer animals (Table 1, Fig. 1). In well fed larvae and adults, srd-1 was expressed in the ASI neurons and str-2 was expressed strongly in the AWC neurons and weakly in the ASI neurons. In dauer larvae, str-2 was expressed in ASI but not AWC neurons, and srd-1 expression was undetectable (Fig. 1). A third ASI-specific fusion gene, str-3, was expressed strongly in dauer larvae, non-dauer larvae, and adults (Fig. 1). Expression of other candidate receptor genes in the repellent-sensing ADL and AWB neurons was unchanged in dauer larvae (data not shown).
Table 1.
Receptor regulation in ASI
| ASI expression
|
|||
|---|---|---|---|
| srd-1∷GFP | str-2∷GFP | str-3∷GFP | |
| Wild-type adults | + | low | + |
| Wild-type dauers | − | + | + |
| Activity mutants | |||
| osm-6 | − | low | + |
| che-3 | − | low | + |
| tax-2 | − | low | + |
| tax-4 | − | low | + |
| Dauer pheromone | |||
| daf-22 (daf-d) | + | + | + |
| daf-22 + wild type | + | low | + |
| Wild type + pheromone | − | − | − |
Figure 1.
Chemosensory receptor expression is altered in the dauer stage. Promoter-GFP fusion genes were used to monitor expression of three chemosensory receptors, srd-1, str-2, and str-3 in adult (A, B, and C) and dauer (D, E, and F) animals. The head region is shown in a lateral view. White arrows denote ASI cell bodies. Adults express srd-1 and str-3 strongly and str-2 weakly in ASI (not detectable in B). Dauer larvae express str-2 and str-3 but not srd-1 in ASI. Yellow arrow in B denotes AWC cell body, which expresses str-2 in adults but not dauer larvae. The ASI neuron pair is bilaterally symmetric; in most panels, only one ASI is apparent, but in E the contralateral ASI neuron is visible. Each neuron extends a dendrite to the tip of the nose and a single U-shaped axon. Anterior is at left and dorsal is up. (Scale bars, 10 μM.)
To ask whether dauer-induced changes in expression were reversible, str-2 expression was examined in animals that had recovered from the dauer stage. Dauer animals that expressed str-2 strongly in the ASI neurons were recovered at low density on food to allow resumption of normal development. Within 24 h of recovery from dauer, the pattern of str-2 expression was restored to that of uninduced animals, with weak expression in ASI and strong expression in AWC (n = 15).
Dauer Pheromone Regulates srd-1, str-2, and str-3 Receptor Expression.
Entry into the dauer larval stage is induced by dauer pheromone, a constitutive signal of nematode density, and enhanced at high temperatures. To ask whether chemosensory receptor expression is regulated directly by sensory stimuli, we examined expression of srd-1, str-2, and str-3 in different environmental conditions. Exposing either larval or adult animals to high temperature (27–28°C) did not alter expression of any of the receptor genes in ASI [srd-1 and str-3 were on in >95% of animals; str-2 expression was low (n > 25 each)]. Thus, temperature did not appear to regulate receptor expression in non-dauer stages. By contrast, adult animals grown at high density repressed srd-1 and str-3 expression [srd-1, 32% ON; str-3, 67% ON (n > 25 for each value)], even when the density was not high enough to induce dauer formation. These results suggest that crowding and perhaps dauer pheromone regulate expression of receptor genes.
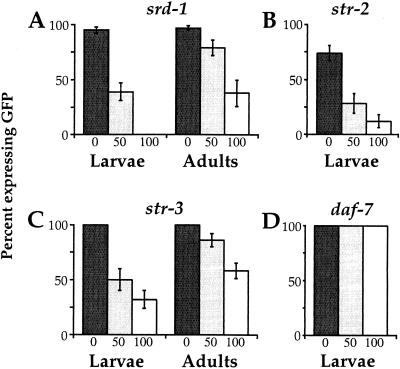
To determine whether crowding-induced effects on receptor expression were caused by the dauer pheromone, we raised animals at low density in the presence of different concentrations of a crude dauer pheromone preparation (13, 14). Well fed animals were exposed to dauer pheromone throughout development and receptor expression was examined in larvae and adults. Although the levels of pheromone used in this experiment were not sufficient to induce dauer development, a decrease in expression of all three receptor genes was observed with increasing pheromone concentration (Fig. 2). Expression of srd-1 and str-3 decreased with increasing pheromone concentrations in both larvae and adults (Fig. 2 A and C). Expression of str-2 was faint but detectable in most ASI neurons of well fed wild-type animals, and decreased further after pheromone exposure (Fig. 2B). All larval stages and adults exhibited pheromone-induced reduction in expression (data not shown). These results demonstrate that dauer pheromone or another component of the pheromone preparation is able to repress receptor gene expression independent of entry into the dauer stage.
