Abstract
BACKGROUND
The gastrin-releasing peptide receptor (GRPr) is upregulated in early and late-stage human prostate cancer (PCa) and other solid tumors of the mammary gland, lung, head and neck, colon, uterus, ovary, and kidney. However, little is known about its role in prostate cancer. This study examined the effects of a heterologous GRPr agonist, bombesin (BBN), on growth, motility, morphology, gene expression, and tumor phenotype of an osteoblastic canine prostate cancer cell line (Ace-1) in vitro and in vivo.
METHODS
The Ace-1 cells were stably transfected with the human GRPr and tumor cells were grown in vitro and as subcutaneous and intratibial tumors in nude mice. The effect of BBN was measured on cell proliferation, cell migration, tumor growth (using bioluminescence), tumor cell morphology, bone tumor phenotype, and epithelial-mesenchymal transition (EMT) and metastasis gene expression (quantitative RT-PCR). GRPr mRNA expression was measured in primary canine prostate cancers and normal prostate glands.
RESULTS
Bombesin (BBN) increased tumor cell proliferation and migration in vitro and tumor growth and invasion in vivo. BBN upregulated epithelial-to-mesenchymal transition (EMT) markers (TWIST, SNAIL, and SLUG mRNA) and downregulated epithelial markers (E-cadherin and β-catenin mRNA), and modified tumor cell morphology to a spindle cell phenotype. Blockade of GRPr upregulated E-cadherin and downregulated VIMENTIN and SNAIL mRNA. BBN altered the in vivo tumor phenotype in bone from an osteoblastic to osteolytic phenotype. Primary canine prostate cancers had increased GRPr mRNA expression compared to normal prostates.
CONCLUSION
These data demonstrated that the GRPr is important in prostate cancer growth and progression and targeting GRPr may be a promising strategy for treatment of prostate cancer.
Keywords: GRPr, EMT, metastasis, canine, bombesin
INTRODUCTION
Prostate cancer (PCa) represents the second leading cause of cancer-related deaths among males with an estimated 27,540 deaths in the USA in 2015 [1]. PCa accounted for 37% of all cancers in African-American males in 2013 [2]. Histopathological studies have shown that up to 75% of men in their eighties will have foci of adenocarcinoma in their prostates [3]. It is important to have a better understanding of the different signaling pathways that play a role in prostate cancer invasion and metastasis. One of these pathways is GRPr signaling that was found to be upregulated in PCa. The gastrin-releasing peptide receptor (GRPr) is a tumor-associated cell membrane receptor that is over-expressed in PCa (62–100%) and many other solid tumors, for example, breast (38–72%), lung (85–100% of small cell type), head and neck (100%), pancreatic (75%), and brain cancer (85% of glioblastomas) [4–8]. Although GRPr is highly expressed in prostate cancer, few studies have investigated the role of the GRPr in the pathogenesis of prostate cancer.
GRPr is a subtype of the bombesin receptor family, which is a member of the G-protein coupled receptor superfamily. GRP receptors have a wide variety of biological functions such as gastrointestinal hormone release (e.g., gastrin), pancreatic and gastric exocrine secretions, and smooth muscle contraction. In many cancers, they have an autocrine growth effect [9–13]. Their preferred agonist is gastrin-releasing peptide (GRP), a bombesin-like peptide that is the natural ligand of GRPr in vivo in mammals [14–16]. GRP and GRP analogues selectively bind to the GRP receptor family. Interestingly, mammalian GRP is functionally and structurally similar to bombesin (BBN). BBN is a tetradecapeptide extracted from amphibian skin that shares C-terminus sequence homology (7 amino acids) with GRP and binds to all GRP receptors with high specificity and affinity similar to GRP. GRP has been shown to promote tumor growth and differentiation in multiple human cancers [17]. GRPr signaling promoted the development of androgen-independent prostate cancer [18,19], and activation of GRPr in the prostate resulted in upregulation of promoters of angiogenesis, which are essential for the development of metastasis [20].
Our hypothesis was that GRPr signaling will increase the proliferation, invasiveness, and tumor growth of prostate cancer. To test our hypothesis, we developed a PCa cell line that expressed recombinant human GRPr, called Ace-1-huGRPr. In this investigation, we determined the impact of GRPr signaling on tumor cell proliferation and migration, the expression of EMT genes after induction of the GRPr signaling pathway and measured the effect of bombesin (GRPr agonist) on tumor growth and phenotype in the subcutis and bone of nude mice. In addition, we measured GRPr expression in primary canine prostate cancers.
MATERIALS AND METHODS
Reagents and Cell Lines
Bombesin (GRPr agonist) was purchased from the Anaspec Company (Fremont, CA). A GRPr antagonist (G-Abz4-STAT), which binds to the receptor with high affinity was obtained from the laboratory of Dr. Tweedle (The Ohio State University). Five frozen tumor specimens from dog primary prostate cancers (OC, CB, FM, Probasco, and LuMa) were obtained from The Ohio State University (OSU) College of Veterinary Medicine Biospecimen Repository. The Ace-1 cell line has been developed in our lab from a primary prostatic carcinoma of an 8-year-old male castrated Labrador retriever [21]. The Ace-1 cells form mixed osteoblastic/osteolytic bone metastases in nude mice [21–24]. The cloned Ace-1-huGRPr cells were provided by coauthor, Dr. Michael Tweedle. A high expressing clone (clone 15) was selected based on cell binding assay using 125I-Tyr4-BBN and expression of huGRPr mRNA. Each cell expressed approximately 690,000 cell membrane receptors. The Ace-1 and Ace- 1-huGRPr cells were stably transfected with luciferase so that the cells could be imaged in vivo using bioluminescence [25]. The cell lines were cultured in DMEM/F12 containing 10% FBS and antibiotics and maintained at 37°C, 5% CO2, and 100% humidity.
Animals
Athymic 5-week-old male nude mice (NCr-nu/nu) were purchased from the OSU Comprehensive Cancer Center (OSUCCC) Target Validation Shared Resource (TVSR). Mice were kept according to the NIH standards established in the “Guidelines for the Care and Use of Laboratory Animals.” All procedures were in accordance with protocols approved by the Institutional Animal Care and Use Committee of The Ohio State University. The mice were subdivided after injection of tumor cells into two equal groups (control and treatment). Bombesin treatment was initiated one day after tumor cell injection. Bombesin (BBN) was injected intraperitoneally twice daily at a dose of 5 μg/kg. After 3 weeks (subcutaneous xenografts) and 2 weeks (intratibial xenografts), the mice were euthanized and tissues were collected.
Ace-1 Xenografts (Subcutaneous and Intratibial Injections)
Subcutaneous injection
Sixteen nude mice were injected subcutaneously (SQ) with 2 × 106 Ace-1- huGRPr cells that were transduced with the luciferase gene and suspended in 0.2 ml sterile Dulbecco’s phosphate-buffered saline (DPBS) with a 25-Ga needle. The mice were monitored daily and the tumor dimensions were recorded three times weekly using digital calipers. The tumor volume was calculated using the formula: length × width × height × 0.5. The tumor dimensions were recorded by two pathologists who were blinded to the treatment groups.
Intratibial injection
Six nude mice were injected intratibially (IT) with 50,0000 Ace-1-huGRPr cells suspended in 10 μl DPBS using a Hamilton syringe and a 27-Ga needle. The mice were anesthetized using 2.5% isoflurane and the right rear limb was sanitized with alcohol. Each mouse was placed in a supine position and the ankle was held using the thumb and index finger. The needle was introduced through the patellar ligament and directed into the tibial marrow space crossing the articular cartilage. The mice were monitored daily and tumor growth was recorded weekly using bioluminescent imaging using the Xenogen IVIS 100 imaging system (Caliper Life Sciences, Hopkington, MA).
