Abstract
Interface tissue engineering involves the development of engineered grafts that promote integration between multiple tissue types. Musculoskeletal tissue interfaces are critical to the safe and efficient transmission of mechanical forces between multiple musculoskeletal tissues e.g. between ligament and bone tissue. However, these interfaces often do not physiologically regenerate upon injury, resulting in impaired tissue function. Therefore, interface tissue engineering approaches are considered to be particularly relevant for the structural restoration of musculoskeletal tissues interfaces. In this article we provide an overview of the various strategies used for engineering musculoskeletal tissue interfaces with a specific focus on the recent important patents that have been issued for inventions that were specifically designed for engineering musculoskeletal interfaces as well as those that show promise to be adapted for this purpose.
Introduction
Tissue interfaces are graded transitional regions between two different types of tissues and are integral to normal tissue function. They are typically characterized by anisotropic mechanical properties and a heterogeneous distribution of extra cellular matrix proteins. Tissue interfaces play a particularly important role in the musculoskeletal system. The unique mechanical and matrix properties of orthopedic tissue interfaces provide seamless structural and functional integration between tissue types with dissimilar mechanical and biochemical properties such as tendon or ligament and bone, cartilage and bone, and muscle and tendon tissues (1, 2). This facilitates the safe transmission of stresses between two different tissues and the maintenance of heterotypic cell populations, both of which are critical to the physiological functioning of the musculoskeletal tissue system (3, 4). There are a number of musculoskeletal injuries that frequently require surgical reattachment of musculoskeletal tissues as in the case of tendon or ligament avulsions at bone insertion sites or osteochondral injuries. However, surgical reconstruction procedures cannot re-establish the interface tissue, leading to high rates of re-injury due to poor integration between tissues at the reconstruction site (5). Furthermore, musculoskeletal interface regions have a poor capacity for regeneration and injury to interface tissue often results in the formation of scar tissue with comparatively inferior mechanical properties (6, 7).
The matrix organization of interface tissue is most likely related to the distribution of mechanical stresses across this region (8). Ligament-bone and tendon-bone interfaces are very similar. Nearly all tendons and most ligaments insert directly into bone; however, some insertions are indirect. For direct insertions, the interfaces are composed of ligament/tendon soft tissue, fibrocartilage, calcified fibrocartilage, and bone. The transition from the soft tissue of ligament to the hard tissue of bone occurs in the space of approximately 1 mm (9). The gradual transition from soft to hard tissue distributes the load placed upon the interface and decreases stress concentrations, making the area especially resistant to damage (10, 11). The interface is much simpler for indirect insertions, where the ligament/tendon is anchored directly to the bone via collagen fibers without a distinct transitional structure, and these fibers typically only go as deep as the periosteum (12).
The cartilage-bone interface is characterized by the transition through four zones of cartilage prior to subchondral bone: superficial, intermediate (middle), deep, and calcified cartilage zones (13). In the superficial zone, chondrocytes are relatively dense, and collagen fibers are aligned parallel to the joint surface. At the intermediate zone, the collagen fibers become randomly aligned and chondrocytes go from flat to oblique in shape. At the deep zone, chondrocytes are spherical and aligned in columns, and the collagen fibers become aligned perpendicular to the joint surface. These collagen fibers penetrate from the deep zone into the calcified cartilage zone and are anchored into the subchondral bone (14).
At the tendon-muscle interface, the muscle cell membrane, called a sarcolemma, is folded into finger-like extensions that serve as anchoring points for collagen fibers to the tendon. At this junction, a specialized extracellular matrix exists at the tendon-muscle interface to help transfer and distribute the force from the muscle to the tendon (15–17). Proteins such as focal adhesion kinase, paxillin, integrin-linked kinase, mitogen-activated protein kinase, and talin are up-regulated at this junction (18–20). These proteins create transmembrane-linkage systems to connect the cytoplasmic actin of the muscle with the extracellular matrix proteins from the tendon (21).
Due to the importance of orthopedic interfaces in the physiological functioning of the musculoskeletal system there is a need to develop an effective clinical therapy that can re-establish these tissue interfaces. Tissue engineering is a promising approach that has the potential to regenerate damaged tissue. However, interface tissue engineering can be particularly challenging using conventional techniques (22). Conventional tissue engineering scaffolds generally have uniform properties and do not effectively recapitulate the anisotropic mechanical and biological properties of interfacial tissue regions, which are essential to the maintenance of multiple resident cell populations present in interfaces.
Over the past two decades, researchers have developed innovative tissue engineering techniques that can mimic the unique organization of native interfacial tissue. This review seeks to provide the reader with an overview of the patents filed over the past 15 years in the field of interface tissue engineering, with a focus on musculoskeletal tissue interfaces. Inventions in this field can largely be grouped into scaffold-based and scaffoldless approaches. Scaffold-based strategies make use of composite, stratified, and gradient scaffolds that can effectively simulate the matrix organization of the native interface tissue. Scaffoldless tissue engineering strategies, on the other hand, largely rely on the principle of cellular self-assembly/self-organization for the formation of a tissue construct without deploying cells into an exogenous scaffold (22).
Multiphase Scaffolds for Interface Tissue Engineering
Multiphase scaffolds are made up of two or more distinct physical regions or phases. It is hypothesized that tailoring the mechanical and structural properties of each phase of the scaffold to support the growth of a specific cell type would result in a tissue construct that is representative of native interfacial tissue. Preferably, the adjoining phases are continuously integrated with one another so as to minimize stress concentrations between adjoining layers (8). Various groups have developed scaffolds with multiple phases for the purposes of engineering the osteochondral interface, the ligament-bone interface and the tendon-bone interface.
A number of polymer-ceramic composite scaffolds have been developed for osteochondral tissue engineering. These scaffolds consist of a distinct polymeric layer adjoining a distinct ceramic layer. Bioactive ceramics such as hydroxyapatite, tricalcium phosphate and other calcium phosphates are known to promote biomineralization, exhibit high mechanical strength, and have been extensively used for bone tissue engineering (31). On the other hand, biodegradable polymers can be fabricated with specific geometries, have tuneable degradation times and can recapitulate the mechanical properties of the native cartilage ECM (31–34). The tissue specific properties of the polymeric and ceramic systems can be taken advantage of by combining them into a single composite scaffold that can promote the growth of a soft-tissue to bone interface.
Brown et al. developed a composite scaffold for osteochondral interface regeneration with a polymeric phase attached to a porous ceramic phase. The polymeric phase was partially infused into the ceramic phase to form an interphase region that provides a smoother transition in structural and mechanical properties of the scaffold (23). The ceramic phase consists of a porous ceramic tablet that was cut from a larger ceramic block using a high-speed diamond-cutting saw. The ceramic tablet was then placed on a set of shims secured in an aluminum mold and a solution of PCL and PLGA was poured into the mold until the bottom surface of the ceramic tablet was submerged in the solution. The mold assembly was finally lyophilized to form a single scaffold. Scanning electron microscopy (SEM) images revealed that the polymeric phase was firmly embedded in the porous ceramic layer.
Parvesio et al. disclosed a composite scaffold for osteochondral tissue engineering composed of a three dimensional matrix of hyaluronic acid derivatives that is structurally integrated with a porous three-dimensional ceramic matrix. The scaffold is infused with a biologically active substance that promotes cell adhesion such as fibronectin, RGD or integrin sequences or growth factors etc. (24). The inventors used mesenchymal stem cells (MSC) to study the potential of their scaffolds to generate osteochondral tissue in vivo using an animal model. MSC were cultured with 10ng/ml TGF-β1 to induce them to differentiate to a chondrocyte lineage while they were exposed to an osteogenic supplement (100nM dexamethasone 10 mM beta-glycerophosphate and 0.05 mM of ascorbic acid-2-phosphate) in order to induce formation of osteocytes. Thereafter, MSCs exposed to TGF-β1 were loaded into a porous sponge composed of hyaluronic acid derivatives in order to form the cartilage component of the osteochondral interface, while MSCs exposed to the osteogenic supplement were loaded into the porous ceramic in order to promote bone formation. The MSC loaded sponge and ceramic phases were joined together using fibrin glue and subsequently implanted into the backs of syngeneic rats. The inventors reported that six weeks after implantation fibrocartilage was distributed throughout the hyaluronic acid matrix, which was partially absorbed, while bone tissue could be seen forming in pores of the ceramic materials. The fibrin glue was completely absorbed by six weeks. Furthermore, it was observed that growth of both bone tissue and cartilage was localized only to their respective scaffold components. Although this underscores the potential of the scaffold to exhibit tissue specific bioactivity, an abrupt change in tissue properties is not representative of native tissue interface structure and may lead to unfavorable stress concentrations in vivo.