Figure 2.
Dauer pheromone suppresses srd-1, str-2, and str-3 expression. The expression of GFP fusion genes with srd-1 (A), str-2 (B), str-3 (C), and daf-7 (D) was examined in animals exposed to different amounts of crude dauer pheromone: 0 μl (dark bars), 50 μl (gray bars), and 100 μl (white bars) in 2 ml of agar. These dauer pheromone levels are lower than those that induce the dauer stage, so no dauer larvae were formed at any concentration. Dauer pheromone suppressed chemosensory receptor expression (A, B, and C) but not daf-7 expression (D) in both larvae and adults, and this suppression was dose-dependent with 100 μl suppressing more than 50 μl. Percentages of animals expressing any GFP, regardless of brightness, were tabulated for each strain. For each column, at least 50 animals were scored and error bars represent standard error of proportion. Adults were not examined for str-2 or daf-7 expression.
To examine recovery of receptor expression after pheromone exposure, we transferred animals that failed to express srd-1 or str-3 from pheromone-containing plates to plates with no pheromone. Gene expression was restored in most animals within 24 h of removal from pheromone, and in all animals within 48 h of removal [24 h: srd-1, 81% (n = 27), str-3, 100% (n = 10); 48 h: srd-1, 100% (n = 24), str-3, 100% (n = 26)]. All larval stages and adults were able to recover when pheromone was removed (data not shown).
The pheromone levels used in this experiment were not sufficient to reduce expression of another ASI-specific gene, daf-7, which has been shown to be repressed by pheromone (Fig. 2D; refs. 20 and 21). This result is consistent with the observation that these pheromone levels were too low to induce dauer formation, and suggests that receptor expression is more sensitive to pheromone repression than daf-7 expression.
A Pheromone-Deficient Mutant Has Increased str-2 Expression in ASI.
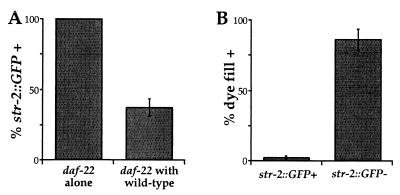
As an independent test of the role of dauer pheromone in receptor expression, we examined receptor expression in daf-22 mutants, which do not produce dauer pheromone. daf-22(m130) mutants have a defect in dauer entry that can be rescued by providing exogenous pheromone (35). daf-22 mutants expressed srd-1 and str-3 strongly in their ASI neurons (Table 1). Surprisingly, they also expressed str-2 at high levels in ASI, instead of the low levels observed in wild-type animals (Table 1). Furthermore, the total percentage of animals expressing str-2 in ASI increased (Fig. 3A, daf-22 alone; compare with Fig. 2). When daf-22 mutant animals were exposed to pheromone-producing wild-type animals, str-2 expression in the daf-22 mutants was reduced to the lower wild-type levels (Fig. 3A). Thus, dauer pheromone secreted by wild-type animals can rescue reduced str-2 expression in daf-22 mutants, suggesting that str-2 regulation is likely to be a direct consequence of the loss of pheromone and not some other effect of the daf-22 mutation. When single wild-type animals were grown in isolation, str-2 expression in ASI neurons was low (data not shown). Because isolated wild-type animals had low str-2 expression but daf-22 mutants had high str-2 expression, the pheromone produced by a single animal may be sufficient to repress str-2 expression in its ASI neurons.
Figure 3.
Low levels of dauer pheromone repress str-2 expression in ASI. (A) str-2 expression in ASI was increased in daf-22 mutants, which fail to produce pheromone (daf-22 alone, n = 89). In addition to the increased percentage of animals expressing str-2 (compare with Fig. 2B), GFP expression in ASI was also much stronger in daf-22 compared with wild-type (data not shown). When daf-22 animals were cocultivated with wild-type, pheromone-producing animals, str-2 expression in ASI was suppressed (daf-22 with wild type, n = 55). (B) As an assay for ASI remodeling, dauer larvae were exposed to the vital dye DiI, which stains exposed ASI neurons. str-2 was highly expressed in dauer larvae that did not stain with DiI (n = 132) and not expressed in dauer larvae that filled with DiI (n = 42). srd-1 and str-3 expression were unaltered in daf-22 mutants compared with wild type (data not shown).