Cell Proliferation Assay
Ace-1 and Ace-1-huGRPr cells (control and BBN-treated) were plated at 3.7 × 103 cells per well in 12- well plates (four replicates for each cell line). After 24 hr. (pre-incubation period), the cells were treated with 1 nM of BBN for 5 days and fresh medium was replaced each day. Cells were harvested at days 3 and 5 and counted using the Cellometer Auto T4 cell counter (Nexcelom Bioscience, Lawrence, MA). Detached cells were removed and wells were washed with DPBS. The adherent cells were trypsinized and diluted 1:1 with trypan blue. Live and dead cells were counted, and cell number and viability were recorded.
Wound Healing Assay
Cell migration was measured using a wound healing assay for Ace-1 and Ace-1-huGRPr cells (control and BBN-treated), which were seeded in 6- well plates at 4 × 104 cells/well and cultured until 100% confluence. The monolayers were wounded (scratched) in a straight line across the well with a 200 μl pipette tip. The wounded monolayers were then washed twice with DPBS to remove cell debris and loosely attached cells. Culture medium with and without 1 nM of BBN was added to the treatment and control wells, respectively. The wound area was subsequently inspected and photographed after 1, 3, 6, and 24 hr using an inverted-phase contrast microscope with digital camera. The wound area was calculated using Wimasis image analysis software (Winscratch; Munich, Germany).
Bombesin Titration and Time Course Experiments
To determine the optimum dose of BBN on gene expression, a titration experiment was performed with different concentrations of BBN (0, 0.1, 0.3, 1, 3, and 10 nM) added to both Ace-1 and Ace-1-huGRPr cells for 24 hr. Six-well plates were used for each cell line and each dose of BBN was performed in triplicate. In addition, a time course experiment was performed to determine the effect of GRPr signaling activation on gene expression. BBN (1.0 nM; the optimum dose determined from the BBN titration experiment) was added to the cell lines (Ace-1 and Ace-1-huGRPr) in triplicate at different time points (0, 30 min, 1, 3, 6, and 24 hr).
RNA Extraction and qRT-PCR
Total RNA was extracted from both Ace-1 and Ace- 1-huGRPr cells using the QuickGene RNA Extraction Kit (AutoGen, Holliston, MA, Cat. No. FK-RC-S2). The RNA was reverse transcribed into complementary DNA (cDNA) using the Superscript II First Strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). Real-time quantitative polymerase chain reaction (qRT-PCR) was performed using a Light Cycler 480 (Roche, IN). Samples were analyzed in triplicate and the relative expression was calculated using the comparative threshold cycle method [26]. qRT-PCR was performed for the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as well as genes that have a role in epithelial-mesenchymal transition (EMT) (VIMENTIN, TWIST, SLUG, SNAIL, CDH1 (E-cadherin), and CTNNB1 (β-catenin), and prostate cancer-bone interaction and prostate cancer metastasis including SRC, bone morphogenic protein- 2 (BMP2), Transforming growth factor beta (TGFβ), myoferlin (MYOF), runt-related transcription factor 2 (RUNX2), receptor activator of NF-κB ligand (RANKL), and osteopontin (SPP1) in addition to both canine and human GRPr. The selection of each primer pair was based on the best amplification and qRT-PCR product melting characteristics from three to four different primer pairs designed for each gene. All primer pairs were designed using Primer- BLAST software (http://www.ncbi.nlm.nih.gov/tools/primer-blast) (Table I). In order to confirm primer specificity, qRT-PCR products for each gene were verified by gel electrophoresis and sequencing.
TABLE I.
Primers Used for qRT-PCR
| Gene | Forward primers | Reverse primers |
|---|---|---|
| BMP2 | TGCGCAGCTTCCACCACGAAGAA | CAAAGGTTCCTGCATCTGTTCCCGA |
| SLUG | GGCAAGGCGTTTTCCAGACCCT | GGGCAAGAAAAAGGCTTCTCCCCAG |
| TWIST | GGCAGGGCCGGAGACCTAGATG | TCCACGGGCCTGTCTCGCTT |
| SNAIL | GTCTGTGGCACCTGCGGGAAG | GAAGGTTGGAGCGGTCGGCA |
| RUNX2 | TGCCTCTGGCCTTCCACTCTCAG | TGCATTCGTGGGTTGGAGAAGCG |
| E-cadherin | GCTGCTGACCTGCAAGGCGA | GGCCGGGGTATCGGGGACAT |
| GAPDH | CCCACTCTTCCACCTTCGAC | AGCCAAATTCATTGTCATACCAGG |
| MYOF | TGCCCCCGAAAGGCTGGGAAT | ACTCCGTGTGCCCTGCGTCT |
| RANKL | TCCGAGCCGCTGTACAAAA | AGTATGAGTCTTGCCCCTCCT |
| VIMENTIN | GAGGACATCATGCGGCTGCGG | CGCTCAAGGTCAAGACGTGCC |
| Osteopontin | GGTTCATATGATGGCCGAGGT | CAGAGGTGCCTCTCACTGTC |
| β-catenin | CAGGTGGCGGACAAGAAGAT | CGCCGGAGATAGAACTCGTC |
| SRC | TATCCAGGCTGAGGAGTGGT | TCCGACACAGAGAGGCAGTA |
| GRPr | TGCCCCATACCCACACTCTA | CGTTTCCGGGATTCAATCTGC |
| TGFβ | AGGAGGTCACCCGCGTGCTAA | GAGCCTCAGCAGGCGCAACT |
Bioluminescent Imaging
Mice were injected intraperitoneally with D-luciferin (100μl at 40mg/ml in PBS) before imaging. Mice were anesthetized and placed in a bioluminescent imaging system for data acquisition. Whole animal imaging was performed to monitor tumor growth and any metastases that occurred. Signal intensities were quantified as the sum of all detected photons in region of interests (ROIs) using Living Image software 2.5 (Caliper Life Sciences, Hopkington, MA). The measured bioluminescence was expressed as photons/sec/cm2. The imaging was performed every 1min until the peak signal intensity was reached (approximately 10 min). The IVIS 100 was adjusted to 1 min exposure, medium binning and F1 stop. The mice were imaged at level D with a distance of 22 cm from the camera.
Faxitron Imaging
High-resolution radiographic images (DICOM format) of mice were obtained using a Faxitron laboratory radiography system LX-60 (Faxitron X-ray Corp., Wheeling, IL). The right leg of each mouse was imaged at 28 KeV for 5 sec on day 14 to qualitatively evaluate the intratibial tumor induced after injection of Ace-1 cells.
Histopathological Studies
The animals were sacrificed at day 21 (SQ experiment) and day 14 (IT experiment). Bone and tissue samples were collected. Tissue specimens were fixed in 10% neutral-buffered formalin at 4°C for 48 hr, embedded in paraffin, cut in 4μm sections, and stained with hematoxylin and eosin (H&E). In addition, bone specimens were decalcified in Mild Decalcifier (formaldehyde, methanol, and formic acid) (Leica Biosystems, Buffalo Grove, IL) at 37°C for 4 hr. Images were acquired using an Olympus BX51 microscope equipped with a Nikon digital camera and quantification of necrotic area in SQ and intratibial tumors was performed using Image J2 (Fiji, Madison, WI) and Photoshop CS6 software (Adobe).