Biodegradable scaffolds composed of multiple polymeric layers have also been used for engineering tissue interfaces (35). It is hypothesized that controlling physical structure and chemical composition of the individual layers can effectively simulate the heterogeneous mechanical properties and matrix composition of tissue interfaces. Athanasiou et al. disclosed an initial patent describing a bi-phasic scaffold system for subchondral bone and cartilage regeneration. It consists of two porous biodegradable polymer layers placed one on top of the other. The stiffness and compressibility of the upper and lower layers was optimized for bone and cartilage tissue respectively. Each of the polymeric layers is formed through a solvent-non solvent phase separation technique. The two polymeric regions are bonded together in a mold subjected to pressure curing (25). However, this fabrication technique does not result in a smooth transition of structural and mechanical properties between the two polymeric layers and may result in a concentration of stresses at the junction between the two layers.
Ateshian et al. disclosed a multiphase scaffold comprised of a hydrogel component and a composite polymeric ceramic component that can provide a functional interface across multiple tissue types (26). As described in their patent, the composite scaffold consists of at least three regions – the first region comprising a hydrogel and the third region comprising a porous composite polymeric-ceramic phase with an intermediary second phase. In a preferred embodiment of the invention the hydrogel component of the scaffold is composed of an agarose hydrogel, while the second phase consists of a composite (PLGA-BG) of poly-lactide-co-glycolide (PLGA) and 45S5 bioactive glass (BG). Previous studies by the inventors showed that agarose hydrogel constructs were able to develop a functional cartilage-like matrix in vivo while PLGA-BG composite scaffolds were biodegradable, promoted osteointegration and osteoblast proliferation and phenotypic expression (36, 37). The second phase, which interfaces the first and third phases, is a mixture of the hydrogel (first phase) and PLGA-BG composite (third phase) with a lower level of calcium phosphate than the third phase. It is thought that this interfacial phase permits interactions between the chondrocytes and osteocytes to promote the formation of an osteochondral interface region. As mentioned by the inventors, the PLGA-BG phase was integrated with agarose hydrogel phase using a custom mold resulting in the formation of the middle interface region. The phase comprising only agarose gel was embedded with chondrocytes while the phase comprising only PLGA-BG was seeded with osteoblasts. The construct was shown to support the growth of both osteoblasts and chondrocytes and maintained a continuous integrated structure over the twenty-day period of the study. Moreover, the constructs were capable of simultaneously supporting discrete matrix zones, which included a glycosaminoglycan rich chondrocyte region, an interphase region rich in GAG and collagen, and an osteoblast region with a mineralized collagen matrix.
Collagen based scaffolds have been known to exhibit a high degree of cell attachment and have been investigated for interface tissue engineering (38). Tampieri et al. described a bioresorbable multilayer collagenous structure developed as an osteochondral substitute (27). The multilayer structure consists of an upper layer of collagen type I matrix, and one or more lower layers composed of a composite matrix of collagen type I and hydroxyapatite. The upper and lower layers are crosslinked and knitted together while in a gel form and subsequently freeze dried (39). The hydroxyapatite in the lower layer is formed by direct nucleation of apatite on the collagen fibrils. This prevents the hydroxyapatite crystals from growing larger than the nanometer dimensions of the collagen fibrils. It was shown previously by the inventors that this direct nucleation process leads to a non-stoichiometric carbonation of hydroxyapatite, which increases the biodegradability and bioactivity of subchondral bone component of the multilayer membrane (40). The inventors evaluated their scaffold in two in vivo studies: an 8-week study in which scaffolds seeded with sheep bone marrow derived stem cells (BMSC) were implanted in an immune suppressed mouse model and a long term (6 month) study in which the scaffolds were implanted into preformed osteochondral lesions in a horse model. The mouse model showed that the hydroxyapatite enriched collagen layers promoted stem cell differentiation toward an osteocyte lineage, while the collagen-only layers promoted differentiation of stem cells toward a chondrocyte lineage. The long-term study showed that the scaffolds promoted the formation of neo-connective tissue that resembled fibrous cartilage tissue.
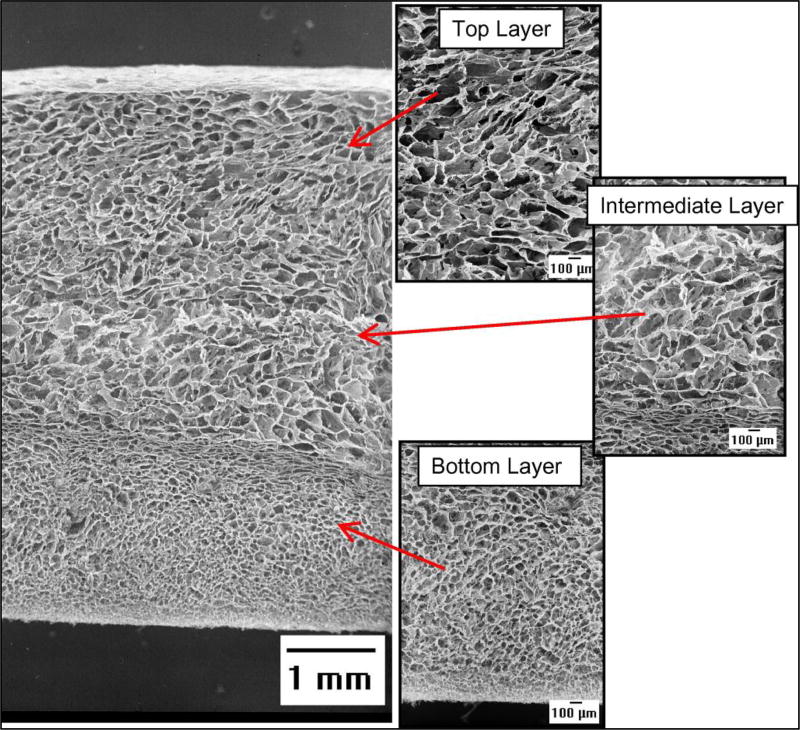
However, the knitting procedures used in the above invention to physically hold the distinct layers together results in the introduction of non-collagenous materials like PLA fibers. Moreover, the layers are freeze-dried in a single step using the same conditions, which results in the formation of voids between the layers and a discontinuous pore architecture. To overcome these drawbacks Gleeson et al. developed an iterative freeze drying process in order to physically integrate multiple collagenous layers (28). The method of fabrication involves freeze drying a first layer of collagen followed by the addition of multiple layers of collagen suspension one on top of the other accompanied by a freeze drying step upon the addition of each successive layer. Before the addition of each new layer the previous freeze-dried layer is rehydrated and then lyophilized once again together with the newly added layer. Scanning electron microscopy (SEM) images show that this iterative freeze-drying process provides seamless structural integration of adjoining layers [Figure 1].
Figure 1.
SEM micrographs of the multi-layer scaffold showing the pore structure, pore interconnectivity, and integration of the component layers. Adapted from (28) with permission.