Sensory Activity in ASI Regulates srd-1 Receptor Expression.
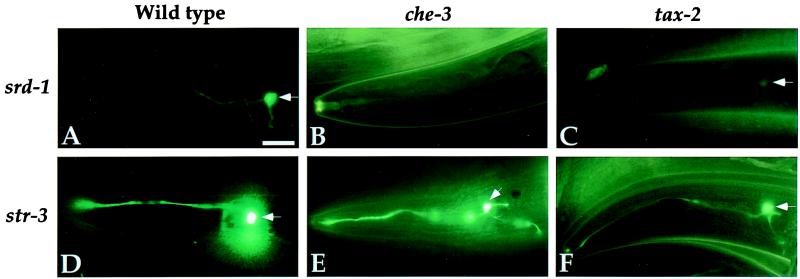
The effect of pheromone demonstrates that a chemosensory cue represses expression of receptor genes. Another way to examine environmental regulation of receptor expression is to characterize expression in mutant backgrounds that deprive animals of the ability to detect sensory cues. The sensory transduction machinery of ASI and other sensory neurons is contained in chemosensory cilia that are exposed to the environment. Mutants with structural defects in the chemosensory cilia are unable to respond to the dauer pheromone or water-soluble attractants, resulting in a dauer-defective phenotype. In osm-6(p811) and che-3(e1124), two mutants with severe truncations of their sensory cilia (36), srd-1 was no longer expressed (Fig. 4, Table 1). This result suggests that sensory input through the ciliated sensory neurons is essential for srd-1 expression.
Figure 4.
Sensory activity regulates srd-1 expression. Animals with sensory defects were examined for alterations in chemosensory receptor expression. srd-1∷GFP was not expressed in che-3 mutant animals, which have defects in their sensory cilia (B), or in tax-2 mutants, which are defective in sensory signal transduction (C). str-3∷GFP expression was similar in wild-type (D), che-3 (E), and tax-2 (F) mutant animals. str-2∷GFP expression in ASI was similar in wild-type, che-3, and tax-2 animals (data not shown). White arrows denote ASI cell bodies. (Scale bars, 10 μm.)
Unlike srd-1, str-2 and str-3 were not affected by cilia defects. Thus, the ASI sensory neurons were present and normally specified in the absence of normal sensory cilia, but were specifically altered in the expression of a single receptor gene.
The phenotype of the cilia mutants suggests that a chemosensory cue stimulates srd-1 expression, antagonizing the pheromone that represses srd-1 expression. If this is true, molecules that transduce chemosensory signals should also regulate srd-1 expression. The ASI neurons, AWC neurons, and several other ciliated neurons express tax-2 and tax-4, which encode a cyclic nucleotide-gated channel required for chemosensation of many water-soluble and volatile odorants (37–39). TAX-2 and TAX-4 are proposed to form a sensory transduction channel, and they are essential for strong expression of str-2 in AWC (32). As observed in the cilia mutants, ASI expression of srd-1 was faint or undetectable in strong tax-2(p691) and tax-4(p678) mutants, whereas expression of str-2 and str-3 in the ASI neurons was unaffected (Fig. 4, Table 1). Thus, sensory signaling by means of the TAX-2/TAX-4 cGMP-gated channel was essential for normal srd-1 expression, but not str-2 or str-3 expression in ASI. These results implicate the subset of ciliated neurons that express tax-2/tax-4 in srd-1 expression.
Sensory neurons in C. elegans may amplify sensory signals by opening voltage-gated Ca2+ channels. Mutations in three different calcium channel subunits, unc-36(e251), egl-19(n582), and unc-2(e55) (40–42), did not alter srd-1, str-2, or str-3 expression in the ASI neurons (data not shown). These results suggest that the regulation of srd-1 expression does not require the opening of voltage-gated Ca2+ channels.
