Abstract
Purpose
We examine the relationship between urine and stone cultures in a large cohort of patients undergoing percutaneous stone removal and compare the findings in infectious vs metabolic calculi.
Materials and Methods
A total of 776 patients treated with percutaneous nephrolithotomy who had preoperative urine cultures and intraoperative stone cultures were included in the study. Statistical analysis used chi-square or logistic fit analysis as appropriate.
Results
Preoperative urine culture was positive in 352 patients (45.4%) and stone cultures were positive in 300 patients (38.7%). There were 75 patients (9.7%) with negative preoperative cultures who had positive stone cultures, and in patients with both cultures positive the organisms differed in 103 (13.3%). Gram-positive organisms predominated in preoperative urine and stone cultures.
Conclusions
Preoperative urine cultures in patients undergoing percutaneous nephrolithotomy are unreliable as there is a discordance with intraoperative stone cultures in almost a quarter of cases. There has been a notable shift toward gram-positive organisms in this cohort of patients.
Keywords: kidney calculi, nephrolithiasis, urinary tract infections, nephrostomy, percutaneous
Renal calculi requiring percutaneous nephrolithotomy commonly harbor infectious organisms even when the stone is metabolic in origin.1–3 Furthermore, a sterile voided urine does not preclude the presence of pathogens in the stone4,5 or the development of postoperative bacteriuria.6 Several recent studies have found that the risk of infectious complications after PCNL correlate better with stone cultures than preoperative voided or renal pelvic cultures.7–9 These studies further noted a poor correlation between voided and stone cultures but are limited by small patient numbers.7–10 Although these groups have recommended the routine collection of stone cultures during PCNL, this is not standard practice for most urologists and the AUA (American Urological Association) guidelines make no recommendations in this regard.4 The European Association of Urology guidelines on urolithiasis state that intraoperative renal stone culture may help to select postoperative antibiotics.11
In this study we determine the ability of preoperative urine cultures to predict the presence of infection in kidney stones and identify the organism(s) present in the stone. Stone composition is analyzed in relation to urine and stone culture findings. We report a shift toward a predominance of gram-positive organisms associated with this complex cohort of patients requiring PCNL. Finally, we present our data regarding sepsis after PCNL, which is among the most significant complications after this procedure. To our knowledge this study represents the largest series of patients undergoing PCNL reported to date evaluating the correlation between preoperative urine culture and stone culture.
METHODS
An institutional review board approved prospectively collected database (IRB#101002243) has been maintained at our institution since April 1999 for all PCNL procedures. Informed consent was obtained from all patients entered in the database. We retrospectively identified 1,295 consecutive patients who underwent PCNL for renal stones at our institution between April 1999 and January 2014. Patients who met the study inclusion criteria had results for preoperative urine culture and intraoperative stone culture.
At our institution a preoperative bladder urine culture is obtained from all patients scheduled to undergo PCNL. All preliminary urine cultures reported as contaminated are requested to be fully speciated by the microbiology laboratory. Patients with a positive urine culture or a history of urosepsis or recurrent urinary tract infections are treated before PCNL for at least 2 weeks with culture specific antibiotics. Those with a negative urine culture are routinely treated for 1 week with oral antibiotics (usually a fluoroquinolone unless contraindicated by patient allergy). All patients receive a prophylactic antibiotic intravenously before the start of surgery.
At our institution PCNL is a single stage procedure. Initially a ureteral catheter is placed. With the patient in the prone position, access is established by the treating urologist under fluoroscopic guidance. The nephrostomy tract is balloon dilated and a 30Fr Amplatz sheath is placed in all instances. Stone material is fragmented using a combination of ultrasonic and pneumatic lithotripsy as indicated. Sterile graspers are used to retrieve stone fragments, which are immediately transferred to a sterile collection cup containing sterile saline. Care is taken with the stone to avoid skin contaminants. The stone fragments are crushed into powder using a sterile needle driver. The specimen is then sent for culture and sensitivity.