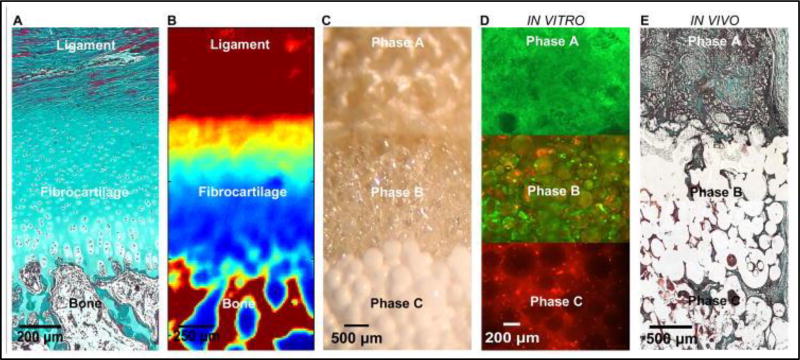
Lu et al developed a triphasic scaffold that has the potential to regenerate the tendon/ligament to bone interface. They hypothesized that a scaffold that can facilitate interactions between fibroblasts and osteoblasts can direct the regeneration of tissue interfaces with heterogeneous matrix properties. Along these lines, Lu et al. disclosed an invention where a graft collar and scaffold are used for promoting fixation of musculoskeletal soft-tissue (ligament and tendon) to bone (29). In a preferred embodiment, the invention provides for a triphasic scaffold for attachment of tendon to bone. The triphasic scaffold can be used as a graft collar or an interference screw. The properties of each of the three phases of the scaffold can be tailored to promote the expansion and maintenance of fibroblasts, chondrocytes and osteoblasts respectively. The invention further provides an apparatus that can induce the formation of fibrocartilage characteristic of the tendon-bone interface by wrapping the graft collar in a polymer fiber mesh in order to apply a compressive force to the graft collar. In a preferred configuration the three phases are of PLAGA woven mesh (Phase A), PLAGA microspheres (Phase B), and PLAGA (85:15)/Bioactive Glass (45S5,BG) (Phase C) composite microspheres, respectively. All phases are continuously joined together by sintering and solvent evaporation. In vitro studies showed that after 28 days there was extensive cell proliferation of fibroblasts and osteobalsts in phases A and B respectively, with both cell types migrating into interphase region of scaffold [Figure 2](8). Thus the triphasic scaffold developed here shows much potential for interface tissue engineering.
Figure 2.
a) The native ACL-bone interface exhibits distinct yet continuous tissue regions, including ligament, fibrocartilage, and bone. (Neonatal Bovine, Modified Goldner Masson Trichrome Stain, bar = 200 µm). b) Fourier Transform Infrared Spectroscopic Imaging or (FTIR-I) revealed that relative collagen content is the highest in the ligament and bone regions, decreases across the fibrocartilage interface from ligament to bone (neonatal bovine, bar=250 µm, with blue to red representing low to high collagen content, respectively). c) A tri-phasic stratified scaffold designed to mimic the three distinct yet continuous interface regions (bar=500 µm). d) In vitro co-culture of fibroblasts and osteoblasts on the tri-phasic scaffold resulted in phase-specific cell distribution and the formation of controlled matrix heterogeneity. Fibroblasts (Calcein AM, green) were localized in Phase A and osteoblasts (CM-DiI, red) in Phase C over time. Both osteoblasts and fibroblasts migrated into Phase B by day 28 (bar = 200 µm). e) In vivo evaluation of the tri-phasic scaffold tri-cultured with Fibroblasts (Phase A), Chondrocytes (Phase B), and Osteoblasts (Phase C) revealed abundant host tissue infiltration and matrix production (week 4, Modified Goldner Masson Trichrome Stain, bar = 500 µm). Adapted from (8) with permission.
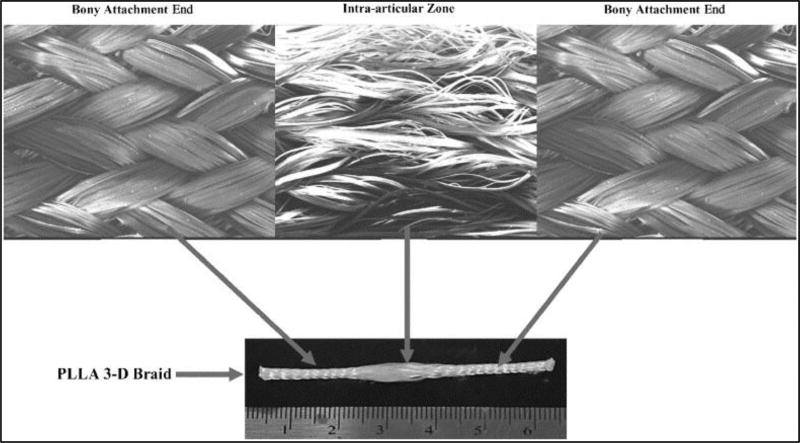
Laurencin et al. developed a multi-region scaffold system that can be used for ligament or tendon repair, regeneration or reconstruction and promote proper fixation to the bone at the implantation site (30). It consists of a quickly degrading polymeric fiber component coupled to a biocompatible slowly degrading (not degraded in less than one year following implantation) polymeric fiber component in the form of a 3-dimensional braided scaffold. The slowly degrading fiber component forms the two ends of the braided scaffold while the faster degrading component forms the middle section of scaffold. The middle section of the scaffold degrades within nine to twelve months and supports ligament or tendon fibroblast ingrowth. The two end-sections, which promote bone ingrowth can be secured into drilled bone tunnels and serve as the bony attachment ends of the scaffold. The middle section of the scaffold can be made more porous and differs in braiding angle and/or polymer composition from the end sections. The 3-dimensional braided structure of the scaffold is formed using a 3-dimensional rotary braiding or row and column method. In a preferred embodiment, the inventors fabricated an 83% PLLA and 17% polyester braided scaffold. The polyester region formed the slowly degrading section of the braid. Ligament reconstruction using this configuration of the scaffold in a sheep model showed that at the end of 12 weeks there was significant tissue infiltration at the site of bone insertion with the presence of fibrous tissue along the soft tissue to bone interface. An early representative version of the 3-dimensional braided scaffold is shown in [Figure 3] (41).
Figure 3.
Representative image of the 3-dimensional braided scaffold developed by Laurencin et al. with distinct fiber organizations in the bony attachment ends and central intra-articular zone. Adapted from (41) with permission.
Development of gradient scaffolds
Gradient scaffolds possess a gradual transition in material properties along one or more spatial dimensions. Scaffolds with graded properties are useful for engineering tissue interfaces since cultured cells differentiate based on the physical and chemical stimuli provided by the scaffold. For instance, the fate of MSC has been shown to be highly sensitive to matrix elasticity. MSC can differentiate into neurons, muscle, or bone simply by changing the stiffness of the substrate (42). Further, scaffolds with a continuous gradient in physical and chemical properties may be able to closely mimic the native extracellular matrix of tissue interfaces, which would promote a convergence of different cell types that duplicates the native composition (43). Thus, gradient scaffolds have the potential to regenerate functional tissue interfaces, including interfaces in the musculoskeletal system.
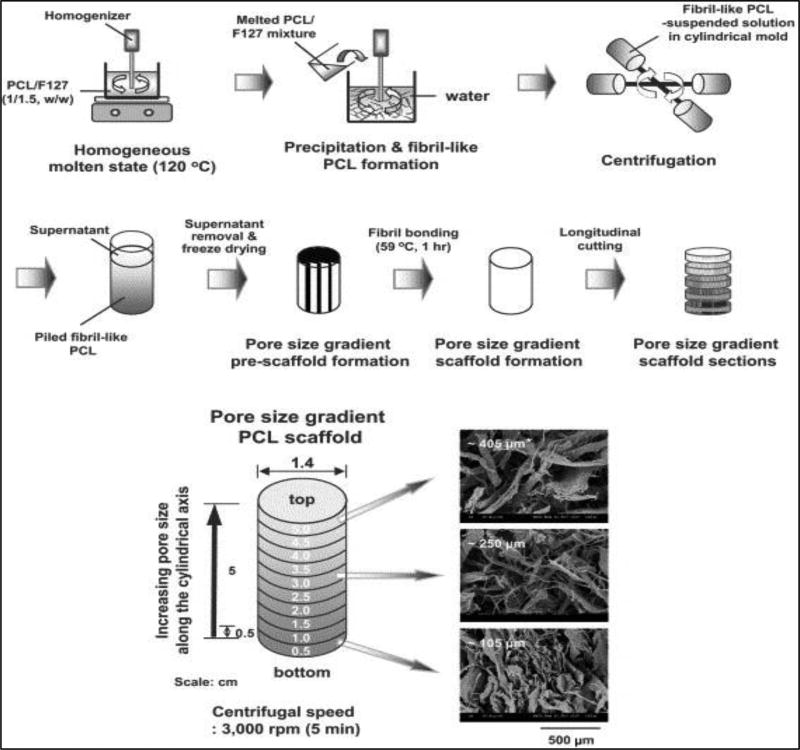
Scaffold physical properties, such as pore size, crosslink density, stiffness, and material composition, directly affect cell behavior and have been incorporated into gradient scaffolds. For instance, different musculoskeletal cell types have been shown to prefer different pore sizes (51). In a study by Oh et al., a gradient of increasing pore sizes was created in a polycaprolactone scaffold using a centrifugation method [Figure 4], and the scaffold was seeded with chondrocytes, osteoblasts, and fibroblasts in vitro. It was found that chondrocytes and osteoblasts showed increased cell count at a pore size of 380–405 µm while fibroblasts were more numerous on the section of the scaffold with a 186–200 µm pore size (52). This has important implications for interface tissue engineering at the transition from ligament and tendon to bone since the scaffold environment must gradually change from one that is favorable for ligament/tendon fibroblasts to one that is favorable for osteoblasts. Thus, gradients in pore size, as well as other physical characteristics, may be useful in guiding cell differentiation and proliferation at musculoskeletal tissue interfaces.