The phenotypes of cilia mutants and tax-2/tax-4 mutants suggest that activity of the ASI neuron may be required for normal srd-1 expression. However, these genes affect other chemosensory neurons in addition to ASI. To achieve a more specific perturbation of ASI activity, we expressed a rat delayed rectifying potassium channel, Kv1.1, in ASI and examined expression of srd-1 and str-3. The Kv1.1 channel should open in response to depolarization of the ASI neuron and allow potassium efflux to repolarize the cell, thereby blunting neuronal excitability (34, 43). Kv1.1 was expressed by using the sra-6 promoter, which is expressed only in ASI, ASH, and PVQ neurons; of these, only ASI expresses tax-2/tax-4. In the animals expressing Kv1.1, str-3 was highly expressed, whereas srd-1 was barely detectable (data not shown). This result supports the hypothesis that neuronal activity of the ASI neurons regulates srd-1 expression in ASI.
Remodeling of the ASI Neurons in the Dauer Stage Induces str-2 and str-3 Expression.
Exposure to dauer pheromone inhibited expression of srd-1, str-2, and str-3, yet str-2 and str-3 were expressed strongly in dauer larvae that were exposed to high levels of pheromone. The dauer-specific changes that occur in ASI morphology could explain this paradox. During the dauer stage, ASI cilia retract from the amphid pore, limiting their access to the environment (12). Pheromone sensation requires intact sensory cilia, so this retraction should diminish pheromone effects on ASI (36). ASI remodeling was first observed by electron microscopy, but we were also able to score this change by the ability of the ASI neurons to take up the vital fluorescent dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate). Animals soaked in DiI or similar dyes exhibit dye-filling of six pairs of amphid neurons including ASI (44). In many dauer larvae, five pairs of neurons filled with DiI but the ASI neurons did not (Fig. 3B). Because the dye-filling pattern was only changed in some dauer larvae, it was possible to ask whether dye-filling correlated with ASI-specific str-2 expression. Dauer animals were filled with DiI and scored for both DiI and GFP fluorescence. A strong correlation between cilia remodeling and GFP expression was observed: ASI neurons that did not fill with DiI, presumably because they had retracted cilia, expressed str-2 strongly in their ASI neurons (Fig. 3B), but ASI neurons that filled well with DiI failed to express str-2. Control experiments demonstrated that DiI filling was not affected by GFP expression in a tax-2∷GFP strain (data not shown). These results suggest that pheromone represses str-2 and str-3 expression in ASI, but relief from this repression occurs when the ASI neurons retract their cilia in the dauer stage.
Discussion
Dauer Pheromone and Sensory Activity Regulate Receptor Expression.
Dauer pheromone is a constitutively secreted chemical that C. elegans uses to assess population density (15). We show here that low levels of dauer pheromone can repress the expression of three candidate chemosensory receptors in ASI: srd-1, str-2, and str-3. These results reveal a striking modification of the chemosensory system by environmental cues. Pheromone regulation of receptor gene expression occurs at pheromone concentrations well below those that induce dauer development, and it also occurs in late developmental stages that can no longer form dauer larvae. Therefore, this form of plasticity is not tightly coupled to the dauer pathway and may modulate chemosensory behavior in non-dauer stages.
The low concentrations of pheromone that altered receptor expression did not repress expression of the transforming growth factor (TGF)-β-encoding daf-7 gene that regulates dauer formation. A commitment to the dauer stage induces a dramatic developmental change of the entire animal, and dauer recovery takes at least a day. This coordinated developmental change may require a stronger sensory input than alterations in receptor expression, which would induce more subtle and rapidly reversible behavioral changes.
Expression of srd-1 in the ASI neurons requires the activity of the TAX-2/TAX-4 channel; the same cGMP-gated transduction channel is also required for expression of daf-7 in ASI (data not shown). Although ASI expression of str-2 and str-3 is repressed by dauer pheromone, it does not depend on the sensory activity of the ASI neurons. These experiments reveal two different mechanisms that regulate gene expression in ASI, one that is tax-2/tax-4-dependent (srd-1, daf-7) and one that is tax-2/tax-4-independent (str-2, str-3). All four genes are repressed by dauer pheromone, which is probably detected by G protein-coupled receptors (45). Pheromone detection by ASI could induce changes in str-2 and str-3 expression by using a G protein signaling pathway that does not require TAX-2/TAX-4. Alternatively, pheromone regulation of str-2 and str-3 in ASI could involve a nonautonomous signal from another sensory neuron that responds to dauer pheromone.
Dauer pheromone inhibits srd-1 expression, but exposed cilia and tax-2/tax-4 are required for srd-1 expression. These results suggest that a second chemosensory input, perhaps a positive input from food, stimulates srd-1 expression. It is also possible that all of the sensory inputs are from pheromones that have complex effects on ASI activity. Because dauer pheromone is a crude extract, the different receptor genes might actually respond to two different pheromones in the extract.