Statistical Analysis
Data were analyzed using Graph Pad Prism 6.0 software (San Diego, CA). All in vitro samples were repeated in triplicate or quadruplicate with at least two independent experiments. qRT-PCR values were normalized to GAPDH mRNA and were expressed as the fold difference between the treatment group and controls (mean ± SD). A P-value <0.05 was considered to be statistically significant. For the in vivo study, the average bioluminescence (photons/sec/cm2), tumor volume, tumor weight, and corresponding standard errors of the mean were determined for each experiment. Data were analyzed using Student’s t-test to compare the results between groups. Dose and time-dependent effects were analyzed using one-way analysis of variance.
RESULTS
Cell Culture Characteristics
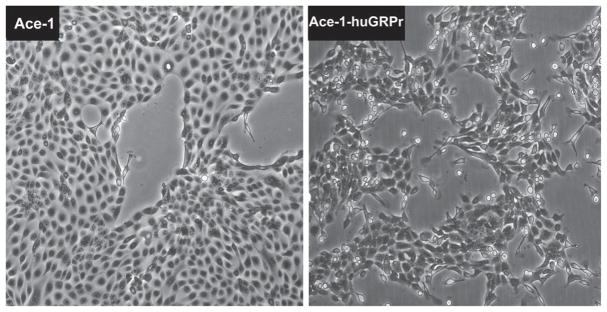
The parent Ace-1 cells had a typical epithelial phenotype in vitro with formation of a cobblestone pattern of adherent polygonal cells (Fig. 1). In contrast, the Ace-1-huGRPr cells had a spindle-shaped morphology and were dissociated from each other (Fig. 1). The cells branched from clusters of cells and the elongated cells migrated out radially.
Fig. 1.
Phase contrast microscopy of Ace-1 and Ace-1-huGRPr cells showing the difference in morphology. The parent Ace-1 cells had a typical epithelial phenotype with formation of a cobblestone pattern of adherent polygonal cells. In contrast, the Ace-1-huGRPr cells had a spindle-shaped morphology and were often dissociated from one another.
Cell Proliferation
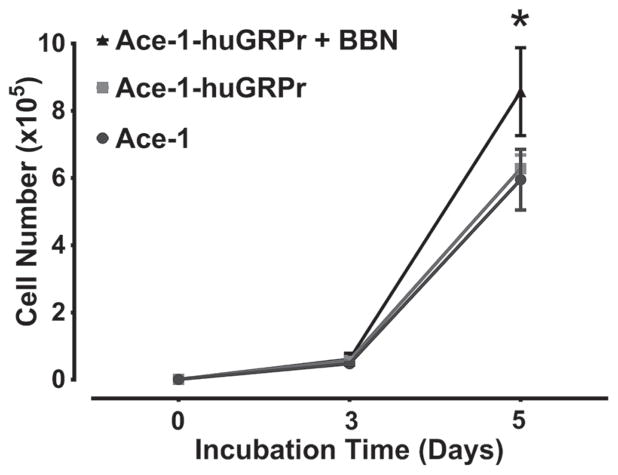
Stimulation of GRPr signaling with bombesin (BBN) increased the growth of Ace-1-huGRPr cells in vitro. After 3 days of incubation, there was no significant difference in the cell number between Ace-1 and Ace-1-huGRPr cells. On day 5, BBN increased the cell number of Ace-1-huGRPr cells compared with treated or untreated Ace-1 and un-treated Ace-1-huGRPr cells. The number of viable cells was 8.6 ± 1.3 × 105 in BBN-treated Ace-1-huGRPr cells, which was significantly greater than control Ace-1-huGRPr cells (6.3 ± 0.4 × 105) or Ace-1 cells (5.9 ± 0.9 × 105) (Fig. 2).
Fig. 2.
In vitro growth curves of Ace-1 and Ace-1-huGRPr cell lines after addition of bombesin (BBN), which served as a GRPr agonist. Data presented as mean ± SD of three replicates for each cell line. Significant differences are indicated as: *P≤0.05 statistically different from control Ace-1 and Ace-1-huGRPr cells.
Wound Healing Assay
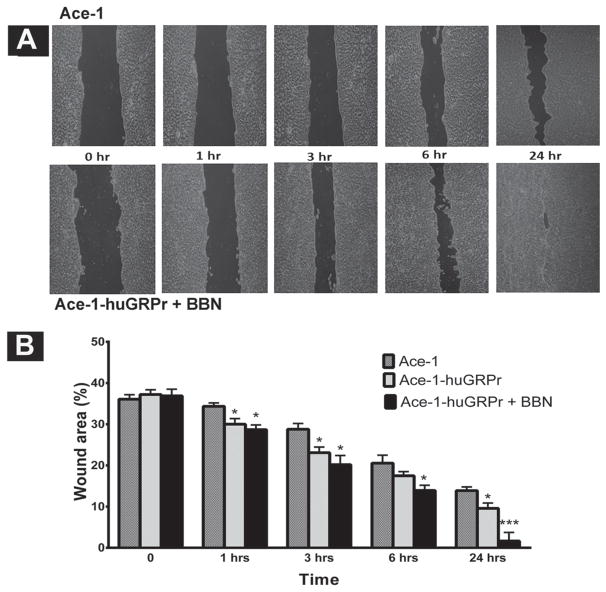
GRPr signaling enhanced cell migration and wound closure of the Ace-1-huGRPr cells. At time 0, the wound area was approximately 37% of the imaged field in all cell lines. After 1 hr, both the treated and untreated Ace-1-huGRPr cells had a statistically significant increase in migration and wound closure (28 ± 1.0% and 30 ± 2.0%, respectively) when compared with the Ace-1 cells (34 ± 1.0%). After 24 hr, the treated Ace-1-huGRPr cells had a marked decrease (P<0.05) in the wound area (2.0 ± 2.0%) when compared with both untreated Ace-1-huGRPr cells (10 ± 1.0%) and Ace-1 cells (13.4 ± 0.5%) (Fig. 3A and B).
Fig. 3.
(A) Representative images of the in vitro wound healing assay that demonstrate the enhancement of migration of Ace-1-huGRPr cells after bombesin (BBN) treatment for 0, 1, 3, 6, and 24 hr compared to Ace-1 cells. (B) The percent wound areas are shown in the bar diagram as mean ± SD from three independent experiments. Significant differences are indicated as: *P≤0.05 and ***P<0.001 different from control Ace-1 cells.
qRT-PCR
GRPr mRNA Expression in Tissues and Different Clones of Ace-1-huGRPr Cells
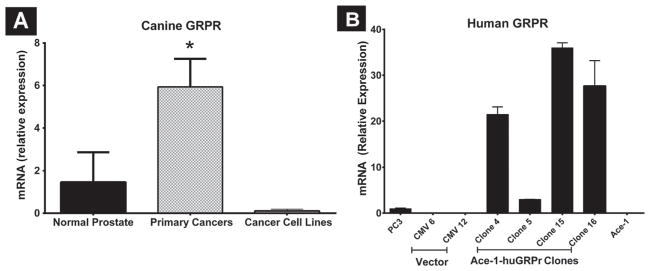
GRPr mRNA expression was significantly greater (4.2-fold) in primary canine prostate cancers when compared with normal prostate, which expressed very low levels of GRPr mRNA. GRPr mRNA expression was decreased in the corresponding cell lines of these primary cancers (Fig. 4A). Ace-1 cells had the lowest canine GRPr mRNA expression. After transfection of Ace-1 cells with human GRPr, clone 15 had the highest GRPr mRNA expression when compared with different clones, parental Ace-1 cells, and human PC-3 cells (Fig. 4B).