Flexible nephroscopy is performed in all cases. A nephrostomy tube, usually a 10Fr Cope loop (Cook Urological, Bloomington, Indiana) is placed at the conclusion of the PCNL procedure in all cases. Noncontrast CT is performed on postoperative day 1. If CT shows no residual stones, a nephrostogram is obtained. If adequate drainage is confirmed, the nephrostomy tube is removed and the patient is discharged from the hospital. If drainage is poor or absent, the patient is discharged home with the nephrostomy tube and scheduled for a repeat nephrostogram a few days later. When remaining stone fragments are identified on CT, second look nephroscopy is performed on postoperative day 2. Patients can generally be discharged home after the secondary procedure. Antibiotics are continued in the postoperative period for at least 1 week. If the results of the stone culture are positive, antibiotics are adjusted based on sensitivities and continued for 3 months after surgery.
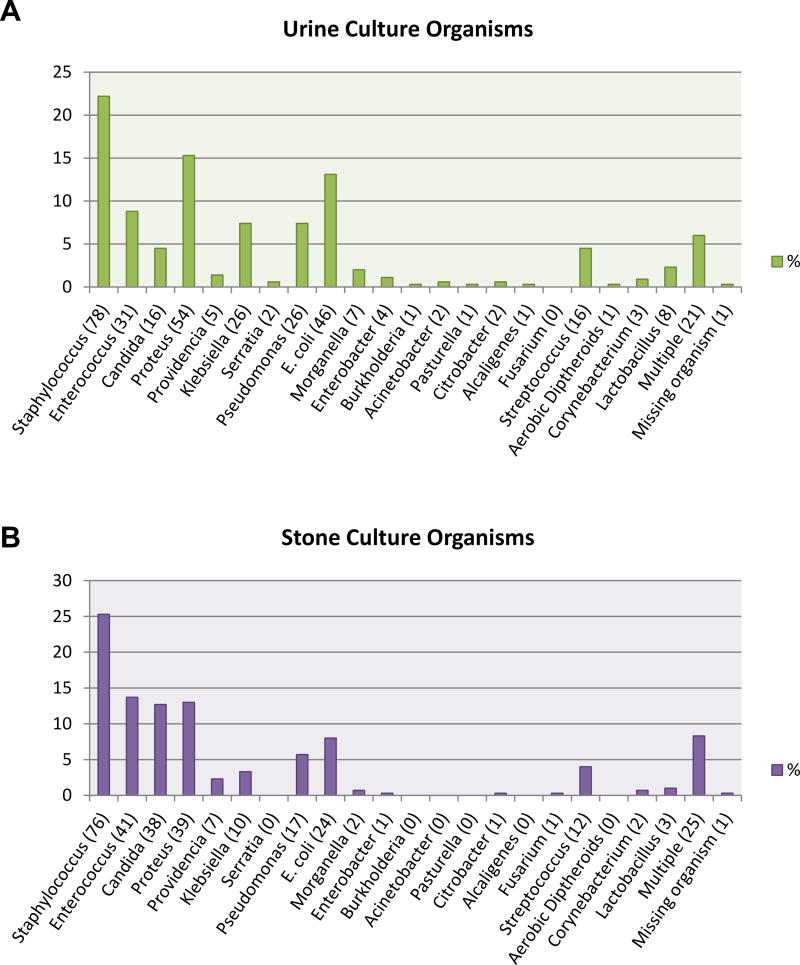
In situations where a culture grew multiple organisms with only one being a urease producing organism, that case was grouped in the category of urea splitting organisms.12 When a culture grew multiple organisms without a urease producing organism or had more than 1 urease producing organism, that case was grouped in multiple organisms. Multiple organisms were cultured in 6% of preoperative urine cultures and 8.3% of stone cultures (see figure). The identity of organisms cultured was missing for 1 patient in each of the preoperative urine culture and stone culture groups. Statistical analysis used chi-square or logistic fit analyses as appropriate. Calculations were done using JMP® software.
Figure.
Types of organisms identified in preoperative urine culture (A) and intraoperative stone culture (B). Numbers in parentheses represent number of patients.
RESULTS
Of 1,295 consecutive patients 828 had results for intraoperative stone culture. Among these patients 776 also had results for preoperative urine culture and were included in the final analysis. Patient and stone demographics are summarized in table 1. Overall 352 (45.4%) patients had a positive preoperative urine culture while 300 (38.7%) had a positive stone culture. The figure illustrates the type and number/percentage of organisms identified in bladder urine (part A) and kidney stones (part B). Staphylococcus was the most common organism cultured from preoperative urine cultures (22.2%) and kidney stones (25.3%). Other common organisms found on preoperative urine culture were proteus species (15.3%), Escherichia coli (13.1%) and enterococcus (8.8%). Other common stone culture pathogens were enterococcus (13.7%), followed by proteus (13%) and candida (12.7%).