Figure 4.
(Top) Schematic diagram showing the fabrication process of a pore size gradient in PCL scaffolds by a centrifugation method and (Bottom) SEM photographs of the top surfaces of the PCL scaffold sections along the longitudinal direction (× 100; *, average pore size). Adapted from (52) with permission.
In a patent by Yannas et al., gradient scaffolds were created with a gradual change in pore size and collagen crosslinking, which may be useful at the interface between tissues with different porosity requirements, such as bone and tendon/ligament (44). The authors have demonstrated the ability to produce collagen fibers at varying degrees of degradation by varying the concentration and temperature of a collagenase soaking solution (53). Thus, collagen fibers with a range of chemical structures and crosslink densities can be produced and incorporated into gradient scaffolds. In addition, pore size has been controlled through the use of various drying procedures on hydrated collagen-glycosaminoglycan membranes (54). The different drying procedures (including freeze drying, critical point drying, and air drying) each produced a different range of pore sizes, which may be useful when developing scaffolds with a porosity gradient. These studies were developed for an artificial skin replacement for the interface between the dermal and epidermal skin layers. However, implants for musculoskeletal tissue interfaces, which possess a gradient change in stiffness and porosity, can also be created with these techniques. For instance, in the transition from ligament to bone, the tissue gradually becomes more stiff and mineralized. A gradient in porosity and crosslink density may be able to accurately mimic this environment and provide the proper cues for enhanced cell infiltration. However, further studies need to be conducted to optimize these techniques for a musculoskeletal tissue interface application.
Vyakarnam et. al. utilized porosity gradients in biocompatible foams and various co-culture techniques to create a composite scaffold for different cell types (43). The patent declares that gradient foams can be formed through the phase separation of a polymer solvent mixture, which is then solidified and lyophilized, resulting in a porous polymer foam. Gradients in the porosity of the foam can be introduced by varying the onset of the lyophilization process. Foams subjected to lyophilization immediately after solidification were reported to form channels through the foam, whereas foams which were allowed to solidify for a longer period of time resulted in larger pores closest to the surface of the foam with smaller pores on the interior. Studies conducted by the authors support the notion that pore size strongly influences cell infiltration. Porous foam scaffolds comprised of block copolymers of lactic acid and caprolactone were fabricated with pore sizes of approximately 80 µm and 100 µm, and significantly higher proliferation of bovine chondrocytes was found on the scaffolds with 100 µm pores. Thus, these foams can be used at the cartilage to bone interface where different chondrocyte densities can be achieved by creating a gradient in pore size. Further, this gradient foam scaffold can be combined with a biodegradable block copolymer film of lactic acid and glyolic acid, which was found to promote proliferation of rat osteoblasts. Additional factors such as proteins, nanoparticles, ceramics, or fibers can also be incorporated into the foam system to impart added functionality to the foam biocomposite. The foams can also be formed in shaped molds to either fit a particular shape or to further control the gradient pore structure of the final scaffold.
Surface modification of fibrous scaffolds has been used extensively to modulate cell attachment, proliferation, and function (55). Polymeric materials can have a large range of properties that are suited for different tissue engineering applications, but each polymer has both advantages and disadvantages. Therefore, composite materials and surface modification of polymers is often used to meet a variety of needs. Surface modification allows a material to retain its mechanical strength and toughness while promoting desirable cell interactions on the outside surface and can be achieved with both covalent and non-covalent linkages. Certain drug delivery applications may favor non-covalent bonding to allow easier dissociation of drug molecules (55). On the other hand, covalent linkages can extend the half-life of a molecule and provide extended bioactivity (55). Different tissues coming together at interfaces may require different surface modifications to promote cell growth. Thus, technologies have been developed to create gradients in surface modifications to promote the development of different musculoskeletal tissues.
Vepari et al. disclosed a method of generating an immobilized protein gradient within a 3-dimensional scaffold that can be used for engineering interfaces between two different tissue types, such as at the bone-ligament interface (47). Either the surface of the scaffold or the protein may be activated to enhance the binding of the protein to the scaffold. Activation in this sense refers to chemical modification of the scaffold or protein with one or more functional groups to promote protein binding. The scaffold is then immersed in a solution containing the protein to enable diffusion and immobilization of the protein within the scaffold structure, thereby forming a gradient in protein concentration. Multiple gradients may be formed within the scaffold with each gradient being associated with a different bioactive agent. The immobilized protein may be an enzyme, a growth factor, cytokines, cell binding motif and/or a cell-signaling factor. Gradients of these agents may be useful in engineering an interface from soft tissue to hard tissue, such as the bone-ligament interface. For instance, gradients in various bone morphogenic proteins (BMPs) immobilized on silk fibroin scaffolds and supplied with osteogenic media were found to exhibit a gradient response in calcium mineral deposition (56). This gradient in calcium mineral may be useful in mimicking the bone-to-tendon/ligament interface, and the concept can be applied to other musculoskeletal interfaces.
In a patent by Hai-Quan et al., surface modification of polymeric fibers is accomplished through covalent conjugation of a combination of biofunctional ligands and growth factors (46), resulting in linkages that are proteolytically stable. Fibers are processed with aminolysis or alkali hydrolysis to produce desirable functional groups, including carboxyl, hydroxyl, and amino groups. Functional ligands and growth factors are then conjugated to these available functional groups in a concentration gradient. This technique was used along with a co-culture technique to produce scaffolds for vascular grafts. Poly(ethylene glycol) scaffolds were conjugated with functional peptides on the inside to promote endothelial cell attachment, while other peptides were conjugated to the outside to promote smooth muscle cell attachment. The attachment of these two cell types was mediated by the changes in peptide composition and hydrophobicity, as determined previously (57). Thus, gradient scaffolds can be fabricated with a spatial gradient change in the surface concentration of one or more ligands, which allows the scaffolds to promote the attachment and differentiation of different cell types. This technique can be modified to promote the formation of musculoskeletal interfaces, such as at the interface between muscle and tendon, but more studies need to be conducted to determine the exact parameters of the required gradients.
Microspheres have been extensively researched as the building blocks for scaffolds and as drug delivery systems (58, 59). Microspheres can be fabricated in a variety of sizes and with a range of mechanical properties, and they can be loaded with bioactive molecules to alter cell behavior or deliver therapeutics. Thus, microspheres can be used to create scaffolds with gradients in both physical and chemical properties. In one example, Wang and coworkers used microspheres to develop growth factor gradients in a scaffold for osteochondral tissue engineering (56). Silk and poly(lactic-co-glycolic) acid microspheres were prepared and loaded with bone morphogenetic protein 2 (BMP-2) and insulin-like growth factor I (lGF-I). A gradient of growth factor concentrations was created using a two-chamber system, which allowed solutions with two different concentrations of growth factors to slowly mix. After imposing a gradient in growth factor uptake in the microspheres, the microspheres are then sintered together to create the final scaffold. After seeding and culturing with human MSC, the cells exhibited both osteogenic and chondrogenic differentiation along the concentration gradients of the two growth factors, suggesting that a bone to cartilage transition can be mediated by growth factor concentration. This and other studies have shown promise for using microspheres in musculoskeletal tissue engineering (60, 61). The patents on microsphere scaffolds describe various techniques that have been used to create gradients in microsphere properties for interfacial tissue engineering.
Microspheres can also be used to create a gradient in physical properties on gradient scaffolds. In a patent by Detamore et al., a plurality of microspheres is linked together via sintering to form a three-dimensional matrix within a tissue-engineered scaffold (48). Sets of microspheres with different properties are distributed at varying spatial distributions within a three-dimensional matrix. Each set of microspheres can have distinct physical characteristics based on composition, particle size, bioactivity, mechanical strength, etc. These microsphere-based gradient scaffolds can be used for a variety of applications in interfacial tissue engineering. Specifically, this microsphere scaffold was used in osteochondal defects in New Zealand White rabbits, and there was histological evidence of de novo bone and cartilage formation in the implant region. Thus, these microsphere scaffolds have the potential to be used for tissue engineering at the interface of bone and cartilage, but they also have the potential to be used at any interface that requires different physical characteristics in the matrix.