Fig. 4.
(A) Expression of canine GRPr mRNA in dog primary prostate cancers (PCa, n = 5), cell lines (n = 4), and normal prostate tissues (n = 3) measured by qRT-PCR. (B) Human GRPr mRNA expression in different clones of Ace-1-huGRPr cells, vector transfected Ace-1 cells, parent Ace-1 and PC-3 cells. The bars represent the relative mRNA expression ± SD. Significant difference is indicated as *P≤0.05.
Bombesin (BBN) Time Course Experiment
Epithelial–mesenchymal transition (EMT) genes
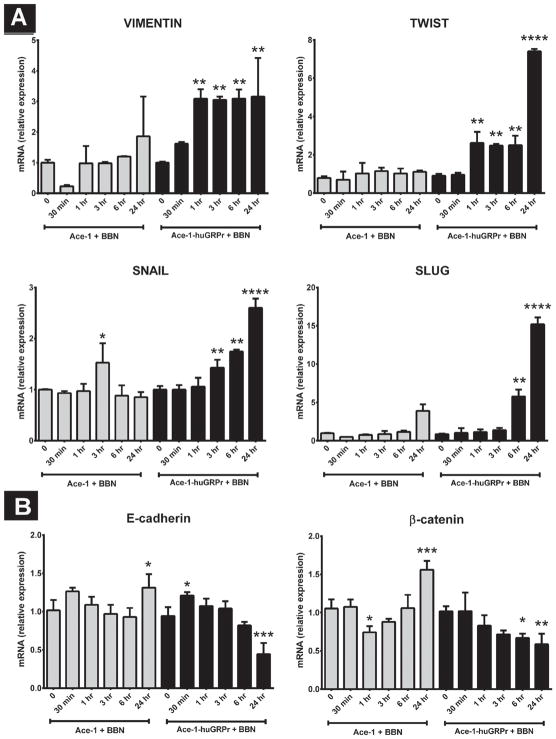
Because Ace-1-huGRPr cells displayed morphological differences (transition to spindle-shaped cells) compared to the parental Ace-1 cells, expression profiling of EMT markers was conducted to investigate the role of GRPr signaling. Ace-1-huGRPr cells expressed higher levels of mesenchymal markers (VIMENTIN, SLUG, TWIST, and SNAIL) and lower levels of epithelial markers (E-cadherin and β-catenin) after treatment with BBN (Fig. 5A and B). In most cases, there was no change in expression of VIMENTIN, SLUG, TWIST, E-cadherin, or β-catenin in the parent Ace-1 cells induced by BBN, except there was mildly increased SNAIL at 3 hr, increased E-cadherin and β-catenin at 24 hr, and decreased β-catenin at 1 hr.
Fig. 5.
The expression of (A) mesenchymal markers (VIMENTIN, SLUG, SNAIL, and TWIST) and (B) epithelial markers (E-cadherin and β-catenin) after treatment with bombesin (BBN). The graphs represent the relative mRNA expression in Ace-1 and Ace-1-huGRPr cells before and after addition of 1nM BBN for different periods of time (0, 30 min, 1, 3, 6, and 24 hr). Significant differences are indicated as: *P≤0.05, **P≤0.01, ***P≤0.001, and ****P≤0.0001 different from control Ace-1 or Ace-1-huGRPr cells.
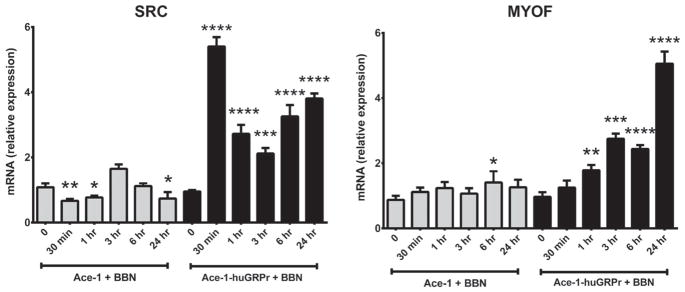
SRC and MYOF genes
Bombesin (BBN) markedly increased the expression of SRC and MYOF mRNA in Ace-1-huGRPr cells (Fig. 6). BBN treatment increased SRC mRNA in Ace-1-huGRPr cells to maximum (5.4- fold) at 30 min, which decreased, but remained increased from 1 to 24 hr. In Ace-1 cells, BBN mildly increased SRC mRNA at 3 hr with mild decreases at 30 min, 1 hr, and 24 hr. BBN increased MYOF mRNA in Ace-1-huGRPr cells progressively from 1 to 24 hr, and there was minimal effect of BBN on the Ace-1 cells.
Fig. 6.
The expression of SRC and MYOF in both Ace-1 and Ace-1-huGRPr cells before and after addition of 1 nM bombesin (BBN) for different time periods (0, 30 min, 1, 3, 6, and 24 hr). Significant differences are indicated as: *P≤0.05, **P≤0.01, ***P≤0.001, and ****P≤0.0001 different from control Ace-1 or Ace-1-huGRPr cells.
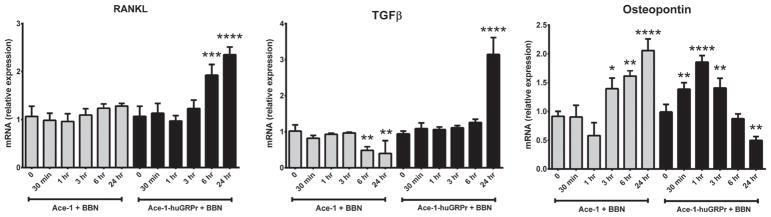
RANKL, TGFβ, and Osteopontin
BBN increased RANKL mRNA in Ace-1-huGRPr cells (2.5-fold) at 6 and 24 hr, and there was no effect in Ace-1 cells. BBN increased TGFβ mRNA in Ace-1-huGRPr cells (3.1-fold) at 24 hr, and decreased TGFβ mRNA in Ace-1 cells at 6 and 24 hr. BBN initially increased Osteopontin mRNA in Ace-1-huGRPr cells (1.3–1.9-fold) at 30 and 60 min, which then decreased from 3 to 24 hr. In contrast, BBN increased Osteopontin mRNA in Ace-1 cells (1.3–2.1-fold) from 3 to 24 hr (Fig. 7).
Fig. 7.
The expression of RANKL, TGFβ, and Osteopontin in both Ace-1 and Ace-1-huGRPr cells before and after addition of 1 nM bombesin (BBN) for different time periods (0, 30 min, 1, 3, 6, and 24 hr). Significant differences are indicated as: *P≤0.05, **P≤0.01, ***P≤0.001, and ****P≤0.0001 different from control Ace-1 or Ace-1-huGRPr cells.
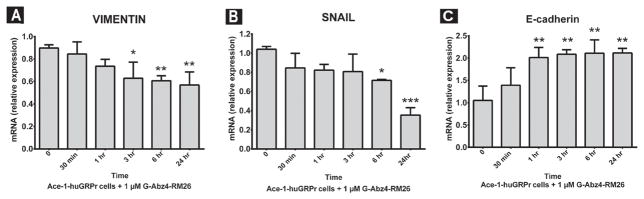
GRPr Antagonist (BBN Analog, G-Abz4-STAT)
Blockade of the GRPr with G-Abz4-STAT induced a MET (mesenchymal to epithelial transition) phenotype by downregulation of SNAIL and VIMENTIN mRNA and upregulation of E-cadherin mRNA in Ace- 1-huGRPr cells (Fig. 8). The BBN antagonist decreased the expression of VIMENTIN mRNA after 3 hr and there was downregulation in SNAIL mRNA expression after 6 hr with a more dramatic effect after 24 hr. E-cadherin (epithelial marker) was upregulated after 1 hr of antagonist treatment and remained high until 24 hr.