Altered Receptor Expression in the Dauer Larva Stage.
Dauer larvae have altered patterns of chemosensory receptor expression that may contribute to the known changes in dauer behavior (15). The expression of the two candidate chemosensory receptor genes, srd-1 and str-2, is altered in dauer larvae in opposite ways. Dauer entry suppresses srd-1 expression in the ASI neurons while inducing str-2 expression in the ASI neurons.
The most intriguing expression pattern was exhibited by str-2, which was expressed predominantly in one AWC neuron in non-dauer animals and exclusively in the ASI neurons in dauer animals. This expression pattern predicts that the chemical sensed by STR-2 could elicit different responses in dauer and non-dauer animals, because AWC and ASI have different synaptic connections (46). AWC senses volatile odors, suggesting that STR-2 recognizes a volatile molecule. Volatile odors, but not water-soluble molecules and pheromones, can be detected by neurons whose endings are retracted from the amphid sensory opening (16). The expression of STR-2 in ASI may allow ASI to sense volatile odors when its sensory endings are retracted from the amphid pore in the dauer stage.
str-3 and str-2 expression are repressed by dauer pheromone in non-dauer animals, but paradoxically their expression is high in dauer animals in the presence of abundant pheromone. Derepression of str-3 and str-2 expression in dauer larvae may result from a retraction of the ASI cilia from the amphid pore, which could relieve the ASI neurons from the inhibitory effects of pheromone. srd-1 is also repressed by pheromone, but its expression remains low in the dauer stage. An activity-regulated pathway that requires normal sensory cilia and the cGMP-gated channel regulates ASI expression of srd-1, but not str-2 or str-3; we suggest that decreased ASI activity maintains srd-1 repression in the dauer stage.
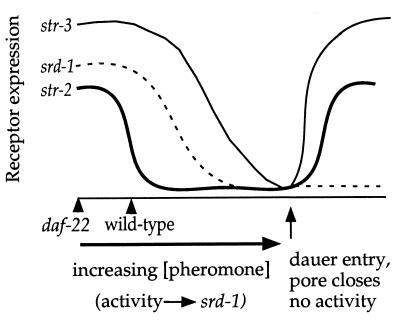
A model for the dynamics of receptor expression during dauer formation is shown in Fig. 5. At low but non-zero pheromone levels, srd-1 and str-3 expression is high, whereas str-2 expression is low. As pheromone concentrations increase, expression of all three receptors decreases, and at the highest level of pheromone expression dauer development occurs. One consequence of dauer development is the retraction of the ASI cilia from the pore, relieving pheromone repression of these genes. ASI retraction allows str-2 and str-3 to be expressed, but the reduced ASI activity caused by its retracted cilia maintains repression of srd-1.
Figure 5.
Model for chemosensory receptor regulation. Pheromone suppresses expression of srd-1, str-2, and str-3 in ASI. Sensory activity is required for srd-1 expression. Under standard conditions, ASI expression of srd-1 and str-3 is high, whereas str-2 expression in ASI is suppressed by endogenous pheromone levels. As pheromone concentrations increase, expression of all three receptors decreases, and if harsh conditions persist, dauer development occurs. One consequence of dauer development is the retraction of the ASI cilia from the pore, relieving pheromone repression. Although this derepression is sufficient for str-2 and str-3 to be expressed, the reduction in ASI activity prevents expression of srd-1.
Other invertebrates and vertebrates alter chemosensory gene expression during development, although they do not have distinct alternative developmental pathways like the dauer pathway. Drosophila larvae and adults display different olfactory behaviors, suggesting that olfactory plasticity accompanies development (47). During fly metamorphosis, many parts of the larval nervous system degenerate, new neurons are born to subserve adult fly behaviors, and new adult chemosensory receptor genes are expressed (48, 49). Zebrafish induce the expression of olfactory receptor genes in temporal waves, providing a possible mechanism for altering odorant sensitivity during development (50).
It might be interesting to examine activity-dependent regulation of vertebrate olfactory receptor genes. Exposing anosmic mice to odors that they do not detect results in an increased peripheral sensitivity to those odors (51). This effect takes several weeks and is not understood, but it could involve activity-dependent receptor gene expression or activity-dependent cell survival.