Table 1.
Patient and stone demographics
| Mean pt age (range) | 53.2 | (3–90) |
| No. gender (%): | ||
| M | 358 | (46.1) |
| F | 418 | (53.9) |
| Unilat stones: | ||
| No. pts | 590 | |
| No. rt side | 252 | |
| No. lt side | 338 | |
| No. size greater than 2 cm (%) | 476 | (61.3) |
| No. size less than 2 cm (%) | 107 | (13.8) |
| No. size not recorded | 2 | |
| Bilat stones: | ||
| No. pts | 186 | |
| No. size greater than 2 cm (%) | 162 | (20.9) |
| No. size less than 2 cm (%) | 22 | (2.8) |
| No. size not recorded | 7 |
Both cultures were negative in 349 (45%) patients. Overall 127 (16.4%) patients with positive preoperative urine cultures had negative stone cultures. On the other hand, a positive stone culture in the presence of sterile urine was identified in 75 (9.7%) patients. There were 225 (29%) cases with positive preoperative urine and stone cultures. The same organisms were identified in both cultures in 122 (54.2%) patients. However, different organisms were isolated between cultures in 103 (45.8%) patients, 13.3% overall. For this analysis the cultures were considered discordant if any pathogen was cultured in the stone that was not cultured in the urine. Based on these data, preoperative urine culture was able to predict the correct organism(s) in kidney stones with 62% sensitivity, 60% specificity and a positive predictive value of only 35% (table 2).
Table 2.
Preoperative bladder urine culture as a predictor of intraoperative stone culture results
| Any Pos Stone Culture |
Same Organism Found in Urine |
|
|---|---|---|
| Sensitivity for predicting stone culture results | 0.75 | 0.62 |
| Specificity for predicting stone culture results | 0.73 | 0.60 |
| Pos predictive value of urine culture | 0.64 | 0.35 |
Sepsis developed in 13 (1.7%) patients in the postoperative period (table 3). Of the 13 patients 9 had discordance between urine and stone culture. In 1 patient the same organism grew in both cultures, 1 had a positive urine culture and a negative stone culture, and 2 had both cultures negative. Of the patients with sepsis 11 had blood cultures obtained, of which 4 were positive. Blood and stone cultures were concordant in 2 patients while none of the blood and urine cultures agreed. Only 1 patient experiencing sepsis had stones composed of struvite and, in this case, both cultures were positive for a proteus organism. The remaining 12 patients in whom sepsis developed after PCNL had metabolic stones.
Table 3.
Operative data comparing patients who did or did not experience sepsis
| Sepsis | No Sepsis | |
|---|---|---|
| Mean mins surgery time (range) | 162 (64–313)* | 128 (28–400) |
| Mean tracts (range) | 2.0 (1–5) | 1.39 (1–7) |
| No./total No. pos blood cultures | 4/11† | Not applicable |
Surgery time not available for 3 patients.
Blood cultures not done in 2 patients.
We further analyzed our urine and stone culture data with respect to stone composition. Patients were separated into a group with metabolic stones and a group whose stones contained mixtures of struvite and/or highly carbonated apatite (table 4). Among patients with metabolic stones 39.1% (252 of 645) had a positive urine culture and 32.7% (211 of 645) had a positive stone culture. Among patients with stones containing struvite and/or carbonate apatite, urine and stone cultures were positive in 79.2% (99 of 125) and in 69.6% (87 of 125), respectively. Thus, the likelihood of a positive urine or stone culture was considerably greater in patients having struvite and/or carbonate apatite stones (p <0.0001). However, 12% of patients with struvite and/or carbonate apatite stones were found to have negative urine and stone cultures. Stone analysis was not performed in 6 patients.
Table 4.