Fig. 8.
The expression of VIMENTIN, SNAIL, and E-cadherin after treatment with a GRPr antagonist (G-Abz4-STAT). The graphs represent the relative mRNA expression of VIMENTIN (A), SNAIL (B), and E-cadherin (C) in Ace-1-huGRPr cells after addition of 1 nM antagonist for different time periods (0, 30 min, 1, 3, 6, and 24 hr). Significant differences are indicated as: *P≤0.05, **P≤0.01, and ***P<0.001 different from control Ace-1-huGRPr cells.
Tumor Growth In Vivo
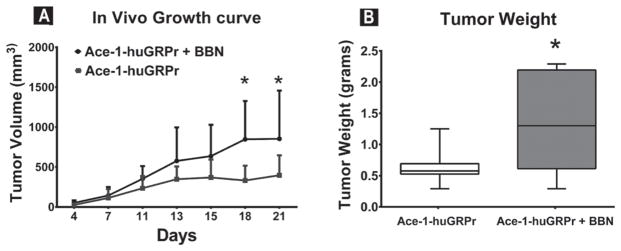
Bombesin (BBN) increased the growth rate of subcutaneous Ace-1-huGRPr tumors. There was a significant increase in the tumor volume in BBN-treated mice at 3 weeks compared to control mice (Fig. 9A). In addition, the tumor weights were 1.1 ± 0.7 g in BBN-treated mice compared to 0.6 ± 0.3 g in the control group (Fig. 9B). These results demonstrated that GRPr signaling accelerated the growth of prostate cancer cells in vivo.
Fig. 9.
In vivo growth of Ace-1-huGRPr subcutaneous tumors in bombesin (BBN)-treated and control mice. (A) Plot represents the tumor volume of subcutaneous xenografts at different time points (4, 7, 11, 13, 15, 18, and 21 day). (B) Box and whisker represents the tumor weights in the two groups at the end of the study. Significant difference is indicated as *P≤0.05.
Bioluminescence
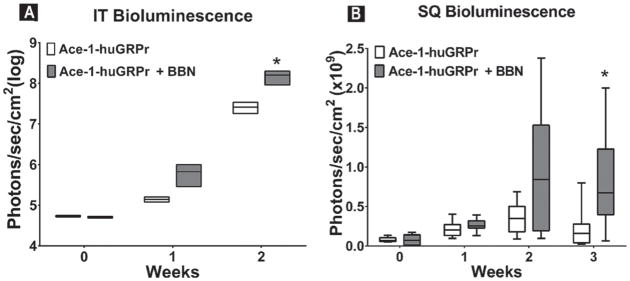
In the intratibial experiment, there was a statistical increase in bioluminescence in BBN-treated mice at day 14 when compared with control mice (Fig. 10A). Bombesin (BBN) also increased the growth rate of viable subcutaneous Ace-1-huGRPr cells in vivo (Figs. 10B and 11A). There was no difference in bioluminescence in BBN-treated and control mice groups at day 7; however, there was an upward trend at day 14. There was a significant increase in tumor bioluminescence in BBN-treated mice at day 21.
Fig. 10.
Box and whisker plots of bioluminescence (photons/sec/cm2) measured from (A) intratibial (IT) and (B) subcutaneous (SQ) Ace-1-huGRPr xenografts in nude mice at different time points. Significant differences are indicated as *P≤ 0.05.
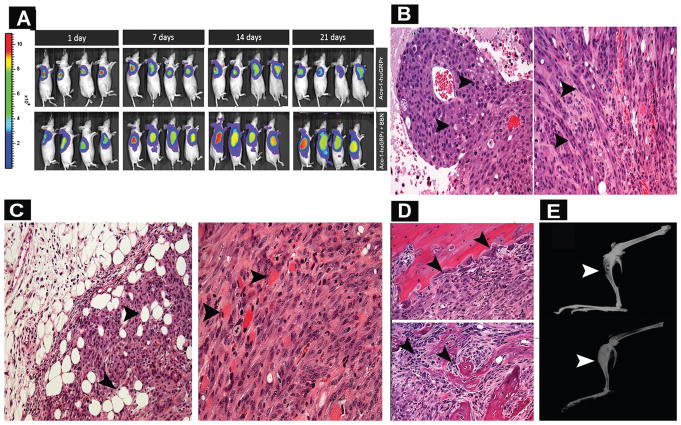
Fig. 11.
(A) Bioluminescence images of representative mice in bombesin (BBN)-treated and control mice with subcutaneous Ace-1- huGRPr tumors at different time points (1, 7, 14, and 21 day). (B) Representative histopathology of Ace-1-huGRPr tumors from bombesin (BBN)-treated and control mice. Neoplastic cells were epithelioid and polyhedral in control mice (left panel; arrowheads) and spindle-shaped in the bombesin-treated mice (right panel, arrowheads). (C) Histopathology of subcutaneous tumors in bombesin-treated mice showing infiltration of neoplastic cells into adipose (left) and muscle (right) tissues (arrowheads). (D) Histopathology of tibias from bombesin-treated and control mice that were injected with Ace-1-huGRPr cells. Note intramedullary tumors with osteoclastic bone resorption in a bombesin-treated mouse (upper panel, arrowheads) and osteoblastic new bone formation in a control mouse (lower panel, arrowheads). (E) Radiographic images of intratibial Ace-1-huGRPr tumors from a bombesin-treated mouse (upper panel) and a control mouse (lower panel) revealing radiolucent osteolytic foci in the tibia of BBN-treated mouse (arrowhead) and a radiodense osteoblastic tumor in the tibia of a control mouse (arrowhead).
Histopathology of Subcutaneous and Intratibial Tumors
The subcutaneous Ace-1-huGRPr tumors in the control mice were characterized by sheets of polyhedral neoplastic cells with areas of coagulation necrosis (24 ± 16%). In contrast, BBN altered the morphology of the Ace-1-huGRPr tumor cells, which were characterized by spindle-shaped cells typical of EMT. The spindle-shaped tumor cells infiltrated into adjacent muscle and adipose tissue. In addition, the subcutaneous tumors in BBN-treated mice had less necrosis (19 ± 6.3%) compared to the control mice (Fig. 11B and C), but this was not statistically significant.
The intratibial tumors in control mice were characterized by tumor growth in the medullary cavity with abundant new woven bone proliferation that is typical of osteoblastic metastases. The woven bone was lined by hypertrophied osteoblasts (Fig. 11D). In contrast, the intratibial tumors in BBN-treated mice were larger (two-fold) in the medullary cavity, had a lack of new woven bone, and had increased osteoclastic bone resorption (Fig. 11D). Therefore, BBN treatment altered the phenotype of the bone tumors from an osteoblastic to an osteolytic pattern.
Faxitron Radiography
The intratibial Ace-1-huGRPr tumors induced osteoblastic metastases with increased radiodensity due to new bone formation in the proximal medullary cavity (Fig. 11E). In contrast, BBN-treated mice had reduced radiodensity of the medullary tumors and increased bone lysis as demonstrated by foci of radiolucency (Fig. 11E).