Functional Implications of Altering Chemosensory Receptor Gene Expression.
Changes in chemosensory receptor gene expression provide a possible strategy for plasticity of olfactory behaviors. In other animals, changes in synaptic connectivity or efficacy have been proposed as the basis of altered olfactory behaviors (52). Altering circuitry and altering gene expression could be two strategies for accomplishing the same task: changing responses to specific odorants.
The structures of the vertebrate and C. elegans olfactory systems suggest distinct advantages for using either a rewiring or a gene expression strategy for olfactory plasticity. In vertebrates, each olfactory neuron expresses a single olfactory receptor, so the neuron always senses the corresponding odor (7). By contrast, individual C. elegans chemosensory neurons express many odorant receptors, all of which are linked to a common behavioral response that is determined by the identity of the neuron (31). Changing the synaptic strength of a single vertebrate neuron would alter responses to a small number of odors sensed by one receptor, but altering the connectivity of a C. elegans olfactory neuron would alter responses to all of the odors sensed by that neuron. Therefore, changes in synaptic strength would not generate an odor-specific behavioral change.
Vertebrate olfactory receptor proteins participate in axon guidance of the olfactory sensory neurons, so altering receptor expression could change the projection of axons to their targets in the olfactory bulb (53, 54). In the nematode, it is less likely that olfactory receptor proteins affect the development of the chemosensory nervous system (28), so manipulating receptor expression could alter olfactory responses to a single odor without affecting neural development or connectivity. Thus, vertebrate olfactory plasticity may be more likely to result from changes in synaptic strength or higher processing centers, whereas odor-specific forms of olfactory plasticity in the nematode may be better achieved by altering expression of receptor genes.
Acknowledgments
We thank Gage Crump for pointing out the density-induced changes in str-3 expression, Paul Wes for comments and discussion, and Shannon Grantner for technical support. This work was supported by a grant from the National Institute for Deafness and Other Communication Disorders. E.L.P. was supported by an American Heart Association Predoctoral Fellowship. E.R.T. was supported by a University of California, San Francisco Chancellor's Fellowship. C.I.B. is an Investigator of the Howard Hughes Medical Institute.
Abbreviation
- GFP
green fluorescent protein
Footnotes
This paper was presented at the Inaugural Arthur M. Sackler Colloquium of the National Academy of Sciences, “Neural Signaling,” held February 15–17, 2001, at the National Academy of Sciences in Washington, DC.
References
- 1.Heisenberg M, Borst A, Wagner S, Byers D. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- 2.de Belle J S, Heisenberg M. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 3.Dubin A E, Heald N L, Cleveland B, Carlson J R, Harris G L. J Neurobiol. 1997;32:214–233. doi: 10.1002/neu.480280208. [DOI] [PubMed] [Google Scholar]
- 4.Shaver S A, Varnam C J, Hilliker A J, Sokolowski M B. Behav Brain Res. 1998;95:23–29. doi: 10.1016/s0166-4328(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 5.Clyne P J, Warr C G, Freeman M R, Lessing D, Kim J, Carlson J R. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 6.Vosshall L B, Amrein H, Morozov P S, Rzhetsky A, Axel R. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts P. Annu Rev Neurosci. 1999;22:487–509. doi: 10.1146/annurev.neuro.22.1.487. [DOI] [PubMed] [Google Scholar]
- 8.Bargmann C I, Mori I. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 717–737. [PubMed] [Google Scholar]
- 9.Jorgensen E M, Rankin C. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 769–790. [PubMed] [Google Scholar]
- 10.Albert P S, Brown S J, Riddle D L. J Comp Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- 11.Swanson M M, Riddle D L. Dev Biol. 1981;84:27–40. doi: 10.1016/0012-1606(81)90367-5. [DOI] [PubMed] [Google Scholar]
- 12.Albert P S, Riddle D L. J Comp Neurol. 1983;219:461–481. doi: 10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]
- 13.Golden J W, Riddle D L. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 14.Golden J W, Riddle D L. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 15.Riddle D, Albert P. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab.Press; 1997. pp. 739–768. [Google Scholar]