Urine and stone cultures with respect to stone composition
| No. Struvite/Highly Carbonated Apatite Stone (%) |
No. Metabolic Stone (%) |
|||
|---|---|---|---|---|
| Both cultures neg | 15 | (12) | 330 | (51.2) |
| Pos urine culture only | 23 | (18.4) | 104 | (16.1) |
| Pos stone culture only | 11 | (8.8) | 63 | (9.8) |
| Both cultures pos | 76 | (60.8) | 148 | (22.9) |
|
|
|
|||
| Totals | 125 | 645 | ||
A subgroup analysis of struvite and/or carbonate apatite stone formers with positive stone cultures was performed. Urease producing organisms were isolated in 75.9% (66 of 87) of patients and nonurease producing organisms were cultured in 23% (20 of 87). The identity of the organism(s) cultured was missing for 1 patient. Overall a negative stone culture or nonurease producing organism was found in 42.4% (53 of 125) of patients with struvite and/or carbonate apatite calculi.
DISCUSSION
There are 2 broad themes suggested by these data. This large cohort confirms the findings of earlier, smaller series that preoperative urine culture is an unreliable predictor of the presence of a positive stone culture. In our data 9.7% of cases had a positive stone culture in the face of a negative preoperative urine culture. Furthermore, discordance between preoperative urine culture and stone culture was common (13.3% of cases), meaning that almost a quarter of the patients in this series undergoing PCNL had organisms identified on stone culture that were not apparent from preoperative urine cultures.
The improper identification of urinary pathogens may result in devastating consequences for patients in whom sepsis develops in the postoperative period. Among the patients with sepsis in this study 9 of 13 (69.2%) had discordance between urine and stone cultures. Although the reported rates of sepsis after PCNL are relatively low, the treatment of severe sepsis with inappropriate antimicrobial coverage may lead to significant morbidity and mortality as well as increased health care expenditures. The American Urological Association Best Practice Policy Statement on Urological Surgery Antimicrobial Prophylaxis makes recommendations regarding perioperative antibiotics for PCNL.13 The 2007 Guideline for the Management of Ureteral Calculi recommends obtaining a urine culture and treating bacteriuria before intervention.14 However, stone culture is not mentioned in either of these publications.
Reports of the occurrence of sepsis after PCNL in the literature range from 0.9% to 4.7%.15 The differences among studies are likely related to highly variable definitions and methods of data collection.15 Often studies addressing the usefulness of stone cultures report the incidence of SIRS after PCNL.7–9 However, SIRS criteria are nonspecific, and may be attributed to any number of etiologies in the postoperative period, including major surgical procedures, cardiogenic events, atelectasis, hypovolemia and pain.16 Therefore, we chose to analyze our data based on the occurrence of septic events which require the presence of a documented infection in addition to SIRS. Our hospital has a tertiary referral practice specializing in complex stones. Such a patient base would presumably increase the number of infectious stones, but nevertheless the sepsis rate in this study was low (1.7%). We attribute much of this to our practice pattern regarding the administration of preoperative, perioperative and postoperative antibiotics, the routine collection of stone cultures, consistent use of flexible nephroscopy to remove fragments and an emphasis on achieving stone-free status through secondary procedures. Resistant organisms and Clostridium difficile infections have not been an issue in our experience, which is consistent with the recent publication from the Mayo Clinic Rochester, where similar perioperative antibiotic protocols are used.17
In addition, it is clear that the microbiology of stone disease has changed dramatically during the last generation from predominantly gram-negative to now predominantly gram-positive organisms.18–21 There are a number of possible explanations. Several groups have documented the decreasing frequency of struvite stones in the adult and pediatric/neurogenic patient population.21,22 In this series proteus and morganella species were identified in only 13.7% (41 of 300) of positive stone cultures and 17.2% (61 of 354) of positive preoperative urine cultures. Clearly the major advances in the urological care of patients with structural and neurogenic disorders have had an impact on reducing stones resulting from ureolysis.
The dramatic developments in the minimally invasive treatment of stone disease (ureteral stenting, extracorporeal shock wave lithotripsy, ureteroscopy, PCNL) may also be a factor in the microbiology of stone disease. Open surgical lithotomy has been relegated to historical status and has been supplanted by approaches using a much larger number of minimally invasive procedures. These entail repeated episodes of instrumentation of the urinary tract, providing opportunities for the introduction of gram-positive organisms.