DISCUSSION
It has been reported that GRPr is upregulated in human prostate intraepithelial neoplasia (PIN) and intraprostatic invasive carcinomas [7,27,28], as well as lymph node and bone metastases [29]. However, the role of the GRPr and its signaling in different stages of prostate cancer and metastasis is unknown. In this study, we used a well described model of canine prostate cancer (Ace-1) that mimics prostate cancer in men (including mixed osteoblastic/osteolytic bone metastases) to investigate the role of the human GRPr on prostate cancer growth and progression [21]. Ace-1 cells are tumorigenic in immunocompromised mice and rats and develop mixed osteoblastic/osteolytic bone metastases after intracardiac injection into the left ventricle [30]. The Ace-1 cells are a valuable model of bone metastasis, including studies on pain associated with bone metastases [31,32]. Although, Ace-1 cells have a low level of canine GRPr mRNA and receptor expression on their cell surface compared to the primary cancer from the dog, they were readily stably transfected with the functional human GRPr. Measurement of GRPr expression in canine primary prostate cancers has shown that GRPrs are upregulated in those tumors when compared to normal prostate gland, which suggests that GRPr signaling is also important in progression of canine prostate cancers as in men with prostate cancer [4–8]. Therefore, we used a clone of Ace-1 cells that was developed to express a high level of functional human GRPr on their cell surface to determine the actions of a heterologous GRPr agonist (bombesin) on prostate cancer in vitro and in vivo.
Based on previous studies, EMT plays an important role in the invasion and metastasis of prostate cancer in men [33–38]. However, the mechanistic link between GRPr activation and EMT gene expression has not been investigated. It is known that invasive prostate carcinomas often lose their epithelial phenotypes (cell polarity and cell-cell adhesion) and gain mesenchymal phenotypes (invasive and migratory properties) that enable them to metastasize to different organs [39–42]. We determined the effect of GRPr signaling on EMT during prostate cancer growth and progression. Interestingly, our study showed that GRPr signaling is important in the promotion of EMT in prostate cancer cells evidenced by upregulation of mesenchymal markers and downregulation of epithelial markers. To our knowledge, this study is the first one to delineate the importance of GRPr signaling in the enhancement of prostate cancer progression through the promotion of EMT.
In order to confirm the importance of GRPr signaling in EMT, a GRPr antagonist was used to block this signaling pathway and the EMT was reversed to a MET process with upregulation of epithelial markers (E-cadherin) and downregulation of mesenchymal markers (VIMENTIN and SNAIL). This change could reduce cancer cell movement and invasion. Similarly, silencing of GRPr signaling led to a marked reduction in the malignant phenotype of the human VCaP and LNCaP prostate cancer cell lines by decreasing cell proliferation, invasion, and the capacity to grow in the absence of cell–cell attachment [43].
GRP and bombesin have been shown to have mitogenic activity and increase proliferation and growth of a variety of cancers including lung [44], prostate [45,46], breast, gastric [47,48], pancreatic [49], colorectal cancers [50], neuroblastoma [51], and glioma [52,53]. Consistent with these data, our studies demonstrated the stimulation of proliferation of Ace- 1-huGRPr cells by bombesin in vitro and increased tumor volume and weight in bombesin-treated mice.
It is well established that SRC is an important cellular factor that promotes proliferation, survival, and migration of tumor cells through transcriptional activation of multiple genes [54–56]. Interestingly, bombesin significantly increased the SRC mRNA expression in Ace-1-huGRPr cells. This may partially explain why the subcutaneous tumors in bombesin-treated mice were larger and heavier than control mice. In addition, tumor bioluminescence (viable cells) was greater in bombesin-treated mice. These findings suggest that upregulation of SRC may be an important downstream effector of GRPr signaling in prostate cancer as demonstrated in other cancers of lung and head and neck [57–59].
We also observed more rapid closure of in vitro wounds after activation of GRPr signaling in Ace-1- huGRPr cells, which indicates that GRPr signaling may have a role in the migration of prostate cancer cells. In some cancers of the colon, breast and brain, bombesin stimulated tumor cell migration [60–62], which is consistent with our findings. We found that GRPr signaling upregulated the expression of SRC, MYOF, and TWIST in prostate cancer cells. Previous studies have shown that these genes are important for inducing migration of various cancer cells [63–65].
Although the role of the GRPr signaling pathway is not completely understood in bone metastasis, it has been shown that bombesin antagonists decreased bone metastasic growth and invasiveness of PC3 prostate cancer cells in nude mice [66]. Radiographic and histopathologic evaluation of tibias with bombesin-treated Ace-1-huGRPr cells showed that GRPr signaling significantly decreased new bone formation induced by the Ace-1 cells as demonstrated by a marked decrease in new woven bone area and an increase in osteoclast numbers. Prior studies have confirmed the importance of RANKL and TGFβ (produced from primary tumors) in the establishment and progression of cancer cells in addition to their role in the induction of bone lysis. RANKL expressed by tumor cells can promote osteoclastogenesis directly without a need for osteoblasts or stromal cells [67]. In this study, a significant increase in the expression of RANKL and TGFβ mRNA expression after bombesin treatment in Ace-1-huGRPr cells was seen. This suggests that GRPr signaling may have a role in the induction of osteoclastic bone resorption at sites of bone metastasis. It is known that osteopontin is important in regulating the normal mineralization of bone extracellular matrix [68]. Interestingly, we observed a lower expression of Osteopontin mRNA in bombesin-treated Ace-1-huGRPr cells compared to controls, which may contribute to the reduction in new bone formation seen in the intratibial tumors.
Overexpression of bombesin by cancer cells correlated with an aggressive histological pattern of colorectal cancer and liver metastasis [69]. Prostate cancer cells that expressed bombesin and MMP-9 were more invasive and had increased metastatic potential [70]. Our study showed that Ace-1- huGRPr cells were smaller and more spindleshaped after treatment with bombesin. This was consistent with the in vivo tumors that contained less differentiated, spindle-shaped tumors cells, which were more invasive into the surrounding adipose tissue and skeletal muscle.
CONCLUSIONS
This investigation demonstrated that GRPr signaling was important for growth, invasion, migration, and progression of prostate cancer. Further investigations will be useful to clarify the molecular mechanisms by which GRPr signaling regulates EMT and increases tumor cell migration, invasion, proliferation, and metastatic phenotype. Blockade or inhibition of GRPr signaling may be a promising strategy for the treatment of the early stages of prostate cancer.
Acknowledgments
Grant sponsor: National Center for Advancing Translational Sciences; Grant number: UL1TR001070; Grant sponsor: National Cancer Institute; Grant number: P30CA016058; Grant sponsor: Ministry of Higher Education and Scientific Research, Egypt; Grant sponsor: The Ohio State University College of Veterinary Medicine Canine Research Funds.