- 16.Bargmann C I, Hartwieg E, Horvitz H R. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 17.Bargmann C I, Horvitz H R. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 18.Georgi L L, Albert P S, Riddle D L. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- 19.Estevez M, Attisano L, Wrana J L, Albert P S, Massague J, Riddle D L. Nature (London) 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- 20.Ren P, Lim C S, Johnsen R, Albert P S, Pilgrim D, Riddle D L. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 21.Schackwitz W S, Inoue T, Thomas J H. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 22.Patterson G I, Koweek A, Wong A, Liu Y, Ruvkun G. Genes Dev. 1997;11:2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thatcher J D, Haun C, Okkema P G. Development (Cambridge, UK) 1999;126:97–107. doi: 10.1242/dev.126.1.97. [DOI] [PubMed] [Google Scholar]
- 24.Inoue T, Thomas J H. Dev Biol. 2000;217:192–204. doi: 10.1006/dbio.1999.9545. [DOI] [PubMed] [Google Scholar]
- 25.Wolkow C A, Kimura K D, Lee M S, Ruvkun G. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 26.Robertson H M. Genome Res. 1998;8:449–463. doi: 10.1101/gr.8.5.449. [DOI] [PubMed] [Google Scholar]
- 27.Robertson H M. Genome Res. 2000;10:192–203. doi: 10.1101/gr.10.2.192. [DOI] [PubMed] [Google Scholar]
- 28.Sengupta P, Chou J H, Bargmann C I. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 29.Troemel E R, Chou J H, Dwyer N D, Colbert H A, Bargmann C I. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 30.The C. elegans Sequencing Consortium. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 31.Troemel E R, Kimmel B E, Bargmann C I. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 32.Troemel E R, Sagasti A, Bargmann C I. Cell. 1999;99:387–398. doi: 10.1016/s0092-8674(00)81525-1. [DOI] [PubMed] [Google Scholar]
- 33.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peckol E L, Zallen J A, Yarrow J C, Bargmann C I. Development (Cambridge, UK) 1999;126:1891–1902. doi: 10.1242/dev.126.9.1891. [DOI] [PubMed] [Google Scholar]
- 35.Golden J W, Riddle D L. Mol Gen Genet. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- 36.Perkins L A, Hedgecock E M, Thomson J N, Culotti J G. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 37.Coburn C M, Bargmann C I. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu H, Mori I, Rhee J S, Akaike N, Ohshima Y. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 39.Mori I, Ohshima Y. BioEssays. 1997;19:1055–1064. doi: 10.1002/bies.950191204. [DOI] [PubMed] [Google Scholar]
- 40.Schafer W R, Kenyon C J. Nature (London) 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- 41.Schafer W R, Sanchez B M, Kenyon C J. Genetics. 1996;143:1219–1230. doi: 10.1093/genetics/143.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee R Y, Lobel L, Hengartner M, Horvitz H R, Avery L. EMBO J. 1997;16:6066–6076. doi: 10.1093/emboj/16.20.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grissmer S, Nguyen A N, Aiyar J, Hanson D C, Mather R J, Guttman G A, Karmilowicz M J, Auperin D D, Chandy K G. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 44.Herman R K, Hedgecock E M. Nature (London) 1990;348:169–171. doi: 10.1038/348169a0. [DOI] [PubMed] [Google Scholar]
- 45.Zwaal R R, Mendel J E, Sternberg P W, Plasterk R H. Genetics. 1997;145:715–727. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White J G, Southgate E, Thomson J N, Brenner S. Philos Trans R Soc London B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 47.Cobb M, Bruneau S, Jallon J M. Proc R Soc London Ser B. 1992;248:103–109. doi: 10.1098/rspb.1992.0048. [DOI] [PubMed] [Google Scholar]
- 48.Tissot M, Gendre N, Hawken A, Stortkuhl K F, Stocker R F. J Neurobiol. 1997;33:281–297. doi: 10.1002/(sici)1097-4695(199703)32:3<281::aid-neu3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 50.Barth A L, Justice N J, Ngai J. Neuron. 1996;16:23–34. doi: 10.1016/s0896-6273(00)80020-3. [DOI] [PubMed] [Google Scholar]
- 51.Wang H W, Wysocki C J, Gold G H. Science. 1993;260:998–1000. doi: 10.1126/science.8493539. [DOI] [PubMed] [Google Scholar]
- 52.Faber T, Joerges J, Menzel R. Nat Neurosci. 1999;2:74–78. doi: 10.1038/4576. [DOI] [PubMed] [Google Scholar]
- 53.Mombaerts P, Wang F, Dulac C, Chao S K, Nemes A, Mendelsohn M, Edmondson J, Axel R. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang F, Nemes A, Mendelsohn M, Axel R. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]