Multiple scaffold phases/materials can be combined with gradients in various proteins and bioactive molecules to achieve a composite scaffold that is customized for any tissue interface. In a patent by Lu et al., microspheres are used for engineering the interface from soft to hard tissues (50). The microspheres are arranged in a gradient of sizes and/or compositions to promote the growth of bone, while a mesh scaffold is used to promote ligament regeneration. The two components are sintered together to produce a ligament-bone interface scaffold, and a calcium phosphate gradient is imposed to differentially affect cell behavior. After co-culture with bovine ACL fibroblasts and osteoblasts, it was found that both cell types expanded upon the initial seeded area. Further, a continuous, confluent culture was observed into the interfacial zone. A mineral gradient was also found across the insertion zone, which correlated with changes in material properties of the initial composite scaffold, proving that material properties can directly affect cell behavior in a controlled manner (50). Although this patent was specifically written for the bone to ligament/tendon interface, the concept of using composite materials and gradients of various properties can also be applied to the other musculoskeletal interfaces.
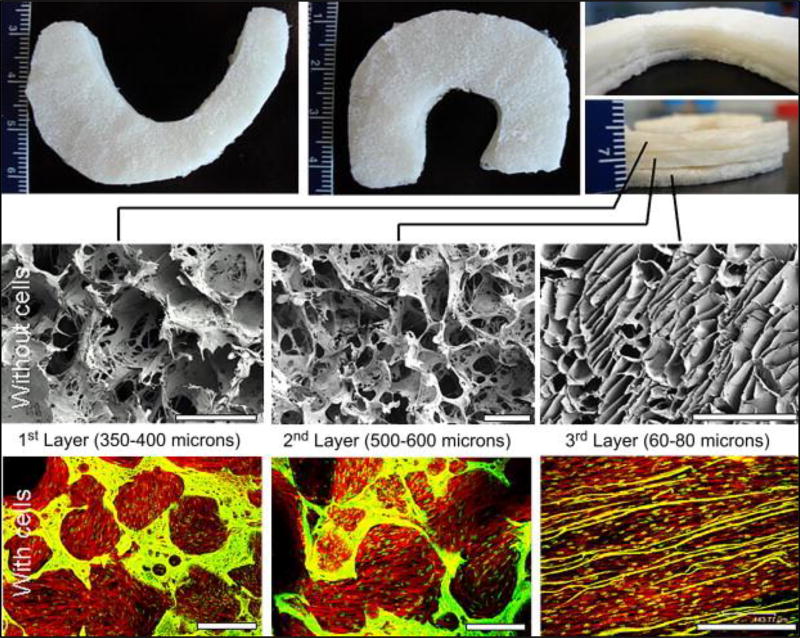
Kaplan et al. disclosed a multilayered silk scaffold with a gradient of pore sizes for meniscus tissue engineering (49). Each layer of the scaffold is comprised of a distinct pore size distribution and orientation that mimics the native architecture of meniscus tissue. In a preferred embodiment the scaffold is comprised of three individual layers. The top two layers are fabricated using a salt leaching method while the third layer is fabricated using a freeze drying technique, thus giving the layers distinct porosity and morphological characteristics. The first two layers of the scaffold are comprised of circular pores with a pore size distribution ranging from 350–400µm and 500–600µm, respectively. The third layer is comprised of partially aligned laminar channels with a pore width distribution of 60–80µm. This distribution of pore sizes is generated to better mimic the native meniscus architecture. Further the multilayer scaffold can be adapted for meniscus regeneration by making each of the layers crescent-shaped or C-shaped. In vitro studies in which human MSC (hMSC) were cultured on the multilayer silk scaffolds showed that the hMSC were able to form an intricate meshwork with their actin filaments in the pores of the first two layers while they exhibited an almost linear arrangement of actin filaments in the third layer [Figure 5]. This indicated that the morphological characteristics of the individual layers of the scaffold had the potential to influence the cytoskeletal organization of cultured hMSC. Further histochemical staining revealed that cells present on the third layer showed a more mature chondrogenic phenotype compared to cells in the other two layers.
Figure 5.
Meniscus shaped scaffolds with three stacked layers (Top). SEM images showing porosity and pore interconnectivity in the individual silk layers without cells (middle) and confocal images of layers with confluent cells (bottom). Scale bar represents 500 microns. Adapted from(49) with permission.
Multi-phase composite scaffolds and gradient scaffolds have shown great promise for engineering tissue interfaces, but challenges still remain in the transition to clinical applications. As with all implants, both the shape of the gradient scaffold and the exact parameters of the gradient must fit the unique geometry of the defect site in each patient. In addition, gradient scaffolds may require seeding with many different cell types from the donor, which is expensive and time consuming. If MSC are used, precise control over the differentiation pathway must be achieved. Future research on scaffold-based approaches should focus on integrating various parameters into a complex scaffold with a combination of different morphologies, porosities, functional groups, and/or growth factors to provide the greatest control over cellular differentiation, phenotype, and intercellular interactions. However, this may require innovative and complex biomaterials, multiple processing steps, and precise spatial and temporal control over administration of multiple biochemical and biomechanical stimuli in order to develop an effective scaffold for tissue engineering at musculoskeletal interfaces.
Scaffoldless Techniques to Engineer Tissue Interfaces
Scaffoldless tissue engineering approaches aim to better approximate embryonic tissue development, which naturally occurs in the absence of biomaterial scaffolds. This bestows a number of distinct advantages over traditional scaffold based tissue engineering, especially as it relates to musculoskeletal interface tissues. The absence of scaffolds alleviates concerns over scaffold biocompatibility, the toxicity of scaffold degradation byproducts, and mismatches between scaffold degradation and new tissue formation (62–64). Each of these factors can negatively influence the mechanical properties and overall healing of implanted tissue constructs. The presence of a scaffold can also significantly alter cell phenotype, negatively influencing the development of interface tissues (62–64). For example, as it relates to musculoskeletal tissues, chondrocytes exhibit a spherical morphology in vivo, however scaffold based cartilage constructs induce cell spreading of seeded chondrocytes, altering the way matrix is secreted from the cells and resulting in malformed tissue constructs (62). Lastly, the presence of a scaffold significantly decreases the amount of cell-cell signaling which can affect matrix production and contribute to stress-shielding, both of which can drastically hinder the development of matrix mechanical properties (62, 65).
Scaffoldless tissue engineering approaches address these shortcomings of scaffold-based techniques. They are further advantageous as they do not subject cells to harsh processing conditions or residual solvents inherent to common scaffolding biomaterials and do not suffer from non-uniform seeding often experienced with cell-seeded scaffolds. Additionally, the high degree of cell-cell contact and intercellular signaling provide a superior biomimetic environment which upregulates the secretion of extracellular matrix (ECM) proteins (65). Because a scaffoldless construct is comprised solely of cells and natural ECM proteins, tissue remodeling can occur through natural enzymatic pathways, as opposed to hydrolytic degradation characteristic of many biodegradable polymers. The mechanisms that drive the formation of scaffoldless tissue constructs rely on the natural ability of cells to sort and assemble themselves according to various individual cell phenotypes and the ability of these cells to collectively organize in response to external stimuli. Together, these phenomena promote the formation of complex tissue types. Many of the scaffoldless protocols described for formation of interface tissues are similar, consisting of high density in vitro suspension cultures secreting their own extracellular matrix. Several recent characteristic examples of patented scaffoldless culture techniques to generate interface tissues are described herein.
Forgacs et al. have developed a method for assembling complex tissue from self-assembling multicellular bodies (66). An extremely concentrated cell suspension is cultured in a non-adherent agarose mold to form a dense cellular body. These multicellular bodies can either be homogenous, consisting of one cell type, or heterogeneous, consisting of multiple cells types, and are used as building blocks for further tissue assembly. Several of these blocks can be placed into contact with one another and cultured to allow fusion between the cell bodies as continued ECM deposition occurs at the interface linking the two multicellular bodies into a larger more complex body of cells. These can be further cultured together around a mold to form more complex architectures to model complex tissue structures. Additionally, Forgacs et al. observed that in heterogeneous cell bodies, that a high degree of self-assembly occurred so that like cell types sorted themselves together within the multicellular bodies, forming multicellular bodies, which even further mimicked naturally occurring tissue types. This method was demonstrated to yield multicellular bodies of human umbilical vein smooth muscle cells (HUVECs), human dermal fibroblasts, and porcine aortic smooth muscle cells which were then further processed into complex tissue architectures, including double-layered vascular tubes. These methods could be applied to interface tissues by using cell types of greater musculoskeletal relevance.