We thank Dr. Holly Borghese for supplying us with the frozen canine primary prostate cancers and Lucas Altstadt for designing RT-PCR primers. The Ohio State College of Veterinary Medicine Biospecimen Repository was supported by the following grants: UL1TR001070 from the National Center for Advancing Translational Sciences and P30CA016058 from the National Cancer Institute to The Ohio State University. The research was supported by the Ministry of Higher Education and Scientific Research, Egypt and The Ohio State University College of Veterinary Medicine Canine Research Funds.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63(3):151–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 3.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2 Pt 1):379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 4.Giacchetti S, Gauville C, de Cremoux P, Bertin L, Berthon P, Abita JP, Cuttitta F, Calvo F. Characterization in some human breast cancer cell lines, of gastrin-releasing peptide-like receptors which are absent in normal breast epithelial cells. Int J Cancer. 1990;46(2):293–298. doi: 10.1002/ijc.2910460226. [DOI] [PubMed] [Google Scholar]
- 5.Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14) Clin Cancer Res. 2002;8(4):1139–1146. [PubMed] [Google Scholar]
- 6.Toi-Scott M, Jones CL, Kane MA. Clinical correlates of bombesin- like peptide receptor subtype expression in human lung cancer cells. Lung Cancer. 1996;15(3):341–354. doi: 10.1016/0169-5002(95)00597-8. [DOI] [PubMed] [Google Scholar]
- 7.Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide and cancer. Biochim Biophys Acta. 2006;1766(1):23–41. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Jensen RT, Moody TW. Bombesin peptides. In: Kastin AJ, editor. Handbook of biologically active peptides. 2. Chapter 69. San Diego: Academic Press; 2013. pp. 506–511. [Google Scholar]
- 9.Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Autocrine growth factors in human small cell lung cancer. Cancer Surv. 1985;4(4):707–727. [PubMed] [Google Scholar]
- 10.Lango MN, Dyer KF, Lui VW, Gooding WE, Gubish C, Siegfried JM, Grandis JR. Gastrin-releasing peptide receptor-mediated autocrine growth in squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 2002;94(5):375–383. doi: 10.1093/jnci/94.5.375. [DOI] [PubMed] [Google Scholar]
- 11.Plonowski A, Nagy A, Schally AV, Sun B, Groot K, Halmos G. In vivo inhibition of PC-3 human androgen-independent prostate cancer by a targeted cytotoxic bombesin analogue, AN-215. Int J Cancer. 2000;88(4):652–657. doi: 10.1002/1097-0215(20001115)88:4<652::aid-ijc21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Sharif TR, Luo W, Sharif M. Functional expression of bombesin receptor in most adult and pediatric human glioblastoma cell lines; role in mitogenesis and in stimulating the mitogen-activated protein kinase pathway. Mol Cell Endocrinol. 1997;130(1–2):119–130. doi: 10.1016/s0303-7207(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang QJ, Knezetic JA, Schally AV, Pour PM, Adrian TE. Bombesin may stimulate proliferation of human pancreatic cancer cells through an autocrine pathway. Int J Cancer. 1996;68(4):528–534. doi: 10.1002/(SICI)1097-0215(19961115)68:4<528::AID-IJC20>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira-Freitas VL, Thomaz LD, Simoneti LE, Malfitano C, De Angelis K, Ulbrich JM, Schwartsmann G, Andrade CF. RC- 3095, a selective gastrin-releasing peptide receptor antagonist, does not protect the lungs in an experimental model of lung ischemia-reperfusion injury. Biomed Res Int. 2015;2015:496378. doi: 10.1155/2015/496378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen RT, Moody T, Pert C, Rivier JE, Gardner JD. Interaction of bombesin and litorin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci USA. 1978;75(12):6139–6143. doi: 10.1073/pnas.75.12.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsuno T, Pradhan TK, Ryan RR, Mantey SA, Hou W, Donohue PJ, Akeson MA, Spindel ER, Battey JF, Coy DH, Jensen RT. Pharmacology and cell biology of the bombesin receptor subtype 4 (BB4-R) Biochemistry. 1999;38(22):7307– 7320. doi: 10.1021/bi990204w. [DOI] [PubMed] [Google Scholar]
- 17.Preston SR, Miller GV, Primrose JN. Bombesin-like peptides and cancer. Crit Rev Oncol Hematol. 1996;23(3):225–238. doi: 10.1016/1040-8428(96)00204-1. [DOI] [PubMed] [Google Scholar]
- 18.Aprikian AG, Han K, Guy L, Landry F, Begin LR, Chevalier S. Neuroendocrine differentiation and the bombesin/gastrin-releasing peptide family of neuropeptides in the progression of human prostate cancer. Prostate Suppl. 1998;8:52–61. [PubMed] [Google Scholar]
- 19.Lee LF, Guan J, Qiu Y, Kung HJ. Neuropeptide-induced androgen independence in prostate cancer cells: Roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol. 2001;21(24):8385–8397. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine L, Lucci JA, Pazdrak B, 3rd, Cheng JZ, Guo YS, Townsend CM, Jr, Hellmich MR. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 2003;63(13):3495– 3502. [PubMed] [Google Scholar]
- 21.LeRoy BE, Thudi NK, Nadella MV, Toribio RE, Tannehill-Gregg SH, van Bokhoven A, Davis D, Corn S, Rosol TJ. New bone formation and osteolysis by a metastatic, highly invasive canine prostate carcinoma xenograft. Prostate. 2006;66(11):1213–1222. doi: 10.1002/pros.20408. [DOI] [PubMed] [Google Scholar]
- 22.Liao J, Li X, Koh AJ, Berry JE, Thudi N, Rosol TJ, Pienta KJ, McCauley LK. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Cancer. 2008;123(10):2267–2278. doi: 10.1002/ijc.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Liao J, Park SI, Koh AJ, Sadler WD, Pienta KJ, Rosol TJ, McCauley LK. Drugs which inhibit osteoclast function suppress tumor growth through calcium reduction in bone. Bone. 2011;48(6):1354–1361. doi: 10.1016/j.bone.2011.03.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise-Milestone L, Akens MK, Rosol TJ, Hojjat SP, Grynpas MD, Whyne CM. Evaluating the effects of mixed osteolytic/osteoblastic metastasis on vertebral bone quality in a new rat model. J Orthop Res. 2012;30(5):817–823. doi: 10.1002/jor.21577. [DOI] [PubMed] [Google Scholar]
- 25.Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL, Keller ET, McCauley LK, Pinzone JJ, Rosol TJ. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate. 2011;71(6):615–625. doi: 10.1002/pros.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999;59(5):1152–1159. [PubMed] [Google Scholar]
- 28.Korner M, Waser B, Rehmann R, Reubi JC. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate. 2014;74(2):217–224. doi: 10.1002/pros.22743. [DOI] [PubMed] [Google Scholar]
- 29.Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69(10):1101–1108. doi: 10.1002/pros.20957. [DOI] [PubMed] [Google Scholar]
- 30.Simmons JK, Elshafae SM, Keller ET, McCauley LK, Rosol TJ. Review of animal models of prostate cancer bone metastasis. Vet Sci. 2014;(1):16–39. [Google Scholar]
- 31.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65(20):9426–9435. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 32.Halvorson KG, Sevcik MA, Ghilardi JR, Rosol TJ, Mantyh PW. Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin J Pain. 2006;22(7):587–600. doi: 10.1097/01.ajp.0000210902.67849.e6. [DOI] [PubMed] [Google Scholar]