The finding of candida in 12% of stone cultures deserves comment for several reasons. Our tertiary referral practice accrues many patients with complex stones requiring percutaneous stone removal who have neurogenic bladders, indwelling catheters of some sort, urinary diversion or who have undergone instrumentation on multiple occasions. These patients have typically received multiple courses of antibiotics before they reach us and, as a group, are at high risk for candida infections. Indeed, 1 of 4 patients with positive blood cultures had candida species grow. For this reason, patients with the previously mentioned risk factors are treated preoperatively with antifungal agents.
Also noteworthy is the finding of enterococcus in 13.7% (41 of 300) of patients with a positive stone culture as this organism is not covered by cephalosporins and is often resistant to fluoroquinolones. AUA guidelines permit the use of cephalosporins as routine prophylaxis before PCNL. Informing this concern is the observation that 2 of 4 cases with sepsis and positive blood cultures grew enterococcus.
Many staghorn stones contain struvite (magnesium ammonium phosphate) and/or carbonate apatite.4 Stones of this composition are associated with microorganisms that produce urease, an enzyme that catalyzes the hydrolysis of urea into ammonia and hydrogen ions.4,5 Stone mineral composition has been proposed as a marker for the presence of infectious organisms in a stone. However, Englert et al showed that among positive stone cultures, urease producing organisms were cultured in 71% of cases with no difference observed among stones of different compositions.23 They concluded that mineral content is an imperfect indicator of the presence of infectious organisms in kidney stones, an observation consistent with the data reported here. It may be that some struvite stones developed in the presence of bacteriuria but no longer contain viable organisms.2,6,24 However, to the surgeon the environment in which a stone formed is less important than the existence of pathogens in a stone at the time of PCNL when the risk of infectious complications is highest.9,22
In this study we found that 32.7% of metabolic stone formers contained infectious organisms in their stones. Interestingly 12 (92.3%) of our patients with sepsis were metabolic stone formers. Despite the popular belief that patients with struvite stones carry a higher risk of severe infectious sequelae, our data suggest that it is the patient with recurrent metabolic stones whose stones often become secondarily infected with resistant organisms after repeated instrumentation and multiple courses of antibiotics who is at higher risk for sepsis after PCNL.
Finally, the process of obtaining a stone culture at PCNL is easy and usually requires less than 1 minute of additional operative time. The cost of a stone culture is minimal compared to the cost of a single septic event. We encourage all urologists to incorporate stone culture at PCNL into routine practice.
CONCLUSIONS
Obtaining an intraoperative stone culture is an easy, inexpensive and valuable practice that should be a routine part of any percutaneous stone removal procedure. The rate of discordance between urine and stone cultures in this study was 23%, consistent with the results of previous smaller studies and strengthened by this large cohort of patients. Gram-positive organisms are considerably more common in the current era of patients requiring PCNL.
Abbreviations and Acronyms
- CT
computerized tomography
- PCNL
percutaneous nephrolithotomy
- SIRS
systemic inflammatory response syndrome
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
References
- 1.Larsen EH, Gasser TC, Madsen PO. Antimicrobial prophylaxis in urologic surgery. Urol Clin North Am. 1986;13:591. [PubMed] [Google Scholar]
- 2.Hugosson J, Grenabo L, Hedelin H, et al. Bacteriology of upper urinary tract stones. J Urol. 1990;143:965. doi: 10.1016/s0022-5347(17)40152-2. [DOI] [PubMed] [Google Scholar]
- 3.McCartney AC, Clark J, Lewi HJ. Bacteriological study of renal calculi. Eur J Clin Microbiol. 1985;4:553. doi: 10.1007/BF02013393. [DOI] [PubMed] [Google Scholar]
- 4.Preminger GM, Assimos DG, Lingeman JE, et al. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005;173:1991. doi: 10.1097/01.ju.0000161171.67806.2a. [DOI] [PubMed] [Google Scholar]