In a similar invention, Nakamura et. al. report a method for developing complex three-dimensional tissues under scaffoldless conditions. Cells of a particular type are cultured in a cell medium that contains an ECM synthesis promoting agent, such as ascorbic acid, which upregulates the amount of ECM proteins which are produced by the cells in culture. These cells are maintained in a suspension culture in molds or culture dishes that mimic the final desired shape of the engineered tissue (67). Cells proliferate and aggregate into dense cultures in vitro until they can be eventually handled and implanted into a particular defect site. To fabricate interface tissues, such as bone-cartilage complexes, two separate suspension cultures are maintained and then transferred to a second culture plate or mold, where the two separate engineered tissue types are allowed to fuse to one another in the same extracellular matrix promoting medium. After the two synthetic tissues have sufficiently integrated to one another in vitro they can be implanted as an interface tissue as desired. This was demonstrated with a cartilage-bone construct, which showed strong adhesion and integration between the two tissues.
Larkin et al. disclosed methods for the development of a contractile, self-organizing, three dimensional skeletal muscle construct that resembles a neuromuscular junction (69). The three-dimensional construct is obtained through a reproducible technique of co-culturing myogenic cells with tendon fibroblast cells. Briefly, according to this technique, a suspension of primary muscle cells is plated onto culture dishes without using an exogenous scaffold. The cells are allowed to form a monolayer by adhering to the culture dish. Tendon tissue in the form of self-organized tendon constructs or segments of adult or fetal rat-tail tendon are brought in contact with the muscle cells. These may be pinned down to constrain the shape of the construct. After about a week the monolayer detaches from the substrate and rolls up to at least partially surround the anchors, forming a self-organized muscle-tendon construct.
The same group disclosed a method for the formation of self-organized bone, ligament and bone-ligament constructs [66]. Briefly, bone marrow derived stromal cells are cultured in growth medium that promotes osteogenic differentiation to form self-organized bone constructs. At the same time, bone marrow derived stromal cells cultured in a fibrogenic growth medium are plated on culture dishes to form a monolayer. As the cells become confluent within this constrained area, the monolayer begins to curl up on itself forming cylindrical ligament constructs. After both the bone and ligament cultures have reached a critical confluence, they can then be cut into strips and placed into contact with one another. The ligament monolayer detaches from the substrate and at least partially surrounds the bone tissue, overlapping to form a three-dimensional self-organized bone-ligament integration site, forming a functional interface tissue construct [66]. Engineered bone-ligament-bone (BLB) constructs were fabricated using the described method and used in MCL replacement in a rat model. After one month, the construct was excised from the animal. The ends of the construct had fused to the bone at the femur and tibia and had increased in size, stiffness and strength. Additionally, the collagen content of the BLB construct was found to resemble that of a native adult MCL [66]. The self-organizing nature of these two techniques allows for a well-integrated interface tissue to be formed between muscle-tendon and ligament-bone that closely approximates naturally occurring interfaces.
Chan et. al. have claimed a method of forming gradient tissues from cultures of encapsulated multipotent or pluripotent stem cells. Mesenchymal or induced pluripotent stem cells are encapsulated in collagen and then separate cultures are maintained under different media conditions, to induce the encapsulated cells to differentiate into two different cell types. These encapsulated cells can then be implanted into a mold or into an in vivo defect site, layered so that one encapsulated cell type is seeded first, a layer of undifferentiated encapsulated cells is seeded on top of that layer, and then the second cell type is seeded last. This will allow a gradient tissue to form between the two desired cell types. This invention is indicated for gradient tissues particularly for bone-cartilage and bone-ligament interfaces. The authors demonstrated the utility of this technique by inducing repair of a full-thickness osteochondral defect in a rabbit model. A three-layered osteochondral construct was fabricated using collagen encapsulated autologous MSCs as described. After implantation and subsequent healing, excellent organization between chondral and subchondral bone structures was observed, demonstrating the strong utility of this technique for interface tissues (70).
Overall there is great promise in emerging techniques for developing scaffoldless interface tissues for musculoskeletal interfaces. Currently, further development and clinical realization of scaffoldless technologies is limited by cell sourcing concerns. Because of the high number of cells that are inherent to these techniques, cell sourcing techniques that are minimally invasive and yield a high number of patient specific or non-immunogenic cells must be identified. It is possible that improved cellular reprogramming and induced pluripotent stem cell technologies may be able to address some of these concerns. Additionally, a limitation of great relevance to interface tissue engineering is the development of increased mechanical properties. In vivo, tissues develop increased mechanical properties as loads are gradually applied to them through the course of development. Because many of the scaffoldless techniques require suspension cultures, it is difficult to apply loads and mechanical stresses evenly to the maturing tissue without disrupting the suspension culture. Thus techniques for increasing mechanical properties by applying novel external stimuli must be identified as well.
Current and Future Developments
This review has provided an overview of the recent technological progress made toward the engineering of functional tissue interfaces. Scaffold based approaches toward interface tissue engineering seek to recapitulate the graded structural, mechanical and biochemical properties of native interface tissue. The use of stratified composite scaffolds and gradient scaffolds can be further incorporated with growth factors as well as biochemical and biomechanical stimulation to promote the maintenance of heterotypic cell populations relevant to tissue interfaces. Alternatively, scaffoldless approaches toward interface tissue engineering have also yielded promising results. Tissue constructs obtained through scaffoldless techniques recapitulate the morphological characteristics of native tissue interfaces.
However, there are a number of challenges to the clinical implementation of the tissue engineering techniques discussed above. A critical factor determining the successful regeneration of interface tissue is the ability of the tissue engineered construct to effectively integrate with the adjacent native tissue. Inadequate integration between the construct and native tissue will result in an adverse concentration of stresses that can be detrimental to both native healthy tissue as well as the tissue engineered construct. Although innovative scaffold designs coupled with growth factor treatments have shown improved integration of tissue-engineered constructs, much work still needs to be done in order for these techniques to be clinically adopted (71–73).
Furthermore, in order to realize the full clinical potential of these methods, it is also essential to gain a deeper understanding of the relationship between the structure and function of tissue interfaces as well the underlying mechanisms of their development. This knowledge can shed light on the importance of heterotypic intercellular interactions and cell types present during interface development. Currently, the mechanism of formation and maintenance of interfacial tissue in vivo and the effect of biochemical and mechanical stimuli on interfacial tissue development are not clearly understood. Optimization of currently available scaffoldless systems can result in reproducible in vitro models that provide useful data regarding native interface tissue development. Moreover, the clinical introduction of these techniques will require the development of reproducible animal models that can be used to establish their effectiveness. Thus, interface tissue engineering is a promising area of research. The development of more biologically relevant scaffold systems, cell co-culture techniques and a deeper understanding of native interfacial tissue development can result in novel techniques for the replacement of damaged interfacial tissue. These would have the potential to significantly improve patient outcomes associated with currently used surgical techniques for treating musculoskeletal tissue injuries.
Table 1.
List of patents surveyed for multi-phase scaffolds
| Patent (Year) | Inventor | Features | Intended Application |
|---|---|---|---|
| Issued, EP 1270025 B1 (2006) | Brown et al. (23) | Lyophilized bilayered polymer-ceramic scaffold | Osteochondral tissue |
| Issued, US 7968111 B2 WO2002070030 A1 (2012) | Parvesio et al. (24) | Composite bilayered hyaluronic acid-ceramic scaffolds | Osteochondral tissue |
| Issued US5607474, WO1993015694 A1 (1993) | Athanasiou et al. (25) | Bilayered polymeric scaffold bonded by pressure curing | Soft tissue/bone interface |
| Application US20060036331, WO2005089127 A2 (2006) | Ateshian et al. (26) | Multilayered polymeric scaffold with hydrogel and composite polymer-ceramic component | Osteochondral tissue |
| Issued, EP 1858562 B1 US20090232875 (2009) | Tampieri et al. (27) | Multilayer collagen scaffolds | Osteochondral tissue |
| Issued, US 8613943 B2 US20120015003 (2013) | Gleeson et al. (28) | Multilayer collagen scaffolds fabricated using iterative freeze drying procedure | Osteochondral tissue |
| Issued US 8753391 B2 (2014) | Lu et al. (29) | Multiphase scaffold with graft collar and interference screw apparatus | Ligament/tendon-bone interface |
| Issued, US 8486143 B2 US 8758437 B2 (2014) | Laurencin et al. (30) | Multiphase braided scaffold for ligament replacement | Ligament-bone interface |
Table 2.