- 33.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98(18):10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: Migrating cancer stem cells—An integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744– 749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 35.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: A coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14(1):29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 36.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 37.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 38.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26(49):6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 40.Hayward SW, Cunha GR. The prostate: Development and physiology. Radiol Clin North Am. 2000;38(1):1–14. doi: 10.1016/s0033-8389(05)70146-9. [DOI] [PubMed] [Google Scholar]
- 41.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253(2):165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 42.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76(6):641– 659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos J, Mesquita D, Barros-Silva JD, Jeronimo C, Henrique R, Morais A, Paulo P, Teixeira MR. Uncovering potential down-stream targets of oncogenic GRPR overexpression in prostate carcinomas harboring ETS rearrangements. Oncoscience. 2015;2(5):497–507. doi: 10.18632/oncoscience.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber S, Zuckerman JE, Bostwick DG, Bensch KG, Sikic BI, Raffin TA. Gastrin releasing peptide is a selective mitogen for small cell lung carcinoma in vitro. J Clin Invest. 1985;75(1):306– 309. doi: 10.1172/JCI111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoosein NM, Logothetis CJ, Chung LW. Differential effects of peptide hormones bombesin, vasoactive intestinal polypeptide and somatostatin analog RC-160 on the invasive capacity of human prostatic carcinoma cells. J Urol. 1993;149(5):1209–1213. doi: 10.1016/s0022-5347(17)36349-8. [DOI] [PubMed] [Google Scholar]
- 46.Nagakawa O, Ogasawara M, Murata J, Fuse H, Saiki I. Effect of prostatic neuropeptides on migration of prostate cancer cell lines. Int J Urol. 2001;8(2):65–70. doi: 10.1046/j.1442-2042.2001.00250.x. [DOI] [PubMed] [Google Scholar]
- 47.Bold RJ, Kim HJ, Ishizuka J, Townsend CM, Jr, Thompson JC. A human gastric cancer cell line possesses a functional receptor for gastrin-releasing peptide. Cancer Invest. 1998;16(1):12–17. doi: 10.3109/07357909809039748. [DOI] [PubMed] [Google Scholar]
- 48.Bold RJ, Lowry PS, Ishizuka J, Battey JF, Townsend CM, Jr, Thompson JC. Bombesin stimulates the in vitro growth of a human gastric cancer cell line. J Cell Physiol. 1994;161(3):519– 525. doi: 10.1002/jcp.1041610315. [DOI] [PubMed] [Google Scholar]
- 49.Avis I, Jett M, Kasprzyk PG, Cuttitta F, Treston AM, Maneckjee R, Mulshine JL. Effect of gastrin-releasing peptide on the pancreatic tumor cell line (Capan) Mol Carcinog. 1993;8(4):214– 220. doi: 10.1002/mc.2940080403. [DOI] [PubMed] [Google Scholar]
- 50.Iishi H, Tatsuta M, Baba M, Yamamoto R, Taniguchi H. Enhancement by bombesin of colon carcinogenesis and metastasis induced by azoxymethane in Wistar rats. Int J Cancer. 1992;50(5):834–839. doi: 10.1002/ijc.2910500529. [DOI] [PubMed] [Google Scholar]
- 51.Ishola TA, Kang J, Qiao J, Evers BM, Chung DH. Phosphatidylinositol 3-kinase regulation of gastrin-releasing peptide-induced cell cycle progression in neuroblastoma cells. Biochim Biophys Acta. 2007;1770(6):927–932. doi: 10.1016/j.bbagen.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores DG, de Farias CB, Leites J, de Oliveira MS, Lima RC, Tamajusuku AS, Di Leone LP, Meurer L, Brunetto AL, Schwartsmann G, Lenz G, Roesler R. Gastrin-releasing peptide receptors regulate proliferation of C6 Glioma cells through a phosphatidylinositol 3-kinase-dependent mechanism. Curr Neurovasc Res. 2008;5(2):99–105. doi: 10.2174/156720208784310240. [DOI] [PubMed] [Google Scholar]
- 53.de Oliveira MS, Cechim G, Braganhol E, Santos DG, Meurer L, de Castro CG, Jr, Brunetto AL, Schwartsmann G, Battastini AM, Lenz G, Roesler R. Anti-proliferative effect of the gastrin-release peptide receptor antagonist RC-3095 plus temozolomide in experimental glioblastoma models. J Neurooncol. 2009;93(2):191–201. doi: 10.1007/s11060-008-9775-2. [DOI] [PubMed] [Google Scholar]
- 54.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61(11):4315–4319. [PubMed] [Google Scholar]
- 56.Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WE, 3rd, Erdem H, Frolov A, Smith CL, Ayala GE, Ittmann MM, Weigel NL. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66(21):10594–10602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 57.Xiao D, Qu X, Weber HC. Activation of extracellular signal-regulated kinase mediates bombesin-induced mitogenic responses in prostate cancer cells. Cell Signal. 2003;15(10):945– 953. doi: 10.1016/s0898-6568(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Q, Thomas SM, Xi S, Smithgall TE, Siegfried JM, Kamens J, Gooding WE, Grandis JR. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer Res. 2004;64(17):6166–6173. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q, Thomas SM, Lui VW, Xi S, Siegfried JM, Fan H, Smithgall TE, Mills GB, Grandis JR. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci USA. 2006;103(18):6901–6906. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee S, Qiao J, Paul P, O’Connor KL, Evers MB, Chung DH. FAK is a critical regulator of neuroblastoma liver metastasis. Onco-target. 2012;3(12):1576–1587. doi: 10.18632/oncotarget.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel M, Kawano T, Suzuki N, Hamakubo T, Karginov AV, Kozasa T. Galpha13/PDZ-RhoGEF/RhoA signaling is essential for gastrin-releasing peptide receptor-mediated colon cancer cell migration. Mol Pharmacol. 2014;86(3):252–262. doi: 10.1124/mol.114.093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao C, Ives K, Hellmich HL, Townsend CM, Jr, Hellmich MR. Gastrin-releasing peptide receptor in breast cancer mediates cellular migration and interleukin-8 expression. J Surg Res. 2009;156(1):26–31. doi: 10.1016/j.jss.2009.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63(1):196–206. [PubMed] [Google Scholar]
- 64.Li R, Ackerman WEt, Mihai C, Volakis LI, Ghadiali S, Kniss DA. Myoferlin depletion in breast cancer cells promotes mesenchymal to epithelial shape change and stalls invasion. PLoS ONE. 2012;7(6):e39766. doi: 10.1371/journal.pone.0039766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pohorelic B, Singh R, Parkin S, Koro K, Yang AD, Egan C, Magliocco A. Role of Src in breast cancer cell migration and invasion in a breast cell/bone-derived cell microenvironment. Breast Cancer Res Treat. 2012;133(1):201–214. doi: 10.1007/s10549-011-1753-2. [DOI] [PubMed] [Google Scholar]
- 66.Stangelberger A, Schally AV, Varga JL, Zarandi M, Szepeshazi K, Armatis P, Halmos G. Inhibitory effect of antagonists of bombesin and growth hormone-releasing hormone on orthotopic and intraosseous growth and invasiveness of PC-3 human prostate cancer in nude mice. Clin Cancer Res. 2005;11(1):49–57. [PubMed] [Google Scholar]
- 67.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107(10):1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKee MD, Addison WN, Kaartinen MT. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cells Tissues Organs. 2005;181(3–4):176–188. doi: 10.1159/000091379. [DOI] [PubMed] [Google Scholar]
- 69.Seretis E, Gavrill A, Agnantis N, Golematis V, Voloudakis-Baltatzis IE. Comparative study of serotonin and bombesin in adenocarcinomas and neuroendocrine tumors of the colon. Ultrastruct Pathol. 2001;25(6):445–454. doi: 10.1080/019131201753343485. [DOI] [PubMed] [Google Scholar]
- 70.Ishimaru H, Kageyama Y, Hayashi T, Nemoto T, Eishi Y, Kihara K. Expression of matrix metalloproteinase-9 and bombesin/gastrin-releasing peptide in human prostate cancers and their lymph node metastases. Acta Oncol. 2002;41(3):289–296. doi: 10.1080/02841860260088845. [DOI] [PubMed] [Google Scholar]