- 5.Bruce RR, Griffith DP. Retrospective follow-up of patients with struvite calculi. In: Smith LH, Robertson WGL, Finlayson B, editors. Urolithiasis Clinical and Basic Research. New York: Plenum Press; 1981. p. 191. [Google Scholar]
- 6.Charton M, Vallancien G, Veillon B, et al. Urinary tract infection in percutaneous surgery for renal calculi. J Urol. 1986;135:15. doi: 10.1016/s0022-5347(17)45500-5. [DOI] [PubMed] [Google Scholar]
- 7.Mariappan P, Smith G, Bariol SV, et al. Stone and pelvic urine culture and sensitivity are better than bladder urine as predictors of urosepsis following percutaneous nephrolithotomy: a prospective clinical study. J Urol. 2005;173:1610. doi: 10.1097/01.ju.0000154350.78826.96. [DOI] [PubMed] [Google Scholar]
- 8.Korets R, Graversen JA, Kates M, et al. Post-percutaneous nephrolithotomy systemic inflammatory response: a prospective analysis of preoperative urine, renal pelvic urine and stone cultures. J Urol. 2011;186:1899. doi: 10.1016/j.juro.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 9.Margel D, Ehrlich Y, Brown N, et al. Clinical implication of routine stone culture in percutaneous nephrolithotomy–a prospective study. Urology. 2006;67:26. doi: 10.1016/j.urology.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Gonen M, Turan H, Ozturk B, et al. Factors affecting fever following percutaneous nephrolithotomy: a prospective clinical study. J Endourol. 2008;22:2135. doi: 10.1089/end.2008.0139. [DOI] [PubMed] [Google Scholar]
- 11.Turk C, Knoll T, Petrik A, et al. Guidelines on Urolithiasis (updated 2015) Available at http://uroweb.org/guideline/urolithiasis/
- 12.Coe FL, Parks JH. Nephrolithiasis: Pathogenesis and Treatment. 2. Chicago: Year Book Medical; 1988. p. 279. [Google Scholar]
- 13.Wolf JS, Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Preminger GM, Tiselius HG, Assimos DG, et al. 2007 Guideline for the management of ureteral calculi. J Urol. 2007;178:2418. doi: 10.1016/j.juro.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 15.Violette PD, Denstedt JD. Standardizing the reporting of percutaneous nephrolithotomy complications. Indian J Urol. 2014;30:84. doi: 10.4103/0970-1591.124213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monga M. Re: Stone and pelvic urine culture and sensitivity are better than bladder urine as predictors of urosepsis following percutaneous nephrolithotomy: a prospective clinical study. J Urol. 2005;174:2069. doi: 10.1097/00005392-200511000-00141. [DOI] [PubMed] [Google Scholar]
- 17.Viers BR, Cockerill PA, Mehta RA, et al. Extended antimicrobial use in patients undergoing percutaneous nephrolithotomy and associated antibiotic related complications. J Urol. 2014;192:1667. doi: 10.1016/j.juro.2014.06.090. [DOI] [PubMed] [Google Scholar]
- 18.Lewi HJ, White A, Hutchinson AG, et al. The bacteriology of the urine and renal calculi. Urol Res. 1984;12:107. doi: 10.1007/BF00257174. [DOI] [PubMed] [Google Scholar]
- 19.Fowler JE., Jr Bacteriology of branched renal calculi and accompanying urinary tract infection. J Urol. 1984;131:213. doi: 10.1016/s0022-5347(17)50311-0. [DOI] [PubMed] [Google Scholar]
- 20.Gnessin E, Mandeville JA, Handa SE, et al. Changing composition of renal calculi in patients with musculoskeletal anomalies. J Endourol. 2011;25:1519. doi: 10.1089/end.2010.0698. [DOI] [PubMed] [Google Scholar]
- 21.Viprakasit DP, Sawyer MD, Herrell SD, et al. Changing composition of staghorn calculi. J Urol. 2011;186:2285. doi: 10.1016/j.juro.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 22.Mandel N, Mandel I, Fryjoff K, et al. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol. 2003;169:2026. doi: 10.1097/01.ju.0000065592.55499.4e. [DOI] [PubMed] [Google Scholar]
- 23.Englert KM, McAteer JA, Lingeman JE, et al. High carbonate level of apatite in kidney stones implies infection, but is it predictive? Urolithiasis. 2013;41:389. doi: 10.1007/s00240-013-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazin D, Andre G, Weil R, et al. Absence of bacterial imprints on struvite-containing kidney stones: a structural investigation at the mesoscopic and atomic scale. Urology. 2012;79:786. doi: 10.1016/j.urology.2011.08.054. [DOI] [PubMed] [Google Scholar]