List of patents surveyed for gradient scaffolds
| Patent Number | Author | Gradient | Intended Application |
|---|---|---|---|
| Application US20060121609 A1 (2006) | Yannas et al. (44) | Pore diameter, chemical composition, crosslink density | Soft tissue-bone interface |
| Application US20050118236 A1 (2005) | Qiu et al. (45) | Porosity, fiber/mesh density | Osteochondral interface |
| Issued, US 6306424 B1, US 6306424 B1 (2006) | Vyakarnam et al. (43) | Porosity of lyophilized foams | Osteochondral interface |
| Issued, US7524513 B2 (2009) | Hai-Quan et al. (46) | Covalently bonded biofunctional ligands and growth factors | Vascular –smooth muscle interface |
| Application US20070212730 A1 US20130230491 A1 (2013) | Vepari et al. (47) | Covalently bonded proteins and enzymes | Bone-ligament interface |
| Issued, US 8277832 B2 (2014) | Detamore et al. (48) | Physical properties of microspheres | Osteochondral interface |
| Issued, US 8986380 B2 (2015) | Kaplan et al. (49) | Multilayered silk scaffold with pore gradient | Meniscus tissue |
| Issued, US7767221 B2 (2010) | Lu et al. (50) | Physical properties of microspheres incorporated into a composite scaffold | Soft tissue-bone interface |
Table 3.
List of Patents Surveyed for Scaffoldless Tissue Engineering Techniques
| Patent | Inventor | Intended Application |
|---|---|---|
| Issued, US 8143055 B2 (2012) | Forgacs et al. (66) | Self-assembled Multi-cellular bodies for various TE applications |
| Issued, WO2005012512 A1, EP 1651756 B1 (2005) | Nakamura et al. (67) | Musculoskeletal interfaces |
| Application, WO2007115336 A3 (2008) | Athanasiou et al. (68) | Fibrocartilage tissue engineering |
| Issued, US8097455 (2012) | Larkin et al. (69) | Musculo-tendon junction tissue engineering |
| Application, WO2011157057 A1 (2011) | Chan et al. (70) | Complex-tissue engineering |
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments -- an adaptation to compressive load. J Anat. 1998;193(Pt 4):481–94. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo SLY, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: injury, healing, and repair. Journal of Biomechanics. 2006;39(1):1–20. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Trotter JA. Structure–function considerations of muscle–tendon junctions. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2002;133(4):1127–33. doi: 10.1016/s1095-6433(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 4.Hoemann CD, Lafantaisie-Favreau C-H, Lascau-Coman V, Chen G, Guzmán-Morales J. The cartilage-bone interface. J Knee Surg. 2012;25(2):85–97. doi: 10.1055/s-0032-1319782. [DOI] [PubMed] [Google Scholar]
- 5.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The Outcome and Repair Integrity of Completely Arthroscopically Repaired Large and Massive Rotator Cuff Tears. J Bone Joint Surg Am. 2004;86(2):219–24. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to Bone Healing: Differences in Biomechanical, Structural, and Compositional Properties Due to a Range of Activity Levels. J Biomech Eng. 2003;125(1):106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 7.Silva MJ, Thomopoulos S, Kusano N, Zaegel MA, Harwood FL, Matsuzaki H, et al. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. Journal of Orthopaedic Research. 2006;24(5):990–1000. doi: 10.1002/jor.20084. [DOI] [PubMed] [Google Scholar]
- 8.Moffat KL, Wang INE, Rodeo SA, Lu HH. Orthopaedic Interface Tissue Engineering for the Biological Fixation of Soft Tissue Grafts. Clin Sports Med. 2009;28(1):157–76. doi: 10.1016/j.csm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiavone Panni A, Fabbriciani C, Delcogliano A, Franzese S. Bone-ligament interaction in patellar tendon reconstruction of the ACL. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):4–8. doi: 10.1007/BF01552150. [DOI] [PubMed] [Google Scholar]
- 10.Schneider H. Structure of tendon attachments. Z Anat Entwicklungsgesch. 1956;119(5):431–56. [PubMed] [Google Scholar]
- 11.Weiler A, Scheffler S, Apreleva M. Healing of Ligament and Tendon to Bone. In: Walsh WR, editor. Repair and Regeneration of Ligaments, Tendons, and Joint Capsule. Orthopedic Biology and Medicine. Humana Press; 2006. pp. 201–31. [Google Scholar]
- 12.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites ('entheses') in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–90. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AP. Articular Cartilage Repair. Am J Sports Med. 1998;26(2):309–24. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 14.Allan KS, Pilliar RM, Wang J, Grynpas MD, Kandel RA. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007;13(1):167–77. doi: 10.1089/ten.2006.0081. [DOI] [PubMed] [Google Scholar]
- 15.Bailey AJ, Shellswell GB, Duance VC. Identification and change of collagen types in differentiating myoblasts and developing chick muscle. Nature. 1979;278(5699):67–9. doi: 10.1038/278067a0. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez F, Rifkin DB. Cell signaling events: a view from the matrix. Matrix Biology. 2003;22(2):101–7. doi: 10.1016/s0945-053x(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 17.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiological reviews. 2004;84(2):649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 18.Chiquet M, Renedo AS, Huber F, Flück M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22(1):73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 19.Flück M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol. 1999;277(1 Pt 1):C152–62. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- 20.Gordon SE, Flück M, Booth FW. Selected Contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol. 2001;90(3):1174–83. doi: 10.1152/jappl.2001.90.3.1174. discussion 65. [DOI] [PubMed] [Google Scholar]
- 21.Charvet B, Ruggiero F, Le Guellec D. The development of the myotendinous junction. A review. Muscles Ligaments Tendons J. 2012;2(2):53–63. [PMC free article] [PubMed] [Google Scholar]
- 22.Lu HH, Thomopoulos S. Functional Attachment of Soft Tissues to Bone: Development, Healing, and Tissue Engineering. Annual Review of Biomedical Engineering. 2013;15(1):201–26. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown KRU, Yuan, Jenny J(US), Yufu LI(US), Zimmermann, Mark C(US) inventor; ETHICON INC (US), assignee. EP1270025 B1. Porous ceramic/porous polymer layered scaffolds for the repair and regeneration of tissue patent. 2006
- 24.Pavesio A, Callegaro L inventors; Fidia Advanced Biopolymers S.R.L. Pavesio, Alessandra Callegaro, Lanfranco, assignee. US7968111 B2. Grafts For The Repair Of Osteochondral Defects patent. 2011
- 25.Athanasiou KA, Boyan, Barbara D inventor; Board Of Regents, The University Of Texas System, assignee. US5607474 A. Multi-phase bioerodible implant/carrier and method of manufacturing and using same patent. 1993
- 26.Lu Helen H (US) Jju, Hung Clark T (US), Guo X Edward (US), Ateshian Gerard (US) inventor; Ethicon, Inc, assignee. Polymer-ceramic-hydrogel composite scaffold for osteochondral repair. US. 2006 [Google Scholar]
- 27.Tampieri A, Pressato D, De Luca C, Di Fede S inventors; Fin-Ceramica Faenza S.P.A., assignee. Cartilaginiform and osteochondral sustitute comprising a multilayer structure and use thereof. 2009 [Google Scholar]
- 28.Gleeson JP, Levingstone, Tanya J, O'brien, Fergal J inventor; Royal College Of Surgeons In Ireland (Dublin, IE), assignee. US8613943 B2. Process for producing a multi-layered scaffold suitable for osteochondral repair patent. 2013
- 29.Lu HHS Jeffrey inventor: The Trustees of Columbia University in the City of New York (New York, NY, US) assignee. US8753391 B2. Fully synthetic implantable multi-phased scaffold patent. 2014
- 30.Laurencin CT, Aronson, Mark T, Nair, Lakshmi Sreedharan inventor; Soft Tissue Regeneration, Inc, assignee. US8486143 B2. Mechanically competent scaffold for ligament and tendon regeneration patent. 2014
- 31.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. Journal of biomechanics. 2007;40(4):750–65. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Niederauer GG, Slivka MA, Leatherbury NC, Korvick DL, Harroff HH, Ehler WC, et al. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000;21(24):2561–74. doi: 10.1016/s0142-9612(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Grogan SP, Rieser F, Winkelmann V, Maquet V, Berge ML, et al. Tissue engineering of biphasic cartilage constructs using various biodegradable scaffolds: an in vitro study. Biomaterials. 2004;25(17):3681–8. doi: 10.1016/j.biomaterials.2003.10.102. [DOI] [PubMed] [Google Scholar]
- 34.Yang PJ, Temenoff JS. Engineering Orthopedic Tissue Interfaces. Tissue Engineering Part B: Reviews. 2009;15(2):127–41. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16(10):2247–70. doi: 10.1111/j.1582-4934.2012.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauck RL, Wang CCB, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis and Cartilage. 2003;11(12):879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Lu HH, El-Amin SF, Scott KD, Laurencin CT. Three-dimensional, bioactive, biodegradable, polymer–bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. Journal of Biomedical Materials Research Part A. 2003;64A(3):465–74. doi: 10.1002/jbm.a.10399. [DOI] [PubMed] [Google Scholar]
- 38.Soo Kim B, Ji Kim E, Suk Choi J, Hoon Jeong J, Hyunchul Jo C, Woo Cho Y. Human collagen-based multilayer scaffolds for tendon-to-bone interface tissue engineering. Journal of Biomedical Materials Research Part A. 2014 doi: 10.1002/jbm.a.35057. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 39.Tampieri A, Sandri M, Landi E, Pressato D, Francioli S, Quarto R, et al. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials. 2008;29(26):3539–46. doi: 10.1016/j.biomaterials.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Tampieri A, Celotti G, Landi E, Sandri M, Roveri N, Falini G. Biologically inspired synthesis of bone-like composite: self-assembled collagen fibers/hydroxyapatite nanocrystals. J Biomed Mater Res A. 2003;67(2):618–25. doi: 10.1002/jbm.a.10039. [DOI] [PubMed] [Google Scholar]
- 41.Laurencin CT, Freeman JW. Ligament tissue engineering: An evolutionary materials science approach. Biomaterials. 2005;26(36):7530–6. doi: 10.1016/j.biomaterials.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 42.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Vyakarnam MN, Zimmerman MC, Scopelianos AG, Chun I, Melican MC, Bazilio CA, et al. inventors; Ethicon Inc. (Somerville, NJ, US), assignee. US7112417 B2. Foam composite for the repair or regeneration of tissue patent. 2006
- 44.Yannas I, Gibson L, O'Brien F, Harley B, Brau R, Samouhos S, et al. inventors; Yannas Ioannis V, assignee. US20060121609 A1. [2006/06/08/];Gradient scaffolding and methods of producing the same patent. 2006
- 45.Qiu Q-Q, Cohen C, Ducheyne P inventors; Gentis Inc, assignee. US20050118236 A1. [2005/06/02/];Bioactive, resorbable scaffolds for tissue engineering patent. 2005
- 46.Hai-Quan M, Kuan CM, Leong KW inventors; Mao Hai-Quan, assignee. US7524513 B2. [2009/04/28/];Biofunctional fibers patent. 2009
- 47.Vepari C, Kaplan DL, Vunjak-novakovic G inventors. Covalently immobilized protein gradients in three-dimensional porous scaffolds. 2013 [Google Scholar]
- 48.Detamore MS, Milind, Scurto, Aaron M, Berkland Cory inventor; The University of Kansas (Lawrence, KS, US) assignee. US8669107 B2. Method of preparing a tissue engineering scaffold comprising a plurality of microspheres linked together patent. 2014
- 49.Kaplan DLM, Biman B inventor; Trustees of Tufts College (Medford, MA, US) assignee. US8986380 B2. Multilayered silk scaffolds for meniscus tissue engineering patent. 2015
- 50.Lu HH, Vaeroy H, Dionisio K inventors; The Trustees of Columbia University in the City of New York (New York, NY, US), assignee. US7767221 B2. Multi-phased, biodegradable and osteointegrative composite scaffold for biological fixation of musculoskeletal soft tissue to bone patent. 2010
- 51.Karande TS, Ong JL, Agrawal CM. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann Biomed Eng. 2004;32(12):1728–43. doi: 10.1007/s10439-004-7825-2. [DOI] [PubMed] [Google Scholar]
- 52.Oh SH, Park IK, Kim JM, Lee JH. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials. 2007;28(9):1664–71. doi: 10.1016/j.biomaterials.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 53.Huang C, Yannas IV. Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J Biomed Mater Res. 1977;11(1):137–54. doi: 10.1002/jbm.820110113. [DOI] [PubMed] [Google Scholar]
- 54.Dagalakis N, Flink J, Stasikelis P, Burke JF, Yannas IV. Design of an artificial skin. Part III. Control of pore structure. J Biomed Mater Res. 1980;14(4):511–28. doi: 10.1002/jbm.820140417. [DOI] [PubMed] [Google Scholar]
- 55.Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Progress in Polymer Science. 2007;32(7):698–725. [Google Scholar]
- 56.Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134(2):81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dewez JL, Lhoest JB, Detrait E, Berger V, Dupont-Gillain CC, Vincent LM, et al. Adhesion of mammalian cells to polymer surfaces: from physical chemistry of surfaces to selective adhesion on defined patterns. Biomaterials. 1998;19(16):1441–5. doi: 10.1016/s0142-9612(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 58.Brown JL, Nair LS, Laurencin CT. Solvent/non-solvent sintering: a novel route to create porous microsphere scaffolds for tissue regeneration. J Biomed Mater Res Part B Appl Biomater. 2008;86(2):396–406. doi: 10.1002/jbm.b.31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi X, Wang Y, Varshney RR, Ren L, Gong Y, Wang D-A. Microsphere-based drug releasing scaffolds for inducing osteogenesis of human mesenchymal stem cells in vitro. Eur J Pharm Sci. 2010;39(1–3):59–67. doi: 10.1016/j.ejps.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Ionescu LC, Lee GC, Sennett BJ, Burdick JA, Mauck RL. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31(14):4113–20. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, et al. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering. J Control Release. 2003;91(3):365–74. doi: 10.1016/s0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- 62.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–21. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix Development in Self-Assembly of Articular Cartilage. PLoS ONE. 2008;3(7) doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13(9):2195–205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 65.Athanasiou KA, Eswaramoorthy R, Hadidi P, Hu JC. Self-Organization and the Self-Assembling Process in Tissue Engineering. Annual Review of Biomedical Engineering. 2013;15(1):115–36. doi: 10.1146/annurev-bioeng-071812-152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forgacs GM, Francoise Suzanne, Norotte Cyrille (Paris, FR) inventor; The Curators of the University of Missouri (Columbia, MO, US), assignee. US8143055 B2. Self-assembling multicellular bodies and methods of producing a three-dimensional biological structure using the same patent. 2012
- 67.Nakamura N, Matsuda H, Sawa Y, Taketani S, Miyagawa S, Yoshikawa H, et al. inventors; Norimasa Nakamura, assignee. WO2005012512 A1. [2005/02/10/];Scaffold-free self-organized 3d synthetic tissue patent. 2005
- 68.Athanasiou KA, Aufderheide A, Hu J inventors; Kyriacos A Athanasiou, assignee. WO2007115336 A3. [2008/05/22/];A shape-based approach for scaffoldless tissue engineering patent. 2008
- 69.Larkin LMA, Ellen M, Calve, Sarah, Kostriminova, Tatiana Y inventor; The Regents Of The University Of Michigan (Ann Arbor, MI, US), assignee. US8097455 B2. System and method for forming skeletal muscle constructs having functional tissue interfaces patent. 2012
- 70.Chan PB, Cheng H, Chik TD, Cheung MK, Luk DK inventors; The University Of Hong Kong, assignee. WO2011157057 A1. [2011/12/22/];Methods for complex tissue engineering patent. 2011
- 71.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. Journal of Biomedical Materials Research Part A. 2008;86A(1):1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 72.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89(11):2485–97. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 73.Manning CN, Kim HM, Sakiyama-Elbert S, Galatz LM, Havlioglu N, Thomopoulos S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011;29(7):1099–105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]